
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Nutr., 04 August 2022
Sec. Nutrition and Food Science Technology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.980429
This article is part of the Research TopicCold Pressed Oils: A Green Source of Specialty Oils, volume IIView all 5 articles
 Aurelio Seidita1
Aurelio Seidita1 Maurizio Soresi1
Maurizio Soresi1 Lydia Giannitrapani1,2
Lydia Giannitrapani1,2 Vita Di Stefano3
Vita Di Stefano3 Roberto Citarrella1
Roberto Citarrella1 Luigi Mirarchi1
Luigi Mirarchi1 Antonella Cusimano2
Antonella Cusimano2 Giuseppa Augello2
Giuseppa Augello2 Antonio Carroccio4,5
Antonio Carroccio4,5 Juan Lucio Iovanna6
Juan Lucio Iovanna6 Melchiorre Cervello2*
Melchiorre Cervello2*For years it has been established that the only truly effective treatment of metabolic syndrome (MS) is lifestyle modification to prevent its cardiovascular (e.g., coronary artery disease and atherosclerosis), metabolic (e.g., diabetes mellitus), and hepatic (e.g., steatosis and non-alcoholic steatohepatitis) complications. The focal points of this approach are to increase physical activity and intake of a diet characterized by high quantities of fruits, vegetables, grains, fish, and low-fat dairy products, the so called mediterranean diet (MD); however, the added value of MD is the presence of extra virgin olive oil (EVOO), a healthy food with a high content of monounsaturated fatty acids, especially oleic acid, and variable concentrations (range 50–800 mg/kg) of phenols (oleuropein, ligstroside, and oleocanthal, and their derivatives, phenolic alcohols, such as hydroxytyrosol and tyrosol). Phenolic compounds not only determine EVOO’s main organoleptic qualities (oxidative stability, specific flavor, and taste features) but, theoretically, make it a source of antioxidant, anti-inflammatory, insulin-sensitizing, cardioprotective, antiatherogenic, neuroprotective, immunomodulatory, and anticancer activity. Although many studies have been carried out on EVOO’s clinical effects and attention toward this dietary approach (healthy and palatable food with strong nutraceutical activity) has become increasingly pressing, there are still many dark sides to be clarified, both in terms of actual clinical efficacy and biochemical and molecular activity. Thus, we reviewed the international literature, trying to show the state of the art about EVOO’s clinical properties to treat MS (along with correlated complications) and the future prospective of its nutraceutical use.
Metabolic syndrome (MS) is an increasingly pressing global health problem, affecting about 31% of the world’s population but predicted to increase over 50% in the next 15 years (1, 2). The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) (3) indicates MS when at least 3 of 5 conditions coexist: abdominal obesity, high triglyceride values, low high-density lipoprotein (HDL) values, high blood pressure, and impaired fasting glucose. These, both individually and in the context of MS, are known risk factors for metabolic and cardiovascular diseases (CVD) (4).
The only established, effective treatment of MS is lifestyle modification through increased physical activity, weight loss, and dietary intake high in fruits, vegetables, grains, fish, and low-fat dairy products: i.e., the mediterranean diet (MD) (4–6). Several studies have shown a direct correlation between MD adherence and overall reduction in mortality and morbidity (6–9).
An MD component believed to contribute a strong beneficial effect is extra virgin olive oil (EVOO), high in monounsaturated fatty acids (MUFAs) and with variable concentrations of phenols. These not only determine EVOO’s main organoleptic qualities (oxidative stability, specific flavor, and taste features) but make it a source of antioxidant, anti-inflammatory, insulin-sensitizing, cardioprotective, antiatherogenic, neuroprotective, and immunomodulatory activity (10). Although many studies have examined EVOO’s clinical effects and MD is seen as increasingly promising, there are still many uncertainties to be clarified regarding its clinical efficacy and biochemical activity.
We reviewed the international literature to summarize the state of the art about EVOO’s clinical properties for treating MS and the future prospects of its nutraceutical use.
Olive oil (OO) has an energetic function, transports fat-soluble vitamins, and makes foods more pleasant.
The composition of OO and EVOO is influenced by tree variety, agronomic conditions, production processes, period, harvesting method, and oil extraction system (11, 12).
Olive oil mostly consists of triglycerides (98–99%) and contains primarily MUFAs in the form of omega-9 oleic acid (C18:1); according to the International Olive Oil Council, its concentration must range from 55 to 83% of total fatty acids.
Olive oil also contains other MUFAs, such as omega-7 palmitoleic acid (C16:1), ranging from 0.3 to 3.5%, and traces of gadoleic/9-eicosenoic (C20:1 ω-11, 0.4%) and heptadecenoic acid (C17:1, 0.3%).
Extra virgin olive oil also contains polyunsaturated fatty acids (PUFAs) including linoleic acid (C18:2, ω-6) and α-linolenic acid (C18:3, ω-3), between 3 and 19% and 0.11 and 1.0%, respectively. EVOO’s lipid profile and high ω6/ω3 ratio have been linked to its protective effects on cardiovascular (CV), autoimmune and inflammatory disorders, but also its anti-thrombotic and blood pressure regulatory qualities, and ensuring oxidative stability for long shelf life (13–15).
Saturated fatty acids participate in the EVOO fatty acid profile: palmitic acid (C16:0, 7.8–17.3%), stearic acid (C18:0, 0.2–3.2%), arachidic acid (C20:0, 0.7%), margaric acid (C17:0, 0.3%), behenic acid (C22:0, 0.2%), lignoceric acid (C24:0, 0.2%), and myristic acid (C14:0, 0.03%).
The minor fraction of EVOO comprises substances responsible for its biological properties and sensory attributes (color, odor, flavor, taste, and aftertaste), primarily present in the mature drupe pulp and pits which are dissolved in the oil via natural or technological processes. Lower quantities of squalene (3–6 g/kg) and phytosterols (β-sitosterol, campesterol, and stigmasterol, in free and esterified forms) (0.8–2.6 g/kg) are present in EVOO. Finally, soluble vitamins (β-carotene and tocopherols), pigments (carotenes and chlorophyll), alcohol triterpene, and especially polyphenols are present in minor quantities.
Phenolic compounds include about 30 molecules from different chemical classes: phenolic alcohols, such as hydroxytyrosol (HT) and tyrosol (Tyr), phenolic acids, flavones, lignans, and secoiridoids. The latter group represents the largest fraction. The principal ones are the aglycone forms of oleuropein and ligstroside, the dialdehydic forms of their decarboxymethylated derivatives, known as oleacein and oleocanthal (Supplementary Table 1). In OO, the content of phenolic compounds ranges from 50 to 1000 mg/kg. The secoiridoids act as natural antioxidants protecting EVOO against autoxidation during storage and are responsible for its bitter and pungent qualities. Much evidence indicates that EVOO’s phenolic compounds can exert biological activities due to their antioxidant, anti-inflammatory, and chemo-preventive properties (16, 17).
In humans, Tyr and HT intestinal absorption occurs in a dose-dependent way with a percentage ranging from 40 to 95% and is strictly dependent on the polarity of their chemical structure (18, 19). Part of these polyphenols, in particular aglycone secoiridoids, can be hydrolyzed at the gastric level, with a time-dependent process, transforming into free Tyr and HT (20); the glycosylated forms do not suffer hydrolysis processes and, together with other polyphenols, pass through the small intestine where they are absorbed by enterocytes via a bidirectional passive diffusion mechanism (19). Once absorbed, polyphenols undergo phase II transformation metabolism, which substantially reduces their bioavailability. The most represented metabolites in plasma are the O-glucuronidated forms of Tyr and HT (21) and, to a lesser extent, homovanillic acid, homovanillic acid sulfate, and HT acetate sulfate (22). Both the unmodified forms and the metabolites of the polyphenol subclasses are ubiquitously distributed in the organism, depositing, in a concentration-dependent way, in certain organs, such as the brain, liver, and kidneys (23).
The clearance of polyphenols and their metabolites essentially occurs via kidney excretion (17, 24).
Based on health studies, in 2011 the European Food Safety Authority (EFSA) authorized a functional health claim on EVOO polyphenols that they “contribute to the protection of blood lipids from oxidative stress.” This benefit emerges with a minimum concentration of 5 mg of HT and its derivatives in 20 g of EVOO (25). Nevertheless, this is a contested point. Originally, Regulation (EC) No 1924/2006 included several health claims for OO polyphenols [for details see European Community, (26)]. In 2011, EFSA was asked about these claims and concluded: “that a cause and effect relationship has been established between the consumption of OO polyphenols and protection of low-density lipoproteins (LDL) particles from oxidative damage” (25). All others health claims were excluded, primarily for inadequate evidence from human studies, because they were “generic and not specific,” or because they did not comply with the criteria in Reg. 1924/2006 (27). In 2012, the European Council updated the regulation to implement this opinion (28). Since then, several studies have analyzed other health claims, reporting new data and raising more issues, including the lack of consistent studies correlating the chemical features and human benefits, especially anti-inflammatory properties of OO phenolic compounds (27).
An overview of some of the beneficial effects of MD and EVOO is presented in Figure 1.
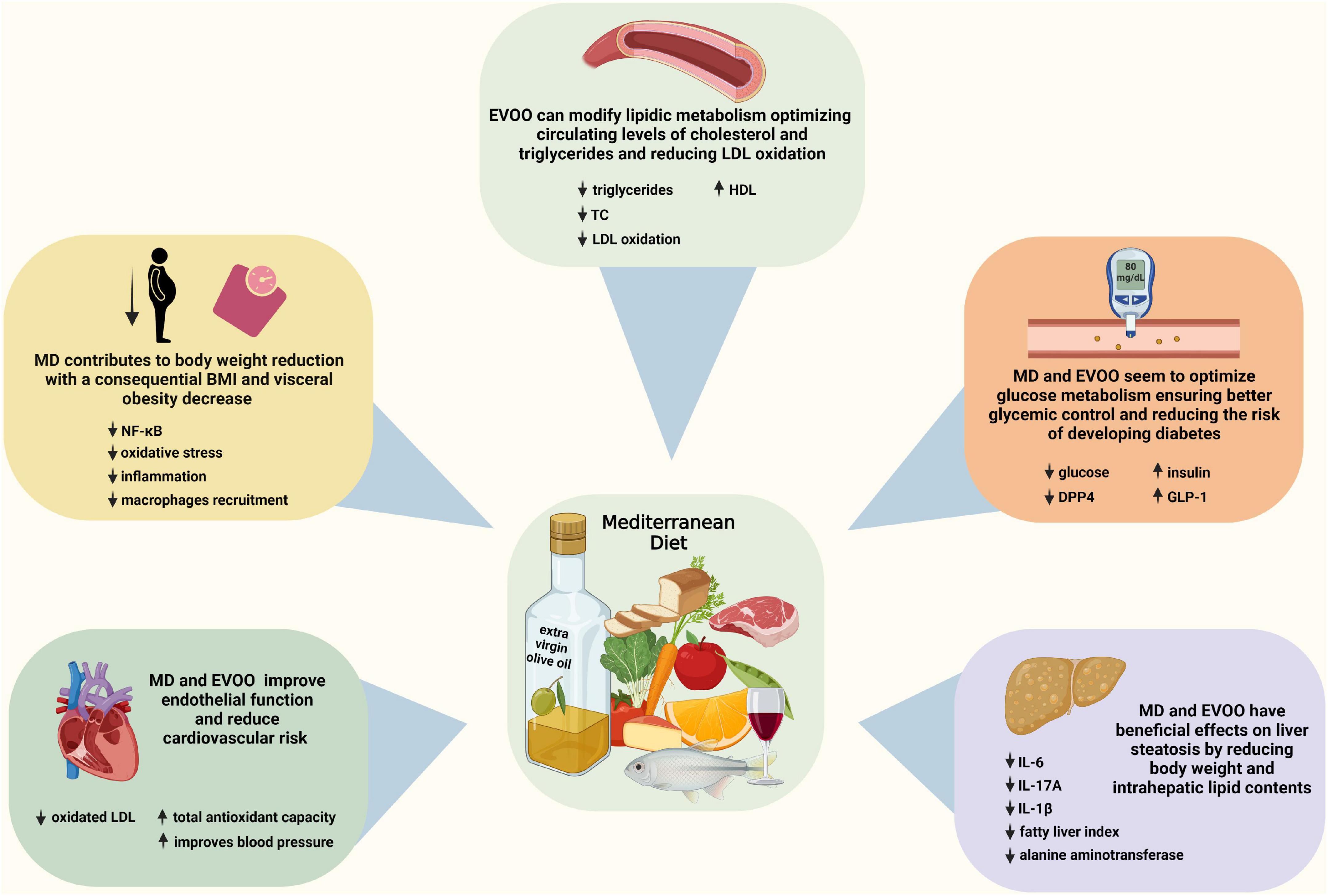
Figure 1. Beneficial effects of the mediterranean diet (MD) and extra virgin olive oil. Some key alterations implicated in MD and EVOO effects are presented. Created with BioRender.com.
Multiple conditions affect EVOO’s biochemical effects: component composition and concentration, absorption, and metabolism. Exposing EVOO to high temperatures or long cooking times might substantially alter its polyphenol content (29). A substantial role could be attributed to the intestinal microbiota, which can modify the metabolism of EVOO’s components and alter their absorption (30).
Several researchers focused on EVOO polyphenols’ reduction of oxidative stress. Some studies demonstrated that polyphenols bind LDLs and prevent their oxidation by free radicals (31, 32). Although the molecular mechanism underlying EVOO’s antioxidant activity is not fully defined, some authors assumed that it could modulate the expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), consequently increasing antioxidant molecule expression (33, 34). This activity would explain the increase in glutathione turnover after a high EVOO content meal, with increased glutathione peroxidase and glutathione reductase activity and the reduction of post-prandial blood levels of lipid peroxide, protein carbonyl, and plasma hydrogen peroxide, probably because of NADPH oxidase activity decrease (34–37).
Moreover, HT and oleocanthal seem to inhibit copper-induced LDL oxidation by chelating metals and scavenging free radicals (38).
Extra virgin olive oil, due to its high MUFA content, significantly reduces concentrations of total cholesterol (TC) and LDL-cholesterol (LDL-C), decreasing TC/HDL and LDL/HDL ratios (6, 39). In this context, polyphenols act synergistically with MUFAs, causing both an inhibition of pancreatic lipases, delaying the post-prandial lipemic peak (40), and rapid lipid clearance (41).
Extra virgin olive oil also exerts anti-inflammatory activity by modulating the activation of pro-inflammatory genes and reducing pro-inflammatory cytokine expression. Several studies proved a reduction in serum phlogosis markers, both immediately after EVOO rich meals and in long-term consumption (6).
Among the downregulated pro-inflammatory molecules are interleukin-6 (IL-6), visfatin, tumor necrosis factor-α (TNF-α), IL-1β, interferon-γ (IFN-γ), and cyclooxygenase-2 (COX-2) which have been analyzed in human (42) and animal (43, 44) models, while IL-1, IL-3, and IL-8 have been analyzed in human peripheral blood mononuclear cells only (45). Finally, TNF-α, IL-6, and IL-17 production was studied in the splenocytes of a mouse model of systemic lupus erythematosus (46). In addition, an increase of anti-inflammatory cytokine IL-10 levels (43) and inhibition of some cell adhesion molecules (VCAM-1 and ICAM-1) have been reported (47). COX-2, LRP1, and MCP-1 are some of the genes modulated in this anti-inflammatory activity. (48). In studies specifically focused on MS patients, EVOO significantly reduced C-reactive protein (CRP) values, IL-6, IL-7, and IL-18 plasma levels (49), and pro-inflammatory molecule gene expression (50).
Extra virgin olive oil-enriched MD intake for 4 weeks helps normal endothelial function by promoting post-prandial vasodilation in patients with hypercholesterolemia (51). Similar effects have been demonstrated in other populations in which a greater bioavailability of nitric oxide (NO) (52) and a reduction of its urinary catabolites were proven. Some authors indicate that chronic EVOO intake can increase endothelial progenitor cells (53). These effects plausibly depend on reducing oxidative stress and the greater stability of the EVOO-induced endothelial cell genome (54). Recent in vitro studies tested EVOO’s effects on endothelial function, showing that HT can increase NO synthesis (55) and that EVOO’s polyphenols can modulate NADPH oxidase activity, reducing vascular endothelial growth factor production and reducing cellular migration and reactive oxygen species genesis (56).
Extra virgin olive oil’s modulation of nuclear factor κB (NF-κB), a transcription factor regulating gene transcription in cytokines, chemokines, adhesion molecules, inflammatory proteins and COX-2 (and several others), plays a significant role. NF-κB is also involved in inflammatory processes related to atherogenesis. EVOO, specifically its phenols, might modulate NF-κB expression, reducing the inflammatory cascade (57).
While many studies agree on these beneficial effects, there are conflicting opinions on EVOO’s ability to modulate platelet aggregation and coagulation. Some studies seem to indicate that both MUFAs and polyphenols could reduce platelet aggregation, probably inhibiting thromboxane A2 synthesis (56, 58–60). EVOO’s MUFAs might reduce factor VII, tissue factor, and plasminogen activator inhibitor-1 procoagulant activity (61–63); however, the small number of studies with discordant results require further analysis to clarify EVOO’s effect on the coagulation cascade.
Since in the 1950s, “The Seven Countries Study” (64) has demonstrated MD’s efficacy in MS treatment. A recent meta-analysis reported beneficial effects on: body weight, body mass index (BMI), waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR) index, TC, LDL, HDL, triglycerides, alanine transaminase, hepatic fat mass, CRP, IL-6, TNF-α, and flow-mediated dilatation. These determined a lower risk of CVD (RR 0.61, 95% CI: 0.42–0.80) and stroke (RR 0.67, 95% CI: 0.35–0.98) (65).
The idea that many of these benefits may be linked not only to a balanced diet but to the added value of EVOO has prompted many further investigations of EVOO.
Table 1 summarizes the results regarding EVOO’s effects on MS from the main human studies.
Mediterranean diet contributes to body weight reduction with a consequent decrease in BMI and visceral obesity (49), but there is insufficient evidence to indicate whether EVOO, by itself, influences these parameters (66). In the PREDIMED study, body weight and waist circumference decreased in patients assigned to MD + EVOO intervention group (67, 68).
The EPIC-PANACEA study found that following an MD including different quantities of EVOO reduced weight gain (69). EVOO may assist in body weight reduction because of its organoleptic qualities which enhance food palatability and promotes satiety (70).
In vitro HT supplementation of cultures of Simpson–Golabi–Behmel syndrome human pre-adipocytes, modulated gene expression, reducing NF-κB and oxidative stress pathway activation, decreasing inflammation and macrophage recruitment (71).
Therefore, EVOO seems to act more as a counter-regulator of adipose tissue inflammation than as a reducer of visceral obesity.
Extra virgin olive oil can modify lipidic metabolism, optimizing circulating cholesterol and triglyceride levels (6, 39) and reducing LDL oxidation (31, 32).
In a randomized trial, MD associated with 8 g/day OO intake for 2 years reduced triglycerides (p = 0.001) and TC (p = 0.02) levels and increased HDL (p = 0.03) (49). It was demonstrated that 1 year of MD + EVOO intake enhanced LDL resistance to oxidation (p = 0.007), reduced changes related to oxidative stress (p < 0.05), increased their size (p = 0.021) and cholesterol content (p = 0.013) compared to a low-fat diet (72). Researchers comparing 200 healthy volunteers divided into three groups, each with an intake of progressively higher polyphenol content OO, demonstrated that HDL levels increased linearly with the concentration of polyphenols; similarly, TC, TC/HDL ratio, and LDL oxidation decreased (32).
Recently a network meta-analysis of OO metabolic effects, as part of MD, evaluated 30 human intervention studies, considering direct and indirect interactions and impact of OO constituents over different metabolic pathways. Effects on glucose, triglycerides, and LDL-C were mediated by adherence to MD, whereas polyphenols increased HDL-C and improved antioxidant and inflammatory status as an independent factor. Interestingly, benefits were more pronounced in subjects with MS or chronic conditions/diseases than healthy subjects (73).
Briefly, we can assume that MD produces a protective effect against lipid-induced atherogenesis by reducing LDL-C, while the added value of EVOO mainly increased HDL-C and prevents LDL oxidation. However, more extensive studies with well-defined EVOO quantities and chemical characteristics (27), are needed in order to confirm these findings.
A meta-analysis of four cohorts and 29 trials associating EVOO with glycemic control found the risk of diabetes mellitus (DM) was inversely associated with EVOO intake (p < 0.01), though non-linearly (p < 0.01) (74). The risk of developing DM decreased by 13% with EVOO intake of 15–20 g/day, but no advantages from further dose increases were proven. EVOO trial analysis showed significantly more evident reductions in HbA1c (p < 0.01) and fasting blood glucose (p < 0.01) than control groups.
The PREDIMED study confirmed that MD + EVOO can improve glucose metabolism, preventing diabetes onset (75). In another subgroup of patients from PREDIMED study MD + EVOO and MD + nuts increased values of adiponectin/leptin ratio (p = 0.001 and p < 0.001, respectively) and adiponectin/HOMA-IR ratio (p = 0.027 and p = 0.069, respectively) compared to baseline (68).
Extra virgin olive oil should influence glucose metabolism, reducing dipeptidyl peptidase-4 activity and resulting in an increase in the glucagon-like peptide-1 incretin-pattern (76). Moreover, EVOO’s polyphenols might partially inhibit carbohydrate digestion and absorption, reducing the hepatic release of glucose and increasing its peripheral uptake (77). Polyphenol antioxidant activity might reduce the production of advanced glycosylated end-products such as HbA1c (78).
Taken together, both MD and EVOO seem to optimize glucose metabolism, ensuring better glycemic control and reducing DM risk. However, EVOO seems to provide additional protection, specifically acting on insulin secretion mechanisms. Nevertheless, most investigations of specific polyphenol mechanisms in regulating glycidic metabolism were conducted in in vitro models.
Many studies investigated the effects of MD and EVOO on cardiovascular risk (49, 79). In the PREDIMED study, patients with the highest EVOO intake showed a 39% reduction in CVD risk. Risk of CVD and CVD death was also reduced by 10 and 7%, respectively, for each 10 g/day increase in EVOO intake (9).
The EPIC cohort study also provides relevant data. Buckland et al. (80) analyzed data from 40,142 coronary disease-event free participants, showing that OO intake during follow-up was negatively associated with coronary disease risk for each 10 g/day OO intake, with a more pronounced effect from EVOO (14% risk reduction, p = 0.072).
More recently, a double-blind randomized, controlled, cross-over study (OLIVAUS) evaluated the effects of high polyphenol EVOO vs. low polyphenol OO on CVD in 50 healthy participants (81). When the population was stratified by CVD risk status, EVOO showed anti-inflammatory and antioxidative effects only during high polyphenol EVOO intake (p = 0.0086). In detail, the subgroup with abdominal obesity showed reduced oxidated LDL and increased total antioxidant capacity (82).
Some authors reported a positive effect of MD + EVOO on blood pressure control; a reduction in SBP values both at 3 and 6 months was reported (83), as well as a negative correlation between changes in NO metabolite concentration and SBP-DBP pressure values compared to baseline in the MD + EVOO group (p = 0.033 and p = 0.044, respectively) (84). The OLIVAUS study showed reduced SBP during high polyphenol EVOO intake rather than low polyphenol OO. These effects disappeared after the challenge ended, connecting blood pressure reduction to continuous EVOO intake (85).
In this context, a randomized, controlled, double-blind, crossover trial on 20 adults with MS evaluated the effect of high polyphenol EVOO vs. a refined oil without polyphenols on endothelial function. Flow-mediated dilatation measurement was used to analyze endothelial function after a single 50 ml dose of one of the 2 OOs. EVOO improved endothelial function (p = 0.0086) compared to refined oil, though it had no significant effects on SBP-DBP; this last data may be related to the single dose and should be analyzed in long-term studies (86).
Although studies report a consistent reduction of CVD risk and mortality, probably related to both MD and EVOO intake, the high variability of EVOO effects on SBP-DBP and its regulation mechanisms (NO and endothelin-1) requires further and larger human studies. Available data do not clarify whether blood pressure modification is acute or chronic, or if there is a threshold effect or tachyphylaxis, or long-term tolerance.
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) represent the hepatic manifestation of MS (87). The link between these two conditions is so high that several researchers prefer the term metabolic associated fatty liver disease (MAFLD) (88).
A 5–7% of body weight decrease can reduce liver steatosis (89). Thus, a balanced MD would seem to have a certain impact on NAFLD/MAFLD. A recent meta-analysis of 18 studies, considering three different dietary patters (western diet, prudent diet, and MD) found an increased risk of NAFLD by 56% for western diet and a reduced risk of this disease by 22 and 23%, respectively in prudent diet and MD (90).
Some studies considered an MD-based dietary intervention compared to a low-fat low-carbohydrate diet in patients with NAFLD (91–93). A recent meta-analysis compared these studies, proving a consistent reduction of the intrahepatic lipid content in intervention groups (mean difference: −0.57, 95% CI: −1.04 to −0.10) (89). However, no difference between groups were proved in alanine aminotransferase and γ-glutamyl transpeptidase level reduction.
Patti et al. (94) analyzed the effectiveness of a 2-month intervention with EVOO (32 g/day) in subjects with MS and associated liver steatosis, showing a reduction of alanine aminotransferase levels (p = 0.029) after intervention, and considered it a possible indirect demonstration of liver steatosis reduction. The PREDIMED cohort randomized patients in three subgroups: MD + EVOO, MD + nuts, and control diet. After 3 years, liver steatosis was present in 8.8, 33.3, and 33.3% of the subgroups (p = 0.027), and mean liver fat content values were 1.2, 2.7, and 4.1% (p = 0.07), respectively (95).
These data indicate that MD has beneficial effects on NAFLD/MAFLD, whereas there is poor, contrasting, and mainly indirect evidence that EVOO could effectively influence liver fat content. Thus, larger randomized studies with specific EVOO quantities and composition are required to clarify its role in MAFLD.
Mediterranean diet is a cornerstone in treating MS and preventing cardiovascular risk. Literature data indicate that an essential component is EVOO which, with high MUFA and polyphenol content constitutes a food with excellent organoleptic properties and a substance with surprising nutraceutical abilities. EVOO, by activating multiple metabolic pathways, could optimize glycemic control and lipid metabolism, reduce endothelial damage and blood pressure, and provide systemic anti-inflammatory activity.
Overall, EVOO seems to play an antiatherogenic and CVD risk reduction role, improving the overall health status of MS patients. Given its ability to modulate inflammatory stress, some studies are evaluating EVOO activity in cancer (e.g., breast cancer) (96).
As of June 2022, 32 trials on EVOO’s effects in several pathological conditions have been registered on ClinicalTrials.gov,1 of which 11 are in the active recruitment phase; the conditions investigated include: CVDs, MS, end-stage renal failure, autoimmune diseases, breast cancer and mitochondrial diseases.
Though the evidence supporting a role of EVOO and its polyphenolic component in MS is increasing rapidly, a recent meta-analysis of 76 trials, found no significant effect of OO, HT, and oleic acid on MS, considered both overall and in its different components. Statistical significance was only shown for OO, HT, and oleic acid’s antioxidant capacity related to components of MS. However, most studies compared OO with other MS treatment approaches, so the lack of statistical significance indicates OO’s non-inferiority rather than non-efficacy (97).
Several doubts remain regarding EVOO’s action mechanisms, the quantities required to optimize its effects and, whether its properties can be separated from those of MD or if the beneficial effects are inextricably linked.
Tsartsou et al.’s (73) meta-analysis provides two compelling indications which need confirmation by prospective studies. First OO’s effects on glucose, triglycerides, and LDL-C were mediated by adherence to MD, whereas polyphenol effects seem to be limited to increasing HDL and modulating oxidative stress and inflammation. Second, polyphenol effects do not seem to be directly correlated to their levels in OO, such that a much lower than previously reported concentration of OO polyphenols can induce protection.
This last result conflicts with recent nutrigenomic studies showing that EVOO cultivars with high polyphenol content can modulate the expression of several transcripts involved in glucose/lipid metabolism, proliferation, inflammation, and cancer, supporting health-promoting effects pathways (70).
We must stress that most human studies of EVOO activity inadequately characterize biochemical features of the EVOO used, especially different phenolic concentrations which consistently differ across varieties (27, 70). This point, together with the differences in daily EVOO intake (which in some studies is not standardized) could influence the results of human clinical trials.
Another shadowy point regards the bioavailability of EVOO compounds (98). Polyphenol absorption in the gut seems to be dose- and time-dependent and is strictly dependent on their chemical structure’s polarity (18–20, 99). Furthermore, the specific individual characteristics of intestinal microbiota can influence the bioavailability of phenolic compounds (30, 100–102). Finally, studies reported that olive cultivars might modify the bio-accessibility and antioxidant activity of EVOO’s phenolic fraction (101, 102).
Thus, considering all these issues, larger, well-structured and standardized (e.g., EVOO quantities and chemical features) studies are required to clarify EVOO’s potential as a nutraceutical product.
AS, MS, and MC: full access to all the data in the study, take responsibility for the integrity of the data, the accuracy of the data analysis, and methodology. AS, VD, and MC: conceptualization. AS, LM, ACu, and GA: investigation and literature research. AS and VD: writing – original draft preparation. AS, MS, LG, VD, RC, ACu, GA, ACa, JI, and MC: writing – review and editing. LG and MS: funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported in part by the project n. 08TP1041100162 named TRIAL “Code IRIS/U GOV 16463” “PO FESR Sicilia 2014-2020” to LG. Publication costs supported in part by the FFR2021 fund of the University of Palermo assigned to MS.
We are grateful to Antonina Azzolina for support in preparing the figure and tables.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.980429/full#supplementary-material
ATP III, Adult Treatment Panel III; COX, cyclooxygenase; CRP, C-reactive protein; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; EFSA, European Food Safety Authority; EVOO, extra virgin olive oil; HDL, high-density lipoproteins; HT, hydroxytyrosol; IFN, interferon; IL, interleukin; LDL, low-density lipoproteins; LDL-C, low-density lipoproteins-cholesterol; MAFLD, metabolic associated fatty liver disease; MD, mediterranean diet; MS, metabolic syndrome; MUFA, monounsaturated fatty acids; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NCEP, National Cholesterol Education Program; OO, olive oil; PUFA, polyunsaturated fatty acids; SBP, systolic blood pressure; TC, total cholesterol; TNF, tumor necrosis factor; Tyr, tyrosol.
1. Fernández-Aparicio Á, Perona JS, Castellano JM, Correa-Rodríguez M, Schmidt-RioValle J, González-Jiménez E. Oleanolic acid-enriched olive oil alleviates the interleukin-6 overproduction induced by postprandial triglyceride-rich lipoproteins in THP-1 macrophages. Nutrients. (2021) 13:3471. doi: 10.3390/nu13103471
2. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. (2020) 323:2526–8. doi: 10.1001/jama.2020.4501
3. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
4. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 65:1415–28. doi: 10.1016/S0140-6736(05)66378-7
5. Rosenzweig JL, Bakris GL, Berglund LF, Hivert MF, Horton ES, Kalyani RR, et al. Primary prevention of ASCVD and T2DM in patients at metabolic risk: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. (2019) [Online ahead of print]. doi: 10.1210/jc.2019-01338
6. Yubero-Serrano EM, Lopez-Moreno J, Gomez-Delgado F, Lopez-Miranda J. Extra virgin olive oil: more than a healthy fat. Eur J Clin Nutr. (2019) 72:8–17. doi: 10.1038/s41430-018-0304-x
7. D’Alessandro A, De Pergola G. Mediterranean diet and cardiovascular disease: a critical evaluation of a priori dietary indexes. Nutrients. (2015) 7:7863–88. doi: 10.3390/nu7095367
8. Gardener H, Wright CB, Gu Y, Demmer RT, Boden-Albala B, Elkind MS, et al. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr. (2011) 94:1458–64. doi: 10.3945/ajcn.111.012799
9. Guasch-Ferré M, Hu FB, Martínez-González MA, Fitó M, Bulló M, Estruch R, et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. (2014) 12:78. doi: 10.1186/1741-7015-12-78
10. Santangelo C, Vari R, Scazzocchio B, De Sanctis P, Giovannini C, D’Archivio M, et al. Anti-inflammatory activity of extra virgin olive oil polyphenols: which role in the prevention and treatment of immune-mediated inflammatory diseases? Endocr Metab Immune Disord Drug Targets. (2018) 18:36–50. doi: 10.2174/1871530317666171114114321
11. Polari JJ, Garcí-Aguirre D, Olmo-García L, Carrasco-Pancorbo A, Wang SC. Impact of industrial hammer mill rotor speed on extraction efficiency and quality of extra virgin olive oil. Food Chem. (2018) 242:362–8. doi: 10.1016/j.foodchem.2017.09.003
12. López-Yerena A, Lozano-Castellón J, Olmo-Cunillera A, Tresserra-Rimbau A, Quifer-Rada P, Jiménez B, et al. Effects of organic and conventional growing systems on the phenolic profile of extra-virgin olive oil. Molecules. (2019) 24:1986. doi: 10.3390/molecules24101986
13. Mariotti M, Peri C. The composition and nutritional properties of extra-virgin olive oil. In: C Peri editor. The Extra-Virgin Olive Oil Handbook. Hoboken, NJ: John Wiley & Sons Ltd (2014). p. 21–34.
14. Sánchez-Villegas A, Sánchez-Tainta A. The Prevention of Cardiovascular Disease through the Mediterranean Diet. Cambridge, MA: Academic Press (2018). p. 59–87.
15. Lombardo L, Grasso F, Lanciano F, Loria S, Monetti E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds. 1st ed. (Vol. 57). Amsterdam: Elsevier (2018).
16. Foscolou A, Critselis E, Panagiotakos D. Olive oil consumption and human health: a narrative review. Maturitas. (2018) 118:60–6. doi: 10.1016/j.maturitas.2018.10.013
17. Emma MR, Augello G, Di Stefano V, Azzolina A, Giannitrapani L, Montalto G, et al. Potential uses of olive oil secoiridoids for the prevention and treatment of cancer: a narrative review of preclinical studies. Int J Mol Sci. (2021) 22:1234. doi: 10.3390/ijms22031234
18. Weinbrenner T, Fitó M, Farré Albaladejo M, Saez GT, Rijken P, Tormos C, et al. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Exp Clin Res. (2004) 30:207–12.
19. Manna C, Galletti P, Maisto G, Cucciolla V, D’Angelo S, Zappia V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. (2000) 470:341–4. doi: 10.1016/s0014-5793(00)01350-8
20. Corona G, Tzounis X, Assunta Dessì M, Deiana M, Debnam ES, Visioli F, et al. The fate of olive oil polyphenols in the gastrointestinal tract: implications of gastric and colonic microflora-dependent biotransformation. Free Radic Res. (2006) 40:647–58. doi: 10.1080/10715760500373000
21. Caruso D, Visioli F, Patelli R, Galli C, Galli G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism. (2001) 50:1426–8. doi: 10.1053/meta.2001.28073
22. Rubió L, Valls RM, Macià A, Pedret A, Giralt M, Romero MP, et al. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. (2012) 135:2922–9. doi: 10.1016/j.foodchem.2012.07.085
23. López de las Hazas MC, Rubió L, Kotronoulas A, de la Torre R, Solà R, Motilva MJ. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol Nutr Food Res. (2015) 59:1395–9. doi: 10.1002/mnfr.201500048
24. Lozano-Castellón J, López-Yerena A, Rinaldi de Alvarenga JF, Romero Del Castillo-Alba J, Vallverdú-Queralt A, Escribano-Ferrer E, et al. Health-promoting properties of oleocanthal and oleacein: two secoiridoids from extra-virgin olive oil. Crit Rev Food Sci Nutr. (2020) 60:2532–48. doi: 10.1080/10408398.2019.1650715
25. EFSA. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/200. EFSA J. (2011) 9:2033.
26. European Community.Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Germany: European Union (2006).
27. De Santis S, Clodoveo ML, Corbo F. Correlation between chemical characterization and biological activity: an urgent need for human studies using extra virgin olive oil. Antioxidants. (2022) 11:258. doi: 10.3390/antiox11020258
28. European Community.Council Regulation No. 432/2012 of 16 May 2012 Establishing a list of Permitted Health Claims Made on Foods, other than those Referring to the Reduction of Disease Risk, to Children’s development, Health. Germany: European Union (2012).
29. Brenes M, García A, Dobarganes MC, Velasco J, Romero C. Influence of thermal treatments simulating cooking processes on the polyphenol content in virgin olive oil. J Agric Food Chem. (2002) 50:5962–7. doi: 10.1021/jf020506w
30. Mosele JI, Martín-Peláez S, Macià A, Farràs M, Valls RM, Catalán Ú, et al. Faecal microbial metabolism of olive oil phenolic compounds: in vitro and in vivo approaches. Mol Nutr Food Res. (2014) 58:1809–19. doi: 10.1002/mnfr.201400124
31. de la Torre-Carbot K, Chávez-Servín JL, Jaúregui O, Castellote AI, Lamuela-Raventós RM, Nurmi T, et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J Nutr. (2010) 140:501–8. doi: 10.3945/jn.109.112912
32. Covas MI, Nyyssönen K, Poulsen HE, Kaikkonen J, Zunft HJ, Kiesewetter H, et al. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med. (2006) 145:333–41. doi: 10.7326/0003-4819-145-5-200609050-00006
33. Zrelli H, Matsuoka M, Kitazaki S, Araki M, Kusunoki M, Zarrouk M, et al. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: role of Nrf2 activation and HO-1 induction. J Agric Food Chem. (2011) 59:4473–82. doi: 10.1021/jf104151d
34. Martín MA, Ramos S, Granado-Serrano AB, Rodríguez-Ramiro I, Trujillo M, Bravo L, et al. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol Nutr Food Res. (2010) 54:956–66. doi: 10.1002/mnfr.200900159
35. Weinbrenner T, Fitó M, de la Torre R, Saez GT, Rijken P, Tormos C, et al. Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J Nutr. (2004) 134:2314–21. doi: 10.1093/jn/134.9.2314
36. Perez-Martinez P, Garcia-Quintana JM, Yubero-Serrano EM, Tasset-Cuevas I, Tunez I, Garcia-Rios A, et al. Postprandial oxidative stress is modified by dietary fat: evidence from a human intervention study. Clin Sci. (2010) 119:251–61. doi: 10.1042/CS20100015
37. Loffredo L, Perri L, Nocella C, Violi F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: focus on extra virgin olive oil and cocoa. Br J Clin Pharmacol. (2017) 83:96–102. doi: 10.1111/bcp.12923
38. Romani A, Ieri F, Urciuoli S, Noce A, Marrone G, Nediani C, et al. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients. (2019) 11:1776. doi: 10.3390/nu11081776
39. Venturini D, Simão AN, Urbano MR, Dichi I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition. (2015) 31:834–40. doi: 10.1016/j.nut.2014.12.016
40. Buchholz T, Melzig MF. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. (2015) 81:771–83. doi: 10.1055/s-0035-1546173
41. Sanders TA, de Grassi T, Miller GJ, Morrissey JH. Influence of fatty acid chain length and cis/trans isomerization on postprandial lipemia and factor VII in healthy subjects (postprandial lipids and factor VII). Atherosclerosis. (2000) 149:413–20. doi: 10.1016/s0021-9150(99)00335-4
42. Fitó M, Cladellas M, de la Torre R, Martí J, Muñoz D, Schröder H, et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr. (2008) 62:570–4. doi: 10.1038/sj.ejcn.1602724
43. Lama A, Pirozzi C, Mollica MP, Trinchese G, Di Guida F, Cavaliere G. Polyphenol-rich virgin olive oil reduces insulin resistance and liver inflammation and improves mitochondrial dysfunction in high-fat diet fed rats. Mol Nutr Food Res. (2017) 61:1600418. doi: 10.1002/mnfr.201600418
44. Jurado-Ruiz E, Varela LM, Luque A, Berná G, Cahuana G, Martinez-Force E. An extra virgin olive oil rich diet intervention ameliorates the nonalcoholic steatohepatitis induced by a high-fat “Western-type” diet in mice. Mol Nutr Food Res. (2017) 61:1600549. doi: 10.1002/mnfr.201600549
45. D’Amore S, Vacca M, Cariello M, Graziano G, D’Orazio A, Salvia R, et al. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim Biophys Acta. (2016) 1861:1671–80. doi: 10.1016/j.bbalip.2016.07.003
46. Aparicio-Soto M, Sánchez-Hidalgo M, Cárdeno A, Rosillo MÁ, Sánchez-Fidalgo S, Utrilla J, et al. Dietary extra virgin olive oil attenuates kidney injury in pristane-induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT, NF-κB and MAPK activation. J Nutr Biochem. (2016) 27:278–88. doi: 10.1016/j.jnutbio.2015.09.017
47. Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, et al. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. (2003) 23:622–9. doi: 10.1161/01.ATV.0000062884.69432.A0
48. Llorente-Cortés V, Estruch R, Mena MP, Ros E, González MA, Fitó M, et al. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis. (2010) 208:442–50. doi: 10.1016/j.atherosclerosis.2009.08.004
49. Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. (2004) 292:1440–6. doi: 10.1001/jama.292.12.1440
50. Camargo A, Ruano J, Fernandez JM, Parnell LD, Jimenez A, Santos-Gonzalez M, et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genomics. (2010) 11:253. doi: 10.1186/1471-2164-11-253
51. Fuentes F, López-Miranda J, Sánchez E, Sánchez F, Paez J, Paz-Rojas E, et al. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann Intern Med. (2001) 134:1115–9. doi: 10.7326/0003-4819-134-12-200106190-00011
52. Yubero-Serrano EM, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, Tasset-Cuevas I, Santos-Gonzalez M, et al. Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age. (2011) 33:579–90. doi: 10.1007/s11357-010-9199-8
53. Marin C, Ramirez R, Delgado-Lista J, Yubero-Serrano EM, Perez-Martinez P, Carracedo J, et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr. (2011) 93:267–74. doi: 10.3945/ajcn.110.006866
54. Gutierrez-Mariscal FM, Perez-Martinez P, Delgado-Lista J, Yubero-Serrano EM, Camargo A, Delgado-Casado N, et al. Mediterranean diet supplemented with coenzyme Q10 induces postprandial changes in p53 in response to oxidative DNA damage in elderly subjects. Age. (2012) 34:389–403. doi: 10.1007/s11357-011-9229-1
55. Storniolo CE, Roselló-Catafau J, Pintó X, Mitjavila MT, Moreno JJ. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. (2014) 2:971–7. doi: 10.1016/j.redox.2014.07.001
56. Calabriso N, Massaro M, Scoditti E, D’Amore S, Gnoni A, Pellegrino M, et al. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J Nutr Biochem. (2016) 28:19–29. doi: 10.1016/j.jnutbio.2015.09.026
57. Joshi AA, Zanwar A. Effect of olive oil on metabolic syndrome. In: VR Preedy, RR Waston editors. Olives and Olive Oil in Health and Disease Prevention. Cambridge, MA: Academic Press (2021). p. 261–72.
58. Vicario IM, Malkova D, Lund EK, Johnson IT. Olive oil supplementation in healthy adults: effects in cell membrane fatty acid composition and platelet function. Ann Nutr Metab. (1998) 42:160–9. doi: 10.1159/000012729
59. Karantonis HC, Antonopoulou S, Demopoulos CA. Antithrombotic lipid minor constituents from vegetable oils. Comparison between olive oils and others. J Agric Food Chem. (2002) 50:1150–60. doi: 10.1021/jf010923t
60. Misikangas M, Freese R, Turpeinen AM, Mutanen M. High linoleic acid, low vegetable, and high oleic acid, high vegetable diets affect platelet activation similarly in healthy women and men. J Nutr. (2001) 131:1700–5. doi: 10.1093/jn/131.6.1700
61. Silva KD, Kelly CN, Jones AE, Smith RD, Wootton SA, Miller GJ, et al. Chylomicron particle size and number, factor VII activation and dietary monounsaturated fatty acids. Atherosclerosis. (2003) 166:73–84. doi: 10.1016/s0021-9150(02)00306-4
62. Bravo-Herrera MD, López-Miranda J, Marín C, Gómez P, Gómez MJ, Moreno JA, et al. Tissue factor expression is decreased in monocytes obtained from blood during Mediterranean or high carbohydrate diets. Nutr Metab Cardiovasc Dis. (2004) 14:128–32. doi: 10.1016/s0939-4753(04)80032-2
63. Delgado-Lista J, Lopez-Miranda J, Cortés B, Perez-Martinez P, Lozano A, Gomez-Luna R, et al. Chronic dietary fat intake modifies the postprandial response of hemostatic markers to a single fatty test meal. Am J Clin Nutr. (2008) 87:317–22. doi: 10.1093/ajcn/87.2.317
64. Menotti A, Puddu PE. How the Seven Countries Study contributed to the definition and development of the Mediterranean diet concept: a 50-year journey. Nutr Metab Cardiovasc Dis. (2015) 25:245–52. doi: 10.1016/j.numecd.2014.12.001
65. Papadaki A, Nolen-Doerr E, Mantzoros CS. The effect of the mediterranean diet on metabolic health: a systematic review and meta-analysis of controlled trials in adults. Nutrients. (2020) 12:3342. doi: 10.3390/nu12113342
66. Mazzocchi A, Leone L, Agostoni C, Pali-Schöll I. The secrets of the mediterranean diet. does [only] olive oil matter? Nutrients. (2019) 11:2941. doi: 10.3390/nu11122941
67. Salas-Salvadó J, Bulló M, Estruch R, Ros E, Covas MI, Ibarrola-Jurado N, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. (2014) 160:1–10. doi: 10.7326/M13-1725
68. Lasa A, Miranda J, Bulló M, Casas R, Salas-Salvadó J, Larretxi I, et al. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur J Clin Nutr. (2014) 68:767–72. doi: 10.1038/ejcn.2014.1
69. Romaguera D, Norat T, Vergnaud AC, Mouw T, May AM, Agudo A, et al. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am J Clin Nutr. (2010) 92:912–21. doi: 10.3945/ajcn.2010.29482
70. De Santis S, Cariello M, Piccinin E, Sabbà C, Moschetta A. Extra virgin olive oil: lesson from nutrigenomics. Nutrients. (2019) 11:2085. doi: 10.3390/nu11092085
71. Scoditti E, Carpi S, Massaro M, Pellegrino M, Polini B, Carluccio MA, et al. Hydroxytyrosol modulates adipocyte gene and miRNA expression under inflammatory condition. Nutrients. (2019) 11:2493. doi: 10.3390/nu11102493
72. Hernáez Á, Castañer O, Goday A, Ros E, Pintó X, Estruch R, et al. The Mediterranean Diet decreases LDL atherogenicity in high cardiovascular risk individuals: a randomized controlled trial. Mol Nutr Food Res. (2017) 61:1601015. doi: 10.1002/mnfr.201601015
73. Tsartsou E, Proutsos N, Castanas E, Kampa M. Network meta-analysis of metabolic effects of olive-oil in humans shows the importance of olive oil consumption with moderate polyphenol levels as part of the Mediterranean diet. Front Nutr. (2019) 6:6. doi: 10.3389/fnut.2019.00006
74. Schwingshackl L, Lampousi AM, Portillo MP, Romaguera D, Hoffmann G, Boeing H. Olive oil in the prevention and management of type 2 diabetes mellitus: a systematic review and meta-analysis of cohort studies and intervention trials. Nutr. Diabetes. (2017) 7:e262. doi: 10.1038/nutd.2017.12
75. Salas-Salvadó J, Bulló M, Babio N, Martínez-González MÁ, Ibarrola-Jurado N, Basora J, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. (2011) 3:14–9. doi: 10.2337/dc10-1288
76. Carnevale R, Loffredo L, Del Ben M, Angelico F, Nocella C, Petruccioli A, et al. Extra virgin olive oil improves post-prandial glycemic and lipid profile in patients with impaired fasting glucose. Clin Nutr. (2017) 36:782–7. doi: 10.1016/j.clnu.2016.05.016
77. Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. (2010) 11:1365–402. doi: 10.3390/ijms11041365
78. Xiao JB, Högger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr Med Chem. (2015) 22:23–38. doi: 10.2174/0929867321666140706130807
79. Mitjavila MT, Fandos M, Salas-Salvadó J, Covas MI, Borrego S, Estruch R, et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin Nutr. (2013) 32:172–8. doi: 10.1016/j.clnu.2012.08.002
80. Buckland G, Travier N, Barricarte A, Ardanaz E, Moreno-Iribas C, Sánchez MJ, et al. Olive oil intake and CHD in the European prospective investigation into cancer and nutrition Spanish cohort. Br J Nutr. (2012) 108:2075–82. doi: 10.1017/S000711451200298X
81. Marx W, George ES, Mayr HL, Thomas CJ, Sarapis K, Moschonis G, et al. Effect of high polyphenol extra virgin olive oil on markers of cardiovascular disease risk in healthy Australian adults (OLIVAUS): a protocol for a double-blind randomised, controlled, cross-over study. Nutr Diet. (2020) 77:523–8. doi: 10.1111/1747-0080.12531
82. Sarapis K, George ES, Marx W, Mayr HL, Willcox J, Esmaili T, et al. Extra virgin olive oil high in polyphenols improves antioxidant status in adults: a double-blind, randomized, controlled, cross-over study (OLIVAUS). Eur J Nutr. (2022) 61:1073–86. doi: 10.1007/s00394-021-02712-y
83. Davis CR, Hodgson JM, Woodman R, Bryan J, Wilson C, Murphy KJA. Mediterranean diet lowers blood pressure and improves endothelial function: results from the MedLey randomized intervention trial. Am J Clin Nutr. (2017) 105:1305–13. doi: 10.3945/ajcn.116.146803
84. Storniolo CE, Casillas R, Bulló M, Castañer O, Ros E, Sáez GT, et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur J Nutr. (2017) 56:89–97. doi: 10.1007/s00394-015-1060-5
85. Sarapis K, Thomas CJ, Hoskin J, George ES, Marx W, Mayr HL, et al. The effect of high polyphenol extra virgin olive oil on blood pressure and arterial stiffness in healthy australian adults: a randomized, controlled, cross-over study. Nutrients. (2020) 12:2272. doi: 10.3390/nu12082272
86. Njike VY, Ayettey R, Treu JA, Doughty KN, Katz DL. Post-prandial effects of high-polyphenolic extra virgin olive oil on endothelial function in adults at risk for type 2 diabetes: a randomized controlled crossover trial. Int J Cardiol. (2021) 330:171–6. doi: 10.1016/j.ijcard.2021.01.062
87. Soresi M, Noto D, Cefalù AB, Martini S, Vigna GB, Fonda M, et al. Nonalcoholic fatty liver and metabolic syndrome in Italy: results from a multicentric study of the Italian Arteriosclerosis society. Acta Diabetol. (2013) 50:241–9. doi: 10.1007/s00592-012-0406-1
88. Ng CH, Huang DQ, Nguyen MH. NAFLD versus MAFLD: prevalence, outcomes and implications of a change in name. Clin Mol Hepatol. (2022) [Online ahead of print]. doi: 10.3350/cmh.2022.0070
89. Houttu V, Csader S, Nieuwdorp M, Holleboom AG, Schwab U. Dietary interventions in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. (2021) 8:716783. doi: 10.3389/fnut.2021.716783
90. Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2021) 36:1470–8. doi: 10.1111/jgh.15363
91. Misciagna G, Del Pilar Díaz M, Caramia DV, Bonfiglio C, Franco I, Noviello MR, et al. Effect of a low glycemic index Mediterranean diet on non-alcoholic fatty liver disease. a randomized controlled clinici trial. J Nutr Health Aging. (2017) 21:404–12. doi: 10.1007/s12603-016-0809-8
92. Properzi C, O’Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. (2018) 68:1741–54. doi: 10.1002/hep.30076
93. Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. (2013) 59:138–43. doi: 10.1016/j.jhep.2013.02.012
94. Patti AM, Carruba G, Cicero AFG, Banach M, Nikolic D, Giglio RV, et al. Daily use of extra virgin olive oil with high oleocanthal concentration reduced body weight, waist circumference, alanine transaminase, inflammatory cytokines and hepatic steatosis in subjects with the metabolic syndrome: a 2-month intervention study. Metabolites. (2020) 10:392. doi: 10.3390/metabo10100392
95. Pintó X, Fanlo-Maresma M, Corbella E, Corbella X, Mitjavila MT, Moreno JJ, et al. A mediterranean diet rich in extra-virgin olive oil is associated with a reduced prevalence of nonalcoholic fatty liver disease in older individuals at high cardiovascular risk. J Nutr. (2019) 149:1920–9. doi: 10.1093/jn/nxz147
96. Markellos C, Ourailidou ME, Gavriatopoulou M, Halvatsiotis P, Sergentanis TN, Psaltopoulou T. Olive oil intake and cancer risk: a systematic review and meta-analysis. PLoS One. (2022) 17:e0261649. doi: 10.1371/journal.pone.0261649
97. Pastor R, Bouzas C, Tur JA. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: systematic review and meta-analysis. Free Radic Biol Med. (2021) 20:372–85. doi: 10.1016/j.freeradbiomed.2021.06.017
98. De Santis S, Clodoveo ML, Cariello M, D’Amato G, Franchini C, Faienza MF, et al. Polyphenols and obesity prevention: critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit Rev Food Sci Nutr. (2021) 61:1804–26. doi: 10.1080/10408398.2020.1765736
99. Venkatakrishnan K, Chiu HF, Wang CK. Extensive review of popular functional foods and nutraceuticals against obesity and its related complications with a special focus on randomized clinical trials. Food Funct. (2019) 10:2313–29. doi: 10.1039/c9fo00293f
100. Aponte M, Ungaro F, d’Angelo I, De Caro C, Russo R, Blaiotta G, et al. Improving in vivo conversion of oleuropein into hydroxytyrosol by oral granules containing probiotic Lactobacillus plantarum 299v and an Olea europaea standardized extract. Int J Pharm. (2018) 543:73–82. doi: 10.1016/j.ijpharm.2018.03.013
101. Castro-Barquero S, Lamuela-Raventós RM, Doménech M, Estruch R. Relationship between mediterranean dietary polyphenol intake and obesity. Nutrients. (2018) 10:1523. doi: 10.3390/nu10101523
Keywords: extra virgin olive oil (EVOO), nutraceuticals, functional foods, metabolic syndrome, cardiovascular disease, insulin resistance
Citation: Seidita A, Soresi M, Giannitrapani L, Di Stefano V, Citarrella R, Mirarchi L, Cusimano A, Augello G, Carroccio A, Iovanna JL and Cervello M (2022) The clinical impact of an extra virgin olive oil enriched mediterranean diet on metabolic syndrome: Lights and shadows of a nutraceutical approach. Front. Nutr. 9:980429. doi: 10.3389/fnut.2022.980429
Received: 28 June 2022; Accepted: 18 July 2022;
Published: 04 August 2022.
Edited by:
Nicola Cicero, University of Messina, ItalyReviewed by:
Filomena Corbo, University of Bari Aldo Moro, ItalyCopyright © 2022 Seidita, Soresi, Giannitrapani, Di Stefano, Citarrella, Mirarchi, Cusimano, Augello, Carroccio, Iovanna and Cervello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melchiorre Cervello, bWVsY2hpb3JyZS5jZXJ2ZWxsb0BpcmliLmNuci5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.