- 1Department of Food Science and Technology, School of Food & Wine, Ningxia University, Yinchuan, China
- 2Ningxia Red Power Goji Co., Ltd., Zhongwei, China
- 3Ningxia Engineering Research Center for Goji Biological Fermentation & Milling, Zhongwei, China
Traditional herbal therapy made from Lycium barbarum leaves has been said to be effective in treating metabolic diseases, while its exact processes are yet unknown. Natural flavonoids are considered as a secure and reliable method for treating obesity. We thus made an effort to investigate the processes by which flavonoids from L. barbarum leaves (LBLF) reduce obesity. To assess the effectiveness of the intervention following intragastric injection of various dosages of LBLF (50, 100, and 200 mg/kg⋅bw), obese model mice developed via a high-fat diet were utilized. Treatment for LBLF may decrease body weight gain, Lee’s index, serum lipids levels, oxidative stress levels, and hepatic lipids levels. It may also enhance fecal lipids excretion and improve glucose tolerance. Additionally, LBLF therapy significantly restored gut dysfunction brought on by a high-fat diet by boosting gut bacterial diversities and altering the composition of the gut bacterial community by elevating probiotics and reducing harmful bacteria.
Introduction
Obesity is becoming one of the biggest global dangers to public health and quality of life due to its rising prevalence (1). Obesity is a significant risk factor for metabolic illnesses and is closely related to our ways of life, dietary habits, environment, and genetic factors (2, 3). The overconsumption of a high-calorie diet dominated by high fat and saccharides causing an imbalance between energy intake and expenditure, thereby driving excessive fat accumulation as triglycerides (TG) in white adipose cells takes primarily charge of the development of obesity (4, 5). When obesity first develops, high blood pressure, low-grade inflammation, non-alcoholic fatty liver, hyperlipidemia, insulin resistance, and cancer are also frequently present (6-8).
Weight-reducing medications like orlistat have recently been thought of as one of the traditional methods for managing obesity. However, they have significant side effects such as increased gastrointestinal exhaust, greasy stools, diarrhea, and hepatotoxicity (9, 10). Given that their characteristics are safe, effective, and have few side effects, active components from the plant kingdom must be found to build functional foods or novel anti-obesity medications. As a well-known traditional medicine, Lycium barbarum leaves have a variety of tonic benefits on people, including tonifying liver and kidney, improving eyesight, and removing toxic heat. Recent research found that L. barbarum leaves’ abundance of nutrients and natural bioactive substances, such as polysaccharides, flavonoids, minerals, alkaloids, vitamins, and trace elements, significantly improved immunological function, promoted cell proliferation, and decreased blood glucose levels (11, 12).
It has been determined how high oxidative stress promotes the etiology of obesity. Increasing cellular oxidative stress levels will permanently damage macromolecules, including lipids, DNA, and proteins, impairing cellular transport and metabolism and eventually triggering the development of chronic illnesses (13, 14). In contrast, excessive fat storage may boost reactive oxygen species (ROS), causing systemic oxidative stress by raising endoplasmic reticulum stress and NADPH oxidase activity in adipocytes (15). Therefore, obesity is a result of oxidative stress as well as a risk factor for it. However, studies found that flavonoids greatly improved antioxidant capacity in rats fed a high-fat diet (16) and reduced the generation of ROS in cells (17). Another important regulating aspect in the development of obesity is the makeup of the gut microorganisms, which are thought of as a “new organ” at this time (18). Growing attention has recently been given to the roles that gut microorganisms play in the pathogenesis of metabolic disorders and how bioactive dietary ingredients reduce obesity by changing the makeup and functionality of intestinal microbes. Studies have shown that flavonoids might alter the composition of the gut bacterial population in conjunction with increased probiotics and diminished harmful bacteria, lowering the formation of endotoxins and enhancing intestinal barrier function (19). Gut microbial dysbiosis is frequently caused by aberrant changes in host homeostasis (20), which raises the risk of metabolic diseases. In light of these findings, gut microorganisms in conjunction with bioactive dietary ingredients can be considered a cutting-edge method of treating obesity.
There are currently few publications on the effectiveness of L. barbarum leaves extract in treating obesity. Thus, the effects of the LBLF supplement were studied in mice fed a high-fat diet better to understand the processes behind LBLF’s anti-obesity properties.
Materials and methods
Extraction and purification of LBLF
The dried L. barbarum leaves (Yinchuan Yuxin Co., Ltd., Yinchuan, China) were crushed and sieved into uniform powder size. The powder (1 g) was mixed with 70 volumes of 70% ethanol (v/v) solution and extracted two times at 70°C (2 h each time). The extraction was filtered using a vacuum pump (SHZ-III, Shanghai, China) and then merged. The ethanol was recycled by rotary evaporation (Re-52AA, Shanghai, China) at 55°C to collect the extract. Next, the petroleum ether was used to remove chlorophyll and lipids in the extract. Finally, the petroleum ether was recycled by rotary evaporation to collect the ultimate crude extract. The crude flavonoids were obtained by vacuum freeze-drying (JDG-0.2, Lanzhou, China).
The 0.375 g of the crude flavonoids powder and 2 g of D101 macroporous resin (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China) were put into a 250 ml triangular bottle containing 100 ml ultrapure water. Then, the triangular bottle is fixed on the shaking table to oscillate at room temperature for 24 h. Under the same experimental conditions, then filtered and dried macroporous resin (2 g) was mixed with 100 ml of 70% ethanol (v/v) solution so that flavonoids could be eluted. The eluent was evaporated, concentrated, and freeze-dried to collect the flavonoid product. The total flavonoid content was measured by Al(NO3)3-NaNO2 colorimetric using rutin (≥98%) as reference (21).
Experimental animals and groups
Healthy male ICR mice, weighing 26 ± 1 g and aging 6–7 weeks, were purchased from the Experimental Animal Centre of Ningxia Medical University (animals certificate number: SYXK 2020-0001). All animal experiments were permitted by the Animal Welfare and Ethics Committee of Ningxia Medical University (No. IACUC-NYLAC-2020-179). During the experiment, the animals were housed in a controlled environment (SPF) with a humidity range of 40–70%, a temperature range of 20–25°C, and a light and dark cycle for 12 h. All animals could be free to obtain food and water. After 1 week of acclimatization, the mice (a total of 40 animals) were randomly divided into two groups: the normal control group (NC) and the obese model construction group (OMC). Without intragastric administration of LBLF, OMC’s mice were given a high-fat diet for 30 days. After fasting for 14 h, blood was taken from the orbit by a capillary tube to detect serum lipids. If there was a notable difference in serum levels of TC, TG, HDL-C, and LDL-C between the NC and OMC groups, it indicated that the model was successfully established. Afterward, the OMC’s mice were randomly divided into four groups (eight animals per group): high-fat diet group (HFD), low-dosage group of 50 mg/kg⋅bw LBLF (LBLFL), medium-dosage group of 100 mg/kg⋅bw LBLF (LBLFM) and a high-dosage group of 200 mg/kg⋅bw LBLF (LBLFH). In the next 8 weeks, the mice in LBLFL, LBLFM, and LBLFH groups were gavage-given different doses of total flavonoids of L. barbarum leaves at 2:00 p.m., respectively. Daily diet provision: NC group given basic diet, HFD and LBLF-dosage groups given a high-fat diet. High-fat diet (Shuangshi Co., Ltd., Suzhou, China) formula contained basic diet 58.0%, casein 8.4%, lard 20.4%, sucrose 7%, sodium cholate 1.3% and cholesterol 1.6% (w/w). The collected blood was centrifuged at 3,500 rpm/min for 10 min to obtain serum. All tissue samples and feces were stored at −20°C.
Serum biochemical analysis as well as hepatic and fecal lipids measurement
The serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels were assayed with AU400 Clinical Biochemistry Analyzer (Olympus Co., Ltd., Tokyo, Japan). The serum lipase (LPS) and non-esterified fatty acid (NEFA) levels were assayed by reagent kits (Nanjing Jiancheng Co., Ltd., Nanjing, China). The contents of triglyceride (TG) and cholesterol (TC) in the liver and feces were also assayed by reagent kits (Nanjing Jiancheng Co., Ltd., Nanjing, China). The improved soxhlet extraction method was used to measure feces’ total fat (TF).
Oral glucose tolerance test
After fasting for 10 h, the respective blood was collected from tail veins at 0, 30, 90, and 120 min after orally giving glucose (2.0 g/kg⋅bw) and then measured with a Sannuo glucometer and glucose strips (22). Finally, drawing the plot of glucose concentration with time aimed to obtain the areas under each curve (AUC).
Detection of oxidative stress markers
The serum and liver activity of catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD), contents of malondialdehyde (MDA) and protein carbonyl (PC), and total antioxidant capacity (T-AOC) and quantitative protein were assayed by reagent kits (Nanjing Jiancheng Co., Ltd., Nanjing, China).
Histopathological analysis
Fresh liver and abdominal adipose tissue were fixed with 10% paraformaldehyde, embedded in paraffin wax after gradient dehydration, sliced into 5 μm sections, and then stained with hematoxylin and eosin. Finally, morphological characteristics of the tissue sections were observed by microscope (Motic Co., Ltd., China).
Analysis of intestinal bacterial composition
The total genomic DNA of the fecal samples was acquired by DNA extraction kit (Omega Engineering Inc., United States). Using 1% agarose gel electrophoresis to detect DNA concentrations, the DNA was diluted to 1 ng/μl using Milli-Q water. The barcoded primers hyper-variable V3-V4 region of the 16S rRNA gene of the diluted DNA was amplified by a primer combination of 515F (5’-GTGCCAGCMGCCGCGGTAA-3’), and 806R (5’-GGACTACHVGGTWTCTAAT-3’). All PCR reactions were operated in a total volume of 50 μl reaction system including 0.2 μM of forward and reverse primers, 15 μl of Phusion High-Fidelity PCR Master Mix, and approximately 10 ng template DNA, as well as PCR-grade water, was used to adjust the volume. The use of Qiagen Gel Extraction Kit (Qiagen Co., Ltd., CA, United States) to purify mixture PCR products. All the samples were sequenced by NovaSeq 6000 (Illumina Co., Ltd., San Diego, CA, United States).
Statistical analysis
All data were expressed as means ± SD. The multiple comparisons were analyzed with LSD or Duncan test using one-way ANOVA analysis, while the paired comparisons were carried out by t-test. p-value < 0.05 (* or #) exhibited a significant difference and p < 0.01 (** or ##) exhibited an extremely significant difference. All figures were drawn with Graphpad Prism 8.0 or origin 2021 tools. Spearman’s method was used for correlation analysis using origin 2021.
Results
The establishment of obese mice model after 30 days of high-fat diet intervention
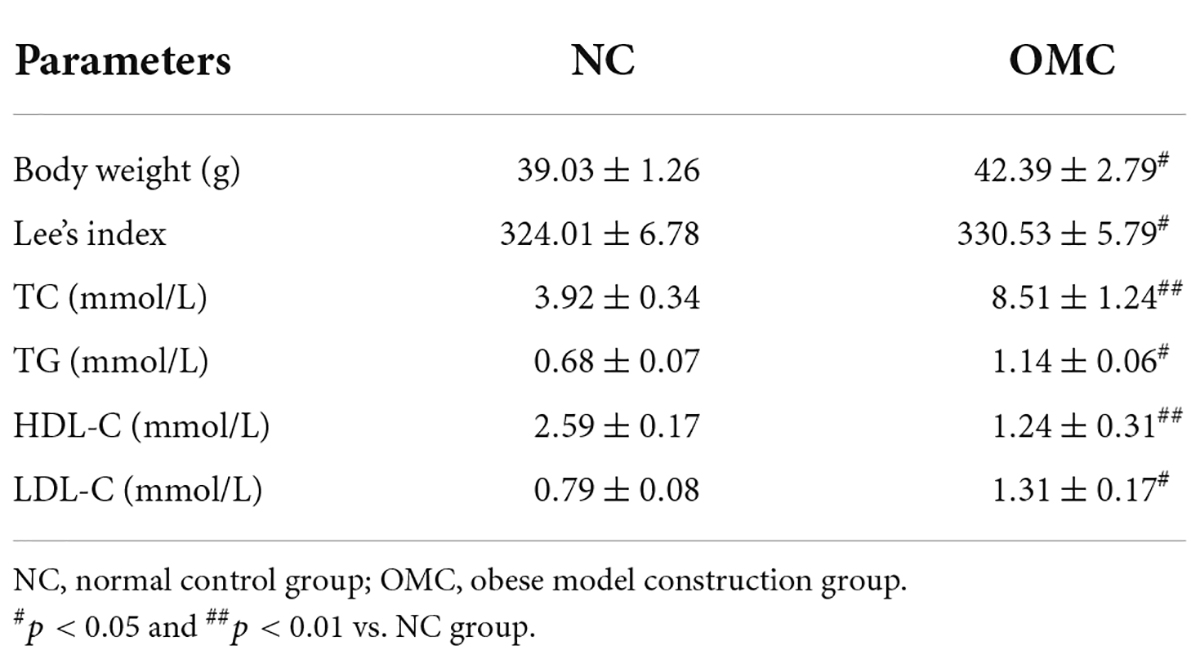
As shown in Table 1, the OMC group saw significant increases in body weight and Lee’s index relative to the NC group (p < 0.05). Meanwhile, the serum levels of TG, TC, and LDL-C were noticeably elevated, whereas the serum level of HDL-C noticeably decreased (p < 0.05). These findings showed that obese mouse models with abnormalities of lipids metabolism were effectively developed.
Effects of LBLF on the food intake, water intake, body weight increase, and Lee’s index
The HFD group saw the most pronounced increases in body weight, as seen in Figure 1. In contrast to the NC group, the HFD and LBLFL groups showed an apparent rise in body weight after 8 weeks of treatment with LBLF (p < 0.05), while the LBLFM and LBLFH groups exhibited no significant difference (Figure 1C) (p > 0.05). Apparently, Lee’s index was significantly increased in the HFD group, which was statistically different from the other groups (Figure 1D) (p < 0.01). Lee’s index in the LBLFL group showed a notable rise that was statistically different (p < 0.05) from the NC group, whereas that of the LBLFM and LBLFH groups exhibited no remarkable difference (Figure 1D) (p > 0.05).
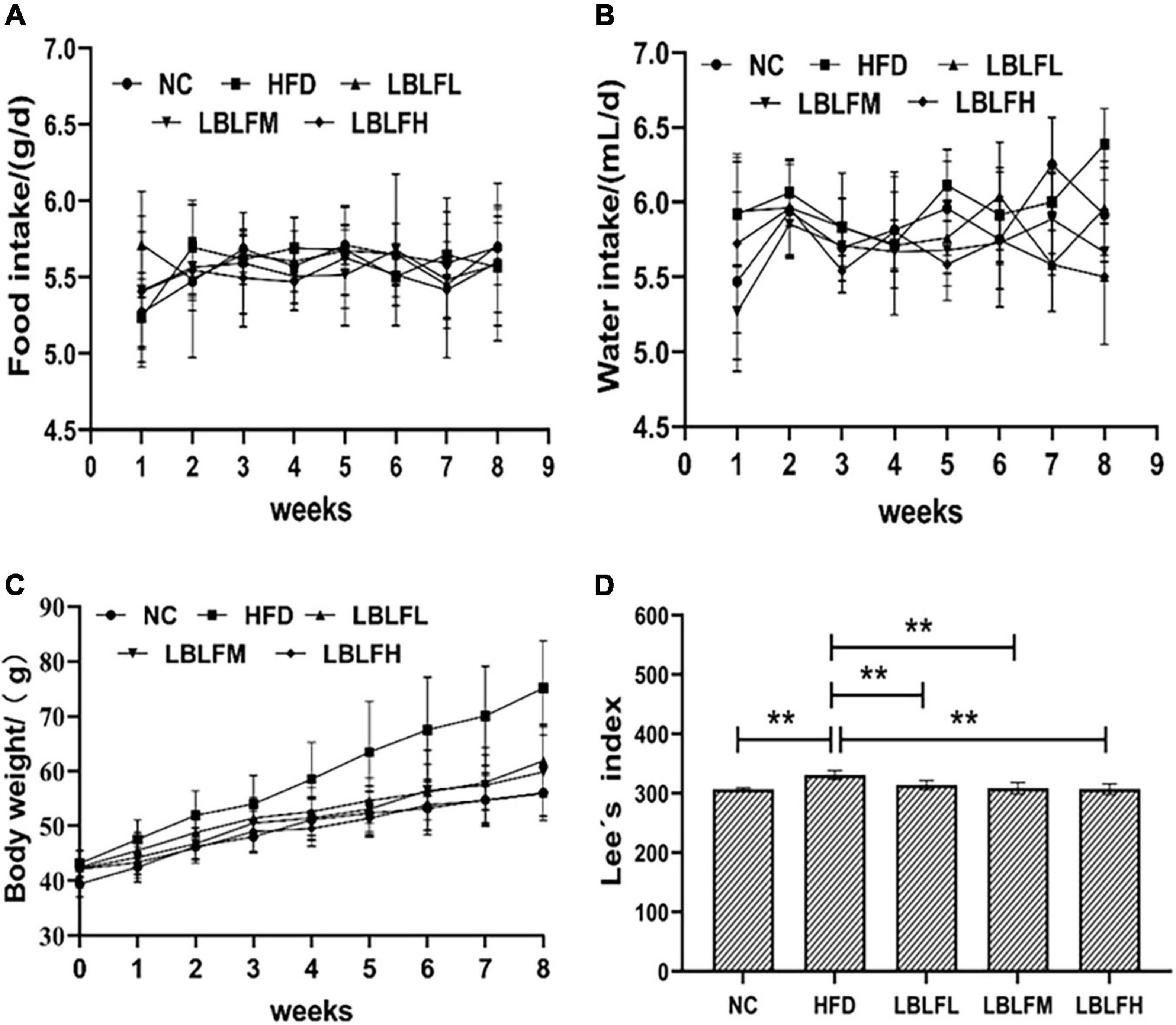
Figure 1. Effects of LBLF supplement on (A) food intake, (B) water intake, (C) body weight, and (D) Lee’s index in obese mice. (*p < 0.05) and (**p < 0.01) vs. HFD group.
Effects of LBLF on serum biochemical markers, hepatic lipids, and oral glucose tolerance in obese mice
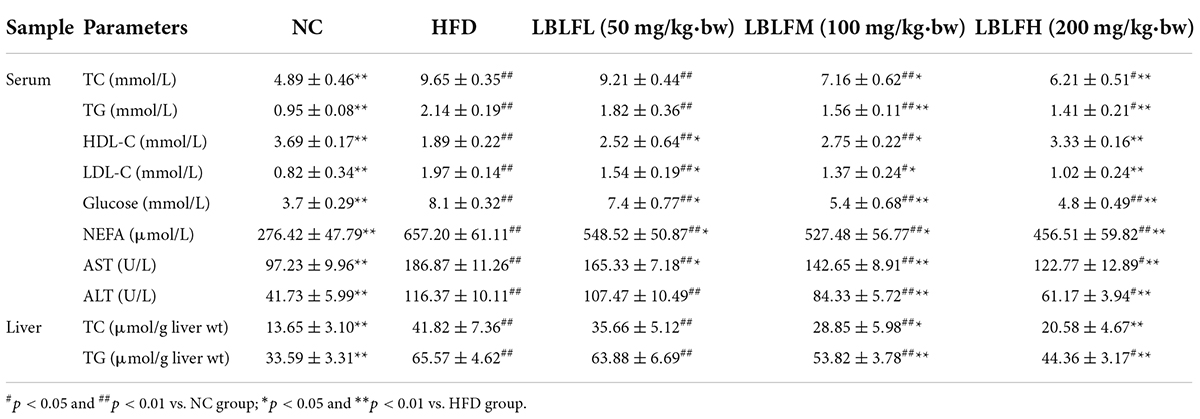
As shown in Table 2, in contrast to the NC group, serum TC, TG, LDL-C, AST, ALT, NEFA, and blood glucose levels in the HFD group showed a significant rise, while the serum HDL-C level showed a marked drop (p < 0.01). However, LBLF administration, especially for the medium and high dosages of LBLF, caused a lower serum AST, ALT, TG, TC, LDL-C, NEFA, blood glucose levels, and a higher serum level of HDL-C than the HFD group, and the intervention efficacy of LBLF treatment groups was similar (p < 0.05 or p < 0.01).
Table 2 indicated that hepatic lipids, including TG and TC, in the HFD group were higher than the in NC group with remarkable differences (p < 0.01). However, LBLF administration, especially for the medium and high dosages of LBLF, caused a remarkable reduction of TC and TG in comparison with the HFD group (p < 0.05), and a more remarkable reduction was shown in the high doses of the LBLF group (p < 0.01).
Figure 2 showed that the AUC of blood glucose concentration with time in the HFD group was notably increased (p < 0.05) in contrast with the NC group. However, the AUC in the LBLF-dose groups was notably reduced (p < 0.05) in contrast to the HFD group.
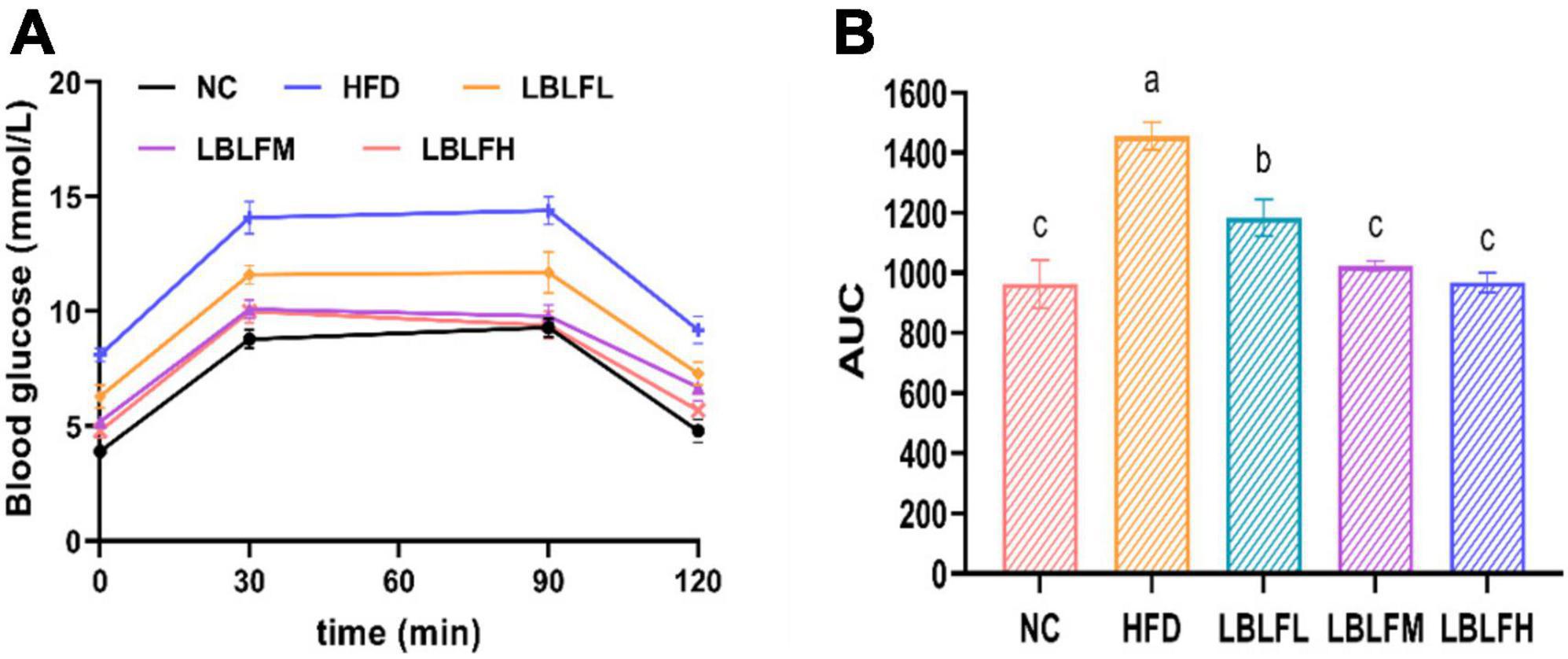
Figure 2. Effects of LBLF supplement on glucose tolerance in obese mice. (A) Oral glucose tolerance test (OGTT); (B) the integrated areas under each curve (AUC) after oral glucose. Different lowercase letters with significant differences (p < 0.05).
The regulation of LBLF on content of fecal lipids and activity of serum lipase in obese mice
Figure 3 showed the findings from a measurement of the lipids, including TC, TG, and TF, in feces. The HFD group had higher TC, TG, and TF contents, which was statistically different (p < 0.01) from the NC group. However, in contrast to the HFD group, LBLF treatment, particularly for the medium and high doses of LBLF, induced a noticeable elevation of TC, TG, and TF in feces (Figures 3A–C). Furthermore, with higher LBLF administration, serum LPS activity in LBLF-dose groups was decreased (p < 0.01) (Figure 3D). The findings mentioned above showed that LBLF intervention could successfully raise fat contents in feces.
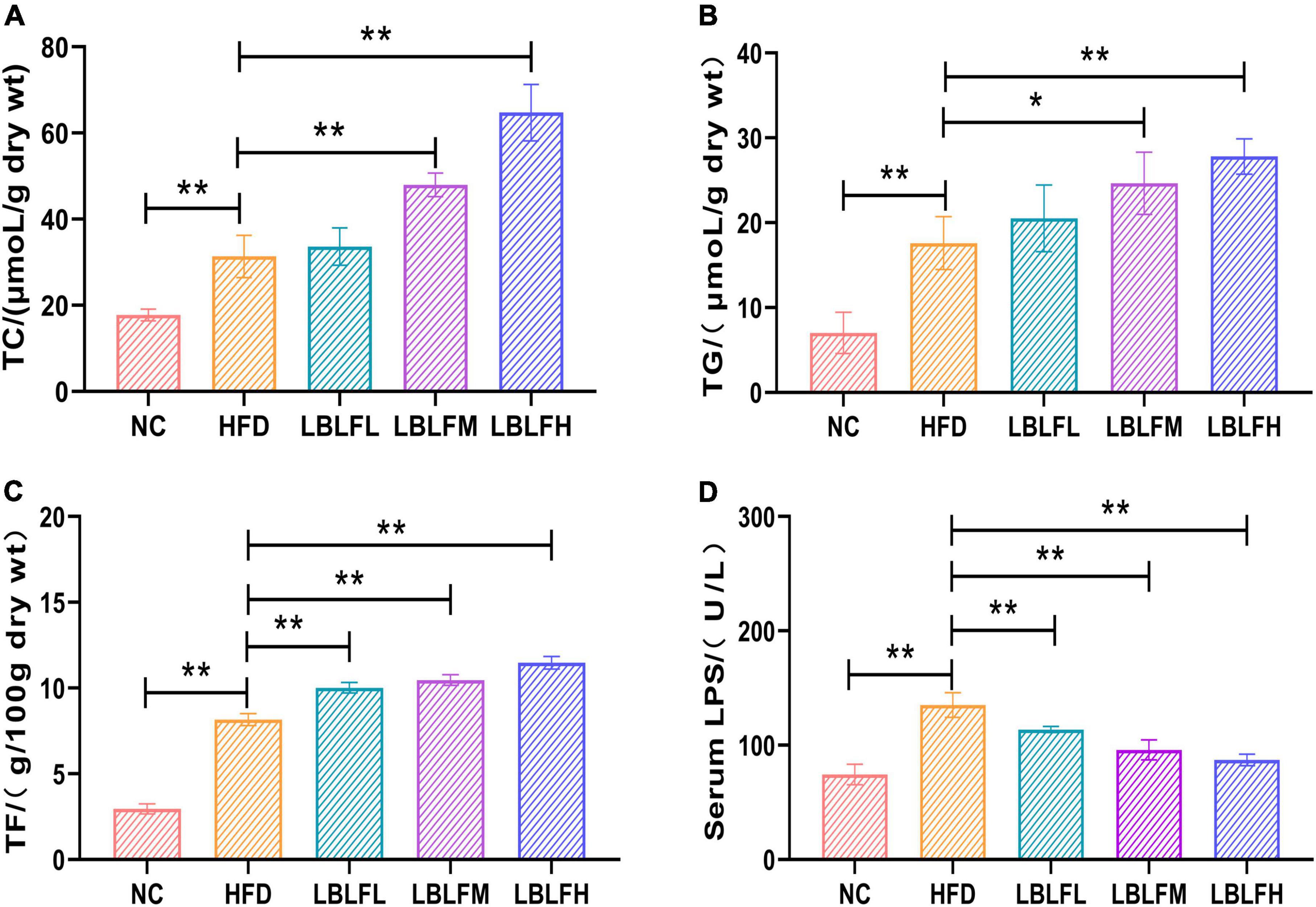
Figure 3. Effects of LBLF supplement on fecal lipids content and serum LPS (lipase) activity in obese mice. (A) Mean content of TC (total cholesterol); (B) mean content of TG (total triglyceride); (C) mean content of TF (total fat); (D) mean activity of serum LPS (lipase). (*p < 0.05) and (**p < 0.01) vs. HFD group.
LBLF suppresses hepatic and serous oxidative stress in obese mice
The serous and hepatic antioxidant enzyme activities (GSH-Px, CAT, SOD), MDA, PC contents, and T-AOC were measured to evaluate oxidative stress levels in mice and the results are seen in Figure 4. In contrast to the NC group, serum GSH-Px, CAT, SOD activities, and T-AOC in the HFD group were remarkably reduced (p < 0.01), while MDA and PC contents were remarkably increased (p < 0.01), which was almost the same variations in those of liver. In contrast with the HFD group, serum GSH-Px, SOD, and CAT activities, and T-AOC in medium and high doses of LBLF exhibited a remarkable rise (p < 0.01 or p < 0.05), while MDA and PC contents showed a notable drop (p < 0.01 or p < 0.05), which was similar alterations in those of liver.
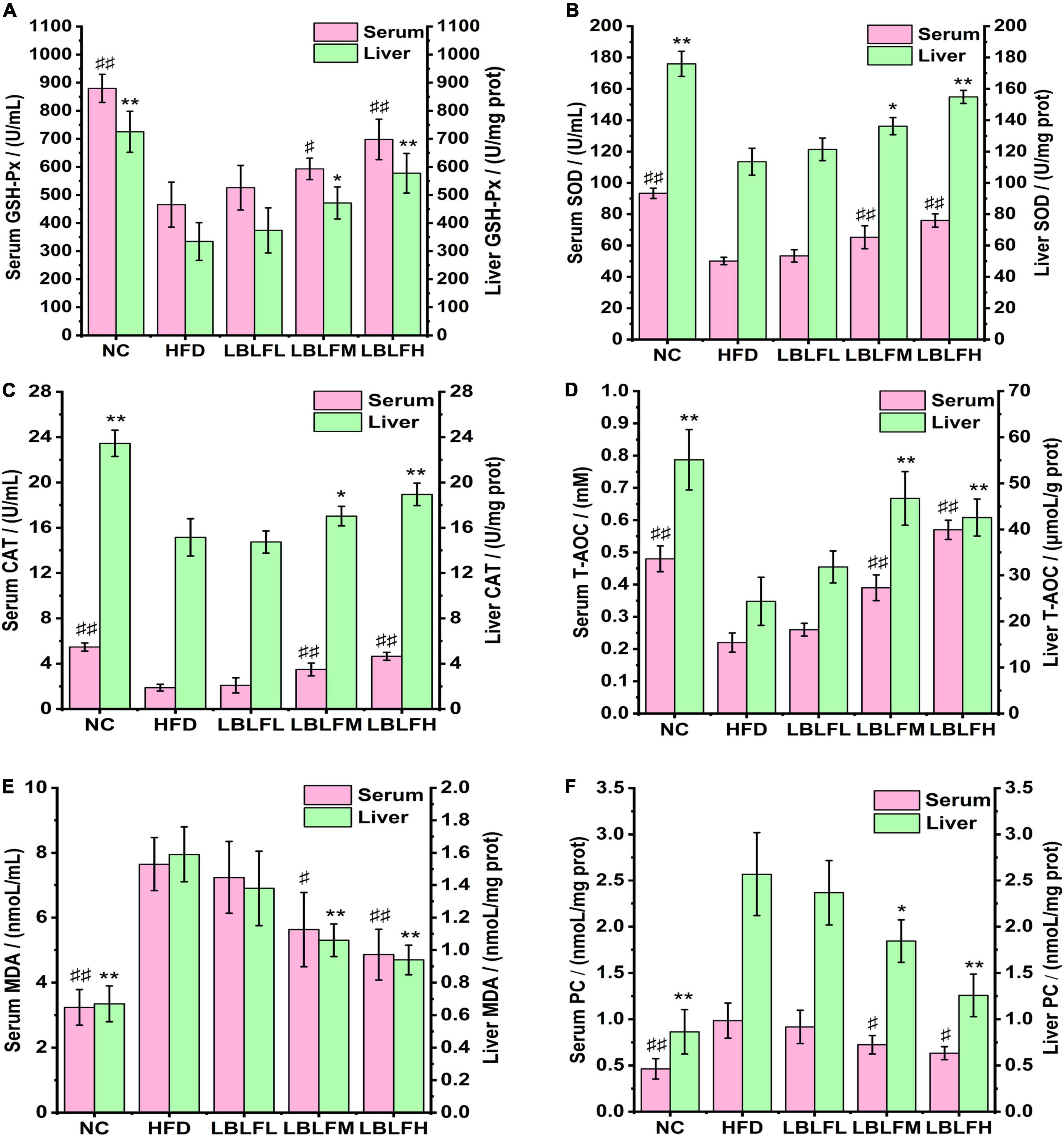
Figure 4. Effects of LBLF supplement on markers of oxidative stress, including (A) GSH-Px, (B) SOD, (C) CAT, (D) T-AOC, (E) MDA, and (F) PC, in serum and liver. Serum markers: (#p < 0.05) and (##p<0.01) vs. HFD group. Liver markers: (*p < 0.05) and (** p < 0.01) vs. HFD group.
Pathological analysis of liver and adipose tissues
As shown in Figure 5, HE staining revealed that the adipocytes in the HFD group were inhomogeneous in size and figure. Moreover, other histopathological features including cellular rupture, fusion, and denaturation also appeared in the HFD group (Figure 5B). Obviously, high-fat diet-induced multiple histopathological symptoms were effectively lightened after administration with LBLF. In contrast to the NC group, hepatocytes filled with a considerable number of varying sizes of fat vacuoles were observed in the HFD group. Nevertheless, the number of adipocytes showed a notable reduction in the liver tissues of the LBLFM and LBLFH groups compared to the HFD group (Figure 5A). High-fat diet intervention caused a notable increase in LI (Figure 5C) and AFI (Figure 5D) (p < 0.05), while the LI and AFI showed a noticeable reduction with the increased LBLF dosages and the significant differences of the LBLFM and LBLFH groups were found (p < 0.05) from the HFD group.
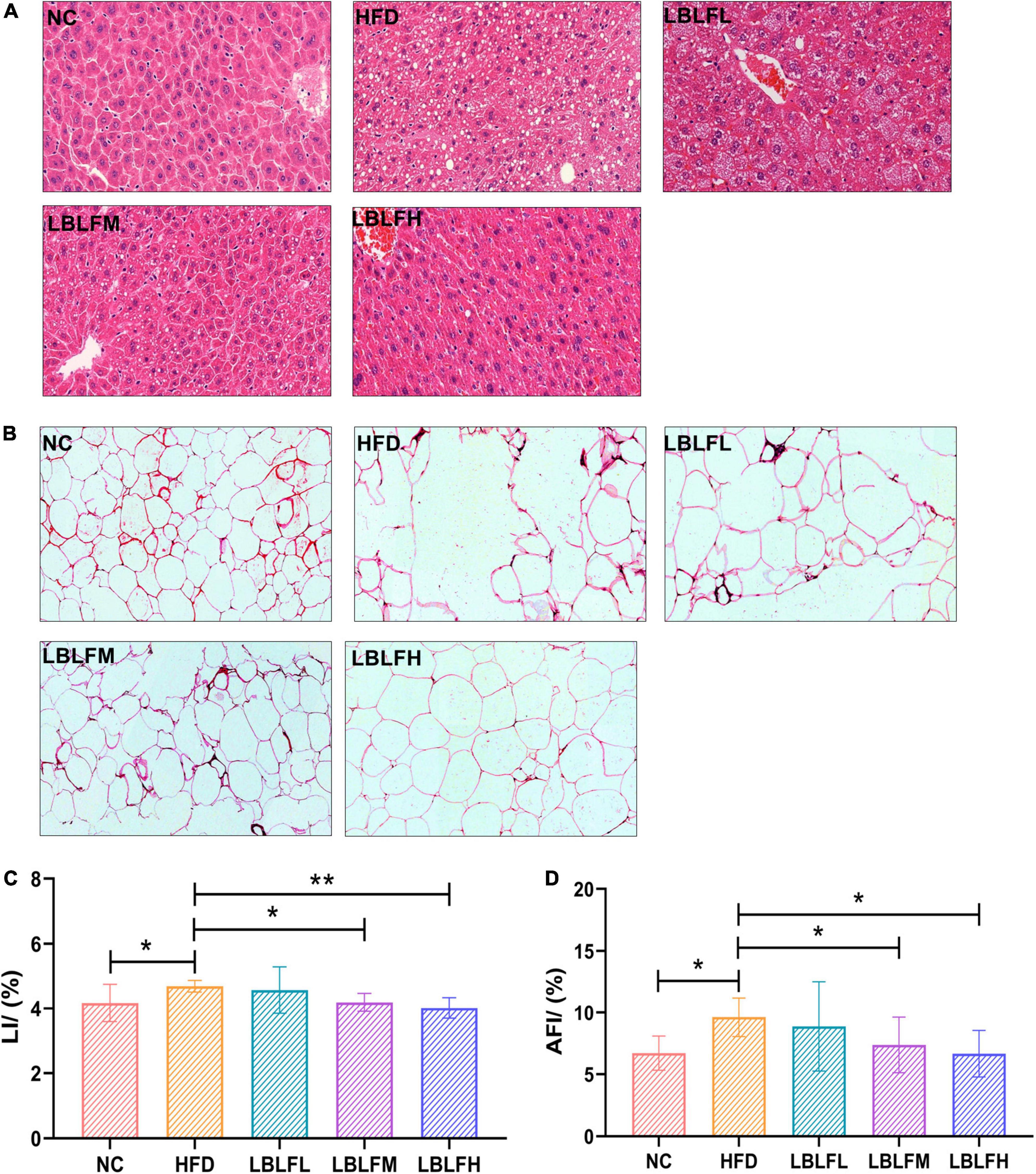
Figure 5. Effects of LBLF supplement on photographs of HE-stained sections of tissues and their related indexes in obese mice. (A) HE-stained sections of liver (×200); (B) HE-stained sections of abdominal fat tissues (×200); (C) LI (liver index); (D) AFI (abdominal fat index). (*p < 0.05) and (**p < 0.01) vs. HFD group.
Effects of LBLF on the gut bacterial composition in obese mice
Figure 6 illustrates indices linked to α-diversities to display the variety of gut bacteria. Additionally, a heatmap of the β-diversity analysis by distance matrix was employed to provide a profile of the extent of the similarities of the intestinal bacterial compositions across groups. High-fat diet intervention resulted in a more pronounced loss in the diversity of the bacterial community than in the NC group, according to the α-diversities related indexes (Figures 6A–C). Nevertheless, after LBLF therapy, the gut bacterial diversities were gradually restored. Additionally, LBLF administration enhanced the extent of similarity of microbial community compositions between LBLF treatment groups and NC groups with decreased difference coefficient on heatmap (Figure 6D), according to the β-diversity analysis.
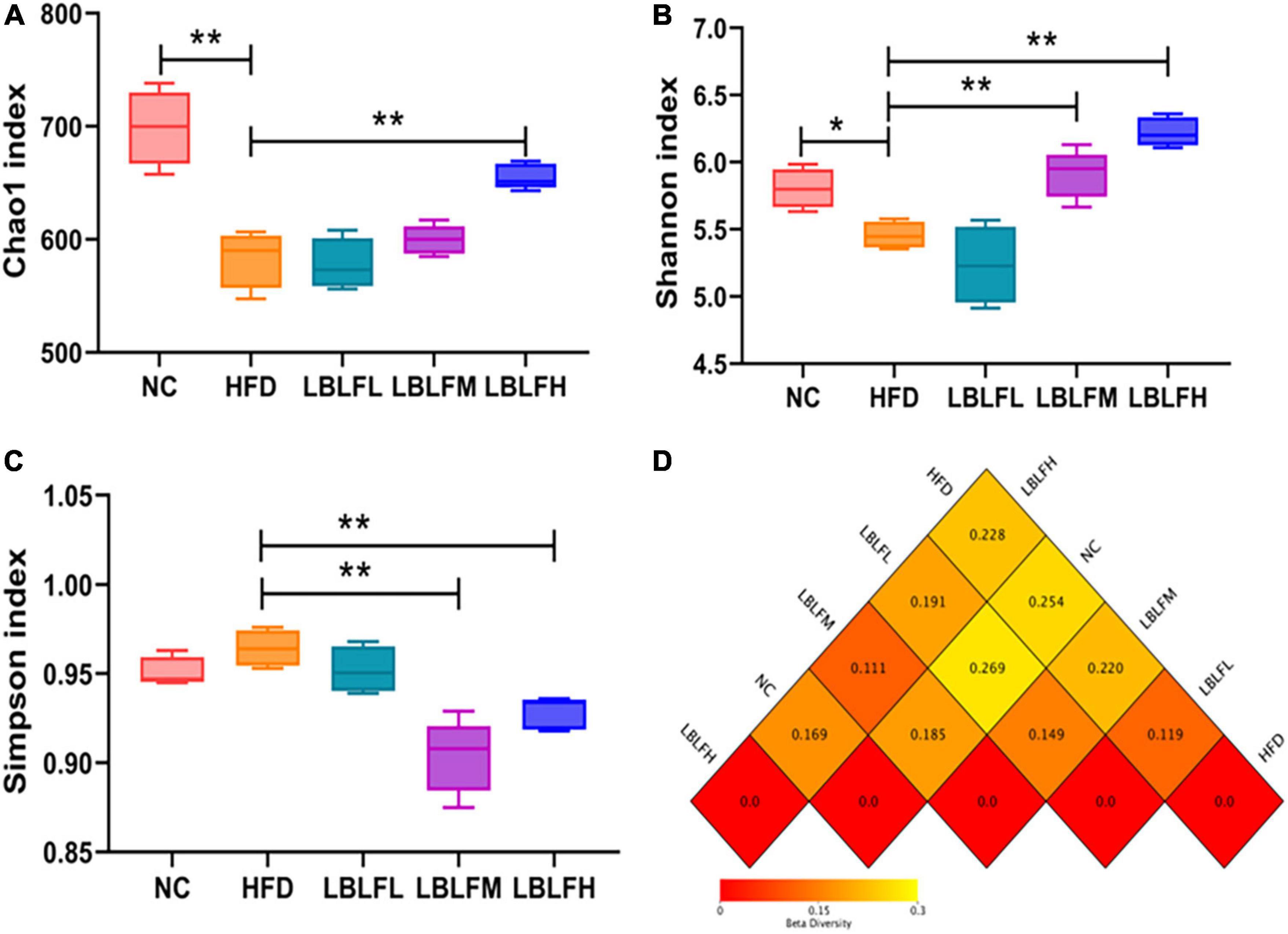
Figure 6. Effects of LBLF supplement on gut microbial α-diversity and β-diversity in obese mice. (A) Chao1 index, (B) Shannon index, and (C) Simpson index as the estimators of α-diversity of gut microbiota. Increased α-diversity with the increase of Chao1 index and Shannon index as well as the reduction of Simpson index. (D) Distance matrix heatmap as the estimators of β-diversity of gut microbes showed the extent of the similarities of the intestinal microbial compositions. A smaller difference coefficient means a bigger extent of the similarity (*p < 0.05) and (**p < 0.01) vs. HFD group.
As shown in Figure 7, higher relative abundances of Lachnoclostridium, Alistipes, Lachnospiraceae_NK4A136_group, Desulfovibrio, Odoribacter, Turicibacter, and lower relative abundances of Dubosiella, Lactobacillus, Akkermansia, Parasutterella, Bifidobacterium, and Parabacteroides were exhibited in the HFD group than the NC group, while LBLF administration partially reversed these changes. The relative contents of the remaining three microbial genera including Bacteroides, Clostridium_sensu_stricto_1, and Muribaculaceae in the HFD group exhibited no obvious difference in contrast with the NC group. Furthermore, relative abundances of Bacteroides and Muribaculaceae were obviously increased concerning the supplement of LBLF. Meanwhile, the relative content of Clostridium_sensu_stricto_1 was obviously reduced with increased LBLF dosages.
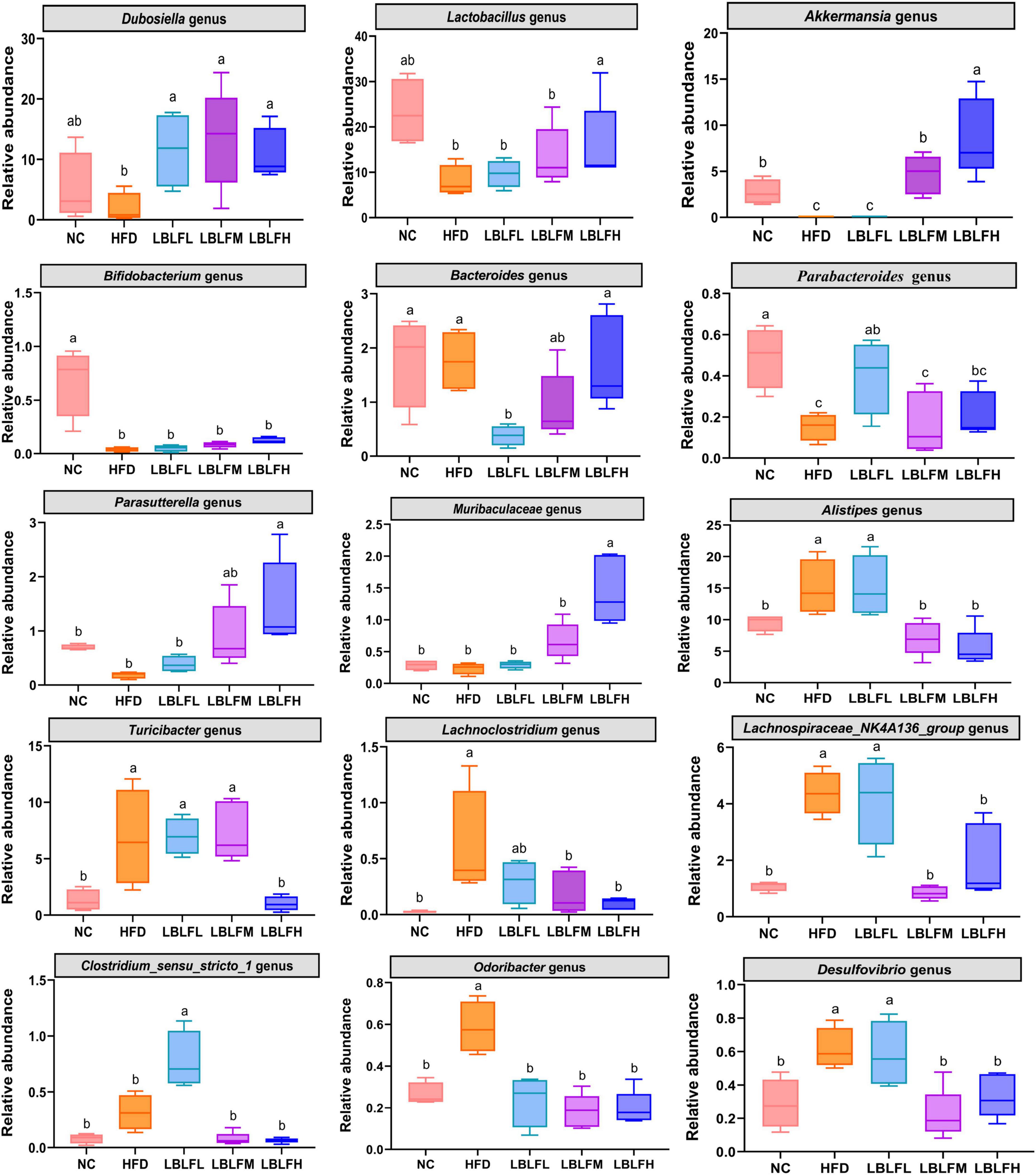
Figure 7. Comparison of relative abundances of the 15 vital intestinal microbes at the genus level. Different lowercase letters with significant differences (p < 0.05).
Additionally, we used Spearman’s correlation analysis to determine the relationships between changes in filtered 15 key microbial genera and high-fat diet-related indexes (the body weight, Lee’s index, liver index, abdominal fat index, serum lipids level, serum transaminase levels, hepatic lipids levels, blood glucose levels, and oxidative stress levels) in order to investigate the impact of gut microbial compositions on the high-fat diet-induced obesity (Figure 8). The relative abundances of seven microbes, including Lachnoclostridium, Alistipes, Lachnospiraceae_NK4A136_group, Desulfovibrio, Odoribacter, Clostridium_sensu_stricto_1, and the Turicibacter, were positively correlated with high-fat diet-associated indexes. Instead, the relative abundances of 6 microbes that benefit from LBLF, including Dubosiella, Lactobacillus, Akkermansia, Parasutterella, Bifidobacterium, Bacteroides, and Muribaculaceae, were inversely correlated with high-fat diet-associated indexes.
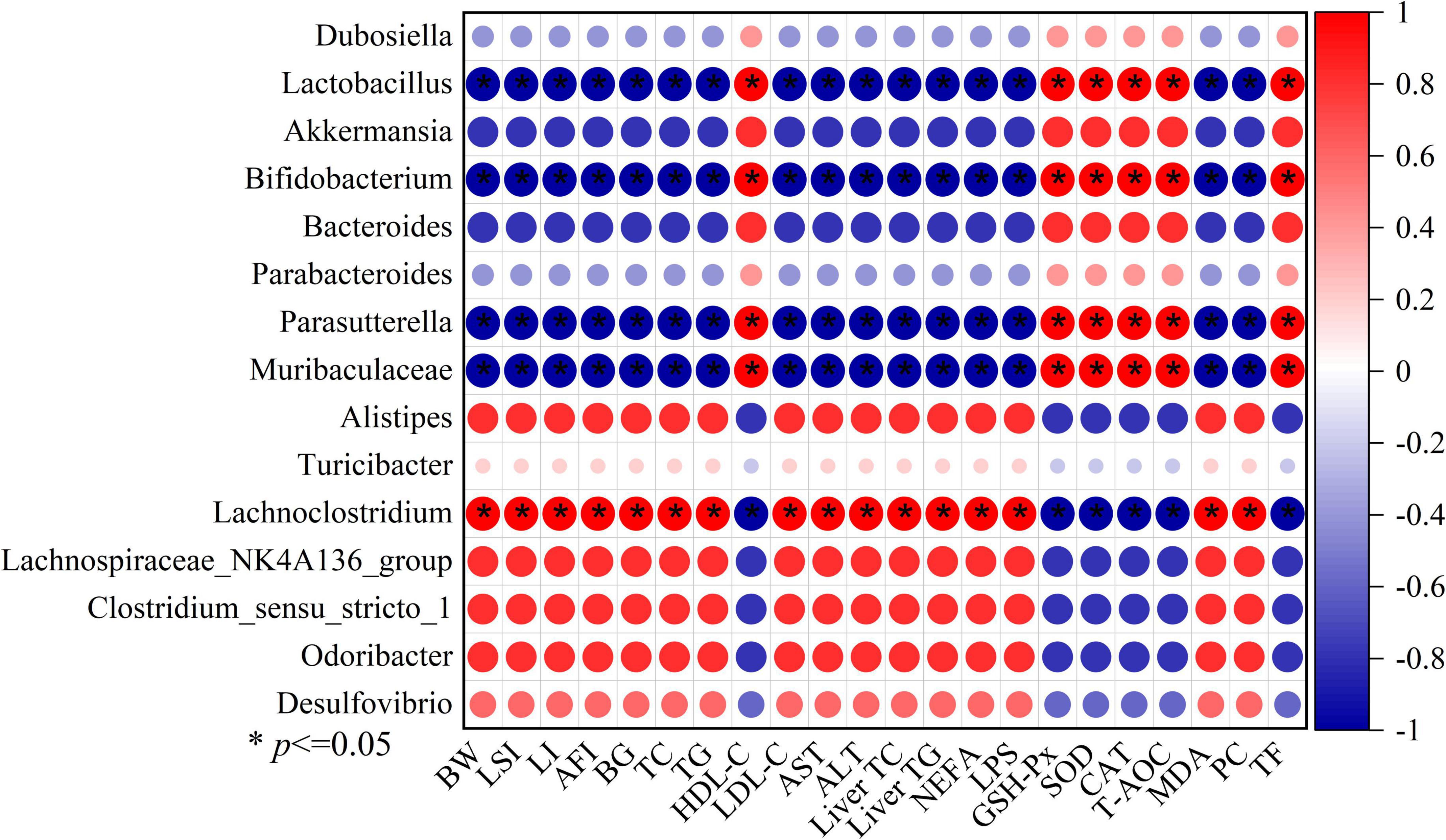
Figure 8. Spearman’s correlation analysis between 15 key intestinal microbes and 22 obesity-related indexes, including BW (body weight), LSI (Lee’s index), LI (liver index), AFI (abdominal fat index), BG (blood glucose), serum TC, TG, HDL-C, LDL-C, AST, ALT, liver TC, liver TG, NEFA (serum non-esterified fatty acids), LPS (serum lipase), serum GSH-Px, SOD,CAT, T-AOC, MDA, PC (protein carbonyl), and TF (total fecal fat). Significant correlations with (* p < 0.05).
Discussion
One heterocyclic ring (C) and two phenolic rings (A and B) make up the fundamental chemical structure of flavonoids, and hundreds of other compounds may be defined by modifying this structure (23). The anti-obesity, anti-hyperlipidemia, hepatoprotective, and antioxidative properties of plant-derived flavonoids have been demonstrated to offer significant therapeutic promise (24, 25). Our previous research detected five phenolic monomers (rutin, chlorogenic acid, kaempferol, coumaric acid, and caffeic acid) from total flavonoid extract in L. barbarum leaves (26). In addition, the content of LBLF was detected by Al(NO3)3-NaNO2 colorimetric, and exceeded 83% in this study. Experimental animals on a high-fat diet frequently develop intestinal bacterial disorders in addition to an abnormal rise in body weight, serum lipids levels, and blood glucose levels (27). Through a high-fat diet intervention, we successfully created obese model mice with excessive body weight and lipids metabolism abnormalities. In high-fat diet-induced rats with hyperlipidemia, flavonoids were shown to lower serum TG, TC, LDL-C, AST, and ALT levels as well as body weight (28), which was consistent with our findings. After being exposed to a high-fat diet, the liver—one of the primary metabolic organs in vivo—deposited too many adipose tissues, damaging hepatocytes and then raising the risk of obesity (29). As anticipated, LBLF supplementation effectively attenuated fatty liver and its related metabolic alterations in obese mice.
We discovered that LBLF significantly raised the amount of TC, TG, and TF in feces while successfully inhibiting serum LPS activity. According to growing evidence, the primary enzyme for digesting lipids, pancreatic lipase, may hydrolyze approximately between 50 and 70% of all ingested fat in the intestinal tract (30). Some of the findings revealed that natural products, particularly flavonoids, might be a class of important inhibitors of pancreatic lipase (31, 32). For instance, Hou et al. (33) revealed that in vitro pancreatic lipase activity was strongly inhibited by flavonoids from Cortex Mori Radicis. We previously looked at the potent inhibitory effects of LBLF on pancreatic lipase in vitro, with a half-inhibitory concentration (IC50) of (0.910 ± 0.008) mg/ml, and reversible and non-competitive inhibition as the kind of inhibition (34). We might therefore conclude that LBLF’s inhibitory impact on pancreatic lipase activity, similar to the effects of weight-loss medications like orlistat, may be responsible for the increased fecal fat contents.
Increased oxidative stress was caused by a high-calorie diet and high dietary saturated fatty acids by stimulating multiple intracellular mechanisms including decreased antioxidant defenses, NADPH oxidases mediated superoxide production, oxidative phosphorylation in mitochondria, glycoxidation, protein kinase C, chronic inflammation, and postprandial ROS generation, etc (35). High-level oxidative stress could stimulate the excessive deposition of adipose tissues, thereby accelerating the development of obesity by promoting the pre-adipocytes proliferation and increase of differentiated adipocytes in size (36, 37). However, natural flavonoids were responsible for ameliorating oxidative stress via stimulating enzyme activities or participating in several signaling pathways associated with oxidative stress in vivo, which had been fully elucidated in previous research (38, 39).
The antioxidant enzymes (GSH-Px, CAT, and SOD) as one of the principal members of the antioxidant defense system effectively relieved oxidative damage (40). The GSH-Px and SOD are mainly responsible for scavenging lipids peroxides such as MDA, blocking the chain reaction of free radicals. The superoxide ion free radicals can effectively be decomposed into hydrogen peroxide under SOD actions. The CAT can decompose harmful hydrogen peroxide. Hu et al. (41) demonstrated that flavonoids could improve antioxidant capacity via the increase of gene expression levels related to antioxidation such as SOD-3.
Flavonoids’ outstanding antioxidant abilities seem to be primarily attributed to their structural traits, which include their metal-chelating properties, the stereochemical features of the molecule, and their hydrogen donating properties of hydroxyl groups in ring B (42, 43). By chelating pro-oxidant metal ions like Cu(II), Zn(II), and Fe(II), for instance, flavonoids prevented the formation of ROS and free radicals, thereby blocking the process of lipids peroxidation and proteins oxidation (44), which may explain the reduction of MDA and protein carbonyl, one of the final markers of lipids and proteins peroxidation, respectively, caused by LBLF. Additionally, flavonoids’ hydrogen-donating abilities prevented free radical chain reactions. HDL-C was reported to exert an anti-atherogenic effect through reversely transporting cholesterol, but it was easily oxidized due to its excellent antioxidative actions (45). It is clear that LBLF can shield HDL-C from oxidative deterioration. Thus, using flavonoids to prevent the harm caused by free radicals and oxidants may be a successful therapeutic strategy for treating obesity.
Correlation analysis between intestinal bacteria and high-fat diet-associated indices in our study indicated that gut microecology, a crucial internal environmental element, had significant effects on the development of obesity. In addition to improving antioxidant capacity and increasing fecal lipids excretion, Lactobacillus, Bifidobacterium, Akkermansia, Muribaculaceae, and Parasutterella also decreased serum and hepatic lipids levels. Instead, Turicibacter, Lachnoclostridium, Alistipes, Lachnospiraceae_NK4A136_group, Desulfovibrio, and Odoribacter significantly exacerbated the detrimental metabolic alterations in a high-fat diet. By producing metabolites and enzymes such as bile acids, indole, short-chain fatty acids, and caseinolytic proteas B as signal molecules, gut microorganisms may interact with the intestinal barrier’s operation and other metabolic processes (46, 47). LBLF intervention obviously increased the relative content of Dubosiella genus, which was convinced to be a potential gut beneficial bacterium due to its anti-inflammatory and anti-oxidation properties. Researchers discovered that by increasing the activity of bile brine hydrolysis enzymes, Lactobacillus and Bifidobacterium might encourage the breakdown of hepatic lipids and enhance the excretion of fecal lipids (48). Numerous studies showed that Lactobacillus and Bifidobacterium enhanced the generation of tryptophan-derived metabolites, which were directly related to the body weight loss in rats and the reduction of liver damage via effects on numerous essential tryptophan catabolic enzymes (49, 50). Additionally, by promoting the growth of epithelial cells and the synthesis of antimicrobial peptides, tryptophan-derived indole metabolites might reduce intestinal inflammatory damage and improve intestinal barrier integrity (51). Therefore, increased concentrations of Lactobacillus and Bifidobacterium might reduce obesity brought on by a high-fat diet. The Akkermansia genus had been reported to have the excellent ability to improve gut barrier function and reduce body weight (52). Several members of Muribaculaceae could lengthen the colon while lowering inflammatory cytokine levels, including IL-6, TNF-α, and IL-1β (53). One of the notable side effects of high-fat diet-induced obesity was the burst of inflammatory cytokines, directly related to energy management and metabolism (54). Inflammatory cytokines were also a significant contributor to the development of obesity-related comorbidities (55). Nevertheless, the relative abundance of Muribaculaceae was obviously raised by LBLF ingestion. Researchers found that decreased lipid levels in the serous and hepatic tissues were strongly connected with elevated parasutterella levels (56), although the precise mechanism is yet unknown. Short-chain fatty acid manufacturers including Lactobacillus, Bifidobacterium, and Bacteroides genera have been found to inhibit obesity and control the transcription and expression of enzymes involved in lipids metabolism (57, 58). Overall, some of the gut microbes that contribute to the mitigation of obesity and its related metabolic changes are selectively enriched after LBLF intervention.
An earlier study revealed that consuming a high-fat diet was directly related to the enrichment of the Alistipes genus in mice models and humans (59). Yang et al. (60) also found that enrichment of several members of Alistipes genus seriously impaired gut barrier function. Additionally, according to Gao et al. (61), the enrichment of the proinflammatory bacteria of the Alistipes genus might cause hepatic inflammation by managing mRNA expression of intestinal tight junctions proteins such as ZO-1, occludin that would then increase intestinal permeability. One of the specific microorganisms in the HFD group and a role in the serum rise of TC and TG were said to be the Turicibacter genus (62). Several microbial species from the Lachnoclostridium genus have been identified as possible colorectal cancer biomarkers (63). The transfer of lipopolysaccharide, one of the primary causes of obesity-related disorders, into the blood from the digestive tract may help some members of the Lachnospiraceae family to promote the development of insulin resistance and obesity (64). After LBLF administration, several gut bacteria that encourage obesity and its associated metabolic alterations were selectively decreased. Overall, it is predicted and stated that these gut bacteria might have a role in preventing or promoting obesity: 1) A long-term high-fat diet could increase intestinal permeability and damage gut barrier function in the host (65) as well as enhance relative contents of gut pathogenic bacteria such as Alistipes, Lachnoclostridium, etc. 2) Bacterial endotoxins, such as lipopolysaccharide, may be produced by gut pathogenic bacteria, leading to the formation of inflammation and oxidative stress (66). Additionally, the movement of endotoxin from the digestive tract into the blood circulation system was facilitated by impairing gut barrier function. 3) In response to the LBLF, some of the gut microbes such as Akkermansia, Lactobacillus, Bifidobacterium, etc might act as potential intestinal probiotics. These microbes may improve intestinal barrier function, relieve oxidative stress, inhibit the production of inflammatory factors, participate in cellular biochemical processes, and promote lipids metabolism via bacteria-mediated metabolites.
In conclusion, LBLF could prominently inhibit the development of high-fat diet-associated obesity and its related metabolic alterations with the reduction of body weight increase, Lee’s index, serum lipids, blood glucose, hepatic lipids, and oxidative stress levels, and increase of fecal lipids contents. Furthermore, LBLF increased gut bacterial community diversities, and the relative contents of beneficial bacteria while decreasing pathogenic bacteria. Taken together, the LBLF may offer a secure and innovative method for preventing foodborne obesity.
Data availability statement
The sequences data presented in this study are deposited in the NCBI Sequence Read Archive (SRA) repository, accession number: PRJNA855047.
Ethics statement
The animal study was reviewed and approved by Animal Welfare and Ethics Committee of Ningxia Medical University, Yinchuan, China.
Author contributions
JL designed the experimental scheme, performed the experiment operation, analyzed the data, and finished the manuscript. YF guided and supervised the whole experimental process. JG participated in partial experiments. YN, TF, and FW gave some constructive advice on experimental design and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research has gained financial support from the National Natural Science Foundation of China (32160534), Ningxia Key Research and Development Program (2020BBF03014), China, and the Natural Science Foundation of Ningxia, China (2022AAC03018).
Conflict of Interest
FW was employed by Ningxia Red Power Goji Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Financial assistance from the Natural Science Foundation of Ningxia and National Natural Science Foundation of China are sincerely thanked.
References
1. Liu HN, Hu WL, Li X, Hu FW, Xi YN, Su Z, et al. Do perfluoroalkyl substances aggravate the occurrence of obesity-associated glucolipid metabolicdisease. Environ Res. (2021) 202:111724. doi: 10.1016/j.envres.2021.111724
2. Alkhudhayri DA, Osman MA, Alshammari GM, Maiman SAA, Yahya MA. Moringa peregrina leaf extracts produce anti-obesity, hypoglycemic, anti-hyperlipidemic, and hepatoprotective effects on high-fat diet fed rats. Saudi J Biol Sci. (2021) 28:3333–42. doi: 10.1016/j.sjbs.2021.02.078
3. Guaraldi G, Milic J, Sebastiani G, Raggi P. Sarcopenic obesity at the crossroad of pathogenesis of cardiometabolic diseases. Atherosclerosis. (2021) 335:84–6. doi: 10.1016/j.atherosclerosis.2021.09.006
4. Hao YK, Zhou FB, Dong JJ, Wang Y, Lang ZF, Li S, et al. Study on the role of flavonoids derived extract from seed residues of hippophae rhamnoides on high-fat diet induced obese mice. J King Saud Univ Sci. (2020) 32:1597–603. doi: 10.1016/j.jksus.2019.12.017
5. Bamforda NJ, Pottera SJ, Baskervillea CL, Harrisb PA, Baileya SR. Effectof increased adiposity on insulin sensitivity and adipokine concentrations indifferent equine breeds adapted to cereal-rich or fat-rich meals. Vet J. (2016) 214:14–20. doi: 10.1016/j.tvjl.2016.02.002
6. Lui PPY, Yung PSH. Inflammatory mechanisms linking obesity and tendinopathy. J Orthop Transl. (2021) 31:80–90. doi: 10.1016/j.jot.2021.10.003
7. Wilson S, Higgins V, Adeli K. Postprandial inflammation and metabolic dysfunction in adolescents with obesity and insulin resistance. Am Heart J. (2021) 242:171–2. doi: 10.1016/j.ahj.2021.10.065
8. Lim S, Kim JW, Targher G. Links between metabolicsyndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrin Met. (2021) 32:500–14. doi: 10.1016/j.tem.2021.04.008
9. Chakrabarti M, Ghosh I, Jana A, Ghosh M, Mukherjee A. Genotoxicity of antiobesity drug orlistat and effect of cafeine intervention: an in vitro study. Drug Chem Toxicol. (2016) 40:339–43. doi: 10.1080/01480545.2016.1236128
10. George G, Sridhar S.N.C., Paul AT. Investigation of synergistic potential of green tea polyphenols and orlistat combinations using pancreatic lipase assay-based synergy directed fractionation strategy. S Afr J Bot. (2016) 135:50–7. doi: 10.1016/j.sajb.2020.08.009
11. Liu HH, Fan YL, Wang WH, Liu N, Zhang H, Zhu ZY, et al. Polysaccharides from Lycium barbarum leaves: isolation, characterization and splenocyteproliferation activity. Int J Biol Macromol. (2012) 51:417–22. doi: 10.1016/j.ijbiomac.2012.05.025
12. Zhang B, Wang MZ, Wang C, Yu TT, Wu Q, Li Y, et al. Endogenous calcium attenuates the immunomodulatory activity of a polysaccharide from Lycium barbarum L. leaves by altering the global molecular conformation? Int J Biol Macromol. (2019) 123:182–8. doi: 10.1016/j.ijbiomac.2018.11.067
13. Valkoa M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J, et al. Free radicals and antioxidants in normal physiological functions and human disease. IJBCB. (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
14. Li WX, Wang LT, Huang WJ, Skibba M, Fang QL, Xie LT, et al. Inhibition of ROS and inflammation by animidazopyridine derivative X22 attenuate high fat diet-induced arterial injuries. Vasc Pharmacol. (2015) 72:153–62. doi: 10.1016/j.vph.2015.05.006
15. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J.Clin.Invest. (2004) 114:1752–61. doi: 10.1172/JCI200421625
16. Chen L, Ma X-B, Liang Y-H, Pei S-C, Feng Y-P, Wei M, et al. Effect of persimmon leaf total flavonoid on enzyme of lipoprotein metabolis and antioxidation in hyperlipidemia rats. Chin J Nat Med. (2011) 9:74–7. doi: 10.1016/S1875-5364(11)60024-1
17. Teng J, Li J, Zhao YL, Wang MF. Hesperetin, a dietary flavonoid, inhibits AGEs-induced oxidative stress and inflammation in RAW264.7 cells. J Funct Foods. (2021) 81:104480. doi: 10.1016/j.jff.2021.104480
18. Ye J, Zhao Y, Chen XM, Zhou HY, Yang YC, Zhang XQ, et al. Pu-erh teaameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res Int. (2021) 144:110360. doi: 10.1016/j.foodres.2021.110360
19. Zhang YL, Xu YN, Zhang L, Chen YJ, Wu T, Liu R, et al. Licorice extract ameliorates hyperglycemia through reshaping gut microbiota structure and inhibiting TLR4/NF-κB signaling pathwayin type 2 diabetic mice. Food Res Int. (2022) 153:110945. doi: 10.1016/j.foodres.2022.110945
20. Wan Y, Tang J, Li JM, Li J, Yuan JH, Wang FL, et al. Contribution of diet to gut microbiota and related host cardiometabolic health: diet-gut interaction in human health. Gut Microbes. (2020) 11:603–9. doi: 10.1080/19490976.2019.1697149
21. Chen JH, Kou TT, Fan YL, Niu YH. Antioxidant activity and stability of the flavonoids from Lycium barbarum leaves during gastrointestinal digestion in vitro. Int J Food Eng. (2020) 16:417–22. doi: 10.1515/ijfe-2019-0315
22. Guo ZT, Hu B, Zhu LY, Yang YL, Liu CY, Liu F, et al. Microbiome-metabolomics insights into the feces of high-fat diet mice to reveal the anti-obesity effects of yak (Bos grunniens) bone collagen hydrolysates. Food Res Int. (2022) 156:111024. doi: 10.1016/j.foodres.2022.111024
23. Oteiza PI, Fraga CG, Galleano M. Linking biomarkers of oxidative stress and disease with flavonoid consumption: from experimental models to humans. Redox Biol. (2021) 42:101914. doi: 10.1016/j.redox.2021.101914
24. Umar A, Iskandar G, Aikemu A, Zhou WT, Berké B, Begaud B, et al. Effects of Cydonia oblonga Miller leaf and fruit flavonoids on blood lipids and anti-oxydant potential in hyperlipidemia rats. J Ethnopharmacol. (2015) 169:239–43. doi: 10.1016/j.jep.2015.04.038
25. Liu S, Chang X, Yu J, Xu W. Cerasus humilis cherry poly-phenol reduces high-fat diet-induced obesity in C57BL/6 mice by mitigating fat deposition, infammation, and oxidation. J Agr Food Chem. (2020) 68:4424–36. doi: 10.1021/acs.jafc.0c01617
26. Niu YH, Chen JH, Fan YL, Kou TT. Effect of flavonoids from Lycium barbarum leaves on the oxidation of myofibrillar proteins in minced mutton during chilled storage. J Food Sci. (2021) 86:1766–77. doi: 10.1111/1750-3841.15728
27. Xiao SW, Zhang ZM, Chen MJ, Zou JF, Jiang S, Qian DW, et al. Xiexin Tang ameliorates dyslipidemia in high-fat diet-induced obese rats via elevating gut microbiota-derived short chain fatty acids production and adjusting energy metabolism. J Ethnopharmacol. (2019) 241:112032. doi: 10.1016/j.jep.2019.112032
28. Ling Y, Shi Z, Yang XL, Cai ZW, Wang LX, Wu XM, et al. Hypolipidemic effect of pure total flavonoids from peel of Citrus (PTFC) on hamsters of hyperlipidemia and its potential mechanism. Exp Gerontol. (2020) 130:110786. doi: 10.1016/j.exger.2019.110786
29. Gisela G, Mónica CS, María DRP, María LGS, Pilar M. Wine pomace product modulates oxidative stress and microbiota in obesity high-fat diet-fed rats. J Funct Foods. (2020) 68:103903. doi: 10.1016/j.jff.2020.103903
30. Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. (2007) 12:879–89. doi: 10.1016/j.drudis.2007.07.024
31. Huang R, Zhang Y, Shen SY, Zhi ZJ, Cheng H, Chen SG, et al. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: an in vitro study. Food Chem. (2020) 326:126785. doi: 10.1016/j.foodchem.2020.126785
32. Zhang HL, Wu QX, Wei X, Qin XM. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Biosci. (2020) 37:100682. doi: 10.1016/j.fbio.2020.100682
33. Hou XD, Ge GB, Weng ZM, Dai ZR, Leng YH, Ding LL, et al. Natural constituents from Cortex Mori Radicis as new pancreatic lipase inhibitors. Bioorg Chem. (2018) 80:577–84. doi: 10.1016/j.bioorg.2018.07.011
34. Liao JL, Fang T, Fan YL. Inhibitory effects of Lycium Barbarum leaves flavonoids on pancreatic lipase activity. J Chi Ins Food Sci Tec. (2022) 22:43–53. doi: 10.16429/j.1009-7848.2022.05.006
35. Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. (2013) 14:10497–538. doi: 10.3390/ijms140510497
36. Rani V, Deep G, Singh RK, Palle K, Yadav UCS. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. (2016) 148:183–93. doi: 10.1016/j.lfs.2016.02.002
37. Lee HM, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion*. J Biol Chem. (2009) 284:10601–9. doi: 10.1074/jbc.M808742200
38. Shen N, Wang TF, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. (2022) 383:132531. doi: 10.1016/j.foodchem.2022.132531
39. Yang YC, Lii CK, Lin AH, Ye YW, Yao HT, Li CC, et al. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidativestress. Free Radical Bio Med. (2011) 51:2073–81. doi: 10.1016/j.freeradbiomed.2011.09.007
40. Olsvik PA, Kristensen T, Rosseland BO, Tollefsen KE, Baeverfjord G, Berntssen MHG. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of atlantic salmon (salmo salar) exposed to hyperoxic water during smoltification. Comp Biochem Phys C. (2005) 141:314–23. doi: 10.1016/j.cbpc.2005.07.009
41. Hu Q, Liu ZG, Guo YJ, Lu S, Du HZ, Cao Y. Antioxidant capacity of flavonoids from Folium Artemisiae argyi and the molecular mechanism in caenorhabditis elegans. J Ethnopharmacol. (2021) 279:114398. doi: 10.1016/j.jep.2021.114398
42. Martin MA, Ramos S. Cocoa polyphenols in oxidative stress: potential health applications. J Funct Foods. (2016) 27:570–88. doi: 10.1016/j.jff.2016.10.008
43. Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. (2010) 31:435–45. doi: 10.1016/j.mam.2010.09.006
44. Kada S, Bouriche H, Senator A, Demirtaş I, Özen T, Toptanci BC, et al. Protective activity of hertia cheirifolia extracts against DNA damage, lipid peroxidation and protein oxidation. Pharm Biol. (2017) 55:330–7. doi: 10.1080/13880209.2016.1261907
45. Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. JCEM. (2004) 89:4963–71. doi: 10.1210/jc.2004-0305
46. Wang JC, Xu WJ, Wang RJ, Cheng RR, Tang ZQ, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extra cellular availability through TLR2 signalling. Food Funct. (2021) 12:3597–610. doi: 10.1039/D1FO00115A
47. Kang YB, Kang X, Yang H, Liu HX, Yang XD, Liu QQ, et al. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol Res. (2022) 175:106020. doi: 10.1016/j.phrs.2021.106020
48. Yang XB, Mo WJ, Zheng CJ, Li WZ, Tang J, Wu XY. Alleviating effects of noni fruit polysaccharide on hepatic oxidative stress and inflammation in rats under a high-fat diet and its possible mechanisms. Food Funct. (2022) 11:2953–68. doi: 10.1039/D0FO00178C
49. Li XP, Suo JQ, Huang XG, Dai HF, Bian HW, Zhu MY, et al. Whole grain qingke attenuates high-fat diet-induced obesity in mice with alterations in gut microbiota and metabolite profile. Front Nutr. (2021) 8:761727. doi: 10.3389/fnut.2021.761727
50. Piotr K, Marek K, Marta GK, Piotr P, Agnieszka S, Marcin U, et al. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, reduces weight gain in rats. Nutrients. (2019) 11:591. doi: 10.3390/nu11030591
51. Wang XT, Naruhisa O, Paolo M, Lance K, Jose ZS, Celine E, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. (2014) 514:237–41. doi: 10.1038/nature13564
52. Liu ZB, Chen ZC, Guo HW, He DP, Zhao HR, Wang ZY, et al. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice†. Food Funct. (2016) 12:4869–79. doi: 10.1039/C6FO01439A
53. Wang Yi, Tao HX, Huang HM, Xiao YQ, Wu XX, Li MX, et al. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. (2021) 7:3142–58. doi: 10.1039/D0FO03061A
54. Naidu PB, Uddandrao VVS, Naik RR, Suresh P, Meriga B, Begum MS, et al. Ameliorative potential of gingerol: promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol. (2016) 419:139–47. doi: 10.1016/j.mce.2015.10.007
55. Su J, Liu XY, Li HQ, Cheng X, Shi SS, Li N, et al. Hypoglycaemic effect and mechanism of an RG-II type polysaccharide purified from Aconitum coreanum in diet-induced obese mice. Int J Biol Macromol. (2020) 149:359–70. doi: 10.1016/j.ijbiomac.2020.01.209
56. Mu HN, Zhou Q, Yang RY, Zeng J, Li XH, Zhang RR, et al. Naringin attenuates high fat diet induced non-alcoholic fatty liver disease and gut bacterial dysbiosis in mice. Front Microbiol. (2020) 11:585066. doi: 10.3389/fmicb.2020.585066
57. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Gibson GR, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. (2007) 50:2374–83. doi: 10.1007/s00125-007-0791-0
58. Donohoe DR, Garge N, Zhang XX, Sun W, O’Connell TM, Bunger MK, et al. The microbiome and butyrate regulate energy metabolism and autophag y in the mammalian colon. Cell Metab. (2011) 13:517–26. doi: 10.1016/j.cmet.2011.02.018
59. Wan Y, Wang FL, Yuan JH, Li J, Jiang DD, Zhang JJ, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. (2019) 68:1417–29. doi: 10.1136/gutjnl-2018-317609
60. Yang J, Wei H, Zhou YF, Szeto CH, Li CG, Lin YF, et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. (2022) 62:135.e–49.e. doi: 10.1053/j.gastro.2021.08.041
61. Gao SL, He YY, Zhang LP, Liu LN, Qu CF, Zheng Z, et al. Conjugated linoleic acid ameliorateshepatic steatosis by modulating intestinal permeability and gut microbiota in ob/ob mice. Food Nutr Res. (2022) 66:*p doi: 10.29219/fnr.v66.8226
62. Li TT, Tong AJ, Liu YY, Huang ZR, Wan XZ, Zhao C, et al. Polyunsaturated fattyacids from microalgae Spirulina platensis modulates lipid metabolism disorders and gut microbiota in high-fat diet rats. Food Chem Toxicol. (2019) 131:110558. doi: 10.1016/j.fct.2019.06.005
63. Liang JQY, Li T, Nakatsu G, Chen YX, Yau TO, Chu E, et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. BMJ. (2020) 69:1248–57. doi: 10.1136/gutjnl-2019-318532
64. Kameyama K, Itoh K. Intestinal Colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. (2014) 29:427–30. doi: 10.1264/jsme2.ME14054
65. Wang GL, Yang XY, Wang J, Zhong DY, Zhang RG, Zhang YN, et al. Walnut green husk polysaccharides prevent obesity, chronic inflammatory responses, nonalcoholic fatty liver disease and colonic tissue damage in high-fat diet fed rats. Int J Biol Macromol. (2021) 182:879–98. doi: 10.1016/j.ijbiomac.2021.04.047
Keywords: Lycium barbarum leaves, flavonoids, obesity, oxidative stress, gut bacteria
Citation: Liao J, Guo J, Niu Y, Fang T, Wang F and Fan Y (2022) Flavonoids from Lycium barbarum leaves attenuate obesity through modulating glycolipid levels, oxidative stress, and gut bacterial composition in high-fat diet-fed mice. Front. Nutr. 9:972794. doi: 10.3389/fnut.2022.972794
Received: 19 June 2022; Accepted: 04 July 2022;
Published: 28 July 2022.
Edited by:
Zhaojun Wei, Hefei University of Technology, ChinaReviewed by:
Yuqing Duan, Jiangsu University, ChinaLei Wang, State Key Laboratory of Biomacromolecules, Institute of Biophysics (CAS), China
Copyright © 2022 Liao, Guo, Niu, Fang, Wang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: YanLi Fan, ZmFueWFubGkubnh1QHFxLmNvbQ==
 JiaLe Liao
JiaLe Liao Jia Guo
Jia Guo YinHong Niu
YinHong Niu Tian Fang
Tian Fang FangZhou Wang
FangZhou Wang YanLi Fan
YanLi Fan