- 1Nutritional Epidemiology Institute and School of Public Health, Tianjin Medical University, Tianjin, China
- 2First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3School of Public Health of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 4Institute of Radiation Medicine, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China
- 5Health Management Centre, Tianjin Medical University General Hospital, Tianjin, China
- 6Tianjin Key Laboratory of Environment, Nutrition and Public Health, Tianjin, China
- 7Center for International Collaborative Research on Environment, Nutrition and Public Health, Tianjin, China
Background: Prospective studies on the association between Helicobacter pylori (H. pylori) infection and subclinical hyperthyroidism are limited. We, therefore, designed a large-scale cohort study to explore the association between H. pylori infection and the risk of subclinical hyperthyroidism in women.
Methods: This prospective cohort study investigated 2,713 participants. H. pylori infection was diagnosed with the carbon 13 breath test. Subclinical hyperthyroidism was defined as serum thyroid-stimulating hormone levels are low or undetectable but free thyroxine and tri-iodothyronine concentrations are normal. Propensity score matching (PSM) analyses and Cox proportional hazards regression models were used to estimate the association between H. pylori infection and subclinical hyperthyroidism.
Results: A total of 1,025 PS-matched pairs of H. pylori infection women were generated after PSM. During 6 years of follow-up, the incidence rate of subclinical hyperthyroidism was 7.35/1,000 person-years. After adjusting potential confounding factors (including iodine intake in food and three main dietary patterns score), the multivariable hazard ratio (HR; 95% confidence intervals) of subclinical hyperthyroidism by H. pylori infection was 2.49 (1.36, 4.56). Stratified analyses suggested a potential effect modification by age, the multivariable HR (95% confidence intervals) was 2.85 (1.45, 5.61) in participants aged ≥ 40 years and 0.70 (0.08, 6.00) in participants aged < 40 years (P for interaction = 0.048).
Conclusion: Our prospective study first indicates that H. pylori infection is significantly associated with the risk of subclinical hyperthyroidism independent of dietary factors among Chinese women, especially in middle-aged and older individuals.
Clinical Trial Registration:https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031137, identifier UMIN000027174.
Introduction
Thyroid hormones (THs), including triiodothyronine (T3) and thyroxine (T4), are produced and released by the thyroid gland (1). They are key regulators of basal metabolic rate and are essential for the normal growth and development of a body. Synthesis and secretion of THs are finely modulated by the hypothalamic–pituitary–thyroid (HPT) axis. Thyroid-stimulating hormone (TSH) is produced by the anterior pituitary, stimulating the synthesis and secretion of THs by negative feedback inhibition of the HPT axis (2). Subclinical hyperthyroidism (SHyper) is defined biochemically: TSH concentrations are low or undetectable but free thyroxine (FT4) and tri-iodothyronine (FT3) concentrations are normal (3). The common causes of subclinical hyperthyroidism include toxic multinodular goiter, toxic adenoma, Graves’ disease, and some exogenous causes (4). The prevalence of subclinical hyperthyroidism in the general population is 1%–3% (5, 6), which varies by age, sex, race, genetic predisposition, iodine status, and the definition of subclinical hyperthyroidism (7). Women, older individuals, and those living in iodine-deficient regions were more frequent (8). In the past decade, the adverse effects of subclinical hyperthyroidism on health have been explored extensively, which has previously been associated with an increased risk for cardiovascular disease, bone loss, fractures, and dementia, even may progression to overt hyperthyroidism (4), which have become an important public health problem.
Helicobacter pylori (H. pylori) is a major human pathogen that specifically colonizes the gastric epithelium and infects the stomach of 44.3% (50.8% in developing countries vs. 34.7% in developed countries) of the world population (9, 10). Although most infected individuals do not develop obvious clinical sequelae, H. pylori is a Group I carcinogen according to the International Agency for Research on Cancer (IARC), with 89% of all gastric cancers being attributable to this infection (9). Helicobacter pylori infection can also involve some extra gastric diseases, such as respiratory (bronchiectasis and asthma), cardiovascular (atherosclerosis, myocardial infarction), Parkinson’s disease, metabolic syndrome, fatty liver disease, and immune-mediated allergic diseases (11, 12). Evidence suggests that H. pylori infection plays an important role in the pathogenesis of thyroid autoimmune diseases due to its ability to mimic antigen distribution on thyroid cell membranes (13, 14). In addition, De Luis et al. (15) showed that the titer of anti- H. pylori immunoglobulin G (IgG) antibody in patients with subclinical hyperthyroidism was significantly higher than that in the control group. Furthermore, a recent meta-analysis (16) involving 862 patients showed that H. pylori infection was significant in Graves’ disease but not in Hashimoto thyroiditis.
To date, specific data linking H. pylori infection with subclinical hyperthyroidism in human populations has been limited and conflicting, and most of the thyroid diseases show woman predilection (17), therefore, we conducted a large prospective cohort study to investigate whether baseline H. pylori infections were associated with subclinical hyperthyroidism in woman adults. Moreover, evidence has illustrated that dietary factors may play a crucial role in subclinical hyperthyroidism (18), which also directly influences H. pylori colonization or virulence (19). Therefore, while studying the association between H. pylori infection and subclinical hyperthyroidism, we must consider the interference of dietary factors in the association. Owing to it being a retrospective cohort, propensity score matching (PSM) was applied to reduce selection bias when comparing the clinical outcomes between patients with and without H. pylori infections.
Materials and methods
Participants
Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study is a prospective dynamic cohort study of participants over 18 years of age, focusing on the relationship between chronic low-grade systemic inflammation and health status (20, 21). This is an ongoing study that was launched in 2007, where the participants underwent health examinations and completed a questionnaire survey to assess their diet and lifestyle factors till May 2013 (22). Refer to previous reports for detailed information (23).
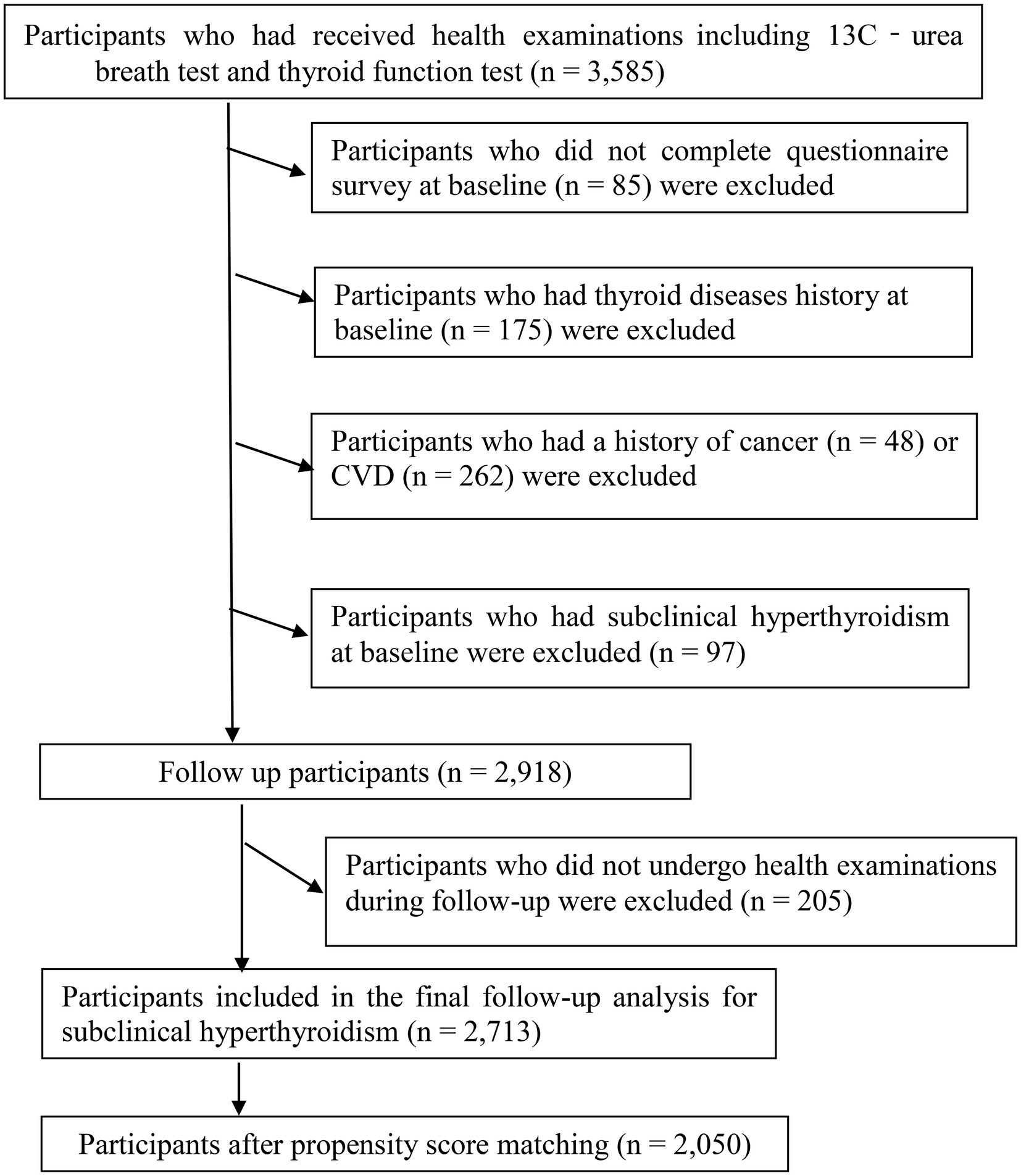
A total of 3,585 women had received at least one health examination and questionnaire survey, including blood tests, thyroid function tests, 13C-urea breath test (13C-UBT), and lifestyle factors. During the research period. Participants who did not complete the questionnaire survey at baseline (n = 85) were excluded. In addition, we excluded participants who had a history of cardiovascular disease (n = 262) or cancer (n = 48), given that cardiovascular disease and cancer can significantly affect the lifestyle of participants. We also excluded participants who had a history of thyroid disease (n = 175) at baseline, subjects with subclinical hyperthyroidism at baseline (n = 97), and 205 participants who were lost to follow-up examinations. The final cohort analysis comprised 2,713 participants (follow-up rate, 93.0%). The participant selection process is described in Figure 1. All participants have agreed to participate and provided written informed consent. The protocol of the study is in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Tianjin Medical University.
Thyroid function tests
After fasting for 8–12 h, fasting blood samples were collected by elbow vein puncture in the morning. Serum FT3, FT4, and TSH were measured by chemiluminescence immunoassay using the ADVIA Centaur FT3, FT4, and TSH3-Ultra analyzer (Siemens Healthcare Diagnostics, New York, NY). The ranges of measurement for FT3, FT4, and TSH were 0.3–30.8 and 1.3–155 pmol/L, and 0.001–150 mIU/L, respectively. Based on the previous reports and national guidelines (23, 24), the normal ranges of FT3, FT4, and TSH were 3.50–6.50 and 11.50–22.70 pmol/L, and 0.55–4.78 mIU/L, respectively. We used a uniform thyrotropin cutoff level for subclinical hyperthyroidism definition: TSH < 0.55 IU/mL and normal FT4 levels.
Assessment of Helicobacter pylori infection
The diagnosis of H. pylori infection was based on the result of fasting 13C-UBT, which is based on the simple principle that the abundantly expressed urease of H. pylori can rapidly hydrolyze isotopically labeled urea solutions. A baseline breath sample was obtained by blowing into a 20-mL container, and a capsule containing 75 mg of 13C-urea was given to patients with 100 ml of water. Two breath samples were collected within a 30-min interval. Patient samples were analyzed by gas chromatography and H. pylori infection was considered present if the difference between the 30-min value and baseline value divided by the baseline value exceeded 4.0‰. 13C-UBT is a mature diagnostic test for H. pylori infection due to its rapid, non-invasive, high sensitivity, and specificity (25).
Assessment of other variables
Data on potential confounders-such as demographic characteristics, socioeconomic status, lifestyle factors, reproductive factors, and family history of CVD, hypertension, diabetes, and hyperlipidemia were obtained from a standardized health-related questionnaire survey. The total energy intake for each participant and dietary intake was assessed using a 100-item Food Frequency Questionnaire (FFQ). Spearman rank correlation coefficient for energy intake between two FFQs administered 3 months apart was 0.68, and for food groups (e.g., fruits, vegetables, and soft drinks) ranged from 0.62 to 0.79. Details of the FFQ have been described elsewhere (26). Factor analysis with principal components based on the FFQ was used to derive three dietary patterns (fruit and sweet foods dietary pattern, vegetable dietary pattern, and animal foods dietary pattern; Supplementary Table 1) (27). Anthropometric parameters, blood pressure, and blood tests are done by trained staff at the hospital. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Metabolic syndrome (MetS) was defined according to the 2009 American Heart Association scientific criteria (28). Details of the various tests have been described in a previous study (29).
Statistical analysis
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, United States). We summarized baseline characteristics based on H. pylori infection using the mean and 95% confidence interval (CI) for continuous variables and counts (with percentages) for categorical variables.
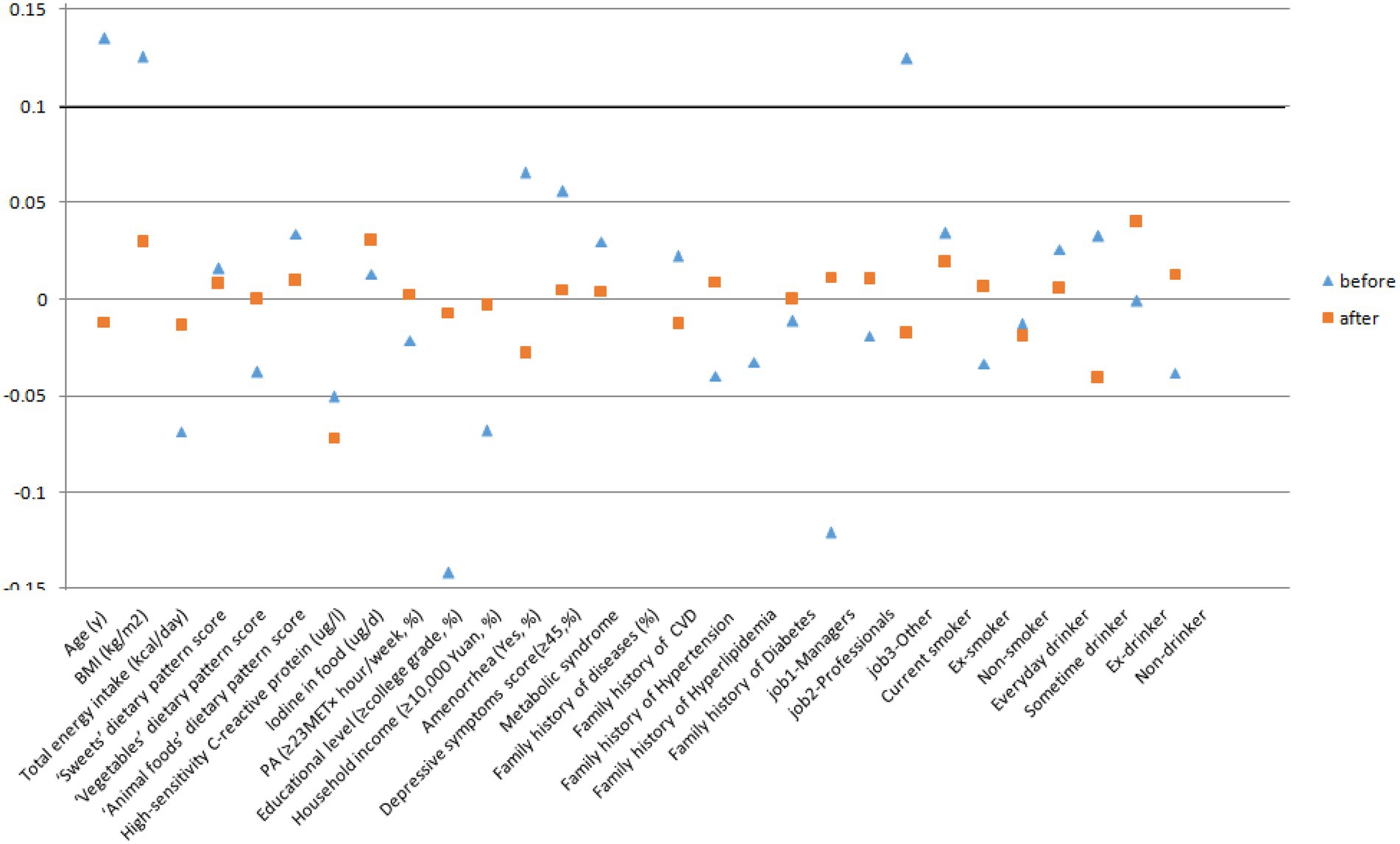
Propensity scores were calculated using a logistic regression model and the main covariates (refer to Table 1). We used nearest neighbor matching to match H. pylori infection and H. pylori uninfected patients in a 1:1 ratio with a caliper distance of 0.2 of the standard deviation of the logit of the PS (30). Standardized differences were used to assess the covariate balance after matching. An absolute standardized difference of less than 0.1 was considered negligible in the groups (31). After the cases and controls were randomly sorted, the control with the closest propensity score was selected for the first case. If the propensity score difference between the two was within the caliper range, the matching was successful; if it was outside the caliper range, the matching failed. If successful, the case and control were removed from the original database and matched to the next case. If no suitable matched control was found for the last case, it was deleted.
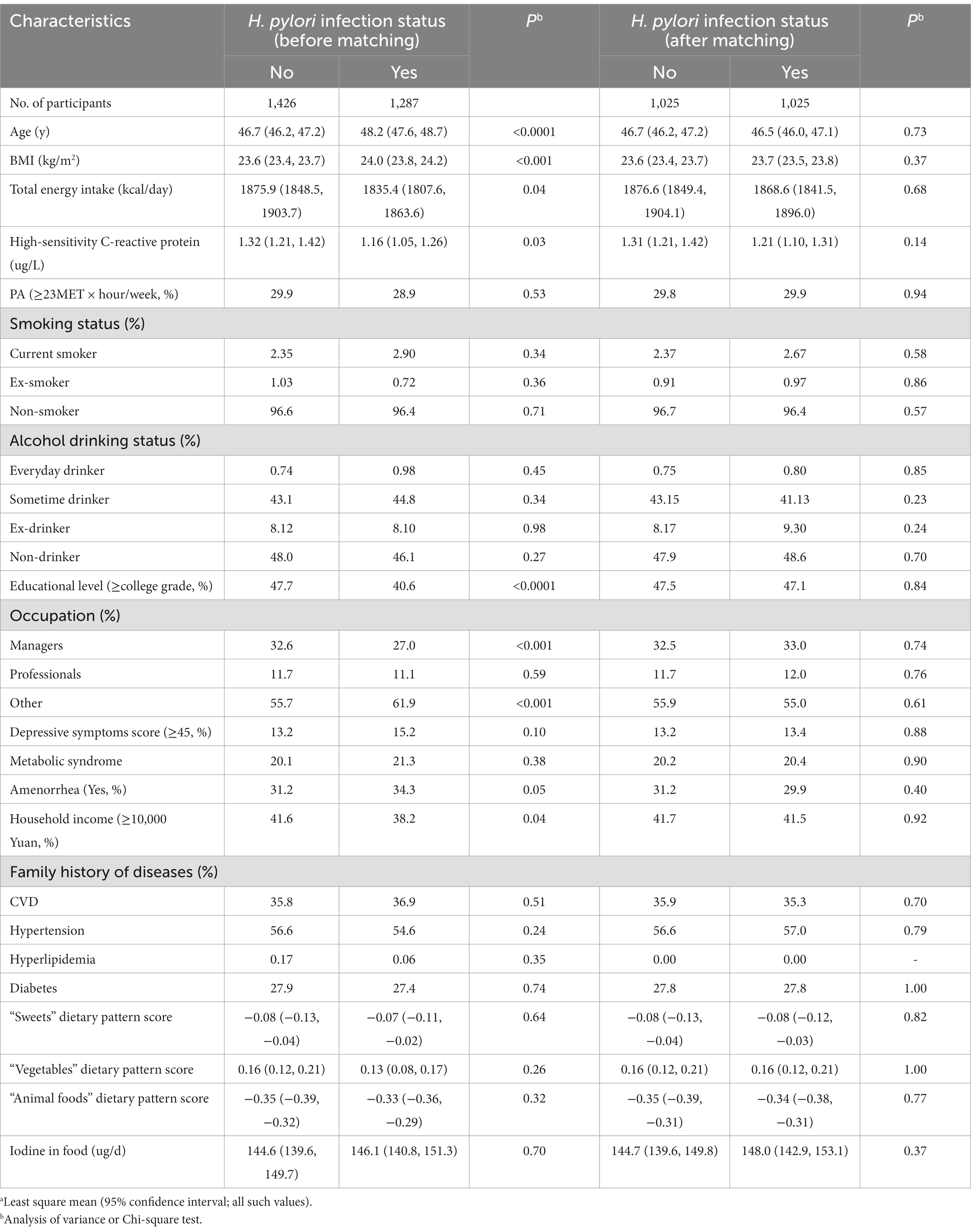
Table 1. Characteristics of the participants according to Helicobacter pylori infection status before and after matching in women.a
Follow-up time was calculated from the date of measuring the baseline measurement of H. pylori infection to the date of the first diagnosis of subclinical hyperthyroidism, or the end of follow-up (31 December 2019), or loss to follow-up, whichever was earliest. Cox proportional hazards models were applied to calculate hazard ratios (HRs) and 95% CIs for the association between H. pylori infection and the risk of subclinical hyperthyroidism. The incidence of subclinical hyperthyroidism was used as the dependent variable. Three models were developed. In Model 1, we adjusted for age, BMI, smoking status, drinking status, education levels, employment status, household income, depressive symptoms score, PA, family history of diseases (including CVD, hypertension, hyperlipidemia, and diabetes), total energy intake, high-sensitivity C-reactive protein (hsCRP), MetS, and amenorrhea status. The total iodine intake in food accounts for a high proportion of iodine intake, as high as 42.7% in the Tianjin population (21), thus, in Model 2, we further adjusted the total iodine intake in food. A study (32) has shown that diet in daily life will have an impact on H. pylori infection, thus, we have adjusted dietary patterns in a final model.
Potential interactions between H. pylori infection and main covariates were assessed by adding cross-product terms to the multivariable Cox models. We stratified the participants by potential effect modifiers including age, BMI, physical activity, amenorrhea status, education level, household income, and Mets status. To examine the robustness of our results, we conducted two sensitivity analyses: (1) we substituted adjustment for waist circumference (WC) was measured with BMI to investigate whether abdominal adiposity, compared with overall adiposity, changed the observed associations. (2) We included participants who had a history of cancer or CVD. All tests were two-tailed and p < 0.05 was defined as statistically significant.
Results
Characteristics of participants according to H. pylori infection status before and after PSM are shown in Table 1 and Figure 2. Among 2,713 participants who were available to be analyzed before PSM, 47.4% were classified as infecting H. pylori. Participants with H. pylori infection status tended to be older, and have higher BMI. Moreover, they had lower total energy intake, hsCRP, educational level, and monthly household income, and they were more likely to be managers and had amenorrhea status. After PSM, there were 1,025 PS-matched pairs of H. pylori infection women were generated and showed no significant baseline differences in any characteristic. Between May 2013 and December 2019, a total of 2,050 participants completed the follow-up analysis for subclinical hyperthyroidism. In the total population, 55 first incident cases of subclinical hyperthyroidism occurred, the incidence rate of subclinical hyperthyroidism was 7.56 per 1,000 person-years across the 6-year follow-up period (range: 2–6 years; median: 3.55 years).
Table 2 presents the associations between H. pylori infection and the risk of subclinical hyperthyroidism. In the analysis of H. pylori infection status before PSM, after adjustment for age, BMI, sociodemographic and lifestyle factors as well as for Mets status (Model 1), HR (95% CI) for subclinical hyperthyroidism was 2.07 (1.06, 4.03; p = 0.033). Further adjustments were made for total iodine intake in food, HRs (95% CIs) for subclinical hyperthyroidism were 2.05 (1.05, 4.02; p = 0.036), and we observed similar results. In the fully adjusted model further adjusted for the dietary quality (three major dietary patterns), these associations were not altered, and HR (95% CI) for subclinical hyperthyroidism was 2.06 (1.05, 4.03; p = 0.035). After H. pylori infection status PSM, we observed more obvious associations between H. pylori infection and the risk of subclinical hyperthyroidism. In Model 1, HR (95% CI) for subclinical hyperthyroidism was 2.34 (1.28, 4.27; p = 0.006). In Model 2, HR (95% CI) for subclinical hyperthyroidism was 2.41 (1.32, 4.40; p = 0.004). In the fully adjusted model further adjusted for the dietary quality (three major dietary patterns), these associations were not altered, HR (95% CI) for subclinical hyperthyroidism was 2.49 (1.36, 4.56; p = 0.003).
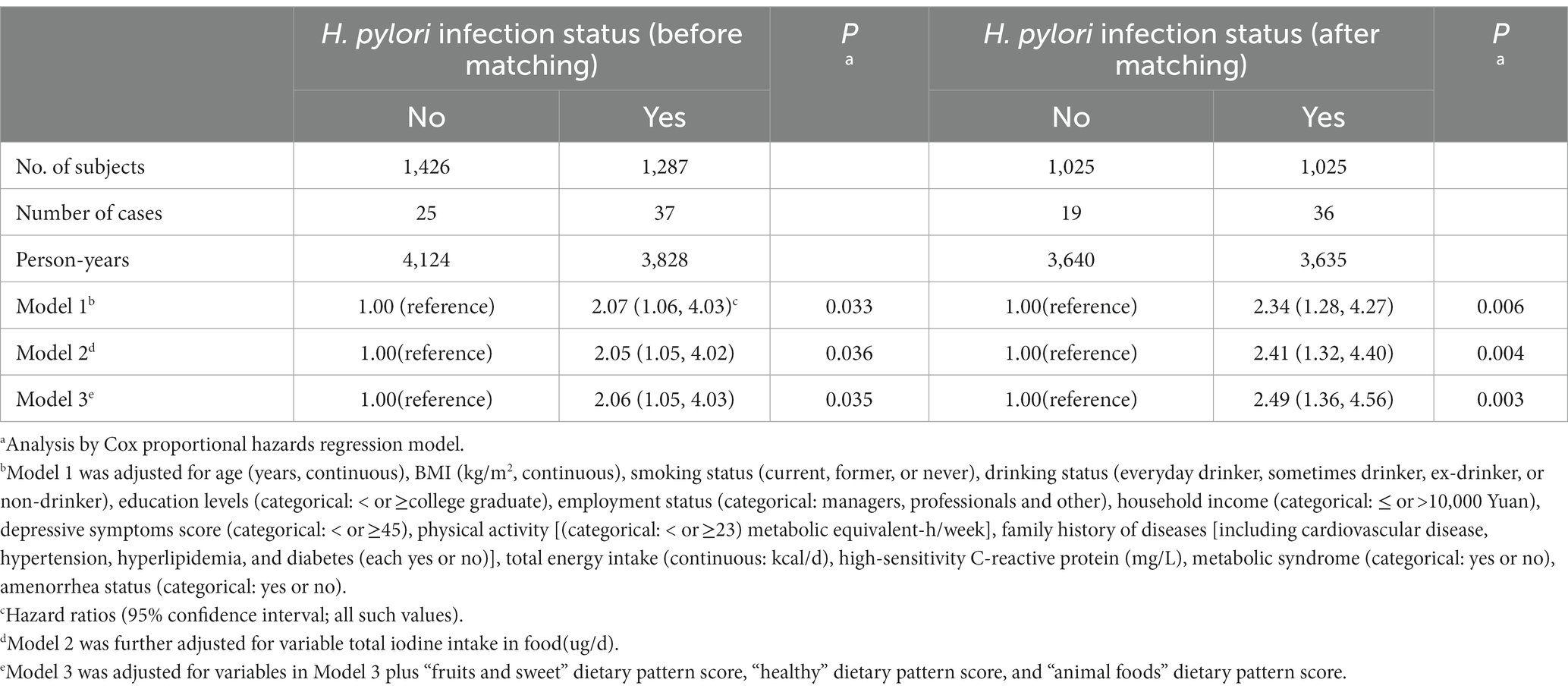
Table 2. Association between Helicobacter pylori infection status and subclinical hyperthyroidism before and after matching in women.
The analyses were stratified by age (<40 or ≥40 years), BMI (< 24.0 or ≥24.0), physical activity (<23 or ≥23 MET-h/wk), MetS (yes or no), amenorrhea status (yes or no), an education level (college graduate or not), and household income (<10,000 or ≥10,000 yuan). The associations between H. pylori infection status and the risk of subclinical hyperthyroidism were generally similar across all subgroups (Table 3). All interactions were not statistically significant (P for interaction > 0.05), except for age (P for interaction = 0.05). Stratified analyses indicated that H. pylori infection was associated with subclinical hyperthyroidism in individuals aged ≥ 40 but not in individuals aged < 40. Similar results were also found in the sensitivity analysis (Table 4). When we adjusted for WC instead of BMI, the full model-adjusted HRs 95% CI for subclinical hyperthyroidism in women were 2.48 (1.36, 4.54; p = 0.003), and the associations did not substantially change. When we included participants with a history of cancer or CVD, the analyses yielded very similar results, the adjusted HRs (95% CIs) of subclinical hyperthyroidism were 2.03 (1.13, 3.64; p = 0.019).
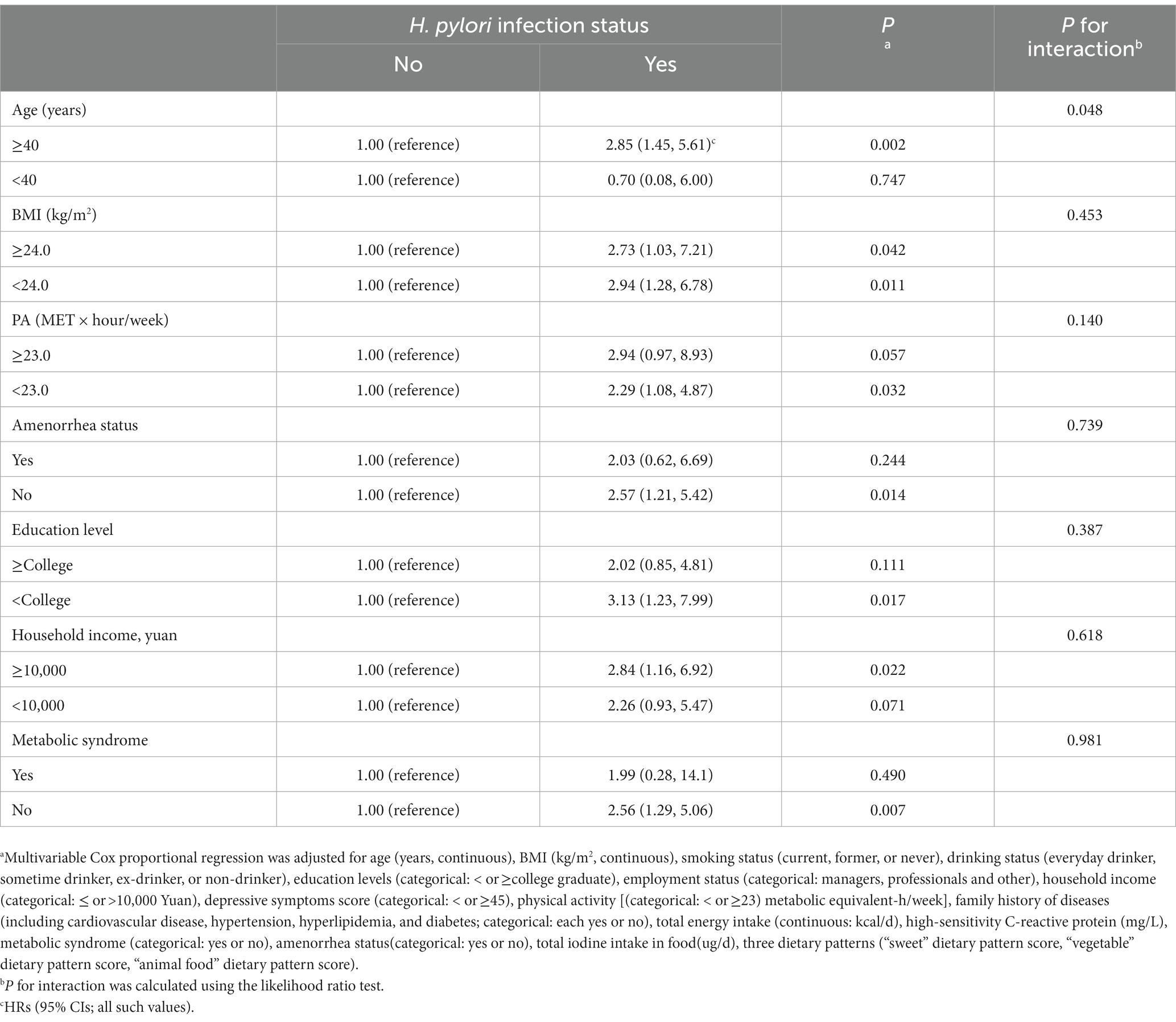
Table 3. Association between Helicobacter pylori infection status and risk of Subclinical hyperthyroidism in women stratified by major covariates.
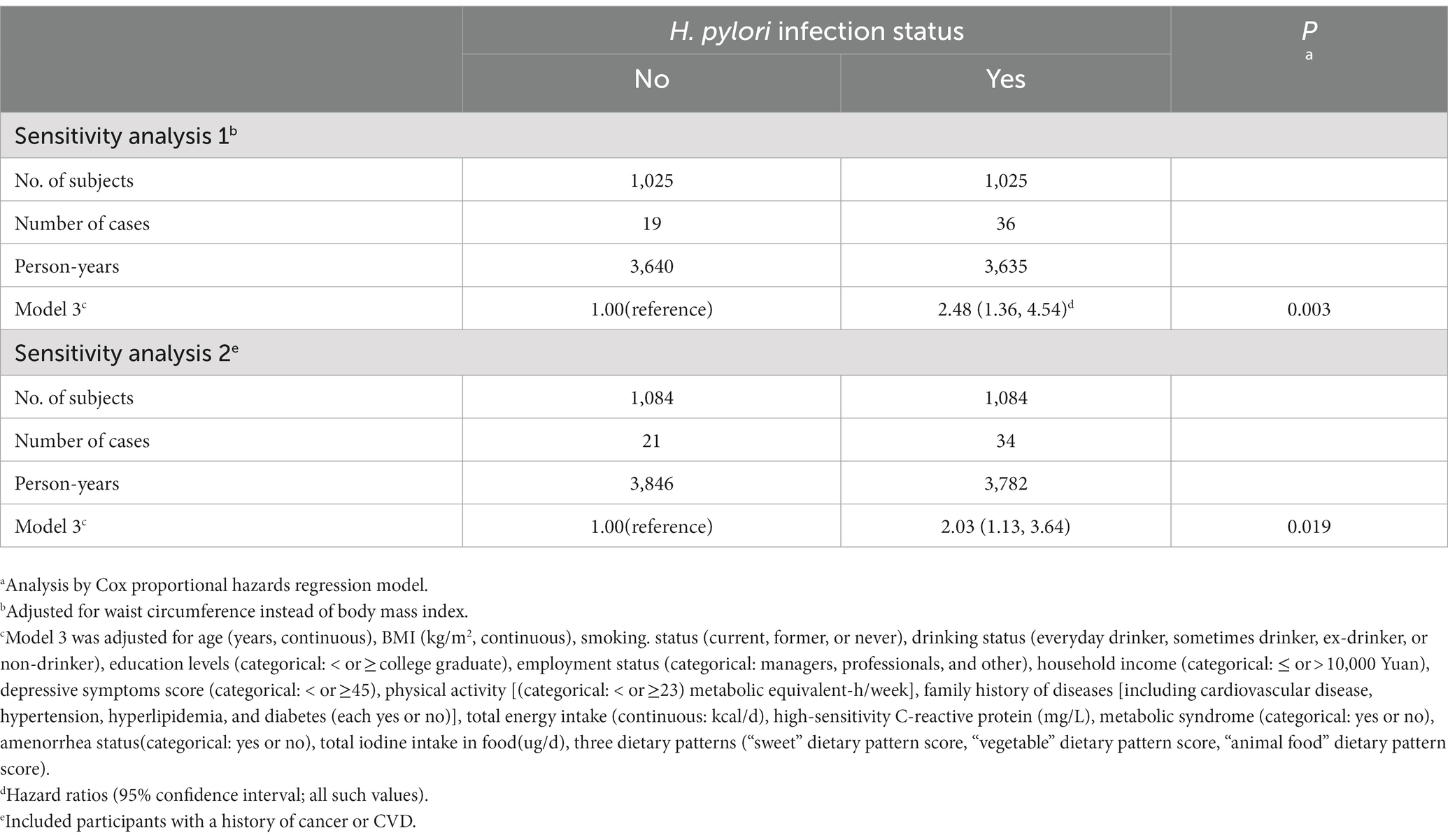
Table 4. Sensitivity analysis of the association between Helicobacter pylori infection status and Subclinical hyperthyroidism in women.
Discussion
In this large-scale prospective study of a Chinese adult population, we have assessed the association between H. pylori infection and the incidence of subclinical hyperthyroidism in women. Considering the potential effect of iodine on subclinical hyperthyroidism, we conducted this cohort study in Tianjin, an iodine-replete area, and further adjusted for total iodine intake in food in Model 2. Meanwhile, dietary factors are not only associated with H. pylori infection (33) but also affect the risk of subclinical hyperthyroidism (34), however, no previous cohort study adjusted for dietary intake when exploring the associations between H. pylori infection and the incidence of subclinical hyperthyroidism, indicating that the risk effect of H. pylori infection on incident subclinical hyperthyroidism might be misestimated. In this large prospective cohort study, we adjusted for dietary factors in the form of three dietary patterns in Model 3, thus, can prevent the influence of dietary factors on the association between H. pylori infection and subclinical hyperthyroidism. We found that H. pylori infection was associated with an increased risk of incidents of subclinical hyperthyroidism independent of dietary factors in women, a series of sensitivity and subgroup analyses supported these results. To the best of our knowledge, this study is the first prospective cohort investigation regarding the association between H. pylori infection and subclinical hyperthyroidism independent of dietary factors in women.
The present results suggested that H. pylori infection was significantly associated with subclinical hyperthyroidism in women, which seems to be in agreement with previous studies (16, 35). Although the exact mechanisms of H. pylori infection in subclinical hyperthyroidism have not been elucidated, some evidence may explain the association. First, it was recently discovered that H. pylori strains can express fucosylated Lewis determinants, which are widely shared by different host tissues and may stimulate an autoimmune response that could potentially damage the thyroid gland (36). Second, a previous study has shown that a homologous 11-residue peptide in both gastric parietal cell antigen and thyroid peroxidase suggests the existence of an epitope common to both antigens (37). Therefore, antibodies are produced during H. pylori infection may cross-react with thyroid antigens, resulting in subclinical hyperthyroidism (38). Furthermore, evidence that H. pylori infection can increase the risk of subclinical hyperthyroidism also comes from the strong correlation between IgG anti-H. pylori antibodies and thyroid autoantibodies, as well as the observation that thyroid auto-antibodies levels gradually decrease after eradication of H. pylori infection (39). Taken together, these studies provide potential explanations linking H. pylori infection and subclinical hyperthyroidism.
We found a significant association between H. pylori infection and subclinical hyperthyroidism in middle-aged and elderly women but not in younger women. This association may be caused by two reasons. First, the successful colony of H. pylori in the stomach requires age-related gastric physiology and special characteristics related to the host (40), and H. pylori-related diseases are increasing with increasing age (41). Second, the thyroid gland would undergo important functional changes during aging, the clinical course of thyroid diseases is different between the elderly and the young. A previous study showed a degree of insensitivity in thyroid cells in the anterior pituitary gland in older adults, occurring with age, mainly manifested as the decrease of TSH secretion of thyrotropin-releasing hormone (TRH) in the elderly, and the serum TSH level is usually higher in older than in young people in response to the decrease of thyroid hormone concentration (42). Further exploration is required to clarify this issue.
The major strengths of this study are it first assessed the association between H. pylori infection and subclinical hyperthyroidism in a large-scale adult population from an iodine-replete area. It is a prospective dynamic study design, which allowed us to examine the long-term effect of H. pylori infection on thyroid function before the occurrence of subclinical hyperthyroidism. Furthermore, we applied the PSM in the H. pylori-infected and -uninfected so we could observe the balance of confounding factors between groups more intuitively in comparing matched groups. Thus, the distribution of covariates between the two groups was balanced and the bias in the observational study was reduced. Moreover, we performed several sensitivity analyses to confirm the robustness of the findings. Nonetheless, several biases and limitations should be considered in the present study. First, although our analyses adjusted for a considerable number of confounders, we could not rule out residual confounding by other unmeasured or unknown factors. Second, the clinical outcome of H. pylori infection may be determined by a combination of virulence among H. pylori strains, duration of infection, host genetic polymorphisms, specific host–microbe interactions, and environmental factors (43). However, our study was based on data from health check-ups; H. pylori strains, eradication of H. pylori infection, and genome-wide association studies (GWAS) were not investigated. CagA-positive strains are endowed with an enhanced inflammatory potential (44) and affect patients with hyperthyroid Graves’s disease more frequently (45). Previous studies supported the finding that eradicating H. pylori infection could lead to the restitution of the cellular immune response (46) and reduce thyroid autoantibodies (14). In addition, one study found the presence of a gene encoding an endogenous peroxidase in the dissected chromosome of an H. pylori strain (47). Therefore, the lack of data on H. pylori might modify the strength of the study results, underestimating the observed associations. Third, although subjects with a self-reported history of all types of thyroid disease, including positive thyroid autoantibodies, were excluded based on a detailed structured questionnaire, serum thyroid autoantibodies were not measured in the current study. Previous reviews (48) have shown that positive thyroid autoantibodies can predict the risk of future thyroid dysfunction, and studies have shown that thyroid autoantibodies reduce the risk of H. pylori infection after eradication (39), suggesting that the lack of data on thyroid autoantibodies may lead to underestimation of the risk of H. pylori infection to subclinical hyperthyroidism. Finally, H. pylori infection rates vary according to geographic region (49), and our study was performed in the general adult Chinese population, therefore, it is possible that our results cannot be generalized to other populations. Further studies are needed to verify the results in other populations.
Conclusion
In conclusion, the findings of this population-based cohort study suggested that H. pylori infection was associated with the risk of subclinical hyperthyroidism independent of dietary factors in adult women. More studies should be performed on different populations to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by UMIN Clinical Trials Registry. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JZ and XH analyzed data and wrote the paper. SW, FZ, YG, GM, QZ, LL, HW, SZ, TZ, XW, SS, MZ, QJ, and KS conducted research. KN designed research and had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81941024, 81872611, 82103837, and 81903315), Tianjin Major Public Health Science and Technology Project (No. 21ZXGWSY00090), National Health Commission of China (No. SPSYYC 2020015), Food Science and Technology Foundation of Chinese Institute of Food Science and Technology (No. 2019-12), 2014 and 2016 Chinese Nutrition Society (CNS) Nutrition Research Foundation—DSM Research Fund (Nos. 2016-046, 2014-071 and 2016-023), China.
Acknowledgments
The authors gratefully acknowledge all the people that have made this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1002359/full#supplementary-material
Abbreviations
H. pylori, Helicobacter pylori; Subclinical hyperthyroidism, Subclinical thyroid dysfunction; FT3, Free triiodothyronine; FT4, Free thyroxine; HR, Hazard ratio; THs, Thyroid hormones; T3, Triiodothyronine; T4, Thyroxine; TSH, Thyroid-stimulating hormone; CI, Confidence interval; CVD, Cardiovascular disease; AITDs, Autoimmune thyroid diseases; TCLSIH, Tianjin Chronic Low-grade Systemic Inflammation and Health; 13C-UBT, 13C urea breath test; BMI, Body mass index; PA, Physical activity; FFQ, Food Frequency Questionnaire; WC, Waist circumference; FBG, Fasting blood glucose; TG, Triglycerides; TC, Total cholesterol; LDL, Low-density lipoprotein cholesterol; HDL, High-density lipoprotein cholesterol; BP, Blood pressure.
References
1. Mullur, R, Liu, YY, and Brent, GA. Thyroid hormone regulation of metabolism. Physiol Rev. (2014) 94:355–82. doi: 10.1152/physrev.00030.2013
2. Chiamolera, MI, and Wondisford, FE. Minireview: thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. (2009) 150:1091–6. doi: 10.1210/en.2008-1795
3. Cooper, DS, and Biondi, B. Subclinical thyroid disease. Lancet. (2012) 379:1142–54. doi: 10.1016/S0140-6736(11)60276-6
4. Biondi, B, and Cooper, DS. Subclinical hyperthyroidism. N Engl J Med. (2018) 378:2411–9. doi: 10.1056/NEJMcp1709318
5. Alwan, H, Villoz, F, Feller, M, Dullaart, RPF, and Bakker, SJL. Subclinical thyroid dysfunction and incident diabetes: a systematic review and an individual participant data analysis of prospective cohort studies. Eur J Endocrinol. (2022) 187:S35–s46. doi: 10.1530/EJE-22-0523
6. Wang, X, Zhao, X, and Huang, X. Association of Subclinical Thyroid Dysfunction with chronic kidney disease: a systematic review and meta-analysis. Endocr Res. (2020) 45:41–9. doi: 10.1080/07435800.2019.1645164
7. Vidili, G, Delitala, A, and Manetti, R. Subclinical hyperthyroidism: the cardiovascular point of view. Eur Rev Med Pharmacol Sci. (2021) 25:3264–71. doi: 10.26355/eurrev_202104_25735
8. Tsai, K, and Leung, AM. Subclinical hyperthyroidism: a review of the clinical literature. Endocr Pract. (2021) 27:254–60. doi: 10.1016/j.eprac.2021.02.002
9. Hooi, JKY, Lai, WY, Ng, WK, Suen, MMY, Underwood, FE, Tanyingoh, D, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. (2017) 153:420–9. doi: 10.1053/j.gastro.2017.04.022
10. Zamani, M, and Ebrahimtabar, F. Systematic review with meta-analysis: the worldwide prevalence of helicobacter pylori infection. Aliment Pharmacol Ther. (2018) 47:868–76. doi: 10.1111/apt.14561
11. Fiorini, G, Zullo, A, Castelli, V, Lo Re, G, Holton, J, and Vaira, D. Role of helicobacter pylori infection in the thyroid diseases. J Gastrointestin Liver Dis. (2013) 22:261–3.
12. Pellicano, R, Ianiro, G, Fagoonee, S, Settanni, CR, and Gasbarrini, A. Review: Extragastric diseases and helicobacter pylori. Helicobacter. (2020) 25:e12741. doi: 10.1111/hel.12636
13. Valtonen, VV, Ruutu, P, Varis, K, Ranki, M, Malkamäki, M, and Mäkelä, PH. Serological evidence for the role of bacterial infections in the pathogenesis of thyroid diseases. Acta Med Scand. (1986) 219:105–11.
14. Hou, Y, Sun, W, Zhang, C, Wang, T, Guo, X, Wu, L, et al. Meta-analysis of the correlation between helicobacter pylori infection and autoimmune thyroid diseases. Oncotarget. (2017) 8:115691–700. doi: 10.18632/oncotarget.22929
15. de Luis, DA, Varela, C, de La Calle, H, Cantón, R, de Argila, CM, San Roman, AL, et al. Helicobacter pylori infection is markedly increased in patients with autoimmune atrophic thyroiditis. J Clin Gastroenterol. (1998) 26:259–63. doi: 10.1097/00004836-199806000-00008
16. Shi, WJ, Liu, W, Zhou, XY, Ye, F, and Zhang, GX. Associations of helicobacter pylori infection and cytotoxin-associated gene a status with autoimmune thyroid diseases: a meta-analysis. Thyroid. (2013) 23:1294–300. doi: 10.1089/thy.2012.0630
18. Ihnatowicz, P, Drywień, M, Wątor, P, and Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto's thyroiditis. Ann Agric Environ Med. (2020) 27:184–93. doi: 10.26444/aaem/112331
19. Raei, N, Behrouz, B, Zahri, S, and Latifi-Navid, S. Helicobacter pylori infection and dietary factors act synergistically to promote gastric cancer. Asian Pac J Cancer Prev. (2016) 17:917–21. doi: 10.7314/APJCP.2016.17.3.917
20. Fan, L, Tan, L, Chen, Y, Du, C, Zhu, M, Wang, K, et al. Investigation on the factors that influence the prevalence of thyroid nodules in adults in Tianjin, China. J Trace Elem Med Biol. (2018) 50:537–42. doi: 10.1016/j.jtemb.2018.03.004
21. Hou, C, Liu, Z, Cui, Y, Wang, Y, Fu, G, Liu, H, et al. An investigation of iodine intake and iodine nutritional status of adults in Tianjin City[in Chinese]. Chin J Endemiol. (2016) 35:138–42.
22. Zhang, S, Fu, J, Zhang, Q, Liu, L, Meng, G, Yao, Z, et al. Association between nut consumption and non-alcoholic fatty liver disease in adults. Liver Int. (2019) 39:1732–41. doi: 10.1111/liv.14164
23. Gu, Y, Zheng, L, Zhang, Q, Liu, L, Meng, G, Yao, Z, et al. Relationship between thyroid function and elevated blood pressure in euthyroid adults. J Clin Hypertens. (2018) 20:1541–9. doi: 10.1111/jch.13369
24. Chaker, L, Bianco, AC, Jonklaas, J, and Peeters, RP. Hypothyroidism. Lancet. (2017) 390:1550–62. doi: 10.1016/S0140-6736(17)30703-1
25. Logan, RP. Urea breath tests in the management of helicobacter pylori infection. Gut. (1998) 43:S47–50.
26. Li, H, Gu, Y, Wu, X, Rayamajhi, S, Bian, S, Zhang, Q, et al. Association between consumption of edible seaweeds and newly diagnosed non-alcohol fatty liver disease: the TCLSIH cohort study. Liver Int. (2021) 41:311–20. doi: 10.1111/liv.14655
27. Xia, Y, Xiang, Q, Gu, Y, Jia, S, Zhang, Q, Liu, L, et al. A dietary pattern rich in animal organ, seafood and processed meat products is associated with newly diagnosed hyperuricaemia in Chinese adults: a propensity score-matched case-control study. Br J Nutr. (2018) 119:1177–84. doi: 10.1017/S0007114518000867
28. Alberti, KG, Eckel, RH, Grundy, SM, Zimmet, PZ, Cleeman, JI, Donato, KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
29. Wu, H, Liu, M, Chi, VTQ, Wang, J, Zhang, Q, Liu, L, et al. Handgrip strength is inversely associated with metabolic syndrome and its separate components in middle aged and older adults: a large-scale population-based study. Metab Clin Exp. (2019) 93:61–7. doi: 10.1016/j.metabol.2019.01.011
30. Austin, PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. (2011) 10:150–61. doi: 10.1002/pst.433
31. Austin, PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
32. Xia, Y, Meng, G, Zhang, Q, Liu, L, Wu, H, Shi, H, et al. Dietary patterns are associated with helicobacter pylori infection in Chinese adults: a cross-sectional study. Sci. Rep. (2016) 30:32334. doi: 10.1038/srep32334
33. Monno, R, De Laurentiis, V, Trerotoli, P, Roselli, AM, Ierardi, E, and Portincasa, P. Helicobacter pylori infection: association with dietary habits and socioeconomic conditions. Clin Res Hepatol Gastroenterol. (2019) 43:603–7. doi: 10.1016/j.clinre.2018.10.002
34. Zhang, J, Gu, Y, Meng, G, Zhang, Q, Liu, L, Wu, H, et al. Association between dietary onion intake and subclinical hypothyroidism in adults: a population-based study from an iodine-replete area. Endocrine. (2021) 74:616–24. doi: 10.1007/s12020-021-02790-2
35. Figura, N, Di Cairano, G, Lorè, F, Guarino, E, Gragnoli, A, Cataldo, D, et al. The infection by helicobacter pylori strains expressing CagA is highly prevalent in women with autoimmune thyroid disorders. J Physiol Pharmacol. (1999) 50:817–26.
36. Wirth, HP, Yang, M, Karita, M, and Blaser, MJ. Expression of the human cell surface glycoconjugates Lewis x and Lewis y by helicobacter pylori isolates is related to cagA status. Infect Immun. (1996) 64:4598–605. doi: 10.1128/iai.64.11.4598-4605.1996
37. Elisei, R, Mariotti, S, Swillens, S, Vassart, G, and Ludgate, M. Studies with recombinant autoepitopes of thyroid peroxidase: evidence suggesting an epitope shared between the thyroid and the gastric parietal cell. Autoimmunity. (1990) 8:65–70. doi: 10.3109/08916939008998434
38. Choi, YM, Kim, TY, Kim, EY, Jang, EK, Jeon, MJ, Kim, WG, et al. Association between thyroid autoimmunity and helicobacter pylori infection. Korean J Intern Med. (2017) 32:309–13. doi: 10.3904/kjim.2014.369
39. Bertalot, G, Montresor, G, Tampieri, M, Spasiano, A, Pedroni, M, Milanesi, B, et al. Decrease in thyroid autoantibodies after eradication of helicobacter pylori infection. Clin Endocrinol. (2004) 61:650–2. doi: 10.1111/j.1365-2265.2004.02137.x
40. Huang, JY, Sweeney, EG, Sigal, M, Zhang, HC, Remington, SJ, Cantrell, MA, et al. Chemodetection and destruction of host urea allows helicobacter pylori to locate the epithelium. Cell Host Microbe. (2015) 18:147–56. doi: 10.1016/j.chom.2015.07.002
41. Goh, KL, Chan, WK, Shiota, S, and Yamaoka, Y. Epidemiology of helicobacter pylori infection and public health implications. Helicobacter. (2011) 16:1–9. doi: 10.1111/j.1523-5378.2011.00874.x
42. Lewiński, ASE, and Karbownik, M. Aging processes and the thyroid gland In: M Karasek, editor. Aging and age-related diseases: The basics. New York: Nova Science Publishers, Inc (2006). 131–72.
43. Shukla, SK, Singh, G, Ahmad, S, and Pant, P. Infections, genetic and environmental factors in pathogenesis of autoimmune thyroid diseases. Microb Pathog. (2018) 116:279–88. doi: 10.1016/j.micpath.2018.01.004
44. Collodel, G, Moretti, E, Campagna, MS, Capitani, S, Lenzi, C, and Figura, N. Infection by CagA-positive helicobacter pylori strains may contribute to alter the sperm quality of men with fertility disorders and increase the systemic levels of TNF-alpha. Dig Dis Sci. (2010) 55:94–100. doi: 10.1007/s10620-008-0704-1
45. Figura, N, Di Cairano, G, and Moretti, E. Helicobacter pylori infection and autoimmune thyroid diseases: the role of virulent strains. Antibiotics. (2019) 9:12. doi: 10.3390/antibiotics9010012
46. Astl, J, and Šterzl, I. Activation of helicobacter pylori causes either autoimmune thyroid diseases or carcinogenesis in the digestive tract. Physiol Res. (2015) 64:S291–301. doi: 10.33549/physiolres.933118
47. Tomb, JF, White, O, Kerlavage, AR, Clayton, RA, Sutton, GG, Fleischmann, RD, et al. The complete genome sequence of the gastric pathogen helicobacter pylori. Nature. (1997) 388:539–47. doi: 10.1038/41483
48. Prummel, MF, and Wiersinga, WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab. (2005) 19:1–15. doi: 10.1016/j.beem.2004.11.003
Keywords: Helicobacter pylori, subclinical hyperthyroidism, dietary factors, propensity score matching, cohort study
Citation: Zhang J, Hai X, Wang S, Zhu F, Gu Y, Meng G, Zhang Q, Liu L, Wu H, Zhang S, Zhang T, Wang X, Sun S, Zhou M, Jia Q, Song K and Niu K (2023) Helicobacter pylori infection increase the risk of subclinical hyperthyroidism in middle-aged and elderly women independent of dietary factors: Results from the Tianjin chronic low-grade systemic inflammation and health cohort study in China. Front. Nutr. 10:1002359. doi: 10.3389/fnut.2023.1002359
Edited by:
Zeinab Ghorbani, Guilan University of Medical Sciences, IranReviewed by:
Ehab Tousson, Tanta University, EgyptDaoyan Wu, Guizhou Medical University, China
Asankha Pallegedara, Wayamba University of Sri Lanka, Sri Lanka
Copyright © 2023 Zhang, Hai, Wang, Zhu, Gu, Meng, Zhang, Liu, Wu, Zhang, Zhang, Wang, Sun, Zhou, Jia, Song and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yeqing Gu, bml1a2FpanVuQHRtdS5lZHUuY24=; Kaijun Niu, bmtqMDgwOUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Juanjuan Zhang1†
Juanjuan Zhang1† Yeqing Gu
Yeqing Gu Hongmei Wu
Hongmei Wu Qiyu Jia
Qiyu Jia Kaijun Niu
Kaijun Niu