
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 03 October 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.999995
This article is part of the Research Topic Nutritional Status in Disease: Development, Evaluation, Prognosis and Individualized Treatment in Clinical Practice View all 23 articles
Background: Lipid metabolism disorders contribute to the risk factor of prostatic hyperplasia. Lipid ratios have also attracted a lot of attention. Yet, research about the correlation of lipid ratios with prostatic hyperplasia is limited. Hence, the aim of this study was to investigate the association of lipid ratios with the risk of benign prostatic hyperplasia (BPH) in Chinese male subjects.
Methods: Healthy men who underwent routine health check-ups from January 2017 to December 2019 were recruited. Twenty-four thousand nine hundred sixty-two individuals were finally enrolled in this research. Binary logistic regression analysis was performed to investigate the relationship between lipid ratios and BPH in Chinese adults.
Results: After health examinations for more than 2 years, 18.46% of subjects were ascertained as incident BPH cases. Higher age, body mass index (BMI), prostate-specific antigen (PSA), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, total cholesterol to high-density lipoprotein cholesterol (TC/HDL-C) ratio, and lower high-density lipoprotein cholesterol (HDL-C) were significantly associated with BPH risk, while total cholesterol (TC) was not significant. When quartiles of TG/HDL-C and TC/HDL-C were analyzed in multivariable model, higher TG/HDL-C and TC/HDL-C were associated with a risk of BPH (odds ratio [OR] = 2.11; 95% confidence interval [CI]: 1.89, 2.36; P-trend < 0.001; and OR = 1.67; 95% CI: 1.50, 1.85; P-trend < 0.001, respectively). In addition, stratified analyses based on the general population exhibited that with increasing age (≥35 years) the relationship of TG/HDL-C ratio with BPH risk was dominantly positive (all P-trend < 0.001, P-interaction = 0.001), and significant associations were also found in blood pressure strata and FBG strata (all P-trend < 0.001), except men with BMI ≥ 28 kg/m2 were slightly weakened (OR = 2.01, 95% CI: 1.41, 2.85; P-trend = 0.04). Moreover, there were significant associations between quartiles of TC/HDL-C and the risk of BPH was observed mainly in age 55–64 years, BMI 18.5–23.9 Kg/m2, blood pressure strata, and FBG strata. However, the P-value for a linear trend among those with BMI ≥ 28 Kg/m2 in which participants at the highest quartile of TC/HDL-C had an OR of 1.45 (95% CI: 1.09, 1.93) was 0.594. Additionally, higher TG/HDL-C ratio (≥0.65) may be a risk factor for BPH in China adults of different age decades (≥35 years) with normal TG and HDL-C.
Conclusions: TG/HDL-C and TC/HDL-C were associated with BPH risk, TG/HDL-C was a powerful independent risk factor for BPH in Chinese adults, and higher TG/HDL-C ratio should be valued in male subjects with normal TG and HDL-C levels.
With the development of society and economy, the rapidly aging problem is prominent, especially in China. BPH is a common disease in elderly men and has become an important public health challenge (1). BPH, also known as benign prostatic obstruction, is a histologic diagnosis that refers to non-malignant proliferation and unregulated growth of the epithelial cells and stromal cells (2). Lower urinary tract symptoms (LUTSs) secondary to BPH (BPH/LUTS) include urinary retention, bifurcation of urination, and pause in urine stream (3, 4), among others. Thus, if the control of BPH has been ineffective, the probability is that LUTS will rise accordingly. Furthermore, BPH increased from 2.17 to 31.11% within the age ranging from 40 to 80 or older (5), and kidneys may become damaged over time as the symptoms of BPH/LUTS intensify. China’s seventh national census shows that the proportion of the population aged over 60 years is 18.70% and increased by 5.44% than 10 years ago (6). This is undoubtedly a powerful reserve for the prostatic hyperplasia population. In addition to that, the epidemiological status of BPH, high prevalence of LUTS/BPH, the severity of the disease, treatment methods, etc. (7–9), all of which may lead to the cost of illness (COI) becoming a major challenge in future. Hence, strengthening the health management and early intervention of BPH will significantly reduce the burden on the elderly population and society.
Currently, the pathogenesis of BPH still remains unclear, except that age is the strongest factor. Various studies (2, 3, 10) have been devoted to revealing the underlying molecular etiology of this common disease, such as observational study, genetic analysis, and biochemical and histochemical analyses. Fujita et al. pointed out that the white blood cell (WBC) count and the neutrophil (NEUT) count were correlated with prostate volume (11), and De Nunzio et al. reported that the mediators of inflammation played a role in the course of the development and progression of BPH (12). Insufficient physical activity, poor eating habits, and obesity are the most common causes of metabolic syndrome (MetS). This phenomenon has triggered focus attention to the role of the Mets in the life cycle. Data have shown a possible relationship between MetS and BPH, such as hyperlipemia, adiposity, hyperglycemia, hypertension (13–16). Especially low HDL-C and hypertriglyceridemia levels were proved to be closely correlated with BPH (17–19). Inflammatory response may be the pathogenesis of BPH caused by dyslipidemia (20). Although, the association of MetS with prostatic hyperplasia has been researched for many years. An in-depth description of the role of lipid components in BPH remains promising to open new perspectives into the occurrence and progression of disease.
BPH risk is associated with an aberrant lipid profile in many races. Previous research in Turkish male subjects showed that TG/HDL-C was significantly related to prostate volume (21). However, the TC/HDL-C ratio was not assessed, and the correlation between TG/HDL-C and prostatic hyperplasia was also not further analyzed in different age decades and in Turkish adults with normal TG and HDL-C levels. To deeply elucidate the relationships of lipid ratios with BPH in the general population and in participants only with normal TG and HDL-C, and to investigate the association between BPH and lipid ratios in different age decades, we conducted a survey using a population-based dataset in China.
A total of 30,481 health screening population who visited the Health Promotion Center of the First Affiliated Hospital of Nanjing Medical University were recruited for this study between January 2017 and December 2019. The participants were predominantly from China, and all of the subjects were classified into BPH group and non-BPH group. The gold standard for prostate examination is prostate pathological cytology; however, it is not recommended for large-scale population studies due to its invasiveness, the risk of complications, and high cost. Expensive MRI examination has also not found an increasingly wide utilization in detecting prostate except for patients with suspected prostate cancer. Herein, the criteria for inclusion in this trial are abdominal ultrasound imaging with no suspicion of prostate cancer, and the volume of the prostate is determined by height (cm) × width (cm) × length (cm) × π/6 based on the abdominal ultrasound measurements, the normal volume is 20–30 mL, the secretion of PSA is influenced by prostate volume size, so there is no special requirement for PSA levels (22). It is noteworthy that the individuals enrolled in the group had undergone health examinations for more than 2 years, which was helpful to exclude inappropriate candidates. The participants with prostatitis, severe liver and kidney dysfunction, cardiac insufficiency, and taking anti-lipidemic drugs or glucose-lowering medication, hormonal and weight-loss drugs, blood pressure-lowering drugs were excluded, as were those subjects with incomplete information. Finally, 24,962 participants were enrolled in this study (Figure 1).
All participants gave written consent to participate. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (ethics committee approval number: 2019-SR-478), also based on the Declaration of Helsinki (as revised in 2013).
Data collection was performed by well-trained staff from the above hospital. BMI was calculated by dividing the weight (kg) by the square of height (m), according to the statement of Working Group on Obesity in China, BMI greater than or equal to 28 kg/m2 is defined as obesity (23). The electronic sphygmomanometer was used to measure blood pressure for three times. Blood samples were collected from subjects after being fasted overnight for more than 8 h. Biochemical parameters like PSA, fasting blood glucose (FBG), TG, TC, LDL-C, and HDL-C were assessed with the Chemistry Analyzer AU5800 (Olympus Corporation, Tokyo, Japan).
P–P plots were used to determine the normal distribution of the variables. Continuous variables were presented as mean ± SD (standard deviations) and compared using an independent t-test. Mann–Whitney U test was used to analyze the skewed variables represented as the median and interquartile range (IQR).
The associations of lipid ratios with BPH were further determined by binary logistic regression analysis. In model 1, age and PSA were adjusted. Model 2 was based on Model 1 by adding BMI and FBG. WBC, NEUT, and hypertension were additionally calibrated in Model 3. These gradual multivariable models were carried out to determine whether the relationships of lipid ratios with BPH were robust. Linear trends were performed with the use of quartiles of the lipid ratios as a continuous variable by calculating the corresponding median TG/HDL-C and TC/HDL-C for each quartile, respectively.
Additionally, stratified analysis was further conducted on all participants. To decrease the impact of outliers, the subjects were stratified into five age groups (<35, 35–44, 45–54, 55–64, ≥65 years), three BMI groups (18.5–23.9, 24–27.9, ≥28 Kg/m2), two blood pressure groups (normal, hypertension), and two FBG groups (<6.1, ≥6.1 mmol/L), and each subgroup was classified into quartiles according to TG/HDL-C and TC/HDL-C levels, with the lowest quartile being the reference group. The stratified analysis of TG/HDL-C on the risk of BPH was also performed among male subjects with normal TG and HDL-C levels by age (<35, 35–44, 45–54, 55–64, ≥65 years), BMI (<18.5, 18.5–23.9, 24–27.9, ≥28 Kg/m2), blood pressure levels (normal and hypertension), and FBG (<6.1 and ≥6.1 mmol/L). Model a: WBC, NEUT, PSA, hypertension, FBG, and BMI were adjusted. Model b: age, WBC, NEUT, PSA, hypertension, and FBG were adjusted. Model c: age, WBC, NEUT, PSA, FBG, and BMI were adjusted. Model d: age, WBC, NEUT, PSA, hypertension, and BMI were adjusted. The likelihood ratio test (LRT) was used to investigate the interactions between lipid ratios and other variables.
All of the data were carried out using SPSS version 23.0 (IBM, Chicago, IL, USA). A P-value < 0.05 (two-sided) was considered statistically significant. The significance of multicollinearity phenomena in multivariate regression analyses was assessed by calculating the tolerance and variance inflation factor (VIF).
Baseline characteristics of 24,962 participants included in this study were displayed in Table 1. The mean age, TC, HDL-C, BMI, and the median TG, PSA of the study population were 46.15 ± 13.24 years, 5.06 ± 0.8 mmol/L, 1.22 ± 0.24 mmol/L, 25.04 ± 2.96 kg/m2, 1.45 mmol/L, 0.88 ng/ml, respectively.
There were 18.46% participants with BPH and had higher levels of age, NEUT, FBG, TG, LDL-C, PSA, SBP, DBP, BMI, but lower HDL-C levels. In addition, the values of TG/HDL-C and TC/HDL-C were also notably different between the BPH group and non-BPH group. However, no significant difference was found in TC between the two groups.
The associations of TG/HDL-C and TC/HDL-C ratios with BPH were evaluated in models 1–3 as shown in Table 2. According to TG/HDL-C and TC/HDL-C levels, 24,962 participants were classified into quartiles, respectively. In the unadjusted model, the ORs (95% CI) of BPH across increasing quartiles of TG/HDL-C and TC/HDL-C were 1.00, 1.59 (1.44–1.76), 1.82 (1.65–2.00), 1.98 (1.80–2.18), and 1.00, 1.21 (1.10–1.33), 1.47 (1.34–1.61), and 1.58 (1.44–1.73) (all P-trend < 0.001), respectively. Higher TG/HDL-C and TC/HDL-C were associated with a significantly high risk of BPH when adjusting for age and PSA; the ORs (95% CI) were 2.25 (2.03–2.50) and 1.83 (1.65–2.02) (all P-trend < 0.001), respectively. The associations were still significant after further adjustment for potential confounders (WBC, NEUT, FBG, BMI, and hypertension); the ORs (95% CI) were 2.11 (1.89–2.36), and 1.67 (1.50–1.85) (all P-trend < 0.001), respectively.
To further investigate the associations of TG/HDL-C and TC/HDL-C with BPH risk, stratified multivariable logistic regression analyses were conducted to calculate the adjusted ORs (95% CI). Taking into account the study power of the stratified analyses, each subgroup is categorized by age strata, BMI strata, blood pressure strata, and FBG strata, respectively. A significant positive correlation between TG/HDL-C and risk of BPH was found in 11 different subgroups except for men aged below 35 years (Table 3), and a linear trend among quartiles of TG/HDL-C and BPH risk in these 11 subgroups was also significant. Moreover, only six different subgroups (age 55–64 years, BMI 18.5–23.9 Kg/m2, normal blood pressure or hypertension, FBG < 6.1 or ≥ 6.1 mmol/L) showed that the relationship of TC/HDL-C with BPH risk was significant. The multivariate-adjusted ORs (95% CI) of BPH in men aged 65 years and above or BMI 18.5–23.9 Kg/m2 or normal blood pressure or FBG < 6.1 mmol/L for the highest quartile of TG/HDL-C compared with the lowest were 2.04 (1.55–2.68), 2.61 (2.15–3.16), 2.21 (1.92–2.55), and 2.09 (1.85–2.37), while the ORs (95% CI) of BPH were 1.52 (1.19–1.95), 2.24 (1.86–2.69), 1.56 (1.36–1.78), and 1.55 (1.38–1.75) for TC/HDL-C. Additionally, the interactions of TG/HDL-C with age strata, BMI strata, and FBG strata were all significant, while a test of interaction between TG/HDL-C with blood pressure strata on BPH was not (P-interaction = 0.379). And the siginificant interaction effects were also detected between TC/HDL-C and age strata, BMI strata, FBG strata, and blood pressure strata.
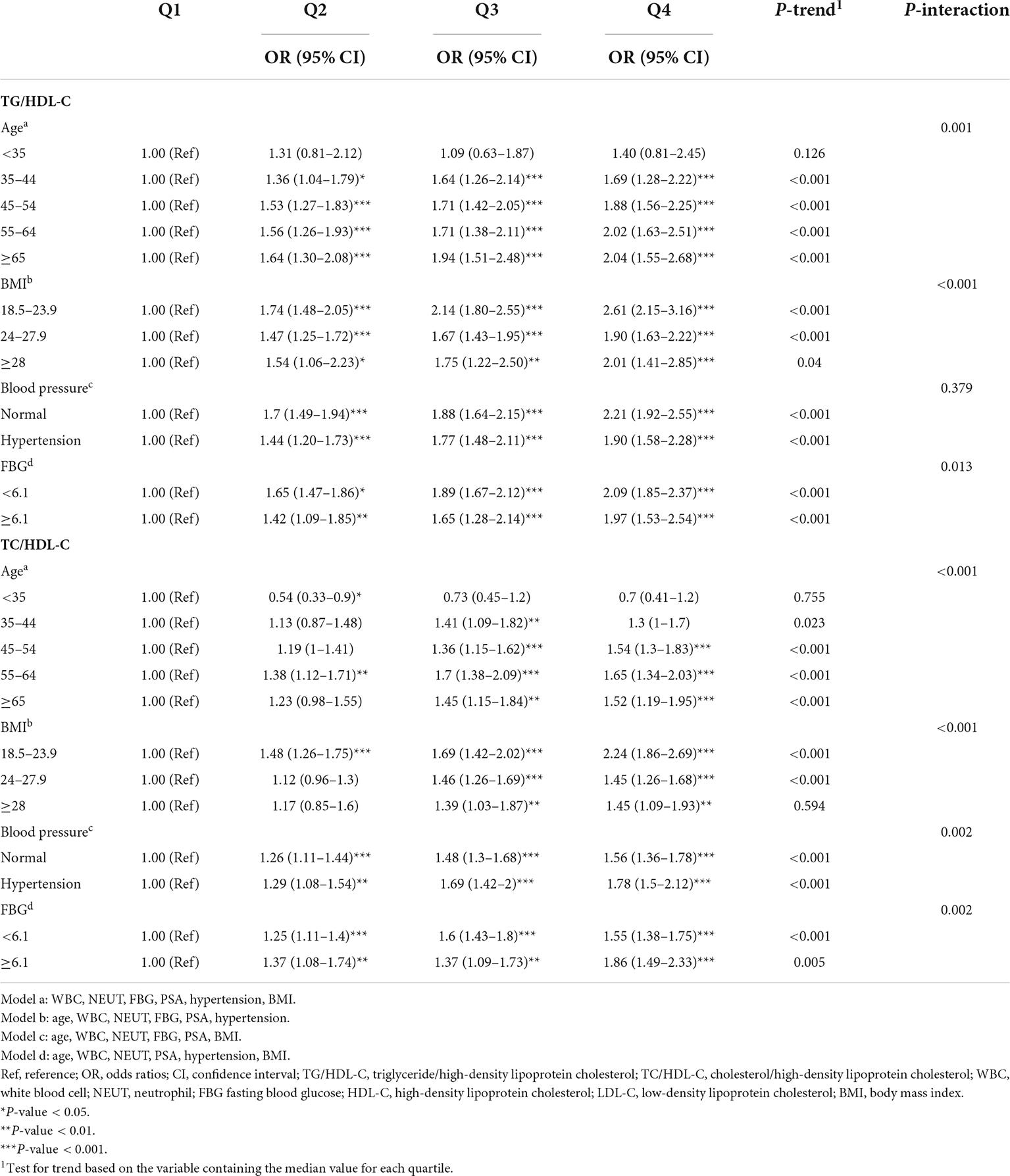
Table 3. ORs of BPH by quartiles of TG/HDL-C and TG/HDL-C stratified by age, BMI, blood pressure, and FBG.
Additionally, 13,983 subjects with normal TG (<1.7 mmol/L) and HDL-C (≥1.0 mmol/L) levels were also divided into strata based on defining features (age, BMI, blood pressure, FBG). The quartiles of TG/HDL-C were calculated [Q1 (<0.65), Q2 (0.65–0.84), Q3 (0.85–1.09), and Q4 (≥ 1.1)] in these participants. Based on the estimated effects of other determinants, binary logistic regression models were used to determine the association between TG/HDL-C and BPH. The logistic analyses showed that TG/HDL-C and BPH were statistically significant except in men aged ≤ 44 years and obese subjects (Figure 2). Again, significant interaction effects were observed between TG/HDL-C and age strata and TG/HDL-C and BMI strata on BPH (P-interaction = 0.003, P-interaction < 0.001, respectively), while no interactions were observed between TG/HDL-C and blood pressure strata and TG/HDL-C and FBG strata in participants.
In all logistic regression models, the test of multicollinearity suggested no collinearity with a range of the variance inflation factor (VIF) between 1.08 and 4.56 and the tolerance between 0.22 and 0.93.
Benign prostatic hyperplasia is one of the most common chronic non-communicable diseases in aging male subjects, and the burden of BPH is now increasing. In this research, the data showed that there were significant associations between TG/HDL-C and TC/HDL-C ratios and BPH risk in a large Chinese population. TG/HDL-C was a powerful independent risk factor for BPH, even though individuals did not have abnormal TG and HDL-C. These findings indicate that the risk of BPH is affected by the value of TG/HDL-C, regardless of the levels of TG and HDL-C. Thus, male subjects with higher TG/HDL-C should be given health guidance or lipid-lowering therapy to reduce the BPH risk.
Clinical and epidemiology studies have already documented that lipid ratio is a useful lipid parameter associated with diseases. For instance, Sánchez-Íñigo et al. (24) evidenced that triglycerides-related variables had the advantage to evaluate the risk of developing hypertension in the Spanish population. Wei et al. (25) found that a high concentration of lipid ratios worked synergistically with hypertension to affect the incidence of ischemic stroke. A lot of research pointed out that TG/HDL-C was favorable to identify increased urinary albumin-to-creatinine ratio, insulin resistance, diabetes risk, incident hypertension, and cardiovascular events (26–31). By far, there has been little study on the relationship between lipid ratios and BPH, except for the Turkish research in which 400 patients were enrolled (21). Yet, only TG/HDL-C was included in the Turkish analysis, and the sample size was of course very small. Our study suggested that TG/HDL-C and TC/HDL-C were related to BPH risk. Notably, the span of TG and HDL-C levels in the Chinese subjects exceeded that of the Turkish sample, and the age groups covered were also relatively broad. These guarantee sufficient power in the investigation of complicated interactions between TG/HDL-C and TC/HDL-C and other confounding factors.
Age is an important risk factor for the occurrence of BPH. As expected, significant positive relationships were observed in TG/HDL-C and BPH in different age decades except for men aged below 35 years, in which the power of the correlation between TG/HDL-C ratio and BPH increased with increasing subjects’ age. Men aged 35 years or over with a normal range of TG and HDL-C also need to take into account the risk of BPH from the higher TG/HDL-C ratio (≥0.65). However, a robust association between TC/HDL-C and the risk of BPH was not found in the stratified analysis by age.
In the stratified multivariable logistic regression analysis by blood pressure and FBG, the relationships between the ratios of TG or TC to HDL-C and BPH were all significant. However, an interesting phenomenon was observed between lipid ratios and BPH across BMI subgroups, the effect of TG/HDL-C on BPH risk was slightly attenuated with increasing BMI values, and the linear trend was not significant between the highest quartile of TC/HDL-C and the risk of BPH. Moreover, the correlation between TG/HDL-C and BPH was not significant in obese individuals whose TG and HDL-C were both within the normal range. This may be related to the increased autonomic hyperactivity caused by increased BMI, which affects BPH growth (32). So the effect of lipid ratios on BPH risk in men with increasing BMI may be blunted by this mechanism.
At present, the growing demand for health services lies in the fact that the incidence of non-communicable diseases is increasing and showing a trend in youth. It is necessary to meet the growing health needs of the people. The purpose of this article was to identify the relationships between lipid ratios and BPH that should be considered in practical work to improve health management services. And it should be used more widely to recognize high-risk populations. This is also clinically important because dyslipidemia is treatable.
In this research, the major strengths are (1) the cohort is based on a large population sample size of 24,962 participants, (2) the effects of TG/HDL-C and TC/HDL-C on BPH risk were both analyzed, (3) further analyze the association between TG/HDL-C and BPH in adults with normal TG and HDL-C levels, and (4) this is the first study to show these findings in China. This study offers reliable and powerful information for further studies about the relationships of disease with blood lipids. Nevertheless, there are several limitations to the study. First, the diagnosis of BPH is mainly based on transabdominal sonography, not prostate biopsy, and might have introduced inaccuracies into this study. Second, the current study was mainly conducted on the Chinese male population; similar results may not be available for other races. Third, this single-institution study may lead to selection bias and limit its generalizability, unadjusted variables including education, diet, regional environment, work situation, marital status, etc. also may result in potential bias and limit the validity of the results. Future research should consider the potential effects of these confounding factors more carefully. In addition, due to the inherent character of the cross-sectional study, this study can only assess the relationship rather than causality.
In this study, the TG/HDL-C and TC/HDL-C ratios are correlated with the risk of BPH in the Chinese population. TG/HDL-C was a powerful independent risk factor for BPH. Furthermore, a higher TG/HDL-C value is useful to assess BPH risk in adults with normal TG and HDL-C levels. Hence, more attention should be paid to those with a higher TG/HDL-C in the health management process, and lifestyle modifications or lipid-lowering agents to help prevent BPH risk in middle-aged and older adults.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Ethics Committee approval number: 2019-SR-478). The patients/participants provided their written informed consent to participate in this study.
CZ and QZ contributed to the conception, design, data analysis, and drafting of the manuscript. YW, JW, and WG participated in the study design. JL, WZ, XL, and NX contributed to the conduct of the study and data collection. All authors read and approved the final manuscript.
This work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB320009) and the Young Scholars Fostering Fund of the First Affiliated Hospital of Nanjing Medical University (No. PY2021008).
All authors appreciated all participants who took part in this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yin Z, Yang JR, Rao JM, Song W, Zhou KQ. Association between benign prostatic hyperplasia, body mass index, and metabolic syndrome in Chinese men. Asian J Androl. (2015) 17:826–30. doi: 10.4103/1008-682X.148081
2. Xiang P, Liu D, Guan D, Du Z, Hao Y, Yan W, et al. Identifcation of key genes in benign prostatic hyperplasia using bioinformatics analysis. World J Urol. (2021) 39:3509–16. doi: 10.1002/jcp.28592
3. Langan RC. Benign prostatic hyperplasia. Prim Care. (2019) 46:223–32. doi: 10.1016/j.pop.2019.02.003
4. Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. (2016) 43:289–97. doi: 10.1016/j.ucl.2016.04.001
5. Yue L, Wang T, Ge Y, Ge M, Zhang C, Hou Q, et al. Prevalence and heritability of benign prostatic hyperplasia and LUTS in men aged 40 years or older in Zhengzhou rural areas. Prostate. (2019) 79:312–9. doi: 10.1002/pros.23737
6. Tu WJ, Zeng X, Liu Q. Aging tsunami coming: the main finding from China’s seventh national population census. Aging Clin Exp Res. (2022) 34:1159–63. doi: 10.1007/s40520-021-02017-4
7. Xiong Y, Zhang Y, Li X, Qin F, Yuan J. The prevalence and associated factors of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males. Aging Male. (2020) 23:1432–9. doi: 10.1080/13685538.2020.1781806
8. Xiong Y, Zhang Y, Tan J, Qin F, Yuan J. The association between metabolic syndrome and lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males: evidence based on propensity score matching. Transl Androl Urol. (2021) 10:384–96. doi: 10.21037/tau-20-1127
9. Miernik A, Gratzke C. Current treatment for benign prostatic hyperplasia. Dtsch Arztebl Int. (2020) 117:843–54. doi: 10.3238/arztebl.2020.0843
10. Kopp W. Diet-induced hyperinsulinemia as a key factor in the etiology of both benign prostatic hyperplasia and essential hypertension? Nutr Metab Insights. (2018) 11:1178638818773072. doi: 10.1177/1178638818773072
11. Fujita K, Hosomi M, Nakagawa M, Tanigawa G, Imamura R, Uemura M, et al. White blood cell count is positively associated with benign prostatic hyperplasia. Int J Urol. (2014) 21:308–12. doi: 10.1111/iju.12243
12. De Nunzio C, Presicce F, Tubaro A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol. (2016) 13:613–26. doi: 10.1038/nrurol.2016.168
13. Fu Y, Zhou Z, Yang B, Zhang K, He L, Zhang X. The relationship between the clinical progression of benign prostatic hyperplasia and metabolic syndrome: a prospective study. Urol Int. (2016) 97:330–5. doi: 10.1159/000448484
14. Grzesiak K, Rył A, Baranowska-Bosiacka I, Rotter I, Dołêgowska B, Słojewski M, et al. Comparison between selected hormone and protein levels in serum and prostate tissue homogenates in men with benign prostatic hyperplasia and metabolic disorders. Clin Interv Aging. (2018) 13:1375–82. doi: 10.2147/CIA.S168146
15. Ngai HY, Yuen KS, Ng CM, Cheng CH, Chu SP. Metabolic syndrome and benign prostatic hyperplasia: an update. Asian J Urol. (2017) 4:164–73. doi: 10.1016/j.ajur.2017.05.001
16. Cakir SS, Ozcan L, Polat EC, Besiroglu H, Kocaaslan R, Ötunctemur A. Statins are effective in the treatment of benign prostatic hyperplasia with metabolic syndrome. Aging Male. (2020) 23:538–43. doi: 10.1080/13685538.2018.1541979
17. Zou C, Gong D, Fang N, Fan Y. Meta-analysis of metabolic syndrome and benign prostatic hyperplasia in Chinese patients. World J Urol. (2016) 34:281–9. doi: 10.1007/s00345-015-1626-0
18. Besiroglu H, Ozbek E, Dursun M, Otunctemur A. Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients: a cross-sectional study. Aging Male. (2018) 21:40–7. doi: 10.1080/13685538.2017.1365833
19. Gacci M, Corona G, Vignozzi L, Salvi M, Serni S, De Nunzio C, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int. (2015) 115:24–31. doi: 10.1111/bju.12728
20. Vignozzi L, Gacci M, Cellai I, Santi R, Corona G, Morelli A, et al. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate. (2013) 73:789–800. doi: 10.1002/pros.22623
21. Besiroglu H, Dursun M, Otunctemur A, Ozbek E. The association between triglyceride high density lipoprotein cholesterol ratio and benign prostate hyperplasia in non-diabetic patients: a cross-sectional study. Aging Male. (2017) 20:198–204. doi: 10.1080/13685538.2017.1303828
22. Meng J, Liu Y, Guan SY, Ma H, Zhang X, Fan S, et al. Age, height, BMI and FBG predict prostate volume in ageing benign prostatic hyperplasia: evidence from 5285 patients. Int J Clin Pract. (2019) e13438. [Epub ahead of print]. doi: 10.1111/ijcp.13438
23. Chinese Society of Health Management, Chinese Nutrition Society, Reproductive Med&ine Branch of China International Exchange and Promotion Association for Medicine and Healthcare, China Health Promotion Foundation, Zhejiang Provincial Clinical Nutrition Center. Expert consensus&standard on weight management for overweight or obese people. Chin J Health Manage. (2018) 12:200–4. doi: 10.3760/cma.i.Issn.1674.0815.2018.03.003
24. Sánchez-Íñigo L, Navarro-González D, Pastrana-Delgado J, FernándezMontero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. (2016) 34:1257–65. doi: 10.1097/HJH.0000000000000941
25. Wei L, Sun J, Xie H, Zhuang Q, Wei P, Zhao X, et al. Interaction analysis of abnormal lipid indices and hypertension for ischemic stroke: a 10-year prospective cohort study. Front Cardiovasc Med. (2022) 9:819274. doi: 10.3389/fcvm.2022.819274
26. Xue J, Wang Y, Li B, Yu S, Wang A, Wang W, et al. Triglycerides to high-density lipoprotein cholesterol ratio is superior to triglycerides and other lipid ratios as an indicator of increased urinary albumin-to-creatinine ratio in the general population of China: a cross-sectional study. Lipids Health Dis. (2021) 20:13. doi: 10.1186/s12944-021-01442-8
27. Kheirollahi A, Teimouri M, Karimi M, Vatannejad A, Moradi N, Borumandnia N, et al. Evaluation of lipid ratios and triglyceride-glucose index as risk markers of insulin resistance in Iranian polycystic ovary syndrome women. Lipids Health Dis. (2020) 19:235. doi: 10.1186/s12944-020-01410-8
28. Liu H, Yan S, Chen G, Li B, Zhao L, Wang Y, et al. Association of the ratio of triglycerides to high-density lipoprotein cholesterol levels with the risk of type 2 diabetes: a retrospective cohort study in Beijing. J Diabetes Res. (2021) 2021:5524728. doi: 10.1155/2021/5524728
29. Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, et al. Association of triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. (2020) 19:33. doi: 10.1186/s12944-020-01213-x
30. Liu D, Guan L, Zhao Y, Liu Y, Sun X, Li H, et al. Association of triglycerides to high-density lipoprotein-cholesterol ratio with risk of incident hypertension. Hypertens Res. (2020) 43:948–55. doi: 10.1038/s41440-020-0439-8
31. Chen Z, Chen G, Qin H, Cai Z, Huang J, Chen H, et al. Higher triglyceride to high-density lipoprotein cholesterol ratio increases cardiovascular risk: 10-year prospective study in a cohort of Chinese adults. J Diabetes Investig. (2020) 11:475–81. doi: 10.1111/jdi.13118
Keywords: benign prostatic hyperplasia, dyslipidemia, lipid ratios, triglycerides, high-density lipoprotein cholesterol
Citation: Zhu C, Wu J, Wu Y, Guo W, Lu J, Zhu W, Li X, Xu N and Zhang Q (2022) Triglyceride to high-density lipoprotein cholesterol ratio and total cholesterol to high-density lipoprotein cholesterol ratio and risk of benign prostatic hyperplasia in Chinese male subjects. Front. Nutr. 9:999995. doi: 10.3389/fnut.2022.999995
Received: 21 July 2022; Accepted: 14 September 2022;
Published: 03 October 2022.
Edited by:
Ming Yang, Sichuan University, ChinaReviewed by:
Qiao Huang, Wuhan University, ChinaCopyright © 2022 Zhu, Wu, Wu, Guo, Lu, Zhu, Li, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Zhang, d2VuemkyMDEwMDMwNUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.