- 1Student Research Committee, Department of Clinical Nutrition and Dietetics, Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
- 3College of Kinesiology, University of Saskatchewan, Saskatoon, SK, Canada
- 4St. George's Hospital, London, United Kingdom
- 5Department of Paediatrics, University of Otago, Dunedin, New Zealand
- 6Department Pharmacology, College of Graduate Health Sciences, The University of Tennessee Health Science Center, Memphis, TN, United States
- 7Department of Biorepository, Biomedical Research Administration, King Fahad Medical City, Riyadh, Saudi Arabia
Although there is a consensus on beneficial effects of a low calorie diet in management of non-alcoholic fatty liver disease, the optimal composition of diet has not yet been elucidated. The aim of this review is to summarize the results of current randomized controlled trials evaluating the effects of low fat diet (LFD) vs. low carbohydrate diet (LCD) on NAFLD. This is a systematic review of all the available data reported in published clinical trials up to February 2022. The methodological quality of eligible studies was assessed, and data were presented aiming specific standard measurements. A total of 15 clinical trial studies were included in this systematic review. There is an overall lack of consensus on which dietary intervention is the most beneficial for NAFLD patients. There is also an overall lack of consensus on the definition of the different restrictive diets and the percentage of macronutrient restriction recommended. It seems that low calorie diets, regardless of their fat and carbohydrate composition, are efficient for liver enzyme reduction. Both LCD and LFD have similar effects on liver enzymes change; however, this improvement tends to be more marked in LFD. All calorie restrictive dietary interventions are beneficial for reducing weight, liver fat content and liver enzymes in individuals with NAFLD. Low fat diets seem to be markedly successful in reducing transaminase levels. Further research is needed to explore diet intensity, duration and long-term outcome.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent liver diseases worldwide (20–30%) (1). Individuals with NAFLD may develop liver injury with a subset developing progressive fibrosis (non-alcoholic steatohepatitis), cirrhosis and complications including end-stage liver failure and hepatocellular carcinoma (2). Fatty liver is one of the causes of liver transplantation (3). In many aspects, the pathophysiology of NAFLD is similar to that of obesity, dyslipidemia and diabetes (4).
Diet and exercise are first line treatments for NAFLD. Studies have shown that patients with NAFLD consume excessive amounts of total energy, refined carbohydrates (including fructose), fibers and antioxidants (vitamin C and vitamin E), cholesterol and saturated fats (SFA) with an insufficient intake of polyunsaturated fats (PUFA) (5, 6). Since there is no pharmacotherapy currently available for patients with NAFLD, lifestyle changes remain the fundamental management option (7, 8). In patients with NAFLD (overweight and obese), calorie restriction drives the reduction of liver fat, body weight, and histological improvement of non-alcoholic steatohepatitis (NASH) (9, 10). However, there is conflicting data on which hypocaloric dietary plan should be adopted according to the macronutrient composition (11, 12). Nordmann et al. (13) showed that low-fat diets are more effective than low-carbohydrate diets in reducing total cholesterol (TC) and low-density lipoprotein (LDL) cholesterol concentrations. In contrast, low-carbohydrate diets can be more effective than low-fat diets in increasing high-density lipoprotein (HDL) cholesterol concentrations and reducing triglyceride (TG) and transaminase levels with a further decrease in 24-h circulating blood insulin concentrations in both isocaloric and hypocaloric conditions (14–17).
Current evidence suggests a change in diet composition alone can reduce hepatic fat infiltration. Studies in animal models and humans have shown that reducing intake of carbohydrate (CHO) sources such as added sugars, high glycemic grains, and fructose may be an effective approach to reverse fatty liver by significantly reducing insulin resistance and inflammation (18–20). A randomized clinical trial in adults demonstrated that a CHO restricted diet (<20 g/day) compared to a low-fat diet resulted in similar weight loss but greater reduction in hepatic fat (−55 vs. −28%, p < 0.001) (21). This suggests a clear metabolic advantage for CHO-restriction, independent of overall weight loss, in adults with NAFLD. A study showed that a low-carbohydrate diet was more effective than a low-fat diet in improving obesity (22, 23). Moreover, another paper explored the impacts of low carbohydrate vs. low-fat/low-calorie diets in achieving weight control and found that the low-carbohydrate diet is more effective than the low-fat/low-calorie diet (24). Furthermore, low-fat diets effectively reduce intrahepatic fat content (24). NAFLD is a significant independent risk factor for cardiovascular disease and type 2 diabetes because of concurrent dyslipidemia and insulin resistance (25), therapeutic diets are believed to be effective for reversal of changes seen with NAFLD (26).
However, despite these data, there is little evidence surrounding an all-inclusive diet for NAFLD. This systematic review focuses on randomized controlled trials (RCTs) of low carbohydrate diets compared to low fat diets, to assess their impact on NAFLD and liver enzymes.
Methods
Conduct of systematic review
A meta-analysis was conducted based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Search strategy
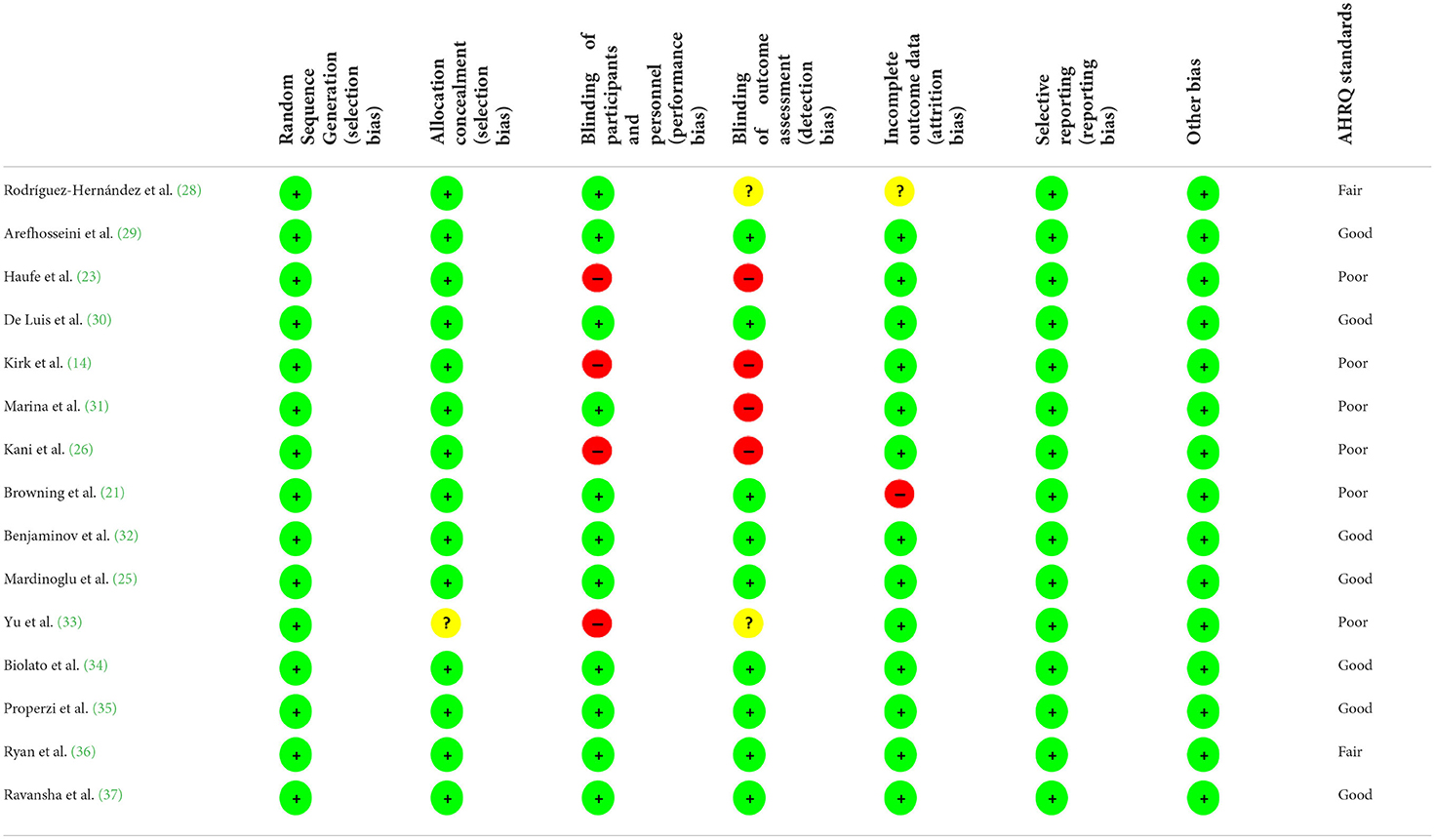
PubMed/Medline, Web of science, Scopus, and Cochrane library databases were searched for relevant articles about the intervention low fat diet or low carbohydrate diet in patients with NAFLD published up to February 2022; without any language restriction. The combination of MESH and non-MESH keywords were used (Supplementary Table 1). Furthermore, all reference lists of related articles, reviews, and meta-analyses were manually checked to avoid missing any studies. The quality of eligible trials was evaluated using the Cochrane quality assessment tool (27), which is comprised of the following: allocation concealment, blinding of participants and personnel, random sequence generation, incomplete outcome data, blinding of outcome assessment, selective reporting and other probable sources of biases (Table 1).
Eligibility criteria
The following criteria were regarded to select eligible studies based on PICO; (1) participants (p): studies that included adult subjects (≥18 years of age) with NAFLD, (2) Intervention (I): examined the effects of low fat diet or low carbohydrate diet in NAFLD patients, (3) comparison: compared with control or baseline value, (4) outcome: those that reported sufficient data related to liver. In addition, studies were excluded if they; (1) were executed on children, pregnant women, other diseases or animals, (2) were not trials, (3) did not provide sufficient information of NAFLD outcomes, or (4) investigated the effects of low fat diet or low carbohydrate diet along with other dietary changes. Unpublished documents and gray literature like conference papers, pilot studies, dissertations, and patents were also excluded.
Data extraction
Two independent investigators (FP and HKV) undertook the study selection whereas a chief investigator (AH) provided further comment if any disagreements. Contact was made with the corresponding authors of any studies where insufficient data was available.
The following data was extracted from eligible studies: first author's name, year of publication and journal, study country, gender of participants, mean age, number of subjects in each group, trial duration, type and energy content of each diet intervention with percent of macronutrient, study design, and the mean and standard deviation of outcome measures at baseline and the end-of-trial.
Definition of low carbohydrate and low fat diet
Low carbohydrate diet definition is very inconsistent, but according to Seidelmann et al. new study (38) optimal proportion of carbohydrate for a healthy diet is 50–55%. Therefore, we considered ≤ 50% as a low carbohydrate diet (LCD) and according to other studies (13, 39) we defined low fat diet (LFD) as a maximum of 30% of the calorie intake from fat.
Results
Literature search and study characteristics
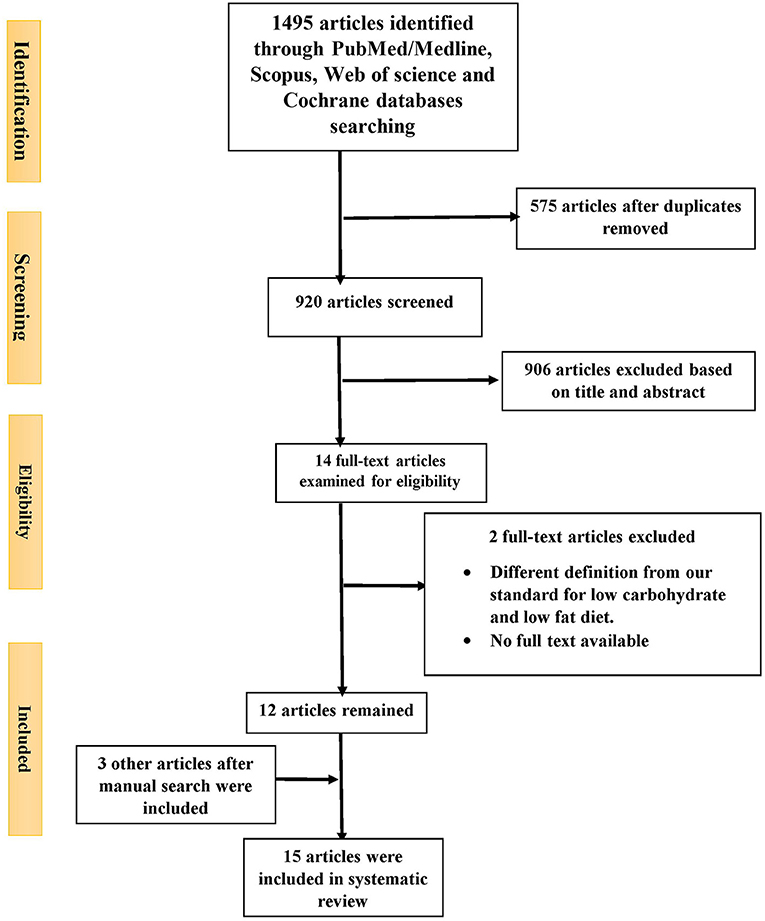
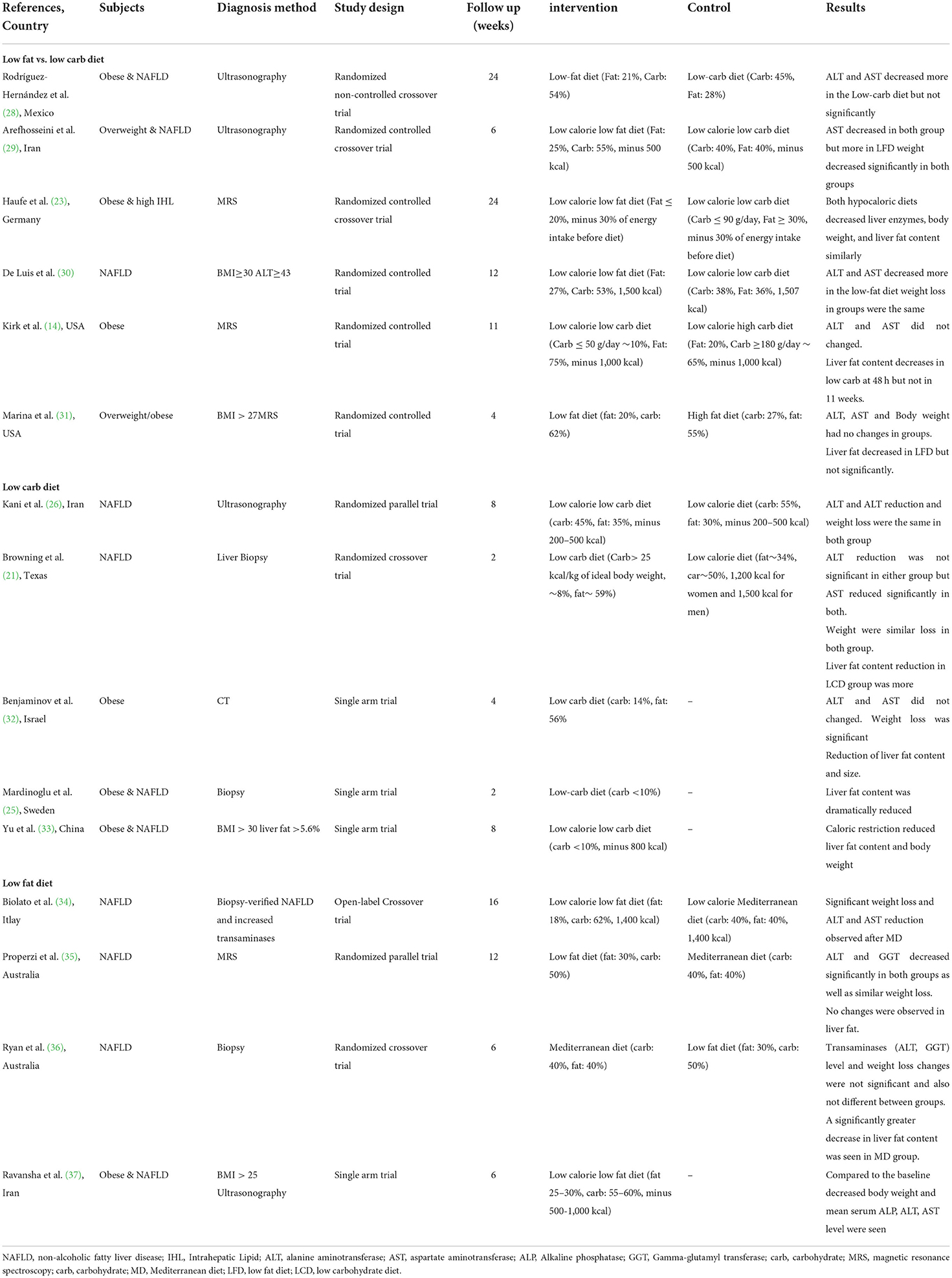
Out of 1,495 articles identified from PubMed/Medline (n = 375), Scopus (474), Web of Science (465) and Cochrane databases (n = 181), 575 duplicate articles were excluded. A further 906 were excluded based on the title and abstract screening approach. The remaining 14 articles were reviewed with two independent authors by reading the full text. Two additional studied were excluded for the following reasons: no full text available (40) and different definition for low carbohydrate and low fat diet (low fat diet; ≤ 30% of total calorie from fat and low carbohydrate diet; ≤ 50% of total calorie from carbohydrate) (4) (Figure 1). After manual search three other articles were also included (14, 29, 32) (Table 2). Finally, 15 articles were included in the current study. Five studies reported LCD vs. other dietary patterns (21, 25, 26, 33), four reported LFD vs. other dietary patterns (34–37), six reported LCD vs. LFD (14, 23, 28–31) and from them nine reported the effects of LCD or LFD on liver fat content (14, 21, 23, 25, 31–33, 35, 36). Fifteen included studies were published between 2005 and 2019. Sample sizes varied from 8 to 140 patients across studies. Mean ages and mean baseline BMIs ranged from 32 to 55 years and 28–45.9 kg.m2, respectively. One trial was performed exclusively in women (28) and others included both genders. Intervention periods were as wide as 2–24 weeks. According to dietary interventions, carbohydrate percentage in LCD was varied from 8 to 45% and 15 to 30% for fat content in LFD.
Quality assessment
The results of the quality assessment of the eligible studies are presented in Table 1. Most studies had a good quality, two had fair quality (28, 36), and six had a poor quality (14, 21, 23, 26, 31, 33) (an unclear risk of bias for in the allocation concealment and blinding of outcome assessments).
Effects of LCD or LFD on liver enzymes and body weight
LCD vs. other dietary patterns.
Two studies compared LCD (<30% from total calorie intake) with low calorie diet. Kani et al. (26) conducted a randomized parallel trial for 30 patients with NAFLD. After intervention with low calorie (−200 to 500 kcal) and low calorie LCD (−200 to 500 kcal, CHO: 45%, FAT: 35%) diets, both ALT and AST decreased. ALP reduction was only seen in low calorie group. However, the changes in liver enzymes and body weight were not significantly different between these two groups.
Browning et al. (21) compared a very LCD (CHO: 8%, FAT: 59%) with low calorie diet (CHO: 50%, FAT: 34%) in 18 patients with NAFLD. A similar weight loss was observed in both groups. Regardless of the dietary intervention, AST but not ALT changed significantly after 2 weeks weight loss.
In a single arm study, Benjaminov et al. (32) showed that a 4 week intervention with a very LCD (CHO: 14%, FAT: 56%) did not alter ALT and AST. Significant weight reduction was seen. Yu et al. (33) conducted a low calorie, very LFD (CHO <10%, 800 kcal) intervention for patients with obesity and NAFLD. In this single arm study, body mean weight reduction after 8 weeks was 6.8 kg (7% of the pre-intervention weights) (p = 0.001).
LFD vs. other dietary patterns
From four studies, three of them compared LFD with Mediterranean diet and one study had no control group. In a crossover study, 20 patients with NAFLD underwent 16 weeks of MD (CHO: 40%, FAT: 40%), 16 weeks of wash-out period, and 16 weeks of LFD (FAT: 18%, CHO: 62%) (34). Both diet interventions were calorie restricted (1,400 kcal). At the end of the MD period, significant weight loss and ALT and AST reduction were observed. But in the LFD group, there were no significant changes in body weight or transaminases. Serum transaminases and body weight at the beginning of LFD period were the same as the end point of MD period: consequently the conditions at the start of the LFD period differed from that of the MD group. These results may reflect the sequence of the dietary intervention.
Properzi et al. (35) compared LFD (FAT: 30%, CHO: 50%) and MD (CHO: 40%, FAT: 35–40%) in a parallel-group RCT. Fifty-six patients with NAFLD were recruited and after 12 weeks intervention data from 49 subjects were available for analysis. At the end of the study, ALT and GGT decreased significantly in both groups. Similar weight loss amounts were seen also, with no no significant differences seen at the final analysis. In a study by Ryan et al. (36) patients with NAFLD randomly received MD (CHO: 40%, FAT: 40%) or LF-HCD (FAT: 30%, CHO: 50%) for 6 weeks. After a 6 week washout period, the subjects swapped to the second diet. ALT and GGT changes were not appreciated in either diet. Similarly, weight loss was not different between two groups (p = 0.22).
Ravanshad et al. (37) designed a single arm randomized trial for obese patients with NAFLD.A low calorie LFD (FAT: 25–30%, CHO: 55–60%) for 6 weeks resulted in decreased body weight and mean serum ALP, ALT, AST levels. Although this study appeared to show beneficial effects for LFD with restricted calorie, the differential effects of calorie and fat restriction were able to be demonstrated.
LCD vs. LFD
Six interventional studies evaluated liver enzymes levels by comparing LCD and LFD. Rodríguez-Hernández et al. (28) enrolled 31 obese women with NAFLD in a randomized crossover study. They showed that 24 weeks of LCD (CHO: 45%, FAT: 28%) decreased ALT and AST more than LFD (FAT: 21%, CHO: 54%), but there was no statistical significance. Since both diets were hypocaloric, the authors claimed that weight loss diminished aminotransferase levels regardless of fat or carbohydrate percentage.
Arefhosseini et al. (29) compared LCD (CHO: 40%, FAT: 40%) with LFD (FAT: 25%, CHO: 55%) in 44 overweight patients with NAFLD patients over 6 weeks. The results arising showed that, regardless of the type of diet, calorie deficit (−500 kcal/day) can reduce AST. Unlike the study conducted by Hernández et al. (28), it was only significant for the LFD. Haufe et al. (23) randomized 102 obese patients including 45% with NAFLD into a LCD (CHO: 25%, FAT 45%) and a LFD (FAT: 27%, CHO: 52%). Both diets were hypocaloric and weight loss was observed in both groups. There were no significant differences in liver enzyme reduction between groups. De Luis et al. (30) assessed 12 weeks of hypocaloric diet with LFD (FAT: 27%, CHO: 53%) and LCD (CHO: 38%, FAT: 36%). Diet therapy improved ALT and GGT inboth groups, but AST decreased only with LFD. Compared with LCD, AST and ALT levels were significantly reduced in the LFD group. Weight losses were the same in both groups (−4 kg).
Kirk et al. (14) designed a randomized clinical trial to compare two types of diet: high carbohydrate diet (=LFD) (FAT: 20%, CHO: 65%) and LCD (CHO: 10%, FAT: 75%). Twenty-two obese patients were enrolled and about 50% of them also had NAFLD. Both diets were hypocaloric (−1,000 kcal/day). After 48 h and ~11 weeks dieting, ALT and AST did not change with either diet. Regardless of the diet group, all subjects lost weight. In a similar study design, Marina et al. (31) evaluated a high fat diet (=LCD) (CHO: 27%, FAT: 55%) and LFD (FAT: 20%, CHO: 62%). About 54% of patients were NAFLD. No changes in liver enzymes or body weight were seen between groups but there was an increasing trend for GGT in LFD.
Overall, high heterogeneity in carbohydrate and fat percent was observed in the designed diets. Low carbohydrate and low fat diets are not well-defined and there is no consensus definition for them and as mentioned in studies percent from total calorie is ranged 8–45% for low carbohydrate diet and 15–30% for low fat diet. Furthermore, most studies combined carbohydrate or fat restriction with calorie restriction preventing clear determination of the effects of the two interventions.
According to the available studies, both LCD and LFD have same effects on liver enzymes in patients with NAFLD. It seems that low caloric diets regardless of their fat and carbohydrate composition are more effective for reduction in liver enzymes. Hypocaloric diets are associated with insulin resistance and metabolic syndrome improvement, so they are effective in reversal of the changes seen with NAFLD. Both LCD and LFD were able to reduce serum transaminase levels but greater improvement appears to be seen with LFD (30).
Effects of LCD or LFD on liver fat content
Haufe et al. (23) in their comparative study showed that the reduction of liver fat following hypocaloric LCD (CHO: 25%, FAT 45%) and hypocaloric LFD (FAT: 27%, CHO: 52%) was similar. Patients with higher baseline intrahepatic fat (IHF) achieved a greater reduction of IHF in this study. Kirk et al. (14) showed that after 48 h, IHF decreased more with the LCD (CHO: 10%, FAT: 75%) than LFD (FAT: 20%, CHO: 65%) but after ~11 weeks the reduction was similar in both groups.
Mardinoglu et al. (25) performed an isocaloric very LCD (CHO <10) diet for 10 obese patients with NAFLD. From the first day of intervention, liver fat content was dramatically educed and the mean reduction was 43.8% after 2 weeks. Yu et al. (33) conducted a single arm study (CHO <10%, 800 kcal) and demonstrated a liver fat content reduction of two thirds after 8 weeks (p = 0.004).
Marina et al. (31) in a comparative study showed LFD (fat = 20%) could reduce liver triglyceride content but it was not significant in comparison to a high fat diet (LCD).
Browning et al. (21) concluded that after 2 weeks liver fat content decreased significantly with weight loss (P < 0.001) but decreased significantly more in LCD (P = 0.008). In a study involving XX subjects, managed with a 4 week intervention with very LCD (CHO = 14%), Benjaminov et al. (32) also showed liver fat content reduction.
In comparing LFD and MD, Ryan et al. (36), in a 6-week crossover design incorporating LFD (FAT: 30%, CHO: 50%) or MD (CHO: 40%, FAT: 35–40%), showed a significantly greater reduction in IHF with MD than LFD (p = 0.03). However, in a parallel design Properzi et al. (35) observed no difference in liver fat content between groups (p = 0.32) with mean (SD) relative reductions in LFD and MD being 25.0 and 32.4%, respectively. Regardless of energy intake, it seems both LFD and LCD can reduce IHF in NAFLD patients.
Discussion
Principal findings
This systematic review evaluating low fat vs. low carbohydrate diets for alcoholic fatty liver disease has demonstrated several major findings. Firstly, considering that NAFLD is one of the commonest causes of liver disease worldwide, there is an overall lack of consensus on which dietary intervention is most beneficial for these patients. Secondly, there is also an overall lack of consensus on the definition of the different restrictive diets and the percentage of macronutrient restriction recommended. Moreover, although most of the included papers relate to trials in middle- or high-income counties the diagnostic methods for NAFLD varied. With these caveats in mind, it appears that both LCD and LFD have the same effects on liver transaminases in individuals with NAFLD and that low calorie diets, regardless of their fat and carbohydrate composition, are more likely to read to reduced transaminases.
Hypocaloric diets are associated with improvements in insulin resistance and metabolic syndrome, and consequently have beneficial effects in NAFLD. Both LCD and LFD are able to reduce serum transaminase levels, however, this improvement tends to be more marked in LFD (30). Additionally, both LFD and LCD seemed to have report weight loss results in patient groups. Moreover, two studies reported fever impact of low free sugar diet on NAFLD in adolescent boys (41) and adults (42). Lastly, there remains insufficient evidence to deduce what duration, combination or intensity of treatment is most effective in these patients.
Limitations
There are several limitations present in this review. Firstly, the included studies are not homogenous. The authors defined low carbohydrate, low fat and low-calorie diets using different parameters. In the included studies, the percentage restrictions ranged from 8 to 45% for LCD and 15 to 30% for LFD. This can affect the reliability of this systematic review. Moreover, it may be difficult to distinguish results between a low calorie, low carbohydrate and low-fat diet, as most of these diets restricted calories and both macronutrients. Another limitation of this systematic review is that most studies did not explore or categorize patients according to different characteristics. For example, patients with different severities of NAFLD may have responded differently to different types of diet adjustments. Similarly, patients with higher body mass indexes may have more marked reductions in weight or liver fat content than their thinner counterparts. This is an increasingly important limitation now that medicine continues to become more individualized. Lastly, the follow up time period varied amongst included studies, with the shortest being 2 weeks and the longest 24 weeks. Therefore, the long-term clinical outcomes of these diets including weight loss, liver enzymes, liver fat content and patient compliance, remain unexplored.
Comparison with previous literature
The current results agree with previous reviews in that calorie restricted diets are overall successful in reducing, but rarely resolving, NAFLD (43, 44). One previous trial compared hypocaloric LFD and LCD for intrahepatic fat reduction in NAFLD and found no significant difference between the beneficial effects of these two diets over a period of 6 months (23). The current results reflect these outcomes and reiterate the noteworthy impact that dietary modification can have on these markers of liver disease.
Additional large studies have also demonstrated that multidisciplinary lifestyle modification, including exercise and diet, can have an even more pronounced impact on NAFLD with one cohort achieving a 64% remission rate after 12 months (45, 46). Nevertheless, other reviews mention the difficulty of managing NAFLD patients with dietary interventions outside of highly controlled trial settings. These interventions require high-intensity specialist services that may not be available, and, whose cost-effectiveness may need to be explored (43).
This systematic review has contributed to existing literature by summarizing the evidence on LCD vs. LFD and confirming that both of these are demonstrated to be successful interventions to promote weight loss and improve hepatic markers in NAFLD.
Suggestions for further research
Future research is needed to answer the questions elicited by the limitations of this report. For example, the intensity of macronutrient restriction that generates the most beneficial response in patients. Secondly, the duration of treatment needed, and therefore, both the feasibility and effectiveness of long-term dietary interventions for the management of NAFLD. Thirdly, the current review suggests that LFD may be more successful in reducing transaminases in these patients, therefore, further exploration of this effect is warranted. Another important component of lifestyle intervention targeting NAFLD is physical exercise. Future research will need to further explore the ideal combination between physical exercise and dietary interventions to ensure long term positive outcomes for these patients.
Conclusions
All calorie restrictive dietary interventions are beneficial for reducing weight, liver fat content and liver enzymes in individuals with NAFLD. Low fat diets seem to be markedly successful in reducing transaminase levels. Further research is needed to explore diet intensity, duration and long-term outcome.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
EK, KA, HV, and AH designed the study. MM, MA, FA, AA, and YS contributed to the literature search, screening data, and data extraction. AA-Z and HK carried out the quality assessment. GR and AS analyzed and interpreted data. HV, FP, AS, AH, and AA-Z wrote and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Student Research Committee, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran (Grant's ID: 1398/9924).
Acknowledgments
The authors sincerely thank Shahid Beheshti University of Medical Sciences for all moral and material supports.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.987921/full#supplementary-material
References
1. Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. (2010) 28:155–61. doi: 10.1159/000282080
2. Adams LA, Lymp JF, Sauver JS, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. (2005) 129:113–21. doi: 10.1053/j.gastro.2005.04.014
3. Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. (2012) 57:675–88. doi: 10.1016/j.jhep.2012.04.015
4. Jang EC, Jun DW, Lee SM, Cho YK, Ahn SB. Comparison of efficacy of low-carbohydrate and low-fat diet education programs in non-alcoholic fatty liver disease: a randomized controlled study. Hepatol Res. (2018) 48:E22–9. doi: 10.1111/hepr.12918
5. Mouzaki M, Allard JP. The role of nutrients in the development, progression, and treatment of nonalcoholic fatty liver disease. J Clin Gastroenterol. (2012) 46:457–67. doi: 10.1097/MCG.0b013e31824cf51e
6. Zelber-Sagi S, Ivancovsky-Wajcman D, Isakov NF, Webb M, Orenstein D, Shibolet O, et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. (2018) 68:1239–46. doi: 10.1016/j.jhep.2018.01.015
7. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. (2012) 55:2005–23. doi: 10.1002/hep.25762
8. George ES, Forsyth A, Itsiopoulos C, Nicoll AJ, Ryan M, Sood S, et al. Practical dietary recommendations for the prevention and management of nonalcoholic fatty liver disease in adults. Adv Nutr. (2018) 9:30–40. doi: 10.1093/advances/nmx007
9. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. (2010) 51:121–9. doi: 10.1002/hep.23276
10. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. (2015) 149:367–78. e5. doi: 10.1053/j.gastro.2015.04.005
11. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. (2012) 56:255–66. doi: 10.1016/j.jhep.2011.06.010
12. Boden G. High-or low-carbohydrate diets: which is better for weight loss, insulin resistance, and fatty livers? Gastroenterology. (2009) 136:1490–2. doi: 10.1053/j.gastro.2009.03.019
13. Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Brehm BJ, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. (2006) 166:285–93. doi: 10.1001/archinte.166.3.285
14. Kirk E, Reeds DN, Finck BN, Mayurranjan MS, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. (2009) 136:1552–60. doi: 10.1053/j.gastro.2009.01.048
15. Ryan MC, Abbasi F, Lamendola C, Carter S, McLaughlin TL. Serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care. (2007) 30:1075–80. doi: 10.2337/dc06-2169
16. McLaughlin T, Carter S, Lamendola C, Abbasi F, Yee G, Schaaf P, et al. Effects of moderate variations in macronutrient composition on weight loss and reduction in cardiovascular disease risk in obese, insulin-resistant adults. Am J Clin Nutr. (2006) 84:813–21. doi: 10.1093/ajcn/84.4.813
17. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. (2003) 348:2082–90. doi: 10.1056/NEJMoa022207
18. Jensen-Urstad AP, Semenkovich CF. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta Mol Cell Biol Lipids. (2012) 1821:747–53. doi: 10.1016/j.bbalip.2011.09.017
19. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. (2005) 115:1343–51. doi: 10.1172/JCI23621
20. Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. (2003) 29:478–85. doi: 10.1016/S1262-3636(07)70061-7
21. Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. (2011) 93:1048–52. doi: 10.3945/ajcn.110.007674
22. Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obesity Rev. (2009) 10:36–50. doi: 10.1111/j.1467-789X.2008.00518.x
23. Haufe S, Engeli S, Kast P, Böhnke J, Utz W, Haas V, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. (2011) 53:1504–14. doi: 10.1002/hep.24242
24. Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, et al. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am Coll Gastroenterol. (2006) 101:2247–53. doi: 10.1111/j.1572-0241.2006.00719.x
25. Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Räsänen SM, et al. An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metab. (2018) 27:559–71. e5. doi: 10.1016/j.cmet.2018.01.005
26. Kani AH, Alavian SM, Esmaillzadeh A, Adibi P, Azadbakht L. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: a parallel randomized trial. Nutrition. (2014) 30:814–21. doi: 10.1016/j.nut.2013.11.008
27. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
28. Rodríguez-Hernández H, Cervantes-Huerta M, Rodríguez-Moran M, Guerrero-Romero F. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. Ann Hepatol. (2016) 10:486–92. doi: 10.1016/S1665-2681(19)31517-0
29. Arefhosseini SR, Ebrahimi-Mameghani M, Naeimi AF, Khoshbaten M, Rashid J. Lifestyle modification through dietary intervention: Health promotion of patients with non-alcoholic fatty liver disease. Health Promot Perspect. (2011) 1:147. doi: 10.5681/hpp.2011.016
30. De Luis D, Aller R, Izaola O, Gonzalez Sagrado M, Conde R. Effect of two different hypocaloric diets in transaminases and insulin resistance in nonalcoholic fatty liver disease and obese patients. Nutr Hosp. (2010) 25:730–5.
31. Marina A, von Frankenberg A, Suvag S, Callahan H, Kratz M, Richards T, et al. Effects of dietary fat and saturated fat content on liver fat and markers of oxidative stress in overweight/obese men and women under weight-stable conditions. Nutrients. (2014) 6:4678–90. doi: 10.3390/nu6114678
32. Benjaminov O, Beglaibter N, Gindy L, Spivak H, Singer P, Wienberg M, et al. The effect of a low-carbohydrate diet on the nonalcoholic fatty liver in morbidly obese patients before bariatric surgery. Surg Endosc. (2007) 21:1423–7. doi: 10.1007/s00464-006-9182-8
33. Yu H, Jia W, Guo Z. Reducing liver fat by low carbohydrate caloric restriction targets hepatic glucose production in non-diabetic obese adults with non-alcoholic fatty liver disease. J Clin Med. (2014) 3:1050–63. doi: 10.3390/jcm3031050
34. Biolato M, Manca F, Marrone G, Cefalo C, Racco S, Miggiano GA, et al. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J Gastroenterol. (2019) 25:509. doi: 10.3748/wjg.v25.i4.509
35. Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. (2018) 68:1741–54. doi: 10.1002/hep.30076
36. Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. (2013) 59:138–43. doi: 10.1016/j.jhep.2013.02.012
37. Ravanshad S, Amirkalali B, Saberfirozi M, Zare N, Maram E. Therapeutic effects of restricted diet in obese patients with Non-alcoholic Fatty Liver Disease (NAFLD). Pak J Med Sci. (2005) 21:472–5. Available online at: https://www.pjms.com.pk/issues/octdec05/article/article19.html
38. Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health. (2018) 3:e419–28. doi: 10.1016/S2468-2667(18)30135-X
39. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. (2008) 359:229–41. doi: 10.1056/NEJMoa0708681
40. Xu JH, Ding YM, Wang B, Fu HF, Xu YZ. Impact of low-carbohydrate diet on the clinical indicators of non-alcoholic fatty liver disease. Chin J Clin Nutr. (2013) 21:287–91. doi: 10.3760/cma.j.issn.1674-635X.2013.05.005
41. Schwimmer JB, Ugalde-Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA. (2019) 321:256–65. doi: 10.1001/jama.2018.20579
42. Khodami B, Hatami B, Yari Z, Alavian SM, Sadeghi A, Varkaneh HK, et al. Effects of a low free sugar diet on the management of nonalcoholic fatty liver disease: a randomized clinical trial. Eur J Clin Nutr. (2022) 76:987–994. doi: 10.1038/s41430-022-01081-x
43. Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. (2017) 4:e000139. doi: 10.1136/bmjgast-2017-000139
44. Eslamparast T, Tandon P, Raman M. Dietary composition independent of weight loss in the management of non-alcoholic fatty liver disease. Nutrients. (2017) 9:800. doi: 10.3390/nu9080800
45. Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. (2013) 59:536–42. doi: 10.1016/j.jhep.2013.04.013
46. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. (2012) 55:885–904. doi: 10.1007/s00125-011-2446-4
Keywords: non-alcoholic fatty liver disease, low fat diet, low carbohydrate diet, NAFLD, liver fat
Citation: Varkaneh HK, Poursoleiman F, Al Masri MK, Alras KA, Shayah Y, Masmoum MD, Alangari FA, Alras AA, Rinaldi G, Day AS, Hekmatdoost A, Abu-Zaid A and Kutbi E (2022) Low fat diet versus low carbohydrate diet for management of non-alcohol fatty liver disease: A systematic review. Front. Nutr. 9:987921. doi: 10.3389/fnut.2022.987921
Received: 06 July 2022; Accepted: 25 July 2022;
Published: 16 August 2022.
Edited by:
Mihnea-Alexandru Găman, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Meysam Zarezadeh, Tabriz University of Medical Sciences, IranSeyed Mohammad Mousavi, Tehran University of Medical Sciences, Iran
Copyright © 2022 Varkaneh, Poursoleiman, Al Masri, Alras, Shayah, Masmoum, Alangari, Alras, Rinaldi, Day, Hekmatdoost, Abu-Zaid and Kutbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azita Hekmatdoost, YV9oZWttYXQyMDAwQHlhaG9vLmNvbQ==; Ahmed Abu-Zaid, YWFidXphaWRAbGl2ZS5jb20=
†These authors have contributed equally to this work
‡ORCID: Ahmed Abu-Zaid orcid.org/0000-0003-2286-2181
 Hamed Kord Varkaneh
Hamed Kord Varkaneh Faezeh Poursoleiman1†
Faezeh Poursoleiman1† Ahmed Abu-Zaid
Ahmed Abu-Zaid