
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 02 September 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.975718
Background: Soy consumption has health benefits, but the relationship between soy and uric acid remains uncertain. This meta-analysis and systematic review evaluated the effects of soy intake on plasma uric acid.
Methods: PubMed, Embase, CNKI, and the Cochrane Library were searched for studies evaluating the effects of soy, soy products, soy protein, and soy isoflavones on uric acid levels. The primary outcome was serum or plasma uric acid concentration. Study quality was evaluated by the Cochrane Collaboration and SYRCLE risk-of-bias tools.
Results: A total of 17 studies were included. Qualitative analysis of three human clinical studies of acute effects revealed that soy consumption increased serum uric acid concentration; however, soy-derived products, including tofu, bean curd cake, and dried bean curd sticks, had no significant effect on serum uric acid. A meta-analysis of five long-term human studies (10 data sets) revealed that soy protein and soy isoflavones had no significant effects on uric acid levels [weighted mean difference (WMD) = –2.11; 95% confidence interval (CI): –8.78, 4.55; p = 0.53]. However, most epidemiological data revealed that soy intake is inversely associated with uric acid levels. Meta-analysis of nine animal trials (29 data sets) revealed that soy protein and soy isoflavones significantly reduced serum uric acid concentrations (vs. controls; MD = –38.02; 95% CI: –50.60, –25.44; p < 0.001).
Conclusion: Soy and its products have different effects on serum uric acid. Soy products like tofu, bean curd cake, and dried bean curd sticks could be high-quality protein sources for individuals with hyperuricemia or gout. It can be beneficial to nutritionists and healthcare decision-makers reconsider their conceptions about the relationship between soy and uric acid levels according to the latest and further scientific study results.
Systematic review registration: [www.crd.york.ac.uk/PROSPERO], identifier [CRD42022331855].
Soybeans, a member of the Fabaceae (legume) family, contain all eight essential amino acids (1, 2). In Asian countries, soy and its products have been consumed for centuries. In recent decades, the production and consumption of soy foods in Western countries have grown due to their reported health benefits (3). Some countries had approved health claims for soy protein, including the United States, Japan, South Africa, Philippines, Brazil, Malaysia, South Korea, Indonesia, and the United Kingdom (4, 5). Soy is a popular food due to its nutritional value, and it provides a wide range of health benefits.
Intake of soy protein or isoflavones is often used as an index of soy consumption because of the relevance of protein and isoflavone fractions and their variability in soy foods (6). Daily soy protein intake of up to 17 g was reported among women with plant-based dietary habits in Shanghai (7). Among adult people in Japan, the average daily intake range of soy protein, between 6 and 11 g, and isoflavone intake is 23 and 54 mg (8). The average soy, soy protein, and soy isoflavone intake of 47 Japanese prefectures from 1980 to 1985 was reported to be 66.8 g, 6.5 g, and 27.8 mg, respectively (9). In Japan, the daily median intake of daidzein and genistein (10) was reported to be 9–12 and 15–20 mg, respectively (11). Daily isoflavone intake has been estimated at 0.3–4.5 mg in European countries and approximately 1–3 mg in the United States (12–16). Moreover, sales of United States soy foods doubled in 6 years, from $2 billion in 1999 to $4.3 billion in 2005. Food manufacturers in the United States introduced more than 2,700 new foods with soy as an ingredient from 2000 to 2007, including 479 in 2006 alone (17).
Hyperuricemia, a risk factor for gout (18), results from increased uric acid production or/and impaired renal uric acid excretion. Uric acid is the final enzymatic product of purine metabolism. Ingesting foods rich in purines may increase serum and plasma uric acid concentrations (19).
The purine content in soy is approximately 137 mg/100 g (20, 21). Soy foods are generally considered to have moderate purine content, at 50–150 mg/100 g (22). High-protein foods also have high purine content. Adenine and guanine concentrations aid in controlling protein biosynthesis (23). Soy contains 35–40% protein by dry weight. Because soy proteins contain all essential amino acids, soy products have essentially equivalent protein value to that of animal sources but have less saturated fat and no cholesterol (24). In addition to its nutritional value, soy protein shows various biological functions, for example, anti-obesity effects and cholesterol-lowering, and may aid help to reduce the severity of lifestyle-related diseases (25–28). However, due to the relationship between protein and purine, soy protein is usually associated with increased serum or plasma uric acid.
Soy is a rich source of isoflavones (29–31). The primary isoflavones contained in soy are daidzein and genistein (32). Some studies (33, 34) revealed that a diet rich in isoflavones is associated with diminished risks of cancers, osteoporosis, and cardiovascular disease. The molecular structures of isoflavones are similar to that of the female hormone estrogen and result in a weak but similar action. Adamopoulos et al. discovered that exogenous estrogen reduced blood uric acid and promoted uric acid excretion in both men and women (35). Flavonoids have been reported to inhibit xanthine oxidase activity in vitro (36, 37). Soy isoflavones may help to reduce serum and plasma uric acid levels.
Although epidemiological studies have linked many potential benefits to soy intake (38), health professionals, and the general public in Asia broadly believe that soy foods increase the risk of gout. To date, little research has investigated the effects of soy and its related substances on serum or plasma uric acid. Moreover, findings concerning the relationship of soy and its related substances with serum and plasma uric acid levels, hyperuricemia, and gout are inconsistent. Accordingly, this review evaluates the effects of soy, soy products, soy protein, and soy isoflavones on serum and plasma uric acid levels through meta-analyses and a systematic evaluation of literature from Asian and Western countries with high soy consumption.
This systematic review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (39). We conducted a thorough literature search of PubMed, Embase, CNKI, and the Cochrane Library to identify human and animal studies on the effects of soy consumption on serum or plasma uric acid. Our search terms included combinations of the following keywords and their variants: uric acid, soy, isoflavones, soy protein, daidzein, and genistein. No language or date restrictions were applied. The keyword search yielded a comprehensive list of titles and abstracts, which were screened for relevance against the study selection criteria mentioned in the following section. Two reviewers (YD and QQ) individually considered the full text for inclusion, and any discrepancies were resolved through consensus.
To meet the inclusion criteria, studies must be (a) clinical studies or animal control trials of (b) participants or animals with normal uric acid levels, (c) group serum or plasma uric acid levels must be reported as means ± standard deviations (SD; clinical trials: at baseline and the end of the trial; animal trials: at the end of the trial), and (d) data on soy, soy product, soy protein, or isoflavone intake must be reported. Meeting summaries, letters to the editor, patents, review articles, unpublished articles, articles with incomplete data, and articles without available full texts were excluded. A flowchart of the screening and selection process is depicted in Figure 1.
The risk of bias was evaluated using the Cochrane Collaboration tool (40) for randomized clinical trials and the Systematic Review Center for Laboratory animal Experimentation (SYRCLE’s) Risk of Bias (RoB) tool (41) for animal studies. The Cochrane Collaboration tool assesses (a) outcome reporting, (b) blinding, (c) allocation concealment, (d) outcome data completeness, (e) randomization, (f) and other sources of bias. The SYRCLE risk-of-bias tool is a version of the Cochran tool modified for animal intervention studies. It assesses (a) baseline characteristics, (b) sequence generation, (c) allocation concealments (d) housing randomness, (e) blinding (performance bias), (f) blinding (detection bias), (g) outcome assessment randomness, (h) outcome data completeness, (i) outcome reporting, and (j) other sources of bias.
The two reviewers independently evaluated the risk of bias for each eligible study and disagreements on scores were resolved through consensus.
The two reviewers independently identified the titles and abstracts that potentially met the inclusion criteria. Then, the full-text articles were read for complete assessment and selection. Each reviewer performed these steps independently, and disagreements on inclusion were resolved through consensus. Data for each included article were recorded, including the year of publication; name of the first author; study period; group size; age; sex; and exposure to soy, soy products, soy protein, or soy isoflavones.
RevMan v5.4 (Cochran Tech, London, United Kingdom) was used for meta-analysis and the creation of forest plots. The units of uric acid concentration were converted to μmol/L.
For animal studies, random effects models were used throughout due to the possible heterogeneity from sources, such as differences in the time at which the endpoints, were evaluated. In addition, the I2 statistic was used to assess heterogeneity within subgroups. I2 values > 25% and > 75% represented moderate and high heterogeneity, respectively. Mean difference (MD) and 95% confidence intervals (CIs) were used to determine the effect.
For clinical studies, heterogeneity between trials was detected with the chi-square and I2 tests. In the situation of significant heterogeneity (I2 > 50% and p < 0.1), a random-effects model was performed for analysis; otherwise, a fixed effects model was performed. The weighted mean difference (WMD) and 95% CI were used to determine the effects. If the mean or SD was unavailable, it was calculated from other mean values and standard error by using the following formulas (assuming a correlation coefficient R = 0.5 (42):
where mean1 is the preintervention mean; mean2 is the postintervention mean; n1 is the preintervention sample size; n2 is the postintervention sample size; SD1 is the preintervention SD; and SD2 is the postintervention SD. Studies with p < 0.05 were considered statistically significant, and two-sided 95% CIs were employed. For studies that could not be analyzed through meta-analysis, descriptive analysis and evaluation (i.e., qualitative analysis) were performed.
Eight (43–50) human clinical studies met the inclusion criteria. These were categorized into those assessing acute or long-term effects based on the study period. Specifically, three (43–45) assessed acute effects and five (46–50) assessed long-term effects. Table 1 displays the details of these studies.
Of the three studies of acute effects, two (43, 44) focused on the effects of soy or soy products on uric acid and one (45) focused on the effects of soy protein. Due to differences in research design (randomized controlled trial, before-after study in the same patient, and randomized controlled crossover trial, respectively), reliable meta-analysis was impossible; therefore, qualitative analysis was performed.
The first study (43) considered was a randomized controlled study conducted by Zhang et al. to detect the serum uric acid concentrations of 60 healthy men after they ingested whole soy, one of four soy products (soy milk, soy powder, bean curd cake, or dried bean curd sticks), or water. The results revealed no significant changes from baseline in serum uric acid concentration after the ingestion of dried bean curd sticks, bean curd cake, or water (p > 0.05). After ingestion of whole soy, serum uric acid concentration significantly increased in the short term, at 21.4 μmol/L after 60 min and 16.3 μmol/L after 120 min; although uric acid remained high through 180 min, this difference was not statistically significant from baseline. The same significant results occurred after the ingestion of soy powder. For soy milk, serum uric acid concentration had increased and was found to be 38.1, 34.4, and 24.1 μmol/L at 60, 120, and 180 min after ingestion, respectively. However, bean curd cake induced a significant decrease in serum uric acid at 60 min in participants with high serum uric acid concentrations.
Another study (44) examined the effect of tofu ingestion on the uric acid metabolism of 8 healthy participants and 10 participants with gout, all aged 30–50 years. Plasma levels (mg/dL) at 0, 60, 120, and 180 min after the ingestion of 4 g/kg of tofu by healthy participants and participants with gout were 5.56, 5.59, 5.83, 5.73, and 8.10, 8.21, 8.27, and 8.12 mg/dL, respectively. Plasma uric acid levels in healthy patients increased statistically after 120 min and 180 min compared with that at baseline, whereas the participants with gout exhibited no significant differences. The plasma uric acid concentrations of the healthy participants and those with gout also did not differ significantly. These results suggest that tofu is a preferable source of protein.
Garrel et al. (45) examined the acute effects of ingesting 80 g of casein, lactalbumin, or soy isolate protein on serum uric acid concentrations. The cohort comprised 10 healthy participants aged 22–27 years. Serum uric acid levels at 0, 60, 120, and 180 min after soy isolate protein ingestion were 283, 307, 319, and 314 μmol/L (p < 0.01), respectively.
The results of these three studies revealed that ingesting soy or soy protein rapidly increases serum uric acid. However, tofu, bean curd cake, and driven bean curd sticks had no significant effect on uric acid concentration.
Five studies of long-term effects met the inclusion criteria. One (46) focused on soy protein and four (47–50) focused on soy isoflavones; of these four, two (47, 48) studied unspecified soy isoflavones, one (49) studied daidzein, and one (50) studied genistein. Some studies employed multiple doses or intervention expectations, and we treated these as individual trials. Thus, this category comprised 10 trials. Table 2 presents the data extracted from each study.
Of the 10 trials, only one revealed significant differences between the control and intervention groups. In the study by Qim et al., uric acid concentration decreased by 23 μmol/L after intervention with 80 mg of daidzein for 24 weeks (intervention group, n = 60; control group, n = 59, p = 0.001; 95% CI: –40.91, –5.09). The 10 trials (with a total of 940 participants) that compared a soy protein or soy isoflavone group with a blank or placebo control group were meta-analyzed. The heterogeneity results were x2 = 14.89, p = 0.09, and I2 = 40%. A fixed-effects model analysis indicated no statistically significant differences in the long-term effects of soy protein and soy isoflavones on the uric acid levels of intervention groups compared with those of the blank and placebo groups (p = 0.53; Figure 2).
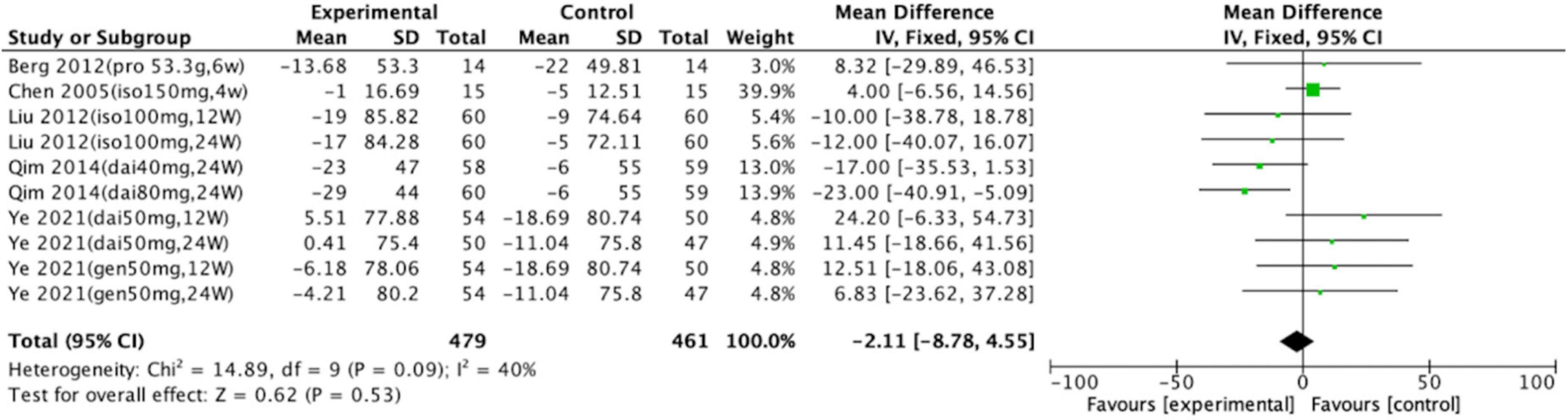
Figure 2. Forest plot of mean change in uric acid concentration after intervention with soy protein and soy isoflavones (SD, standard deviation; CI, confidence interval; IV, inverse variance; μmol/L).
Subgroup analyses were conducted to assess the effects of isoflavones on serum uric acid levels (Figure 3). Groups consuming daidzein (intervention group, n = 222; control group, n = 215; p = 0.07; WMD = –10.07; 95% CI: –21.11, 0.97), genistein (intervention group, n = 108; control group, n = 97; p = 0.38; WMD = 9.66; 95% CI: –11.91, 31.23), or soy isoflavones (intervention group, n = 135; control group, n = 135; p = 0.87; WMD = –0.75; 95% CI: –8.59, 10.09) did not significantly differ from the control groups. Total meta-analysis revealed that soy isoflavones had no effect on uric acid levels (intervention group, n = 465; control group, n = 447; p = 0.48; WMD = –2.44; 95% CI: –9.21, 4.33).
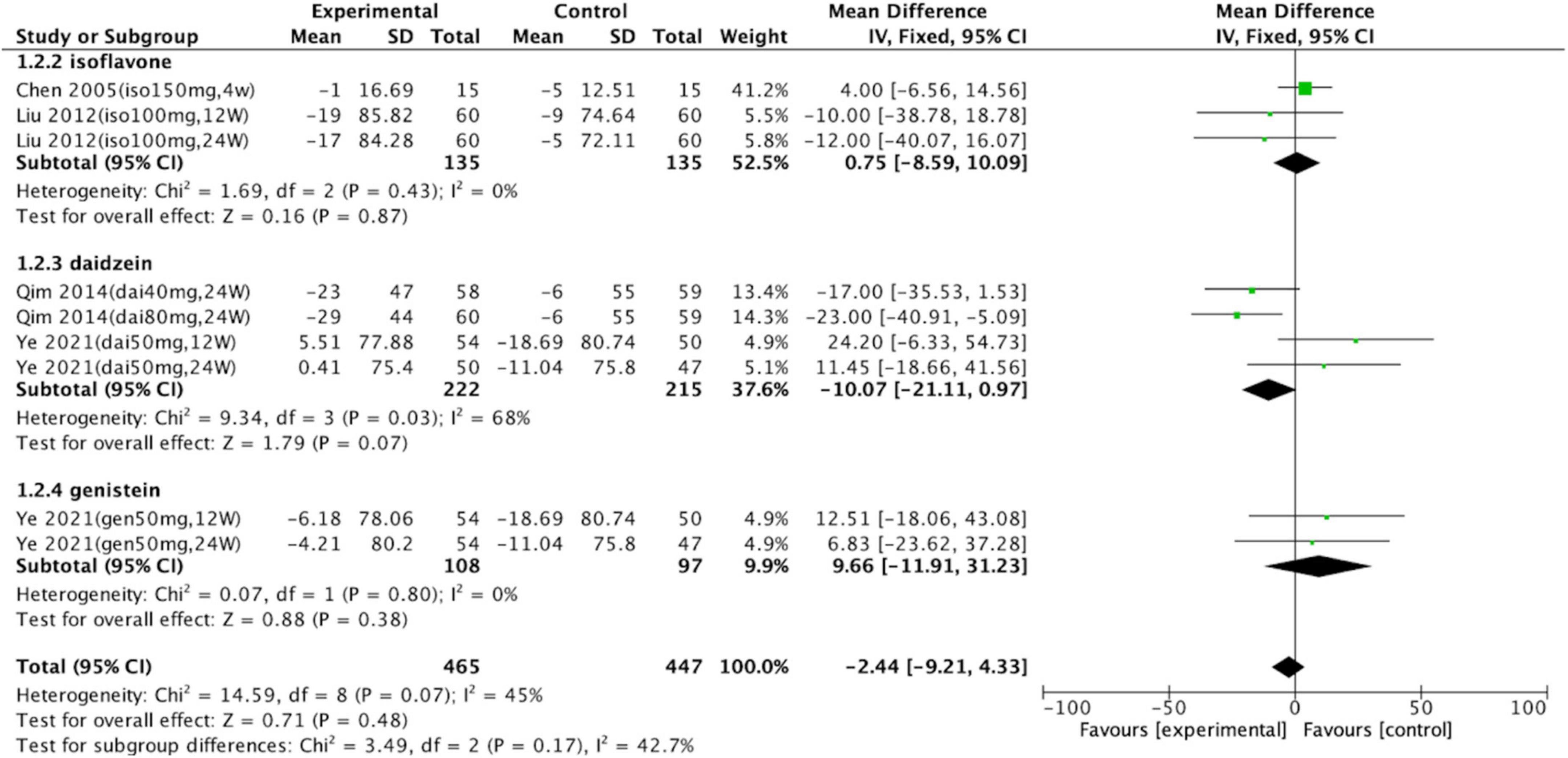
Figure 3. Forest plot of mean change in uric acid concentration after intervention with soy isoflavones. Subgroup analyses evaluated the effects of daidzein, genistein, and unspecified soy isoflavones (SD, standard deviation; CI, confidence interval; IV, inverse variance; μmol/L).
According to the Cochrane Collaboration risk-of-bias assessment, the five studies included failed to achieve all seven benchmarks. The overall risks for each type of bias are presented in Figure 4, and the risks of each bias for each included study are presented in Figure 5. One study (20%) exhibited high risks of performance bias and detection bias. Three studies (60%) demonstrated unclear risks of selection bias (allocation concealment).
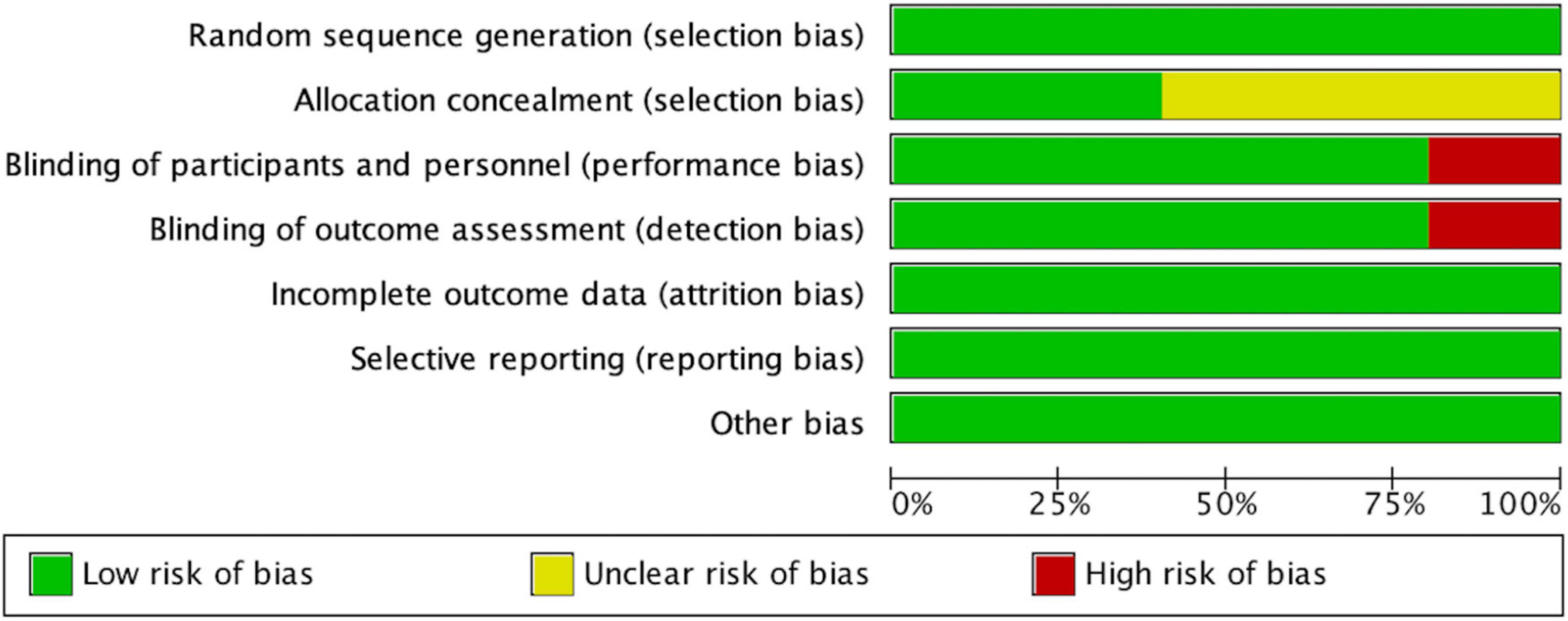
Figure 4. Risk of bias graph, with risk of each type of bias presented as a percentage among included studies).
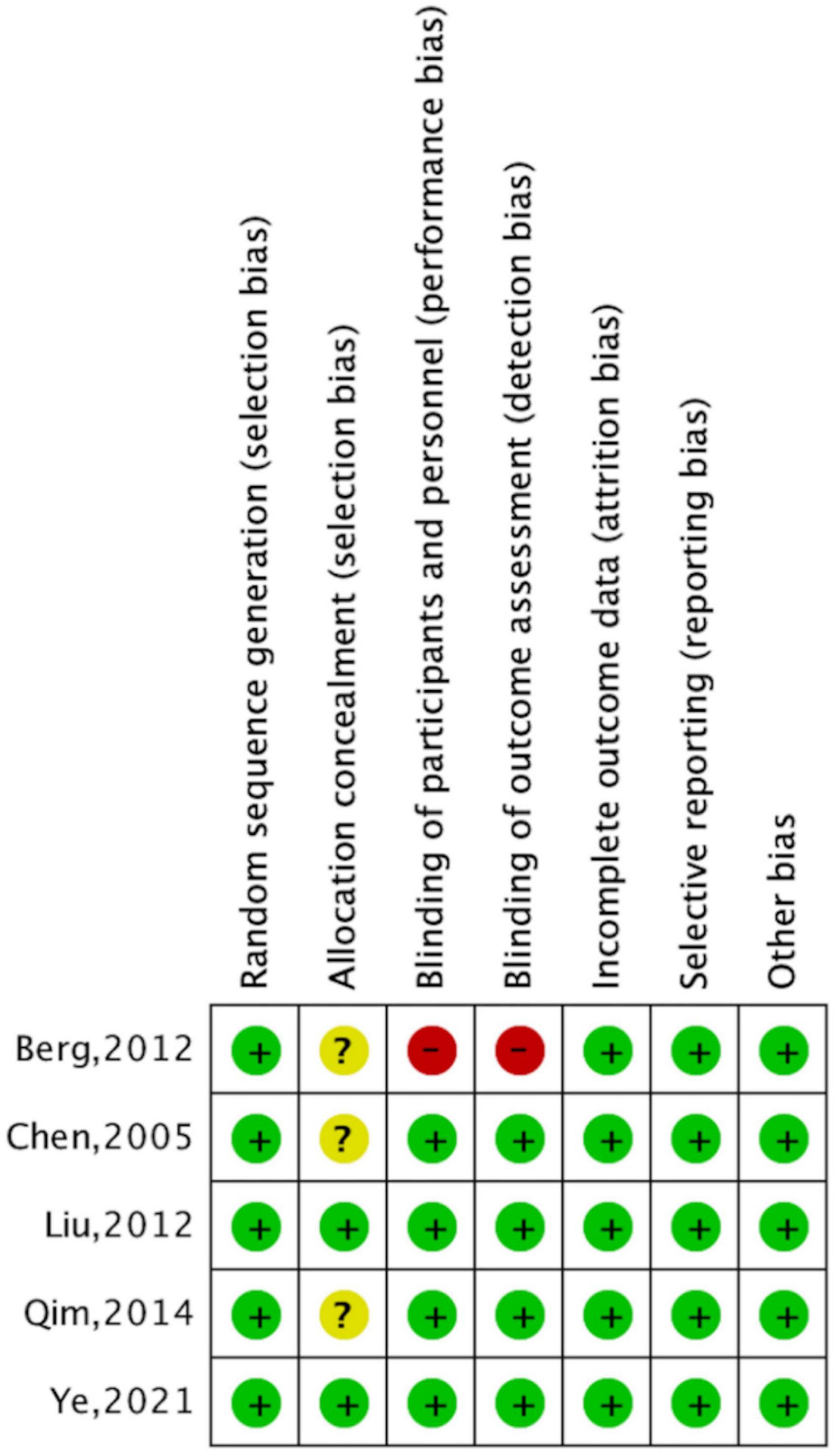
Figure 5. Risk of bias summary, with risk of each type of bias in included studies presented separately. Note: +, ?, – indicate high, uncertain, and low bias, respectively.
To better evaluate the relationship between soy consumption and serum uric acid in real life, we reviewed the epidemiological data. An earlier study conducted by Pan et al. interviewed 59 non-vegetarian medical students and 55 vegetarian Taiwanese Buddhists (51). In which, non-vegetarians consumed one serving of soy food 1 day, while vegetarians consumed 3.5 servings. The results revealed that the plasma uric acid level of vegetarians was significantly lower than that of non-vegetarians (226 ± 59 mmol/L and 258 ± 54 mmol/L, respectively) in women, but, no such difference in men. Villegas et al. conducted a cross-sectional study among 3,978 men in Shanghai (52). The results revealed that intake of soy products (tofu, fried tofu, vegetarian chicken, and tofu cake) was associated with a decreased risk of hyperuricemia. Another cross-sectional study by Liu et al. was conducted among 2,939 adults aged 50–75 in Guangzhou (53). The participants were divided into quartiles according to their intake amount of soy protein. Soy protein from soy, soy drink, and other soy products was negatively correlated with the risk of hyperuricemia. Tang et al. divided 120 volunteers aged 54–56 years into a control group and a genistein-rich group (54) and observed a significant difference in serum uric acid levels between the two groups (5.34 ± 1.13 mg/dL in the control group and 4.75 ± 1.21 mg/dL in genistein-rich group). A large cohort study (55) collected data on the eating habits of 63,257 Singaporean Chinese aged 45–74 years, and found that soy was associated with a decreased risk of gout (hazard ratio = 0.86, 95% CI: 0.75–0.98). However, inconsistent with these results, a previous study by Yu et al. (56) assessed the intake of soy beverages among 987 men and 1,189 women and revealed that the intake of soy beverages was not associated with the risk of hyperuricemia. Liu et al. (57) also conducted a cross-sectional study about soy isoflavones supplementation among 183 Chinese adults. Their study observed no significant difference in serum uric acid levels between low isoflavones groups with the consumption of isoflavones of 4.6 mg/d and high isoflavones groups with 23.6 mg/d (274.67 ± 99.00 μmol/L and 269.01 ± 84.88 μmol/L, respectively).
Nine studies (58–66) met the inclusion criteria. Two (58, 59) studied the effects of soy protein and the remaining seven (60–66) studied the effects of soy isoflavones on uric acid. Among the seven studies on isoflavones, three (61, 64, 65) focused on daidzein, three (60, 62, 63) on genistein, and one (66) on unspecified soy isoflavones. As with long-term clinical studies, some studies employed different doses and intervention periods, and we divided the corresponding research into individual trials. Thus, this category comprised 29 trials. Table 3 displays the details of these trials.
The effects of soy protein and soy isoflavones on serum and plasma uric acid levels were reported for the 29 data sets of the nine studies (intervention group, n = 267; control group, n = 67). The meta-analysis indicated that, compared with the levels in the controls, soy protein and isoflavones were resulted in lower serum and plasma uric acid levels (p < 0.001; MD = –38.02; 95% CI: –50.60, –25.44).
We performed further subgroup analysis. First, a meta-analysis was carried out on rats, mice, laying hens, and broiler chickens. Six rat trials (control group, n = 44; intervention group, n = 44) were analyzed. Four trials revealed differences between the control and intervention groups. Two intervention groups exhibited significantly increased serum or plasma uric acid levels, whereas the other two intervention groups exhibited reduced serum and plasma uric acid concentrations. A meta-analysis of six rat trials revealed that the effect of the intervention group on reducing uric acid was non-significant (p = 0.14). Six mouse trials (n = 60 in control group; n = 60 in intervention group) were analyzed. In five of the six trials, mice receiving soy protein or isoflavones exhibited reduced uric acid levels; in the remaining trial, no differences were observed between the intervention and control animals. Overall, soy protein or soy isoflavones reduced serum and plasma uric acid (MD = –71.68; 95% CI: –121.53, –21.83; p = 0.005) in mice; this result differs from that of the rat trials. Eleven laying hen trials were included in the meta-analysis. In five of these trials, soy protein or soy isoflavones reduced uric acid levels, whereas none was observed in the other six trials. Of 133 intervention and 133 control laying hens, soy protein or soy isoflavones reduced uric acid levels by a mean of 46.05 μmol/L (95% CI: –70.98, –21.12; p < 0.001). Six broiler chicken trials (n = 30 in the control group; n = 30 in the intervention group) analyzed the effect of soy protein or soy isoflavones on uric acid levels. In three of these trials, broilers receiving soy protein or soy isoflavones exhibited reduced uric acid levels, whereas no differences were observed between the intervention and control animals in the other three trials. Meta-analysis of these broiler trials revealed that soy protein or soy isoflavones had no effect on serum or plasma uric acid levels (p = 0.15; Figure 6).
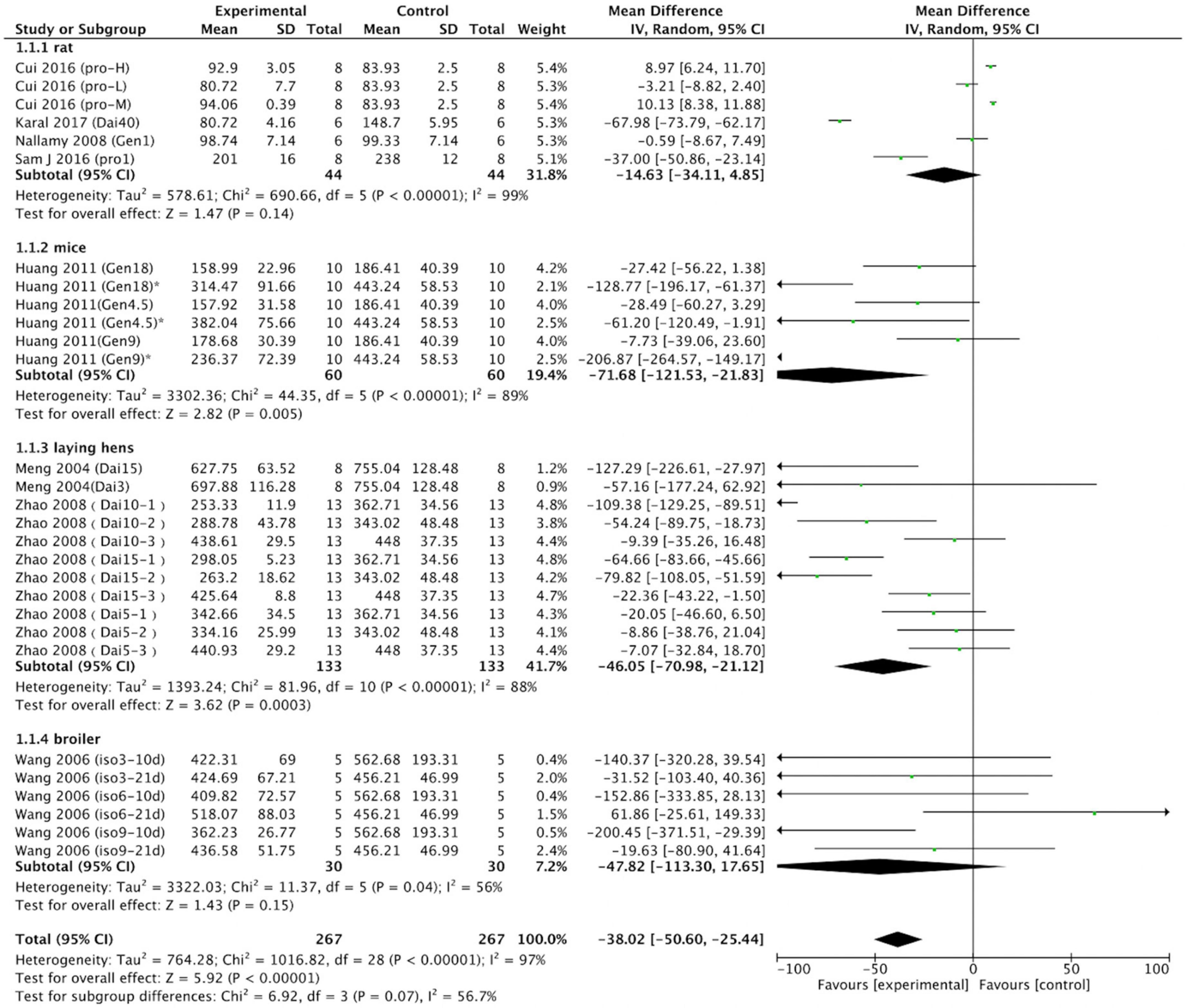
Figure 6. Forest plot of the effects of soy protein and soy isoflavones on serum and plasma uric acid concentration (grouped by animal species; SD, standard deviation; CI, confidence interval; IV, inverse variance; μmol/L).
We separated the data into those on soy protein and those on soy isoflavones for meta-analysis. Soy protein (p = 0.79; MD = –1.13; 95% CI: –9.58, 7.33) had no effect on uric acid concentration (Figure 7).
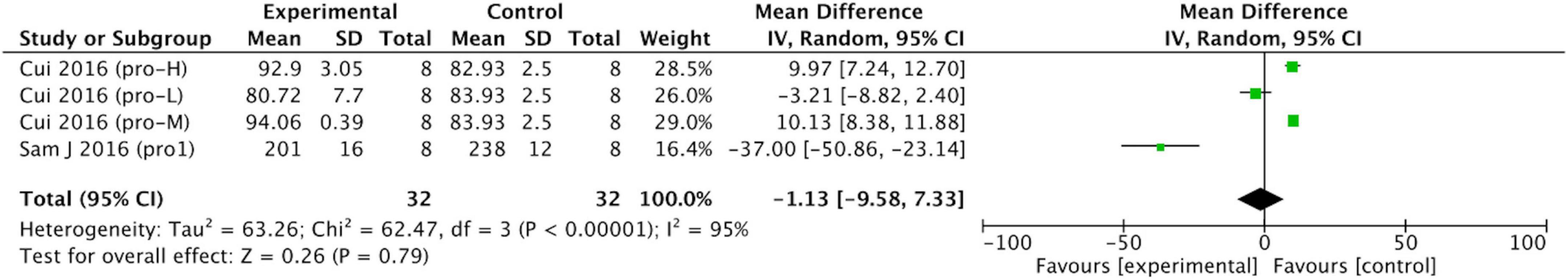
Figure 7. Forest plot of the effects of soy protein (SD, standard deviation; CI, confidence interval; IV, inverse variance; μmol/L).
Subgroup analyses were performed to evaluate the effects of soy isoflavones on serum and plasma uric acid levels. Unspecified soy isoflavones (n = 30 in control group; n = 30 in intervention group; p = 0.15; MD = –47.82; 95% CI: –113.30, 17.65) did not significantly reduce serum or plasma uric acid concentrations. However, daidzein (n = 139 in control group; n = 139 in intervention group; p < 0.001; MD = –47.90; 95% CI: –67.64, –28.15) and genistein (n = 66 in control group; n = 66 in intervention group; p = 0.003; MD = –58.03; 95% CI: –96.90, –19.16) significantly reduced serum or plasma uric acid concentrations (Figure 8).

Figure 8. Forest plot of the effects of soy on serum and plasma uric acid concentration. Subgroup analyses evaluated the effects of daidzein, soy protein, genistein, and unspecified soy isoflavones (SD, standard deviation; CI, confidence interval; IV, inverse variance; μmol/L).
Eight studies (89%) mentioned random group allocation, but no studies described the randomization process. Five studies (56%) provided research animals’ baseline characteristics. None mentioned allocation concealment. Eight studies described random housing. No study described blinding (performance bias) or random outcome assessment. All studies achieved and reported complete outcome data. No other sources of bias affected any of the studies. The methodological quality of each study is displayed in Table 4.
The relationship between soy and health has gradually become a topic of concern. The relationship between soy and uric acid levels is controversial. A common belief is that people with high serum or plasma uric acid levels should avoid eating soy due to the high purine content of soy protein, which increases the production of uric acid. However, the expected increase caused by daily intake in individuals of Asian descent is almost clinically irrelevant (67). The British Society for Rheumatology also pointed out that patients with gout should be encouraged to add vegetable- and soy-derived protein to their diets (68).
In this study, we systematically reviewed the effects of soy, soy products, soy protein, and soy isoflavones on serum and plasma uric acid levels in clinical and animal studies. Soy consumption had no significant effect on human uric acid concentration. Studies of the acute effects of soy intake on uric acid demonstrated that whole soy and soy protein increased uric acid levels in the short term. However, soy products such as tofu, tofu cake, and dried tofu had no effect. Zhang et al. (43) found that whole soy intake increased serum uric acid concentration by 6.8% after 60 min. Results from the study by Garrel et al. (45) showed that soy protein intake increased serum uric acid concentration by 12% after 120 min. This difference in serum uric acid concentration may be related to the amount of protein, with the intervention of 40 g soy protein in Zhang’s study and 80 g soy protein isolate in Garrel’s study. Consistent with the study by Garrel et al., another study (69), which was excluded from the final meta-analysis due to no report of serum uric acid concentration, observed that intake of 80 g soy protein increased serum uric acid concentration by 10% after 120 min. It is noteworthy that many studies adopted large amounts of protein (40–80 g) as intervention, which is equivalent to the daily amount of protein recommended by the Dietary Guidelines for Chinese residents, exceeding the amount typically consumed in a meal, and let alone from one protein source. In addition, Garrel’s study also examined the acute effects of casein and lactalbumin on uric acid levels. In contrast to the results of soy protein, the serum uric acid concentration of participants decreased significantly at 3 h after ingestion of casein and lactalbumin. Long-term clinical studies mainly presented evidence of the effects of soy protein and soy isoflavones on uric acid; soy had no effect on uric acid levels.
A cross-over study designed by Serrano et al. (70) investigated the effects of soy beverages on glucose homeostasis and monitored changes in serum uric acid levels. Their results revealed a significant increase in uric acid levels at 60 min after consuming soy beverage, as well as an increase in insulin levels. However, another 3-month open randomized controlled trial designed by Zhang et al. (71) revealed that soy products and fruits may help reduce the risk of asymptomatic hyperuricemia by reducing insulin resistance. Enhanced insulin sensitivity can promote the resecretion of uric acid in renal tubular epithelial cells and increase the excretion of uric acid (72). Consistent with the result, many epidemiological studies also revealed that soy consumption is inversely associated with uric acid levels.
The included animal studies evaluated the effects of soy isoflavones and soy protein on uric acid levels. For normal rats and broilers, no differences were found in uric acid concentrations between the soy intervention group and controls. For mice and laying hens, the effects of soy intervention on uric acid levels were significant, and soy isoflavones reduced uric acid concentrations. In subgroup analysis according to the type of soy, soy protein and soy isoflavones had no effect on uric acid concentration. However, daidzein and genistein, which are the main components of soy isoflavones, both significantly reduced uric acid concentrations in animal studies.
Purine in the form of purine nucleotides participates in human energy metabolism and is a crucial component of genetic material (73). Guanine and adenine are two bases of DNA and RNA (74). Protein formation depends on DNA and RNA and thus on two purines. Adenine, especially adenosine triphosphate (ATP), provides energy and can be transformed into hypoxanthine, and guanine and hypoxanthine directly synthesize xanthine. Xanthine oxidase catalyzes the oxidation of hypoxanthine and xanthine to form uric acid (75). This may explain why soy protein causes significant short-term increases in serum and plasma uric acid concentrations but no significant long-term effects.
Traditional soy products are roughly processed from soybeans as the primary raw material. The purine content of the edible portion of the soybean is affected by the water content added during processing—the lower the water content, the higher the purine content (76). Our study found that uric acid concentration increased rapidly after the intake of whole soy, whereas the intake of soy-derived products had no effect. This difference may be due to the change in purine content during soy processing.
Traditional soy foods contain approximately 3.5 mg of isoflavones per gram of protein (8). Of soy isoflavones, genistein, daidzein, and glycitein make up about 50, 40, and 10%, respectively, (10). Isoflavones have a molecular structure similar to that of estrogen, allowing them to bind to both estrogen receptors (77, 78), and to exert estrogen-like effects under certain conditions. Some studies have investigated the relationship between estrogen and uric acid. Studies have demonstrated that endogenous estrogen may reduce uric acid by inhibiting the production of uric acid reabsorption protein and promoting that of uric acid secretory protein (79). Budhiraja et al. observed the inhibition of xanthine oxidoreductase systems by estradiol in a rat model of hypoxia (80). Although the overall mechanism by which estrogen affects uric acid metabolism remains unclear, numerous studies have demonstrated that an increase in estrogen reduces uric acid levels. Soy isoflavones are commonly referred to as phytoestrogens. Circulating isoflavone levels were three orders of magnitude higher than estrogen levels after ingestion of approximately two servings of soy foods (81). Therefore, soy isoflavones may inhibit uric acid through an estrogen-like effect, and the results of our meta-analysis support this idea.
Soy is rich in protein and isoflavones. One serving of soy food provides approximately 8 g of protein and 25 mg of isoflavones. The effects of soy and its products on uric acid may be the combined effects of isoflavones and soy protein. Isoflavones and soy protein can interact. Genistein and daidzein are the two main components of aglycone-type isoflavones (82). As natural small molecules and active substances, aglycone-type isoflavones can interact with and change the structure and function of soy protein (83). The formation of soy protein and soy isoflavone complexes affects the structure and nutritional function of the protein and increases the antioxidant capacity and bioavailability of isoflavones (84, 85). However, soy protein and isoflavones have different specific effects on uric acid metabolism. Protein rich in purine may increase uric acid concentration, whereas isoflavones may reduce uric acid concentration.
Human clinical studies on the effects of soy consumption on uric acid have mostly focused on individuals with normal uric acid levels. However, the effects of a substance on serum or plasma uric acid levels are often influenced by individual differences. Statins significantly reduce serum and plasma uric acid levels in individuals with coronary heart disease complicated with dyslipidemia, and the decrease of uric acid in individuals with hyperuricemia is greater than that in patients with normal uric acid (86). Some studies have also revealed that, compared with normal rats, hyperuricemic rats exhibit significantly less and slower absorption of hesperidin (87). Therefore, it is prudent that our findings about the relationship between soy consumption and uric acid level were extended to patients with hyperuricemia or gout. Additional research is required to assess the association between soy and serum or plasma uric acid with high uric acid levels, for example, in animal models of hyperuricemia or gout. In addition, animal trials have focused on soy isoflavones, daidzein, and genistein but rarely on soy or soy protein. Accordingly, future research on soy and soy protein should be emphasized, especially in animal models of hyperuricemia and gout.
Our findings indicate that the evidence that soy increases serum or plasma uric acid concentrations remains insufficient; moreover, the relationship differs with soy products. Although soy and its active substances are believed to increase serum and plasma uric acid levels and lead to hyperuricemia or gout in Asia, the latest American College of Rheumatology (2020) and European League Against Rheumatism (2016) guidelines do not specify a maximal soy intake (88, 89). Soy products like tofu, bean curd cake, and dried bean curd sticks may be high-quality protein sources for individuals with hyperuricemia or gout. It can be beneficial to nutritionists and healthcare decision-makers reconsider their conceptions about the relationship between soy and uric acid levels according to the latest and further scientific study results.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
YD, MZ, and HL contributed to the conception and design of the study. YD, QQ, and ZL organized the data, assessed all studies for eligibility, and evaluated the risk of bias in all included studies. YD drafted the manuscript. QQ and ZL performed the statistical analyses. MZ and HL reviewed and edited the manuscript. All authors approved the final version of the manuscript.
This study was supported by the 512 Talent Training Project of Bengbu Medical College (BY51201203).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dudek SG. Nutrition essentials for nursing practice. 4th ed. Philadelphia, PA: Lippincott (2001).
3. Huang H, Krishnan HB, Pham Q, Yu LL, Wang TT. Soy and gut microbiota: Interaction and implication for human health. J Agric Food Chem. (2016) 64:8695–709. doi: 10.1021/acs.jafc.6b03725
4. U.S. Food and Drug Administration. Food labeling: Health claims; soy protein and coronary heart disease. Food and drug administration, HHS. Final rule. Fed Regist. (1999) 64:57700–33.
5. Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr. (2008) 138:1244S–9S. doi: 10.1093/jn/138.6.1244S
6. Rizzo G, Baroni L. Soy, soy foods and their role in vegetarian diets. Nutrients. (2018) 10:43. doi: 10.3390/nu10010043
7. Cui X, Dai Q, Tseng M, Shu XO, Gao YT, Zheng W. Dietary patterns and breast cancer risk in the shanghai breast cancer study. Cancer Epidemiol Biomarkers Prev. (2007) 16:1443–8. doi: 10.1158/1055-9965.EPI-07-0059
8. Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. (2006) 55:1–12. doi: 10.1207/s15327914nc5501_1
9. Nagata C. Ecological study of the association between soy product intake and mortality from cancer and heart disease in Japan. Int J Epidemiol. (2000) 29:832–6. doi: 10.1093/ije/29.5.832
10. Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Analyt Technol Biomed Life Sci. (2002) 777:129–38. doi: 10.1016/s1570-0232(02)00342-2
11. Wakai K, Egami I, Kato K, Kawamura T, Tamakoshi A, Lin Y, et al. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. (1999) 33:139–45. doi: 10.1207/S15327914NC330204
12. Mortensen A, Kulling SE, Schwartz H, Rowland I, Ruefer CE, Rimbach G, et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res. (2009) 2:S266–309. doi: 10.1002/mnfr.200800478
13. van Erp-Baart MA, Brants HA, Kiely M, Mulligan A, Turrini A, Sermoneta C, et al. Isoflavone intake in four different European countries: The VENUS approach. Br J Nutr. (2003) 1:S25–30. doi: 10.1079/BJN2002793
14. Horn-Ross PL, Lee M, John EM, Koo J. Sources of phytoestrogen exposure among non-Asian women in California, USA. Cancer Causes Control. (2000) 11:299–302. doi: 10.1023/a:1008968003575
15. de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, et al. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: The Framingham study(1-4). J Nutr. (2001) 131:1826–32. doi: 10.1093/jn/131.6.1826
16. Boker LK, Van der Schouw YT, De Kleijn MJ, Jacques PF, Grobbee DE, Peeters PH. Intake of dietary phytoestrogens by Dutch women. J Nutr. (2002) 132:1319–28. doi: 10.1093/jn/132.6.1319
17. Soyfoods Association of North America. Soyfood sales and trends. Washington, DC: Soyfoods Association of North America (2008).
18. Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. (2007) 120:442–7. doi: 10.1016/j.amjmed.2006.06.040
19. Schlesinger N. Dietary factors and hyperuricaemia. Curr Pharm Des. (2005) 11:4133–8. doi: 10.2174/138161205774913273
20. Rong S, Zou L, Wang Z, Pan H, Yang Y. Purine in common plant food in China. J Hygiene Res. (2012) 41:101. doi: 10.19813/j.cnki.weishengyanjiu.2012.01.022
21. Kaneko K, Kudo Y, Yamanobe T, Mawatari K, Yasuda M, Nakagomi K, et al. Purine contents of soybean-derived foods and selected Japanese vegetables and mushrooms. Nucleosides Nucleotides Nucleic Acids. (2008) 27:628–30. doi: 10.1080/15257770802138681
22. Brulé D, Sarwar G, Savoiet L. Purine content of selected Canadian food products. J Food Compos Anal. (1988) 1:130–8. doi: 10.1016/0889-1575(88)90016-6
23. Walton GM, Gill GN. Preferential regulation of protein synthesis initiation complex formation by purine nucleotides. Biochim Biophys Acta. (1976) 447:11–9. doi: 10.1016/0005-2787(76)90090-3
24. Young VR. Soy protein in relation to human protein and amino acid nutrition. J Am Diet Assoc. (1991) 91:828–35.
25. Hecker KD. Effects of dietary animal and soy protein on cardiovascular disease risk factors. Curr Atheroscler Rep. (2001) 3:471–8. doi: 10.1007/s11883-001-0037-4
26. Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. (2007) 4:72–82. doi: 10.7150/ijms.4.72
27. Nachvak SM, Moradi S, Anjom-Shoae J, Rahmani J, Nasiri M, Maleki V, et al. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: A systematic review and dose-response meta-analysis of prospective cohort studies. J Acad Nutr Diet. (2019) 119:1483.e–500.e. doi: 10.1016/j.jand.2019.04.011
28. Hashimoto R, Sakai A, Murayama M, Ochi A, Abe T, Hirasaka K, et al. Effects of dietary soy protein on skeletal muscle volume and strength in humans with various physical activities. J Med Invest. (2015) 62:177–83. doi: 10.2152/jmi.62.177
29. Cvejić J, Malencić D, Tepavcević V, Posa M, Miladinović J. Determination of phytoestrogen composition in soybean cultivars in Serbia. Nat Prod Commun. (2009) 4:1069–74.
30. Murai Y, Takahashi R, Rodas FR, Kitajima J, Iwashina T. New flavonol triglycosides from the leaves of soybean cultivars. Nat Prod Commun. (2013) 8:453–6.
31. Maria GC, António HP, Miguel PM, Maria TC, Margarida MC, Paula RO, et al. Comparative analysis of over-the-counter tablet preparations of isoflavones extracted from soy available in Portugal. Nat Prod Commun. (2006) 1:973–80.
32. Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein, and their b-glycoside conjugates: Antitumor isoflavones in soybean food from American and Asian diets. J Agric Food Chem. (1993) 41:1961–7.
33. Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol Ther. (2001) 90:157–77. doi: 10.1016/s0163-7258(01)00137-1
34. Clarkson TB. Soy, soy phytoestrogens and cardiovascular disease. J Nutr (2002) 132:566S–9S. doi: 10.1093/jn/132.3.566S
35. Adamopoulos D, Vlassopoulos C, Seitanides B, Contoyiannis P, Vassilopoulos P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol. (1977) 85:198–208. doi: 10.1530/acta.0.0850198
36. Lin CM, Chen CS, Chen CT, Liang YC, Lin JK. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem Biophys Res Commun. (2002) 294:167–72. doi: 10.1016/S0006-291X(02)00442-4
37. Van Hoorn DE, Nijveldt RJ, Van Leeuwen PA, Hofman Z, M’Rabet L, De Bont DB, et al. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur J Pharmacol. (2002) 451:111–8. doi: 10.1016/s0014-2999(02)02192-1
38. Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. (1995) 333:276–82. doi: 10.1056/NEJM199508033330502
39. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
40. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
41. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
42. Higgins JPT, Green S editors. Cochrane handbook for systematic reviews of interventions version 5.0.2. London: The Cochrane Collaboration (2009).
43. Zhang M, Lin L, Liu H. Acute effect of soy and soy products on serum uric acid concentration among healthy Chinese men. Asia Pac J Clin Nutr. (2018) 27:1239–42. doi: 10.6133/apjcn.201811_27(6).0010
44. Yamakita J, Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Higashino K. Effect of Tofu (bean curd) ingestion and on uric acid metabolism in healthy and gouty subjects. Adv Exp Med Biol. (1998) 431:839–42. doi: 10.1007/978-1-4615-5381-6_161
45. Garrel DR, Verdy M, PetitClerc C, Martin C, Brulé D, Hamet P. Milk- and soy-protein ingestion: Acute effect on serum uric acid concentration. Am J Clin Nutr. (1991) 53:665–9. doi: 10.1093/ajcn/53.3.665
46. Berg A, Schaffner D, Pohlmann Y, Baumstark MW, Deibert P, König D, et al. A soy-based supplement alters energy metabolism but not the exercise-induced stress response. Exerc Immunol Rev. (2012) 18:128–41.
47. Chen CY, Bakhiet RM, Hart V, Holtzman G. Isoflavones improve plasma homocysteine status and antioxidant defense system in healthy young men at rest but do not ameliorate oxidative stress induced by 80% VO2pk exercise. Ann Nutr Metab. (2005) 49:33–41. doi: 10.1159/000084175
48. Liu ZM, Ho SC, Chen YM, Ho YP. The effects of isoflavones combined with soy protein on lipid profiles, C-reactive protein and cardiovascular risk among postmenopausal Chinese women. Nutr Metab Cardiovasc Dis. (2012) 22:712–9. doi: 10.1016/j.numecd.2010.11.002
49. Qin Y, Shu F, Zeng Y, Meng X, Wang B, Diao L, et al. Daidzein supplementation decreases serum triglyceride and uric acid concentrations in hypercholesterolemic adults with the effect on triglycerides being greater in those with the GA compared with the GG genotype of ESR-β RsaI. J Nutr. (2014) 144:49–54. doi: 10.3945/jn.113.182725
50. Ye YB, He KY, Li WL, Zhuo SY, Chen YM, Lu W, et al. Effects of daidzein and genistein on markers of cardiovascular disease risk among women with impaired glucose regulation: A double-blind, randomized, placebo-controlled trial. Food Funct. (2021) 12:7997–8006. doi: 10.1039/d1fo00712b
51. Pan WH, Chin CJ, Sheu CT, Lee MH. Hemostatic factors and blood lipids in young Buddhist vegetarians and omnivores. Am J Clin Nutr. (1993) 58:354–9. doi: 10.1093/ajcn/58.3.354
52. Villegas R, Xiang YB, Elasy T, Xu WH, Cai H, Cai Q, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: The Shanghai men’s health study. Nutr Metab Cardiovasc Dis. (2012) 22:409–16. doi: 10.1016/j.numecd.2010.07.012
53. Liu J, Sun LL, He LP, Ling WH, Liu ZM, Chen YM. Soy food consumption, cardiometabolic alterations and carotid intima-media thickness in Chinese adults. Nutr Metab Cardiovasc Dis. (2014) 24:1097–104. doi: 10.1016/j.numecd.2014.04.016
54. Tang SC, Hsiao YP, Ko JL. Genistein protects against ultraviolet B-induced wrinkling and photoinflammation in in vitro and in vivo models. Genes Nutr. (2022) 17:4. doi: 10.1186/s12263-022-00706-x
55. Teng GG, Pan A, Yuan JM, Koh WP. Food sources of protein and risk of incident gout in the Singapore Chinese health study. Arthritis Rheumatol. (2015) 67:1933–42. doi: 10.1002/art.39115
56. Yu KH, See LC, Huang YC, Yang CH, Sun JH. Dietary factors associated with hyperuricemia in adults. Semin Arthritis Rheum. (2008) 37:243–50. doi: 10.1016/j.semarthrit.2007.04.007
57. Liu B, Qin L, Liu A, Uchiyama S, Ueno T, Li X, et al. Prevalence of the equol-producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. J Epidemiol. (2010) 20:377–84. doi: 10.2188/jea.je20090185
58. Bhathena SJ, Ali AA, Mohamed AI, Hansen CT, Velasquez MT. Differential effects of dietary flaxseed protein and soy protein on plasma triglyceride and uric acid levels in animal models. J Nutr Biochem. (2002) 13:684–9. doi: 10.1016/s0955-2863(02)00227-9
59. Cui B. Effects of Soy protein on Hyperuricemia rat and Metabolic Mechanism. Tianjin: Tianjin Medical University (2016).
60. Palanisamy N, Viswanathan P, Anuradha CV. Effect of genistein, a soy isoflavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren Fail. (2008) 30:645–54. doi: 10.1080/08860220802134532
61. Karale S, Kamath JV. Protective role of daidzein against cyclophosphamide induced nephrotoxicity in experimental rats. Int J Pharm Pharm Sci. (2017) 9: 103–7.
62. Huang J, Wang S, Zhu M, Chen J, Zhu X. Effects of genistein, apigenin, quercetin, rutin and astilbin on serum uric acid levels and xanthine oxidase activities in normal and hyperuricemic mice. Food Chem Toxicol. (2011) 49:1943–7. doi: 10.1016/j.fct.2011.04.029
63. Huang J, Wang S, Zhu M. Studies on anti-inflammatory, analgesic and uric acid lowering effects of Genistein. Northwest Pharmaceutical J. (2011) 26:193–5.
64. Zhao F, Huang Y, Cao J, Che X. Effects of daidzein on growthhormone and physio-blochemical parameters in laying hens. J Nuclear Agric Sci. (2008) 22:912.
65. Meng T, Han Z, Wang G. Effects of diet supplemented with daidzein on several physlological-biochemical parameters in laying hens. Chin J Anim Nutr. (2004) 16:30–2. doi: 10.3382/ps/pew483
66. Wang L, Wang G, Dai J, Wang L, Meng Q. Influence of soybean isoflavones on meat quality and production performance of the broilers. Feed Industry. (2006) 27:30–1.
67. Messina M, Messina VL, Chan P. Soyfoods, hyperuricemia and gout: A review of the epidemiologic and clinical data. Asia Pac J Clin Nutr. (2011) 20: 347–58.
68. Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British society for rheumatology and British health professionals in rheumatology guideline for the management of gout. Rheumatology. (2007) 46:1372–4. doi: 10.1093/rheumatology/kem056a
69. Dalbeth N, Wong S, Gamble GD, Horne A, Mason B, Pool B, et al. Acute effect of milk on serum urate concentrations: A randomised controlled crossover trial. Ann Rheum Dis. (2010) 69:1677–82. doi: 10.1136/ard.2009.124230
70. Serrano JC, Martín-Gari M, Cassanye A, Granado-Serrano AB, Portero-Otín M. Characterization of the post-prandial insulinemic response and low glycaemic index of a soy beverage. PLoS One. (2017) 12:e0182762.
71. Zhang M, Gao Y, Wang X, Liu W, Zhang Y, Huang G. Comparison of the effect of high fruit and soybean products diet and standard diet interventions on serum uric acid in asymptomatic hyperuricemia adults: An open randomized controlled trial. Int J Food Sci Nutr. (2016) 67:335–43. doi: 10.3109/09637486.2016.1153608
72. Dubchak N, Falasca GF. New and improved strategies for the treatment of gout. Int J Nephrol Renovasc Dis. (2010) 3:145–66. doi: 10.2147/IJNRD.S6048
73. Guo H. Specialized ribosomes and the control of translation. Biochem Soc Trans. (2018) 46:855–69. doi: 10.1042/BST20160426
74. Rong S, Zou L, Cui X, Li M, Pan H, Yang Y. Assessment of dietary purine intake in Chinese residents. Acta Nutr Sin. (2015) 37:234. doi: 10.13325/j.cnki.acta.nutr.sin.2015.03.007
75. Ramallo IA, Zacchino SA, Furlan RL. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. Phytochem Anal. (2006) 17:15–9. doi: 10.1002/pca.874
76. Shi Y, Nue X, Zhang B, Lu X. Discussion on the content of purine in traditional soybean products and soybean protein. China Oils Fats. (2020) 45:61–6.
77. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. (1997) 138:863–70.
78. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. (1998) 139:4252–63. doi: 10.1210/endo.139.10.6216
79. Cheng X, Klaassen CD. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos. (2009) 37:2178–85. doi: 10.1124/dmd.109.027177
80. Budhiraja R, Kayyali US, Karamsetty M, Fogel M, Hill NS, Chalkley R, et al. Estrogen modulates xanthine dehydrogenase/xanthine oxidase activity by a receptor-independent mechanism. Antioxid Redox Signal. (2003) 5:705–11. doi: 10.1089/152308603770380007
81. van der Velpen V, Hollman PC, van Nielen M, Schouten EG, Mensink M, Van’t Veer P, et al. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur J Clin Nutr. (2014) 68:1141–7. doi: 10.1038/ejcn.2014.108
82. Ma YR. Effect of heat treatment and in vitro gastrointestinal digestion on the bioactive compounds contents and antioxidant activity of soymilk. GuangDong: South China University of Technology (2014).
83. Zhao S, Jiang L, Wang D, Sui X, Zhou L, Fan Z. The effect mechanism of EGCG on the structure of soybean protein. Food Sci. (2012) 12:67–75.
84. Wang N, Wu C, Li Y, Teng F. Effect of high pressure homogenization on soybean protein isolate-soybean isoflavone interaction and functional properties of their complex. Food Sci. (2020) 41:146–53.
85. Yang J. Preparation of soy protein ingredients for special populations and evaluation of nutritional benefits. GuangDong: South China University of Technology (2015).
86. Yan L, Ye L, Wang K, Zhou J, Zhu C. [Atorvastatin improves reflow after percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction by decreasing serum uric acid level]. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2016) 45:530–5. doi: 10.3785/j.issn.1008-9292.2016.09.12
87. Li K, Wei L, Han Z, Xiong H, Zhang F, Liu X, et al. Comparative pharmacokinetic study of hesperetin after oral administration in normal and hyperuricemia rats by UPLC-MS/MS. Curr Rev Clin Exp Pharmacol. (2021) 16:155–61. doi: 10.2174/1574884715666200702120521
88. FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020 American college of rheumatology guideline for the management of gout. Arthritis Rheumatol. (2020) 72:879–95.
Keywords: animal trials, clinical studies, meta-analysis, soy, systematic review, uric acid
Citation: Duan Y, Qi Q, Liu Z, Zhang M and Liu H (2022) Soy consumption and serum uric acid levels: A systematic review and meta-analysis. Front. Nutr. 9:975718. doi: 10.3389/fnut.2022.975718
Received: 22 June 2022; Accepted: 09 August 2022;
Published: 02 September 2022.
Edited by:
Mauro Lombardo, San Raffaele Telematic University, ItalyReviewed by:
Ceren Gezer, Eastern Mediterranean University, TurkeyCopyright © 2022 Duan, Qi, Liu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaqing Liu, bGhxYmJtY0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.