- 1Digital Health Research Division, Korea Institute of Oriental Medicine, Daejeon, South Korea
- 2School of Korean Convergence Medical Science, University of Science and Technology, Daejeon, South Korea
- 3Gwangju Alzheimer’s Disease and Related Dementias (GARD) Cohort Research Center, Chosun University, Gwangju, South Korea
- 4Department of Biomedical Science, Chosun University, Gwangju, South Korea
- 5Dementia Research Group, Korea Brain Research Institute, Daegu, South Korea
Objective: This study examined the association of whole-body composition and segmental bioimpedance variables with cold pattern (CP) in different sexes.
Methods: We assigned 667 older individuals to a CP group (n = 488) and a non-CP group (n = 179) by using an eight-item self-administered questionnaire. Seven body composition variables and three pairs of segmental bioimpedance variables for the upper and lower extremities, which were obtained from a segmental multifrequency bioimpedance analyzer, were employed to investigate their association with CP. Participants’ characteristics were first described. Then we compared the selected body composition and bioimpedance variables between the CP and non-CP groups. Finally, their association with CP was investigated using univariate and multivariate regression analyses. All analyses were performed separately for women and men.
Results: Both women and men exhibited a comparable mean age in the CP and non-CP groups; however, women with CP had significantly lower blood pressures, whereas men with CP showed a higher proportion of osteoarthritis than those without CP. Compared with the non-CP group, individuals with CP exhibited significantly smaller body sizes indicated by shorter height and smaller weight, lower body mass index, and smaller volume-to-body surface area ratio in both sexes. After controlling for age, height, weight, and other covariates, we found significant reductions in body lean mass such as fat-free mass and body cell mass, basal metabolic rate per unit mass, total body water, and intra-to-extracellular water ratio in the CP group. With regard to segmental bioimpedance analysis, the resistance ratios and phase angles in the upper and lower extremities yield significant associations with CP incidence, as demonstrated by the odds ratio (95% confidence interval) of 1.72 (1.16–2.57), 1.69 (1.18–2.48), 0.60 (0.40–0.89), and 0.57 (0.39–0.82), respectively. However, these results did not emerge in men.
Conclusion: Abnormal cellular water distribution and deterioration in body cell mass and/or cell strength are associated with CP prevalence, regardless of age, height, weight. These findings are similar in the upper and lower extremities and are more pronounced in women. The abovementioned patterns may be considered effective indicators for identifying CP in the older adult population.
Introduction
Cold pattern (CP) is a medical term commonly used to describe cold sensitive symptoms, including aversion to cold, feeling of cold on the extremities or body, feeling comfortable in warm conditions, having a pale face and colorless urine, and relevant characteristics of pulse and tongue (1). These cold hypersensitive symptoms are common in the general population as shown by its prevalence that reaches up to 12%, according to a community-based survey (2) and 60% in the Korean and Japanese populations (3, 4).
From traditional medicine perspective, CP is one of the two fundamental principles that are related to the nature of diseases that manifest from yin-yang transformations (5) and is an important diagnostic factor for several diseases (6). In fact, previous studies reported a number of serious chronic diseases in older adults that have been linked to CP or cold hypersensitivity. For instance, Raynaud’s disease, hypotension, chronic gastritis, and diabetes mellitus are associated with cold hypersensitivity in the hands and feet (7, 8). Women with cold hypersensitivity in their extremities may experience shoulder stiffness, fatigue, low back pain, and headache (9). Additionally, individuals with cold hypersensitivity may encounter many other diseases such as cold-related injuries, rheumatic diseases, nerve injuries, migraines, and vascular diseases, as described in the general population of northern Sweden (10). Noteworthy, individuals with CP have a significantly lower quality of life, as evidenced by significantly lower European Quality of Life Five Dimension indices (11) and poorer sleep quality (12) than those without CP. As it is related to various medical problems, CP has been used to identify pathological patterns by nearly 85% of members of the Association of Korean Medicine when prescribing herbal treatment (13).
From modern medicine perspective, these symptoms reflect the subjective thermal sensations (e.g., feeling cold), thermoregulation (e.g., pale face due to peripheral vasoconstriction), and behavioral adjustments (e.g., seeking warmth) of a person when subjected to a given climatic stimulus (14). The human body is warmed and powered up during various metabolic reactions, which extract energy from chemical bonds in food molecules to maintain homeostasis (15). Figure 1 briefly explains the thermoregulatory mechanisms in the human body. The metabolic rate is therefore related to thermoregulation, in such a way that enhanced thermogenesis results in an elevated basal metabolic rate (16). Indeed, the metabolic rate at rest is considered a potential parameter that has a relationship with CP as it is negatively associated with CP regardless of age, sex, and fat-free mass (FFM) (17).
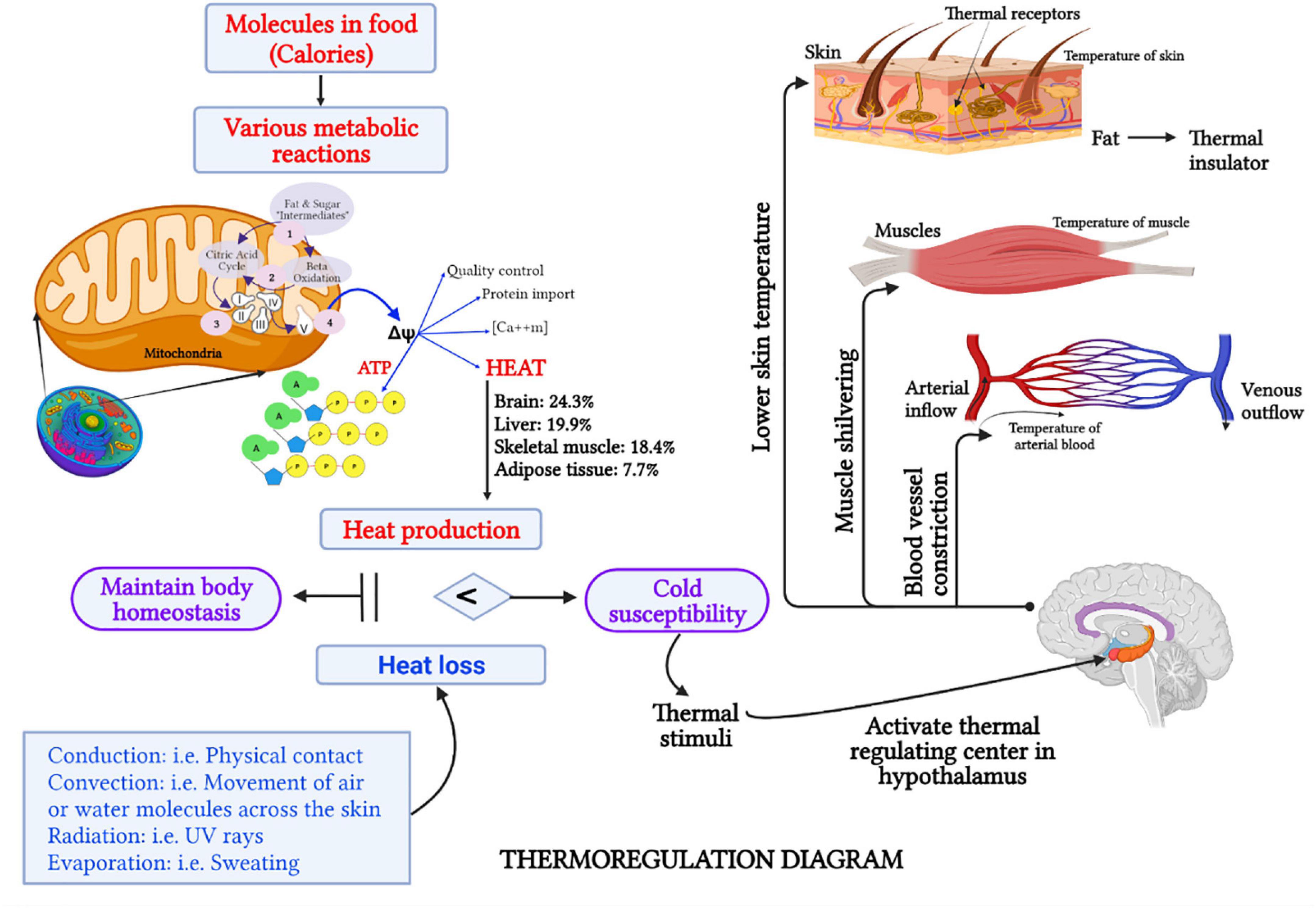
Figure 1. Schematic representation (created with BioRender.com) of the thermoregulation mechanism in the human body. Intracellular mitochondria play a critical role in generating energy (in the form of ATP) and heat, which is sufficient for a cell’s sustenance (52). Healthy cellular mitochondria in the brain, liver, and skeletal muscle mass contribute as much as 63% of the body heat (58). When heat production and heat loss are balanced, human body homeostasis is maintained. However, when heat production is insufficient to compensate heat loss, which occurs via conduction, convection, radiation, or evaporation, the human body is likely to be susceptible to cold (85, 86). For a given thermal stimuli, such as cold wind, the thermoreceptors which are free nerve endings in the skin and other organs will send a signal to the hypothalamus to activate the heat-regulating center (87). Consequently, several responses, such as lowering of skin temperature, shivering of skeletal muscle to produce more heat, and vasoconstriction to prevent heat loss, will take place (35). Therefore, individuals with CP usually experience cold sensations in their hands or feet, have a pale face, and seek a warmer environment.
In relation to the resting metabolic rate, the other body composition variables such as body weight, body mass index (BMI), skeletal muscle mass, intracellular water (ICM), extracellular water (ECM), and body water ratios have been investigated in relations with CP or cold hypersensitivity (18–20, 21). These previous studies found a significant reduction in BMI and skeletal muscle mass in individuals with CP when compared with the heat pattern or warm sensation group (18, 19). Additionally, Mun and colleagues suggested that sex and BMI might be key features when predicting CP in an aging population (21). Nevertheless, these variables cannot reflect changes at the cellular level such as the cellular hydration, cell integrity, or cell membrane strength, which are essential information of cellular health status.
Evidently, information on body cellular health and its hydration status can be demonstrated based on resistance (R), reactance (Xc), and phase-angle (PA) variables obtained from the bioelectrical impedance analysis (BIA) technique. This technique, which is simple, portable, and inexpensive, can be used effectively to evaluate R, Xc, and PA and estimate body composition variables (22). The BIA assumes that the human body is a homogenous conductive cylinder and its impedance quotient has an empirical relationship with the volume of electrolyte water contained in the FFM (23, 24). R is used as a measure of resistivity and is inversely related to the amount of body water (23). Since low frequency (i.e., 5 kHz) current cannot penetrate the cell membrane while high frequency (i.e., 250 kHz) can pass through the cellular environment, R reflects the ECW volume when measured at a low-frequency and represents the total body fluid when measured at a high-frequency current (24, 25). Accordingly, the ratio between a high- and low-frequency R implies the water ratio between the total body and ECW volumes (26, 27). Xc, the capacitance initiated by the cell membranes, indicates the cell mass, volume, or strength. PA is another parameter that can be calculated based on R and Xc, which represents the phase shift between voltage and current and indicates the ability of the cell membrane to hold charges (23). A reduction in the Xc and PA suggests a lower body cell mass (BCM) or function, whereas a reduction in R implies a relative increase in body fluids and/or FFM with regard to fat components (24, 28).
In addition, segmental BIA evaluates the body segmentally by considering the arms, legs, and trunk as five separate cylinders, and provides R, Xc, and PA measurements for each segment independently (Inbody S10 User’s Manual) (29). Previous studies on various diseases among older people suggested different relations with segmental bioimpedance variables between the upper and lower extremities (30, 31). Therefore, examining the segmental R, Xc, and PA in the upper and lower extremities may provide an insight as to how these values alter in persons with prolonged CP or cold hypersensitivity symptoms, especially in the hands and feet. Furthermore, early detection of CP in individuals based on these variables may enable a prompt and effective treatment to prevent the progression of the related diseases (7–10).
However, to our knowledge, only one relevant study in the community of Jeju Haenyeo has investigated and reported a significant association between the whole-body PA and non-CP with an odds ratio (OR) and 95% confidence interval (CI) of 2.40 (1.16–4.97) (11). Thus, the changes in these body compositions as well as bioimpedance variables were not well-investigated. Consequently, more studies are needed on the association of the body composition and bioimpedance variables with the risk of CP. In this study, we primarily aimed to examine the association between CP and several body composition and bioimpedance variables of the body segments as well as the whole body. Particularly, we explored these associations for different sexes, while considering age and potential comorbidities. We hypothesized that individuals with CP might have lower values in the BCM and/or cell strength as indicated by lower Xc and/or PA variables as well as changes in their cellular hydration status.
Materials and methods
Participants
A total of 784 participants (age 55–90 years) were recruited at Chonnam University Hospital (Gwangju City, Republic of Korea). Individuals were excluded from the study if they met one of the following criteria: had obtained less than 3 years of education; had a medical history or ongoing acute or chronic illness that interfered with the intended study design such as neurological diseases, infections, or mental health instability; had a cognitive disorder; or had an abnormal skin condition at the location of the measurement probe. In addition to a general medical examination, the participants underwent a precise clinical assessment to obtain information on demographics and medical history. In the data preprocessing, participants who had non-random missing data (n = 18) and extreme results (sparse data points above or below three times the interquartile range were considered extreme points) for any parameter (n = 8) were excluded from the analysis. According to the principle of multifrequency bioelectrical impedance analysis, correct impedance measurements should have lower values for higher frequencies (i.e., impedance at 1 kHz is greater than impedance at 5 kHz); therefore, observations that did not follow this pattern were excluded (n = 91) (31). All of the participants answered the cold heat pattern questionnaire and were accordingly classified into the CP or non-CP groups. Finally, 667 participants, including 488 CP and 179 non-CP individuals, were included in the final analysis (Figure 2). The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (approval number: CNUH-2019-279). Written informed consent was obtained from all participants. This study was performed in accordance with the principles of the Declaration of Helsinki.
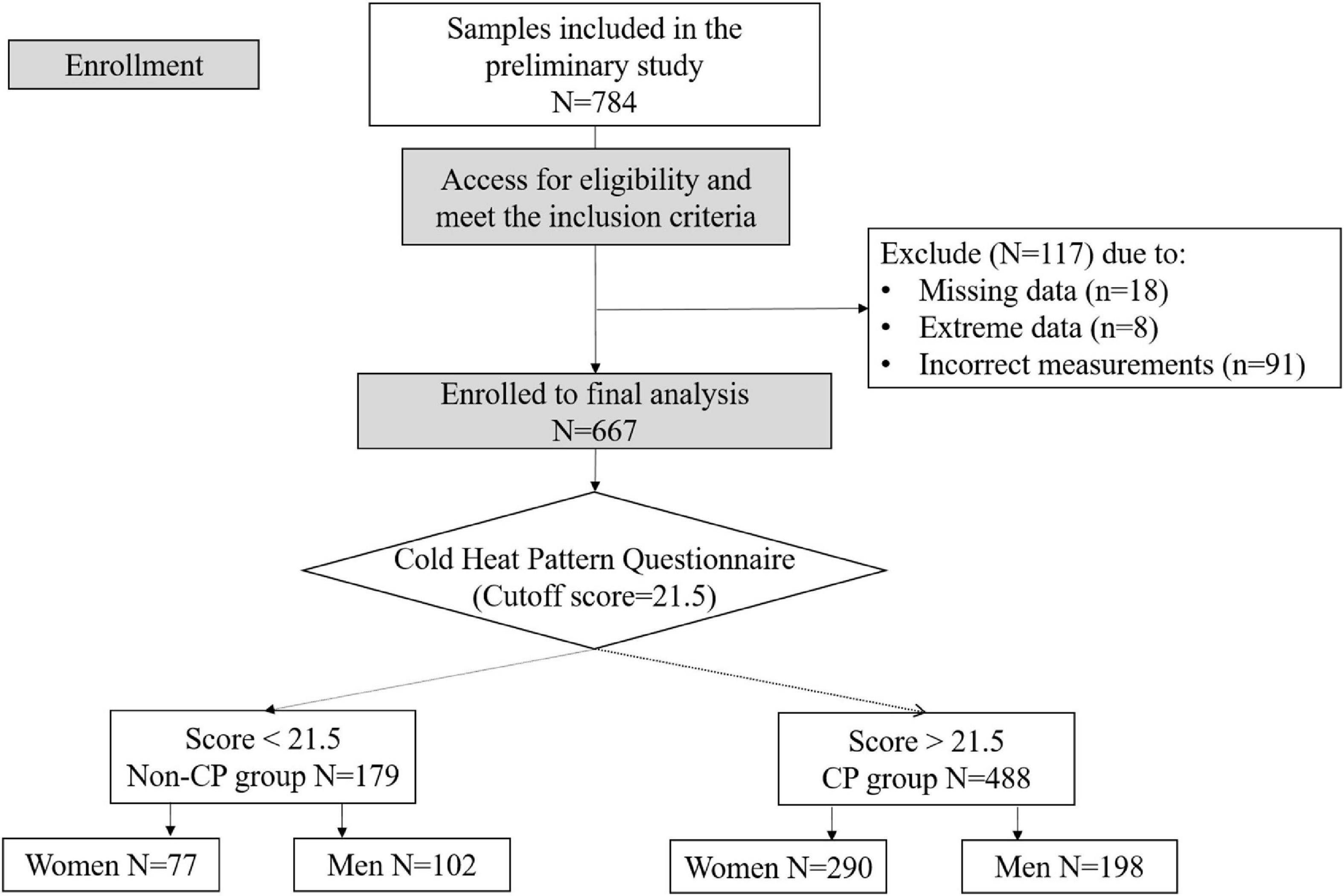
Figure 2. Consolidated standards of reporting trials (CONSORT) diagram illustrating enrollment and exclusion criteria for this study.
Body composition, bioimpedance measurement, and data selection
A segmental multi-frequency BIA device was utilized to measure whole-body composition and segmental bioimpedance variables in this study (Inbody S10, South Korea) (29). The device uses a tetrapolar 8-point tactile electrode to compute segmental impedance at six electrical frequencies (1, 5, 50, 250, 500, and 1,000 kHz) and Xc and PA at three frequencies (5, 50, and 250 kHz). These impedance values were then used to estimate seven whole-body composition variables, including fat mass (FM), FFM, BCM, basal metabolic rate per unit mass (BMR_m), ICW, ECW, and ICW_ECW ratio, which were used in this study. According to the formula of Robert (32), BMR_m was calculated as the BMR divided by the fourth root of the body weight (32). Additionally, body volume per unit of surface area was included as a feature of heat diffusion or conduction in considering the body size (33) and was computed using the Sendroy equations (34) (Supplementary Table 1).
For the anthropometric measurements, height (cm) and weight (kg) were automatically measured using the same BIA device to the nearest 1 mm and 100 g, respectively. BMI was then calculated (kg/cm2). With regard to segmental bioimpedance variables, the R, Xc, and PA were measured separately for each body segment, including the two arms, two legs, and trunk. As peripheral regions such as the arm and leg have appreciable variances, whereas the vital central organs are strongly resisted by thermoregulatory mechanisms (35), the segmental variables for the body trunk were ignored in the analysis. Using these extremity bioimpedance variables, we computed the upper (lower) extremities variables by averaging the corresponding variables in the right and left arms (legs) as suggested in our previous publication (31). In this study, two R ratio variables for the upper and lower extremities (i.e., Ri_Re_upper and Ri_Re_lower) were computed such that the R ratio was the difference between the R at 250 kHz and that at 5 kHz divided by the R at 5 kHz (Supplementary Table 1) (26, 36). Xc (Xc_upper, Xc_lower) and PA (PA_upper, PA_lower) variables in the upper and lower extremities were selected at 50 kHz of frequency. All measurements were performed in the supine position by well-trained staff who followed the instructions in the InBody S10 Manual (InBody S10, South Korea).
Cold heat pattern questionnaire for the cold pattern
A list of eight questions related to cold syndromes was utilized to recognize CP and non-CP as described in Table 1. These eight questions were derived from the cold heat pattern questionnaire for the classification of cold group and the details can be found here (37). Each question has the lowest score of one and the highest score of five; hence, a maximum of forty points can be obtained. The cutoff score of 21.5 accurately predicted people with CP from non-CP participants with an area under the curve of 0.93 (sensitivity = 0.94, specificity = 0.80) and resulted in an agreement with experts’ diagnosis of up to 87.1% (38). Thus, this cutoff value was used as the threshold to classify individuals into either CP or non-CP groups.
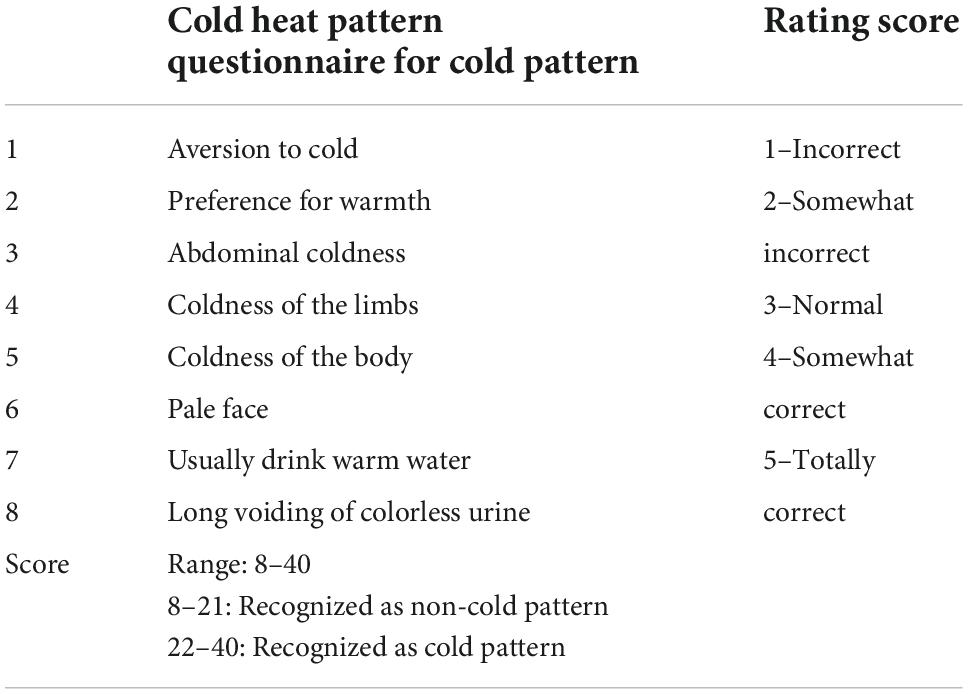
Table 1. Description of the cold heat pattern questionnaire for cold pattern which was used in this study.
Covariates
Several factors were considered as covariates in the multivariate regression analysis model in this study. First, age can influence the change in body composition (39) and the thermoregulatory responses (40–42). For instance, previous studies reported a relation between aging and body composition changes such as increasing body FM in tandem with decreasing lean mass and bone mineral density in older adults (43–45). Furthermore, older adults are unlikely to sense and respond to thermal imbalance appropriately compared to younger individuals (46). Therefore, age was included as a potential covariate in the logistic regression models. Second, previous studies reported significant relationship between educational levels and the changes in body composition, such that highly educated people tend to have lower BMI and FM compared to those with lower level of education (47, 48). To minimize the influence of educational levels since it shows significant difference between CP and non-CP groups, educational years was added as another covariate. Moreover, comorbidities that were significantly different between the two groups were included as additional covariates in the adjustment model and comprise systolic blood pressure, diastolic blood pressure, and osteoarthritis. In the second adjusted model in section “Relationship between the selected bioelectrical impedance analysis variables and cold patterns in women and men,” we considered height and weight as two additional covariates to lessen the difference between the body sizes when examining the associations between CP and the whole-body composition and segmental bioimpedance variables.
Statistical analysis
Information on the participants’ demographics and body composition parameters are summarized as the mean and standard deviation (SD) and median and range (from minimum to maximum values) for continuous variables, and as frequency and proportions for categorical variables in the CP and non-CP groups. As women are more susceptible to CP (49) and have relatively higher FM in tandem with lower FFM than men (50), this study examined the changes in the body composition and impedance variables in association with the CP for women and men separately. A univariate independent two-sample t-test was used to compare the continuous variables between the two groups and Pearson’s chi-square test or Fisher’s exact test was used to check the independence of each categorical variable with CP and non-CP. Cohen’s d test was employed to examine the effect size of differences of each continuous variable between CP and non-CP groups.
Univariate and multivariate regression analyses were applied to investigate the association between CP and body composition variables using the estimated ORs and its 95% CI. Three models were constructed before and after controlling for covariates, including the crude model, first adjusted model, and the second adjusted model. In the crude model, no covariate was adjusted for. Age, systolic blood pressure, diastolic blood pressure, educational years, and osteoarthritis were included as covariates in the first adjusted model. The second adjusted model controlled for those in the first adjusted model, as well as height and weight. All of the continuous parameters were standardized to a mean of zero and a standard deviation (SD) of one before applying these regression analyses. Finally, a two-sample z-test was used to compare these associations between women and men. A p-value of less than 0.05 was considered statistically significant. R version 4.1.2 (The R Project for Statistical Computing; available at: http://www.r-project.org/) was used for all statistical analyses.
Results
Participant characteristics
Table 2 describes participants’ demographic characteristics, anthropometric measurements, and relevant comorbidities. There was a greater proportion of women in the CP group [290/(290+198) = 59.4%] than in the non-CP group [77/(77+102) = 44.3%]. In women, the systolic and diastolic blood pressures were significantly lower in the CP group than in the non-CP group. Among men, the CP group had a higher proportion of osteoarthritis and more years of education than those in the non-CP group. There was no difference in the mean age between the two groups for both women and men. In terms of the anthropometric measurements, with the exception of height, both CP women and men exhibited similar results of significantly lower BMI and less weight. Interestingly, the CP participants exhibited a significantly lower body volume per unit surface area compared to those with non-CP, with mean differences of approximately 1.1 L/m2 in both women and men, respectively.
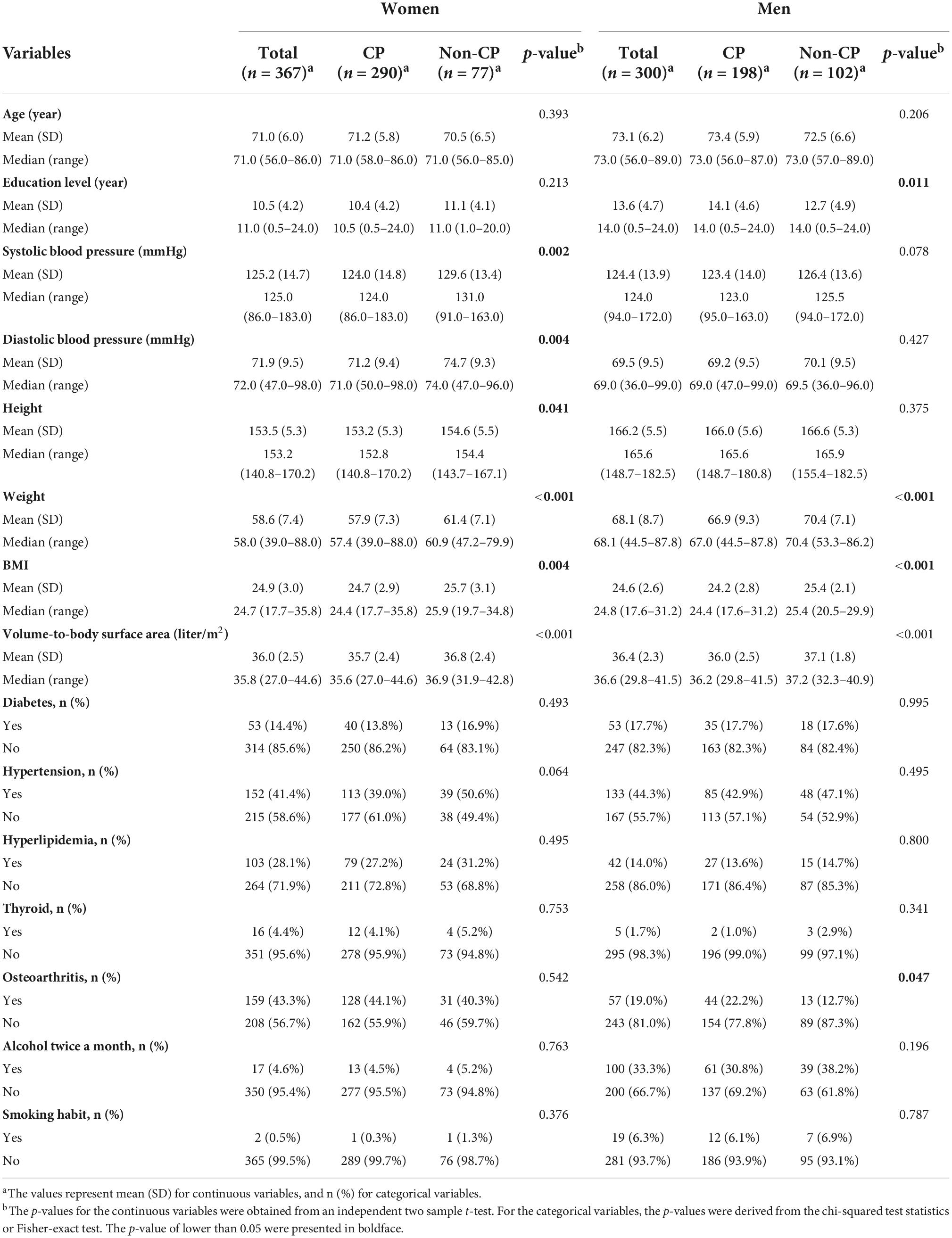
Table 2. Demographic information, anthropometric measurements, and the relevant comorbidities in women and men.
Descriptions of the selected bioelectrical impedance analysis variables in women and men
Figure 3 shows the distributions and comparisons of whole-body composition and segmental bioimpedance variables in the CP and non-CP groups in women and men. In women, for the body composition variables, the CP group demonstrated a significantly lower body lean mass as indicated by the lower values of FFM and BCM compared to those of the non-CP participants. Accordingly, the water volume in the extracellular and intracellular spaces was reduced in the CP group compared with that in the non-CP group. Interestingly, BMR_m was lower in the CP group than in the non-CP group. The differences were prominent in these variables as the effect sizes, indicated by Cohen’s d results, ranged from −0.50 to −0.64. Moreover, ICW_ECW was significantly lower in the CP group than in the non-CP group with a moderate difference of Cohen’s d of −0.36. FM did not significantly differ between the two groups. The abovementioned patterns were similar in women and men except with BMR_m, ICW_ECW, and FM. In men, the effect sizes in FFM, BCM, ICW, and ECW were relatively smaller compared to those in women with Cohen’s d, ranging from −0.26 to −0.29. Nevertheless, men with CP did not show differences in BMR_m and ICW_ECW compared to those in the non-CP group, whereas FM was significantly lower in individuals with CP than in non-CP individuals (d = −0.38).
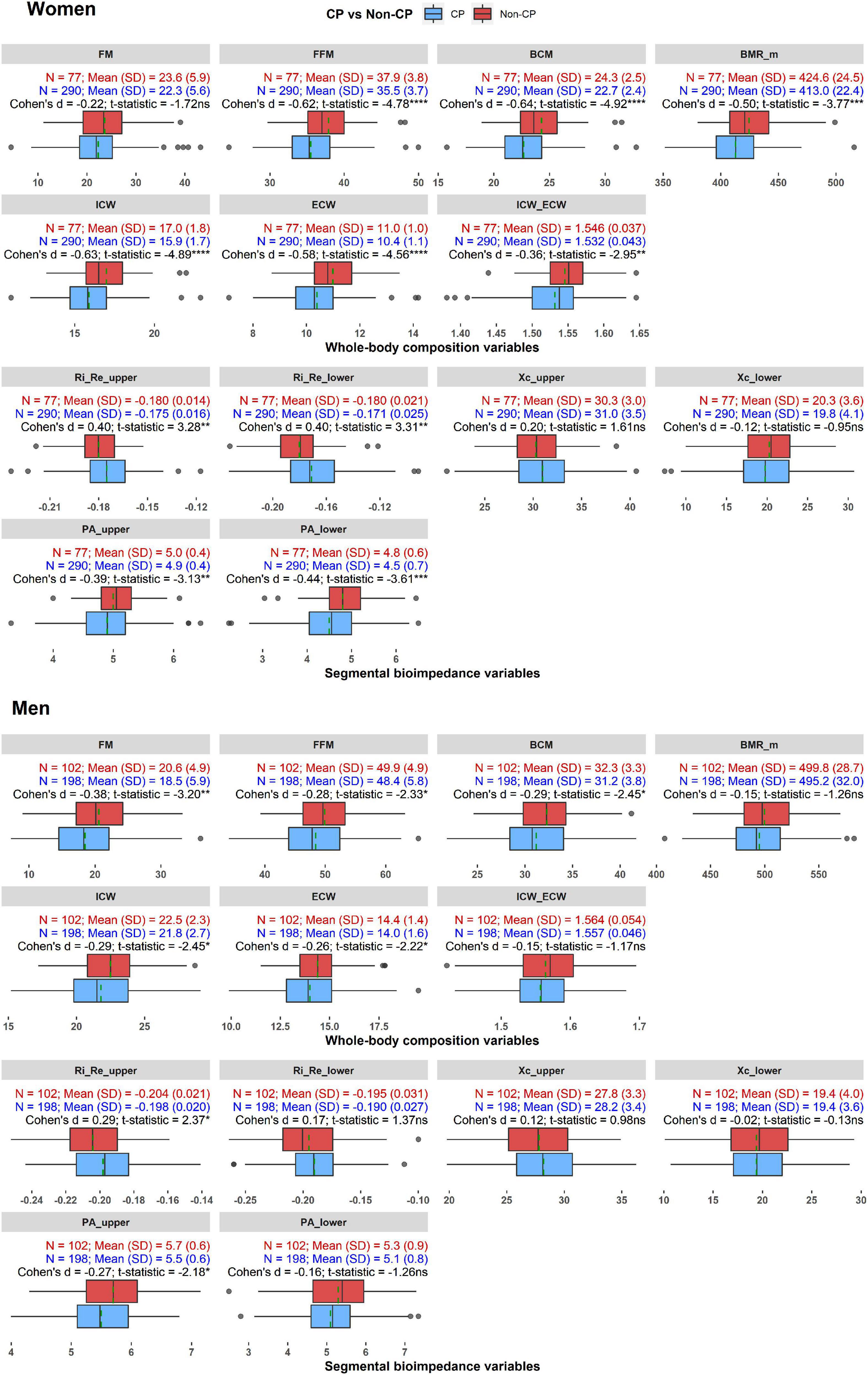
Figure 3. The distributions and comparisons of whole-body composition and segmental bioimpedance variables in the CP and non-CP groups in women and men. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant. Dashed green line indicates mean value of each variable in each group.
With regard to the segmental bioimpedance variables, R ratios and PAs in the upper or lower extremities differed significantly between women with and without CP. Particularly, PA_upper and PA_lower were lower (d = −0.39 and −0.44, respectively) while Ri_Re_upper and Ri_Re_lower were higher in the CP group compared to the non-CP group (d = 0.40). In men, only Ri_Re_upper and PA_upper differed significantly between the CP and non-CP groups (d = 0.29 and −0.27, respectively).
Comprehensive information on the associations depicted in Figure 2 is provided in Supplementary Table 2.
Relationship between the selected bioelectrical impedance analysis variables and cold patterns in women and men
The associations between the selected BIA variables and CP were investigated separately in women and men (Table 3).
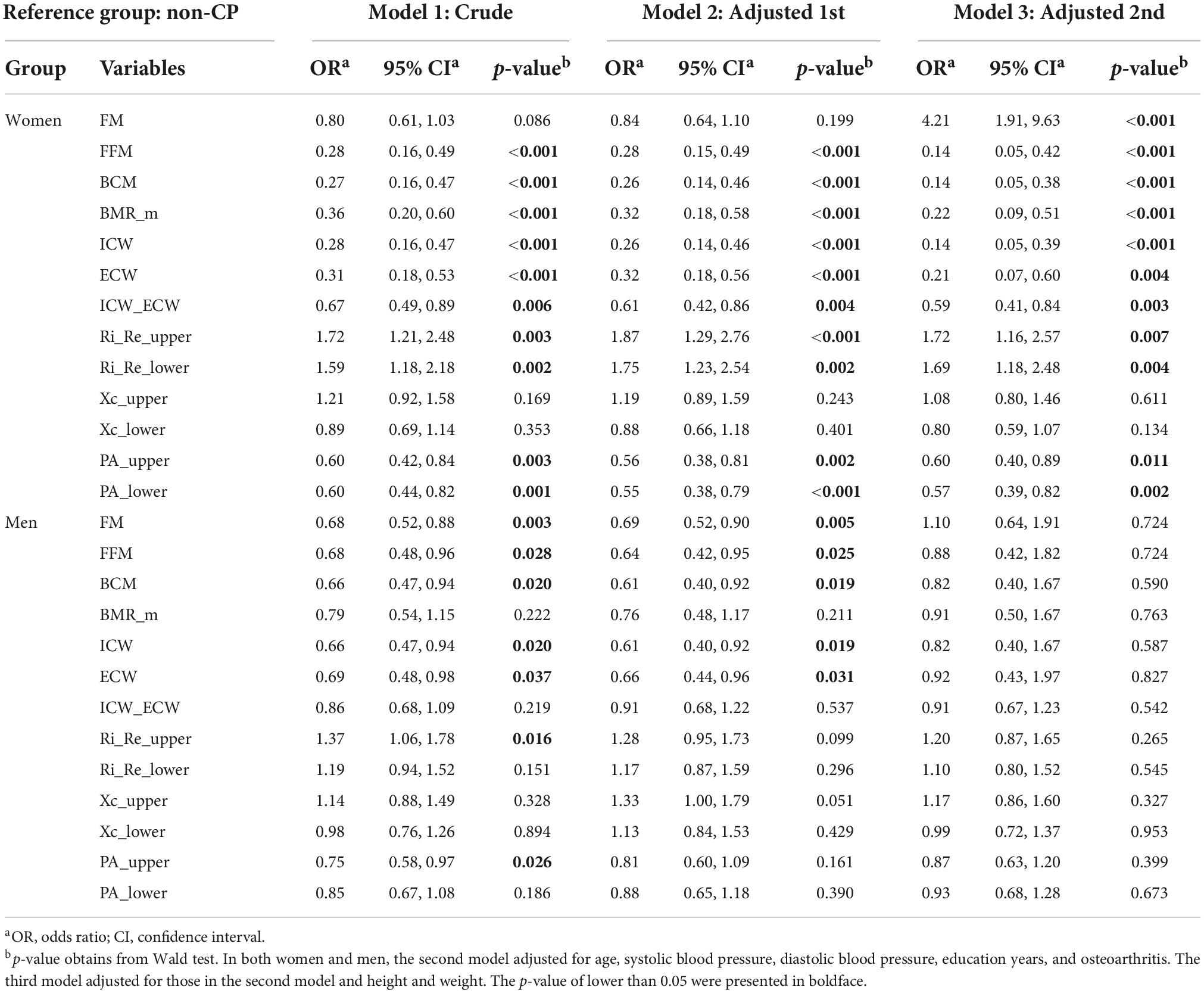
Table 3. Estimated odds ratios and their 95% confidence intervals derived from three logistic regression models in women and men.
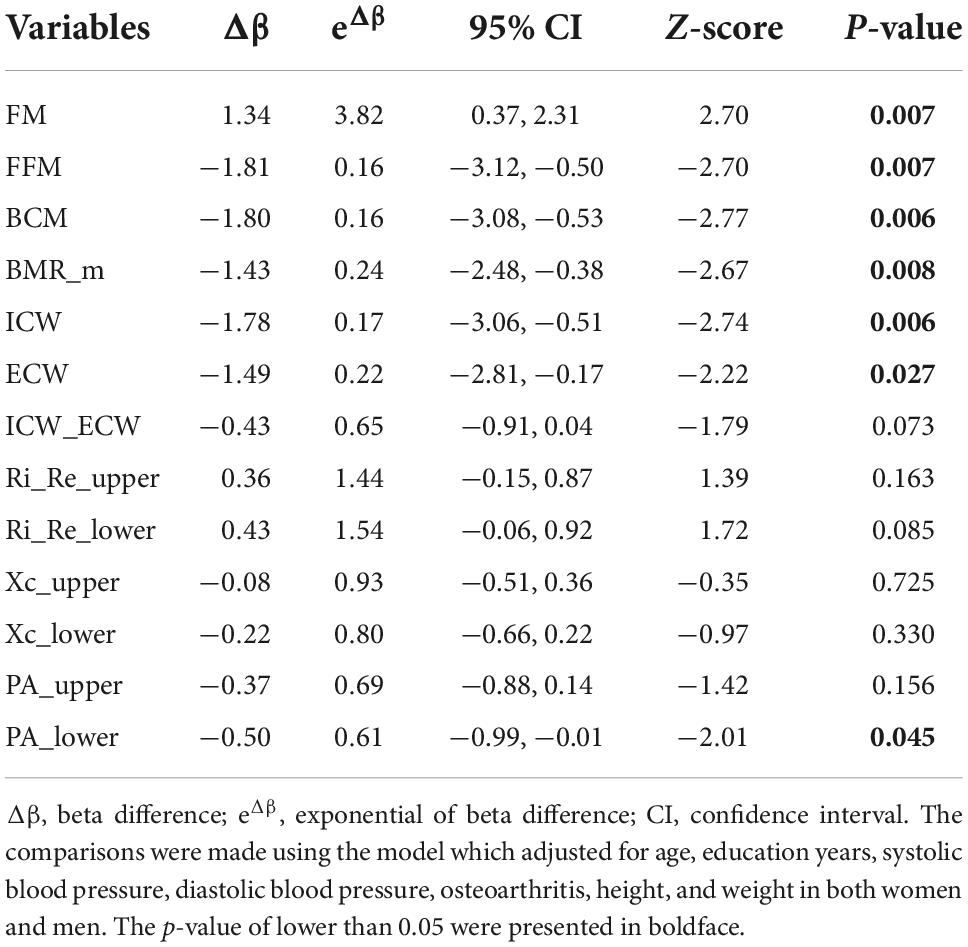
In women, regarding the whole-body composition variables, FFM, BCM, BMR_m, ICW, ECW, and ICW_ECW exhibited negative associations with CP before the covariates were controlled for, with OR (95% CI) ranging from 0.27 to 0.67 (0.16–0.89). One unit decrease in one of these variables corresponded to 33–73% increase the odds of CP. After adjusting for age and comorbidities, these associations remained significant at approximately the same magnitudes. Additionally, when including height and weight in the third model, the associated magnitudes achieved even greater values of OR (95% CI) and range from 0.14 to 0.59 (0.05–0.84). Furthermore, FM, which was not significantly associated with CP in the crude and first adjusted models, was positively associated with CP with OR (95% CI) of 4.21 (1.91–9.63). Among the segmental bioimpedance variables, the R ratios and PAs in the upper and lower extremities were significantly associated with CP in all three models, regardless of age, height, weight, and the other covariates. Particularly, in the last model, PA_upper and PA_lower produced negative associations with CP, with ORs (95% CI) of 0.60 (0.40–0.89) and 0.57 (0.39–0.82), Ri_Re_upper and Ri_Re_lower yielded positive associations with CP, with ORs (95% CI) of 1.72 (1.16–2.57) and 1.69 (1.18–2.48), respectively.
In men, before adjusting for the covariates, FM, FFM, BCM, ICW, and ECW showed approximately similar negative associations with CP, with ORs (95% CI) ranging from 0.66 to 0.69 (0.47–0.98). One unit decrease in one of these variables corresponded to 31–34% increase the odds of having CP. When taking age and other covariates into account in the second model, these associations remained significant at a slightly greater magnitude of OR. However, none of them showed a statistically significant association with CP after incorporating height and weight in the last model. For the segmental bioimpedance variables, only Ri_Re_upper and PA_upper were shown to be linked to CP in the crude model. After controlling for covariates in the second and the last model, all the segmental variables failed to produce an association with CP.
Comparison of these associations between women and men
Differences in the associations with CP were reported between women and men (Table 4). Among the whole-body composition variables, except for ICW_ECW, all showed significantly stronger associations with CP in women than in men. Specifically, one unit increase in FM corresponded with a 282% increased odd of having CP in women with respect to men; whereas one unit decrease in either FFM, BCM, BMR_m, ICW, or ECW corresponded with 76–84% increased odd of having CP in women with respect to men. Most of segmental bioimpedance variables showed insignificant differences between women and men in their associations with CP.
Discussion
In this study, seven whole-body composition variables including FM, FFM, BCM, BMR_m, ICW, ECW, and ICW_ECW and three pairs of segmental bioimpedance variables consisting of R ratios, Xc results, and PAs in the upper and lower extremities were used to examine the associations with CP. Regarding the whole-body composition variables, we found that individuals with CP had significantly smaller body sizes, lesser body lean mass and water volume as expressed by a decrease in weight, FFM, BCM, ICW, and ECW than those with non-CP in both women and men. With respect to the segmental bioimpedance variables, we found significant increase in R ratios and decrease in PAs in the CP group compared with the non-CP group. After controlling for age, height, weight and the other covariates, these variables continued to significantly associate with the CP prevalence, especially in women. From previous studies, some of these variables have been investigated in people with cold hypersensitivity symptoms. Particularly, they reported significantly lower body lean mass indices, FM indices, or ICW_ECW ratios in the cold group when compared with the non-cold or heat group (11, 19, 21). Additionally, whole-body PA was found to be negatively associated with the incidence of CP (11). Our findings are consistent with these results.
First of all, in terms of the whole-body composition variables, we found significant reductions in BMI, FFM, and BCM in both women and men. In women, the lower BMI might be due to a decrease in FFM or BCM mainly, rather than a decline in FM as indicated by the significantly lower FFM and BCM and indifferent FM when compared with those of the non-CP participants (Figure 3). After controlling for age, height, weight, and the comorbidities, FFM and BCM exhibited significantly negative associations with the CP prevalence. Interestingly, FM, which was not associated with CP in the crude model, became positively associated with CP in the last model (Table 3). In men, BMI reduction may be caused by a decrease in both FFM and FM as demonstrated by the relatively lower FFM and FM in the CP group (Figure 3). However, the reductions in these lean and FM variables might be subtle; therefore, they failed to produce significant associations with CP after controlling for age, height, weight, and other covariates.
The abovementioned observations between lower body lean mass and CP prevalence might be explained by the human thermoregulation and thermal equilibrium mechanisms. Evidently, our human body generates heat internally and maintains a core temperature of approximately 37°C, regardless of the environment (51). The heat generations are mainly functions of healthy cellular mitochondria in the body lean mass (52); thus, individuals with lower body lean mass may have relatively lower amount of heat production. On the contrary, FM tissue has been considered an insulation layer to protect body temperature expenditure (53, 54). Lower FFM and BCM together with a higher FM suggest that the heat production might be insufficient to compensate for the heat loss despite a thicker insulative layer, as shown in our CP individuals. Consequently, CP individuals are more sensitive to a given thermal stimulus (e.g., cold wind) whereby the nervous system is activated and enhances peripheral vasoconstriction to protect the core temperature (55). Therefore, CP individuals with low body lean mass tend to feel aversion to cold, especially on their extremities, where the fluctuation of temperature is more perceptible than in the body trunk. Moreover, when heat production is insufficient, the body trunk temperature might be deficient; therefore, these individuals may also experience abdominal discomfort with cold. Subsequently, individuals with CP are likely to have lower body lean mass and be more susceptible to ambient thermal fluctuations than those among the non-CP individuals, as shown in our studies with women regardless of age, height, and weight.
In addition, BMR which is highly correlated with the body lean mass variables was also decreased in the CP group (56, 57). In this study, we calculated BMR with respect to the body weight (BMR_m) as proposed by a previous report (32). Physiologically, BMR_m reflects the actual rate of metabolism during heat production (56) such that the higher the BMR_m, the more the heat that will be generated. Our result of BMR_m reduction confirmed the inferiority of the heat production in individuals with CP. However, since human body produces heat mainly from healthy cells in lean mass such as the brain, liver, or skeletal muscle mass, but hardly from the adipose tissue (58, 59), future studies with a mass-specific BMR might provide more insight into how different body masses manipulate BMR changes in individuals with CP.
Another variable was included to investigate the changes in body volume in relation to the body surface area, named as the volume-to-body surface area, between the CP and non-CP groups. From the above discussions, the CP individuals have a smaller body size as expressed by the lower weight and height and lesser body lean mass; thus, they produce less heat compared to the non-CP individuals. As revealed by the significantly lower body volume per surface area, our results suggested that the CP individuals not only have a smaller body size but also a relatively higher body surface area compared to the non-CP individuals. According to Bergmann’s rule, individuals with small body volume and higher body surface area are unlikely to be able to withstand cold temperature due to the imbalance between the lesser heat production and greater heat loss (33, 60). The findings of this study are consistent with the Bergmann’s rule.
Based on the water-related variables, we found that individuals with CP had a reduced total body water volume as indicated by the decrease in both ICW and ECW. ICW reflects the BCM, whereas, ECW is associated with the nutritional status of the individual (61, 62). Nevertheless, the decrease in body lean mass might cause the reduction in ICW and ECW. Moreover, we found an abnormal cellular water distribution as demonstrated by the decrease in ICW_ECW ratio in CP individuals compared to the non-CP individuals. ICW_ECW has been considered a prognostic factor of several pathological conditions such as edema, sarcopenia, cancer, and cognitive decline, and is associated with muscle strength and gait speed in older populations (27, 63–66). Decreased ICW_ECW suggests more serious reduction in the intracellular area water compared to that in the extracellular space and might have been caused by the lower BCM, body cell volume, or a malnutrition status in the CP individuals.
Regarding the segmental bioimpedance variables, the R ratios and PAs in the upper and lower extremities differed significantly between the CP and non-CP groups, especially in women, after controlling for the covariates; however, the results were not significant in men. The R ratios were calculated to reflect the changes in ICW/ECW between the upper and lower extremities, such that its positive association with the CP incidence suggested a decrease in ICW compared to ECW (26, 36). This finding is in line with the result of whole-body ICW/ECW ratio described above. The OR results indicated comparable magnitudes of changes of the abnormal cellular water distributions in the upper and lower extremities. Furthermore, the PAs in the upper and lower extremities were negatively associated with the incidence of CP. PA has been considered a useful indicator of BCM and cell membrane integrity in many clinical situations (23, 67, 68) and in relation with the hydration status of the body (69, 70). Marini and colleagues reported a positive correlation between PA and ICW/ECW ratio (or negatively correlated with ECW/ICW ratio) in that the water volume was measured by the dilution technique which is the gold standard to access body fluid (69). In addition, PA appeared to link with the body lean mass quantities such as FFM, FFM index, or skeletal muscle mass (70, 71). Since the majority of FFM is ICW (72), PA also positively correlates with ICW rather than with ECW, especially in men (69). Thus the decrease of PAs corresponds with the lower ICW or ICW with respect to ECW (ICW/ECW), and is associated with the reduction of body lean mass. These aforementioned variables are highly correlated to each other (73). In this relationship, we found that both the whole-body lean mass quantities, ICW/ECW ratio, and segmental PAs exhibited similar negative associations with CP prevalence. Our findings were consistent with these results and indicated a lower BCM quantitatively, or an impairment of the body cell membrane capacitance and integrity in the CP group compared to the non-CP individuals. Notably, after considering BCM as an additional covariate, these associations between segmental bioimpedance variables and CP prevalence diminished (Supplementary Table 3).
From the perspective of sex differences, the associations obtained from the whole-body composition and segmental bioimpedance variables with CP prevalence differed significantly between women and men. Previous studies have suggested that women have a lower metabolic heat production and greater insulative response during cold stress experiences than men (74). A smaller body lean mass and higher burden of FM might be the cause for these observations. Compared to men, women have relatively lower surface area and larger peripheral heat sink; hence, they are less capable of acclimating with heat (75). Furthermore, despite having a greater insulation layer from body FM, women maintain a constant rectal temperature at a greater metabolic cost than men (76). Furthermore, in terms of thermal sensations, women require a higher thermal comfort zone and tend to experience more discomfort than men when exposed to cooling situations, especially in their extremities (77, 78). For these reasons, women may be more susceptible with CP, thus they presented more pronounced associations with CP than the men.
According to age, thermal sensations in relation to CP susceptibility vary across different age groups (79). A common agreement is that older adults are more susceptible to cold hypersensitivity than younger people (40, 41). Older adults have relatively low heat production and a narrow thermoneutral zone that may be due to impaired thermal perception and weakened autonomic and behavioral thermoregulatory responses (41, 42). Compared to younger individuals, older individuals are unlikely to sense and respond to a thermal imbalance appropriately (46). Thus, physiological aging can influence the probability of having CP in older people. In this study, the impact of age as well as comorbidities seemed negligible, which might be due to the statistical indifference in the mean age between groups.
Women in the CP group had slightly lower systolic and diastolic blood pressure than those in the non-CP group. Similarly, although the difference did not reach the significant threshold of 0.05, men with CP exhibited a trend of lower systolic blood pressure than those without CP. Previous studies reported a lower level of blood pressure in the individuals with cold extremities syndromes, especially in women (9, 80). They suggested a relationship between blood pressure and feeling of cold in the extremities, as in the Flammer syndrome. This syndrome explains a phenomenon of when people react to stimuli like cold by altering their blood pressure (80, 81). The mechanisms of the relationship between body weight and height with blood pressure are complex (82). Previous studies suggested that body weight and BMI have positive associations with systolic blood pressure whereas body height mainly affects the diastolic blood pressure in a negative correlative manner (83, 84). Nevertheless, the Flammer syndrome or the influence of body size and BMI might contribute to the lower blood pressures in the CP group in our study.
This study provided an insight into how whole-body composition and segmental bioimpedance change in relation to CP incidence for different sexes. The results suggest that individuals with abnormal cellular water distribution, insufficient BCM and/or body cell strength might be susceptible to cold hypersensitivity symptoms. An increase in BCM or overall health status induced by a well-balanced diet and regular physical activities might help protect the body from CP.
In this study, the prevalence of CP was approximately 73% (448/667), which is higher than the 60% proportion of cold hypersensitivity in the general population in Korea or Japan (3, 4). The selection of participants of older age (55 years of age and above) who are more susceptible to cold hypersensitivity compared to the younger people (40–42) may be one of the reasons for this higher prevalence of CP. Subsequently, younger CP participants may show different relationships between body composition and bioimpedance variables due to their different physiological and pathological conditions. Additionally, the CP questionnaire that was adopted in this study is simple, with only eight questions, and the accuracy might have been improved if a longer questionnaire was used (37, 38). Diagnosing CP using objective criteria can aid in the generalization of these findings.
Conclusion
This study investigated the association between CP and seven whole-body compositions with three pairs of segmental bioimpedance variables. Lean mass was significantly lower, whereas FM was relatively higher in individuals with CP than in those without CP, regardless of age, height, weight, and other covariates. Correspondingly, body volume with respect to the surface area and BMR_m were lower in the CP group than in the non-CP group. Segmental bioimpedance analysis revealed that individuals with CP have abnormal cellular water distributions as well as a significantly lower BCM and/or cell membrane strength in both upper and lower extremities. These findings emerged only in women but not in men. The behaviors in these body lean and segmental bioimpedance variables may be considered as potential markers for identifying CP, especially in women.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Chonnam National University Hospital (approval number: CNUH-2019-279). The patients/participants provided their written informed consent to participate in this study.
Author contributions
DD analyzed the data and wrote the manuscript. KK handled the Institutional Review Board approval and managed the data. SK assisted in the data analysis. SL developed and validated the cold pattern identification questionnaire. KL took care of the data collection and curation. JK designed the study and wrote the manuscript. All authors revised and approved the contents of the manuscript, contributed to the article, and approved the submitted version.
Funding
This study was supported by the Korean Institute of Oriental Medicine (KIOM; Grant No. KSN2022130) funded by the Korean Government.
Acknowledgments
We thank all the contributors of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.975464/full#supplementary-material
References
1. Shi Lin Y. Differentiation of cold and heat patterns: Ambiguities in diagnosis. Clin Acupunct Oriental Med. (2001) 2:102–10. doi: 10.1054/caom.2001.0082
2. Wigley FM. Clinical practice. Raynaud’s Phenomenon. N Engl J Med. (2002) 347:1001–8. doi: 10.1056/NEJMcp013013
3. Ushiroyama T, Kamimoto Y, Sakuma K, Ueki M. Assessment of chilly sensation in Japanese women with laser Doppler fluxmetry and acceleration plethysmogram with respect to peripheral circulation. Bull Osaka Med Coll. (2005) 51:76–84.
4. Yoo J, Lee D, Seon S, Shin M, Lee G, Han I. Cold hypersensitivity in hands and feet. Clin Pract Guideline Korean Med. (2021):10.
5. Wang FL, Zhang WW. Differentiation of syndromes. In Handbook of Traditional Chinese Medicine. (Vol. 3), Singapore: World Scientific (2014). p. 205–73. doi: 10.1142/9789814293839_0015
7. Bae KH, Go HY, Park KH, Ahn I, Yoon Y, Lee S. The association between cold hypersensitivity in the hands and feet and chronic disease: Results of a multicentre study. BMC Complement Altern Med. (2018) 18:40. doi: 10.1186/s12906-018-2082-3
8. Musa R, Qurie A. Raynaud disease. In StatPearls. Treasure Island, FL: StatPearls Publishing (2022).
9. Tsuboi S, Mine T, Tomioka Y, Shiraishi S, Fukushima F, Ikaga T. Are cold extremities an issue in women’s health? Epidemiological evaluation of cold extremities among Japanese women. Int J Womens Health. (2019) 11:31–9. doi: 10.2147/IJWH.S190414
10. Stjernbrandt A, Carlsson D, Pettersson H, Liljelind I, Nilsson T, Wahlström J. Cold sensitivity and associated factors: a nested case-control study performed in Northern Sweden. Int Arch Occup Environ Health. (2018) 91:785–97. doi: 10.1007/s00420-018-1327-2
11. Lee E, Kahye Seul Gee Kim S, Lee S, Cha S, Lee Y, Mun S. An analysis of the relationship between the Cold pattern and Anthropometry, Bio Impedance Analysis (BIA) and Quality of Life in Jeju Haenyeo. J Soc Prev Korean Med. (2016) 20:67–74.
12. Seo B-N, Jeong K, Baek Y, Lee S. Study on the relationship between cold type and sleep quality in koreans. J Physiol Pathol Korean Med. (2021) 35:42–6. doi: 10.15188/kjopp.2021.02.35.1.42
13. Bae K-H, Lee Y, Park K-H, Yoon Y, Mun S, Lee S. Perception of cold and heat pattern identification in diseases: a survey of Korean medicine doctors. Integr Med Res. (2017) 6:26–32. doi: 10.1016/j.imr.2016.10.004
14. Wang Z, de Dear R, Luo M, Lin B, He Y, Ghahramani A, et al. Individual difference in thermal comfort: A literature review. Build Environ. (2018) 138:181–93. doi: 10.1016/j.buildenv.2018.04.040
15. Popson MS, Dimri M, Borger J. Biochemistry, heat and calories. In StatPearls. Treasure Island, FL: StatPearls Publishing (2021).
16. Davis RW. Metabolism and Thermoregulation. In: Marine Mammals. Cham: Springer (2019). doi: 10.1007/978-3-319-98280-9_4
17. Mun S, Kim S, Bae KH, Lee S. Cold and spleen-qi deficiency patterns in korean medicine are associated with low resting metabolic rate. Evid-Based Complement Altern Med. (2017) 2017:9532073. doi: 10.1155/2017/9532073
18. Pham DD, Lee J, Kim G, Song J, Kim J, Leem CH. Relationship of the cold-heat sensation of the limbs and abdomen with physiological biomarkers. Evid-Based Complement Altern Med. (2016) 2016:2718051. doi: 10.1155/2016/2718051
19. Kim J, Park SJ, Yoon J, Lee BJ, Kim KH. Association of cold-heat patterns with tongue features, body composition, anthropometric indices, and blood parameters in Tae-Eum Type. Evid-Based Complement Altern Med. (2018) 2018:2754195. doi: 10.1155/2018/2754195
20. Mun S, Park K, Lee S. Association of cold-heat pattern and anthropometry/body composition in individuals between 50–80 years of age. J Physiol Pathol Korean Med. (2020) 34:209–14. doi: 10.15188/kjopp.2020.08.34.4.209
21. Mun S, Park K, Lee S. Study on the anthropometric and body composition indices for prediction of cold and heat pattern. J Korean Med. (2021) 42:185–96. doi: 10.13048/jkm.21046
22. Yi Y, Baek JY, Lee E, Jung HW, Jang IY. A comparative study of high-frequency bioelectrical impedance analysis and dual-energy x-ray absorptiometry for estimating body composition. Life (Basel). (2022) 12:994. doi: 10.3390/life12070994
23. Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors (Basel). (2014) 14:10895–928. doi: 10.3390/s140610895
24. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis–part I: review of principles and methods. Clin Nutr. (2004) 23:1226–43. doi: 10.1016/j.clnu.2004.06.004
25. Bera TK. Bioelectrical impedance methods for noninvasive health monitoring: A review. J Med Eng. (2014) 2014:381251. doi: 10.1155/2014/381251
26. Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys. (2008) 30:1257–69. doi: 10.1016/j.medengphy.2008.06.009
27. Yamada Y, Nishizawa M, Uchiyama T, Kasahara Y, Shindo M, Miyachi M, et al. Developing and validating an age-independent equation using multi-frequency bioelectrical impedance analysis for estimation of appendicular skeletal muscle mass and establishing a cutoff for sarcopenia. Int J Environ Res Public Health. (2017) 14:809. doi: 10.3390/ijerph14070809
28. Tanaka S, Ando K, Kobayashi K, Seki T, Hamada T, Machino M, et al. Low bioelectrical impedance phase angle is a significant risk factor for frailty. Biomed Res Int. (2019) 2019:6283153. doi: 10.1155/2019/6283153
29. InBody Co Ltd. InBody370 User’s Manual. (1996). Available online at: https://nl.inbody.com/wp-content/uploads/2019/01/InBodyS10_CDmanual_Eng_E.pdf (accessed March 15, 2020).
30. Jun MH, Kim S, Ku B, Cho JH, Kim K, Hoo H-R, et al. Glucose-independent segmental phase angles from multi-frequency bioimpedance analysis to discriminate diabetes mellitus. Sci Rep. (2018) 8:648. doi: 10.1038/s41598-017-18913-7
31. Doan DNT, Ku B, Kim KH, Jun M, Jun M, Lee KH, et al. Segmental bioimpedance variables in association with mild cognitive impairment. Front Nutr. (2022) 9:873623. doi: 10.3389/fnut.2022.873623
32. Robert TB. Chapter 17 - Thermodynamics of Biological Systems. In Modern Engineering Thermodynamics. Cambridge, MA: Academic Press (2011). p. 693–726. doi: 10.1016/B978-0-12-374996-3.00017-8
33. Bergmann C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe (On the proportions of heat economy of animals to their size). Gottingen: Vandenhoeck & Ruprecht (1848).
34. Sendroy J Jr., Collison HA. Determination of human body volume from height and weight. J Appl Physiol. (1966) 21:167–72. doi: 10.1152/jappl.1966.21.1.167
35. Osilla EV, Marsidi JL, Sharma S. Physiology, temperature regulation. In StatPearls. Treasure Island, FL: StatPearls Publishing (2022).
36. Yamada Y, Yoshida T, Yokoyama K, Watanabe Y, Miyake M, Yamagata E, et al. The extracellular to intracellular water ratio in upper legs is negatively associated with skeletal muscle strength and gait speed in older people. J Gerontol A Biol Sci Med Sci. (2017) 72:293–8. doi: 10.1093/gerona/glw125
37. Bae KH, Jang ES, Park K, Lee Y. Development on the questionnaire of cold-heat pattern identification based on usual symptoms - reliability and validation study. J Physiol Pathol Korean Med. (2018) 32:341:346. doi: 10.15188/kjopp.2018.10.32.5.341
38. Bae KH, Yoon Y, Yeo M, Kim HS, Lee Y, Lee S. Development on the questionnaire of cold-heat pattern identification based on usual symptoms for health promotion - focused on agreement study. J Soc Prev Korean Med. (2016) 20:17–26.
39. St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. (2010) 26:152–5. doi: 10.1016/j.nut.2009.07.004
40. Smolander J. Effect of cold exposure on older humans. Int J Sports Med. (2002) 23:86–92. doi: 10.1055/s-2002-20137
41. Kingma B, Frijns A, van Marken Lichtenbelt W. The thermoneutral zone: implications for metabolic studies. Front Biosci (Elite Ed). (2012) 4:1975–85. doi: 10.2741/518
42. Shibasaki M, Okazaki K, Inoue Y. Aging and thermoregulation. J Phys Fit Sport. (2013) 2:37–47. doi: 10.7600/jpfsm.2.37
43. Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ. Cross-sectional age differences in body composition in persons 60 + years of age. J Gerontol. (1995) 50A:M307–16. doi: 10.1093/gerona/50A.6.M307
44. Seidell JC, Visscher TL. Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr. (2000) 54(Suppl 3):S33–9. doi: 10.1038/sj.ejcn.1601023
45. Ponti F, Santoro A, Mercatelli D, Gasperini C, Conte M, Martucci M, et al. Aging and imaging assessment of body composition: from fat to facts. Front Endocrinol. (2020) 10:861. doi: 10.3389/fendo.2019.00861
46. Guergova S, Dufour A. Thermal sensitivity in the elderly: A review. Ageing Res Rev. (2011) 10:80–92. doi: 10.1016/j.arr.2010.04.009
47. Seppänen-Nuijten E, Lahti-Koski M, Männistö S, Knekt P, Rissanen H, Aromaa A, et al. Fat free mass and obesity in relation to educational level. BMC Public Health. (2009) 9:448. doi: 10.1186/1471-2458-9-448
48. Sagarra-Romero L, Gómez-Cabello A, Pedrero-Chamizo R, Vila-Maldonado S, Gusi-Fuertes N, Villa-Vicente JG, et al. Relación entre el nivel educativo y la composición corporal en personas mayores no institucionalizadas: proyecto multi-céntrico EXERNET [Relation between educational level and body composition in non-institutionalized elderly: The elderly EXERNET multi-center study.]. Rev Esp Salud Publica. (2017) 91:e201710041.
49. Kaikaew K, van den Beukel JC, Neggers SJCMM, Themmen APN, Visser JA, Grefhorst A. Sex difference in cold perception and shivering onset upon gradual cold exposure. J Therm Biol. (2018) 77:137–44. doi: 10.1016/j.jtherbio.2018.08.016
50. Bredella MA. Sex differences in body composition. Adv Exp Med Biol. (2017) 1043:9–27. doi: 10.1007/978-3-319-70178-3_2
51. Osilla E, Marsidi J, Sharma S. Physiology, temperature regulation. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2022).
52. Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. (2012) 26:711–23. doi: 10.1016/j.beem.2012.05.003
53. Savastano DM, Gorbach AM, Eden HS, Brady SM, Reynolds JC, Yanovski JA. Adiposity and human regional body temperature. Am J Clin Nutr. (2009) 90:1124–31. doi: 10.3945/ajcn.2009.27567
54. Speakman JR. Obesity and thermoregulation. Handb Clin Neurol. (2018) 156:431–43. doi: 10.1016/B978-0-444-63912-7.00026-6
56. Cunningham JJ. Body composition and resting metabolic rate: the myth of feminine metabolism. Am J Clin Nutr. (1982) 36:721–6. doi: 10.1093/ajcn/36.4.721
57. Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. (1990) 86:1423–7. doi: 10.1172/JCI114857
58. Heymsfield SB, Peterson CM, Bourgeois B, Thomas DM, Gallagher D, Strauss B, et al. Human energy expenditure: advances in organ-tissue prediction models. Obes Rev. (2018) 19:1177–88. doi: 10.1111/obr.12718
59. Wang Z, Bosy-Westphal A, Schautz B, Müller M. Mechanistic model of mass-specific basal metabolic rate: evaluation in healthy young adults. Int J Body Compos Res. (2011) 9:147.
60. Shelomi M, Zeuss D. Bergmann’s and allen’s rules in native european and mediterranean phasmatodea. Front Ecol Evol. (2017) 5:25. doi: 10.3389/fevo.2017.00025
61. Guirao X, Franch G, Gil MJ, García-Domingo MI, Girvent M, Sitges-Serra A. Extracellular volume, nutritional status, and refeeding changes. Nutrition. (1994) 10:558–61.
62. Mager JR, Sibley SD, Beckman TR, Kellogg TA, Earthman CP. Multifrequency bioelectrical impedance analysis and bioimpedance spectroscopy for monitoring fluid and body cell mass changes after gastric bypass surgery. Clin Nutr. (2008) 27:832–41. doi: 10.1016/j.clnu.2008.06.007
63. Lee JY, Ryu HS, Yoon SS, Kim EH, Yoon SW. Extracellular-to-Intracellular fluid volume ratio as a prognostic factor for survival in patients with metastatic cancer. Integr Cancer Ther. (2019) 18:1534735419847285. doi: 10.1177/1534735419847285
64. Serra-Prat M, Lorenzo I, Palomera E, Ramírez S, Yébenes JC. Total body water and intracellular water relationships with muscle strength, frailty and functional performance in an elderly population. J Nutr Health Aging. (2019) 23:96–101. doi: 10.1007/s12603-018-1129-y
65. Hioka A, Akazawa N, Okawa N, Nagahiro S. Increased total body extracellular-to-intracellular water ratio in community-dwelling elderly women is associated with decreased handgrip strength and gait speed. Nutrition. (2021) 86:111175. doi: 10.1016/j.nut.2021.111175
66. Lee J, Shields RK. Extracellular to intracellular body water and cognitive function among healthy older and younger adults. J Funct Morphol Kinesiol. (2022) 7:18. doi: 10.3390/jfmk7010018
67. Barbosa-Silva MC, Barros AJ. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care. (2005) 8:311–7. doi: 10.1097/01.mco.0000165011.69943.39
68. Kumar S, Dutt A, Hemraj S, Bhat S, Manipadybhima B. Phase angle measurement in healthy human subjects through bio-impedance analysis. Iran J Basic Med Sci. (2012) 15:1180–4.
69. Marini E, Campa F, Buffa R, Stagi S, Matias CN, Toselli S, et al. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin Nutr. (2020) 39:447–54. doi: 10.1016/j.clnu.2019.02.016
70. Stagi S, Irurtia A, Rosales Rafel J, Cabras S, Buffa R, Carrasco-Marginet M, et al. Segmental body composition estimated by specific BIVA and dual-energy X-ray absorptiometry. Clin Nutr. (2021) 40:1621–7. doi: 10.1016/j.clnu.2021.02.043
71. Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. (2016) 103:712–6. doi: 10.3945/ajcn.115.116772
72. Tobias A, Ballard BD, Mohiuddin SS. Physiology, water balance. In StatPearls. Treasure Island, FL: StatPearls Publishing (2021).
73. Jaremków A, Markiewicz-Górka I, Hajdusianek W, Gać P. Relationships between body composition parameters and phase angle as related to lifestyle among young people. J Clin Med. (2021) 11:80. doi: 10.3390/jcm11010080
74. Solianik R, Skurvydas A, Vitkauskienė A, Brazaitis M. Gender-specific cold responses induce a similar body-cooling rate but different neuroendocrine and immune responses. Cryobiology. (2014) 69:26–33. doi: 10.1016/j.cryobiol.2014.04.015
75. Burse RL. Sex differences in human thermoregulatory response to heat and cold stress. Hum Factors. (1979) 21:687–99. doi: 10.1177/001872087912210606
76. Wagner JA, Horvath SM. Influences of age and gender on human thermoregulatory responses to cold exposures. J Appl Physiol. (1985) 58:180–6. doi: 10.1152/jappl.1985.58.1.180
77. Karjalainen S. Thermal comfort and gender: A literature review. Indoor Air. (2012) 22:96–109. doi: 10.1111/j.1600-0668.2011.00747.x
78. Schellen L, Loomans MG, de Wit MH, Olesen BW, van Marken Lichtenbelt WD. The influence of local effects on thermal sensation under non-uniform environmental conditions–gender differences in thermophysiology, thermal comfort and productivity during convective and radiant cooling. Physiol Behav. (2012) 107:252–61. doi: 10.1016/j.physbeh.2012.07.008
79. Tuomaala P, Holopainen R, Piira K, Airaksinen M. Impact of individual characteristics - such as age, gender, bmi, and fitness - on human thermal sensation. In Proceedings of the 13th Conference of International Building Performance Simulation Association. Chambery: (2013). p. 26–8. doi: 10.26868/25222708.2013.2240
80. Konieczka K, Ritch R, Traverso CE, Kim DM, Kook MS, Gallino A, et al. Flammer syndrome. EPMA J. (2014) 5:11. doi: 10.1186/1878-5085-5-11
81. Flammer J, Konieczka K. The discovery of the Flammer syndrome: a historical and personal perspective. EPMA J. (2017) 8:75–97. doi: 10.1007/s13167-017-0090-x
82. Shariq OA, McKenzie TJ. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. (2020) 9:80–93. doi: 10.21037/gs.2019.12.03
83. Song YH. The correlation of blood pressure with height and weight in Korean adolescents aged 10-19 years; The Korean National Health and Nutrition Examination Surveys (2009-2011). Korean J Pediatr. (2014) 57:35–40. doi: 10.3345/kjp.2014.57.1.35
84. Hosseini M, Baikpour M, Yousefifard M, Fayaz M, Koohpayehzadeh J, Ghelichkhani P, et al. Blood pressure percentiles by age and body mass index for adults. EXCLI J. (2015) 14:465–77.
86. Koop LK, Tadi P. Physiology, Heat Loss. In StatPearls. Treasure Island, FL: StatPearls Publishing (2021).
Keywords: cold pattern recognition, body composition, segmental bioimpedance analysis, sex difference, cellular water disturbance
Citation: Doan DNT, Kim K, Kim SG, Lee S, Lee KH and Kim J (2022) Segmental bioelectrical impedance analysis for Korean older population with cold pattern. Front. Nutr. 9:975464. doi: 10.3389/fnut.2022.975464
Received: 22 June 2022; Accepted: 18 November 2022;
Published: 01 December 2022.
Edited by:
Christelle Guillet, University of Auvergne, FranceReviewed by:
Francesca Battista, University of Padua, ItalyElisabetta Marini, University of Cagliari, Italy
Copyright © 2022 Doan, Kim, Kim, Lee, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaeuk Kim, amFldWtraW1Aa2lvbS5yZS5rcg==
 Dieu Ni Thi Doan
Dieu Ni Thi Doan Kahye Kim
Kahye Kim Seul Gee Kim2
Seul Gee Kim2 Kun Ho Lee
Kun Ho Lee Jaeuk Kim
Jaeuk Kim