- 1Postdoctoral Research Station, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Department of Endocrinology, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Retinopathy is a chronic inflammatory disease whose prognosis could be improved with dietary interventions. However, the association between a pro-inflammatory diet and the prevalence of retinopathy has not been fully elucidated. We assess the association between the dietary inflammatory index (DII), which is a comprehensive index determining inflammatory potential derived from food parameters according to literature, and the prevalence of retinopathy based on the data from the National Health and Nutritional Examination Survey (NHANES) 2005–2008 involving 2,403 participants. Energy-adjusted DII (E-DII) was not related to the occurrence of retinopathy in the general, non-diabetic, or middle-aged participants. In the diabetic and aged participants, one unit increment of E-DII accounted for 14 and 15% higher the prevalence of retinopathy respectively. The highest E-DII group had a 78 and 79% higher prevalence of retinopathy than the lowest group respectively. After adjusting for several covariables, the highest E-DII group was still associated with a 68% increase in retinopathy in diabetic patients. These results suggest that E-DII is positively associated with the prevalence of diabetic retinopathy among diabetic patients.
Introduction
There is a growing number of people at risk of retinopathy, whose incidence is estimated to be 34.6% in diabetes, which is a major epidemic worldwide (1). The global prevalence of age-related macular degeneration is 8.69% mapped to an age range of 45–85 years (2). Retinopathy causes progressive vision loss, which not only has widespread effect on the quality of life but also poses pressure on the social economy.
Retinopathy is a chronic inflammatory disease manifested by increased vascular permeability, edema, infiltration of inflammatory cells, neovascularization, and expression of angiogenic factors (3). In addition, intravitreal anti-VEGF agent injection has shown promising therapeutic effects, raising the anti-inflammation strategy for retinopathy (4). Recently, it is reported that diet and lifestyle interventions could improve the prognosis of retinopathy (5).
The dietary inflammatory index (DII) is a literature-derived, population-based index to determine the inflammatory potential of diets (6). Forty-five food parameters were identified to be related to six inflammatory biomarkers: IL-1β, IL-4, IL-6, IL-10, TNF-α, and C-reactive protein, based on the dietary algorithm developed from nearly 6,500 articles (6). Although several studies have established the association between DII and Diabetes Mellitus (DM) as well as its complications (7–10), there is no report regarding the relationship between DII and retinopathy. Herein, we assess the association between DII and retinopathy especially DR using the National Health and Nutrition Examination Survey (NHANES) database. Moreover, we did subgroup analysis and interaction tests regarding age, gender, diabetic status, hypertension, and other demographic and metabolic factors, to identify the specific population among whom DII has a relatively independent association with the prevalence of retinopathy.
Methods
Study population
NHANES is a nationwide and ongoing cross-sectional survey managed by the National Center for Health Statistics at the US Centers for Disease Control and Prevention. It conducts a repeated 2-year cycle test with a complex multistage probability sampling design, and all sample weights are designed to represent data for the civilian non-institutionalized US population. The NHANES research protocol was approved by the institutional review board and included the written, informed consent of all participants, following the principles of the Declaration of Helsinki. Data in this study were all obtained from NHANES, publicly available at https://www.cdc.gov/nchs/nhanes/ (accessed date: 27 March 2022).
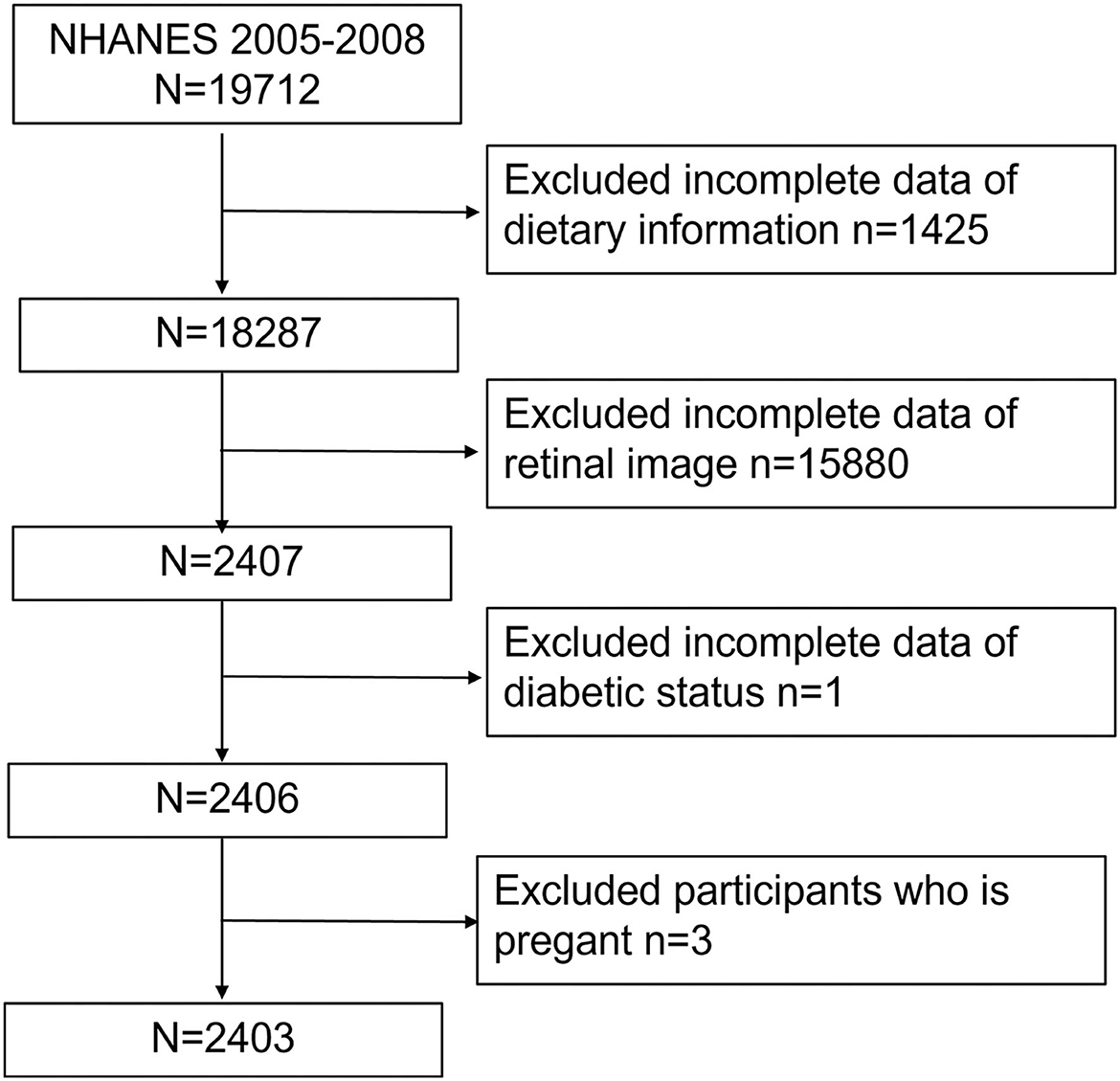
Our study was based on a 4-year NHANES survey cycle, 2005–2008, since this cycle included full information on retinopathy based on a retinal imaging exam. After excluding participants without necessary dietary conditions (n = 1,425), retinal image results (n = 15,880), or diabetic status (from questionnaire and plasma glucose and HbA1c test, n = 1) as well as pregnant women (n = 3), we finally included 2,403 participants (Figure 1).
Definition of participants, exposure, and outcomes
For the present study, subjects who declared to have a previous DM physician-diagnosis or who have fasting glucose ≥126 mg/dL, HbA1c (%) ≥6.5, or OGTT ≥200 mg/dL, were defined as DM. Participants who declared to have a pre-DM physician diagnosis or who have fasting glucose ≥100 mg/dL, HbA1c (%) ≥5.7, or OGTT ≥140 mg/dL were defined as pre-diabetic. Furthermore, subjects who reported no DM or pre-diabetes history with normal glucose and HbA1c test were considered normal. The whole population was constituted of 1,089 normal glucose, 823 prediabetes, and 491 DM participants.
DII was designed as the exposure variable. Dietary intake was documented and validated utilizing the 24-h dietary history interview at the Mobile Examination Center. The dietary data were obtained at the mobile examination center and were validated by the Nutrition Methodology Working Group. The 24-h dietary recall data were used to calculate the DII score according to the calculating protocol published by Shivappa et al. (6). In our study, 27 of the 45 food parameters were available to calculate DII in the 2005–2008 NHANES cycle, including alcohol, protein, fiber, β-carotene, cholesterol, carbohydrates, energy, fats, n-3 fatty acid, n-6 fatty acid, poly-unsaturated fatty acid, mono-unsaturated fatty acid, saturated fat, thiamin, magnesium, zinc, selenium, iron, riboflavin, folic acid, vitamin A, vitamin B-6, vitamin B-12, vitamin C, vitamin D, vitamin E, caffeine, and niacin. DII calculation formula is shown below. Each value of the 27 parameters above was subtracted from the global daily mean intake and divided by its standard deviation, both of which were available in N. Shivappa's paper (6). Then the value was converted to a percentile score, multiplied by 2, and subtracted by 1 to achieve symmetrical distribution. The percentile value of each component was multiplied by the corresponding overall inflammatory effect score, which was summed up to get the overall DII score for each participant. The higher positive DII score indicated a more pro-inflammatory diet, while a lower negative DII score suggested an anti-inflammatory effect of diet. To control for the effect of total energy intake, we used the energy-adjusted dietary inflammatory index (E-DII) wherein we calculated all the food parameters per 1,000 kcal of consumption. In this study, the E-DII score was served as a continuous variable and then categorized into tertiles from the total sample for further analysis.
Calculation formula of DII:
Z score = (24 h intake of each component—global daily intake) / standard deviation of global daily intake
Z score1 = Z score → converted to a percentile score ×2 −1
DII = Σ Z score1 × overall inflammatory effect score of each component
According to the severity scale provided by the Early Treatment for Diabetic Retinopathy Study (ETDRS), retinopathy is characterized by hard exudates, vitreous hemorrhage, cotton-wool spots, and intra-retinal microvascular changes over retinal tissue (11). A non-mydriatic fundus photography (TRC-NW6S; Topcon, Tokyo, Japan) was used for detecting DR in the survey based on the NHANES Digital Grading Protocol. In NHANES, participants aged 40 years and older were eligible for retinal imaging. The detailed information can be found in the NHANES Ophthalmology Procedures Manual available on the NHANES website.
Covariates assessment
We incorporated age, body mass index (BMI, kg/m2), race ethnicity, educational level, smoking status, family poverty-to-income ratio (PIR), marital status, hypertension, and high cholesterol as covariates which were available in NHANES questionnaire data or demographic data. Participants aged 65 years and older were considered as aged. Participants with BMI equal to or higher than 25 were overweight according to the World Health Organization standards. The smoking status was classified as never, former smoker, and current smoker based on the “Smoking—Cigarette Use” section in the NHANES Questionnaire data. PIR < 1.00 means household income below the poverty threshold, while PIR higher than 3.00 means household income more than triple the poverty threshold. Participants who were divorced, widow, or lived separately were considered to be living separately. Hypertension and high cholesterol were considered if the participants were ever told they had high blood pressure or cholesterol level.
Statistical analysis
The descriptive analysis was summarized according to the subjects' retinal status. Continuous variables were presented as mean ± SD and analyzed by Student's t-tests. Categorial variables were presented as percentages and analyzed by Chi-square tests. The relationship between E-DII and retinopathy was examined with a logistic regression model. Subgroup analysis was also conducted by categories including gender, age, race ethnicity, educational level, marital status, family income, smoking status, BMI, diabetic status, hypertension, and high cholesterol. Interaction terms, such as demographic parameters, diabetic status, and so on, were added to test the heterogeneity. Then we categorized the participants into 3 groups by the tertiles of E-DII and did logistic regression analysis for the association as well as the Wald test to assess P for trend. A multivariate linear regression was used for the relationship between E-DII and retinopathy after being stepwise adjusted for the potential confounding factors. Lastly, weighted generalized additive models and smooth curve fittings were used to address the non-linear association between E-DII and DR in DM patients. All analyses were performed with the software package R (http://www.R-project.org, The R Foundation, access on 26 April 2022) and EmpowerStats (www.empowerstats.com access on 26 April 2022).
Results
Baseline characteristics of participants
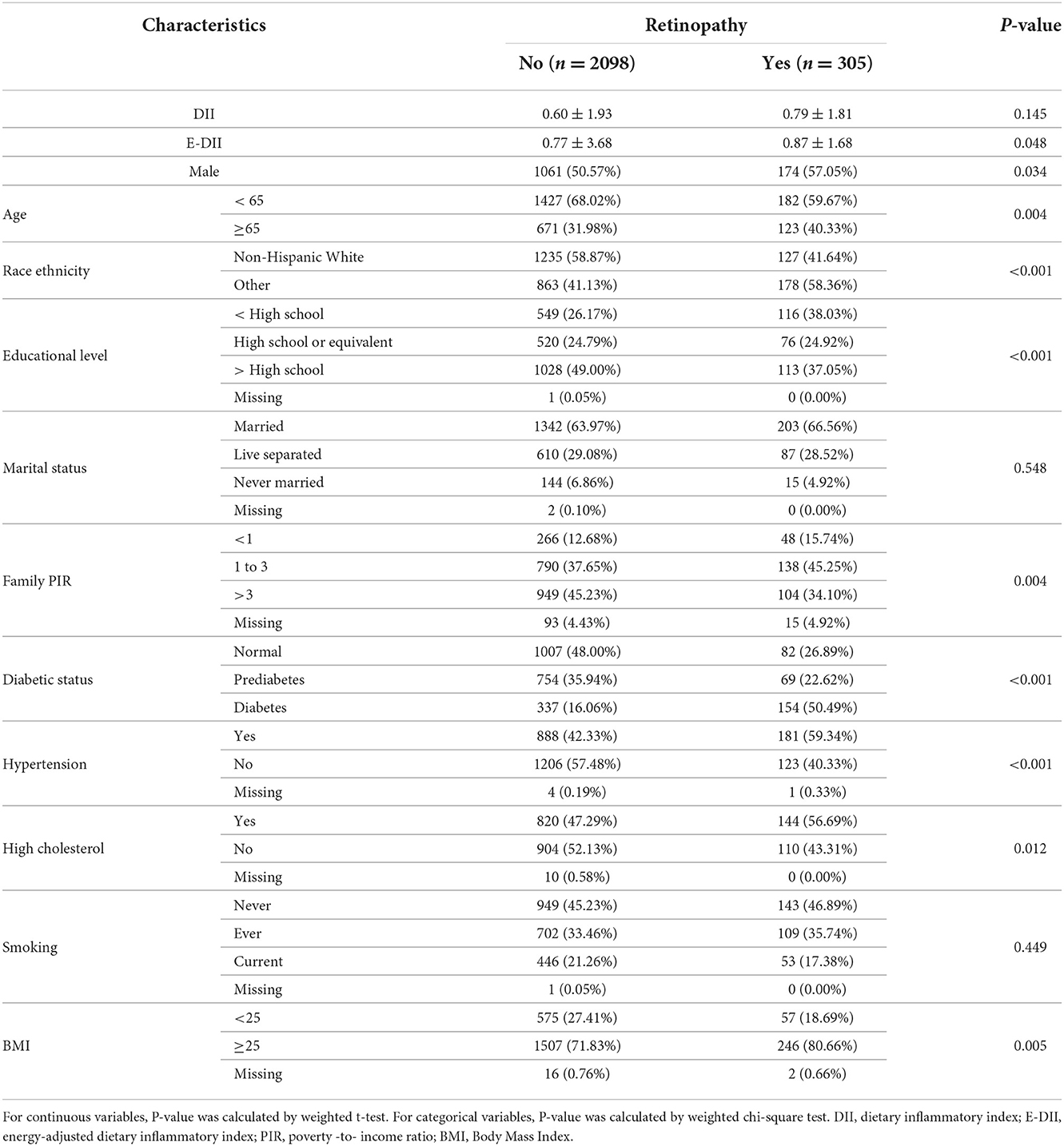
Table 1 lists characteristics information of 2,403 eligible participants with (n = 305) and without retinopathy (n = 2,098). Participants with retinopathy tended to be older, low-educated, and overweight with no high income. The prevalence of DM, hypertension, or dyslipidemia was higher in people with retinopathy than in those without. Non-Hispanic Whites had a lower prevalence of retinopathy than other race ethnicities. E-DII was significantly higher in participants with retinopathy than in those without. There was no obvious difference between retinopathy and non-retinopathy in marital and smoking status. In Supplementary Table S1, we found that there were significant differences in gender, race ethnicity, educational level, marital status, family PIR, diabetic status, hypertension, and smoking status among participants with different levels of E-DII.
Association between E-DII score and retinopathy
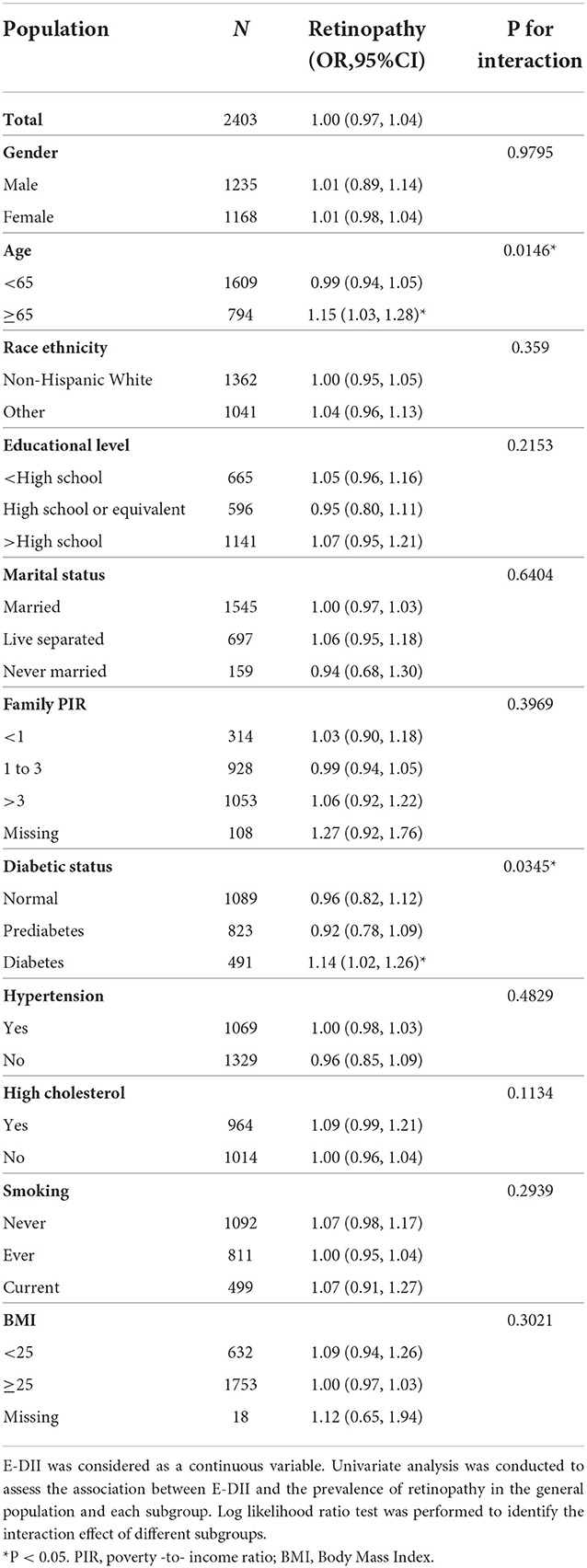
As shown in Table 2, in the whole population, E-DII was not significantly associated with the prevalence of retinopathy when it was taken as a continuous variable (OR = 1, 95%CI: 0.97–1.04). Then, we did subgroup analysis according to gender, age, race ethnicity, educational level, marital status, family income, diabetic status, hypertension, dyslipidemia, smoking status, and BMI. E-DII was significantly associated with the prevalence of retinopathy in DM and aged (age ≥ 65) participants. One unit increase in E-DII significantly accounted for 14 and 15% increments in the prevalence of retinopathy in DM patients (OR = 1.14, 95%CI: 1.02–1.26) and aged participants (OR = 1.15, 95%CI: 1.03, 1.28), respectively. The log likelihood ratio test proved that there was a significant interaction between E-DII and retinopathy among different diabetic status and age groups (both P for interaction <0.05), while we found no significant difference among other groups (all P for interaction > 0.05).
Then we categorized aged and DM participants into three groups by tertiles of E-DII. The highest E-DII group had a 78 and 79% higher prevalence of DR as compared to the lowest group (OR = 1.78, 95%CI:1.11–2.85 and OR = 1.79, 95%CI:1.11–2.88) in DM patients and aged participants, respectively (Table 3). Furthermore, the occurrence of retinopathy had a significant increasing trend across the tertiles of E-DII for both DM and aged participants (both P for trend < 0.05).
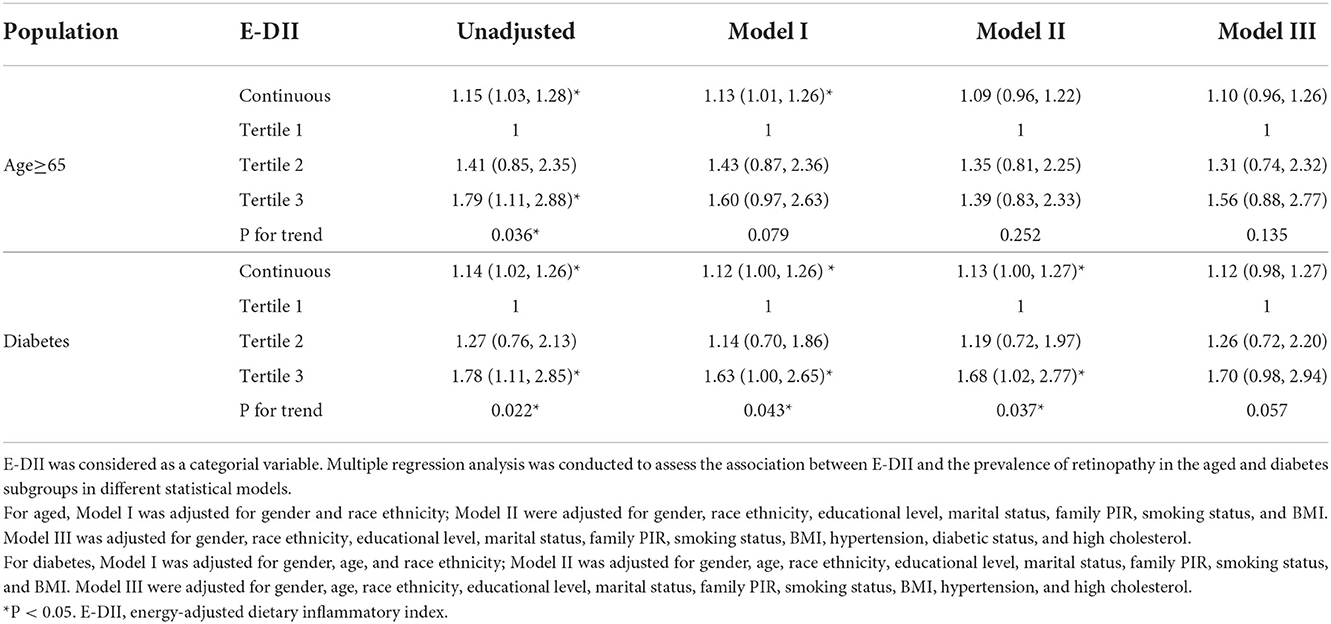
Table 3. Association between E-DII and retinopathy in the aged and diabetes participants according to different models.
After adjusted for age, gender, and race ethnicity, 1 unit increment in the E-DII score accounted for a 12% (OR = 1.12 95%CI: 1–1.26) increment in the prevalence of DR in DM people. The prevalence of DR in the highest tertile is 63% higher than in the lowest tertile group (OR=1.63 95%CI: 1–2.65). After further adjusted for educational level, marital status, family income, smoking status, and BMI, the statistical significance remains with 1 unit increment in the DII score accounting for a 13% (OR = 1.13 95% CI, 1.00–1.27) increase in the prevalence of DR. The prevalence of DR in the highest tertile is 68% higher than in the lowest tertile (OR = 1.68 95% CI, 1.02–2.77). There is a significantly increased risk for retinopathy across the tertiles of E-DII in all the models above for DM patients (all P for trend < 0.05). However, the association between E-DII and DR lost significance with hypertension and dyslipidemia included in the covariables although E-DII induced a 12% increase in the prevalence of DR with a 1 unit increment (OR = 1.12 95% CI, 0.98–1.27). In aged participants, 1 unit increment in the E-DII score accounted for a 13% (OR = 1.13, 95%CI: 1.01–1.26) increment in the prevalence of retinopathy after adjusted for gender and race ethnicity. However, E-DII was not significantly associated with retinopathy with additional covariables whenever it was taken as a continuous or categorial variable in aged participants.
We conducted a log-likelihood ratio test comparing the one-line linear regression model with a two-piecewise linear model in DM patients, which indicated a non-linear relationship between DII and retinopathy (P < 0.01) (Figure 2, Table 4). When E-DII is < −0.87, there is a significant positive correlation between E-DII and retinopathy. When E-DII is higher than −0.87, the curve tends to be flat, and E-DII does not give rise to a higher prevalence of DR.
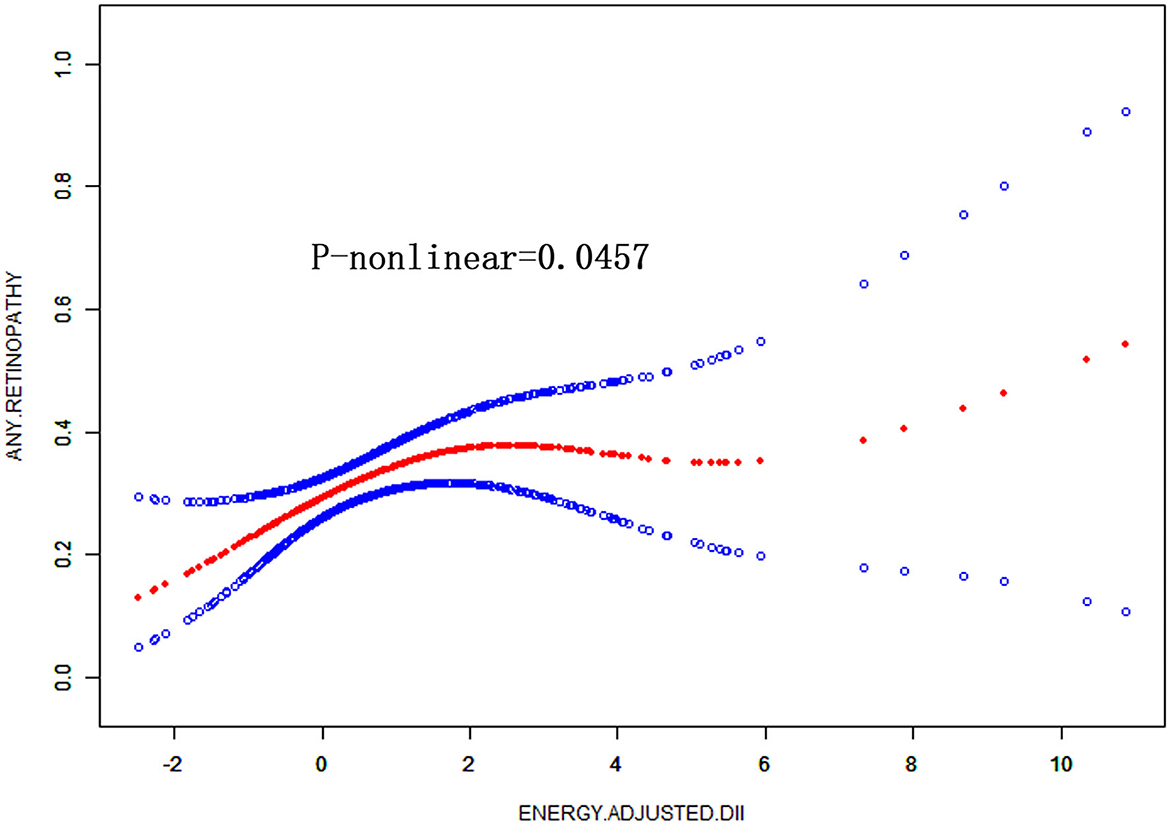
Figure 2. Non-linear relationship between DII and the prevalence of retinopathy in diabetes by the generalized additive model after adjusted for gender, age, race ethnicity, educational level, marital status, family PIR, smoking status, and BMI.
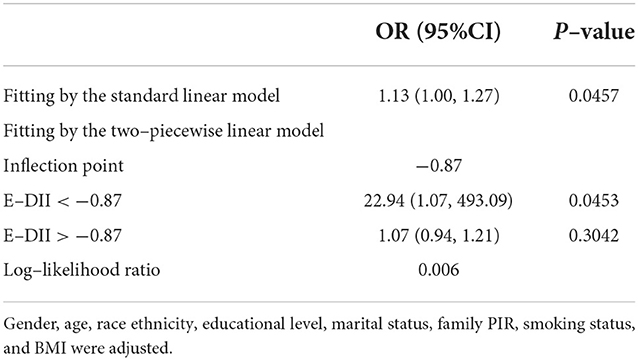
Table 4. Threshold effect analysis of E-DII on retinopathy using the two-piecewise linear regression model.
Discussion
In the study based on NHANES data, we observed no significant association between E-DII and the prevalence of retinopathy in the general population. In the subgroup analysis, one unit increase of E-DII gives rise to a significantly higher prevalence of retinopathy in aged and DM participants, respectively. The most pro-inflammatory diet induced a higher prevalence of retinopathy than the most anti-inflammatory diet group in aged and DM participants, respectively. The relationship remains significant after adjusted for age, gender, race ethnicity, educational level, marital status, PIR, smoking status, and BMI in DM patients, which is a non-linear relationship.
Consistent with previous studies, which showed an anti-inflammatory diet alleviated retinopathy in aged or diabetic participants, we found a diet with anti-inflammatory potential was associated with a decreased prevalence of retinopathy in DM or aged participants. Adherence to a Mediterranean diet or antioxidant supplement with Omega-3 unsaturated fatty acids and vitamins could improve age-related macular degeneration and DR (12–17). DII is an integrated index to evaluate dietary inflammatory potential, which might synthetically reflect consumption lifestyle and give instructions to patients at high risk of retinopathy.
Both DR and age-related macular degeneration are chronic inflammatory diseases that involves the activation of the microglia, monocytes-macrophages, neutrophils, and lymphocytes as well as the secretion of inflammatory mediators, including IL-6, TNF-α, MCP-1, and VEGF (18, 19). All of these cause damage to endothelium, pericytes, and ganglion cells, which promotes vascular permeability and neovascularization (20, 21). DM is closely associated with gut microbiota dysbiosis, which destructs gut mucosa barrier integrity, enhances intestinal permeability, and increases plasma endotoxins (22, 23). Recently, DII was found to be negatively associated with enterolignans, a potential marker for microbiota diversity, which indicated anti-inflammatory diet might protect against DR through the improvement of gut flora and systematic inflammation (24).
DII is crudely associated with retinopathy in aged participants when it is taken as a categorial or continuous variable. After adjusted for gender and race ethnicity, one unit increment of E-DII significantly accounted for 13% increase in the prevalence of retinopathy, but it lost statistical significance after adjusted for other confounding factors in other models. DII is associated with multiple metabolic diseases including obesity, DM, and cardiovascular disease (25). In our study, DII was crudely and positively associated with BMI, hypertension, and high cholesterol, which are risk factors for DR (26, 27). Although after adjusted for demographic factors, smoking status, and BMI, the association between E-DII and DR still exists. The association lost statistical significance with hypertension and high cholesterol included in the covariables. Hence, the association between E-DII and DR cannot rule out the effect of hypertension and high cholesterol. In addition, E-DII is not only associated with the prevalence of DM but also positively related to the severity of DM and insulin resistance (7, 8). Our study provides evidence that E-DII is associated with DR, which is a diabetic complication found in 22.27% of DM patients (28). Furthermore, E-DII was not associated with the presence of retinopathy in normal glucose and prediabetes, which implied that the presence of retinopathy was induced by the synergistic effect of inflammation and hyperglycemia or insulin resistance (7).
To our knowledge, this is the first study exploring the association between DII and retinopathy. However, there are some inevitable limitations. Firstly, out of the 45 parameters, we took the 27 parameters available in NHANES, which might not reflect the whole dietary inflammatory potential. In addition, DII originated from 24-h dietary intake recall, which would give rise to recall bias. We did not include glucose control levels in the covariates, as the ideal glucose control level is not consistent for different age groups. So was medication data, which was hard to extract from the NHANES database. So, prospective cohort studies are needed to verify the causation between E-DII and retinopathy in DM patients independent of these confounding factors. Finally, we did not do survey design analysis in this study because we aimed to assess the association between E-DII and retinopathy rather than epidemiology surveys, which focus on the prevalence of some diseases in the whole population. Due to the inclusion and exclusion criteria that excluded lots of samples, our data might not represent the general population of the United States.
Conclusion
A pro-inflammatory diet is positively associated with the prevalence of retinopathy in patients with DM rather than people with normal glucose or prediabetes. To prevent retinopathy, it is advisable for patients with DM to take an anti-inflammatory diet.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: www.cdc.gov/nchs/nhanes.
Ethics statement
The studies involving human participants were reviewed and approved by the studies involving human participants were reviewed and approved by the NCHS Ethic Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SL directed the study. WP designed the study. WP, ZZ, and YZ did the data extraction and analysis. HL plotted the figures. WP and YZ wrote the article. SZ guided and gave constructive advice during revision. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Project of China Postdoctoral Science Foundation (No. 2021M700959), Collaborative Innovation Team Construction Project of Guangzhou University of Chinese Medicine (No. 2021XK15), and National Natural Science Foundation of China for Young Scholars (Nos. 82104791 and 82104621).
Acknowledgments
Great gratitude to the US Centers for Disease Control and Prevention as part of the ongoing National Health and Nutrition Examination Survey (NHANES) which give the public access to all data sets used in the present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.981302/full#supplementary-material
References
1. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T., Chen, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:9. doi: 10.2337/dc11-1909
2. Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Diabetic retinopathy. Lancet Glob Health. (2014) 2:e106–16. doi: 10.1016/S2214-109X(13)70145-1
3. Taurone S, Ralli M, Nebbioso M, Greco A, Artico M, Attanasio G, et al. The role of inflammation in diabetic retinopathy: a review. Eur Rev Med Pharmacol Sci. (2020) 24:10319–29. doi: 10.26355/eurrev_202010_23379
4. Zhang W, Liu H, Rojas M, Caldwell RWB, Caldwell R. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy. (2011) 3:20. doi: 10.2217/imt.11.24
5. Bryl A, Mrugacz M, Falkowski M, Zorena K. The effect of diet and lifestyle on the course of diabetic retinopathy-a review of the literature. Nutrients. (2022) 14:1252. doi: 10.3390/nu14061252
6. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
7. Shu Y, Wu X, Wang J, Ma X, Li H, Xiang Y. Associations of dietary inflammatory index with prediabetes and insulin resistance. Front Endocrinol (Lausanne). (2022) 13:820932. doi: 10.3389/fendo.2022.820932
8. King DE, Xiang J. The dietary inflammatory index is associated with diabetes severity. J Am Board Fam Med. (2019) 32:801–6. doi: 10.3122/jabfm.2019.06.190092
9. Deng FE, Shivappa N, Tang Y, Mann JR, Hebert JR. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: findings from NHANES III. Eur J Nutr. (2017) 56:1085–93. doi: 10.1007/s00394-016-1158-4
10. Mazidi M, Shivappa N, Wirth MD, Hebert JR, Kengne AP. Greater Dietary Inflammatory Index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. (2018) 120:204–9. doi: 10.1017/S0007114518001071
11. Group ETDRSR. Early Treatment diabetic retinopathy study design and baseline patient characteristics. Ophthalmology. (1991) 98:741–56. doi: 10.1016/S0161-6420(13)38009-9
12. Ren J, Ren A, Deng X, Huang Z, Jiang Z, Li Z, et al. Long-Chain polyunsaturated fatty acids and their metabolites regulate inflammation in age-related macular degeneration. J Inflamm Res. (2022) 15:865–80. doi: 10.2147/JIR.S347231
13. Maria Hernandez S., González-Zamora, Valentina Bilbao-Malavé, Manuel Sáenz de Viteri, Jaione Bezunartea, et al. Anti-Inflammatory and anti-oxidative synergistic effect of vitamin d and nutritional complex on retinal pigment epithelial and endothelial cell lines against age-related macular degeneration. Nutrients. (2021) 13:1423. doi: 10.3390/nu13051423
14. Lazzara F, Conti F, Platania CBM, Eandi CM, Drago F, Bucolo C. Effects of vitamin D3 and meso-zeaxanthin on human retinal pigmented epithelial cells in three integrated in vitro paradigms of age-related macular degeneration. Front Pharmacol. (2021) 12:778165. doi: 10.3389/fphar.2021.778165
15. Keenan TD, Agron E, Mares J, Clemons TE, van Asten F, Swaroop A, et al. Adherence to the mediterranean diet and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2. Ophthalmology. (2020) 127:1515–28. doi: 10.1016/j.ophtha.2020.04.030
16. Eynard AR, Repossi G. Role of omega3 polyunsaturated fatty acids in diabetic retinopathy: a morphological and metabolically cross talk among blood retina barriers damage, autoimmunity and chronic inflammation. Lipids Health Dis. (2019) 18:114. doi: 10.1186/s12944-019-1049-9
17. Sala-Vila A, Diaz-Lopez A, Valls-Pedret C, Cofan M, Garcia-Layana A, Lamuela-Raventos RM, et al. Dietary marine omega-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol. (2016) 134:1142–9. doi: 10.1001/jamaophthalmol.2016.2906
18. Garcia-Garcia J, Usategui-Martin R, Sanabria MR, Fernandez-Perez E, Telleria JJ, Coco-Martin RM. Pathophysiology of age-related macular degeneration. Implications for Treatment Ophthalmic Res. (2022). doi: 10.1159/000524942
19. Wagner BD, Patnaik JL, Palestine AG, Frazer-Abel AA, Baldermann R, Holers VM, et al. Association of systemic inflammatory factors with progression to advanced age-related macular degeneration. Ophthalmic Epidemiol. (2022) 29:139–48. doi: 10.1080/09286586.2021.1910314
20. Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Wong CCKWT, et al. Age-related macular degeneration. Nature Rev. (2021) 7:265. doi: 10.1038/s41572-021-00265-2
21. Taurone S, Ralli M, Nebbioso M, Greco A, Artico M, Attanasio G, et al. The role of inflammation in diabetic retinopathy: a review. Eur Rev Med Pharmacol Sci. (2020) 24:10319–29.
22. Jabbehdari S, Sallam AB. Gut microbiome and diabetic retinopathy. Eur J Ophthalmol. (2022) 32:2494–97. doi: 10.1177/11206721221083068
23. Scuderi G, Troiani E, Minnella AM. Gut microbiome in retina health: the crucial role of the gut-retina axis. Front Microbiol. (2021) 12:726792. doi: 10.3389/fmicb.2021.726792
24. Fernandes R, Viana SD, Nunes S, Reis F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:1876–97. doi: 10.1016/j.bbadis.2018.09.032
25. Hariharan R, Odjidja EN, Scott D, Shivappa N, Hebert JR, Hodge A, et al. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes Rev. (2022) 23:e13349. doi: 10.1111/obr.13349
26. Leiden HA., Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Blood pressure, lipids, and obesity are associated with retinopathy: the Hoorn study. Diabetes Care. (2002) 25:1320–4. doi: 10.2337/diacare.25.8.1320
27. Faria JBL. Faria JMLd. The contribution of hypertension to diabetic nephropathy and retinopathy the role of inflammation and oxidative stress. Hypertension Res. (2011) 34:413–22. doi: 10.1038/hr.2010.263
Keywords: diabetes, retinopathy, dietary inflammatory index, diabetic diets, inflammation
Citation: Pan W, Zhang Z, Zhang Y, Lu H, Wang B, Zhao S and Li S (2023) Pro-Inflammatory diet accounts for higher prevalence of retinopathy in diabetes participants rather than normal glucose and prediabetes: Results from NHANES, 2005–2008. Front. Nutr. 9:981302. doi: 10.3389/fnut.2022.981302
Received: 29 June 2022; Accepted: 06 September 2022;
Published: 11 January 2023.
Edited by:
Lubia Velázquez López, Instituto Mexicano del Seguro Social, MexicoReviewed by:
Abril Violeta Muñoz Torres, National Autonomous University of Mexico, MexicoEmmanouella Magriplis, Agricultural University of Athens, Greece
Copyright © 2023 Pan, Zhang, Zhang, Lu, Wang, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saimei Li, bHNtQGd6dWNtLmVkdS5jbg==; Wenjun Pan, d2VuanVucGFucGVubnk5MUBzaW5hLmNvbQ==
 Wenjun Pan
Wenjun Pan Zhuqi Zhang2
Zhuqi Zhang2 Yuzhuo Zhang
Yuzhuo Zhang