- Immunogenomics Laboratory, Department of Human Genetics and Molecular Medicine, School of Health Sciences, Central University of Punjab, Bathinda, Punjab, India
Purpose: As an immune-modulator, vitamin D is known to regulate immune response and is implicated in disease pathogenesis. Celiac disease (CD) is a systemic autoimmune disease and susceptibility conferred by vitamin D metabolism is under investigation. Studies on the association of vitamin D metabolism and genetic polymorphisms are expected to explain CD pathogenesis. We performed a systematic review–based meta-analysis to investigate the 25(OH)D serum levels and susceptibility conferred by the genetic variants of VDR in CD.
Methods: Systematic review was conducted through a web-based literature search following stringent study inclusion–exclusion criteria. The Newcastle–Ottawa Scale and GRADE tools were used to assess the quality of evidence in studies and the study outcome. Cohen's κ value was estimated to access the reviewer's agreement. RevMan 5.4.1 was used to perform the meta-analyses. Weighted mean difference and Meta p-value was assessed for 25(OH)D serum levels. Meta-odds ratio and Z-test p-value were evaluated to estimate the allelic susceptibility of VDR variants.
Results: A total of 8 out of 12 studies were evaluated for “25(OH)D” serum level, while four studies were found eligible for SNPs (Bsm1, Apa1, Fok1, and Taq1) of VDR. Significantly higher levels [WMD = 5.49, p < 0.00001] of 25(OH)D were observed in healthy controls than in patients with CD. rs2228570-T (Fok1) [Meta-OR = 1.52, p = 0.02] was confirmed to be predisposing allele for CD.
Conclusion: Reduced serum level of 25(OH)D and association of Fok1 T-allele of VDR confirmed in this study plays a critical role in immunomodulation and maintaining barrier integrity, which is majorly implicated in CD.
1. Introduction
Celiac disease (CD) is an immune-mediated gluten enteropathy affecting almost 1% of the population worldwide in individuals carrying HLA DQ2/8 susceptibility alleles, which are encoded by DQA1*0501-DQB1*02 and DQA1*0301-DQB1*0302 (1, 2). Almost 94.94% of the CD subjects are positive for this specific HLA-DQB1*02 allele (2). Though the etiology of CD is not well understood but is marked by the presence of inflammatory cytokines IL18, IL17, TNF-α, IL12, IL21, and IL15 (3). Vitamin D was found to be associated with reducing the effects of inflammatory molecules (4). Components of the immune system, such as B-lymphocytes, T-lymphocytes, and dendritic cells, are influenced by the regulatory effects of vitamin D and expressed vitamin D receptor (VDR), which is involved in the biological activity of 1,25(OH)2D3, and these cells also have the capability of locally synthesizing active 1,25(OH)2D3 (5). This active form of vitamin D exerts its effects by binding to the nuclear receptor VDR. The 1,25(OH)2D3-VDR complex dimerizes with the retinoid X receptor (RXR), and the 1,25(OH)2D3-VDR-RXR heterodimer translocates to the nucleus where it binds Vitamin D Response Element (VDRE) in the promoter regions of vitamin D responsive genes and induces the expression of vitamin D responsive genes (6). Some of the remarkable effects of vitamin D in immune system regulation were the suppression of Th1/Th17 CD4+ T cell proliferation and subsequent alteration of the cytokine responses (5). Thus, it is worth studying the association between vitamin D metabolism and various immune-mediated disorders.
Duodenal epithelial damage in CD is caused by the cytokines released by the activated T-cells upon exposure to gliadin peptides, and vitamin D is reported to suppress the proliferation of T-cells (7, 8). In vitro study performed on Caco-2 cell layers reported the protective role of 1,25(OH)2D3 on the damage of tight junction, which was induced by the pepsin–trypsin digested gliadin (PT–G). Vitamin D was observed to increase the expression of the tight junction–associated proteins and was also able to minimize epithelial permeability (9). Notably, vitamin D deficiency increases the risk of severe intestinal damage, which is a prominent symptom of CD (10). During the early infant stage, 25(OH)D concentrations between <30 and >75 nmol/L were associated with an increased risk of developing CD in genetically predisposed children. The non-linear relationship raises the need for more studies on the possible role of 25(OH)D in the onset of CD (11). In vivo studies have shown a positive response to vitamin D supplementation in the celiac mice model (12).
In the association study in the Spanish Basque population, four polymorphisms of VDR (Bsm1-rs1544410, Apa1-rs7975232, Taq1-rs731236, and Fok1-rs2228570) were genotyped. Fok1 was reported to be a risk genotype in 25.64% of CD cases as compared to 9.89% of controls (p = 0.01, OR = 3.45) (13). Another association study on the Norwegian cohort did not find any association with the VDR marker (Bsm1) or serum vitamin D level (14). Two significant SNPs, Fok1 and Bsm1, were reported in the Russian Tomsk group, p = 0.009 and p = 0.001, respectively (15). A total of 92 Viennese CD patients and controls were genotyped for Apa1 and Taq1 SNPs of the VDR gene; however, no significant risk association was observed (16). A recent meta-analysis study by Lu et al. (17) did not find any association with VDR genotype but reported lower levels of 25(OH)D in CD patients than in controls.
In this systematic review and meta-analysis, we intended to assess an association of serum level of 25(OH)D and VDR gene polymorphism with CD. Four SNPs (Bsm1-rs1544410, G>A; Apa1-rs7975232, C>A; Taq1-rs731236, T>C; and Fok1-rs2228570, C>T) of VDR were evaluated.
2. Methods
2.1. Search strategy
For the search and retrieval of relevant published literature, various web search engines for scientific databases such as Google scholar, NCBI (PubMed/MEDLINE), SCOPUS, EMBASE, and Web of Science were used. All the published literature until May 2022 on the association of vitamin D serum concentration and VDR gene polymorphism with celiac disease was searched. For this literature search, keywords were used as follows: “Celiac disease” AND “serum vitamin D concentration” OR “serum 25(OH)D concentration”. For the genotype association study, the key terms used were as follows: “Celiac disease” AND “VDR polymorphism” OR “VDR genotype” OR “VDR variants” OR “Bsm1 polymorphism” OR “Apa1 polymorphism” OR “Fok1 polymorphism” OR “Taq1 polymorphism”.
2.2. Inclusion and exclusion criteria
To limit the screening to relevant articles, the inclusion and exclusion criteria were defined. Articles were considered eligible for the study if they met the following inclusion criteria: (i) published full-text original research article, (ii) case–control study design, (iii) mean serum 25(OH)D concentration can be obtained, (iv) VDR gene polymorphism association study with celiac disease and healthy controls, and (v) Only articles written in the English language. Our study was not restricted to any specific population or ethnicity, and all the relevant articles available till May 2022 were considered.
The exclusion criteria for the study were as follows: (i) studies other than case–control, (ii) if the CD patients were on a gluten-free diet or vitamin D supplementation, and (iii) review articles. Apart from this, duplicate publications were also excluded.
2.3. Data extraction and evaluation of confined studies
Two researchers independently performed a literature search checked the eligibility criteria, and extracted data from the shortlisted literature. From all the studies which we considered eligible according to our inclusion and exclusion criteria, relevant data for serum vitamin D concentration and VDR genotype in patients with CD and healthy controls were retrieved along with the name of the author and the year of publication. For the concentration of vitamin D, the serum levels of 25(OH)D [mean ± standard deviation (SD)] in patients with CD and healthy controls were extracted from every eligible article, and for the vitamin D concentration, the units considered were in ng/ml, data obtained in other units (such as nmol/L) were converted to ng/ml (1 ng/ml = 2.5 nmol/L). Few other information about the study population such as the mean age of the patients with CD and healthy controls and the male:female ratio in the study were also obtained.
For the VDR genotype association studies, VDR SNPs data of CD cases and controls were obtained. Four SNPs of the VDR gene: Bsm1-rs1544410, Apa1-rs7975232, Fok1-rs2228570, and Taq1-rs731236 were analyzed, and the genotype frequencies in CD cases and controls were obtained. After data extraction, to carry out a systematic review, standard checklists were used to analyze methodological quality and strength of association, which also included the risk of bias evaluation in observational studies as recommended by the Cochrane handbook (https://training.cochrane.org/handbook). The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement was followed for reporting the findings (http://www.prisma-statement.org/) (18). The PRISMA statement is provided in Supplementary Table 1.
2.4. Quality assessment
Cohen's kappa (κ) value was calculated in order to estimate the extent of concordance between two reviewers, who performed a literature search, and checked eligibility and data extraction (19). Based on the percentage of agreement and Cohen's κ score, values were classified as poor, slight, fair, moderate, substantial, or almost perfect. Sensitivity analysis was used to evaluate the impact of each study on the meta-analysis result by removing one study at a time from the combined dataset. Funnel plots were analyzed in order to detect any study biasness. If any of the studies fell outside the funnel plot or gave rise to an asymmetric funnel plot, then that study was considered to be biased. Irrelevant studies were ruled out after a thorough analysis. The Newcastle–Ottawa Scale (NOS) tool was used to assess the quality of the eligible studies (i.e., study participant selection, comparability, and outcome) (20). Studies were graded on a scale of 0 (lowest) to 9 (highest), with low (stars 7–9), moderate (stars 4–6), and high (stars 0–3) risk of bias. The analysis was limited to studies with low risk.
GRADEpro.v.3.6 was used to assess the quality of evidence (QoE) for each of the outcomes using The Grades of Research, Assessment, Development, and Evaluation (GRADE) tool (21). Based on the proposed criteria, the included study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias, and evidence were grouped into four main categories: high, moderate, low, and very low.
2.5. Statistical analysis
For the meta-analysis of the serum 25(OH)D level, the mean difference was used to analyze the pooled continuous data of serum 25(OH)D concentration which was obtained as mean ± standard deviation (SD). To evaluate the combined MD, mean differences (MD) with 95% confidence intervals (CIs) were presented for all relevant studies on a forest plot. A collective study was carried out without dividing the study based on age group due to very few studies were obtained with relevant data in the adult age group. The dichotomous data of the alleles of the four SNPs of the VDR gene were utilized to calculate Meta-OR using Mantel–Haenszel (M–H) method with 95% CI). The statistical analysis for this study was carried out using the RevMan (version 5.4.1, The Cochrane Collaboration) software. For this meta-analysis, the assessment of study heterogeneity was done by chi-square p-value and I2 value. The meta-analysis was carried out using the DerSimonian and Laird random effect model for I2 > 50% and p < 0.05, and for I2 < 50% and p > 0.05, the fixed effect model was used.
3. Results
3.1. Features of enclosed studies
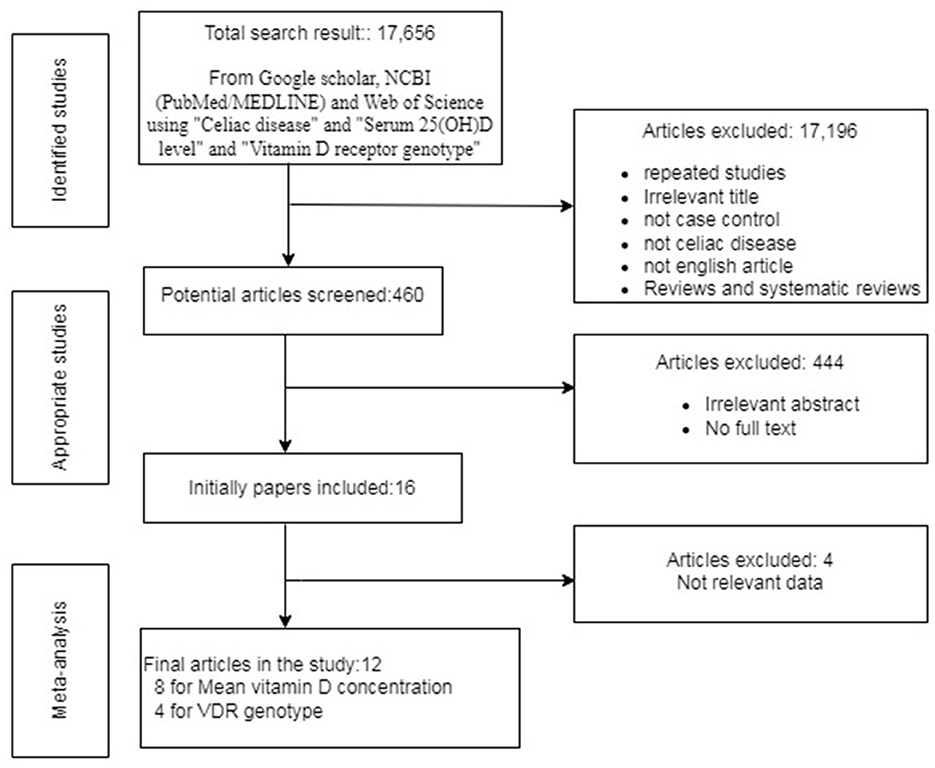
After employing all the above-described literature retrieval strategies, we got a total of 17,656 studies. Overall, 17,196 studies were excluded based on the inclusion and exclusion criteria because of irrelevant titles or because they were review articles or were not in the English language. After screening the rest of the articles, 444 articles were excluded from our study because of duplicate publications, irrelevant abstracts (in silico, in vitro, in vivo studies, case studies), and articles without full text. Initially, a total of 16 studies were considered for this study but due to the lack of relevant data, four articles were excluded. Finally, 12 studies that satisfied our inclusion and exclusion criteria and had the data of serum 25(OH)D level and VDR genotype in CD cases and control were included in this meta-analysis as shown in Figure 1. All these data of mean serum 25(OH)D concentration and the genotype of VDR SNPs Bsm1 rs1544410, Apa1 rs7975232, Fok1 rs2228570, Taq1 rs731236, and their summary statistics are provided in Tables 1, 2.
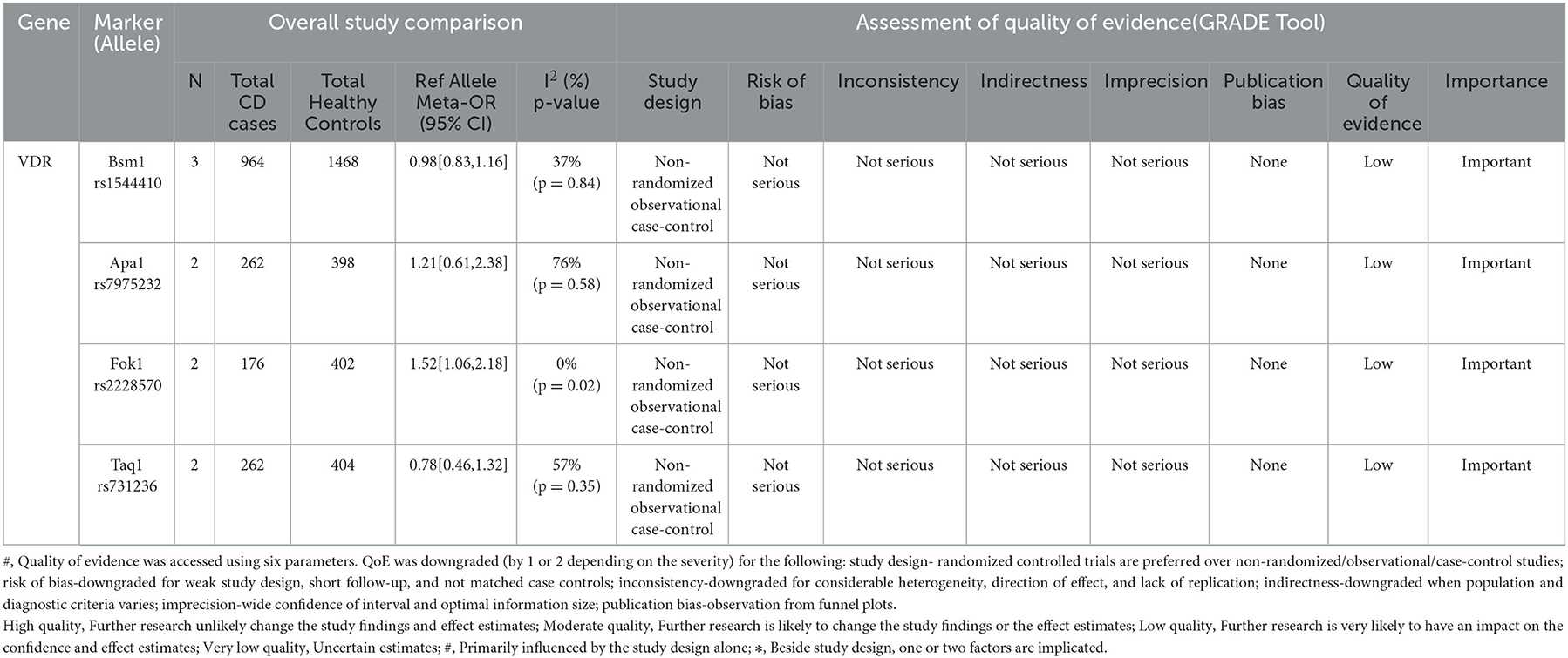
Table 1. Summary table of the alleles included in the meta-analysis and the quality of evidences as graded by the GRADE tool.
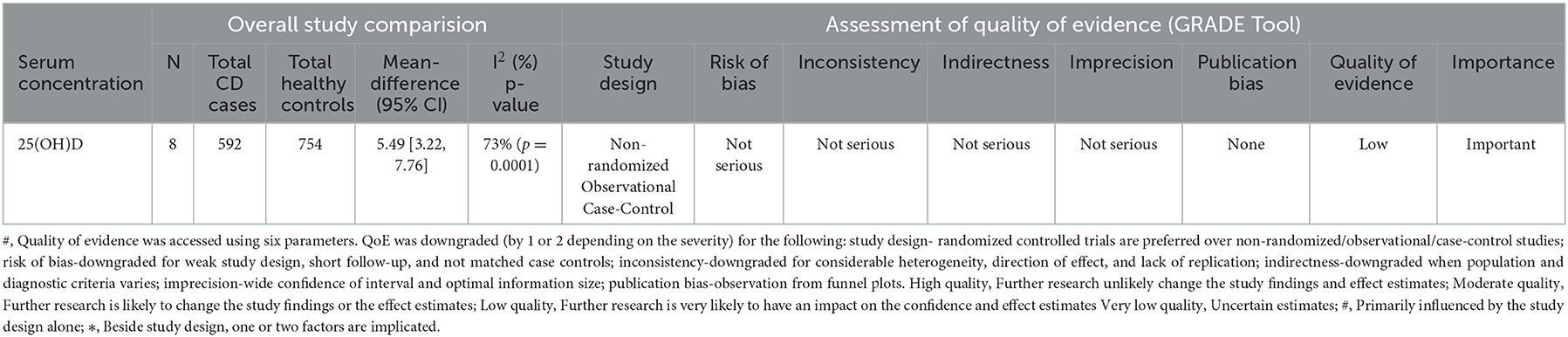
Table 2. Summary table of mean 25(OH)D concentration included in the meta-analysis and the quality of evidences as graded by the GRADE.
3.2. Publication bias evaluation and sensitivity analysis
In order to detect any study biasness in this meta-analysis, the funnel plots of all the studies were analyzed. Upon this analysis, the exclusion of any study was not done as study biasness was not detected. When it came to the inclusion and exclusion of relevant and irrelevant articles from this systematic review, reviewers were almost unanimous (Cohen's κ = 0.96; % agreement 98.28). All the 12 eligible studies included in this meta-analysis are given in Supplementary Figure 1.
3.3. Quality of the studies included and risk of biasness
Based on the evaluation of the quality of evidence, all 12 studies were identified to have a low risk of bias (NOS = 7–8) (Supplementary Table 2). Because of concise criteria for evaluation and homogeneous populations, GRADE's approach observation indicated that none of the 12 studies increased the possibility of bias, and the indirectness of the findings was not a concern. The evidence quality was insufficient to rule out any of the studies that were included. As a result, the entire study was deemed important and rated as low risk in Supplementary Table 2.
3.4. Concentration of 25(OH)D in CD patients and control
A total of eight studies were evaluated in this meta-analysis with mean serum 25(OH)D concentration of patients with CD and controls, the list of same is given in Supplementary Table 3 (22–29). It constitutes 592 patients with CD and 754 controls. This meta-analysis was carried out using the Random effect model. Significant associations were defined as Z-test p-values (i.e., Meta-p-values) of <0.05. The mean difference was evaluated for all the studies included, and a forest plot was plotted using the mean differences with 95% CI, of each of these studies to estimate the combined mean difference (Figure 2). The outcome of this meta-analysis exhibited that the mean 25(OH)D concentration in the healthy controls was 5.49 ng/ml higher than that of CD patients (WMD = 5.49, 95% CI = 3.22–7.76). The observed meta-p-value was significant (p < 0.00001) (Figure 2).

Figure 2. Forest plot to show meta-analysis of vitamin D serum concentration in CD patients and control.
3.5. VDR allele association with CD patients and controls
For this meta-analysis of allelic association, four studies were included in which the allelic frequencies for the four VDR SNPs (Bsm1 rs1544410, Apa1 rs7975232, Fok1 rs2228570, and Taq1 rs731236) were given for patients with CD and healthy controls, basic information of which is provided in Supplementary Table 4. The meta-analysis for the SNPs Bsm1 rs1544410 and Fok1 rs2228570 was performed using the fixed effect model for insignificant heterogeneity (I2 < 50% and p > 0.05), whereas for Apa1 rs7975232 and Taq1 rs731236, the random effect model was used because of a high degree of data heterogeneity. Significant associations were defined as Z-test p-values (i.e., Meta-p-values) <0.05. The Meta-OR was used to predict risk.
3.5.1. Bsm1
Three studies were evaluated (13–15). The G allele of this marker rs1544410 was found to be protective for CD [Meta-OR = 0.98 (0.83–1.16), p = 0.20]. The meta p-value was declared insignificant (Figure 3A).
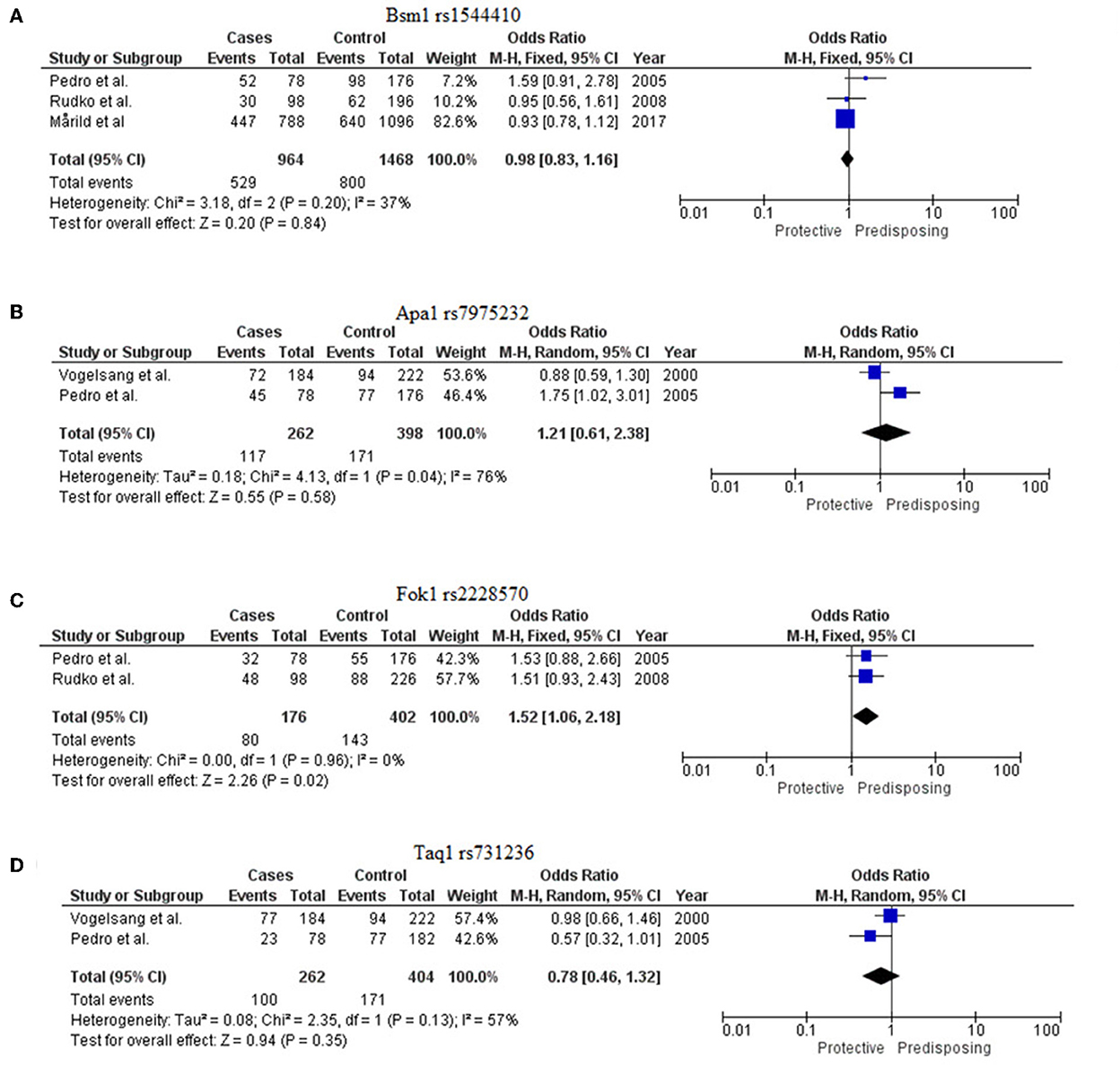
Figure 3. Forest plots to show meta-analysis of VDR SNPs [(A) Bsm1 rs1544410, (B) Apa1 rs7975232, (C) Fok1 rs2228570, and (D) Taq1 rs731236].
3.5.2. Apa1
Two studies were considered for this VDR marker (13, 16). The C allele of this marker rs7975232 was found to confer risk for CD [Meta-OR = 1.21 (0.61–2.38), p = 0.58] but with insignificant p-value (Figure 3B).
3.5.3. Fok1
Two eligible studies were included for this marker (13, 14). The T allele of rs2228570 was identified to be significantly predisposing for the disease [Meta-OR = 1.52 (1.06–2.18), p = 0.02] (Figure 3C).
3.5.4. Taq1
Two studies were evaluated for this meta-analysis (13, 16). T allele of rs731236 was found to be protective [Meta-OR = 0.78 (0.46–1.32), p = 0.13]. But no significant association was found (Figure 3D).
4. Discussion
Multisystem CD is characterized by circulating innate lymphoid cells and increased levels of IL-18, IFN-γ, and innate lymphoid cell precursors were noted (3). A reduced vitamin D level has been reported to be correlated with higher IFN-γ and innate lymphoid cell precursor (4). Vitamin D deficiency is shown to induce T-cell-mediated pro-inflammatory immune responses that are pivotal in CD (30, 31). This gave an insight into dietary supplementation of vitamin D as a therapeutic approach to inhibit cytokine IFN-γ production (4). Several cross-section studies concluded the association of vitamin D deficiency with immune-related diseases (32–34). In vitro and in vivo studies on induced CD-like conditions reported vitamin D supplementation rescue from cellular and tissue damage, which directly indicated the protective role of vitamin D in CD (8, 35, 36).
This meta-analysis suggests the association of the reduced serum level of 25(OH)D [MD = 5.49; P < 0.00001] and rs2228570-T (Fok1) [Meta-OR = 1.52, p = 0.02] with CD. All the studies were performed in the last decade and on a modest sample size (Figure 2). Cross-section studies with case-control study design, which was included in this meta-analysis, are however unable to comment on the cause-effect relationship between VDD and CD. Nevertheless, this finding suggests vitamin D supplements to subjects with CD to restore the normal duodenal mucosal barrier and suppress inflammatory immune responses as illustrated in Figure 4. 25(OH)D is the most stable form of vitamin D, and its transport and stability are determined by the availability of vitamin D binding protein (VDBP) in the serum. To date, no reports are available on the association of serum VDBP with CD.
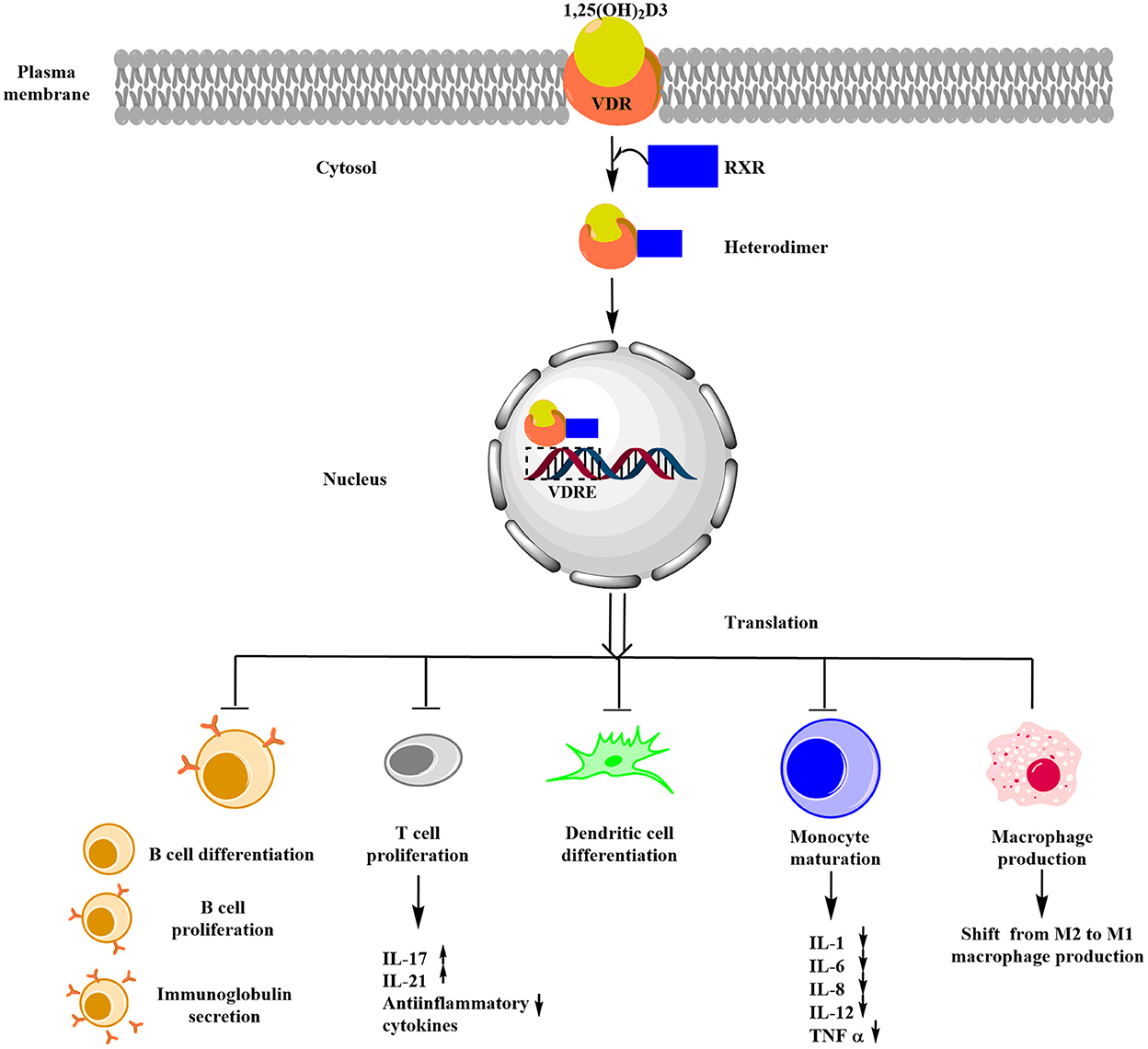
Figure 4. Schematic representation of the role of vitamin D in immune regulation in CD. Upon binding of vitamin D3 with VDR, RXR is recruited and results in the formation of a heterodimer known as VDR-RXR complex, whose translated product (formed by binding of the heterodimer with VDRE) exerts immune modulation by inhibiting differentiation and proliferation of B-cell, T-cell, and dendritic cells and also inhibits immunoglobulin secretion.
The extra-calcium role of vitamin D and its involvement in immune modulation suggested that in genetically predisposed individuals, vitamin D deficiency can be an underlying cause for the onset of CD in children. Moreover, vitamin D deficiency can lead to dysregulated immune responses that result in abnormal intestinal mucosa and a greater risk of developing acute gastrointestinal infection (37). Several reports are available to suggest significant improvements in subjects with CD following vitamin D supplementation alongside gluten-free diet (GFD) (38).
Very limited genetic association studies were performed on CD to determine the contribution of vitamin D metabolism to the disease. Only four research articles were available on VDR, and all the studies were performed in populations with European ancestry (Supplementary Table 4). Four polymorphisms namely, Bsm1 (rs154441, G>A), Apa1 (rs7975232, C>A), Fok1 (rs2228570, C>T) and Taq1 (rs731236, T>C) were considered where at two studies were available for the meta-analysis. The association of the Fok1-T allele with CD (OR = 1.52, p = 0.02) suggested the putative role of this gene in the disease pathogenesis (Figure 3C).
The Fok1 polymorphism of VDR is associated with several other immune-mediated disorders such as type 2 diabetes (T2DM) (39). The Fok1 polymorphism also known as the start codon polymorphism (SCP), in exon 2 of the VDR has been shown to alter the structure of the VDR. The change in C > T also represented as F > f leads to a threonine to methionine substitution and provides two possible sites for the initiation of translation (40). The shorter VDR form, that is, 424 amino acid protein (encoded by the common allele C) in the FF genotype appears to be more effective in binding 1,25(OH)2D3 and has a higher binding capacity (41), while rs2228570-T (in ff genotype) leads to the production of 427 amino acid protein product, which is comparatively 1.7 times less efficient at the binding of 1,25(OH)2D3. Reduced binding efficiency with 1,25(OH)2D3 thus restricts VDR activation, and therefore, may limit regulating the expression of specific genes that are implicated in immune regulation.
5. Conclusion
In this meta-analysis, lower levels of serum 25(OH)D were observed in patients with CD, which indicates that deficiency of vitamin D may play a significant role in the pathogenesis of CD. The SNPs of the VDR gene (Bsm1-rs1544410, Apa1-rs7975232, and Taq1-rs731236) did not show any significant association with CD, but Fok1 (rs2228570-T) was identified to be providing significant risk for CD. However, due to limitations in the number of studies performed on the association of VDR gene polymorphism and CD, strong evidence to support this association is still lacking.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SS conceptualized the study. SS and TS designed the study and performed the initial literature search. PB and TS performed the systematic review. SS and PB performed the analysis and wrote the manuscript. All the authors read the final manuscript and approved it for publication.
Funding
The authors acknowledge the support from the Scientific and Engineering Research Board (SERB), New Delhi (ECR/2016/001660).
Acknowledgments
The authors acknowledge DST-FIST Funded Department of Human Genetics and Molecular Medicine for providing facilities. PB acknowledges DST-INSPIRE for fellowship (DST/INSPIRE-Fellowship/2019/IF190501).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.996450/full#supplementary-material
Supplementary Figure 1. Funnel plot analysis of all eligible studies for serum vitamin D concentration and VDR gene SNPs.
Supplementary Table 1. PRISMA checklist.
Supplementary Table 2. The Newcastle-Ottawa Scale (NOS) assessment for all the twelve studies found eligible for this study ensuring the quality of evidence.
Supplementary Table 3. Mean difference of vitamin D [25(OH)D] concentration in serum.
Supplementary Table 4. Allele frequencies of four VDR SNPs those were included in the study of healthy controls and CD patients.
References
1. Lindfors K, Ciacci C, Kurppa K, Lundin KE, Makharia GK, Mearin ML, et al. Coeliac disease. Nat. Rev. Dis. Primers. (2019) 5:1–8. doi: 10.1038/s41572-018-0054-z
2. Poddighe D, Rebuffi C, De Silvestri A, Capittini C. Carrier frequency of HLA-DQB1* 02 allele in patients affected with celiac disease: A systematic review assessing the potential rationale of a targeted allelic genotyping as a first-line screening. World J. Gastroenterol. (2020) 26:1365. doi: 10.3748/wjg.v26.i12.1365
3. Yu X, Vargas J, Green PH, Bhagat G. Innate lymphoid cells and celiac disease: current perspective. Cell Mol Gastroenterol Hepatol. (2021) 11:803–14. doi: 10.1016/j.jcmgh.2020.12.002
4. Ercolano G, Moretti A, Falquet M, Wyss T, Tran NL, Senoner I, et al. Gliadin-reactive vitamin D-sensitive proinflammatory ILCPs are enriched in celiac patients. Cell Rep. (2022) 39:110956. doi: 10.1016/j.celrep.2022.110956
5. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. (2010) 88:441–50. doi: 10.1007/s00109-010-0590-9
6. Aranow C. Vitamin D and the immune system. J Invest Med. (2011) 59:881–6. doi: 10.2310/JIM.0b013e31821b8755
7. Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis. (2014) 210:1296–305. doi: 10.1093/infdis/jiu235
8. Vici G, Camilletti D, Polzonetti V. Possible role of vitamin D in celiac disease onset. Nutrients. (2020) 12:1051. doi: 10.3390/nu12041051
9. Dong S, Singh TP, Wei X, Yao H, Wang H. Protective Effect of 1,25-Dihydroxy Vitamin D3 on pepsin-trypsin-resistant gliadin-induced tight junction injuries. Dig Dis Sci. (2018) 63:92–104. doi: 10.1007/s10620-017-4738-0
10. Malaguarnera L. Vitamin D and microbiota: Two sides of the same coin in the immunomodulatory aspects. Int Immunopharmacol. (2020) 79:106112. doi: 10.1016/j.intimp.2019.106112
11. Andrén Aronsson C, Liu X, Norris JM, Uusitalo U, Butterworth MD, Koletzko S, et al. 25(OH)D levels in infancy is associated with celiac disease autoimmunity in at-risk children: a case-control study. Front. Nutr. (2021) 8:720041. doi: 10.3389/fnut.2021.720041
12. Trasciatti S, Piras F, Bonaretti S, Marini S, Nencioni S, Biasci E, et al. Effect of oral cholecalciferol in a murine model of celiac disease: A dose ranging study. J Steroid Biochem Mol Biol. (2022) 220:106083. doi: 10.1016/j.jsbmb.2022.106083
13. Pedro JS, Bilbao JR, Perez de. Nanclares G, Vitoria JC, Martul P, Castano L. Heterogeneity of vitamin D receptor gene association with celiac disease and type 1 diabetes mellitus. Autoimmunity. (2005) 38:439–44. doi: 10.1080/08916930500288455
14. Mårild K, Tapia G, Haugen M, Dahl SR, Cohen AS, Lundqvist M, et al. Maternal and neonatal vitamin D status, genotype and childhood celiac disease. PLoS ONE. (2017) 12:e0179080. doi: 10.1371/journal.pone.0179080
15. Rudko AA, Kondratieva EI, Yankina GN, Loshkova EV, Puzyrev VP. Association of polymorphisms of immune response modifier genes with celiac disease and its clinical forms in the Tomsk population. Mol Biol. (2008) 42:37–43. doi: 10.1134/S0026893308010056
16. Vogelsang H, Suk EK, Janisiw M, Stain C, Mayr WR, Panzer S. Calcaneal ultrasound attenuation and vitamin-D-receptor genotypes in celiac disease. Scand J Gastroenterol. (2000) 35:172–6. doi: 10.1080/003655200750024344
17. Lu C, Zhou W, He X, Zhou X, Yu C. Vitamin D status and vitamin D receptor genotypes in celiac disease: a meta-analysis. Crit Rev Food Sci Nutr. (2021) 61:2098–106. doi: 10.1080/10408398.2020.1772716
18. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Chinese edition). J Chin Integr Med. (2009) 7:889–96. doi: 10.3736/jcim20090918
19. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). (2012) 22:276–82. doi: 10.11613/BM.2012.031
20. Sandberg F, Viktorsdóttir MB, Salö M, Stenström P, Arnbjörnsson E. Comparison of major complications in children after laparoscopy-assisted gastrostomy and percutaneous endoscopic gastrostomy placement: a meta-analysis. Pediatr Surg Int. (2018) 34:1321–7. doi: 10.1007/s00383-018-4358-6
21. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
22. Setty-Shah N, Maranda L, Nwosu BU. Increased risk for vitamin d deficiency in obese children with both celiac disease and type 1 diabetes. Gastroenterology Research and Practice. 2014; 2014. doi: 10.1155/2014/561351
23. Nwosu BU, Maranda L. Vitamin D status and adiposity in pediatric malabsorption syndromes. Digestion. (2015) 92:1–7. doi: 10.1159/000381895
24. Björck S, Brundin C, Karlsson M, Agardh D. Reduced bone mineral density in children with screening-detected celiac disease. J Pediatr Gastroenterol Nutr. (2017) 65:526–32. doi: 10.1097/MPG.0000000000001568
25. Piatek-Guziewicz A, Ptak-Belowska A, Przybylska-Felus M, Pasko P, Zagrodzki P, Brzozowski T, et al. Intestinal parameters of oxidative imbalance in celiac adults with extraintestinal manifestations. World J Gastroenterol. (2017) 23:7849. doi: 10.3748/wjg.v23.i44.7849
26. Tokgöz Y, Terlemez S, Karul A. Fat soluble vitamin levels in children with newly diagnosed celiac disease, a case control study. BMC Pediatr. (2018) 18:1–5. doi: 10.1186/s12887-018-1107-x
27. Işikay S, Işikay N, Per H, Bora Çarman K, Kocamaz H. Restless leg syndrome in children with celiac disease. Turk J Pediatr. (2018) 60:70–75. doi: 10.24953/turkjped.2018.01.010
28. Lionetti E, Galeazzi T, Dominijanni V, Acquaviva I, Catassi GN, Iasevoli M, et al. Lower level of plasma 25-hydroxyvitamin d in children at diagnosis of celiac disease compared with healthy subjects: a case-control study. J Pediatr. (2021) 228:132–7. doi: 10.1016/j.jpeds.2020.08.089
29. Uyanikoglu A, Cindioglu C, Ciftci A, Koyuncu I, Eren MA. The value of 25 (OH) and 1, 25 (OH) vitamin D serum levels in newly diagnosed or on diet adult celiac patients: A case-control study. Int Med. (2021) 3, 37-42 doi: 10.5455/im.10207
30. Lerner A, Shapira Y, Agmon-Levin N, Pacht A, Ben-Ami Shor D, López HM, et al. The clinical significance of 25OH-vitamin D status in celiac disease. Clin Rev Allergy Immunol. (2012) 42:322–30. doi: 10.1007/s12016-010-8237-8
31. Ciccocioppo R. Frulloni L. Immunomodulatory role of vitamin D in coeliac disease. (2020) 3:122–7. doi: 10.30455/2611-2876-XXXX
32. Komisarenko YI, Bobryk MI. Vitamin D deficiency and immune disorders in combined endocrine pathology. Front Endocrinol. (2018) 9:600. doi: 10.3389/fendo.2018.00600
33. Heidari B, Hajian-Tilaki K, Babaei M. Vitamin D deficiency and rheumatoid arthritis: epidemiological, immunological, clinical and therapeutic aspects. Mediterr J Rheumatol. (2019) 30:94–102. doi: 10.31138/mjr.30.2.94
34. Pittas AG, Jorde R, Kawahara T, Dawson-Hughes B. Vitamin D supplementation for prevention of type 2 diabetes mellitus: to D or not to D? J Clin Endocrinol Metabol. (2020) 105:3721–33. doi: 10.1210/clinem/dgaa594
35. Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1, 25 (OH) 2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. (2012) 12:1–4. doi: 10.1186/1471-230X-12-1
36. Chen S, Zhu J, Chen G, Zuo S, Zhang J, Chen Z, et al. 1, 25-Dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-α induced injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling pathway. Biochem Biophys Res Commun. (2015) 460:873–8. doi: 10.1016/j.bbrc.2015.03.125
37. Tanpowpong P., Camargo C.A. 2014. Early-life vitamin D deficiency and childhood-onset coeliac disease. Public Health Nutr. 17:823–826. doi: 10.1017/S1368980013003510
38. Rondanelli M, Faliva MA, Gasparri C, et al. Micronutrients dietary supplementation advices for celiac patients on long-term gluten-free diet with good compliance: a review. Medicina (Kaunas). (2019) 55:337. doi: 10.3390/medicina55070337
39. Wang Q, Xi B, Reilly KH, Liu M, Fu M. Quantitative assessment of the associations between four polymorphisms (Fok1, ApaI, BsmI, TaqI) of vitamin D receptor gene and risk of diabetes mellitus. Mol Biol Rep. (2012) 39:9405–14. doi: 10.1007/s11033-012-1805-7
40. Hasan HA. Ra'ed OA, Muda WA, Mohamed HJ, Samsudin AR. Association of Vitamin D receptor gene polymorphisms with metabolic syndrome and its components among adult Arabs from the United Arab Emirates Diabetes & metabolic syndrome. Clin Res Rev. (2017) 11:S531–7. doi: 10.1016/j.dsx.2017.03.047
Keywords: celiac disease, vitamin D deficiency, vitamin D receptor, Fok1 polymorphism, meta-analysis, autoimmunity
Citation: Shree T, Banerjee P and Senapati S (2023) A meta-analysis suggests the association of reduced serum level of vitamin D and T-allele of Fok1 (rs2228570) polymorphism in the vitamin D receptor gene with celiac disease. Front. Nutr. 9:996450. doi: 10.3389/fnut.2022.996450
Received: 17 July 2022; Accepted: 16 December 2022;
Published: 19 January 2023.
Edited by:
Jasmina Debeljak Martacic, University of Belgrade, SerbiaReviewed by:
Wei Wei, China Academy of Chinese Medical Sciences, ChinaShaden Haddad, Damascus University, Syria
Copyright © 2023 Shree, Banerjee and Senapati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabyasachi Senapati,  c2FieWFzYWNoaTEwMTJAZ21haWwuY29t
c2FieWFzYWNoaTEwMTJAZ21haWwuY29t
 Tanya Shree
Tanya Shree Pratibha Banerjee
Pratibha Banerjee Sabyasachi Senapati
Sabyasachi Senapati