- 1Clinical Laboratory Center, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Geriatrics Department, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
Background: Low back pain is the leading cause of years lived with disability worldwide. The aim of this study was to evaluate whether dried fruit intake causally protects against low back pain using two-sample Mendelian randomization (MR).
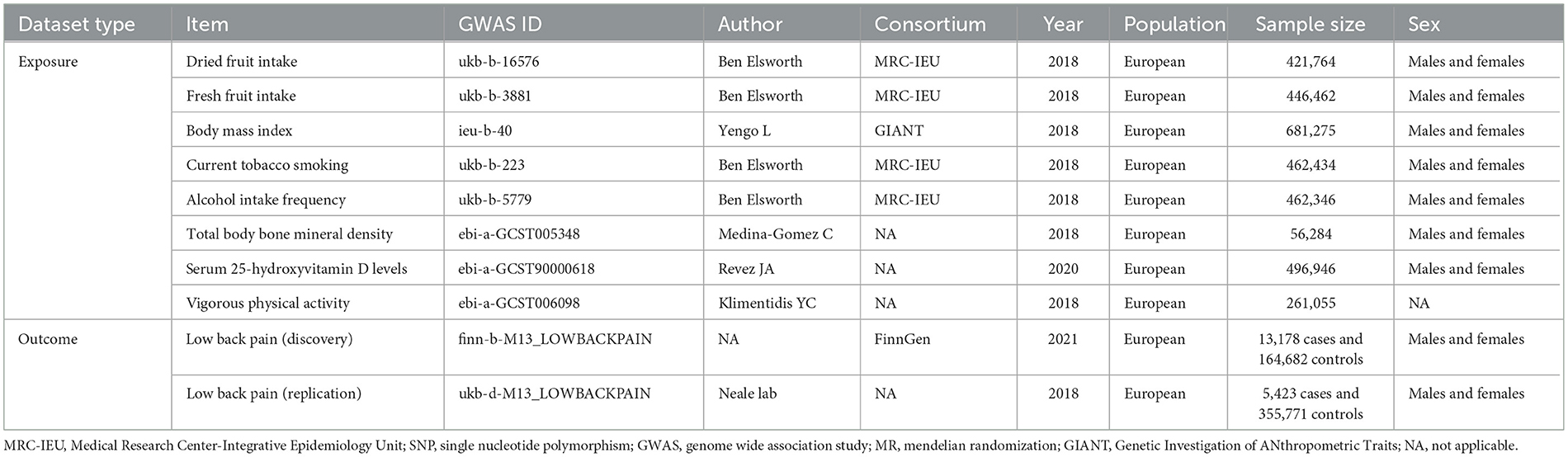
Methods: We obtained summary-level data for dried fruit intake (N = 421,764) from the IEU Open GWAS Project. Forty-one independent genetic variants proxied dried fruit intake. The corresponding data for low back pain were derived from the FinnGen project (13,178 cases and 164,682 controls; discovery data) and the Neale lab (5,423 cases and 355,771 controls; replication data). We conducted univariable and multivariable MR analyses.
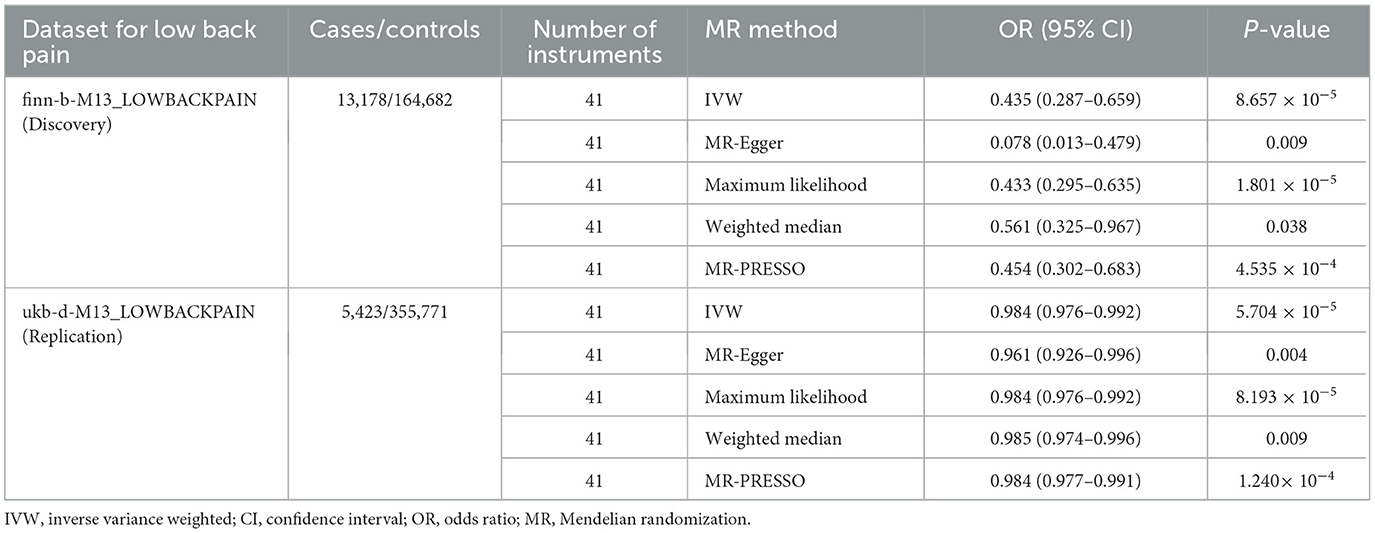
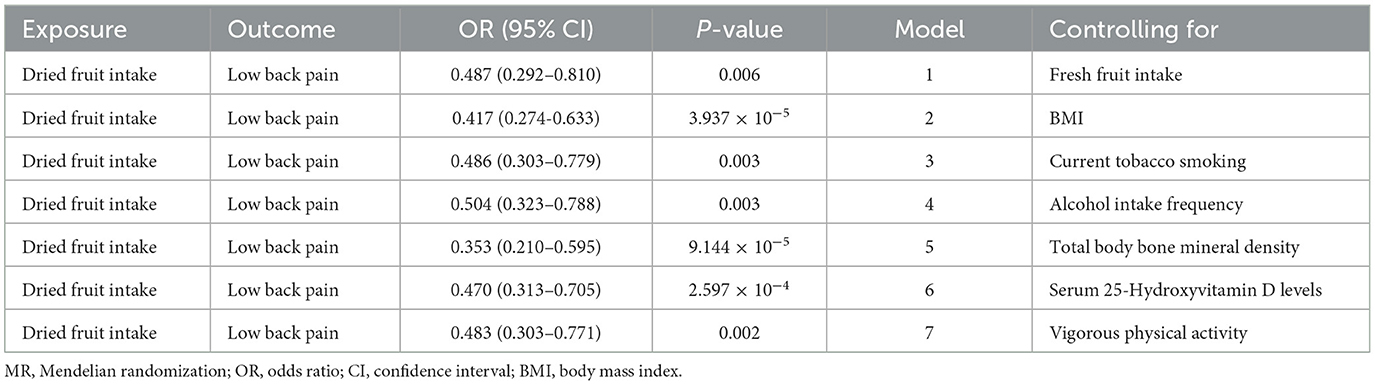
Results: In the univariable MR analysis, the inverse variance weighted estimate showed that greater dried fruit intake was associated with decreased risk of low back pain [odds ratio (OR) = 0.435, 95% confidence interval (CI): 0.287–0.659, P = 8.657 × 10−5]. Sensitivity analyses using the MR-Egger (OR = 0.078, 95% CI: 0.013–0.479, P = 0.009), maximum likelihood (OR = 0.433, 95% CI: 0.295–0.635, P = 1.801 × 10−5), weighted median (OR = 0.561, 95% CI: 0.325–0.967, P = 0.038) and Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) (OR = 0.454, 95% CI: 0.302–0.683, P = 4.535 × 10−4) methods showed consistent results. No evidence of directional pleiotropy was identified according to the Egger intercept (intercept P-value = 0.065) or applying the MR-PRESSO method (global test P-value = 0.164). The replication analysis yielded similar results. The multivariable MR revealed that the inverse association between dried fruit intake and low back pain was consistent after adjustment for fresh fruit intake, body mass index, current tobacco smoking, alcohol intake frequency, total body bone mineral density, serum 25-hydroxyvitamin D levels, and vigorous physical activity.
Conclusion: This MR study provides evidence to support that dried fruit intake causally protects against low back pain.
Introduction
Low back pain is a leading contributor to disability worldwide among all musculoskeletal disorders (1). For the majority of patients, low back pain is non-specific, because precise identification of the specific nociceptive source is not possible. If lacking proper diagnosis and therapy, acute low back pain cases are at risk for the development of chronic pain. This can lead to more frequent healthcare visits, increased financial costs, activity limitation, high rates of disability, and reduced quality of life. The pathogenesis of low back pain is complex and multifactorial. Numerous risk factors contribute to its pathogenesis, including an older age, unhealthy lifestyles, physical factors, musculoskeletal tissue structural failure, and psychological factors (1). In addition, low back pain can be associated with some diseases such as tumors and infections. Epidemiological studies show that low back pain affects about 80% of people in Western countries during their lifetime (2). In Europe, the prevalence of low back pain has ranged between 6 and 11% in the general population (3). The social and economic costs related to low back pain are enormous. It is estimated that the annual economic loss caused by low back pain is about 2.8 billion pounds in the United Kingdom and 8.9 billion euros in Spain (1, 4). Low back pain has become a significant burden for society and health systems, and this burden is still rising due to an aging population.
Dried fruits are shelf-stable forms of fresh fruits, which contain low water content. They represent a small but significant proportion of human diets in modern populations. Traditional dried fruits include prunes, pears, peaches, apples, dates, raisins, mulberries, figs, and apricots. The Middle East and North Africa region has the highest per capita dried fruit consumption (>30 kg per year) (5). In contrast, dried fruit consumption is low in Europe (5). For instance, per capita consumption of dried grapes is about 1.08 kg per year in Germany.1 Dried fruits are enriched in a variety of dietary fibers, and they are good sources of a number of micronutrients, including magnesium, potassium, iron, calcium, phosphorus, zinc, niacin, vitamin K, vitamin B6, vitamin E, and choline (6). In addition, dried fruits contain many bioactive compounds, including polyphenols, carotenoids, and flavonoids (6). Over the two past decades, experimental research and human clinical studies have reported beneficial effects of dried fruit intake on reducing inflammation, body weight, blood pressure, and glycated hemoglobin levels (7–11). In addition, dried fruits have been shown to exert anti-oxidative, anti-cancer, and anti-aging properties (5, 12–15). However, it remains unclear whether dried fruit intake has a beneficial effect on low back pain. Mendelian randomization (MR) is a statistical method using single nucleotide polymorphisms (SNPs) as instruments for inferring causality between risk factors and a disease outcome. MR can greatly reduce the risk of confounding and reverse causation, which are shortcomings of conventional epidemiological studies (16). Recently, Jin et al. (17) used inverse variance weighted MR and weighted median methods to evaluate the causality between dried fruit intake and 11 site-specific cancers, finding that dried fruit intake had protective effects against some site-specific cancers such as breast cancer and lung cancer. In the present study, we aimed to analyze the potential causal effect of genetically predicted dried fruit intake on low back pain applying the MR framework.
Methods
Ethical approval
Our study was exempt from ethical approval, because we only analyzed publicly available summary-level data of genome-wide association studies (GWAS). All GWAS summary statistics used in our MR study were obtained from the IEU Open GWAS Project2 (18).
Genetic instruments
Summary-level data for dried fruit intake were obtained from a GWAS study (N = 421,764) of the UK Biobank, using the GWAS-ID “ukb-b-16576.” The UK Biobank is a large population-based cohort of more than 500,000 participants who were recruited at ages 40 to 69 years across England, Wales, and Scotland from 2006 to 2010 (19). In the UK Biobank, dried fruit intake was available from a question “About how many pieces of dried fruit would you eat per day? (Count one prune, one dried apricot, 10 raisins as one piece; put 0 if you do not eat any)” included in the touchscreen questionnaire3 Participants who answered >100 were rejected. Genotyping of the participants was performed using Affymetrix UK Biobank Axiom array. Extensive centralized quality control was applied for the genetic data (19). We identified SNPs robustly associated with dried fruit intake at genome-wide significance (P < 5 × 10−8) as instrumental variables. We restricted instrumental variables to independent SNPs without linkage disequilibrium (R2 < 0.001) to minimize MR biases by using the clump_data function of the R package “TwoSampleMR” version 0.5.64 (18, 20). The European panel of 1,000 Genomes data was used as the reference panel (21). Palindromic SNPs with intermediate allele frequencies were not used, because they may invert the direction of causality.
Analyses were adjusted for fresh fruit intake, body mass index (BMI), current smoking status, alcohol intake frequency, total body bone mineral density, serum 25-hydroxyvitamin D levels, and vigorous physical activity applying multivariable MR. Summary-level data for these exposures were obtained from the IEU Open GWAS Project. The detailed information are shown in Table 1.
Summary-level data for low back pain
Summary-level data for low back pain in individuals of European descent were derived from the FinnGen study (13,178 cases and 164,682 controls) using the GWAS-ID “finn-b-M13_LOWBACKPAIN” (Table 1). The FinnGen study is a nationwide cohort launched in 2017. It aims to collect and evaluate genome and health data from 500,000 Finnish biobank participants (22). Low back pain was identified according to International classification of diseases (ICD) codes retrieved from nationwide registries in Finland. The effect alleles in dried fruit intake and low back pain datasets were harmonized using the harmonize_data function from the TwoSampleMR R package.
For replication analyses, summary statistics for low back pain were obtained from the Neale lab study with the GWAS-ID “ukb-d-M13_LOWBACKPAIN”, including 5,423 cases and 355,771 controls (Table 1).
Statistical analysis
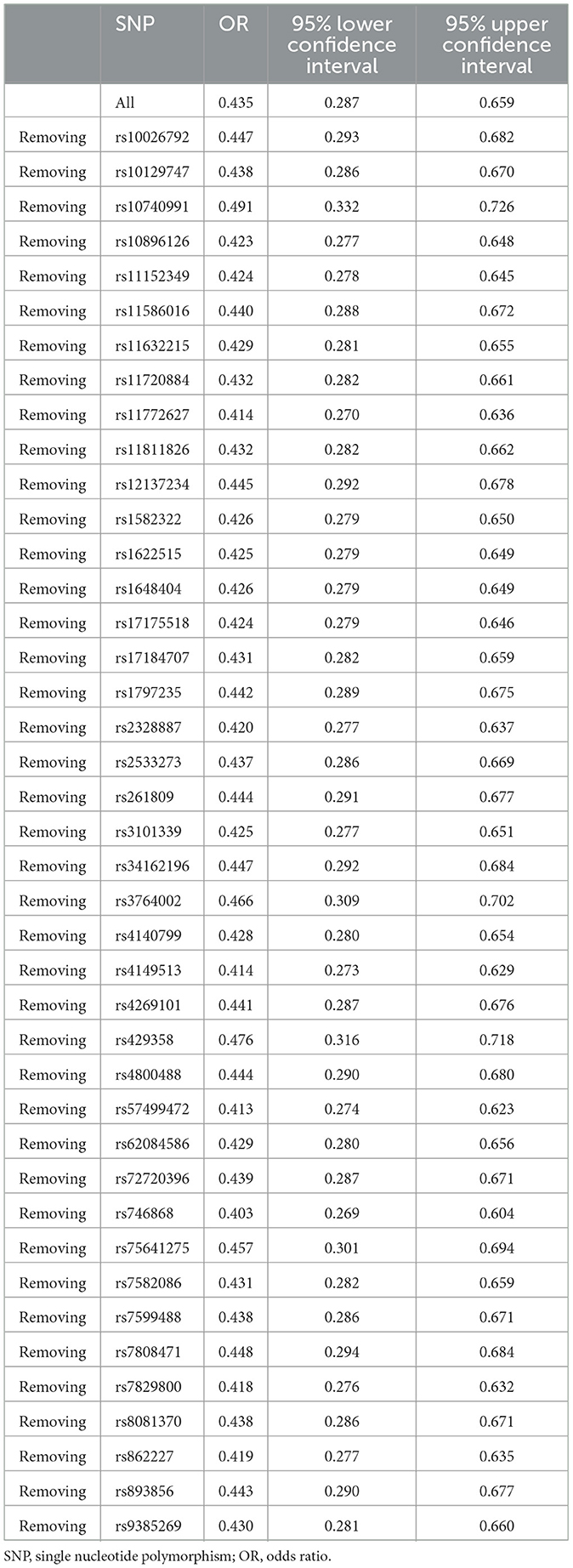
In this two-sample MR analysis, we used the inverse variance weighted method implemented in the TwoSampleMR R package as the primary MR method. This method gives reliable causal assessments and has the highest statistical power if the selected SNPs meet the instrumental variable assumptions (23). We then conducted sensitivity analyses using the MR-Egger, weighted median, weighted mode, simple mode, and maximum likelihood methods for assessing the robustness of the findings. These methods relax different MR assumptions regarding pleiotropy. For instance, the MR-Egger method can give unbiased assessments even when the exclusion restriction assumption is violated, but it has comparatively low statistical power (24). The weighted median method stipulates that at least 50% of the information is from valid instrumental variables (25). To assess the presence of horizontal pleiotropy, we used the MR-Egger regression intercept (24). If the intercept term is significantly different from zero, this is taken as evidence for horizontal pleiotropy (24). We applied the Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) method to detect and correct for potentially pleiotropic outliers (26). We carried out leave-one-out sensitivity analysis to evaluate whether individual instrumental variables drive observed causal associations. A PhenoScanner5 search was performed to identify phenotypes related to the selected instrumental variables. For evaluating the presence of heterogeneity between variant-specific estimates, we used the Cochran's Q statistical test. Besides univariable MR, we performed multivariable MR to control for potential confounders including fresh fruit intake, BMI, current smoking status, alcohol intake frequency, total body bone mineral density, serum 25-hydroxyvitamin D levels, and vigorous physical activity. Seven models for multivariable MR were taken into account: (a) model 1: adjustment for fresh fruit intake; (b) model 2: adjustment for BMI; (c) model 3: adjustment for current tobacco smoking; (d) model 4: adjustment for alcohol intake frequency; (e) model 5: adjustment for total body bone mineral density; (f) model 6: adjustment for serum 25-hydroxyvitamin D levels; and (g) model 7: adjustment for vigorous physical activity. We carried out all MR analyses using the TwoSampleMR (version 0.5.6) and MR-PRESSO (version 1.0) packages in R version 4.0.4. Statistical significance was set at P < 0.05.
Results
Forty-one independent SNPs were selected as instrumental variables in the assessment of dried fruit intake (Supplementary Table S1). These instrument SNPs explained 0.63% of the variance in dried fruit intake. According to the study by Jin et al. (17), the F-statistics of individual SNPs ranged between 17.5 and 47.9, indicating adequate instrument strength.
The inverse variance weighted MR estimate showed that greater dried fruit intake was significantly associated with decreased risk of low back pain (OR = 0.435, 95% CI: 0.287–0.659, P = 8.657 × 10−5) (Table 2 and Figure 1). Sensitivity analyses using the MR-Egger (OR = 0.078, 95% CI: 0.013–0.479, P = 0.009), maximum likelihood (OR = 0.433, 95% CI: 0.295–0.635, P = 1.801 × 10−5), weighted median (OR = 0.561, 95% CI: 0.325–0.967, P = 0.038) and MR-PRESSO (OR = 0.454, 95% CI: 0.302–0.683, P = 4.535 × 10−4) methods also revealed an inverse association between greater dried fruit intake and low back pain (Table 2). The Cochran's Q statistical test did not provide evidence for statistically significant heterogeneity in the causal estimate amongst instrumental variables (Q = 48.801, P = 0.160). No evidence of directional pleiotropy was identified according to the Egger intercept (intercept P-value = 0.065) or applying the MR-PRESSO method (global test P-value = 0.164). Results of the leave-one-out sensitivity analysis demonstrated that the observed causal associations were not driven by individual instrumental variables (Table 3). We further conducted a PhenoScanner search to identify SNPs related to other potential confounders at genome-wide significance (P < 5 × 10−8) (Supplementary Table S2). The result of the inverse variance weighted analysis was not significantly altered after removing these SNPs (OR = 0.567, 95% CI: 0.339–0.950, P = 0.031).
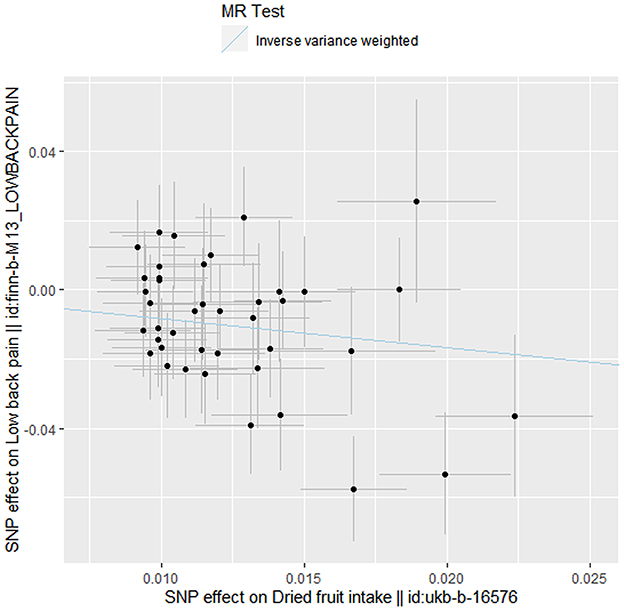
Figure 1. Scatter plot showing Mendelian randomization effect estimates of dried fruit intake over low back pain. Each variant-low back pain association is plotted against variant-dried fruit intake association, and the corresponding Mendelian randomization estimate for inverse variance weighted is plotted.
We replicated the protective effect of dried fruit intake on low back pain using a validation sample from the Neale lab study (GWAS ID: ukb-d-M13_LOWBACKPAIN). Consistent with the primary analysis, the replication analysis showed that greater dried fruit intake was causally associated with decreased low back pain risk (Table 2). Besides dried fruit intake, we evaluated the casual association of fresh fruit intake, BMI, current tobacco smoking, alcohol intake frequency, total body bone mineral density, serum 25-hydroxyvitamin D levels, and vigorous physical activity with low back pain using the inverse variance weighted method (Supplementary Table S3). The instrumental variables for these exposures and their association with low back pain are shown in Supplementary Tables S4–S10.
To verify whether the protective effect of dried fruit intake on low back pain was independent of fresh fruit intake, BMI, current tobacco smoking, alcohol intake frequency, total body bone mineral density, serum 25-hydroxyvitamin D levels, and vigorous physical activity, we performed multivariable MR analyses. Results of multivariable MR analyses supported that greater dried fruit intake was protective against low back pain (Table 4).
Discussion
To our knowledge, this is the first MR study to evaluate the casual association between dried fruit intake and low back pain. Using genetic data from individuals of European descent, our analyses showed that greater dried fruit intake was associated with decreased low back pain risk. This association was consistent after adjustment for fresh fruit intake, BMI, current tobacco smoking, alcohol intake frequency, total body bone mineral density, serum 25-hydroxyvitamin D levels, and vigorous physical activity.
MR is an effective analytic method for causal inference. It is less affected by certain fundamental shortcomings of traditional observational investigations. Recently, MR studies have been performed to assess potential risk factors for low back pain. In 2020, Elgaeva and colleagues applied inverse variance weighted meta-analysis as the main method for evaluating the causal association between BMI and back pain (27). Summary statistics for BMI were obtained from the GIANT consortium (N = 322,154), and the corresponding data for back pain and chronic back pain were derived from a large European sample (N = 453,860). They found that 1-standard deviation (4.65 kg/m2) increase in BMI was associated with a significant increase in back pain (OR = 1.15, 95% CI: 1.06–1.25, P = 0.001) and chronic back pain (OR = 1.20, 95% CI: 1.09–1.32, P = 0.0002); the significant causal association remained in secondary analysis and sensitivity analyses. These results suggested that a higher BMI may be a risk factor for back pain. Consistent with their findings, Zhou and colleagues found a casual association between BMI and low back pain (OR = 1.28, 95% CI: 1.18–1.39, P = 6.60 × 10−9) using a two-sample MR design (28). An MR study by a Chinese research group recently evaluated the causal effect of plasma omega-3 levels on low back pain risk, finding that up-regulated plasma omega-3 levels were linked with reduced low back pain risk using the inverse variance weighted method (β = −0.366, OR = 0.694, P = 0.049) (29). However, this link was not supported by sensitivity analyses using the weighted mode (P = 0.281) and MR-Egger (P = 0.228) methods. In 2022, Williams et al. applied inverse weighted variance, Causal Analysis Using Summary Effect (CAUSE), and sensitivity analyses to evaluate risk factors for chronic back pain (30). Their study demonstrated that several life style factors including greater alcohol intake (inverse variance weighted OR = 1.29, 95% CI: 1.17–1.43, P = 7.2 × 10−7) and smoking (inverse variance weighted OR = 1.27, 95% CI: 1.19–1.35, P = 7.0 × 10−15) increased the risk of chronic back pain (30).
In our study, we applied a two-sample MR design to evaluate the causal association between dried fruit intake and low back pain. Consistent with previous MR studies, we used the inverse variance weighted method in the primary MR analysis. The result of the inverse variance weighted-based estimate was statistically significant; no single instrument SNPs drove the causal estimate. Multiple sensitivity analyses using various methods revealed consistent and stable results. To verify the causal association found in the discovery dataset, we used a validation sample for low back pain from another European population. The replication analysis provided similar results and suggested a causal association between dried fruit intake and low back pain. Furthermore, to adjust for potential confounding factors including fresh fruit intake, BMI, current tobacco smoking, alcohol intake frequency, total body bone mineral density, serum 25-hydroxyvitamin D levels, and vigorous physical activity, we used multivariable MR. The effect of dried fruit intake remained after adjustment for these factors. In summary, the above-mentioned efforts enhanced the robustness of the results in our study.
The mechanisms involved in the causal association between dried fruit intake and low back pain remain unclear. Dried fruits are obtained from fresh fruits by using various drying techniques. They are important healthful snacks and are rich sources of dietary fibers, minerals, vitamins, and a variety of bioactive compounds such as flavonoids and carotenoids (6). Dried fruits exert multiple biological effects, including anti-oxidative, anti-inflammatory, anti-atherosclerosis, and anti-cancer effects (5, 7, 10, 12, 14, 15, 31). Experimental research showed that dried fruit intake suppressed proinflammatory cytokines and promoted functions of the musculoskeletal system (32–34). In clinical studies, numerous authors found that daily intake of dried fruits had protective effects on musculoskeletal health in both men and women (35–37). The protective effect of dried fruit intake on low back pain might be related to the micronutrients and bioactive compounds dried fruits contain. For instance, vitamin E could improve musculoskeletal health by maintaining bone mineral density and reducing oxidative stress and inflammation (38, 39). In addition, some authors found that flavonoids had antioxidant and antinociceptive activities, which may be used to relieve pain (40, 41). However, since the evidence regarding the underlying mechanisms is limited, further experimental and clinical studies are required.
One limitation of this MR study is that we did not have access to individual-level data to adjust for medication usage. It has been reported that some medications such as non-steroidal anti-inflammatory drugs and opioids have beneficial effects for low back pain. Another limitation is that we only studied participants of European descent in this MR study. The association between dried fruit intake and low back pain in Asians such as Chinese and Indians remains unclear.
In summary, this MR study using genetic data from individuals of European descent provided evidence to support that greater dried fruit intake was associated with decreased risk of low back pain. The results highlighted the importance of evaluating dried fruit intake for the prevention of low back pain. Further validations using randomized controlled trials with large sample sizes are warranted.
Data availability statement
Publicly available datasets were analyzed in this study. They can be obtained at https://gwas.mrcieu.ac.uk/.
Ethics statement
Our Mendelian randomization study analyzing publicly available summary-level data was exempt from ethical approval.
Author contributions
JH contributed to study concept and design, acquisition and interpretation of data, statistical analyses, and manuscript writing and revision. Z-FX supervised the study, contributed to data interpretation, and assisted in reviewing the manuscript. Both authors read and approved the final manuscript.
Acknowledgments
We thank the IEU Open GWAS Project (https://gwas.mrcieu.ac.uk/) for providing summary statistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1027481/full#supplementary-material
Footnotes
1. ^https://gfa.org.ge/wp-content/uploads/2019/05/Dried-Fruits-market-research.pdf
2. ^https://gwas.mrcieu.ac.uk/
3. ^https://biobank.ndph.ox.ac.uk/ukb/field.cgi?id=1319
References
1. Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. (2021) 398:78–92. doi: 10.1016/S0140-6736(21)00733-9
2. Orrillo E, Vidal Neira L, Piedimonte F, Plancarte Sanchez R, Astudilllo Mihovilovic S, Narvaez Tamayo MA, et al. What is new in the clinical management of low back pain: a narrative review. Cureus. (2022) 14:e22992. doi: 10.7759/cureus.22992
3. Juniper M, Le TK, Mladsi D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opin Pharmacother. (2009) 10:2581–92. doi: 10.1517/14656560903304063
4. Alonso-García M, Sarría-Santamera A. The economic and social burden of low back pain in spain: a national assessment of the economic and social impact of low back pain in spain. Spine. (2020) 45:E1026–32. doi: 10.1097/BRS.0000000000003476
5. Mossine VV, Mawhinney TP, Giovannucci EL. Dried fruit intake and cancer: a systematic review of observational studies. Adv Nutr. (2020) 11:237–50. doi: 10.1093/advances/nmz085
6. Alasalvar C, Salvadó JS, Ros E. Bioactives and health benefits of nuts and dried fruits. Food Chem. (2020) 314:126192. doi: 10.1016/j.foodchem.2020.126192
7. Puglisi MJ, Vaishnav U, Shrestha S, Torres-Gonzalez M, Wood RJ, Volek JS, et al. Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids Health Dis. (2008) 7:14. doi: 10.1186/1476-511X-7-14
8. Anderson JW, Weiter KM, Christian AL, Ritchey MB, Bays HE. Raisins compared with other snack effects on glycemia and blood pressure: a randomized, controlled trial. Postgrad Med. (2014) 126:37–43. doi: 10.3810/pgm.2014.01.2723
9. Bays H, Weiter K, Anderson J, A. randomized study of raisins versus alternative snacks on glycemic control and other cardiovascular risk factors in patients with type 2 diabetes mellitus. Phys Sportsmed. (2015) 43:37–43. doi: 10.1080/00913847.2015.998410
10. Di Lorenzo C, Sangiovanni E, Fumagalli M, Colombo E, Frigerio G, Colombo F, et al. Evaluation of the anti-inflammatory activity of raisins (Vitis vinifera L) in human gastric epithelial cells: a comparative study. Int J Mol Sci. (2016) 17:1156. doi: 10.3390/ijms17071156
11. Chaiwong S, Chatturong U, Chanasong R, Deetud W, To-On To-On K, Puntheeranurak S, et al. Dried mulberry fruit ameliorates cardiovascular and liver histopathological changes in high-fat diet-induced hyperlipidemic mice. J Tradit Complement Med. (2021) 11:356–68. doi: 10.1016/j.jtcme.2021.02.006
12. Kountouri AM, Gioxari A, Karvela E, Kaliora AC, Karvelas M, Karathanos VT. Chemopreventive properties of raisins originating from Greece in colon cancer cells. Food Funct. (2013) 4:366–72. doi: 10.1039/c2fo30259d
13. Chen ZJ, Yang YF, Zhang YT, Yang DH. Dietary Total Prenylflavonoids from the Fruits of Psoralea corylifolia L. Prevents age-related cognitive deficits and down-regulates Alzheimer's markers in SAMP8 Mice. Molecules. (2018) 23:196. doi: 10.3390/molecules23010196
14. Kowalska J, Kowalska H, Marzec A, Brzeziński T, Samborska K, Lenart A. Dried strawberries as a high nutritional value fruit snack. Food Sci Biotechnol. (2018) 27:799–807. doi: 10.1007/s10068-018-0304-6
15. Donno D, Mellano MG, Riondato I, De Biaggi M, Andriamaniraka H, Gamba G, et al. Traditional and unconventional dried fruit snacks as a source of health-promoting compounds. Antioxidants. (2019) 8:396. doi: 10.3390/antiox8090396
16. Budu-Aggrey A, Paternoster L. Research techniques made simple: using genetic variants for randomization. J Invest Dermatol. (2019) 139:1416–21. doi: 10.1016/j.jid.2019.03.1138
17. Jin C, Li R, Deng T, Lin Z, Li H, Yang Y, et al. Association between dried fruit intake and pan-cancers incidence risk: a two-sample Mendelian randomization study. Front Nutr. (2022) 9:899137. doi: 10.3389/fnut.2022.899137
18. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
19. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
20. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
21. 1000 Genomes Project Consortium; Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. (2015) 526:68–74. doi: 10.1038/nature15393
22. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv. (2022) 3:1360. doi: 10.1101/2022.03.03.22271360
23. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
24. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
25. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
26. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
27. Elgaeva EE, Tsepilov Y, Freidin MB, Williams FMK, Aulchenko Y, Suri P, et al. Prize in clinical science 2020. Examining causal effects of body mass index on back pain: a Mendelian randomization study. Eur Spine J. (2020) 29:686–91. doi: 10.1007/s00586-019-06224-6
28. Zhou J, Mi J, Peng Y, Han H, Liu Z. Causal associations of obesity with the intervertebral degeneration, low back pain, and sciatica: a two-sample Mendelian randomization study. Front Endocrinol. (2021) 12:740200. doi: 10.3389/fendo.2021.740200
29. Zhou S, Zhu G, Xu Y, Gao R, Li H, Han G, et al. Mendelian randomization study on the putative causal effects of omega-3 fatty acids on low back pain. Front Nutr. (2022) 9:819635. doi: 10.3389/fnut.2022.819635
30. Williams FMK, Elgaeva EE, Freidin MB, Zaytseva OO, Aulchenko YS, Tsepilov YA, et al. Causal effects of psychosocial factors on chronic back pain: a bidirectional Mendelian randomisation study. Eur Spine J. (2022) 31:1906–15. doi: 10.1007/s00586-022-07263-2
31. Moazen S, Amani R, Homayouni Rad A, Shahbazian H, Ahmadi K, Taha Jalali M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: a randomized double-blind controlled trial. Ann Nutr Metab. (2013) 63:256–64. doi: 10.1159/000356053
32. Shahnazari M, Turner RT, Iwaniec UT, Wronski TJ Li M, Ferruzzi MG, et al. Dietary dried plum increases bone mass, suppresses proinflammatory cytokines and promotes attainment of peak bone mass in male mice. J Nutr Biochem. (2016) 34:73–82. doi: 10.1016/j.jnutbio.2016.04.007
33. Kim KY, Ku SK, Lee KW, Song CH, An WG. Muscle-protective effects of Schisandrae Fructus extracts in old mice after chronic forced exercise. J Ethnopharmacol. (2018) 212:175–87. doi: 10.1016/j.jep.2017.10.022
34. Wallace TC. Dried plums, prunes, and bone health: a comprehensive review. Nutrients. (2017) 9:401. doi: 10.3390/nu9040401
35. Jensen GS, Attridge VL, Benson KF, Beaman JL, Carter SG, Ager D. Consumption of dried apple peel powder increases joint function and range of motion. J Med Food. (2014) 17:1204–13. doi: 10.1089/jmf.2014.0037
36. Arjmandi BH, Johnson SA, Pourafshar S, Navaei N, George KS, Hooshmand S, et al. Bone-protective effects of dried plum in postmenopausal women: efficacy and possible mechanisms. Nutrients. (2017) 9:496. doi: 10.3390/nu9050496
37. Hooshmand S, Gaffen D, Eisner A, Fajardo J, Payton M, Kern M. Effects of 12 months consumption of 100 g dried plum (prunes) on bone biomarkers, density, and strength in men. J Med Food. (2022) 25:40–7. doi: 10.1089/jmf.2021.0080
38. Kim N, Kang Y, Choi YJ, Lee Y, Park SJ, Park HS, et al. Musculoskeletal health of the adults over 50 years of age in relation to antioxidant vitamin intakes. Clin Nutr Res. (2022) 11:84–97. doi: 10.7762/cnr.2022.11.2.84
39. Kim M, Eo H, Lim JG, Lim H, Lim Y. Can low-dose of dietary vitamin E supplementation reduce exercise-induced muscle damage and oxidative stress? A meta-analysis of randomized controlled trials. Nutrients. (2022) 14:1599. doi: 10.3390/nu14081599
40. Guilhon-Simplicio F, Machado TM, do Nascimento LF, Souza RDS, Koolen HHF, da Silva FMA, et al. Chemical composition and antioxidant, antinociceptive, and anti-inflammatory activities of four amazonian byrsonima species. Phytother Res. (2017) 31:1686–93. doi: 10.1002/ptr.5884
Keywords: Mendelian randomization, low back pain, dried fruit intake, genome-wide association studies, summary statistics
Citation: Huang J and Xie Z-F (2023) Dried fruit intake causally protects against low back pain: A Mendelian randomization study. Front. Nutr. 10:1027481. doi: 10.3389/fnut.2023.1027481
Received: 09 September 2022; Accepted: 20 February 2023;
Published: 23 March 2023.
Edited by:
Luís Pedro Rato, Instituto Politécnico da Guarda, PortugalReviewed by:
Tuo Deng, The First Affiliated Hospital of Wenzhou Medical University, ChinaNelson Andrade, Chemistry and Technology Network (REQUIMTE), Portugal
Copyright © 2023 Huang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng-Fu Xie, eGllX3poZW5nZnVAeWVhaC5uZXQ=
 Jian Huang
Jian Huang Zheng-Fu Xie2*
Zheng-Fu Xie2*