
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 08 December 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.996004
This article is part of the Research Topic Nutrition in Prevention and Management of Non-alcoholic Fatty Liver Disease View all 8 articles
 Nora A. Alfadda1
Nora A. Alfadda1 Ghadeer S. Aljuraiban1
Ghadeer S. Aljuraiban1 Hadeel M. Awwad2
Hadeel M. Awwad2 Mohammad S. Khaleel2
Mohammad S. Khaleel2 Abdulrahman M. Almaghamsi3
Abdulrahman M. Almaghamsi3 Suphia M. Sherbeeni4
Suphia M. Sherbeeni4 Adel N. Alqutub5
Adel N. Alqutub5 Abdullah S. Aldosary6
Abdullah S. Aldosary6 Assim A. Alfadda2,7*
Assim A. Alfadda2,7*Background: Non-alcoholic fatty liver disease (NAFLD) is an overlooked complication of type 2 diabetes (T2D). Current recommendations for the management of NAFLD are mainly focused on weight reduction, overlooking the role of macronutrient composition. Although dietary carbohydrates play a major role in intrahepatic fat synthesis, their association with the progression of liver steatosis has not been fully investigated in patients with T2D.
Aim: To investigate the association between higher carbohydrate intake and the presence of liver steatosis in patients with T2D.
Methods: This cross-sectional study included men and women aged 18–60 years diagnosed with T2D. Anthropometric measurements, hepatic steatosis assessment using the controlled attenuation parameter (CAP), blood samples, and dietary data were analyzed. Participants were divided into two groups: NAFLD and NAFLD-free. A two-sample t-test was used to evaluate the differences between the two groups. Stepwise multiple linear regression models adjusted for potential confounders were used to determine the association between CAP values and higher carbohydrate intake.
Results: In total, 358 participants were included. NAFLD was present in 79.3% of the participants. Body mass index, waist circumference, ALT, HbA1c, and triglycerides showed direct, while HDL-Cholesterol revealed inverse associations with CAP values. No significant relationship was found between carbohydrate intake and steatosis in the total study sample; however, multiple linear regression analysis revealed a significant relationship between carbohydrate intake and CAP values in patients aged ≤50 years.
Conclusion: In patients with T2D, higher carbohydrate intake was associated with liver steatosis in those aged 50 years and below. Further studies are required to confirm the causality between carbohydrate intake and liver steatosis.
Type 2 diabetes (T2D) is one of the fastest-growing diseases worldwide, representing a great healthcare challenge at present (1). Recent reports have demonstrated that T2D is rising in parallel with the increasing incidence of non-alcoholic fatty liver disease (NAFLD) (2), an overlooked complication of T2D. NAFLD is the most widespread form of chronic liver disease (2) and represents a spectrum of chronic hepatic diseases caused by fat accumulation in more than 5% of hepatocytes in the absence of significant alcohol abuse (3, 4). The global pooled prevalence of NAFLD in T2D is 59.67% as reported in a meta-analysis (5). In Saudi Arabia (SA), the prevalence of NAFLD was estimated at 72.8% among patients with T2D (6).
In patients with T2D, chronic increased levels of glucose, insulin, and free fatty acids contribute to resistance to insulin-stimulated glucose uptake in skeletal muscles and adipose tissue, in addition to resistance to the insulin-mediated suppression of lipolysis in adipose tissue (7). Moreover, elevated blood glucose concentrations lead to increased glucose uptake by the liver, resulting in increased conversion of glucose to fatty acids via de novo lipogenesis. As a result of the high blood glucose and free fatty acid concentrations, and to prevent the lipotoxicity of free fatty acids, fat is mildly accumulated in the liver as an adaptive response. As free fatty acids continue to flow into the liver, hepatic intracellular triglycerides will increase, leading to excessive hepatic lipid accumulation. Unlike healthy individuals, the exportation of hepatic fat by very low-density lipoprotein (VLDL) is either insufficient or impaired in patients with NAFLD and/or T2D (7, 8). Patients with T2D usually experience increased liver lipogenesis (9, 10), along with reduction in fatty acid oxidation (11) and triglycerides secretion through VLDL (12). A patient with T2D who has developed NAFLD is exposed to many complications, such as increased microvascular comorbidities of T2D (13, 14), increased risk of cardiovascular events (15), and the progression of the fatty liver into more severe and fatal conditions such as fibrosis, cirrhosis, and hepatocellular carcinoma. These adverse health conditions pose an extra burden on the national healthcare systems, especially with the increasing rates of T2D in SA (16, 17).
The current literature suggests weight reduction as a primary approach to managing NAFLD (4, 18, 19). However, these recommendations are mainly focused on calorie deficit, overlooking the role of dietary macronutrient composition. Macronutrient composition, and more specifically carbohydrate content, may modulate the success of intrahepatic fat reduction, as suggested by multiple studies (20–22). Theoretically, increased intake of dietary carbohydrates is known to be a strong contributor to the development of NAFLD, since they stimulate de novo lipogenesis and induce the synthesis of intrahepatic fat (23, 24). However, research in this area is limited and evidence is still scarce, which may be attributed to methodological limitations in collecting dietary data and achieving a representative sample size (25). Moreover, limited data is available to support the role of carbohydrate intake on the progression of NAFLD in patients with T2D. Knowledge achieved in this area will sufficiently contribute to the current dietary guidelines for patients with T2D, thus helping prevent NAFLD and its advanced stages. Therefore, in this cross-sectional study, we aim to investigate the relationship between carbohydrate intake and the presence of hepatic steatosis in patients diagnosed with T2D. We hypothesize that higher carbohydrate intake is associated with hepatic steatosis in patients diagnosed with T2D.
This is a cross-sectional analysis conducted on a sub-sample randomly selected from the CORDIAL cohort study (non-alcoholic fatty liver disease in a Saudi Cohort with type 2 diabetes mellitus) at the Obesity Research Center, King Saud University, Riyadh, Saudi Arabia. Details on the study have been published previously (26). In brief, the CORDIAL study is a large ongoing prospective cohort started in 2015 that aims to identify the history of hepatic steatosis in patients with T2D over 10 years, with 1,000 participants currently recruited.
The present study was focused on analyzing anthropometric measurements, liver imaging (FibroScan®), blood samples, and completing and analyzing dietary data for recruited participants to explore associations between carbohydrate intake and the presence of liver steatosis in patients with T2D. Signed consent forms were collected from each participant prior to their inclusion in the study and participants had the right to withdraw at any time. The study was approved by the Local Research Ethics Committee in King Fahad Medical City (IRB 12-344).
The sample size was calculated based on local data (6, 27) to determine the prevalence of NAFLD among patients with T2D with a two-sided significance level of 5% and a confidence level of 95%. The required sample size was 368 participants. Considering a 10% dropout rate, the final sample size was approximately 405 participants.
The CORDIAL study included men and women aged 18–60 years and diagnosed with T2D. Subjects were excluded if they showed evidence of hepatic decompensation, or if they have preexisting hepatocellular carcinoma, causes of fatty liver other than NAFLD, or a presence of significant alcohol intake (i.e., daily intake of ≥30 g for men and ≥20 g for women). For the current analyses, we excluded participants who did not complete three non-consecutive days of the 24-h dietary recall or had missing liver imaging data. The recruitment process is illustrated in Figure 1.
Dietary data were assessed using a detailed 24-h recall assessment method for three non-consecutive days, two on weekdays and one on weekends. The 24-h recall data were collected by trained clinical dietitians utilizing the United States Department of Agriculture 5-step multiple-pass technique (28, 29). Owing to the COVID-19 restrictions, the protocol was adjusted to one dietary assessment conducted in an in-person visit and two by phone.
During the dietary assessment, three-dimensional measurement aids (such as food models and household measures) and two-dimensional measurement aids (such as food photographs) were used to estimate portion sizes of consumed food. Moreover, participants were provided a brochure that contains photographs and descriptions of different portion sizes and household measures to be utilized in phone interviews. Most of the two-dimensional measurement aids were obtained from the Nutritional Assessment Guide for Saudi Arabia (30), which contains comprehensive information on different local dishes displayed in various amounts and sizes. All dietary data were transformed into metric units and analyzed using ESHA’s Food Processor® Nutrition Analysis software (ESHA Research, OR, USA) (31) to estimate macronutrient compositions. Saudi local dishes that were not available in ESHA’s database were entered as separate ingredients depending on the original recipe, using the Nutritional Assessment Guide for Saudi Arabia. In case a food item was unavailable, this was added manually if the nutritional information of that food was available, or the most similar available food item was used. Trained dietitians followed a study-specific operational manual and a standard protocol for data coding and entry.
To detect liver steatosis, a controlled attenuation parameter (CAP) test using FibroScan® (Echosens Ltd., Paris, France) was conducted by a trained specialist. CAP is a valid, non-invasive measuring method that is used to diagnose and quantify hepatic steatosis. It can provide higher accuracy in diagnosing and assessing steatosis compared to circulating biomarkers or ultrasonography (32). Hepatic steatosis was determined based on FibroScan® CAP cutoff values that range from S0, which indicates no steatosis, to S3, indicating severe steatosis. Steatosis stages were defined as follows: (i) S0 for no steatosis (CAP values less than 248 dB/m); (ii) S1 for mild steatosis (CAP values from 248 to <268 dB/m, indicating ≥10% of hepatocytes with fat); (iii) S2 for moderate steatosis (CAP values from 268 to <280 dB/m, indicating ≥33% hepatocytes with fat); (iv) S3 for severe steatosis (CAP values equal to 280 dB/m or more, indicating ≥66% hepatocytes with fat) (33).
Anthropometric parameters [height, weight, and waist circumference (WC)] were recorded once, using a standard protocol, with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as kg/m2. All blood samples were collected after overnight fasting, well-contained and stored in the main study site. Blood samples were collected mainly to measure liver enzymes, lipid profile, and glycated hemoglobin (HbA1c). Anthropometrics and blood samples were collected by a certified nurse. Serum lipids and liver enzymes were measured using Abbott – Architect Plus, a clinical chemistry autoanalyzer (Abbott, Abbott Park, IL, USA). Glycated hemoglobin (HbA1c) determination was performed using D-100®, a high-performance liquid chromatography analyzer (Bio-Rad Laboratories, Hercules, CA, USA).
A normality test was performed for all variables and data were checked for outliers and data transfer errors. All included data were normally distributed. Participants were divided into two groups, NAFLD and NAFLD-free, and a two-sample t-test was used to evaluate the differences between the two groups. Continuous data were presented as mean ± standard deviation (SD) and categorical data were presented as frequencies and percentages.
Pearson’s correlation test was used to measure the association between continuous variables. Moreover, participants were stratified into subgroups based on steatosis severity (S0–S3). Analysis of variance (ANOVA) and χ2 tests were used to analyze differences between continuous and categorical variables, and Tukey’s post-hoc test was performed to detect the significant differences between groups.
We used stepwise multiple linear regression models adjusted for potential confounders to determine associations with CAP values for each 2 SD higher carbohydrate intake. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for demographic data (marital status, education, employment, and income) and smoking. Model 3 was additionally adjusted for BMI. Model 4 is model 2 adjusted for LDL-cholesterol (LDL-C) and HDL-cholesterol (HDL-C). Finally, model 5 is model 2 adjusted for triglycerides. Additionally, we evaluated potential effect modifications by age, sex, BMI, and WC. P-values of <0.05 were considered statistically significant for all analyses. Logistic regression analysis was also conducted to investigate the association between carbohydrate intake and the presence/absence of NAFLD, using the previously defined models. Moreover, receiver operating characteristic (ROC) curve analysis was performed to assess the models. All statistical analyses were performed using the Statistical Package for Social Science (IBM SPSS®) version 23.0 (SPSS Inc., IBM, Armonk, NY, USA).
A total of 358 participants were included in the current study. Descriptive characteristics of the study sample are presented in Table 1. All variables were normally distributed. Approximately 79% of participants were diagnosed with NAFLD, of which 97.2% were overweight or obese. BMI was significantly higher in the NAFLD group (33.4 ± 5.1 kg/m2) compared to the NAFLD-free group (30.9 ± 6.3 kg/m2, P < 0.001). Similarly, the mean WC was higher in the NAFLD group (107.1 ± 13.0 cm) compared to the NAFLD-free group (103.3 ± 12.9 cm, P = 0.03). Significant differences were found between the NAFLD and NAFLD-free groups in ALT (25.6 ± 14.1 U/L vs. 21.1 ± 8.9 U/L, P = 0.009), HDL-C (1.1 ± 0.3 mmol/L vs. 1.2 ± 0.3 mmol/L, P = 0.04), and triglycerides (1.6 ± 0.8 mmol/L vs. 1.3 ± 0.6 mmol/L, P = 0.001) (Table 1).
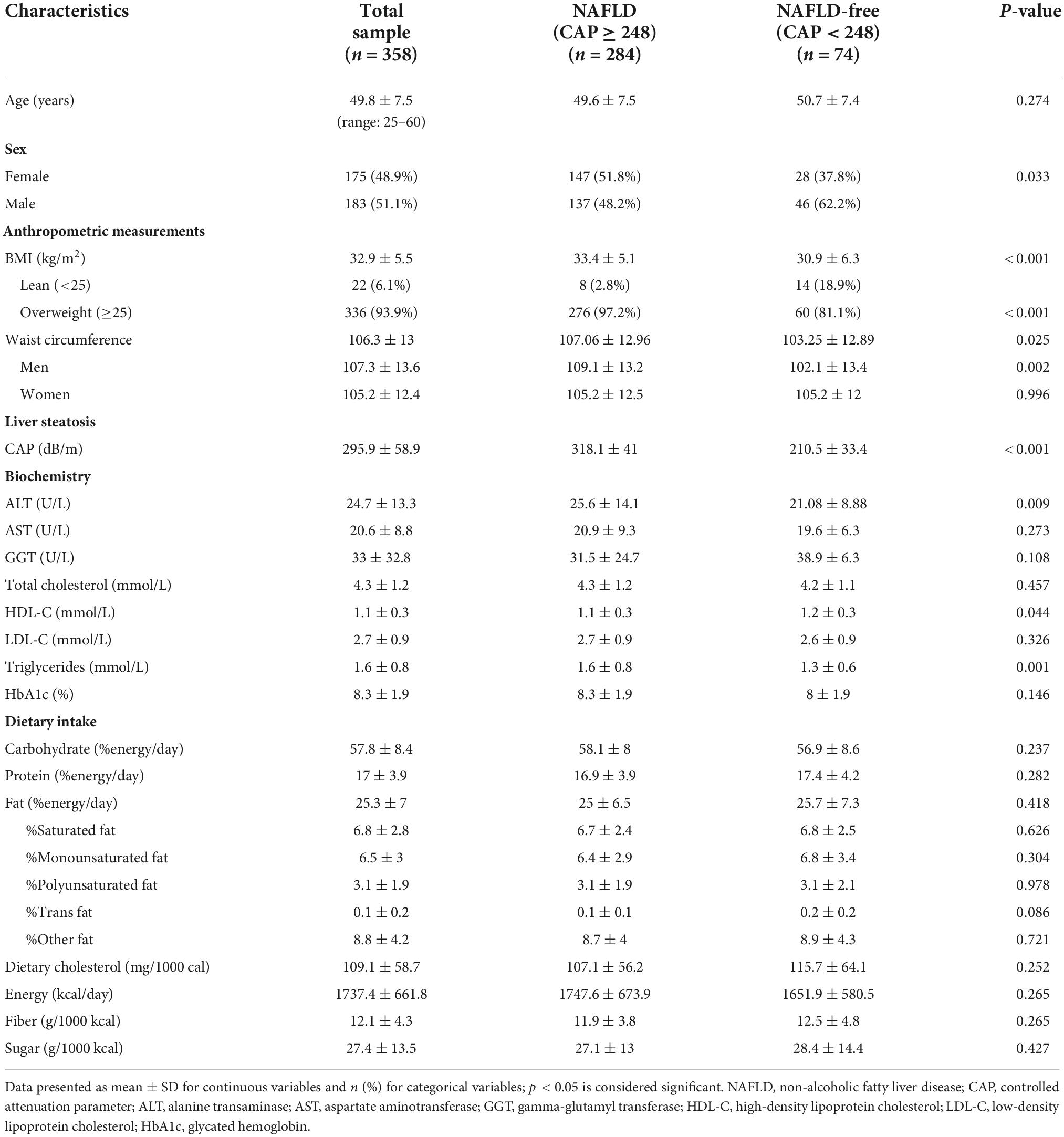
Table 1. Baseline characteristics of the study sample stratified by the presence of non-alcoholic fatty liver disease (NAFLD), n = 358.
In the total study sample, CAP values were significantly and directly correlated with BMI (r = 0.2, P < 0.001), WC (r = 0.2, P < 0.001), and being overweight (r = 0.3, P < 0.001). CAP values were significantly correlated with higher ALT levels (r = 0.3, P < 0.001), triglyceride levels (r = 0.2, P = 0.003), and lower HDL-C levels (r = −0.2, P = 0.002) in the total study sample. Moreover, greater HbA1c levels were significantly correlated with higher CAP values (r = 0.1, P = 0.02). No significant associations were found between NAFLD and nutrient intake (Tables 1, 2).
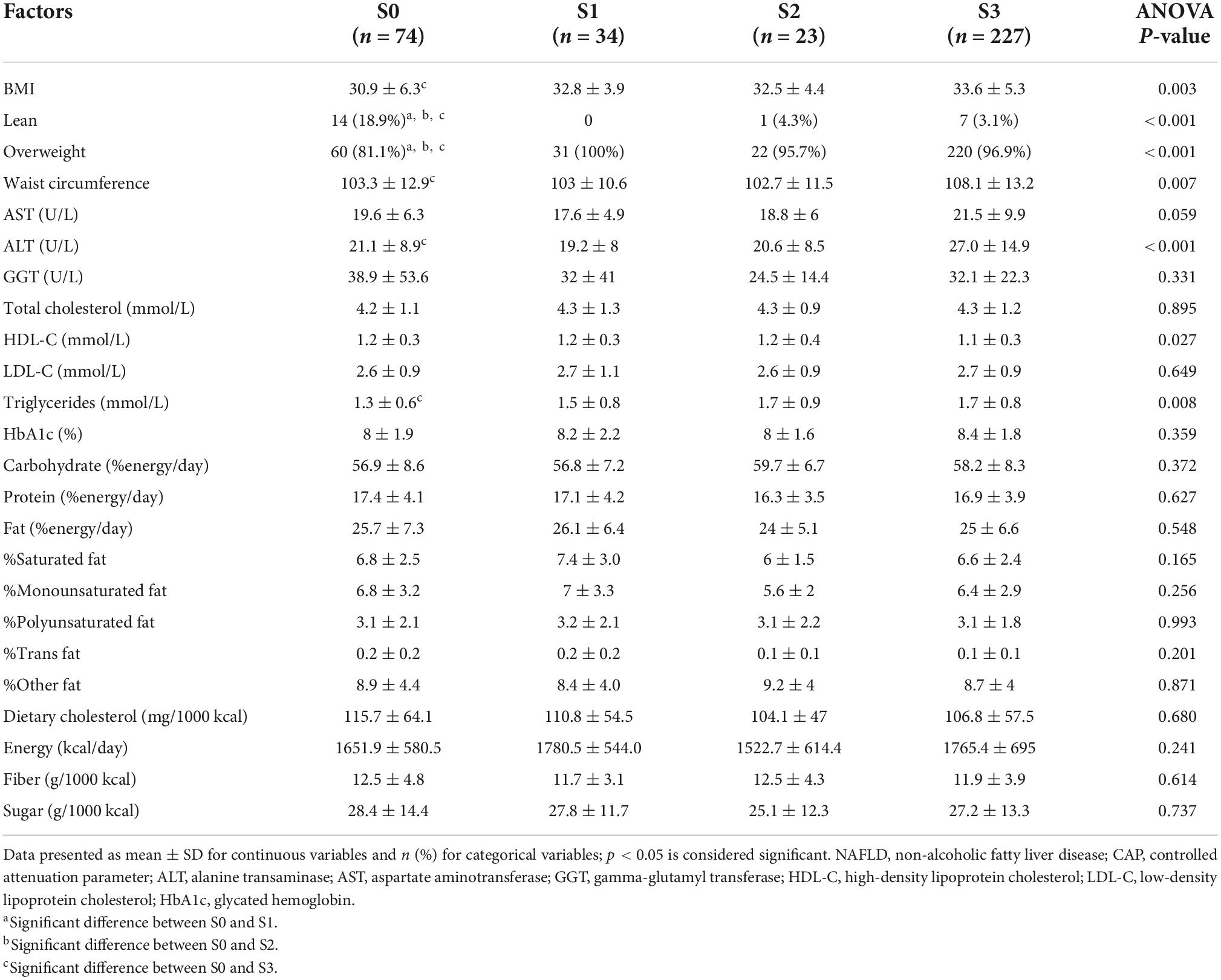
Table 2. Analysis of variance between hepatic steatosis severity and weight, biochemical, and dietary data, n = 358.
When the means of steatosis severity groups were compared to each other, the ANOVA test revealed significant differences in BMI, WC, overweight, ALT, triglycerides, and HDL-C. In Tukey’s post-hoc analysis, participants with no steatosis have significantly lower BMI, WC, ALT, and triglycerides compared to the severe steatosis group (P = 0.001, P = 0.03, P = 0.004, and P = 0.005, respectively) (Table 2).
Linear regression analysis showed no statistically significant association between carbohydrate intake and CAP in all models for the total study sample (Supplementary Table 1).
Test of interaction revealed a significant effect of age as a modifier; therefore, we stratified the study sample by median age (51 years) and repeated the regression analysis (Supplementary Table 1). In participants aged 50 years and below, carbohydrate intake was significantly associated with CAP in model 3 (B = 1.249, P = 0.034) and model 4 (B = 1.400, P = 0.025) (Table 3). No significant associations were observed in the other age group (>50 years) (Supplementary Table 1). In the logistic regression analysis, carbohydrate intake did not have any significant contribution in all models (Supplementary Table 2). In the total sample, ROC curve analysis revealed significant results in models 2–5 (P < 0.001) (Supplementary Figure 1 and Supplementary Table 3). Similar findings were observed in participants aged ≤50 and >50 years (Supplementary Figures 2, 3 and Supplementary Table 3).
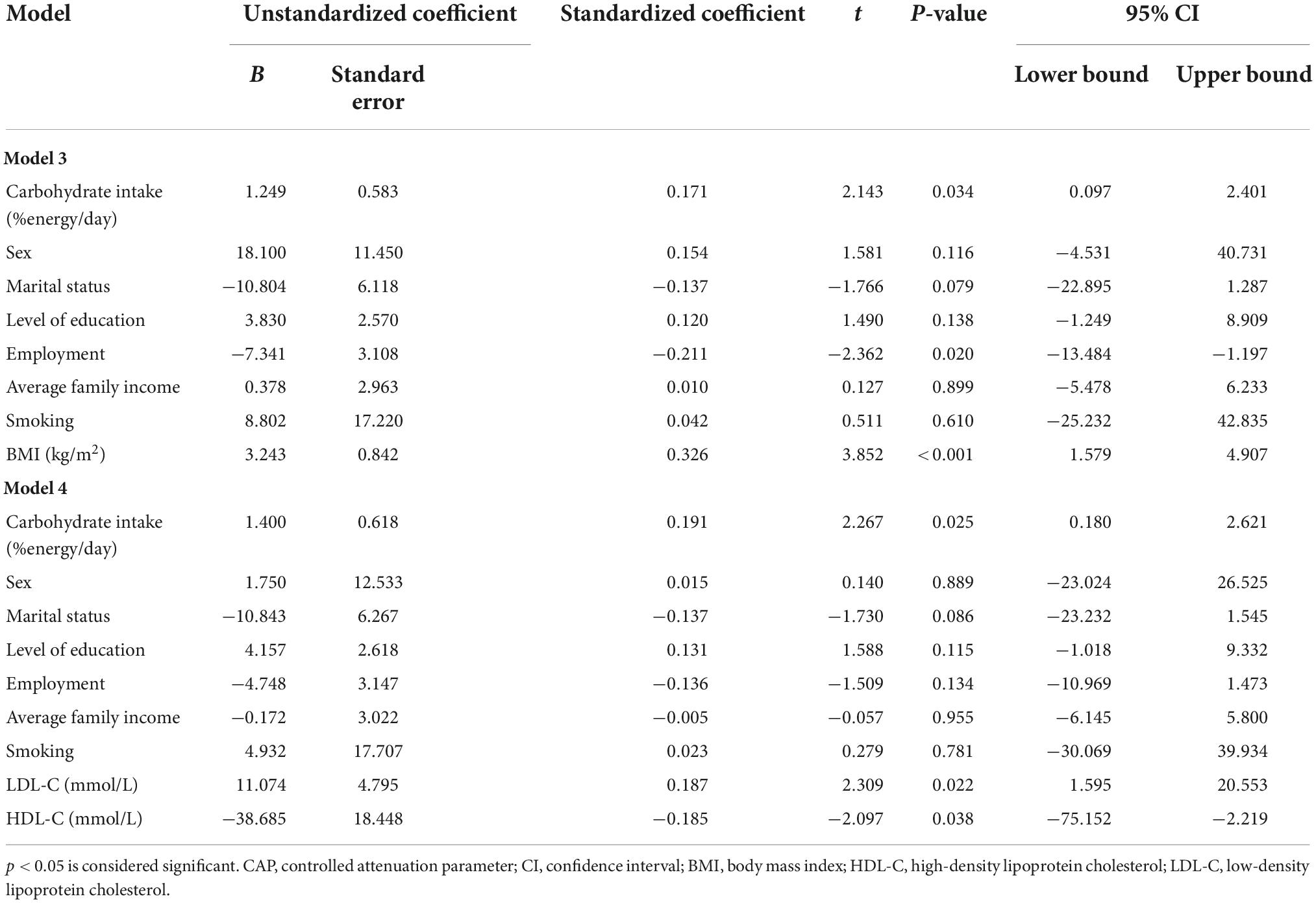
Table 3. Stepwise multiple linear regression analysis between CAP values and two standard deviation higher carbohydrate intake in participants aged 50 years and below, n = 163.
To the best of our knowledge, this is the first national study investigating carbohydrate intake in relation to CAP-confirmed NAFLD in participants diagnosed with T2D. In this study, hepatic steatosis was associated with higher carbohydrate consumption in participants aged ≤50 years in the linear regression model 3, which was adjusted for sex, demographic data, smoking, and BMI, and model 4, which was adjusted for sex, demographic data, smoking, LDL-C, and HDL-C. Moreover, we found significant associations between hepatic steatosis and higher BMI, WC, triglycerides, ALT, HbA1c, and lower HDL-C in the total study sample.
Non-alcoholic fatty liver disease was present in 79.3% of the study participants. Similarly, a study conducted in Abha city (2018) showed that NAFLD was present in 72.8% of 245 participants previously diagnosed with T2D (6). Another study in Jeddah (2003) showed that the prevalence of NAFLD among 116 patients with T2D was 55% (34). Both SA studies used abdominal ultrasound examination to diagnose NAFLD, whereas FibroScan® was used in our study. Globally, the pooled prevalence of NAFLD in patients with T2D in a meta-analysis that included 24 studies involving 35,599 patients with T2D was 59.67% (5). Another meta-analysis of 17 studies involving 10,897 patients with T2D reported an overall NAFLD prevalence of 54% (35). In both reports, the highest prevalence rates were reported in Romania (87.1%), India (87%), and Italy (75%). In our study, the detected frequency of NAFLD among patients with T2D is thought to be in line with the recent local and global data.
Although participants in the NAFLD group consumed slightly more carbohydrates than the NAFLD-free group, this was not statistically significant. Moreover, the results of our study showed no significant association between carbohydrate intake and CAP values and with steatosis stages in the total study sample. Several studies found otherwise (21, 36, 37); less carbohydrate intake was associated with lower intrahepatic fat. It is noteworthy that some of these studies used absolute consumption in grams instead of energy-adjusted intake (21).
Interestingly, linear regression models revealed a significant association between carbohydrate intake and CAP in participants aged ≤50 years, after adjusting for demographic variables, smoking, BMI, LDL-C, and HDL-C. The underlying mechanism remains unclear; however, this may suggest that younger individuals with T2D are more likely to benefit from lower carbohydrate intake to prevent the development of NAFLD. Similar to our findings, the Rotterdam study did not find an association between steatosis and carbohydrate intake in their predominantly older study sample (38). Although the studies that found a desirable effect of less carbohydrate consumption on liver steatosis were not focused on those diagnosed with T2D, it is noticeable that the mean age of the included participants in some of these studies was comparable to the mean age of our younger group (43.2 ± 5.8 years) (20, 36, 39).
In our study, higher weight was associated with the presence of hepatic steatosis in patients with T2D. These findings contribute to the accumulating evidence that NAFLD is associated with increased body weight and central obesity (40–43). According to a meta-analysis of 16 studies, BMI and WC were both independently associated with NAFLD (44). Individuals with central obesity had a higher risk of NAFLD than individuals with general obesity (44, 45), as central obesity may interrupt the secretion of adipose tissue-derived adipokines, leading to an increase in harmful and a decrease in protective adipocytokines (46, 47), which may accelerate the occurrence of NAFLD (48, 49).
The present study showed that HbA1c was significantly associated with CAP values. This suggests that poor diabetes control could be a significant contributor to the development of liver steatosis. It has been suggested that in T2D, hyperglycemia induces the generation of oxidative stress markers and inflammatory mediators leading to cell dysfunction (50, 51). In patients with both T2D and NAFLD, the emission of inflammatory mediators takes place in an early stage of the disease prior to liver damage. Therefore, to prevent further progression of NAFLD into its more severe stages, more focus should be given to monitoring blood glucose levels, HbA1c, and lipid profile, in addition to encouraging a healthy lifestyle (51).
We found that liver steatosis in patients with T2D was correlated with dyslipidemia (i.e., high triglycerides and low HDL-C), which was observed in previous studies as well (51, 52). It is known that T2D-induced hyperglycemia is likely to enhance the pathogenicity of NAFLD by inducing dyslipidemia (53). Furthermore, increased serum triglyceride level is associated with insulin resistance, which also leads to hepatocyte fat deposition. With respect to liver enzymes, ALT was correlated with hepatic steatosis, which is comparable to previous studies (40, 54). Ultrasonography-detected NAFLD was the most common cause of abnormal liver biochemistry, according to a large prospective cohort study from the United Kingdom (55). In this study, participants diagnosed with NAFLD had significantly higher WC, triglycerides, and HbA1c and lower HDL-C, which are the characteristics of metabolic syndrome (56). NAFLD is currently considered a hepatic manifestation of metabolic syndrome in patients with T2D (57, 58). The links between NAFLD, metabolic syndrome, and T2D are probable due to the shared pathogenic factors (59).
This is the first national study to investigate carbohydrate intake in relation to CAP-confirmed NAFLD in participants diagnosed with T2D in Saudi Arabia and the first to identify age as a potential effect modifier. A key strength of our study is the use of a non-invasive diagnostic tool to assess liver steatosis, the CAP. Moreover, we performed a comprehensive, detailed dietary assessment using the best available method (24-h recall), which was conducted on three non-consecutive days to allow for the correction of within-subject variability in nutrient intake (60). Furthermore, dietary data were analyzed using a validated food analysis software, with reliance on local food guides to estimate the nutritional values of the traditional dishes. On the contrary, this study has potential limitations that need to be considered. The cross-sectional nature of the study makes it difficult to infer causality. Further limitations include residual confounding and measurement errors and the possibility of misreporting the actual dietary intake, which is a common limitation of the 24-h recall method.
In adult patients who are diagnosed with T2D, the results of our study support our hypothesis that higher carbohydrate intake is associated with liver steatosis in patients aged ≤50 years. Further studies are needed to confirm causality and help make dietary recommendations for patients with T2D and/or NAFLD. Future studies are needed to investigate whether younger individuals with T2D are more likely to benefit from lower carbohydrate intake to prevent the development of NAFLD.
The datasets presented in this article are not readily available. Requests to access the datasets should be directed to corresponding author.
The studies involving human participants were reviewed and approved by the Local Research Ethics Committee in King Fahad Medical City, Riyadh, Saudi Arabia. The participants provided their written informed consent to participate in this study.
NA, GA, and AAA contributed to the design of the study. AAA, SS, ANA, ASA, and AMA contributed to participant recruitment. NA and HA collected and analyzed the dietary data. MK collected the clinical data. NA and GA wrote the manuscript. All authors participated in interpretation of the data and reviewed and approved the final manuscript.
This work was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH) and King Abdul-Aziz City for Science and Technology, Kingdom of Saudi Arabia (Project No. 08-MED513-02).
We thank the participants and the Obesity, Endocrine and Metabolism Center, the Department of Gastroenterology and Hepatology, and the Department of Medical Imaging administration personnel at King Fahad Medical City, who assisted with the study. We also thank Shahid Nawaz Mohammad Nawaz, Ousman Mahmood Ousman, Arthur Isnani, Sulieman Mohammed Althuwaini, and Kenneth Cudera Domero for their assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.996004/full#supplementary-material
1. Unnikrishnan R, Pradeepa R, Joshi SR, Mohan V. Type 2 diabetes: demystifying the global epidemic. Diabetes. (2017) 66:1432. doi: 10.2337/db16-0766
2. Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. (2016) 17:774. doi: 10.3390/ijms17050774
3. Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. (2017) 9:715–32. doi: 10.4254/wjh.v9.i16.715
4. Alswat KA, Fallatah HI, Al-Judaibi B, Elsiesy HA, Al-Hamoudi WK, Qutub AN, et al. Position statement on the diagnosis and management of non-alcoholic fatty liver disease. Saudi Med J. (2019) 40:531–40. doi: 10.15537/smj.2019.6.23980
5. Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine. (2017) 96:e8179. doi: 10.1097/MD.0000000000008179
6. Alsabaani AA, Mahfouz AA, Awadalla NJ, Musa MJ, Al Humayed SM. Non-alcoholic fatty liver disease among type-2 diabetes mellitus patients in Abha City, South Western Saudi Arabia. Int J Environ Res Public Health. (2018) 15:2521. doi: 10.3390/ijerph15112521
7. Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. (2007) 86:285–300. doi: 10.1093/ajcn/86.2.285
8. Xia MF, Bian H, Gao X. Nafld and diabetes: two sides of the same coin? Rationale for gene-based personalized nafld treatment. Front Pharmacol. (2019) 10:877. doi: 10.3389/fphar.2019.00877
9. Tian J, Goldstein JL, Brown MS. Insulin induction of srebp-1c in rodent liver requires Lxrα-C/Ebpβ complex. Proc Natl Acad Sci. (2016) 113:8182–7. doi: 10.1073/pnas.1608987113
10. Linden AG, Li S, Choi HY, Fang F, Fukasawa M, Uyeda K, et al. Interplay between Chrebp and Srebp-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J Lipid Res. (2018) 59:475–87. doi: 10.1194/jlr.M081836
11. Schmid AI, Szendroedi J, Chmelik M, Krššák M, Moser E, Roden M. Liver atp synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. (2011) 34:448–53. doi: 10.2337/dc10-1076
12. Kamagate A, Dong HH. Foxo1 integrates insulin signaling to Vldl production. Cell Cycle. (2008) 7:3162–70. doi: 10.4161/cc.7.20.6882
13. Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. (2018) 14:99–114. doi: 10.1038/nrendo.2017.173
14. Ix JH, Sharma K. mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of Fetuin-a, Adiponectin, and Ampk. J Am Soc Nephrol. (2010) 21:406–12. doi: 10.1681/asn.2009080820
15. Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. (2007) 30:2119–21. doi: 10.2337/dc07-0349
16. Alhowaish AK. Economic costs of diabetes in Saudi Arabia. J Fam Commun Med. (2013) 20:1–7. doi: 10.4103/2230-8229.108174
17. Robert AA, Al Dawish AM, Braham R, Musallam AM, Al Hayek AA, Al Kahtany HN. Type 2 diabetes mellitus in Saudi Arabia: major challenges and possible solutions. Curr Diabetes Rev. (2017) 13:59–64. doi: 10.2174/1573399812666160126142605
18. Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, et al. Association of weight loss interventions with changes in biomarkers of nonalcoholic fatty liver disease: a systematic review and meta-analysis. JAMA Intern Med. (2019) 179:1262–71. doi: 10.1001/jamainternmed.2019.2248
19. Zelber-Sagi S, Godos J, Salomone F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: a review of observational studies and intervention trials. Therap Adv Gastroenterol. (2016) 9:392–407. doi: 10.1177/1756283X16638830
20. Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. (2004) 49:1578–83. doi: 10.1023/B:DDAS.0000043367.69470.b7
21. Gonzalez C, de Ledinghen V, Vergniol J, Foucher J, Le Bail B, Carlier S, et al. Hepatic steatosis, carbohydrate intake, and food quotient in patients with Nafld. Int J Endocrinol. (2013) 2013:428542. doi: 10.1155/2013/428542
22. Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. (2012) 96:727–34. doi: 10.3945/ajcn.112.038695
23. Worm N. Beyond body weight-loss: dietary strategies targeting intrahepatic fat in Nafld. Nutrients. (2020) 12:1316.
24. Parks EJ. Dietary carbohydrate’s effects on lipogenesis and the relationship of lipogenesis to blood insulin and glucose concentrations. Br J Nutr. (2002) 87(Suppl. 2):S247–53. doi: 10.1079/BJNBJN/2002544
25. Neuschwander-Tetri BA. Carbohydrate intake and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. (2013) 16:446–52. doi: 10.1097/mco.0b013e328361c4d1
26. Alfadda AA, Sherbeeni SM, Alqutub AN, Aldosary AS, Aldaghri NM, Taylor-Robinson SD, et al. Transient elastography for the prevalence of non-alcoholic fatty liver disease in patients with type 2 diabetes: evidence from the cordial cohort study. Saudi J Gastroenterol. (2022) 28:426–33. doi: 10.4103/sjg.sjg_73_22
27. Elmakki E, Aqeely H, Bani I, Omer H, Solan Y, Taher A, et al. Nonalcoholic fatty liver disease (Nafld) in Saudi patients with T2dm in Jazan region: prevalence and associated factors. Br J Med Med Res. (2015) 5:872–9. doi: 10.9734/bjmmr/2015/13077
28. Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the usda five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. (2004) 104:595–603. doi: 10.1016/j.jada.2004.01.007
29. Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the Us department of agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. (2003) 77:1171–8. doi: 10.1093/ajcn/77.5.1171
30. Alfadda AA. The nutritional assessment guide for Saudi Arabia. Olaya: King Fahad National Library (2018).
31. Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, et al. Agreement on nutrient intake between the databases of the first national health and nutrition examination survey and the esha food processor. Am J Epidemiol. (2002) 156:78–85. doi: 10.1093/aje/kwf003
32. Sirli R, Sporea I. Controlled attenuation parameter for quantification of steatosis: which cut-offs to use? Can J Gastroenterol Hepatol. (2021) 2021:6662760. doi: 10.1155/2021/6662760
33. Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (Fibroscan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease – where do we stand? World J Gastroenterol. (2016) 22:7236–51. doi: 10.3748/wjg.v22.i32.7236
34. Akbar DH, Kawther AH. Nonalcoholic Fatty liver disease in saudi type 2 diabetic subjects attending a medical outpatient clinic: Prevalence and general characteristics. Diabetes Care. (2003) 26:3351–2. doi: 10.2337/diacare.26.12.3351-a
35. Amiri Dash Atan N, Koushki M, Motedayen M, Dousti M, Sayehmiri F, Vafaee R, et al. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. (2017) 10(Suppl. 1):S1–7.
36. Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. (2011) 93:1048–52. doi: 10.3945/ajcn.110.007674
37. Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. (2013) 59:138–43. doi: 10.1016/j.jhep.2013.02.012
38. Alferink LJ, Kiefte-de Jong JC, Erler NS, Veldt BJ, Schoufour JD, de Knegt RJ, et al. Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: the rotterdam study. Gut. (2019) 68:1088–98. doi: 10.1136/gutjnl-2017-315940
39. Haufe S, Engeli S, Kast P, Bohnke J, Utz W, Haas V, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. (2011) 53:1504–14. doi: 10.1002/hep.24242
40. Mansour-Ghanaei F, Joukar F, Mobaraki SN, Mavaddati S, Hassanipour S, Sepehrimanesh M. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: a cross-sectional study in North of Iran. Diabetes Metab Syndr. (2019) 13:1591–6. doi: 10.1016/j.dsx.2019.03.009
41. Koda M, Kawakami M, Murawaki Y, Senda M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J Gastroenterol. (2007) 42:897–903. doi: 10.1007/s00535-007-2107-z
42. Almahmoud MH, Al Khawaja NM, Alkinani A, Khader Y, Ajlouni KM. Prevalence of fatty liver disease and its associated factors among jordanian patients with type 2 diabetes mellitus: a cross-sectional study. Ann Med Surg. (2021) 68:102677. doi: 10.1016/j.amsu.2021.102677
43. Properzi C, O’Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. (2018) 68:1741–54. doi: 10.1002/hep.30076
44. Pang Q, Zhang J-Y, Song S-D, Qu K, Xu X-S, Liu S-S, et al. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol. (2015) 21:1650–62. doi: 10.3748/wjg.v21.i5.1650
45. Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-pacific? J Gastroenterol Hepatol. (2007) 22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x
46. Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. (2011) 17:2801–11. doi: 10.3748/wjg.v17.i23.2801
47. Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue–emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. (2005) 2:273–80. doi: 10.1038/ncpgasthep0186
48. Borst SE. The role of tnf-alpha in insulin resistance. Endocrine. (2004) 23:177–82. doi: 10.1385/endo:23:2-3:177
49. Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. (2008) 43:811–22. doi: 10.1007/s00535-008-2213-6
50. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. (2019) 11:45–63. Epub 2019/07/25.
51. Shams MEE, Al-Gayyar MMH, Barakat EAME. Type 2 diabetes mellitus-induced hyperglycemia in patients with Nafld and normal Lfts: relationship to lipid profile, oxidative stress and pro-inflammatory cytokines. Sci Pharm. (2011) 79:623–34. doi: 10.3797/scipharm.1104-21
52. Mansour-Ghanaei R, Mansour-Ghanaei F, Naghipour M, Joukar F. Biochemical markers and lipid profile in nonalcoholic fatty liver disease patients in the persian guilan cohort study (Pgcs), Iran. J Fam Med Prim Care. (2019) 8:923–8. doi: 10.4103/jfmpc.jfmpc_243_18
53. Jin HB, Gu ZY, Yu CH, Li YM. Association of nonalcoholic fatty liver disease with type 2 diabetes: clinical features and independent risk factors in diabetic fatty liver patients. Hepatobiliary Pancreat Dis Int. (2005) 4:389–92.
54. Mandal A, Bhattarai B, Kafle P, Khalid M, Jonnadula SK, Lamicchane J, et al. Elevated liver enzymes in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Cureus. (2018) 10:e3626–e. doi: 10.7759/cureus.3626
55. Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. (2012) 56:234–40. doi: 10.1016/j.jhep.2011.03.020
56. Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. (2006) 444:881–7. doi: 10.1038/nature05488
57. Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. (2009) 13:9–19.
58. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. (2006) 43:S99–112. doi: 10.1002/hep.20973
59. Gan L, Chitturi S, Farrell GC. Mechanisms and implications of age-related changes in the liver: nonalcoholic fatty liver disease in the elderly. Curr Gerontol Geriatr Res. (2011) 2011:831536. doi: 10.1155/2011/831536
Keywords: type 2 diabetes, non-alcoholic fatty liver disease, carbohydrate, diet, nutrition
Citation: Alfadda NA, Aljuraiban GS, Awwad HM, Khaleel MS, Almaghamsi AM, Sherbeeni SM, Alqutub AN, Aldosary AS and Alfadda AA (2022) Higher carbohydrate intake in relation to non-alcoholic fatty liver disease in patients with type 2 diabetes. Front. Nutr. 9:996004. doi: 10.3389/fnut.2022.996004
Received: 16 July 2022; Accepted: 14 November 2022;
Published: 08 December 2022.
Edited by:
Domenico Sergi, University of Ferrara, ItalyReviewed by:
Hiroshi Okada, Matsushita Memorial Hospital, JapanCopyright © 2022 Alfadda, Aljuraiban, Awwad, Khaleel, Almaghamsi, Sherbeeni, Alqutub, Aldosary and Alfadda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Assim A. Alfadda, YWFsZmFkZGFAa3N1LmVkdS5zYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.