
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 11 August 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.970364
This article is part of the Research TopicThe Role of Soy in Human Health and DiseaseView all 5 articles
Soy is a hotly debated and widely discussed topic in the field of nutrition. However, health practitioners may be ill-equipped to counsel clients and patients about the use of soyfoods because of the enormous, and often contradictory, amount of research that has been published over the past 30 years. As interest in plant-based diets increases, there will be increased pressure for practitioners to gain a working knowledge of this area. The purpose of this review is to provide concise literature summaries (400–500 words) along with a short perspective on the current state of knowledge of a wide range of topics related to soy, from the cholesterol-lowering effects of soy protein to the impact of isoflavones on breast cancer risk. In addition to the literature summaries, general background information on soyfoods, soy protein, and isoflavones is provided. This analysis can serve as a tool for health professionals to be used when discussing soyfoods with their clients and patients.
A substantial amount of soy-related research has been conducted over the past 2–3 decades, as about 2,000 papers are indexed in PubMed annually. Much of this research has been conducted because the soybean is a uniquely rich source of isoflavones (1). Isoflavones have been purported to exert several health benefits, from reducing cancer risk (2, 3) to alleviating menopausal symptoms (4) and improving memory (5). On the other hand, isoflavones are also routinely classified in the scientific literature as endocrine disruptors (6–16), despite the conclusions of a recently published comprehensive technical review that neither soy nor isoflavones warrant such classification (17). Consequently, even though soyfoods have been consumed for centuries by Asian populations (18) and have become increasingly mainstream outside of Asia, they are not without controversy.
In addition to the traditional Asian soyfoods, questions have been raised about the healthfulness of soy protein ingredients (concentrated sources of soy protein) because of the processing they undergo (19, 20) and about soybean oil, because of its high omega-6 (n-6) polyunsaturated fatty acid (PUFA) content (21–23). Soy protein ingredients are the base for manufacturing modern soy products such as soy-based meat alternatives and are widely used by the food industry for their functional properties (24). Both soy protein and soybean oil play a huge role in the US food supply (17, 25).
Between 1998 and 2009, many concerns about soy were raised (26–31) and challenges to the proposed health benefits were made (32–34). These developments led to lay publications claiming soy is detrimental to health (35–37). This may explain why, according to a 2021 survey of 1,500 US consumers, fewer than half of baby boomers consider soyfoods to be somewhat/very healthy compared to 68% of generation Z-ers, as the latter demographic was not exposed to this information as adults (38). Hesitancy about soy exists despite worldwide recommendations emphasizing the personal and planetary health benefits of plant-based diets (39, 40).
Many health professionals may not be in a position to accurately advise their clients and patients about soy because of the vast amount of research that has to be reviewed in order to gain a working understanding of the subject. This review will provide concise literature summaries (400–500 words) along with a short perspective on the current state of knowledge of the more hotly debated and widely discussed soy-related topics, so that health practitioners will be able to provide well-informed recommendations and counsel. Prior to presenting these summaries, general background information on soyfoods, soy protein, and isoflavones is provided.
Regarding the perspectives, because there is some subjectivity involved in the evaluation of the literature when conducting narrative reviews, a conservative approach has been adopted when reaching conclusions about the strength of the data. As much as possible, emphasis was placed on systematic reviews, meta-analyses, and the positions of independent health agencies when formulating the perspectives. Generally speaking, clinical trials influenced conclusions more than observational studies, but some nuance is still required. Small, short-term clinical trials evaluating markers of disease risk may carry less weight than large, prospective, observational studies with long follow-ups evaluating disease outcome.
Historical records suggest the use of soybeans as a food originated in China possibly around 2,000 years ago (41), although archeological evidence indicates soybean domestication may have occurred several thousand years earlier (42). From China the soybean spread to Japan and other Southeast Asian countries although recent data suggest there may well have been multiple independent efforts to domesticate wild soybeans (43). There are two general categories of Asian soyfoods, fermented and unfermented. Fermented soyfoods include natto, tempeh and miso whereas unfermented foods include soymilk and tofu (Table 1). Tempeh is a more recent creation having been developed in Indonesia around the 1600s (44). Globally, most soy is consumed in the unfermented form (excluding soy sauce, which is a condiment, not a food) (45).
There is a vast array of foods made using concentrated sources of soy protein, often referred to as soy protein ingredients, as a base. The starting point for these ingredients are soybean flakes, which are produced by crushing soybeans and removing the oil using a food grade solvent. The primary soy protein ingredients are soy protein isolate (SPI), soy protein concentrate (SPC) and soy flour, which are comprised of ≥90, 65–90, and 50–65% protein, respectively (46).
These ingredients have been extensively used by the food industry for decades. They are used in a wide range of foods because of their functional properties such as solubility, gelation, hydrating capacity, emulsification, adhesion/cohesion, and foaming (25, 47). Because they are added to foods in such small quantities when used in this way, their contribution to protein intake is negligible. More relevant from a nutritional perspective is the use of these products in the manufacture of dairy and meat alternatives, and as a means of delivering high quality protein in a variety products such as cereals, energy bars and infant formula (25).
The protein digestibility corrected amino acid score (PDCAAS) is the method for determining protein quality accepted by most regulatory bodies including the Food and Agriculture Organization (FAO) of the United Nations and the US Food and Drug Administration (FDA). PDCAAS is determined by comparing the profile (mg/g protein) of indispensable amino acids (IAAs) in a protein with the biological requirement for the IAAs and then correcting for digestibility based on the digestibility of protein at the end of the large intestine (fecal digestibility) in rats.
Considerable soy protein quality research has been conducted, although until recently that research focused mostly on concentrated sources of soy protein such as SPI and SPC. Soy protein is well-digested and has an IAA pattern that closely matches human requirements. The limiting amino acids in soy protein are the sulfur-containing amino acids (SAA), methionine and cysteine. SPI and SPC have a PDCAAS of ~1.0 (scores are truncated at 1.0 or 100% using this methodology) (48–50). Research published in 2011 showed the PDCAASs for SPI and SPC are slightly higher than for beef (0.92) and much higher than for other plant proteins (e.g., pea protein concentrate, 0.73; kidney beans, 0.68; pinto beans, 0.63; rice, 0.53; wheat gluten, 0.25).
However, beginning in 2011, the FAO convened a series of meetings of experts in protein quality methodology. The reports of these meetings recommend gradually shifting from the PDCAAS to one of five potential methods for assessing protein quality. The most well-known is the digestible indispensable amino acid score (DIAAS), which has been widely used in the animal feed industry and has received the most support. Since some methodological issues remain to be resolved, and limited data exist on the quality of proteins using this method, it will likely be many years before regulatory bodies adopt the DIAAS (51). Nevertheless, it is now quite common to see in the literature protein quality data based on DIAAS.
In contrast to the PDCAAS, when using the DIAAS, protein quality scores are not truncated (i.e., scores can be above 1.0 or 100%), and digestibility is based on the digestibility of individual IAAs determined at the end of the small intestine (ileal digestibility) using pigs or humans. Additionally, the FAO developed three new IAA scoring patterns for evaluating protein quality (birth to 6 months; 6 months to 3 years and >3 years [which includes older child, adolescent, and adult]). Recently, tofu (52), soymilk (52), and a popular soy-based burger (53) received scores using the DIAAS of 97, 117, and 107%, respectively when using the IAA reference pattern for the older child, adolescent, and adult. In comparison, when using this same reference pattern, 80% lean ground beef and a popular pea protein-based burger received scores of 110 and 83%, respectively (53).
There has been considerable investigation of the ability of soy protein to stimulate muscle protein synthesis (MPS) and to promote gains in muscle mass and strength in response to resistance exercise training (RET). Whey protein, which represents ~20% of the protein in cow's milk, has traditionally been considered the optimal protein for building lean body mass (54–56) because it is high in the branched chain amino acid leucine, an important trigger for MPS (57). Acute feeding studies (~4 h) show consuming whey protein stimulates MPS to a greater extent than soy protein (58–64). However, the entire hypertrophic period following resistance exercise is unlikely to be captured by short-term studies. Therefore, acute studies identifying differences among protein sources measuring MPS may not predict long-term changes in gains in muscle mass and strength (65, 66). The results of a recently published meta-analysis that included nine clinical trials supports this conclusion in that it was found soy protein promotes gains in muscle mass and strength similarly to whey and other animal proteins (67).
Based on their systematic review, meta-analysis and meta-regression, Morton et al. (68) concluded that protein source likely plays a minor, if any, role in determining RET-induced gains in fat-free mass and strength over a period of weeks. Recently, Morgan and colleagues (69) determined that protein quality likely has a significant, although small benefit in both young and older adults on indices of muscle protein anabolism. Their analysis found that higher protein quality was associated with superior strength gains in response to RET but not with changes in lean body mass (69). While there may be some uncertainty about the precise impact of protein quality, there is little disagreement that the protein requirements of individuals engaged in endurance and RET exceed those of the general population by anywhere from as little as an additional 50% to as high as 250% (68, 70–74).
There is considerable interest in the role of protein in body weight management (75, 76). Some evidence suggests that dietary protein is the most effective macronutrient at providing a satiating effect (77). After adjustments for a wide range of factors, Lieberman et al. (78) recently found that among 14 developed countries, protein intake, regardless of demographic and lifestyle factors, was consistently ~16% of total energy, which is nearly twice the amount needed to meet the adult protein recommended dietary allowance (RDA) (79). In contrast, there were relatively large variations in the amount of carbohydrate and fat consumed by country.
Lieberman and colleagues (78) proposed that protein intake is tightly regulated by biological control mechanisms. This proposal concurs with the protein leverage hypothesis (PLH). (80). According to the PLH, there is a strong biological propensity to regulate the quantity of protein consumed (81). According to this hypothesis, diets with a lower protein content as a percentage of calories, could stimulate caloric intake. However, some researchers have concluded that “… no individual nutrient is a friend or a foe when it comes to weight loss and its maintenance.” (82).
A review published in 2008 found that soy is as good as other protein sources for promoting weight loss (83). More recently, the authors of a double-blind, randomized cross-over study involving 17 healthy adults concluded that consuming soy protein exerts comparable effects to whey protein on appetite profile, energy metabolism, and subsequent energy intake. For this study, during each of the three testing visits, the participants were given one of three breakfast meals and an ad libitum lunch, while appetite ratings and metabolic testing were assessed for the following 3 h. Energy intake at lunch was measured at 180 min after completion of breakfast. (84). These findings align with other research, which indicates there is little evidence suggesting one source of protein is more effective than another as an aid for weight management (85–89).
Isoflavones, which are diphenolic molecules, naturally occur in plants and act by binding to both estrogen receptors (ER), ERα and ERβ, thereby influencing gene transcription.
The potency of isoflavones relative to estrogen is difficult to assess. Potency for compounds that bind to ERs is typically discussed in terms of relative binding affinity (RBA) and compared to 17β-estradiol, with the potency of the latter arbitrarily set at 100. Depending on the isoflavone and ER, estimates range from isoflavones being only about 1/1,000 as potent (90) to nearly as potent (91). However, RBA does not completely capture potency. The physiological effect of ligands binding to ERs will depend upon the conformational shape of the ligand-receptor complex, the relative ratio of the two ERs, and the types of co-repressors and co-activators in cells. All of these factors can strengthen or weaken the biological activity of the ligand. Also, there may be isoflavone metabolites formed within cells that are more or less potent than their parent compound (92, 93).
Isoflavones were identified as ER agonists in the 1950s (94, 95) and in the 1960s, as possible ER antagonists (anti-estrogens) (96). The main dietary source of isoflavones are legumes from the family Fabaceae (97), namely soybeans (Glycine max). Mean isoflavone intake in Japan among older adults ranges from ~30 to 50 mg/d (45, 98) whereas daily per capita intake in the United States (99–101) and Europe (102, 103) is <3 mg, although recent reports suggested daily intake may be as high as 7 mg in France (104) and was estimated at 4–6 mg among British adults although little of that came from soyfoods (103).
The three soybean isoflavone aglycones, genistein, daidzein, and glycitein, have molecular weights (g/mol) of 270, 254.2, and 284.3, respectively. These three aglycones and their glycosides (the predominant form in unfermented soy) account for about 50, 40, and 10%, respectively, of total isoflavone content (105). In plants isoflavones function as phytoalexins and as such accumulate during stress, such as during microbe attacks (106). Isoflavones also play a role in nitrogen fixation, thereby reducing the need for nitrogen fertilization (107, 108).
There is no precise estimate of the bioavailability of isoflavones although the European Food Safety Authority (EFSA) concluded it is low (109). In humans, there is a biphasic appearance of isoflavones in the plasma and urine following isoflavone ingestion. Isoflavones levels in the plasma occur 1–2 h, and then again at 4–8 h, following consumption (110–114).
Genistein inhibits the growth of a wide range of cancer cells in vitro via mechanisms unrelated to its ability to bind to ERs, although inhibition typically occurs at concentrations that are not achievable in vivo in response to intake within the dietary range (115). The demonstration that in comparison to ERα, soybean isoflavones preferentially bind to ERβ (91, 116), provides a molecular explanation for classifying isoflavones as selective estrogen receptor modulators (117). In general, activation of ERα and ERβ is seen as exerting proliferative and anti-proliferative effects, respectively (118).
Some of the proposed benefits of soyfoods, such as the promotion of bone health, alleviation of hot flashes and improvement of cognitive function, may be due to the estrogen-like effects of isoflavones. Finally, even if one accepts a low potency estimate for isoflavones relative to estrogen, it does not rule out possible physiological effects because blood levels of isoflavones in individuals consuming 30 to 100 mg/d exceed circulating estrogen levels in premenopausal women by many hundreds to 1,000-fold (119, 120).
Fermented soyfoods are frequently heralded over unfermented ones on social media because fermentation reduces the content of compounds that potentially inhibit nutrient absorption and the mistaken belief that Asians eat primarily fermented soyfoods. However, the clinical significance of this rather modest reduction is unclear. Furthermore, most soy consumed globally is unfermented, as fermented soyfoods play a small role in the cuisines of ethnic Chinese (Table 2) (45, 123). Worthy of note is that in the Shanghai Men's Health Study (SHMS), which comprehensively evaluated soy intake, the food frequency questionnaire included only questions about unfermented soyfoods because fermented soy intake is so low in Shanghai (124).
Whether fermentation affects isoflavone content is unclear, but it does affect isoflavone form. To varying degrees, fermentation converts isoflavone glycosides to aglycones. Murphy et al. (125) found that about one-third of the isoflavones in the fermented soyfoods miso and tempeh were in the aglycone form whereas in tofu, which is unfermented, typically <10% was in aglycone form. The degree to which this conversion occurs depends upon the bacteria used and the duration of fermentation (126, 127). Some studies have reported fermentation causes a decrease in total isoflavone content (128) whereas others have not (129). There are also conflicting data on the extent to which absorption is affected by the isoflavone form. Aglycones are absorbed more quickly, but total absorption may not be affected (114, 130, 131). The health implications of a faster absorption rate, and possibly higher peak circulating levels, are unclear.
Fermentation reduces protease inhibitor (PI) content (132), but its effect on protein digestion is unclear as the digestibility of protein from traditional unfermented soyfoods (52), soy protein ingredients (48) and foods made using these ingredients (53) is quite good. Older rat research suggests protein digestion is appreciably affected only when ~50% of the residual PI content remains (133). Fermentation also reduces phytate content, but its effect on mineral absorption is unclear (132). Phytate adversely impacts the absorption of calcium from soy (134); nevertheless, the absorption of calcium from calcium-set tofu (135) and calcium-fortified soymilk (136, 137) is similar to that of cow's milk.
Importantly, the results of single meal studies, which are typically used to determine bioavailability, may exaggerate the effect of enhancers and inhibitors of mineral absorption (138). Also, in contrast to older research (139), there may be adaptation to the inhibitory effects of phytate on iron absorption with chronic consumption of a high-phytate diet (140). Even so, the US iron RDA for vegetarians is 1.8-fold higher than for non-vegetarians because of the assumed lower bioavailability of non-heme iron in plant foods (141).
Observational studies tend to show tofu is more likely than miso to be associated with reduced risk of chronic disease (e.g., cardiovascular disease and various cancers), although it is difficult to control for all confounding variables that might be associated with possible differing patterns of use associated with these foods (142–145). And, some evidence indicates miso intake increases risk of developing gastric cancer (146, 147), although miso was recently found to be associated with an improved survival from this disease (148). Natto may benefit bone health (149–151) because of its high vitamin K content due to fermentation with Bacillus subtilis natto (152). Natto also contains nattokinase, an enzyme secreted by Bacillus subtilis natto (153), which exhibits fibrinolytic activity (154, 155). Furthermore, fermented soyfoods may function as probiotics, but this depends upon whether the product is pasteurized after the inoculum has been added (44, 156). Finally, fermentation has been shown to create antioxidants not present in unfermented soyfoods, but the clinical relevance of these molecules is unclear (157, 158).
Perspective: Overall, there appears to be little evidence that fermentation of soyfoods results in clinical benefit beyond that derived from unfermented soyfoods, but this issue has not been rigorously investigated. Natto is a notable exception because of its high vitamin K and nattokinase content. Data do not support general recommendations to choose fermented soyfoods over traditional soyfoods such as soymilk and tofu although fermented foods (tempeh, miso, natto) are based on the whole soybean whereas this is true of only some unfermented soyfoods (e.g., edamame, soynuts).
The historically low incidence rates of BCa in countries in which soyfoods have been a traditional part of the diet (159) helped fuel speculation that isoflavones exert anti-estrogenic effects thereby potentially offering protection against this disease (160). However, research published beginning in the late 1990s showed that genistein (28) and isoflavone-rich SPI (161) stimulated the growth of existing ER-positive mammary tumors in ovariectomized athymic mice. In addition, in this model isoflavones inhibited the efficacy of the breast cancer drugs tamoxifen (162, 163) and letrozole (164). These findings drew attention to the ER agonistic properties of isoflavones and led to clinicians advising their BCa patients to limit or avoid soy (165).
In contrast to the studies in mice, beginning in 1999 (166), clinical trials consistently showed that neither soy nor isoflavone consumption affected markers of BCa risk (167), including mammographic density (168–170) and in vivo breast cell proliferation (120, 166, 171–174) [Cells that proliferate more quickly are more likely to be transformed into cancer cells (175)]. These studies involved women with BCa, women at high risk of BCa and healthy women. In many cases, isoflavone intake greatly exceeded typical intake in Japan. In contrast to the lack of effect of isoflavones on cell proliferation, combined hormone therapy (CHT, estrogen plus progestin) increases proliferation (176, 177). CHT, although not estrogen alone, is known to increase risk of BCa (178). Since isoflavones do not possess progestin-like activity (179), the effect of CHT is potentially relevant to soy (179).
In 2009, the first prospective observational study to examine the impact of post-diagnosis soy intake on the prognosis of BCa patients was published (180). Among women participating in the Shanghai Breast Cancer Survival Study, post-diagnosis soy intake was associated with a significantly decreased risk of recurrence and BCa-specific mortality. Subsequently published observational studies conducted in the US (181, 182) and China (183, 184) aligned with these findings as was summarized by meta-analyses published in 2013 (185) and 2019 (186). Protective effects were observed in both ER-positive and ER-negative patients. No mechanisms for the protective effects have been proposed.
In 2012 [reaffirmed in 2021 (187)], the American Cancer Society (188) and the American Institute for Cancer Research (189); in 2014, the World Cancer Research Fund International (190), and in 2015, the Canadian Cancer Society (191), all concluded that women diagnosed with BCa can safely consume soy. However, the positions of these organization were based primarily on the epidemiologic data, not a comprehensive review of the literature. On the other hand, in 2015, the EFSA concluded isoflavone supplements (soyfoods were not examined) do not affect breast tissue in postmenopausal women (109). This conclusion was based on the animal, clinical and epidemiologic data. In 2018, the Permanent Senate Commission on Food Safety of the German Research Foundation (SKLM) reached a similar conclusion (192).
Perspective: The absence of clinical trials examining the impact of soy on BCa recurrence or mortality precludes claims that the soy and BCa controversy has been definitively resolved. However, given that clinical data are supportive of safety and the observational data are suggestive of benefit, the totality of the evidence is aligned with the positions of health agencies that women diagnosed with BCa can safely consume soyfoods. Although suggestive, the observational data do not provide a sufficient basis for recommending BCa patients begin consuming soy specifically to improve prognosis.
The historically low incidence rates of PCa in countries in which soyfoods have been a traditional part of the diet (193, 194) helped fuel speculation that isoflavones are protective against this disease, speculation which is biologically plausible since prostate tissue isoflavone concentrations exceed those in the blood (195). Several animal studies published in the 1990s provided support for a role of soy in PCa prevention. For example, in 1997, rats fed isoflavone-rich soy protein developed fewer chemically-induced prostate tumors than rats fed casein (196). Also, Zhou et al. (197) found soy protein plus isoflavones dose-dependently suppressed tumor formation in severe combined immune-deficient mice subcutaneously inoculated with prostate cancer cells. And, dietary genistein inhibited the progression of prostate tumors in a transgenic mouse model of PCa (198). Whether isoflavones exert protective effects in these models via the androgen receptor is unclear (199).
Several clinical trials also found that soy and isoflavone intake decreased prostate specific antigen (PSA) levels in men with PCa (200). PSA is a marker of prostate tumor growth (201). Of the eight trials identified in an older review involving men with PCa, four reported isoflavones slowed the rise in PSA levels and in four there was no effect (200). Although a few subsequently published studies found isoflavones or soy lowered PSA levels (202, 203), more recent work has not shown this to be the case (204, 205). The lack of efficacy is supported by a systematic review that included four studies, which were published in 2004, 2010, 2011, and 2013 (206).
Of the clinical trials that failed to show efficacy, two are especially notable because of their size and duration, One, which was stopped early at ~2 years because of a lack of efficacy, involved 177 men at high risk of recurrence after radical prostatectomy for PCa (204). Men were randomized to receive either 20 g/d SPI or casein. SPI provided 43 mg total isoflavones, of which 25 mg was genistein. Arguably, isoflavone exposure was low for a study examining PCa progression. In the other study, 300 men with confirmed high-grade prostatic intraepithelial neoplasia were randomized to receive daily a placebo or 40 g/d SPI (estimated isoflavone content, 100 mg) plus vitamin E and selenium for 3 years (205). The primary end point was time to development of invasive PCa. The lack of efficacy cannot be attributed to a low isoflavone dose, but the possible carcinogenic effects of vitamin E and selenium may have countered any protective effects of isoflavones (207, 208). However, there is also evidence that selenium reduces PCa risk (208).
Some observational evidence supports a protective effect of soy against PCa. For example, in 2018, a meta-analysis of 30 population studies found that both soyfood and soy protein intake were associated with a decreased PCa risk (2). However, the most robust findings were based on case-control, not prospective studies. The former carry less weight within the epidemiologic community. In contrast, another meta-analysis also published in 2018, which analyzed the combined results of the Japan Collaborative Cohort Study and the Japan Public Health Center-based prospective Study, found that serum genistein and daidzein concentrations were not significantly associated with PCa risk, although the odds ratios (ORs) for both isoflavones were below 1.0, which is suggestive of a protective effect (209). Finally, a population-based prospective study involving 43,580 Japanese men aged 45–74 years with no history of cancer found that over the median follow-up period of 16.9 years, isoflavone and soy intake was associated with a statistically significant increase in risk of PCa mortality (210).
Perspective: There is suggestive evidence that soyfoods reduce risk of developing PCa, but the data are too inconsistent to reach firm conclusions. Nevertheless, health professionals advising clients or patients concerned about developing PCa are justified in recommending that soyfoods be part of a dietary approach aimed at addressing this concern. Continued research is warranted but effects on PCa development or progression should not currently be a sole basis for recommending soy intake.
The relationship between dietary protein intake and bone health is complex (211). Overall, studies suggest dietary protein has a neutral to possibly small beneficial effect on bone (212), but whether this depends upon the type of protein consumed is unclear (211, 213).
Early interest in the anti-osteoporotic effect of soyfoods stemmed from studies showing soy protein to be less hypercalciuric than animal protein (214, 215), an effect attributed to the lower SAA content (mg/g protein) of the former (216–218). However, the notion that animal protein causes bone dissolution as a result of its high SAA content (219, 220) has lost support (221–224), as has the hypothesis that soy protein improves calcium balance when compared to animal protein (225, 226). Nevertheless, the presence of isoflavones in soybeans continues to attract interest in the possible skeletal benefits of soyfoods because of the well-established skeletal benefits of estrogen (227).
Large prospective cohort studies from Shanghai (228) and Singapore (229), reported that soy intake was associated with a reduced fracture risk among women. In addition, a US prospective study involving Seventh-day Adventist (SDA) postmenopausal women, found soymilk intake was inversely related to risk of osteoporosis, although this may have resulted from calcium, rather than isoflavone intake (230). Interestingly, a recently published analysis of the SMHS found that high soy isoflavone intake (>45.2 mg/d vs. <21.7 mg/d) was associated with a significant reduction in risk of osteoporotic, but not non-osteoporotic, fracture (231). These findings conflict with the aforementioned Singaporean study which found soy intake was protective in women but not men. However, the Singaporean study did not sub-analyze the data according to fracture type (229). It is possible that the lack of effect in Singaporean men may have resulted from their lower isoflavone intake relative to men from Shanghai, although this lower intake did not prevent protective effects of isoflavones from being observed in Singaporean women.
In 1998, isoflavones were first shown clinically to improve bone mineral density (BMD) in postmenopausal women. (232). Over the past two decades clinical trials that examined markers of bone resorption and/or formation or BMD have produced inconsistent results. However, the authors of a meta-analysis of the clinical data that was published in 2021, reported a trend of isoflavones to increase bone formation markers such as bone alkaline phosphatase and osteocalcin (233). Additionally, there was a trend toward lower levels of pyridinoline and deoxypyridinoline, two bone resorption markers. In this analysis, ~1,000 women consuming a placebo were compared to over 1,000 women who consumed on average a daily dose of nearly 100 mg isoflavones. The trials ranged in duration from 3 to 24 months.
However, of the 4 large (n ≥ 100), long-term (≥2 y) trials that evaluated postmenopausal BMD (234–237), only one showed significant benefit (Table 3) (237, 238). This specific trial intervened with genistein in aglycone form and included osteopenic women (237) whereas the other three trials included healthy postmenopausal women and intervened with isoflavones in glycoside form. Finally, research published in 2015 (239), which involved the use of novel methodology to study bone loss in postmenopausal women found isoflavones to be efficacious and showed a more moderate isoflavone dose (~100 mg/d total isoflavones) increased bone calcium retention more than higher isoflavone doses (240). Two of the three aforementioned large, long-term trials showing no effect of isoflavones used daily doses of >100 mg (Table 3).
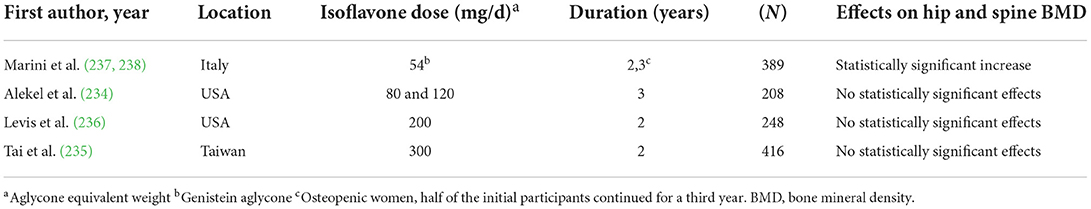
Table 3. Effects of isoflavones on bone mineral density in postmenopausal women in large, long-term clinical trials.
Perspective: The role of estrogen in bone health provides a theoretical basis for isoflavones to exert skeletal benefits, although isoflavones and estrogen differ at the molecular and clinical level [For example, unlike estrogen, isoflavones do not exert proliferative effects on endometrial tissue (241, 242) and as noted previously, isoflavones preferentially bind to ERβ (91, 116)]. The results of the observational and clinical data warrant additional research being conducted. However, only the results of sufficiently powered trials at least 2 years in duration hold the potential to meaningfully impact the current state of knowledge. At this point, it is premature to recommend isoflavone intake specifically for the purpose of improving bone health. Nevertheless, given that adequate protein is needed for bone health, isoflavones may have skeletal benefits, and some soyfoods and soy products are fortified with calcium, soyfoods can certainly be viewed as foods to emphasize for those concerned about bone health.
Results of the Honolulu-Asia Aging Study (HAAS) published in 2000 (26) raised concern that soy intake might impair cognition. This study found higher midlife tofu intake among men was associated with indicators of cognitive impairment and brain atrophy in late life. A post-hoc analysis using the men's intake as a surrogate showed the relationship between cognitive decline and tofu intake also applied to their spouses. It was theorized that soy isoflavones were acting as ER antagonists; at that time evidence suggested estrogen therapy might reduce the risk of developing dementia and Alzheimer's disease (243, 244). However, there were many limitations to this observational study, including that the study was designed to investigate coronary heart disease (CHD) not cognitive function and the dietary assessment included only 26 foods.
In contrast to the HAAS, the results of several small clinical trials published between 2001 and 2006 suggested that soy isoflavones provided primarily in the form of supplements exerted cognitive benefits (245–249), although this was not the case for a large 1-year study that intervened with 25 g/d SPI that provided 99 mg isoflavones (250). In 2008, the controversy that was started by the HAAS was reignited by an Indonesian cross-sectional involving older women and men that reported the consumption of tofu was associated with worse memory. In contrast, tempeh intake was associated with better memory, especially among those >68 years of age (31).
However, a follow-up study published in 2011 by the research group who conducted the cross-sectional study from Indonesia found positive linear associations of weekly tofu and tempeh consumption with immediate recall, which were significant in those with an average age of 67 years (251). Furthermore, in those with an average age of 80 years, the earlier reported negative association of tofu with immediate recall was no longer significant (251). More recently, a Japanese prospective observational study found higher midlife genistein intake was associated with cognitive impairment (252) although these results contrast with an earlier prospective observational study from Japan that found soyfood and isoflavone intake decreased risk of cognitive impairment in elderly women (253). They also contrast with the results of a large prospective Japanese study involving more than 40,000 adult men and women which found soyfood intake was unrelated to the development of disabling dementia over the approximate 20 year follow up period (254).
In 2014, a comprehensive examination of the animal, clinical and observation evidence found there was insufficient data to reach conclusions about the relationship between soy or isoflavone intake and cognitive function among older adults (255). However, in 2015, a meta-analysis of 10 placebo-controlled randomized trials that involved over 1,000 postmenopausal women, reported that isoflavones improved cognitive function and visual memory (256). Two years later, an analysis concluded that isoflavone supplementation improved executive function and memory domains of cognitively normal older adults in half of the studies evaluated (257). Finally, in 2020, a meta-analysis of 16 trials (1386 participants, mean age 60 years) found soy isoflavones improved memory and overall cognitive function (5). In the trials included in these meta-analyses, isoflavones were typically provided either in the form of supplements or an isoflavone-rich, concentrated source of soy protein. As to potential mechanisms for the observed benefits, a cross-over study involving older men and women reported that the consumption for 16 weeks of 67 g/d soynuts that provided ~25.5 g protein and 174 mg isoflavones, increased psychomotor speed performance, likely as a result of the increase in cerebral blood flow in 4 brain clusters, although executive function and memory were unaffected (258).
Perspective: The clinical data suggest isoflavones benefit cognitive function. Therefore, there is some evidence to recommend soyfood consumption as a means of delaying cognitive impairment. However, the totality of the evidence is too inconsistent to draw meaningful conclusions.
Hot flashes are the most common menopause-related symptom experienced by women (259). A hot flash is a transient vasomotor event consisting of a sensation of warmth, typically accompanied by sweating, flushing, palpitations, and sometimes anxiety (260). Adlercreutz et al. (261) proposed in 1992 that isoflavones possess sufficient estrogen-like activity to mitigate the drop in circulating estrogen as women transition through menopause to alleviate hot flashes. Three years later the first clinical trial to evaluate this hypothesis was published (262). Over the years, many reviews and analyses of the results of the numerous soy/isoflavone-hot flash trials have been published but with contrasting conclusions. Most have suggested isoflavones are not efficacious (33, 263, 264) or offer at best modest benefits (265–269), whereas a smaller number have been more supportive of efficacy (270–274). Given that hot flashes are typically subjectively determined, there is a large and variable placebo effect (275), and since there are also large intra-individual differences in isoflavone metabolism (276), the inconsistent data are not completely unexpected.
However, in 2012, Taku et al. (4) offered an explanation for the inconsistency based on the differing genistein, but not total, isoflavone content of the supplements used in the clinical trials. A previously published narrative review had hinted at the importance of genistein content (272). In general, two types of soy isoflavone supplements have been used in clinical trials; one is derived from whole soybeans and has an isoflavone profile similar to that found in soybeans and soyfoods, that is, genistein is the predominant isoflavone. In contrast, the other type is made from the hypocotyledon (or germ) portion of the soybean and is quite low in genistein (~10% of total isoflavone content).
In the meta-analysis by Taku et al. (4), which included nine trials that evaluated hot flash severity (n = 988 women) and 13 trials (n = 1,196 women) that evaluated frequency, the net effect, that is the decrease in response to isoflavones minus the effect in the placebo group, was a 26.19% (p = 0.001) decrease in severity and a 20.62% (p = 0.00001) reduction in frequency. However, sub-analysis revealed that among studies that provided more than 18.8 mg/d genistein (median for all studies) the net reduction in frequency was 29.13% whereas it was only 12.47% among trials that intervened with supplements providing less than the median genistein intake (difference between groups, p = 0.03). One year later, a Cochrane review did not sub-analyze the data according to the isoflavone profile of the supplement, but did call for further investigation of the benefits of genistein for alleviating hot flashes (277). Clinical trials published after the meta-analysis by Taku et al. (4) are generally supportive of the efficacy of isoflavones (278–281).
Perspective: Clinical trials evaluating the efficacy of isoflavones to alleviate hot flashes, which date back nearly 30 years, have produced conflicting results, which are reflected in the reviews and analyses of the data published over this time. However, nearly all of these reviews have not considered the differing isoflavone profiles of the supplements used in the clinical trials. Trials that provide at least 20 mg genistein and at least 50–60 mg total isoflavones consistently show isoflavones to be efficacious for reducing the frequency and severity of hot flashes. Whether other endpoints which have been inconsistently affected in clinical trials involving isoflavones may be due in part to the differing genistein content of the supplement has not been examined.
Research of the effects of soy intake on thyroid function in rats was first published nearly 100 years ago (282). More relevant is the publication almost three decades later of several case reports describing goiter in infants fed soy infant formula (SIF) (283–285). However, the concern raised by these reports was allayed when the mineral iodine was added to the formula. In the 1990s, isoflavones were shown in vitro to serve as an alternate substrate to tyrosine for iodination (thereby potentially exacerbating thyroid function when iodine intake is marginal) and to inhibit the activity of thyroid peroxidase (TPO) in vitro and in rats (27). This enzyme is required for the production of both thyroxine (T4) and triiodothyronine (T3). In 2004, Conrad et al. (286) concluded that infants fed SIF had prolonged increases in thyroid stimulating hormone (TSH) levels based on a retrospective analysis of infants with congenital hypothyroidism. These increases were thought to be due to the inhibition of levothyroxine absorption by soy protein, not as a result of isoflavones exerting a systemic effect (287).
In 2015, the EFSA (109) and in 2018, the SKLM (192), concluded isoflavones do not affect thyroid function in postmenopausal women (the only group studied). In 2019, the first meta-analysis to examine the effect of soy and isoflavones on thyroid hormones, which included 18 clinical trials, found no effect on free levels of T4 or T3 (288). The analysis included trials that intervened with soy isoflavones, soy extracts, soy protein, daidzein-rich isoflavones or isolated genistein in doses ranging from 40 to 200 mg/d.
The above-referenced meta-analysis did find a very modest increase in TSH levels, although the authors of this work were unclear as to whether the increase was of clinical significance (288). TSH levels increased by only 0.248 mIU/L; normal reference values for TSH are 0.5 to 4.5 mIU/L. Furthermore, an examination of the forest plot from this paper shows that only four studies, all by the same research group, were responsible for the results showing an increase in TSH (289–292). Nevertheless, Tonstad et al. (293) found when comparing the 5th vs. 1st intake quintiles, there was an association between isoflavone and soy protein intake and elevated TSH levels (>5 mIU/l) among SDA women (n = 548), but not among men (n = 295). However, these findings are a bit surprising given the moderate intake; midpoint isoflavone and soy protein intakes among women in the 5th quintile were only 25.46 mg/d and 6.92 g/d, respectively. That is equivalent to approximately only one serving of a traditional soyfood daily.
As already noted, concern has been raised that soy intake may exacerbate thyroid function in individuals whose iodine intake is marginal. However, research published in 2012 involving 35 participants indicates this is unlikely because supplementation with 80 mg/d isoflavones for 3 months led to only negligible amounts of iodinated isoflavones (~0.01%) in urine samples (294). Finally, in subclinical hypothyroid patients, one cross-over study reported that 16 mg/d isoflavones provided by 30 g/d SPI for 8 weeks increased the likelihood of progressing from subclinical to overt hypothyroidism (291). However, a follow up study by this same research group in which participants consumed for 8 weeks the identical amount of SPI but that provided 66 mg/d isoflavones did not confirm these findings (295).
Perspective: Extensive evidence indicates isoflavones do not affect T4 or T3 levels in euthyroid individuals. There are conflicting data about the effects on TSH levels. Limited evidence indicates isoflavones are unlikely to impair thyroid function in individuals with subclinical hypothyroidism or whose iodine intake is marginal. Soy protein likely inhibits the absorption of levothyroxine, a drug used to treat hypothyroidism, but this is true for food in general and many dietary supplements, herbs and drugs (296). Hypothyroid patients do not have to avoid all soy as long as there is a sufficient time interval between soy consumption and levothyroxine ingestion. Recommendations are to consume the medication ~1 h before breakfast or to wait as long as fours after eating (297). Alternatively, as long as soy intake occurs in a consistent manner, the dose of levothyroxine can be adjusted appropriately so if necessary (298).
The classification of isoflavones as phytoestrogens underlies theoretical concerns raised about effects on male fertility, which coincided with rising apprehension that environmental estrogens play a role in the declining sperm count occurring among men worldwide (299–301) and possibly contribute to the observed decline in testosterone levels (302, 303). Among US men, there has been a marked increase in testosterone testing, new initiation of testosterone administration, and even initiation of testosterone administration without recent testing, all of which is associated with exposure to televised direct-to-consumer advertising (304).
A few animal studies published around the turn of the century appeared to lend credence to concerns about soy. For example, in 1998, Strauss et al. (305) reported that genistein reduced serum and testicular testosterone concentrations and prostate weight in mice; in 2001, Weber et al. (306) found that an isoflavone-rich diet lowered testosterone levels in adult male Sprague-Dawley rats and in 2002, Sharpe et al. (307) observed that the neonatal feeding of SIF suppressed testosterone levels in marmosets.
More germane than the animal studies, is the publication of two case-control studies each describing single individuals who experienced feminizing effects [erectile dysfunction (29, 308), increased estrogen levels (29), loss of libido (29, 308), gynecomastia (29), low testosterone (308)] in response to excessive isoflavone intake (360 mg/d) and a pilot US case-control study which found an inverse association between soy intake and sperm concentration (but not count) among male partners of couples attending a fertility clinic (309). Sperm concentration was decreased largely because of an observed increase in ejaculate volume associated with soy intake, a finding that does not seem biologically plausible, especially considering that median genistein intake in the highest intake group was only 7.48 mg/d.
In contrast to these suggestive data, in 2021 a meta-analysis of 41 clinical trials conducted mostly in Western populations, found no effects of soy or isoflavones on reproductive hormone levels in men (310). Trials intervened with either isoflavone-rich soy protein or isoflavone supplements and involved men of all ages Total testosterone and free testosterone levels were measured in 1,753 and 752 men, respectively, and estradiol and estrone levels were measured in 1,000 and 239 men, respectively. Sub-analysis of the data according to isoflavone dose (<75 mg/d vs. ≥75 mg/d) and study duration ( ≤ 12 weeks vs. >12 weeks) also showed no effects. In addition to there being no effects on hormone levels, none of the three clinical trials to evaluate the impact of isoflavone intake on sperm or semen parameters showed any adverse effects (311–313), although one of these was not published in full manuscript form (313). Isoflavone doses ranged from 40 to 480 mg/d for durations from ~2 to 3 months.
In addition, neither of the two placebo-controlled clinical trials that evaluated the effects of isoflavones on breast tissue in men found evidence of gynecomastia. One of these studies, which involved 200 men, intervened with 66 mg/d isoflavones for 3 months (292) and the other ~100 mg/d for 3 years and involved >300 men (205). Finally, a study that included 184 men from couples undergoing in vitro fertilization, found that neither the intake of soyfoods nor isoflavones by the male partners was related to fertilization rates or a host of other fertility measures (314).
Perspective: Extensive clinical trial data show no effect of soy or isoflavones on testosterone or estrogen levels in men even when exposure markedly exceeds typical Japanese intake. More limited but consistent clinical evidence indicates no adverse effects of soy or isoflavones on sperm or semen parameters or risk of developing gynecomastia.
Concerns that isoflavones might affect circulating reproductive hormone levels in women, and in particular, raise estrogen levels, arose because isoflavones are classified as phytoestrogens. Isoflavones, can in theory, influence estrogen levels by virtue of effects on enzymes involved in steroid metabolism (315–318). They could also impact biologically active levels of hormones by affecting sex hormone binding globulin (SHBG) concentrations (319). However, evidence that isoflavones affect hormone levels in women is unimpressive, although via their interaction with ERs isoflavones can potentially affect biological processes affected by the hormone estrogen without affecting circulating estrogen concentrations.
A meta-analysis by Hooper et al. (320) published in 2009, found that based on 35 clinical trials involving postmenopausal women, that there were no effects of soy or isoflavone intake on estradiol, estrone, SHBG, follicle stimulating hormone (FSH) or luteinizing hormone (LH). In 11 studies involving premenopausal women, these interventions also had no effect on estradiol, estrone or SHBG concentrations. In contrast, FSH and LH levels were significantly reduced by about 20% based on seven studies (n = 73 women) using standardized mean differences (mean divided by the standard deviation of differences), but not mean differences. However, in sensitivity analysis when only studies at low risk of bias were retained, the results were no longer statistically significant. Furthermore, subsequent to this analysis, a 6-month study by Khan et al. (120), found no effect of isoflavones (235 mg/d) on FSH (LH was not examined) in 53 premenopausal women. Thus, the evidence does not suggest FSH is affected by isoflavones. In general, studies published subsequent to the meta-analysis by Hooper et al. (320) show a lack of effect of isoflavone exposure on hormone levels in women (8, 120, 236, 321–326).
Research on the impact of soy on menstrual cycle length (MCL) was first published by Cassidy et al. (327, 328) in 1994/95. The findings of this work led to concerns about infertility because MCL was increased as a result of a change in follicular phase length (~first 2 weeks of the menstrual cycle). However, ovulation was not prevented. In the meta-analysis by Hooper et al. (320), on the basis of 10 studies, soy/isoflavone intake was found to increase MCL by 1.05 d. Menstrual cycle function is suggested to be an indication of fertility (329, 330). However, short, but not long, menstrual cycles have been linked to 11–36% longer time to pregnancy (331–333).
No clinical research examining the impact of soy on MCL published subsequent to the meta- analysis by Hooper et al. (320) was identified. Limited older evidence suggested that longer cycles might be protective against the development of breast cancer (334). If the increase is due to an increase in follicular phase length as was noted for soy, in theory, women will spend less of their lifetime in the luteal phase, a period during which breast cells are more actively proliferating (335).
Perspective: Considerable clinical evidence indicates neither isoflavones nor soy impact circulating reproductive hormone concentrations in women. Less clear are the effects on FSH and LH. The impact of soy intake on MCL has not been studied for 15 years but MCL may be increased by soy by ~1 day, although ovulation is not prevented. The increased MCL is not expected to affect fecundity.
The effect of soy consumption on the onset of puberty has been the subject of limited investigation. This relationship is of interest in part because throughout the world pubertal characteristics are occurring at an earlier age (336–347); however, this trend is apparent in countries where soyfoods are part of the traditional diet as well as in countries that historically have not consumed soy (348). Two case-control studies found urinary isoflavone levels in Korean girls with precocious puberty were higher than in children without this condition (349, 350). However, there were several experimental design weaknesses to these studies (17) and the findings contrast with the results of a US cross-sectional study involving 327 SDA girls 12–18 years old that examined the impact of soy intake on age of menses onset (AOM) (351).
For this study, soy intake at their current age was used as a proxy for the soy intake of girls prior to the onset of menses. Neither total soy product intake nor the intake of soy-based meat alternatives, tofu/traditional soy, or soy beverages, was significantly related to AOM or the likelihood of early (<12 y) or late (≥14 y) AOM. (351). A similarly designed study involving 248 SDA boys age 12–18 y found (mean isoflavone, puberty onset) moderate (10.1 mg/d, 12.58 y) and high (54.9 mg/d, 12.50 y) isoflavone intake was significantly associated with earlier adjusted median age at pubarche (based on pubic hair development) in comparison to low-soy consumers (0.8 mg/d, 13.00 y) (352). However, in contrast, isoflavone intake was unrelated to a secondary measure of puberty, facial hair onset. Furthermore, in boys consuming the most soy, puberty onset was actually later than the average is for boys in the US (353).
Two small US clinical trials (354, 355) and one Japanese population-based cross-sectional study (356) examined the impact of soy intake on hormone levels in children. In the Japanese study, which involved 230 boys and 198 girls aged 3–6 y, after adjusting for potential confounding variables, higher soy intake was inversely related to urinary estrone and estradiol in boys and positively related to urinary testosterone and androstenediol in girls (356). Similar findings were reported for isoflavone intake. In contrast, no effects of isoflavone intake on hormone levels were noted in either of the two clinical intervention studies conducted. In one, estrogen levels were measured in 17 US girls aged 8–14 years, who consumed one serving of soy daily (average isoflavone intake, ~27 mg/d) for 8 weeks (354). The other study measured estrogen levels in eight girls and testosterone levels in four boys (aged 5–11 years) after consumption of a daily tablet containing16 mg or 48 mg isoflavones or a placebo for 8 weeks each in a randomized crossover design separated by 2 week washout periods (355).
Perspective: Limited evidence indicates there is no clear association between puberty onset and the intake of soyfoods. Additional observational and clinical research is warranted.
There are two issues to consider when addressing the impact of maternal soy consumption during pregnancy. One is the effect on the mother and the other the effect on the fetus. Neither issue has been examined extensively. Asian women consume soy during pregnancy as they do throughout other periods of life (357–359). For example, Miyake et al. (358) reported that the genistein and daidzein intake of 1,002 pregnant Japanese women participating in the Japan Osaka Maternal and Child Health Study was 15.0 ± 10.1 mg/d and 9.0 ± 6.1 mg/d (mean ± SD), respectively. These values are in alignment with those reported by Nagata et al. (360) (21.7 ± 13.7 mg/d), who also studied the total isoflavone intake of pregnant Japanese women (n = 194).
Wang et al. (361) examined the association between the soy intake of pregnant women between 13 and 24 weeks of gestation in southwest China and risk of gestational diabetes mellitus (GDM) and cesarean section (CS). Participants in this prospective study were divided into the insufficient soy intake group (<40 g soy/d) and control group (≥40 g soy/d), as the latter is the amount recommended by the Chinese Nutrition Society. Among the 224 participants, there were 36 cases of GDM and 120 cases of CS. After adjustment, consumption of <40 g/d was associated with an increased risk of GDM (OR, 2.116; 95% confidence interval [CI]: 1.228, 7.907; p = 0.017), but not with CS.
These results align with the findings from another prospective Chinese study which involved 1,495 pregnant women, 529 of whom were diagnosed with GDM (362) At 6–14 gestational weeks, dietary information was collected by trained interviewers by 24-h dietary recall for 3 days including 2 weekdays and 1 weekend day. Mean soy intake was 8.7 ± 16.6 g/d (the assumption is that this value refers to g soy protein). When compared with non-soyfood consumers, the third soyfood intake tertile was associated with a decrease in risk of GDM (Relative Risk [RR] 0.73; 95% CI: 0.54, 0.99, p = 0.049).
Protective effects were also noted in a prospective Japanese study involving 84,948 women; during the follow up period, 1,904 developed GDM (363). After adjustment, compared with those in the lowest isoflavone intake quintile (mean, 8.4 mg/d), women in the highest quintile (mean, 64.0 mg/d) were significantly less likely to have GDM (RR, 0.82; 95% CI: 0.70, 0.95; p for trend = 0.05). Additionally, miso soup and natto, but not tofu intake, were inversely associated with GDM.
Possible clinical support for the observational data comes from a 6-week Iranian study involving 68 women with GDM who were randomly assigned to consume the control diet containing 0.8 g protein/kg body weight (70% animal and 30% plant protein) or a diet containing the same amount of protein but comprised of 35% animal protein, 35% textured soy protein containing 75 mg isoflavones and 30% other plant proteins (364). Compared to the those consuming the soy-containing diet, the control group had significantly higher fasting plasma glucose, serum insulin levels and the homeostasis model of assessment-insulin resistance. Somewhat parenthetically, the control group had a higher incidence of newborn hyperbilirubinemia (32.4% vs. 8.8%, p = 0.01) and newborn hospitalization rates (20.6% vs. 2.9%, p = 0.02).
Finally, Schiattarella et al. (365) recently concluded there is some evidence a vegetarian diet as well as a plant-based diet reduces risk of developing GDM and/or some symptoms of this condition. This conclusion aligns with recent work by Wang et al. (366), who found that among 2,099 Chinese women participating in the Tongji Maternal and Child Health Cohort, after adjusting for social-demographic characteristics and lifestyle factors, women in the highest quartile of plant-based dietary index (PDI) were less than half as likely to develop GDM. Soy products typically comprise a large part of the bean intake category, which represents a significant portion of the PDI.
Perspective: Intriguing although limited evidence indicates soy consumption during pregnancy reduces risk of developing GDM. Research aimed at better understanding this relationship is warranted.
Despite the practice among Asians of consuming soy during pregnancy, concern has arisen that in utero isoflavone exposure could adversely impact the fetus (367, 368). In 2010, Balakrishnan et al. (369) demonstrated in an ex-vivo human placental perfusion model that genistein can transfer across the human placenta. Twenty years earlier, Adlercreutz et al. (370), reported maternal plasma isoflavone and cord and amniotic fluid values for seven Japanese women at delivery. Several other investigators have also provided data on isoflavone amniotic fluid and/or cord blood concentrations (360, 371–376). These data, which were recently reviewed by Messina et al. (17), led the authors to conclude that “.. in utero isoflavone concentrations are markedly lower than estrogen concentrations” and that because of this difference, “isoflavones are unlikely to exert an estrogenic effect on the fetus.”
Nevertheless, in 2000, a British prospective study, which included 7,928 boys born to mothers taking part in the Avon Longitudinal Study of Pregnancy and Childhood, found that mothers who drank soymilk [yes or no; OR, 3.67; 95% CI: 0.87, 15.44)] or who ate soy “meat” (≥1x/wk vs. never; OR, 2.95; 95% CI: 0.90, 9.68) during pregnancy were about 3-fold more likely to give birth to boys with hypospadias (a birth defect where the opening of the penis is on the underside of the organ) (377). However, these associations were not statistically significant (377). The authors speculated that isoflavones might be responsible for the apparent association; however, legume (dried peas, beans, lentils, and chick peas) intake was associated with a 7-fold increased risk of hypospadias (≥4x/wk vs. never; OR, 7.56; 95% CI: 2.25, 25.42), despite non-soy legumes containing negligible amounts of isoflavones (125, 378). Soy meat analogs, which typically contain very low levels of isoflavones, were also associated with an increased risk (125).
A large Japanese nationwide birth cohort study represents the most direct examination of the relationship between risk of hypospadias and isoflavone intake. Women were recruited for this study during early pregnancy (379).
The intake of genistein (median, 15.3 mg/d) was based on a self-administered food-frequency questionnaire. There were 51 cases of hypospadias among the more than 40,000 women who delivered singleton live male births. In comparison with mothers in the 11th−89th percentiles of genistein intake, those women in the low intake group ( ≤ 10th percentile) were nearly three times as likely to report having a son with hypospadias. In contrast, there was no relationship between the highest genistein intake (≥90th percentile) and risk of hypospadias. These findings led the authors to conclude that low isoflavone intake during early pregnancy may increase hypospadias risk. In addition to the genistein findings, low intake of tofu and natto, were each associated with about a 2-fold increase in risk.
Finally, Song et al. (380) reported that after adjusting for 10 potentially confounding variables, maternal intake during early pregnancy of several foods, one of which was soy, was associated with a reduced risk of ventricular septal defects (VSDs) in the offspring. The highest intake category was consumption ≥6x/wk. In China, where this study was conducted, VSDs occur in ~2.5 births out every 1,000.
Perspective: Only limited research has evaluated the impact of maternal soy intake on the fetus. According to one school of thought, in utero isoflavones concentrations are too low to an exert effects on the fetus. Older speculation that maternal isoflavone intake increases risk of hypospadias is contradicted by more recent research showing the opposite effect.
Equol was first isolated from equine urine in 1932 (381) and identified 50 years later in human urine (382). Twenty years later, it was proposed that those individuals whose large intestine host the microbiota capable of converting the isoflavone daidzein into equol, are more likely to benefit from soyfood consumption than those do not (383). Approximately 50% of Japanese fall into the equol-producing category, whereas only about 30% of Westerners do (384, 385). Equol is reported to be a more potent ER agonist than its precursor daidzein, providing a potential role for microbiota metabolism on the ultimate consequences of dietary exposure to daidzein (385).
So where does the equol hypothesis stand today, 20 years after it was first proposed? While it still remains to be proven, the hypothesis received support from a recently-published Japanese study that examined the relationship between isoflavone intake and the volume of white matter lesions among 91 cognitively normal elderly Japanese (386). Blood isoflavone and equol levels were analyzed ~6 to 9 years prior to the determination of white matter lesions. Circulating isoflavone levels were unrelated to the volume of white matter lesions; however, among the 23 study participants with the highest circulating equol levels, lesion volume was reduced by ~50%. White matter is found in the deeper tissues of the brain and contains nerve fibers that affect brain function and learning. White matter lesions disrupt brain function and are associated with an increased risk for cognitive impairment and Alzheimer's disease.
These Japanese results are biologically plausible because equol has been shown to reduce arterial stiffness, a significant determinant of white matter lesion volume in the elderly (387). The stiffer and harder the blood vessel walls, the more the heart must work to pump blood into the arteries. However, the impact on arterial stiffness cannot be the entire explanation, because clinical trials indicate that isoflavones (not just equol) also reduce arterial stiffness (388).
Since only a few clinical trials have directly administered equol to participants, the question that arises is whether equol is simply reflective of some unidentified phenotype or characteristic that leads to a different response to soy than the response of non-producers. While that is a distinct possibility, equol is biologically active in humans as clinical trials have shown equol alleviates menopausal symptoms (389). However, the same is also true for genistein, a soybean isoflavone which is not converted into equol (4).
Nothing about the equol hypothesis suggests that isoflavones do not exert beneficial effects in equol producers and in non-producers alike. Both equol and isoflavones can exert physiological effects. If equol does have benefits independent of isoflavones, a reasonable question is whether steps can be taken to convert non-producers into equol producers Some evidence indicates vegetarians are more likely to be equol producers than non-vegetarians (390, 391), which suggests diet potentially affects the intestinal bacteria in a way that can lead to equol production.
Perspective: The hypothesis proposed two decades ago that equol producers are more likely to benefit from soyfood consumption than non-producers remains intriguing, but unproven, and one that warrants continued investigation.
Much is known about the health effects of soy protein ingredients because these products are typically studied in animal and clinical trials, rather than traditional Asian soyfoods. For example, most information about soy protein quality (49, 50, 392–397), and its ability to lower cholesterol (398–405), and to promote gains in muscle mass and strength in response to RET (67) is based on studies involving SPI or SPC. Nevertheless, these concentrated sources of soy protein have raised concerns because of evidence suggesting they may increase levels of insulin-like growth factor-1 (IGF-1) (24) and for the extensive processing they undergo (19, 20).
Although there is a critical role for IGF-1 in normal growth and development, some evidence indicates elevated levels of this hormone may be a factor in the development of some cancers (406) and adversely impact longevity (407–410) On the other hand, higher IGF-1 levels have been linked with protection against cardiovascular disease (CVD) (411, 412). Additionally, higher levels of IGF-1 have been associated with a reduced risk of developing type 2 diabetes (413) in some studies although not all data concur (408). While some, but not all, evidence (414) indicates that soy protein may slightly increase IGF-1 concentrations, increases have been observed only at intake levels exceeding 25 g/d (415, 416). Other proteins, especially high-quality proteins, have also shown to increase IGF-1 (417), although there is some disagreement on this point (414).
As to the effects of processing, the manufacturing of SPI and SPC from soybeans results in a marked reduction in the fat and fiber content, as well as in most instances, an isoflavone concentration that is decreased by 80 to 90% (105, 418). Therefore, these ingredients should be viewed primarily as sources of protein. Ironically, recommendations to limit the intake of these products are sometimes made in an attempt to avoid excessive isoflavone intake (419).
Foods made using the soy protein ingredients, such as meat and dairy alternatives, are classified as group 4, ultra-processed foods (UPFs), according to the NOVA food classification system (420). However, a recent review concluded that the major criticisms of UPFs do not apply to these foods more so than to they do to their animal-based counterparts, meat and cow's milk, which are classified as group 1 foods or unprocessed/minimally processed foods (421).
Finally, as noted previously, the starting point for manufacturing concentrated sources of soy protein are soybean flakes, which are produced by crushing soybeans and removing the oil using a food grade solvent such as hexane. As such, claims have been made that residual hexane in products using these ingredients is a health risk (422). However, a review of residual levels of hexane in soy-based foods found “there is no evidence to substantiate any risk or danger to consumer health when foods containing trace residual concentrations of hexane are ingested.” (423). Also, it has been estimated that over a million soy burgers would need to be consumed daily before reaching hexane levels in rats shown to cause neurological problems (424).
Perspective: Nutritionists typically emphasize consuming whole foods, whether it be whole grains rather than refined grains, or whole fruit rather than fruit juice. This same approach can be applied to recommendations regarding foods based on soy protein ingredients. Nutritionists are justified in emphasizing the consumption of whole soyfoods (tempeh, edamame, soynuts) and minimally processed soyfoods (tofu, soymilk). However, foods based on concentrated sources of soy protein are convenient ways to obtain ample amounts of high-quality protein that for many people may be the only acceptable way to incorporate soy into the diet. The potential benefits and safety of concentrated sources of soy protein have been rigorously evaluated.
The cholesterol-lowering effect of soy protein has been studied clinically for more than 50 years (425). A meta-analysis of the clinical data published in 1995 found, on the basis of 31 trials involving 564 participants, that soy protein reduced low-density-lipoprotein cholesterol (LDL-C) an estimated 12.9% (426). In 1999, after conducting its own analysis of the literature, the US Food and Drug Administration (FDA) approved a health claim for soyfoods and CHD (427). The FDA established 25 g/d as the threshold intake for cholesterol reduction. In contrast to the 1995 meta-analyses, more recent meta-analyses of the clinical data published between 2003 and 2019 show a range in LDL-C reduction to be a more modest level of between 3.2 and 6% (398–405).
The effect of soy protein is independent of the fatty acid content of soyfoods although the high PUFA content of traditional soyfoods represents a second mechanism by which incorporating soyfoods into the diet can potentially lower blood cholesterol (398). Soy protein may also lower blood triglyceride levels and slightly raise high-density-lipoprotein cholesterol levels, although the health claim is unrelated to these effects (399). Although no mechanism for the cholesterol-lowering effect of soy protein has been definitively identified, some authors have suggested that peptides formed from the digestion of soy protein upregulate hepatic LDL (428) and VLDL (429) receptors.
In 2007, the FDA announced its intention to reevaluate evidence in support of the health claim (34) and in 2017 (430), it announced its intention, pending public comment, to revoke the claim because the data were considered to no longer be sufficiently consistent to support an unqualified health claim (Unqualified health claims require significant scientific agreement). Of the 46 studies included in the FDA analysis, 19 (41%) reported that soy protein statistically significantly lowered LDL-C. While the data are inconsistent, they are no more so than they are for oat β-glucan (431) and phytosterols/stanols (432), both of which have unqualified CHD claims based on their cholesterol-lowering effects.
The FDA did not meta-analyze the results of the 46 studies it considered in its review. When this was done by Blanco Mejia et al. (405), soy protein was found to significantly lower LDL-C by 3.2%. Further, it was established via a cumulative meta-analysis, that at no time since the health claim was approved was the effect of soy protein on LDL-C not statistically significant (433). Like the FDA, in its review Health Canada also found a minority of studies (33%) reported a statistically significant reduction in LDL-C; however, it determined that most studies (81%) showed a reduction even if not statistically significant (404). Hence, it was concluded the direction of effect was consistent and for this reason, in 2015, Health Canada approved a cholesterol-lowering health claim for soy protein (432).
Finally, it is notable that as part of the process for evaluating efficacy, the FDA conducted a comprehensive safety review. In addition to examining the literature, the FDA addressed hundreds of comments submitted during the open comment period, many of which dealt with safety concerns. Although the FDA efficacy analysis focused on soy protein, most of the public concerns centered on isoflavones. These concerns were rejected as the FDA concluded that “the use of soy protein at the levels [25 g/d] necessary to justify a [health] claim has been demonstrated, to our satisfaction, to be safe…” (430).
Perspective: Soy protein has a modest, yet clinically relevant, cholesterol-lowering effect. The FDA is currently scheduled to make a final decision about the existing health claim in August of 2023. If this highest level claim is revoked, speculation is that it will be replaced with a strongly worded qualified health claim, such as the one that exists for soybean oil and CHD (434).
Gout, the most common form of inflammatory arthritis worldwide, is caused by deposition of monosodium urate crystals in joints and various other tissues and appears in relation to chronic hyperuricemia (435). Estimates are that more than 9 million Americans have gout and more than 32 million have hyperuricemia (436). Worldwide an estimated 41 million people have gout (437). Age-standardized incidence rates of gout in South Asia, Southeast Asia, and East Asia, are moderately lower (~10%) than in Western Europe and North America (437). Gout and hyperuricemia can be considered components of metabolic syndrome, as insulin resistance leads to renal underexcretion of uric acid (438, 439). In the Third National Health and Nutrition Examination Survey (NHANES, 1988–1994), the prevalence of metabolic syndrome was 62.8% in patients with gout, compared with 25.4% in non-gout patients (440). Elevated uric acid levels may also increase risk of CVD (441).
A common perception among health professionals in Asia is that soyfoods increase risk of gout and potentially precipitate acute attacks in patients with this disease (361, 442, 443). For example, among the health professionals surveyed, 69, 46, and 27% in Singapore, Indonesia, and Thailand, respectively, consider consumption of soyfoods as a gout risk factor. This belief exists despite, with few exceptions, soyfoods not having an especially high purine content (444). To prevent gout, the Japanese Society of Gout and Nucleic Acid Metabolism recommends limiting purine intake to 400 mg/d (445).
However, the importance of patients with gout maintaining a low-purine diet has been deemphasized in recent years (446). A cross-sectional study involving >6,000 elderly participants with metabolic syndrome, found that non-soy legumes, despite being a purine-rich food, were inversely related to serum uric acid levels and the prevalence of hyperuricemia (447). One possible explanation for this lack of association is that serum uric acid levels are affected differently by purine bases and metabolites involved in the endogenous synthesis of purines (448). For this reason, dietary recommendations should be based more on how a food affects plasma urate, rather than on the purine content of a food (449).
Importantly, the results of intervention trials show that soy protein intake at levels as much as three times higher than the typical intake of older Japanese (~8–9 g/d) (45, 98) does not exert meaningful effects on blood uric acid levels (215, 450–457). It is noteworthy that the uric acid rising potential of soy purines (mainly adenosine and guanine) is much lower than those in meat and fish (higher proportion as hypoxanthine) (444, 448). This may be why a prospective study in gout patients reported that the impact of plant purine on gout attacks was substantially smaller than purine from animal sources (458).
Furthermore, population data suggest that soy intake may reduce risk of developing gout (459–462) and the guidelines of the British Society for Rheumatology for the management of gout include a recommendation to consume soybeans and other vegetable sources of protein (463); these guidelines align with the diet goals set forth by Beyl et al. (446) to consume tofu for the management of gout flares.
Perspective: Extensive clinical and limited observational data indicate that soyfoods do not increase risk of gout or appreciably affect serum uric levels. To the contrary, some recommendations call for increasing the consumption of soyfoods to manage gout. Aside from its effect on uric acid, soyfood intake may be advantageous for gout patients because some evidence suggests it favorably affects parameters of metabolic syndrome (464) and potentially decreases risk of CVD (465–467).
Kidney stones refer to the presence of renal calculi which results from an imbalance between the precipitation and solubility of salts in the kidneys and urinary tract. An analysis of data from 2013–2014 NHANES indicates that about 10% of Americans have a history of kidney stones (468). In most industrialized countries, ~80% of the kidney stones are composed of calcium salts, such as calcium oxalate (469). Risk factors for kidney stone formation include (1) higher body mass index (2) low fluid intake (3) low intake of calcium/low-fat dairy products (4) high intake of sugar sweetened beverages (5) low intake of fruits and vegetables (6) high sodium intake and (7) high animal protein intake (470). Dietary recommendations for at risk individuals (hyper-absorbers of oxalate, history of kidney stones), typically call for limiting oxalate intake to between 50 (471) and 100 mg/d (472). The Academy of Nutrition and Dietetics classifies foods containing >10 mg oxalate/serving as high-oxalate foods. As much as 50% of the oxalate in the urine comes from food when a typical diet containing 10 to 250 mg dietary oxalate is consumed, the other half coming from endogenous synthesis (473, 474).
Nevertheless, research shows a high oxalate diet is not associated with increased risk of kidney stones in the general population (475). Also, oxalate intake poorly reflects urinary oxalate levels (476, 477), likely because intestinal oxalate absorption and liver oxalate production varies among individuals (478, 479). Historically, patients were advised to decrease calcium intake to limit diet-dependent intestinal absorptive hypercalciuria (480, 481). However, prospective studies show low calcium intake increased risk of kidney stone formation (482–485). When calcium and oxalate are consumed together, a calcium-oxalate complex forms within the intestinal tract limiting the intestinal absorption and subsequent urinary excretion of free oxalate (481, 486).
Many soyfoods are relatively low in oxalate; one analysis found that of the 22 types of tofu examined only one contained >10 mg per serving, and both soymilks examined contained <6 mg/serving (487). Ellis and Lieb (488), reported values of ~5.3 mg and 4.0 mg per cup for two different soymilks. These values are much lower than high-oxalate foods such as spinach, rhubarb, peanuts, and chocolate, which contain >100 mg/100 g (489). However, several soyfoods contain >10 mg/serving including tempeh, soynuts and edamame; and some concentrated sources of soy protein are also quite high (487). It is difficult to generalize about the oxalate content of soyfoods because it varies among soyfoods and among different types of the same soyfood. Differences in oxalate values for a single food may be due to analytical methods, and/or biological variation from several sources, including cultivar, time of harvest, and growing conditions (489).
Of potential relevance is that soyfoods are high in phytate, a metal chelator. Absorbed phytate is excreted in the urine (490, 491) and may mitigate oxalate-induced kidney stone formation (483, 487). It is a strong inhibitor of calcium oxalate crystal formation in vitro (492) and in the Nurses' Health Study II, over an 8-year period women consuming the most phytate were 37% less likely to develop kidney stones (483). However, recent clinical work shows that despite phytate being excreted in the urine following supplementation, no changes in any of the well-established urinary risk factors for calcium renal stone formation were observed (493).
Massey et al. (494) found that oxalate absorption (mean ± SD) from soyfoods ranged from 2.1 ± 2.1% to 5.4 ± 4.2%, which is similar to other foods, and urinary oxalate excretion increased by 19.6 ± 23.3 to 124 ± 156 μmol (1.7 ± 2.1 to 10.9 ± 13.8 mg) during the 8 h following consumption of seven different soyfoods. They concluded that frequent soy product consumption may increase risk of kidney stones in susceptible individuals. However, normal urinary oxalate excretion is 110 to 440 μmol (10 to 39 mg) daily (494); therefore, it would appear that as long as other high-oxalate foods are avoided, soyfoods will not lead to hyperoxaluria.
Perspective: The oxalate content of soyfoods is likely not a concern for people not prone to developing kidney stones. For those who are, it is difficult to make recommendations about consumption because of the variation in the concentration of compounds in soyfoods potentially involved in the etiology of kidney stone formation including oxalate, protein, sodium, phytate and calcium. The decision to incorporate a given soyfood into the diet should be based on the specifics of that food placed within the context of the overall diet.
Food allergies are defined as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.” (495). IgE-mediated food allergy is a significant public health issue that affects an estimated 3 to 10% of adults and 8% of children worldwide (496–499).
Although IgE from patients allergic to soy may bind to as many as 28 proteins from soy (500, 501), only eight (Gly m 1 to Gly m 8) have been registered by the International Union of Immunological Societies Allergen Nomenclature Sub-Committee (502, 503). β-conglycinin (Gly m 5) and glycinin (Gly m 6) are the major storage proteins as they account for about 70% of the whole soybean protein. These proteins are associated with severe allergic reactions in European soy-allergic individuals (504). Importantly, Gly m Bd 30 K, which is also known as P34, is viewed as the protein most likely to cause allergic reactions in soy-sensitive people (505).
Since over 200 foods have been shown to be allergenic (506), regulatory agencies have recognized the need to focus allergen labeling regulations on a limited set of priority allergens. In the US, soy is one of eight foods (Big 8) designated as a priority allergen that must be called out as an allergen on product labels when present in a food (507). The eight foods (milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, and soybeans) requiring labeling are thought to account for 90% of food allergies among Americans. In Canada, the priority allergens are the same as in the US with the addition of sesame (508), which will officially soon be added to the US list (509). Somewhat parenthetically, the increased popularity of concentrated sources of pea protein is also resulting in more cases of allergic reactions to pea (510).
In Japan, seven food allergens, of which soy is not one, require mandatory labeling whereas in Europe, 14 foods, one of which is soy, require labeling (511). However, due to the lack of data on prevalence, severity and/or potency, or due to regional consumption of some foods, in 2021, the ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens recommended soy not be listed as a global priority allergen (512).
Five large surveys of food allergy prevalence in the US or Canada based on parent- or self-reported data, which likely overestimates prevalence, have been published over the past decade (498, 499, 513–515). These surveys highlight the variation in prevalence that exists among the major allergens. Among adults, soy allergy prevalence was reported to be 0.1, 0.16, 0.35 and 0.6%, which, in each case, made it the lowest prevalence of any of the major allergens. Among children and adolescents, these values were 0.25, 0.32 and 0.5%, which as in the case of adults, made soy the lowest prevalence.
Finally, estimates based on clinical experience, are that about 70% of children outgrow their soy allergy by age 10 years (516). Because highly refined soybean oil contains only negligible amounts of soy protein (517–522), soybean oil does not elicit allergic reactions in individuals sensitive to soy protein (523) and as such, does not fall under the allergy labeling regulation. In contrast, because it may contain small amounts of protein, this is not the case for lecithin derived from soybeans as it can potentially elicit allergic reactions (524–526). However, evidence suggests most soybean-allergic individuals do not react adversely to the ingestion of soybean lecithin.
Perspective: Soy protein is currently classified as a major allergen in the US although its prevalence is low relative to other major food allergens. Estimates are that about 3 out of every 1,000 adults and about 5 out of every 1,000 children, are allergic to soy protein. These individuals need to avoid all soy products containing protein but can consume highly refined soybean oil.
Soybean oil is the most widely consumed edible oil in the US (527) and the world (528). It accounts for over 7% of US caloric intake and over 40% of the intake of both essential fatty acids (529). Soybean oil is comprised of ~16% saturated fat, 22% monounsaturated fat and 62% PUFA (530). In 2017, the FDA approved a qualified health claim for soybean oil and reduced risk of CHD based on its ability to lower blood cholesterol levels when replacing saturated fat in the diet (434). In contrast to some vegetable oils high in n-6 fatty acids (e.g., corn oil, sesame oil), soybean oil contains considerable amounts of the essential n-3 fatty acid α-linolenic acid (ALA). A recent meta-analysis of prospective studies found ALA intake and tissue ALA concentrations were inversely related to all-cause mortality (531).
Despite the health claim, there are assertions that the intake of soybean oil, as well as other oils high in n-6 fatty acids, and that have a high n-6:n-3 fatty acid ratio, causes inflammation (21–23, 532) and that the US dietary n-6:n-3 ratio, of about 10:1 (529), is not compatible with optimal health. Some experts recommended dietary ratios as low as 2:1, (533) which is considerably lower than the ratio of ~7:1 in soybean oil. However, the notion that a higher n-6:n-3 ratio is harmful has been rejected by leading health agencies; instead, the emphasis is on making sure sufficient amounts of each type of fatty acid is consumed (534–542). Reviews of the clinical data show linoleic acid (LA) intake does not increase markers of inflammation (543, 544). This conclusion aligns with research showing that none of the seven clinical trials that evaluated the effects of soybean oil on inflammation found a statistically significant increase (545–551).
Beyond inflammation, there is concern that LA intake increases the oxidative susceptibility of LDL-C, thereby raising risk of CHD (21). PUFA are particularly susceptible to oxidation because of their multiple double bonds (552). The hypothesis that oxidized LDL-C promotes atherosclerosis was proposed more than 40 years ago (553, 554). Nevertheless, this hypothesis remains controversial (555, 556).
Furthermore, it is not clear that high-PUFA diets promote LDL-C oxidation more than high-saturated fat diets (557), although relative to PUFA, monounsaturated fat may increase LDL-C oxidation lag time (558). However, other dietary factors, such as antioxidants like vitamin E (559, 560), can greatly influence oxidation time, and likely have more impact than fatty acid intake (561). Of the three clinical trials (545, 562, 563) that examined the impact of soybean oil on oxidative markers, only one reported an increase (563). But in the context of the substantial LDL-C and small-dense LDL-C (sdLDL-C) lowering that occurred, and the in vitro assay used to assess time to sdLDL-C oxidation, the biological significance of this finding is unclear.
Finally, and most importantly, LA intake is not only associated with a decreased risk of CHD (564), but with diabetes (565), cancer and all-cause mortality (566). In addition, among participants of the European Prospective Investigation into Cancer and Nutrition, erythrocyte LA levels were inversely associated with risk of rheumatoid arthritis, an autoimmune and inflammatory disease (567).
Perspective: LA intake does not increase inflammation, a finding consistent with clinical trials showing soybean oil does not affect inflammation. In contrast, LA intake is associated with decreased risk of CHD and diabetes. Emphasis should be placed on making sure recommended intakes of both n-6 and n-3 fatty acids are met. When soybean oil replaces foods high in saturated fat, evidence indicates risk of CHD, and perhaps risk of other chronic diseases is reduced. The totality of the evidence indicates the susceptibility of high PUFA to oxidize is not a cause for concern. Although the data are more limited, research shows soybean oil ingestion does not increase oxidative stress.
There are few if any soyfood intake recommendations from independent health organizations. As noted previously, the US FDA established 25 g/d soy protein as the threshold intake for cholesterol reduction (427). However, this threshold was established for regulatory (labeling) purposes and is not intended to suggest all hypercholesterolemic individuals should consume soyfoods. The Chinese Nutrition Society recommends pregnant women consume at least 40 g of soy daily, but this recommendation is made primarily because soy is an inexpensive means to increase protein intake in a region where meat is relatively expensive and intake is low (361). These conditions are not applicable to pregnant women in much of the developed world. He et al. (568) recently reported that the Chinese dietary guidelines call for consuming ≥10 g soybeans/1,000 kcal; however, in an older paper, Liu et al. (123) listed the Chinese dietary guidelines for soybeans as calling for the consumption of 50 g/d. In any event, it is not possible to determine from these Chinese recommendations an amount of soy protein or isoflavones to consume since, on a weight basis, soyfoods do not provide equal amounts of these components.
One basis for formulating an intake recommendation is to mimic the intake of regions that have traditionally consumed soyfoods, especially China and Japan, given their long history of consumption. Interestingly, national surveys in China indicate soy intake increased over a recent 40-year period (568) whereas in Japan, data indicate soy intake decreased, especially in relation to total protein intake (45, 569). Despite the increase in China, He et al. (568) reported that <30% of the Chinese population meets the ≥10 g soybeans/1,000 kcal recommendation. However, in comparison to Japan, dietary habits in China are much more heterogenous (123, 570). In some regions, relatively little soy is consumed whereas Shanghai likely represents the highest soyfood-consuming region in the world.
In the SMHS (n = 54,219) (571) and the Shanghai Women's Health Study (SWHS, n = 45,694) (572), mean (±SD) soy protein intake was 12.5 ± 7.94 g/d (~16.0% of total protein intake) (573) and 8.8 ± 6.3 g/d (13.4% of total protein intake), respectively. Mean (±SD) isoflavone intake (mg/d) in the SMHS and SWHS was 36.2 ± 24.4 and 40.8 ± 28.7, respectively. One analysis of the SWHS is particularly informative because data were provided on the extremes of intake. The mean isoflavone intake of the ~2.2% of the women who consumed ≥25 g/d soy protein was 145.7 mg/d whereas the mean isoflavone intake of the nearly 9% of the women who consumed <2.5 g/d soy protein was 7.4 mg/d (572).
In Japan, the mean second soy protein intake tertile was 9.8 g/d for male (n = 5,883) and 9.4 g/d for female (n = 7,638) participants of the Takayama prospective study (98). These values represent about 11% (men) and 13% (women) of total protein intake, assuming a total protein intake of 90 and 70 g/d for men and women, respectively, using intake values from the NIPPON DATA80/90 Nutrition Study (574) (Adult men and women in the US (575) and Europe (78) consume similar amounts of protein as Shanghainese and Japanese men and women). Mean isoflavone intake in the Japan Public Health Center–Based prospective study, which involved 83,064 men and women was 37.5 mg/d (576).
Importantly, because mean intake may not represent optimal intake, another approach for formulating intake recommendations is to consider the intakes associated with desirable health outcomes in observational studies. Although there is often a monotonic response, in most instances when statistically significant associations are found, the highest intake is associated with the largest protective effect. For example, in a recent analysis of the SMHS, the hazard ratios for osteoporotic fracture risk associated with isoflavone intake (mg/d) for quartiles 1 (reference, <21.7), 2 (21.7–32.1), 3 (32.2–45.2), and 4 (>45.1) were 1.00, 0.89, 0.91, and 0.73, respectively (231). Similarly, in the SWHS, there was a dose-response between soy protein intake and systolic and diastolic blood pressure, with the reduction being greatest in women consuming ≥25 g/d (572).
Finally, and perhaps most importantly, insight about intake can potentially be gained from the amount of soy protein and isoflavones that produce benefits in clinical trials. For the alleviation of menopause-related hot flashes, the previously referenced meta-analysis by Taku et al. (4) found that ~50 mg/d isoflavones is needed for efficacy. Studies included in a meta-analysis that found isoflavones improve flow mediated dilation (FMD) in postmenopausal women with low baseline FMD levels intervened with between ~50 and 100 mg/d (577). On the other hand, studies included in a meta-analysis involving mostly postmenopausal women that found isoflavones improve cognitive function intervened with between 60 and 160 mg/d (5).
In general, the clinical trials suggest the dose of isoflavones required for efficacy is greater than suggested by the observational studies. However, there are two important caveats regarding the interpretation of the clinical trials. One is that none of the individual studies included in the above-mentioned meta-analyses intervened with more than one isoflavone dose, and for the most part, the meta-analyses did not attempt to determine dose-response relationships. Two, although entirely speculative, it is possible that long-term consumption, as reflected in observational studies, can produce efficacious results in response to lower intakes than is needed for efficacy in relatively short-term intervention studies.
Based on the above discussion, a reasonable adult intake recommendation of 15–25 g/d soy protein and 50 to 100 mg/d isoflavones appears to be appropriate. Consuming amounts that exceed these recommendations is not associated with adverse effects, but there is little historical precedent for consuming more than these amounts. Also, given the dietetic principles of moderation and variation, and the benefit from consuming nutrients provided by other dietary sources of protein, it is reasonable to recommend that soy protein not account for more than ~25 to 30% of total protein intake. For average European (78) and American (575) men and women, this would be about 25 g/d soy protein.
MM wrote the initial draft of the manuscript with contributions from JE, JK, VM, AD, and HL. All authors reviewed and commented on subsequent drafts of the manuscript and read and approved the final manuscript.
The authors wish to thank Lisa M. Balbes, Ph.D., of Balbes Consultants LLC for editorial support.
MM was employed by the Soy Nutrition Institute Global, an organization that receives funding from the United Soybean Board (USB) and from industry members who are involved in the manufacture and/or sale of soyfoods and/or soybean components. JK was employed by Medifast Inc., a nutrition and weight-management company based in Baltimore, Maryland, that uses soy protein in many of its products. JE and AD are scientific advisors to the Soy Nutrition Institute Global. VM is married to MM, and employed by Nutrition Matters, which receives no funding from the soy industry.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Huang MH, Norris J, Han W, Block T, Gold E, Crawford S, et al. Development of an updated phytoestrogen database for use with the SWAN food frequency questionnaire: intakes and food sources in a community-based, multiethnic cohort study. Nutr Cancer. (2012) 64:228–44. doi: 10.1080/01635581.2012.638434
2. Applegate CC, Rowles JL, Ranard KM, Jeon S, Erdman JW. Soy consumption and the risk of prostate cancer: an updated systematic review and meta-analysis. Nutrients. (2018) 10:40. doi: 10.3390/nu10010040
3. Okekunle AP, Gao J, Wu X, Feng R, Sun C. Higher dietary soy intake appears inversely related to breast cancer risk independent of estrogen receptor breast cancer phenotypes. Heliyon. (2020) 6:e04228. doi: 10.1016/j.heliyon.2020.e04228
4. Taku K, Melby MK, Kronenberg F, Kurzer MS, Messina M. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: systematic review and meta-analysis of randomized controlled trials. Menopause. (2012) 19:776–90. doi: 10.1097/gme.0b013e3182410159
5. Cui C, Birru RL, Snitz BE, Ihara M, Kakuta C, Lopresti BJ, et al. Effects of soy isoflavones on cognitive function: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2020) 78:134–44. doi: 10.1093/nutrit/nuz050
6. Lee A, Beaubernard L, Lamothe V, Bennetau-Pelissero C. New evaluation of isoflavone exposure in the French population. Nutrients. (2019) 11:2308. doi: 10.3390/nu11102308
7. Bar-El DS, Reifen R. Soy as an endocrine disruptor: cause for caution? J Pediatr Endocrinol Metab. (2010) 23:855–61. doi: 10.1515/jpem.2010.138
8. Chung MK, Buck Louis GM, Kannan K, Patel CJ. Exposome-wide association study of semen quality: systematic discovery of endocrine disrupting chemical biomarkers in fertility require large sample sizes. Environ Int. (2019) 125:505–14. doi: 10.1016/j.envint.2018.11.037
9. Fernandez-Lopez A, Lamothe V, Delample M, Denayrolles M, Bennetau-Pelissero C. Removing isoflavones from modern soyfood: why and how? Food Chem. (2016) 210:286–94. doi: 10.1016/j.foodchem.2016.04.126
10. Beszterda M, Franski R. Endocrine disruptor compounds in environment: as a danger for children health. Pediatr Endocrinol Diabetes Metab. (2018) 24:88–95. doi: 10.18544/PEDM-24.02.0107
11. Patisaul HB. Endocrine disruption by dietary phyto-oestrogens: impact on dimorphic sexual systems and behaviours. Proc Nutr Soc. (2017) 76:130–44. doi: 10.1017/S0029665116000677
12. Salsano S, Perez-Deben S, Quinonero A, Gonzalez-Martin R, Dominguez F. Phytoestrogen exposure alters endometrial stromal cells and interferes with decidualization signaling. Fertil Steril. (2019) 112:947–58 e3. doi: 10.1016/j.fertnstert.2019.06.014
13. Kwack SJ, Kim KB, Kim HS, Yoon KS, Lee BM. Risk assessment of soybean-based phytoestrogens. J Toxicol Environ Health A. (2009) 72:1254–61. doi: 10.1080/15287390903212212
14. Xiao Y, Zhang S, Tong H, Shi S. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother Res. (2018) 32:384–94. doi: 10.1002/ptr.5966
15. Rietjens I, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol. (2017) 174:1263–80. doi: 10.1111/bph.13622
16. Min J, Wang Z, Liang C, Li W, Shao J, Zhu K, et al. Detection of phytoestrogen metabolites in breastfed infants' urine and the corresponding breast milk by LC-MS/MS. J Agric Food Chem. (2020) 68:3485–94. doi: 10.1021/acs.jafc.9b08107
17. Messina M, Mejia SB, Cassidy A, Duncan A, Kurzer M, Nagato C, et al. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: a technical review of the observational and clinical data. Crit Rev Food Sci Nutr. (2021) 62:5824–85. doi: 10.1080/10408398.2021.1895054
18. Johnson LA, Myers DJ, Burden DJ. Soy protein's history, prospects in food, feed. Inform. (1992) 3:429–44.
19. Monteiro CA, Cannon G, Levy R, Moubarac J-C, Jaime P, Martins AP, et al. NOVA. The star shines bright. [Food classification. Public health]. World Nutr. (2016) 7:28–38.
20. Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. (2013) 14(Suppl. 2):21–8. doi: 10.1111/obr.12107
21. DiNicolantonio JJ, O'Keefe JH. Omega-6 vegetable oils as a driver of coronary heart disease: the oxidized linoleic acid hypothesis. Open Heart. (2018) 5:e000898. doi: 10.1136/openhrt-2018-000898
22. Maki KC, Eren F, Cassens ME, Dicklin MR, Davidson MH. Omega-6 polyunsaturated fatty acids and cardiometabolic health: current evidence, controversies, and research gaps. Adv Nutr. (2018) 9:688–700. doi: 10.1093/advances/nmy038
23. Sanders TAB. Omega-6 fatty acids and cardiovascular disease. Circulation. (2019) 139:2437–9. doi: 10.1161/CIRCULATIONAHA.119.040331
24. Khalil DA, Lucas EA, Juma S, Smith BJ, Payton ME, Arjmandi BH. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J Nutr. (2002) 132:2605–8. doi: 10.1093/jn/132.9.2605
25. Thrane M, Paulsen PV, Orcutt MW, Krieger TM. Soy protein: impacts, production, and applications. In: Nadathur SR, Wanasundara JPD, Scanlin L, editors. Sustainable Protein Sources. Cambridge, MA: Academic Press (2017). p. 23–46.
26. White LR, Petrovitch H, Ross GW, Masaki K, Hardman J, Nelson J, et al. Brain aging and midlife tofu consumption. J Am Coll Nutr. (2000) 19:242–55. doi: 10.1080/07315724.2000.10718923
27. Doerge D, Chang H. Inactivation of thyroid peroxidase by soy isoflavones, in vitro and in vivo. J Chromatogr B Anal Technol Biomed Life Sci. (2002) 777:269–79. doi: 10.1016/S1570-0232(02)00214-3
28. Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor- positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. (1998) 58:3833–8.
29. Martinez J, Lewi JE. An unusual case of gynecomastia associated with soy product consumption. Endocr Pract. (2008) 14:415–8. doi: 10.4158/EP.14.4.415
30. Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. (2009) 117:1883–9. doi: 10.1289/ehp.0900923
31. Hogervorst E, Sadjimim T, Yesufu A, Kreager P, Rahardjo TB. High tofu intake is associated with worse memory in elderly Indonesian men and women. Dement Geriatr Cogn Disord. (2008) 26:50–7. doi: 10.1159/000141484
32. Balk E, Chung M, Chew P, Ip S, Raman G, Kuplenick B, et al. Effects of Soy on Health Outcomes. Evidence Report/Technology Assessment No. 126 (Prepared by Tufts-New England Medical Center Evidence-Based Practice Center under Contract No. 290-02-0022.) AHRQ Publication No. 05-E024-2. Rockville, MD Agency for Healthcare Research and Quality (2005).
33. Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. (2006) 113:1034–44. doi: 10.1161/CIRCULATIONAHA.106.171052
34. Department Department of Health and Human Services Food and Drug Administration (docket no. 2007N−0464]. Health Claims and Qualified Health Claims; Dietary Lipids and Cancer, Soy Protein and Coronary Heart Disease, Antioxidant Vitamins and Certain Cancers, and Selenium and Certain Cancers; Reevaluation; Opportunity for Public Comment.
36. Daniels K. The Whole Soy Story: The Dark Side of America's Favorite Health Food. Washington, DC: NewTrends Publishing, Inc. (2005).
37. Fallon S, Enig MG. Tragedy and hype - the third international soy symposium. Nexus. (2000) 17–22, 74–5.
39. Willett W, Rockstrom J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. (2019) 393:447–92. doi: 10.1016/S0140-6736(18)31788-4
40. Gardner CD, Hartle JC, Garrett RD, Offringa LC, Wasserman AS. Maximizing the intersection of human health and the health of the environment with regard to the amount and type of protein produced and consumed in the United States. Nutr Rev. (2019) 77:197–215. doi: 10.1093/nutrit/nuy073
41. Hymowitz T, Shurtleff W. Debunking soybean myths and legends in the historical and popular literature. Crop Sci. (2005) 45:473–6. doi: 10.2135/cropsci2005.0473
42. Lee GA, Crawford GW, Liu L, Sasaki Y, Chen X. Archaeological soybean (Glycine max) in East Asia: does size matter? PloS ONE. (2011) 6:e26720. doi: 10.1371/journal.pone.0026720
43. Sedivy EJ, Wu F, Hanzawa Y. Soybean domestication: the origin, genetic architecture and molecular bases. New Phytol. (2017) 214:539–53. doi: 10.1111/nph.14418
44. Ahnan-Winarno AD, Cordeiro L, Winarno FG, Gibbons J, Xiao H. Tempeh: a semicentennial review on its health benefits, fermentation, safety, processing, sustainability, and affordability. Compr Rev Food Sci Food Saf. (2021) 20:1717–67. doi: 10.1111/1541-4337.12710
45. Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. (2006) 55:1–12. doi: 10.1207/s15327914nc5501_1
46. Codex General Standard for Soy Protein Products, Codex Standard 175-1989. (1989). Available online at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiU28Td7535AhUrhIkEHadFAHoQFnoECAcQAQ&url=https%3A%2F%2Fwww.chinaoils.cn%2Fuploads%2Fsoft%2F20201216%2F1608079304454577.pdf&usg=AOvVaw3zD_3OKAgBfR40HqsDVLA6
47. Morr CV. Current status of soy protein functionality in food systems. J Am Oil Chemists Soc. (1990) 67:265–71. doi: 10.1007/BF02539674
48. Hughes GJ, Ryan DJ, Mukherjea R, Schasteen CS. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: criteria for evaluation. J Agric Food Chem. (2011) 59:12707–12. doi: 10.1021/jf203220v
49. Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. (2015) 145:372–9. doi: 10.3945/jn.114.195438
50. Mathai JK, Liu Y, Stein HH. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br J Nutr. (2017) 117:490–9. doi: 10.1017/S0007114517000125
51. FAO. Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAO Expert Consultation, Vol. 92. Rome: FAO Food and Nutrition Paper (2013).
52. Reynaud Y, Buffiere C, Cohade B, Vauris M, Liebermann K, Hafnaoui N, et al. True ileal amino acid digestibility and digestible indispensable amino acid scores (DIAASs) of plant-based protein foods. Food Chem. (2020) 338:128020. doi: 10.1016/j.foodchem.2020.128020
53. Fanelli NS, Bailey HM, Thompson TW, Delmore R, Nair MN, Stein HH. Digestible indispensable amino acid score (DIAAS) is greater in animal-based burgers than in plant-based burgers if determined in pigs. Eur J Nutr. (2022) 61:461–75. doi: 10.1007/s00394-021-02658-1
54. van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr. (2015) 145:1981–91. doi: 10.3945/jn.114.204305
55. Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. (2015) 80(Suppl. 1):A8–15. doi: 10.1111/1750-3841.12802
56. Hulmi JJ, Lockwood CM, Stout JR. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: a case for whey protein. Nutr Metab. (2010) 7:51. doi: 10.1186/1743-7075-7-51
57. Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. (2014) 44(Suppl. 1):S71–7. doi: 10.1007/s40279-014-0152-3
58. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. (2007) 85:1031–40. doi: 10.1093/ajcn/85.4.1031
59. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. (2009) 107:987–92. doi: 10.1152/japplphysiol.00076.2009
60. Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab. (2012) 9:57. doi: 10.1186/1743-7075-9-57
61. Mitchell CJ, Della Gatta PA, Petersen AC, Cameron-Smith D, Markworth JF. Soy protein ingestion results in less prolonged p70S6 kinase phosphorylation compared to whey protein after resistance exercise in older men. J Int Soc Sports Nutr. (2015) 12:6. doi: 10.1186/s12970-015-0070-2
62. Gran P, Larsen AE, Bonham M, Dordevic AL, Rupasinghe T, Silva C, et al. Muscle p70S6K phosphorylation in response to soy and dairy rich meals in middle aged men with metabolic syndrome: a randomised crossover trial. Nutr Metab. (2014) 11:46. doi: 10.1186/1743-7075-11-46
63. Rittig N, Bach E, Thomsen HH, Moller AB, Hansen J, Johannsen M, et al. Anabolic effects of leucine-rich whey protein, carbohydrate, and soy protein with and without beta-hydroxy-beta-methylbutyrate (HMB) during fasting-induced catabolism: a human randomized crossover trial. Clin Nutr. (2017) 36:697–705. doi: 10.1016/j.clnu.2016.05.004
64. Luiking YC, Engelen MP, Soeters PB, Boirie Y, Deutz NE. Differential metabolic effects of casein and soy protein meals on skeletal muscle in healthy volunteers. Clin Nutr. (2011) 30:65–72. doi: 10.1016/j.clnu.2010.06.012
65. Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrao ME, Jannig PR, et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol. (2016) 594:5209–22. doi: 10.1113/JP272472
66. Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, et al. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PloS ONE. (2014) 9:e89431. doi: 10.1371/journal.pone.0089431
67. Messina M, Lynch H, Dickinson JM, Reed KE. No difference between the effects of supplementing with soy protein versus animal protein on gains in muscle mass and strength in response to resistance exercise. Int J Sport Nutr Exerc Metab. (2018) 28:674–85. doi: 10.1123/ijsnem.2018-0071
68. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. (2017) 52:376–84. doi: 10.1136/bjsports-2017-097608
69. Morgan PT, Harris DO, Marshall RN, Quinlan JI, Edwards SJ, Allen SL, et al. Protein source and quality for skeletal muscle anabolism in young and older adults: a systematic review and meta-analysis. J Nutr. (2021) 151:1901–20. doi: 10.1093/jn/nxab055
70. Hudson JL, Wang Y, Bergia Iii RE, Campbell WW. Protein intake greater than the RDA differentially influences whole-body lean mass responses to purposeful catabolic and anabolic stressors: a systematic review and meta-analysis. Adv Nutr. (2020) 11:548–58. doi: 10.1093/advances/nmz106
71. Antonio J. High-protein diets in trained individuals. Res Sports Med. (2019) 27:195–203. doi: 10.1080/15438627.2018.1523167
72. Paddon-Jones D. Protein recommendations for bodybuilders: in this case, more may indeed be better. J Nutr. (2017) 147:723–4. doi: 10.3945/jn.117.247981
73. Rodriguez NR, DiMarco NM, Langley S. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Am Diet Assoc. (2009) 109:509–27. doi: 10.1016/j.jada.2009.01.005
74. Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, et al. International Society of Sports Nutrition Position Stand: protein and exercise. J Internal Soc Sports Nutr. (2017) 14:1–25. doi: 10.1186/s12970-017-0177-8
75. Astrup A. The satiating power of protein–a key to obesity prevention? Am J Clin Nutr. (2005) 82:1–2. doi: 10.1093/ajcn/82.1.1
76. Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr. (2012) 108(Suppl. 2):S105–12. doi: 10.1017/S0007114512002589
77. Morell P, Fisman S. Revisiting the role of protein-induced satiation and satiety. Food Hydrocolloids. (2017) 68:199–210. doi: 10.1016/j.foodhyd.2016.08.003
78. Lieberman HR, Fulgoni VL, Agarwal S, Pasiakos SM, Berryman CE. Protein intake is more stable than carbohydrate or fat intake across various US demographic groups and international populations. Am J Clin Nutr. (2020) 112:180–6. doi: 10.1093/ajcn/nqaa044
79. Wolfe RR, Cifelli AM, Kostas G, Kim IY. Optimizing protein intake in adults: interpretation and application of the recommended dietary allowance compared with the acceptable macronutrient distribution range. Adv Nutr. (2017) 8:266–75. doi: 10.3945/an.116.013821
80. Raubenheimer D, Simpson SJ. Protein leverage: theoretical foundations and ten points of clarification. Obesity. (2019) 27:1225–38. doi: 10.1002/oby.22531
81. Simpson SJ, Raubenheimer D. The power of protein. Am J Clin Nutr. (2020) 112:6–7. doi: 10.1093/ajcn/nqaa088
82. Magkos F. The role of dietary protein in obesity. Rev Endocr Metab Disord. (2020) 21:329–40. doi: 10.1007/s11154-020-09576-3
83. Cope MB, Erdman JW Jr., Allison DB. The potential role of soyfoods in weight and adiposity reduction: an evidence-based review. Obes Rev. (2008) 9:219–35. doi: 10.1111/j.1467-789X.2007.00390.x
84. Melson CE, Nepocatych S, Madzima TA. The effects of whey and soy liquid breakfast on appetite response, energy metabolism, and subsequent energy intake. Nutrition. (2019) 61:179–86. doi: 10.1016/j.nut.2018.11.007
85. Neacsu M, Fyfe C, Horgan G, Johnstone AM. Appetite control and biomarkers of satiety with vegetarian (soy) and meat-based high-protein diets for weight loss in obese men: a randomized crossover trial. Am J Clin Nutr. (2014) 100:548–58. doi: 10.3945/ajcn.113.077503
86. Lang V, Bellisle F, Alamowitch C, Craplet C, Bornet FR, Slama G, et al. Varying the protein source in mixed meal modifies glucose, insulin and glucagon kinetics in healthy men, has weak effects on subjective satiety and fails to affect food intake. Eur J Clin Nutr. (1999) 53:959–65. doi: 10.1038/sj.ejcn.1600881
87. Lang V, Bellisle F, Oppert JM, Craplet C, Bornet FR, Slama G, et al. Satiating effect of proteins in healthy subjects: a comparison of egg albumin, casein, gelatin, soy protein, pea protein, and wheat gluten. Am J Clin Nutr. (1998) 67:1197–204. doi: 10.1093/ajcn/67.6.1197
88. Douglas SM, Lasley TR, Leidy HJ. Consuming beef vs. soy protein has little effect on appetite, satiety, and food intake in healthy adults. J Nutr. (2015) 145:1010–6. doi: 10.3945/jn.114.206987
89. Speaker KJ, Sayer RD, Peters JC, Foley HN, Pan Z, Wyatt HR, et al. Effects of consuming a high-protein diet with or without soy protein during weight loss and maintenance: a non-inferiority, randomized clinical efficacy trial. Obes Sci Pract. (2018) 4:357–66. doi: 10.1002/osp4.278
90. Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, et al. Mechanisms enforcing the estrogen receptor beta selectivity of botanical estrogens. FASEB J. (2013) 27:4406–18. doi: 10.1096/fj.13-234617
91. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. (1998) 139:4252–63. doi: 10.1210/endo.139.10.6216
92. Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys. (1998) 356:133–41. doi: 10.1006/abbi.1998.0783
93. Kulling SE, Honig DM, Metzler M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J Agric Food Chem. (2001) 49:3024–33. doi: 10.1021/jf0012695
94. Carter MW, Matrone G, Smart WWGJ. Effect of genistein on reproduction of the mouse. J Nutr. (1955) 55:639–45. doi: 10.1093/jn/55.4.639
95. Carter MW, Smart Jr. WWG, Matrone G. Estimation of the estrogenic activity of genistein obtained from soybean meal (20693). Proc Soc Exp Biol Med. (1953) 84:506–7. doi: 10.3181/00379727-84-20693
96. Folman Y, Pope GS. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other utero-vaginotrophic compounds of low potency. J Endocrinol. (1966) 34:215–25. doi: 10.1677/joe.0.0340215
97. Dixon RA, Sumner LW. Legume natural products: understanding and manipulating complex pathways for human and animal health. Plant Physiol. (2003) 131:878–85. doi: 10.1104/pp.102.017319
98. Konishi K, Wada K, Yamakawa M, Goto Y, Mizuta F, Koda S, et al. Dietary soy intake is inversely associated with risk of type 2 diabetes in Japanese women but not in men. J Nutr. (2019) 149:1208–14. doi: 10.1093/jn/nxz047
99. Bai W, Wang C, Ren C. Intakes of total and individual flavonoids by US adults. Int J Food Sci Nutr. (2014) 65:9–20. doi: 10.3109/09637486.2013.832170
100. Sebastian RS, Wilkinson Enns C, Goldman JD, Martin CL, Steinfeldt LC, Murayi T, et al. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet among US adults. J Nutr. (2015) 145:1239–48. doi: 10.3945/jn.115.213025
101. Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. (2007) 137:1244–52. doi: 10.1093/jn/137.5.1244
102. Zamora-Ros R, Ferrari P, Gonzalez CA, Tjonneland A, Olsen A, Bredsdorff L, et al. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res Treat. (2013) 139:163–76. doi: 10.1007/s10549-013-2483-4
103. Ziauddeen N, Rosi A, Del Rio D, Amoutzopoulos B, Nicholson S, Page P, et al. Dietary intake of (poly)phenols in children and adults: cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008-2014). Eur J Nutr. (2019) 58:3183–98. doi: 10.1007/s00394-018-1862-3
104. Lee A, Bensaada S, Lamothe V, Lacoste M, Bennetau-Pelissero C. Endocrine disruptors on and in fruits and vegetables: estimation of the potential exposure of the French population. Food Chem. (2021) 373:131513. doi: 10.1016/j.foodchem.2021.131513
105. Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Anal Technol Biomed Life Sci. (2002) 777:129–38. doi: 10.1016/S1570-0232(02)00342-2
106. Dakora FD, Phillips DA. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Mol Plant Pathol. (1996) 49:1–20. doi: 10.1006/pmpp.1996.0035
107. Rípodas C, Via VD, Aguilar OM, Zanetti ME, Blanco FA. Knock-down of a member of the isoflavone reductase gene family impairs plant growth and nodulation in Phaseolus vulgaris. Plant Physiol Biochem. (2013) 68:81–9. doi: 10.1016/j.plaphy.2013.04.003
108. Subramanian S, Stacey G, Yu O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. (2006) 48:261–73. doi: 10.1111/j.1365-313X.2006.02874.x
109. EFSA. EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2015. Scientific opinion on the risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. (2015) 13:4246. doi: 10.2903/j.efsa.2015.4246
110. Fanti P, Sawaya BP, Custer LJ, Franke AA. Serum levels and metabolic clearance of the isoflavones genistein and daidzein in hemodialysis patients. J Am Soc Nephrol. (1999) 10:864–71. doi: 10.1681/ASN.V104864
111. Franke AA, Yu MC, Maskarinec G, Fanti P, Zheng W, Custer LJ. Phytoestrogens in human biomatrices including breast milk. Biochem Soc Trans. (1999) 27:308–18. doi: 10.1042/bst0270308
112. Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. (2003) 77:411–9. doi: 10.1093/ajcn/77.2.411
113. King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. (1998) 67:867–72. doi: 10.1093/ajcn/67.5.867
114. Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. (2003) 77:1459–65. doi: 10.1093/ajcn/77.6.1459
115. Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, et al. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. (2021) 2021:3268136. doi: 10.1155/2021/3268136
116. Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. (1997) 138:863–70. doi: 10.1210/endo.138.3.4979
117. Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. (2008) 74:1656–65. doi: 10.1055/s-0028-1088304
118. Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. (2004) 64:423–8. doi: 10.1158/0008-5472.CAN-03-2446
119. van der Velpen V, Hollman PC, van Nielen M, Schouten EG, Mensink M, Van't Veer P, et al. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur J Clin Nutr. (2014) 68:1141–7. doi: 10.1038/ejcn.2014.108
120. Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, Ivancic D, et al. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res. (2012) 5:309–19. doi: 10.1158/1940-6207.CAPR-11-0251
121. Shirabe R, Saito E, Sawada N, Ishihara J, Takachi R, Abe SK, et al. Fermented and nonfermented soy foods and the risk of breast cancer in a Japanese population-based cohort study. Cancer Med. (2021) 10:757–71. doi: 10.1002/cam4.3677
122. Lee MJ, Kim JH. Estimated dietary isoflavone intake among Korean adults. Nutr Res Pract. (2007) 1:206–11. doi: 10.4162/nrp.2007.1.3.206
123. Liu Z, Li W, Sun J, Liu C, Zeng Q, Huang J, et al. Intake of soy foods and soy isoflavones by rural adult women in China. Asia Pacific J Clin Nutr. (2004) 13:204–9. doi: 10.1201/9781439822203.ch3
124. Cai H, Zheng W, Xiang YB, Xu WH, Yang G, Li H, et al. Dietary patterns and their correlates among middle-aged and elderly Chinese men: a report from the Shanghai Men's Health Study. Br J Nutr. (2007) 98:1006–13. doi: 10.1017/S0007114507750900
125. Murphy PA, Song T, Buseman G, Barua K, Beecher GR, Trainer D, et al. Isoflavones in retail and institutional soy foods. J Agric Food Chem. (1999) 47:2697–704. doi: 10.1021/jf981144o
126. Chien HL, Huang HY, Chou CC. Transformation of isoflavone phytoestrogens during the fermentation of soymilk with lactic acid bacteria and bifidobacteria. Food Microbiol. (2006) 23:772–8. doi: 10.1016/j.fm.2006.01.002
127. Lee IH, Chou CC. Distribution profiles of isoflavone isomers in black bean kojis prepared with various filamentous fungi. J Agric Food Chem. (2006) 54:1309–14. doi: 10.1021/jf058139m
128. Rekha CR, Vijayalakshmi G. Biomolecules and nutritional quality of soymilk fermented with probiotic yeast and bacteria. Appl Biochem Biotechnol. (2008) 151:452–63. doi: 10.1007/s12010-008-8213-4
129. Rekha CR, Vijayalakshmi G. Isoflavone phytoestrogens in soymilk fermented with beta-glucosidase producing probiotic lactic acid bacteria. Int J Food Sci Nutr. (2011) 62:111–20. doi: 10.3109/09637486.2010.513680
130. Yuan B, Zhen H, Jin Y, Xu L, Jiang X, Sun S, et al. Absorption and plasma disposition of genistin differ from those of genistein in healthy women. J Agric Food Chem. (2012) 60:1428–36. doi: 10.1021/jf204421c
131. Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. (2001) 131:1362–75S. doi: 10.1093/jn/131.4.1362S
132. Reddy NR, Pierson MD. Reduction in antinutritional and toxic components in plant foods by fermentation. Food Res Int. (1994) 27:281–90. doi: 10.1016/0963-9969(94)90096-5
133. Rackis JJ, McGhee JE, Booth AN. Biological threshold levels of soybean trypsin inhibitors by rat bioassay. Cereal Chem. (1975) 52:85–92. doi: 10.1007/BF02545077
134. Heaney RP, Weaver CM, Fitzsimmons ML. Soybean phytate content: effect on calcium absorption. Am J Clin Nutr. (1991) 53:745–7. doi: 10.1093/ajcn/53.3.745
135. Weaver CM, Heaney RP, Connor L, Martin BR, Smith DL, Nielsen E. Bioavailability of calcium from tofu vs. milk in premenopausal women. J Food Sci. (2002) 68:3144–7. doi: 10.1111/j.1365-2621.2002.tb08873.x
136. Zhao Y, Martin BR, Weaver CM. Calcium bioavailability of calcium carbonate fortified soymilk is equivalent to cow's milk in young women. J Nutr. (2005) 135:2379–82. doi: 10.1093/jn/135.10.2379
137. Tang AL, Walker KZ, Wilcox G, Strauss BJ, Ashton JF, Stojanovska L. Calcium absorption in Australian osteopenic post-menopausal women: an acute comparative study of fortified soymilk to cows' milk. Asia Pacific J Clin Nutr. (2010) 19:243–9.
138. Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. (2003) 78:633–9S. doi: 10.1093/ajcn/78.3.633S
139. Brune M, Rossander L, Hallberg L. Iron absorption: no intestinal adaptation to a high-phytate diet. Am J Clin Nutr. (1989) 49:542–5. doi: 10.1093/ajcn/49.3.542
140. Armah SM, Boy E, Chen D, Candal P, Reddy MB. Regular consumption of a high-phytate diet reduces the inhibitory effect of phytate on nonheme-iron absorption in women with suboptimal iron stores. J Nutr. (2015) 145:1735–9. doi: 10.3945/jn.114.209957
141. Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press (2001).
142. Shin A, Lee J, Park MS, Park JW, Park SC, Oh JH, et al. Isoflavone and soyfood intake and colorectal cancer risk: a case-control study in Korea. PloS ONE. (2015) 10:e0143228. doi: 10.1371/journal.pone.0143228
143. Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. (2009) 89:1155–63. doi: 10.3945/ajcn.2008.27029
144. Yang WS, Va P, Wong MY, Zhang HL, Xiang YB. Soy intake is associated with lower lung cancer risk: results from a meta-analysis of epidemiologic studies. Am J Clin Nutr. (2011) 94:1575–83. doi: 10.3945/ajcn.111.020966
145. Im J, Park K. Association between soy food and dietary soy isoflavone intake and the risk of cardiovascular disease in women: a prospective cohort study in Korea. Nutrients. (2021) 13:1407. doi: 10.3390/nu13051407
146. Weng KG, Yuan YL. Soy food intake and risk of gastric cancer: a dose-response meta-analysis of prospective studies. Medicine. (2017) 96:e7802. doi: 10.1097/MD.0000000000007802
147. Kim J, Kang M, Lee JS, Inoue M, Sasazuki S, Tsugane S. Fermented and non-fermented soy food consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci. (2011) 102:231–44. doi: 10.1111/j.1349-7006.2010.01770.x
148. Minami Y, Kanemura S, Oikawa T, Suzuki S, Hasegawa Y, Nishino Y, et al. Associations of Japanese food intake with survival of stomach and colorectal cancer: a prospective patient cohort study. Cancer Sci. (2020) 111:2558–69. doi: 10.1111/cas.14459
149. Ikeda Y, Iki M, Morita A, Kajita E, Kagamimori S, Kagawa Y, et al. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J Nutr. (2006) 136:1323–8. doi: 10.1093/jn/136.5.1323
150. Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, et al. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol. (2007) 53:464–70. doi: 10.3177/jnsv.53.464
151. Katsuyama H, Ideguchi S, Fukunaga M, Saijoh K, Sunami S. Usual dietary intake of fermented soybeans (Natto) is associated with bone mineral density in premenopausal women. J Nutr Sci Vitaminol. (2002) 48:207–15. doi: 10.3177/jnsv.48.207
152. Homma K, Wakana N, Suzuki Y, Nukui M, Daimatsu T, Tanaka E, et al. Treatment of natto, a fermented soybean preparation, to prevent excessive plasma vitamin K concentrations in patients taking warfarin. J Nutr Sci Vitaminol. (2006) 52:297–301. doi: 10.3177/jnsv.52.297
153. Hsu RL, Lee KT, Wang JH, Lee LY, Chen RP. Amyloid-degrading ability of nattokinase from Bacillus subtilis natto. J Agric Food Chem. (2009) 57:503–8. doi: 10.1021/jf803072r
154. Fujita M, Hong K, Ito Y, Fujii R, Kariya K, Nishimuro S. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull. (1995) 18:1387–91. doi: 10.1248/bpb.18.1387
155. Fujita M, Nomura K, Hong K, Ito Y, Asada A, Nishimuro S. Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun. (1993) 197:1340–7. doi: 10.1006/bbrc.1993.2624
156. Okada Y, Tsuzuki Y, Sugihara N, Nishii S, Shibuya N, Mizoguchi A, et al. Novel probiotic yeast from Miso promotes regulatory dendritic cell IL-10 production and attenuates DSS-induced colitis in mice. J Gastroenterol. (2021) 56:829–42. doi: 10.1007/s00535-021-01804-0
157. Esaki H, Kawakishi S, Morimitsu Y, Osawa T. New potent antioxidative o-dihydroxyisoflavones in fermented Japanese soybean products. Biosci Biotechnol Biochem. (1999) 63:1637–9. doi: 10.1271/bbb.63.1637
158. Esaki H, Onozaki H, Morimitsu Y, Kawakishi S, Osawa T. Potent antioxidative isoflavones isolated from soybeans fermented with Aspergillus saitoi. Biosci Biotech Biochem. (1998) 62:740–46. doi: 10.1271/bbb.62.740
159. Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. (2002) 97:72–81. doi: 10.1002/ijc.1571
160. Messina M, Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. (1991) 83:541–6. doi: 10.1093/jnci/83.8.541
161. Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. (2001) 61:5045–50.
162. Du M, Yang X, Hartman JA, Cooke PS, Doerge DR, Ju YH, et al. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis. (2012) 33:895–901. doi: 10.1093/carcin/bgs017
163. Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. (2002) 62:2474–7.
164. Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. (2008) 29:2162–8. doi: 10.1093/carcin/bgn161
165. Deng G, Davatgarzadeh A, Yeung S, Cassileth B. Phytoestrogens: science, evidence, and advice for breast cancer patients. J Soc Integr Oncol. (2010) 8:20–30.
166. Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. (1999) 84:4017–24. doi: 10.1210/jc.84.11.4017
167. Finkeldey L, Schmitz E, Ellinger S. Effect of the intake of isoflavones on risk factors of breast cancer—a systematic review of randomized controlled intervention studies. Nutrients. (2021) 13:2309. doi: 10.3390/nu13072309
168. Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. (2010) 16:745–60. doi: 10.1093/humupd/dmq011
169. Wu AH, Spicer D, Garcia A, Tseng CC, Hovanessian-Larsen L, Sheth P, et al. Double-blind randomized 12-month soy intervention had no effects on breast MRI fibroglandular tissue density or mammographic density. Cancer Prev Res. (2015) 8:942–51. doi: 10.1158/1940-6207.CAPR-15-0125
170. Labos G, Trakakis E, Pliatsika P, Augoulea A, Vaggopoulos V, Basios G, et al. Efficacy and safety of DT56a compared to hormone therapy in Greek post-menopausal women. J Endocrinol Invest. (2013) 36:521–6.
171. Sartippour MR, Rao JY, Apple S, Wu D, Henning S, Wang H, et al. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutr Cancer. (2004) 49:59–65. doi: 10.1207/s15327914nc4901_8
172. Palomares MR, Hopper L, Goldstein L, Lehman CD, Storer BE, Gralow JR. Effect of soy isoflavones on breast proliferation in postmenopausal breast cancer survivors. Breast Cancer Res Treatment. (2004) 88(Suppl. 1):4002. doi: 10.3275/8926
173. Cheng G, Wilczek B, Warner M, Gustafsson JA, Landgren BM. Isoflavone treatment for acute menopausal symptoms. Menopause. (2007) 14:468–73. doi: 10.1097/GME.0b013e31802cc7d0
174. Shike M, Doane AS, Russo L, Cabal R, Reis-Filo J, Gerald W, et al. The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. J Natl Cancer Inst. (2014) 106:dju189. doi: 10.1093/jnci/dju189
175. Tomasetti C, Poling J, Roberts NJ, London NR Jr., Pittman ME, et al. Cell division rates decrease with age, providing a potential explanation for the age-dependent deceleration in cancer incidence. Proc Natl Acad Sci USA. (2019) 116:20482–8. doi: 10.1073/pnas.1905722116
176. Conner P, Skoog L, Soderqvist G. Breast epithelial proliferation in postmenopausal women evaluated through fine-needle-aspiration cytology. Climacteric. (2001) 4:7–12. doi: 10.1080/cmt.4.1.7.12
177. Conner P, Soderqvist G, Skoog L, Graser T, Walter F, Tani E, et al. Breast cell proliferation in postmenopausal women during HRT evaluated through fine needle aspiration cytology. Breast Cancer Res Treat. (2003) 78:159–65. doi: 10.1023/A:1022987618445
178. Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the women's health initiative randomized clinical trials. JAMA. (2020) 324:369–80. doi: 10.1001/jama.2020.9482
179. Greco S, Pellegrino P, Zannotti A, Delli Carpini G, Ciavattini A, Reis FM, et al. Phytoprogestins: unexplored food compounds with potential preventive and therapeutic effects in female diseases. Nutrients. (2021) 13:4326. doi: 10.3390/nu13124326
180. Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, et al. Soy food intake and breast cancer survival. JAMA. (2009) 302:2437–43. doi: 10.1001/jama.2009.1783
181. Caan BJ, Natarajan L, Parker B, Gold EB, Thomson C, Newman V, et al. Soy food consumption and breast cancer prognosis. Cancer Epidemiol Biomark Prev. (2011) 20:854–8. doi: 10.1158/1055-9965.EPI-10-1041
182. Guha N, Kwan ML, Quesenberry CP. Jr., Weltzien EK, Castillo AL, et al. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. (2009) 118:395–405. doi: 10.1007/s10549-009-0321-5
183. Zhang YF, Kang HB, Li BL, Zhang RM. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pacific J Cancer Prev. (2012) 13:479–82. doi: 10.7314/APJCP.2012.13.2.479
184. Kang X, Zhang Q, Wang S, Huang X, Jin S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ. (2010) 182:1857–62. doi: 10.1503/cmaj.091298
185. Chi F, Wu R, Zeng YC, Xing R, Liu Y, Xu ZG. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pacific J Cancer Prev. (2013) 14:2407–12. doi: 10.7314/APJCP.2013.14.4.2407
186. Qiu S, Jiang C. Soy and isoflavones consumption and breast cancer survival and recurrence: a systematic review and meta-analysis. Eur J Nutr. (2019) 58:3079–90. doi: 10.1007/s00394-018-1853-4
187. Soy: Intake Does Not Increase Risk for Breast Cancer Survivors. American Institute for Cancer Research (2021). Available online at: https://www.aicr.org/cancer-prevention/food-facts/soy/ (accessed January 18, 2022).
188. Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. (2012) 62:242–74. doi: 10.3322/caac.21142
189. American Institute for Cancer Research. Soy Is Safe for Breast Cancer Survivors (2012). Available online at: http://wwwaicrorg/cancer-research-update/november_21_2012/cru-soy-safehtml (accessed Feburary 5, 2013).
190. World Cancer Research Fund International. Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Breast Cancer Survivors (2014). Available online at: www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf. Accessed December 10, 2014.
191. Eating Well After Breast Cancer (2019). Available online at: https://www.cancer.ca/en/cancer-information/cancer-type/breast/supportive-care/eating-well-after-breast-cancer/?region=on (Accessed October 25, 2019).
192. Huser S, Guth S, Joost HG, Soukup ST, Kohrle J, Kreienbrock L, et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: a comprehensive safety evaluation. Arch Toxicol. (2018) 92:2703–48. doi: 10.1007/s00204-018-2279-8
193. Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. (2005) 41:834–45. doi: 10.1016/j.ejca.2004.12.033
194. Zhang J, Dhakal IB, Zhao Z, Li L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: role of nutrition transition. Eur J Cancer Prev. (2012) 21:480–9. doi: 10.1097/CEJ.0b013e328351c732
195. Grainger EM, Moran NE, Francis DM, Schwartz SJ, Wan L, Thomas-Ahner J, et al. A novel tomato-soy juice induces a dose-response increase in urinary and plasma phytochemical biomarkers in men with prostate cancer. J Nutr. (2019) 149:26–35. doi: 10.1093/jn/nxy232
196. Pollard M, Luckert PH. Influence of isoflavones in soy protein isolates on development of induced prostate-related cancers in L-W rats. Nutr Cancer. (1997) 28:41–5. doi: 10.1080/01635589709514551
197. Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. (1999) 129:1628–35. doi: 10.1093/jn/129.9.1628
198. Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res. (2001) 61:6777–82.
199. Sivonova MK, Kaplan P, Tatarkova Z, Lichardusova L, Dusenka R, Jurecekova J. Androgen receptor and soy isoflavones in prostate cancer. Mol Clin Oncol. (2019) 10:191–204.
200. Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. (2006) 89:1121–34. doi: 10.1093/jaoac/89.4.1121
201. Pentyala S, Whyard T, Pentyala S, Muller J, Pfail J, Parmar S, et al. Prostate cancer markers: an update. Biomed Rep. (2016) 4:263–8. doi: 10.3892/br.2016.586
202. Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, et al. Phase II Trial of Isoflavone in prostate specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. (2008) 8:132. doi: 10.1186/1471-2407-8-132
203. Kwan W, Duncan G, Van Patten C, Liu M, Lim J. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer. (2010) 62:198–207. doi: 10.1080/01635580903305318
204. Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Enk Rueter E, Melamed J, et al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA. (2013) 310:170–8. doi: 10.1001/jama.2013.7842
205. Fleshner NE, Kapusta L, Donnelly B, Tanguay S, Chin J, Hersey K, et al. Progression from high-grade prostatic intraepithelial neoplasia to cancer: a randomized trial of combination vitamin-E, soy, and selenium. J Clin Oncol. (2011) 29:2386–90. doi: 10.1200/JCO.2010.32.0994
206. Ratha P, Neumann T, Schmidt CA, Schneidewind L. Can isoflavones influence prostate specific antigen serum levels in localized prostate cancer? A systematic review. Nutr Cancer. (2021) 73:361–8. doi: 10.1080/01635581.2020.1759660
207. Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, Goodman GE, Minasian LM, Parnes HL, et al et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. (2014) 106:djt456. doi: 10.1093/jnci/djt456
208. Vivarelli F, Canistro D, Cirillo S, Papi A, Spisni E, Vornoli A, et al. Co-carcinogenic effects of vitamin E in prostate. Sci Rep. (2019) 9:11636. doi: 10.1038/s41598-019-48213-1
209. Perez-Cornago A, Appleby PN, Boeing H, Gil L, Kyro C, Ricceri F, et al. Circulating isoflavone and lignan concentrations and prostate cancer risk: a meta-analysis of individual participant data from seven prospective studies including 2,828 cases and 5,593 controls. Int J Cancer. (2018) 143:2677–86. doi: 10.1002/ijc.31640
210. Sawada N, Iwasaki M, Yamaji T, Shimazu T, Inoue M, Tsugane S, et al. Soy and isoflavone consumption and subsequent risk of prostate cancer mortality: the Japan Public Health Center-based Prospective Study. Int J Epidemiol. (2020) 49:1553–61. doi: 10.1093/ije/dyaa177
211. Darling AL, Millward DJ, Lanham-New SA. Dietary protein and bone health: towards a synthesised view. Proc Nutr Soc. (2021) 80:165–72. doi: 10.1017/S0029665120007909
212. Shams-White MM, Chung M, Du M, Fu Z, Insogna KL, Karlsen MC, et al. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr. (2017) 105:1528–43. doi: 10.3945/ajcn.116.145110
213. Shams-White MM, Chung M, Fu Z, Insogna KL, Karlsen MC, LeBoff MS, et al. Animal versus plant protein and adult bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. PloS ONE. (2018) 13:e0192459. doi: 10.1371/journal.pone.0192459
214. Anderson JJB, Thomsen K, Christiansen C. High protein meals, insular hormones and urinary calcium excretion in human subjects. In: Christiansen C, Johansen JS, Riis BJ, editors. Osteoporosis. Viborg: Nørhaven A/S (1987). p. 240–5.
215. Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. (1988) 66:140–6. doi: 10.1210/jcem-66-1-140
216. Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. (1982) 307:652–9. doi: 10.1056/NEJM198209093071104
218. Heaney RP. Protein intake and the calcium economy. J Am Diet Assoc. (1993) 93:1259–60. doi: 10.1016/0002-8223(93)91951-L
219. Fenton TR, Tough SC, Lyon AW, Eliasziw M, Hanley DA. Causal assessment of dietary acid load and bone disease: a systematic review & meta-analysis applying Hill's epidemiologic criteria for causality. Nutr J. (2011) 10:41. doi: 10.1186/1475-2891-10-41
220. Roughead ZK, Johnson LK, Lykken GI, Hunt JR. Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. J Nutr. (2003) 133:1020–6. doi: 10.1093/jn/133.4.1020
221. Calvez J, Poupin N, Chesneau C, Lassale C, Tome D. Protein intake, calcium balance and health consequences. Eur J Clin Nutr. (2012) 66:281–95. doi: 10.1038/ejcn.2011.196
222. Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. (2009) 90:1674–92. doi: 10.3945/ajcn.2009.27799
223. Dawson-Hughes B. Interaction of dietary calcium and protein in bone health in humans. J Nutr. (2003) 133:852–4S. doi: 10.1093/jn/133.3.852S
224. Roughead ZK. Is the interaction between dietary protein and calcium destructive or constructive for bone? J Nutr. (2003) 133:866–9S. doi: 10.1093/jn/133.3.866S
225. Roughead ZK, Hunt JR, Johnson LK, Badger TM, Lykken GI. Controlled substitution of soy protein for meat protein: effects on calcium retention, bone, and cardiovascular health indices in postmenopausal women. J Clin Endocrinol Metab. (2005) 90:181–9. doi: 10.1210/jc.2004-0393
226. Spence LA, Lipscomb ER, Cadogan J, Martin B, Wastney ME, Peacock M, et al. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr. (2005) 81:916–22. doi: 10.1093/ajcn/81.4.916
227. Levin VA, Jiang X, Kagan R. Estrogen therapy for osteoporosis in the modern era. Osteoporos Int. (2018) 29:1049–55. doi: 10.1007/s00198-018-4414-z
228. Zhang X, Shu XO, Li H, Yang G, Li Q, Gao YT, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. (2005) 165:1890–5. doi: 10.1001/archinte.165.16.1890
229. Koh WP, Wu AH, Wang R, Ang LW, Heng D, Yuan JM, et al. Gender-specific associations between soy and risk of hip fracture in the Singapore Chinese Health Study. Am J Epidemiol. (2009) 170:901–9. doi: 10.1093/aje/kwp220
230. Matthews VL, Knutsen SF, Beeson WL, Fraser GE. Soy milk and dairy consumption is independently associated with ultrasound attenuation of the heel bone among postmenopausal women: the Adventist Health Study-2. Nutr Res. (2011) 31:766–75. doi: 10.1016/j.nutres.2011.09.016
231. Cui Y, Cai H, Zheng W, Shu X-O. Associations of dietary intakes of calcium, magnesium and soy isoflavones with bone fracture risk in men: a prospective study. JBMR Plus. (2021) 6:e10563. doi: 10.1002/jbm4.10563
232. Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW Jr. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. (1998) 68:1375–9S. doi: 10.1093/ajcn/68.6.1375S
233. Kanadys W, Baranska A, Blaszczuk A, Polz-Dacewicz M, Drop B, Malm M, et al. Effects of soy isoflavones on biochemical markers of bone metabolism in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2021) 18:5346. doi: 10.3390/ijerph18105346
234. Alekel DL, Van Loan MD, Koehler KJ, Hanson LN, Stewart JW, Hanson KB, et al. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr. (2010) 91:218–30. doi: 10.3945/ajcn.2009.28306
235. Tai TY, Tsai KS, Tu ST, Wu JS, Chang CI, Chen CL, et al. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: a 2-year randomized double-blind placebo-controlled study. Osteoporos Int. (2012) 23:1571–80. doi: 10.1007/s00198-011-1750-7
236. Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch Intern Med. (2011) 171:1363–9. doi: 10.1001/archinternmed.2011.330
237. Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. (2008) 93:4787–96. doi: 10.1210/jc.2008-1087
238. Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. (2007) 146:839–47. doi: 10.7326/0003-4819-146-12-200706190-00005
239. Pawlowski JW, Martin BR, McCabe GP, McCabe L, Jackson GS, Peacock M, et al. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: a randomized crossover trial. Am J Clin Nutr. (2015) 102:695–703. doi: 10.3945/ajcn.114.093906
240. Weaver CM, Martin BR, Jackson GS, McCabe GP, Nolan JR, McCabe LD, et al. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using (41)Ca methodology. J Clin Endocrinol Metab. (2009) 94:3798–805. doi: 10.1210/jc.2009-0332
241. Murray MJ, Meyer WR, Lessey BA, Oi RH, DeWire RE, Fritz MA. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: a pilot trial. Menopause. (2003) 10:456–64. doi: 10.1097/01.GME.0000063567.84134.D1
242. Kaari C, Haidar MA, Junior JM, Nunes MG, Quadros LG, Kemp C, et al. Randomized clinical trial comparing conjugated equine estrogens and isoflavones in postmenopausal women: a pilot study. Maturitas. (2006) 53:49–58. doi: 10.1016/j.maturitas.2005.02.009
243. Waring SC, Rocca WA, Petersen RC, O'Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. (1999) 52:965–70. doi: 10.1212/WNL.52.5.965
244. Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. (1999) 281:1197–202. doi: 10.1001/jama.281.13.1197
245. File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H. Eating soya improves human memory. Psychopharmacology. (2001) 157:430–6. doi: 10.1007/s002130100845
246. File SE, Hartley DE, Elsabagh S, Duffy R, Wiseman H. Cognitive improvement after 6 weeks of soy supplements in postmenopausal women is limited to frontal lobe function. Menopause. (2005) 12:193–201. doi: 10.1097/00042192-200512020-00014
247. Kritz-Silverstein D, Von Muhlen D, Barrett-Connor E, Bressel MA. Isoflavones and cognitive function in older women: the SOy and Postmenopausal Health In Aging (SOPHIA) Study. Menopause. (2003) 10:196–202. doi: 10.1097/00042192-200310030-00004
248. Duffy R, Wiseman H, File SE. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol Biochem Behav. (2003) 75:721–9. doi: 10.1016/S0091-3057(03)00116-3
249. Casini ML, Marelli G, Papaleo E, Ferrari A, D'Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. (2006) 85:972–8. doi: 10.1016/j.fertnstert.2005.09.048
250. Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lampe JW, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. (2004) 292:65–74. doi: 10.1001/jama.292.1.65
251. Hogervorst E, Mursjid F, Priandini D, Setyawan H, Ismael RI, Bandelow S, et al. Borobudur revisited: soy consumption may be associated with better recall in younger, but not in older, rural Indonesian elderly. Brain Res. (2011) 1379:206–12. doi: 10.1016/j.brainres.2010.10.083
252. Svensson T, Sawada N, Mimura M, Nozaki S, Shikimoto R, Tsugane S. Midlife intake of the isoflavone genistein and soy, and the risk of late-life cognitive impairment: the JPHC Saku Mental Health Study. J Epidemiol. (2021) 31:660–8. doi: 10.2188/jea.JE20210199
253. Nakamoto M, Otsuka R, Nishita Y, Tange C, Tomida M, Kato Y, et al. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur J Clin Nutr. (2018) 72:1458–62. doi: 10.1038/s41430-017-0061-2
254. Murai U, Sawada N, Charvat H, Inoue M, Yasuda N, Yamagishi K, et al. Soy product intake and risk of incident disabling dementia: the JPHC Disabling Dementia Study. Eur J Nutr. (2022) doi: 10.1007/s00394-022-02937-5. [Epub ahead of print].
255. Soni M, Rahardjo TB, Soekardi R, Sulistyowati Y, Lestariningsih, Yesufu-Udechuku A, et al. Phytoestrogens and cognitive function: a review. Maturitas. (2014) 77:209–20. doi: 10.1016/j.maturitas.2013.12.010
256. Cheng PF, Chen JJ, Zhou XY, Ren YF, Huang W, Zhou JJ, et al. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause. (2015) 22:198–206. doi: 10.1097/GME.0000000000000290
257. Thaung Zaw JJ, Howe PRC, Wong RHX. Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann N Y Acad Sci. (2017) 1403:150–63. doi: 10.1111/nyas.13459
258. Kleinloog JPD, Tischmann L, Mensink RP, Adam TC, Joris PJ. Longer-term soy nut consumption improves cerebral blood flow and psychomotor speed: results of a randomized, controlled crossover trial in older men and women. Am J Clin Nutr. (2021) 114:2097–106. doi: 10.1093/ajcn/nqab289
259. Nelson HD, Haney E, Humphrey L, Miller J, Nedrow A, Necolaidis C, et al. Management of Menopuase-Related Symptoms. Summary, Evidence Report/Technology Assessment No. 120. (Repared by the Oregon Evidence-based Practice Center, under contract No. 290-02-0024.) AHRQ Pub. No. 05-E016-1. Rockville, MD: Agency for Health Research Quality (2005).
260. Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. (1990) 592:52–86; discussion 123–33. doi: 10.1111/j.1749-6632.1990.tb30316.x
261. Adlercreutz H, Hamalainen E, Gorbach S, Goldin B. Dietary phyto-oestrogens and the menopause in Japan. Lancet. (1992) 339:1233. doi: 10.1016/0140-6736(92)91174-7
262. Murkies AL, Lombard C, Strauss BJ, Wilcox G, Burger HG, Morton MS. Dietary flour supplementation decreases post-menopausal hot flushes: effect of soy and wheat. Maturitas. (1995) 21:189–95. doi: 10.1016/0378-5122(95)00899-V
263. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. (2015). 22:1155–72; quiz 73–4. doi: 10.1097/GME.0000000000000546
264. Krebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. (2004) 104:824–36. doi: 10.1097/01.AOG.0000140688.71638.d3
265. Eden JA. Managing the menopause: phyto-oestrogens or hormone replacement therapy? Ann Med. (2001) 33:4–6. doi: 10.3109/07853890109002054
266. Stearns V, Hayes DF. Cooling off hot flashes. J Clin Oncol. (2002) 20:1436–8. doi: 10.1200/JCO.2002.20.6.1436
267. Franco OH, Chowdhury R, Troup J, Voortman T, Kunutsor S, Kavousi M, et al. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA. (2016) 315:2554–63. doi: 10.1001/jama.2016.8012
268. The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian translational science symposium in Chicago, IL (October 2010). Menopause. (2011) 18:732–53. doi: 10.1097/gme.0b013e31821fc8e0
269. Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. (2006) 295:2057–71. doi: 10.1001/jama.295.17.2057
270. Messina M, Hughes C. Efficacy of soyfoods and soybean isoflavone supplements for alleviating menopausal symptoms is positively related to initial hot flush frequency. J Med Food. (2003) 6:1–11. doi: 10.1089/109662003765184697
271. Bolanos R, Del Castillo A, Francia J. Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: systematic review and meta-analysis. Menopause. (2010) 17:660–6. doi: 10.1097/gme.0b013e3181cb4fb5
272. Williamson-Hughes PS, Flickinger BD, Messina MJ, Empie MW. Isoflavone supplements containing predominantly genistein reduce hot flash symptoms: a critical review of published studies. Menopause. (2006) 13:831–9. doi: 10.1097/01.gme.0000227330.49081.9e
273. Guo PP, Li P, Zhang XH, Liu N, Wang J, Chen DD, et al. Complementary and alternative medicine for natural and treatment-induced vasomotor symptoms: an overview of systematic reviews and meta-analyses. Complement Ther Clin Pract. (2019) 36:181–94. doi: 10.1016/j.ctcp.2019.07.007
274. Chen LR, Ko NY, Chen KH. Isoflavone supplements for menopausal women: a systematic review. Nutrients. (2019) 11:2649. doi: 10.3390/nu11112649
275. Li L, Xu L, Wu J, Dong L, Lv Y, Zheng Q. Quantitative analysis of placebo response and factors associated with menopausal hot flashes. Menopause. (2017) 24:932–7. doi: 10.1097/GME.0000000000000858
276. Wiseman H, Casey K, Bowey EA, Duffy R, Davies M, Rowland IR, et al. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. Am J Clin Nutr. (2004) 80:692–9. doi: 10.1093/ajcn/80.3.692
277. Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. (2013) 12:CD001395. doi: 10.1002/14651858.CD001395.pub4
278. Lopez-Rios L, Barber MA, Wiebe J, Machin RP, Vega-Morales T, Chirino R. Influence of a new botanical combination on quality of life in menopausal Spanish women: results of a randomized, placebo-controlled pilot study. PloS ONE. (2021) 16:e0255015. doi: 10.1371/journal.pone.0255015
279. Barnard ND, Kahleova H, Holtz DN, Del Aguila F, Neola M, Crosby LM, et al. The Women's Study for the Alleviation of Vasomotor Symptoms (WAVS): a randomized, controlled trial of a plant-based diet and whole soybeans for postmenopausal women. Menopause. (2021) 28:1150–6. doi: 10.1097/GME.0000000000001812
280. Chi X-X, Zhang T. The effects of soy isoflavone on bone density in north region of climacteric Chinese women. J Clin Biochem Nutr. (2013) 53:102–7. doi: 10.3164/jcbn.13-37
281. Bitto A, Arcoraci V, Alibrandi A, D'Anna R, Corrado F, Atteritano M, et al. Visfatin correlates with hot flashes in postmenopausal women with metabolic syndrome: effects of genistein. Endocrine. (2017) 55:899–906. doi: 10.1007/s12020-016-0968-8
282. McCarrison R. The goitrogenic action of soya-bean and ground-nut. Ind J Med Res. (1933) XXI:179–81.
283. Van Wyk JJ, Arnold MB, Wynn J, Pepper F. The effects of a soybean product on thyroid function in humans. Pediatrics. (1959) 24:752–60. doi: 10.1542/peds.24.5.752
284. Shepard TH, Gordon EP, Kirschvink JF, McLean CM. Soybean goiter. New Engl J Med. (1960) 262:1099–103. doi: 10.1056/NEJM196006022622201
285. Pinchera A, MacGillivray H, Crawford JD, Freeman AG. Thyroid refractiveness in an athyreotic cretin fed soybean formula. N Engl J Med. (1965) 273:83–7. doi: 10.1056/NEJM196507082730205
286. Conrad SC, Chiu H, Silverman BL. Soy formula complicates management of congenital hypothyroidism. Arch Dis Child. (2004) 89:37–40. doi: 10.1136/adc.2002.009365
287. Persiani S, Sala F, Manzotti C, Colovic M, Zangarini M, Donazzolo Y, et al. Evaluation of levothyroxine bioavailability after oral administration of a fixed combination of soy isoflavones in post-menopausal female volunteers. Drug Res. (2016) 66:136–40. doi: 10.1055/s-0035-1555784
288. Otun J, Sahebkar A, Ostlundh L, Atkin SL, Sathyapalan T. Systematic review and meta-analysis on the effect of soy on thyroid function. Sci Rep. (2019) 9:3964. doi: 10.1038/s41598-019-40647-x
289. Sathyapalan T, Aye M, Rigby AS, Fraser WD, Thatcher NJ, Kilpatrick ES, et al. Soy reduces bone turnover markers in women during early menopause: a randomized controlled trial. J Bone Miner Res. (2017) 32:157–64. doi: 10.1002/jbmr.2927
290. Sathyapalan T, Javed Z, Rigby AS, Kilpatrick ES, Atkin SL. Soy protein improves cardiovascular risk in subclinical hypothyroidism: a randomized double-blinded crossover study. J Endocr Soc. (2017) 1:423–30. doi: 10.1210/js.2016-1068
291. Sathyapalan T, Manuchehri AM, Thatcher NJ, Rigby AS, Chapman T, Kilpatrick ES, et al. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. (2011) 96:1442–9. doi: 10.1210/jc.2010-2255
292. Sathyapalan T, Rigby AS, Bhasin S, Thatcher NJ, Kilpatrick ES, Atkin SL. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: a randomized controlled study. J Clin Endocrinol Metab. (2017) 102:425–33. doi: 10.1210/jc.2016-2875
293. Tonstad S, Jaceldo-Siegl K, Messina M, Haddad E, Fraser GE. The association between soya consumption and serum thyroid-stimulating hormone concentrations in the Adventist Health Study-2. Public Health Nutr. (2016) 19:1464–70. doi: 10.1017/S1368980015002943
294. Sosvorova L, Miksatkova P, Bicikova M, Kanova N, Lapcik O. The presence of monoiodinated derivates of daidzein and genistein in human urine and its effect on thyroid gland function. Food Chem Toxicol. (2012) 50:2774–9. doi: 10.1016/j.fct.2012.05.037
295. Sathyapalan T, Dawson AJ, Rigby AS, Thatcher NJ, Kilpatrick ES, Atkin SL. The effect of phytoestrogen on thyroid in subclinical hypothyroidism: randomized, double blind, crossover study. Front Endocrinol. (2018) 9:531. doi: 10.3389/fendo.2018.00531
296. Virili C, Antonelli A, Santaguida MG, Benvenga S, Centanni M. Gastrointestinal mlabsorption of thyroxine. Endocr Rev. (2019) 40:118–36. doi: 10.1210/er.2018-00168
297. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. (2012) 22:1200–35. doi: 10.1089/thy.2012.0205
298. Zeitler P, Solberg P. Food and levothyroxine administration in infants and children. J Pediatr. (2010) 157:13–4. doi: 10.1016/j.jpeds.2010.05.025
299. Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. (1993) 341:1392–5. doi: 10.1016/0140-6736(93)90953-E
300. Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jegou B, Jensen TK, Jouannet P, Keiding N, et al et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. (1996) 104(Suppl. 4):741–803. doi: 10.1289/ehp.96104s4741
301. Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. (2001) 16:972–8. doi: 10.1093/humrep/16.5.972
302. Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. (2007) 92:196–202. doi: 10.1210/jc.2006-1375
303. Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. (2007) 92:4696–705. doi: 10.1210/jc.2006-2633
304. Layton JB, Kim Y, Alexander GC, Emery SL. Association between direct-to-consumer advertising and testosterone testing and initiation in the United States, 2009-2013. JAMA. (2017) 317:1159–66. doi: 10.1001/jama.2016.21041
305. Strauss L, Makela S, Joshi S, Huhtaniemi I, Santti R. Genistein exerts estrogen-like effects in male mouse reproductive tract. Mol Cell Endocrinol. (1998) 144:83–93. doi: 10.1016/S0303-7207(98)00152-X
306. Weber KS, Setchell KD, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol. (2001) 170:591–9. doi: 10.1677/joe.0.1700591
307. Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, et al. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. (2002) 17:1692–703. doi: 10.1093/humrep/17.7.1692
308. Siepmann T, Roofeh J, Kiefer FW, Edelson DG. Hypogonadism and erectile dysfunction associated with soy product consumption. Nutrition. (2011) 27:859–62. doi: 10.1016/j.nut.2010.10.018
309. Chavarro JE, Toth TL, Sadio SM, Hauser R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod. (2008) 23:2584–90. doi: 10.1093/humrep/den243
310. Reed KE, Camargo J, Hamilton-Reeves J, Kurzer M, Messina M. Neither soy nor isoflavone intake affects male reproductive hormones: an expanded and updated meta-analysis of clinical studies. Reprod Toxicol. (2021) 100:60–7. doi: 10.1016/j.reprotox.2020.12.019
311. Mitchell JH, Cawood E, Kinniburgh D, Provan A, Collins AR, Irvine DS. Effect of a phytoestrogen food supplement on reproductive health in normal males. Clin Sci. (2001) 100:613–8. doi: 10.1042/cs1000613
312. Beaton LK, McVeigh BL, Dillingham BL, Lampe JW, Duncan AM. Soy protein isolates of varying isoflavone content do not adversely affect semen quality in healthy young men. Fertil Steril. (2010) 94:1717–22. doi: 10.1016/j.fertnstert.2009.08.055
313. Messina M, Watanabe S, Setchell KD. Report on the 8th international symposium on the role of soy in health promotion and chronic disease prevention and treatment. J Nutr. (2009) 139:796–802S. doi: 10.3945/jn.108.104182
314. Minguez-Alarcon L, Afeiche MC, Chiu YH, Vanegas JC, Williams PL, Tanrikut C, et al. Male soy food intake was not associated with in vitro fertilization outcomes among couples attending a fertility center. Andrology. (2015) 3:702–8. doi: 10.1111/andr.12046
315. Rice S, Mason HD, Whitehead SA. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. (2006) 101:216–25. doi: 10.1016/j.jsbmb.2006.06.021
316. Lacey M, Bohday J, Fonseka SM, Ullah AI, Whitehead SA. Dose-response effects of phytoestrogens on the activity and expression of 3beta-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. (2005) 96:279–86. doi: 10.1016/j.jsbmb.2005.03.006
317. Mesiano S, Katz SL, Lee JY, Jaffe RB. Phytoestrogens alter adrenocortical function: genistein and daidzein suppress glucocorticoid and stimulate androgen production by cultured adrenal cortical cells. J Clin Endocrinol Metab. (1999) 84:2443–8. doi: 10.1210/jc.84.7.2443
318. Ohno S, Shinoda S, Toyoshima S, Nakazawa H, Makino T, Nakajin S. Effects of flavonoid phytochemicals on cortisol production and on activities of steroidogenic enzymes in human adrenocortical H295R cells. J Steroid Biochem Mol Biol. (2002) 80:355–63. doi: 10.1016/S0960-0760(02)00021-3
319. Loukovaara M, Carson M, Palotie A, Adlercreutz H. Regulation of sex hormone-binding globulin production by isoflavonoids and patterns of isoflavonoid conjugation in HepG2 cell cultures. Steroids. (1995) 60:656–61. doi: 10.1016/0039-128X(95)00089-9
320. Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. (2009) 15:423–40. doi: 10.1093/humupd/dmp010
321. Delmanto A, Nahas-Neto J, Traiman P, Uemura G, Pessoa EC, Nahas EA. Effects of soy isoflavones on mammographic density and breast parenchyma in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Menopause. (2013) 20:1049–54. doi: 10.1097/GME.0b013e3182850270
322. Ye YB, Wang ZL, Zhuo SY, Lu W, Liao HF, Verbruggen MA, et al. Soy germ isoflavones improve menopausal symptoms but have no effect on blood lipids in early postmenopausal Chinese women: a randomized placebo-controlled trial. Menopause. (2012) 19:791–8. doi: 10.1097/gme.0b013e31823dbeda
323. Evans M, Elliott JG, Sharma P, Berman R, Guthrie N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: a multi-center, randomized, placebo-controlled study. Maturitas. (2011) 68:189–96. doi: 10.1016/j.maturitas.2010.11.012
324. Carmignani LO, Pedro AO, Montemor EB, Arias VA, Costa-Paiva LH, Pinto-Neto AM. Effects of a soy-based dietary supplement compared with low-dose hormone therapy on the urogenital system: a randomized, double-blind, controlled clinical trial. Menopause. (2015) 22:741–9. doi: 10.1097/GME.0000000000000380
325. Husain D, Khanna K, Puri S, Haghighizadeh M. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. J Am Coll Nutr. (2015) 34:42–8. doi: 10.1080/07315724.2013.875434
326. Maskarinec G, Ollberding NJ, Conroy SM, Morimoto Y, Pagano IS, Franke AA, et al. Estrogen levels in nipple aspirate fluid and serum during a randomized soy trial. Cancer Epidemiol Biomarkers Prev. (2011) 20:1815–21. doi: 10.1158/1055-9965.EPI-11-0363
327. Cassidy A, Bingham S, Setchell K. Biological effects of isoflavones in young women: importance of the chemical composition of soyabean products. Br J Nutr. (1995) 74:587–601. doi: 10.1079/BJN19950160
328. Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. (1994) 60:333–40. doi: 10.1093/ajcn/60.3.333
329. Mumford SL, Steiner AZ, Pollack AZ, Perkins NJ, Filiberto AC, Albert PS, et al. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. J Clin Endocrinol Metab. (2012) 97:E1871–9. doi: 10.1210/jc.2012-1350
330. Vassena R, Vidal R, Coll O, Vernaeve V. Menstrual cycle length in reproductive age women is an indicator of oocyte quality and a candidate marker of ovarian reserve. Eur J Obstet Gynecol Reprod Biol. (2014) 177:130–4. doi: 10.1016/j.ejogrb.2014.03.027
331. Crawford NM, Pritchard DA, Herring AH, Steiner AZ. Prospective evaluation of luteal phase length and natural fertility. Fertil Steril. (2017) 107:749–55. doi: 10.1016/j.fertnstert.2016.11.022
332. Wesselink AK, Wise LA, Hatch EE, Rothman KJ, Mikkelsen EM, Stanford JB, et al. Menstrual cycle characteristics and fecundability in a North American preconception cohort. Ann Epidemiol. (2016) 26:482–7 e1. doi: 10.1016/j.annepidem.2016.05.006
333. Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sorensen HT, Huybrechts KF, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. (2011) 174:701–9. doi: 10.1093/aje/kwr130
334. Olsson HL, Olsson ML. The menstrual cycle and risk of breast cancer: a review. Front Oncol. (2020) 10:21. doi: 10.3389/fonc.2020.00021
335. Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. (1993) 15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102
336. Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. (2008) 121(Suppl. 3):S172–91. doi: 10.1542/peds.2007-1813D
337. Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. (2010) 126:e583–90. doi: 10.1542/peds.2009-3079
338. Junqueira Do Lago M, Faerstein E, De Souza Lopes C, Werneck GL. Family socio-economic background modified secular trends in age at menarche: evidence from the Pro-Saude Study (Rio de Janeiro, Brazil). Ann Hum Biol. (2003) 30:347–52. doi: 10.1080/0301446031000091783
339. Harris MA, Prior JC, Koehoorn M. Age at menarche in the Canadian population: secular trends and relationship to adulthood BMI. J Adolesc Health. (2008) 43:548–54. doi: 10.1016/j.jadohealth.2008.07.017
340. Hosokawa M, Imazeki S, Mizunuma H, Kubota T, Hayashi K. Secular trends in age at menarche and time to establish regular menstrual cycling in Japanese women born between 1930 and 1985. BMC Womens Health. (2012) 12:19. doi: 10.1186/1472-6874-12-19
341. Cho GJ, Park HT, Shin JH, Hur JY, Kim YT, Kim SH, et al. Age at menarche in a Korean population: secular trends and influencing factors. Eur J Pediatr. (2010) 169:89–94. doi: 10.1007/s00431-009-0993-1
342. Morris DH, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Secular trends in age at menarche in women in the UK born 1908-93: results from the Breakthrough Generations Study. Paediatr Perinat Epidemiol. (2011) 25:394–400. doi: 10.1111/j.1365-3016.2011.01202.x
343. Cabanes A, Ascunce N, Vidal E, Ederra M, Barcos A, Erdozain N, et al. Decline in age at menarche among Spanish women born from 1925 to 1962. BMC Public Health. (2009) 9:449. doi: 10.1186/1471-2458-9-449
344. Herman-Giddens ME. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. Int J Androl. (2006) 29:241–6; discussion 86–90. doi: 10.1111/j.1365-2605.2005.00575.x
345. Himes JH. Examining the evidence for recent secular changes in the timing of puberty in US children in light of increases in the prevalence of obesity. Mol Cell Endocrinol. (2006) 254–255:13–21. doi: 10.1016/j.mce.2006.04.013
346. Talma H, Schonbeck Y, van Dommelen P, Bakker B, van Buuren S, Hirasing RA. Trends in menarcheal age between 1955 and 2009 in the Netherlands. PloS ONE. (2013) 8:e60056. doi: 10.1371/journal.pone.0060056
347. Ohlsson C, Bygdell M, Celind J, Sonden A, Tidblad A, Savendahl L, et al. Secular trends in pubertal growth acceleration in Swedish boys born from 1947 to 1996. JAMA Pediatr. (2019) 173:860–5. doi: 10.1001/jamapediatrics.2019.2315
348. Messina M, Rogero MM, Fisberg M, Waitzberg D. Health impact of childhood and adolescent soy consumption. Nutr Rev. (2017) 75:500–15. doi: 10.1093/nutrit/nux016
349. Kim J, Kim S, Huh K, Kim Y, Joung H, Park M. High serum isoflavone concentrations are associated with the risk of precocious puberty in Korean girls. Clin Endocrinol. (2011) 75:831–5. doi: 10.1111/j.1365-2265.2011.04127.x
350. Yum T, Lee S, Kim Y. Association between precocious puberty and some endocrine disruptors in human plasma. J Environ Sci Health A Tox Hazard Subst Environ Eng. (2013) 48:912–7. doi: 10.1080/10934529.2013.762734
351. Segovia-Siapco G, Pribis P, Messina M, Oda K, Sabate J. Is soy intake related to age at onset of menarche? A cross-sectional study among adolescents with a wide range of soy food consumption. Nutr J. (2014) 13:54. doi: 10.1186/1475-2891-13-54
352. Segovia-Siapco G, Pribis P, Oda K, Sabate J. Soy isoflavone consumption and age at pubarche in adolescent males. Eur J Nutr. (2018) 57:2287–94. doi: 10.1007/s00394-017-1504-1
353. Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. (2012) 130:e1058–68. doi: 10.1542/peds.2011-3291
354. Maskarinec G, Morimoto Y, Novotny R, Nordt FJ, Stanczyk FZ, Franke AA. Urinary sex steroid excretion levels during a soy intervention among young girls: a pilot study. Nutr Cancer. (2005) 52:22–8. doi: 10.1207/s15327914nc5201_3
355. Zung A, Shachar S, Zadik Z, Kerem Z. Soy-derived isoflavones treatment in children with hypercholesterolemia: a pilot study. J Pediatr Endocrinol Metab. (2010) 23:133–41. doi: 10.1515/JPEM.2010.23.1-2.133
356. Wada K, Nakamura K, Masue T, Sahashi Y, Ando K, Nagata C. Soy intake and urinary sex hormone levels in preschool Japanese children. Am J Epidemiol. (2011) 173:998–1003. doi: 10.1093/aje/kwr006
357. Li J, Teng X, Wang W, Chen Y, Yu X, Wang S, et al. Effects of dietary soy intake on maternal thyroid functions and serum anti-thyroperoxidase antibody level during early pregnancy. J Med Food. (2011) 14:543–50. doi: 10.1089/jmf.2010.1078
358. Miyake Y, Sasaki S, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, et al. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: the Osaka Maternal and Child Health Study. J Allergy Clin Immunol. (2005) 115:1176–83. doi: 10.1016/j.jaci.2005.02.016
359. Ishitsuka K, Sasaki S, Yamamoto-Hanada K, Mezawa H, Konishi M, Ohya Y, et al. Changes in dietary intake in pregnant women from periconception to pregnancy in the Japan Environment and Children's Study: a nationwide Japanese birth cohort study. Matern Child Health J. (2020) 24:389–400. doi: 10.1007/s10995-019-02835-z
360. Nagata C, Iwasa S, Shiraki M, Ueno T, Uchiyama S, Urata K, et al. Associations among maternal soy intake, isoflavone levels in urine and blood samples, and maternal and umbilical hormone concentrations (Japan). Cancer Causes Control. (2006) 17:1107–13. doi: 10.1007/s10552-006-0044-4
361. Wang Y, Luo B, Xiang J. The association between soy intake and risk of gestational diabetes mellitus: a prospective cohort study. BMC Pregn Childbirth. (2021) 21:695. doi: 10.1186/s12884-021-04175-9
362. Pang X, Cai C, Dong H, Lan X, Zhang Y, Bai D, et al. Soy foods and nuts consumption during early pregnancy are associated with decreased risk of gestational diabetes mellitus: a prospective cohort study. J Matern Fetal Neonatal Med. (2022) 1–9. doi: 10.1080/14767058.2021.2017872
363. Dong JY, Kimura T, Ikehara S, Cui M, Kawanishi Y, Kimura T, et al. Soy consumption and incidence of gestational diabetes mellitus: the Japan Environment and Children's Study. Eur J Nutr. (2021) 60:897–904. doi: 10.1007/s00394-020-02294-1
364. Jamilian M, Asemi Z. The effect of soy intake on metabolic profiles of women with gestational diabetes mellitus. J Clin Endocrinol Metab. (2015) 100:4654–61. doi: 10.1210/jc.2015-3454
365. Schiattarella A, Lombardo M, Morlando M, Rizzo G. The impact of a plant-based diet on gestational diabetes: a review. Antioxidants. (2021) 10:557. doi: 10.3390/antiox10040557
366. Wang H, Huang L, Lin L, Chen X, Zhong C, Li Q, et al. The overall plant-based diet index during pregnancy and risk of gestational diabetes mellitus: a prospective cohort study in China. Br J Nutr. (2021) 126:1519–28. doi: 10.1017/S0007114521000234
367. Yang J, Nakagawa H, Tsuta K, Tsubura A. Influence of perinatal genistein exposure on the development of MNU- induced mammary carcinoma in female Sprague-Dawley rats. Cancer Lett. (2000) 149:171–9. doi: 10.1016/S0304-3835(99)00357-2
368. Shibayama T, Fukata H, Sakurai K, Adachi T, Komiyama M, Iguchi T, et al. Neonatal exposure to genistein reduces expression of estrogen receptor alpha and androgen receptor in testes of adult mice. Endocr J. (2001) 48:655–63. doi: 10.1507/endocrj.48.655
369. Balakrishnan B, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transplacental transfer and biotransformation of genistein in human placenta. Placenta. (2010) 31:506–11. doi: 10.1016/j.placenta.2010.03.007
370. Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. (1999) 180:737–43. doi: 10.1016/S0002-9378(99)70281-4
371. Jarrell J, Foster WG, Kinniburgh DW. Phytoestrogens in human pregnancy. Obstet Gynecol Int. (2012) 2012:850313. doi: 10.1155/2012/850313
372. Dalais FS, Meliala A, Wahlqvist ML. Maternal and cord blood phytoestrogen levels in Indonesian women. J Nutr. (2000) 130:684S.
373. Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, et al. Fetal exposure to phytoestrogens–the difference in phytoestrogen status between mother and fetus. Environ Res. (2005) 99:195–203. doi: 10.1016/j.envres.2004.11.006
374. Mustafa AM, Malintan NT, Seelan S, Zhan Z, Mohamed Z, Hassan J, et al. Phytoestrogens levels determination in the cord blood from Malaysia rural and urban populations. Toxicol Appl Pharmacol. (2007) 222:25–32. doi: 10.1016/j.taap.2007.03.014
375. Foster WG, Chan S, Platt L, Hughes CL Jr. Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol Lett. (2002) 129:199–205. doi: 10.1016/S0378-4274(02)00018-8
376. Engel SM, Levy B, Liu Z, Kaplan D, Wolff MS. Xenobiotic phenols in early pregnancy amniotic fluid. Reprod Toxicol. (2006) 21:110–2. doi: 10.1016/j.reprotox.2005.07.007
377. North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon longitudinal study of pregnancy and childhood. BJU Int. (2000) 85:107–13. doi: 10.1046/j.1464-410x.2000.00436.x
378. Franke AA, Custer LJ, Wang W, Shi CY. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med. (1998) 217:263–73. doi: 10.3181/00379727-217-44231
379. Michikawa T, Yamazaki S, Ono M, Kuroda T, Nakayama SF, Suda E, et al. Isoflavone intake in early pregnancy and hypospadias in the Japan Environment and Children's Study. Urology. (2019) 124:229–36. doi: 10.1016/j.urology.2018.11.008
380. Song X, Liu Y, Wang T, Zhang S, Sun M, Shu J, et al. Association of maternal dietary habits and MTHFD1 gene polymorphisms with ventricular septal defects in offspring: a case-control study. Front Pediatr. (2021) 9:785440. doi: 10.3389/fped.2021.785440
381. Marrian GF, Haselwood GAD C.X.L.V. Equol, a new active phenol isolated from ketohydroxyestrin fraction of mares urine. Biochem J. (1932) 26:1226–32. doi: 10.1042/bj0261227
382. Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Origin of lignans in mammals and identification of a precursor from plants. Nature. (1982) 298:659–60. doi: 10.1038/298659a0
383. Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. (2002) 132:3577–84. doi: 10.1093/jn/132.12.3577
384. Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. (2010) 140:1355–62S. doi: 10.3945/jn.109.119776
385. Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. (2010) 140:1363–8S. doi: 10.3945/jn.109.119784
386. Sekikawa A, Higashiyama A, Lopresti BJ, Ihara M, Aizenstein H, Watanabe M, et al. Associations of equol-producing status with white matter lesion and amyloid-β deposition in cognitively normal elderly Japanese. Alzheimer's Dementia Transl Res Clin Interv. (2020) 6:e12089. doi: 10.1002/trc2.12089
387. Usui T, Tochiya M, Sasaki Y, Muranaka K, Yamakage H, Himeno A, et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin Endocrinol. (2013) 78:365–72. doi: 10.1111/j.1365-2265.2012.04400.x
388. Man B, Cui C, Zhang X, Sugiyama D, Barinas-Mitchell E, Sekikawa A. The effect of soy isoflavones on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. (2020) 60:603–14. doi: 10.1007/s00394-020-02300-6
389. Daily JW, Ko BS, Ryuk J, Liu M, Zhang W, Park S. Equol decreases hot flashes in postmenopausal women: a systematic review and meta-analysis of randomized clinical trials. J Med Food. (2019) 22:127–39. doi: 10.1089/jmf.2018.4265
390. Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. (2006) 136:2188–93. doi: 10.1093/jn/136.8.2188
391. Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. (2016) 65:63–72. doi: 10.1136/gutjnl-2014-308209
392. Istfan N, Murray E, Janghorbani M, Evans WJ, Young VR. The nutritional value of a soy protein concentrate (STAPRO-3200) for long-term protein nutritional maintenance in young men. J Nutr. (1983) 113:2524–34. doi: 10.1093/jn/113.12.2524
393. Istfan N, Murray E, Janghorbani M, Young VR. An evaluation of the nutritional value of a soy protein concentrate in young adult men using the short-term N-balance method. J Nutr. (1983) 113:2516–23. doi: 10.1093/jn/113.12.2516
394. Scrimshaw NS, Wayler AH, Murray E, Steinke FH, Rand WM, Young VR. Nitrogen balance response in young men given one of two isolated soy proteins or milk proteins. J Nutr. (1983) 113:2492–7. doi: 10.1093/jn/113.12.2492
395. Wayler A, Queiroz E, Scrimshaw NS, Steinke FH, Rand WM, Young VR. Nitrogen balance studies in young men to assess the protein quality of an isolated soy protein in relation to meat proteins. J Nutr. (1983) 113:2485–91. doi: 10.1093/jn/113.12.2485
396. Young VR, Wayler A, Garza C, Steinke FH, Murray E, Rand WM, et al. A long-term metabolic balance study in young men to assess the nutritional quality of an isolated soy protein and beef proteins. Am J Clin Nutr. (1984) 39:8–15. doi: 10.1093/ajcn/39.1.8
397. Beer WH, Murray E, Oh SH, Pedersen HE, Wolfe RR, Young VR. A long-term metabolic study to assess the nutritional value of and immunological tolerance to two soy-protein concentrates in adult humans. Am J Clin Nutr. (1989) 50:997–1007. doi: 10.1093/ajcn/50.5.997
398. Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, et al. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. (2010) 140:2302–11S. doi: 10.3945/jn.110.124958
399. Anderson JW, Bush HM. Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J Am Coll Nutr. (2011) 30:79–91. doi: 10.1080/07315724.2011.10719947
400. Weggemans RM, Trautwein EA. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. Eur J Clin Nutr. (2003) 57:940–6. doi: 10.1038/sj.ejcn.1601628
401. Reynolds K, Chin A, Lees KA, Nguyen A, Bujnowski D, He J. A meta-analysis of the effect of soy protein supplementation on serum lipids. Am J Cardiol. (2006) 98:633–40. doi: 10.1016/j.amjcard.2006.03.042
402. Harland JI, Haffner TA. Systematic review, meta-analysis and regression of randomised controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis. (2008) 200:13–27. doi: 10.1016/j.atherosclerosis.2008.04.006
403. Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. (2005) 81:397–408. doi: 10.1093/ajcn.81.2.397
404. Benkhedda K, Boudrault C, Sinclair SE, Marles RJ, Xiao CW, Underhill L. Food Risk Analysis Communication. Issued By Health Canada's Food Directorate. Health Canada's Proposal to accept a health claim about soy products and cholesterol lowering. Int Food Risk Anal J. (2014) 4:22. doi: 10.5772/59675
405. Blanco Mejia S, Messina M, Li SS, Viguiliouk E, Chiavaroli L, Khan TA, et al. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J Nutr. (2019) 149:968–81. doi: 10.1093/jn/nxz020
406. Brahmkhatri VP, Prasanna C, Atreya HS. Insulin-like growth factor system in cancer: novel targeted therapies. BioMed Res Int. (2015) 2015:538019. doi: 10.1155/2015/538019
407. Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, et al. Interventions to slow aging in humans: are we ready? Aging Cell. (2015) 14:497–510. doi: 10.1111/acel.12338
408. Milman S, Huffman DM, Barzilai N. The somatotropic axis in human aging: framework for the current state of knowledge and future research. Cell Metab. (2016) 23:980–9. doi: 10.1016/j.cmet.2016.05.014
409. Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. (2014) 25:558–66. doi: 10.1016/j.tem.2014.07.002
410. Novosyadlyy R, Leroith D. Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. J Gerontol A Biol Sci Med Sci. (2012) 67:640–51. doi: 10.1093/gerona/gls065
411. Li Y, Shelat H, Wu H, Zhu M, Xu J, Geng YJ. Low circulating level of IGF-1 is a distinct indicator for the development of cardiovascular disease caused by combined hyperglycemia and dyslipidemia. Int J Cardiol. (2014) 171:272–3. doi: 10.1016/j.ijcard.2013.11.091
412. Akanji AO, Smith RJ. The insulin-like growth factor system, metabolic syndrome, and cardiovascular disease risk. Metab Syndr Relat Disord. (2012) 10:3–13. doi: 10.1089/met.2011.0083
413. Rajpathak SN, He M, Sun Q, Kaplan RC, Muzumdar R, Rohan TE, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. (2012) 61:2248–54. doi: 10.2337/db11-1488
414. Schuler R, Markova M, Osterhoff MA, Arafat A, Pivovarova O, Machann J, et al. Similar dietary regulation of IGF-1- and IGF-binding proteins by animal and plant protein in subjects with type 2 diabetes. Eur J Nutr. (2021) 60:3499–504. doi: 10.1007/s00394-021-02518-y
415. Messina M, Magee P. Does soy protein affect circulating levels of unbound IGF-1? Eur J Nutr. (2018) 57:423–32. doi: 10.1007/s00394-017-1459-2
416. Bosland MC, Enk E, Schmoll J, Schlicht MJ, Randolph C, Deaton RJ, et al. Soy protein supplementation in men following radical prostatectomy: a 2-year randomized, placebo-controlled clinical trial. Am J Clin Nutr. (2021) 113:821–31. doi: 10.1093/ajcn/nqaa390
417. Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. (2002) 11:1441–8.
418. Bhagwat S, Haytowitz DB. USDA Database for the Isoflavone Content of Selected Foods, Release 2.1. U.S. Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory Home Page (2015). Available online at: https://data.nal.usda.gov/dataset/usda-database-isoflavone-content-selected-foods-release-21-november-2015/resource/1de757af.
420. Wickramasinghe K, Breda J, Berdzuli N, Rippin H, Farrand C, Halloran A. The shift to plant-based diets: are we missing the point? Global Food Secur. (2021) 29:100530. doi: 10.1016/j.gfs.2021.100530
421. Messina M, Sievenpiper JL, Williamson P, Kiel J, Erdman JW. Perspective: soy-based meat and dairy alternatives, despite classification as ultra-processed foods, deliver high-quality nutrition on par with unprocessed or minimally processed animal-based counterparts. Adv Nutr. (2022) 13:726–38. doi: 10.1093/advances/nmac026
422. Vallaeys C, Kastel M, Fantle W, Buske L. Toxic Chemicals: Banned in Organics But Common in “Natural” Food Production. Soy Protein and Chemical Solvents in Nutrition Bars and Meat Alternatives. Cornucopia, WI: The Cornucopia Institute (2010).
423. Swanson BG. Hexane Extraction in Soyfood Processing (2009). Available online at: https://www.scribd.com/document/110859286/Regulatory-Expert-Document-Barry-Swanson-Revised (accessedd January 19, 2022).
424. Schwarcz J. The right chemistry: hexane residues in soy burgers are no cause for concern. Montreal Gazette (2015).
425. Hodges RE, Krehl WA, Stone DB, Lopez A. Dietary carbohydrates and low cholesterol diets: effects on serum lipids on man. Am J Clin Nutr. (1967) 20:198–208. doi: 10.1093/ajcn/20.2.198
426. Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. (1995) 333:276–82. doi: 10.1056/NEJM199508033330502
427. Food labeling: health claims; soy protein coronary heart disease. Food Drug Administration, HHS. Final rule. Fed Regist. (1999) 64:57700–33.
428. Lovati MR, Manzoni C, Gianazza E, Sirtori CR. Soybean protein products as regulators of liver low-density receptors. I. Identification of active B-conglycinin subunits. J Agr Food Chem. (1998) 46:2474–80. doi: 10.1021/jf980099h
429. Duranti M, Lovati MR, Dani V, Barbiroli A, Scarafoni A, Castiglioni S, et al. The alpha' subunit from soybean 7S globulin lowers plasma lipids and upregulates liver beta-VLDL receptors in rats fed a hypercholesterolemic diet. J Nutr. (2004) 134:1334–9. doi: 10.1093/jn/134.6.1334
430. US Food and Drug Administration. Food labeling: health claims; Soy protein and coronary heart disease. Fed Reg. (2017) 82:50324–46.
431. Ho HV, Sievenpiper JL, Zurbau A, Blanco Mejia S, Jovanovski E, Au-Yeung F, et al. The effect of oat beta-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: a systematic review and meta-analysis of randomised-controlled trials. Br J Nutr. (2016) 116:1369–82. doi: 10.1017/S000711451600341X
432. Summary of Health Canada's Assessment of a Health Claim about Soy Protein and Cholesterol Lowering. Bureau of Nutritional Sciences Food Directorate Health Products and Food Branch. Available online at: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/summary-assessment-health-claim-about-protein-cholesterol-lowering.html.
433. Jenkins DJA, Blanco Mejia S, Chiavaroli L, Viguiliouk E, Li SS, Kendall CWC, et al. Cumulative meta-analysis of the soy effect over time. J Am Heart Assoc. (2019) 8:e012458. doi: 10.1161/JAHA.119.012458
434. Qualified Health Claim Petition – Soybean Oil and Reduced Risk of Coronary Heart Disease (Docket No. FDA-2016-Q-0995). Available online at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjBrrmAqaLsAhWVcc0KHWziCnkQFjABegQIBRAC&url=https%3A%2F%2F%2Fmedia%2F106649%2Fdownload&usg=AOvVaw1OacdW5qPEJwAz-_0yxdGz.
435. Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. (2015) 11:649–62. doi: 10.1038/nrrheum.2015.91
436. Singh G, Lingala B, Mithal A. Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatology. (2019) 79:69–76. doi: 10.1136/annrheumdis-2019-eular.4438
437. Safiri S, Kolahi AA, Cross M, Carson-Chahhoud K, Hoy D, Almasi-Hashiani A, et al. Prevalence, incidence, and years lived with disability due to gout and its attributable risk factors for 195 countries and territories 1990-2017: a systematic analysis of the Global Burden of Disease Study (2017). Arthritis Rheumatol. (2020) 72:1916–27. doi: 10.1002/art.41404
438. Yokose C, McCormick N, Choi HK. The role of diet in hyperuricemia and gout. Curr Opin Rheumatol. (2021) 33:135–44. doi: 10.1097/BOR.0000000000000779
439. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. (2019) 71:991–9. doi: 10.1002/art.40807
440. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. (2007) 57:109–15. doi: 10.1002/art.22466
441. Yu W, Cheng JD. Uric acid and cardiovascular disease: An update from molecular mechanism to clinical perspective. Front Pharmacol. (2020) 11:582680. doi: 10.3389/fphar.2020.582680
442. Chang H-J. A study on the farmer's life style and dietary habits of the hyperuricemia patients and their recognition on the symptom of gouty arthritis. J Chin Nutr Soc. (1991) 16:191–209.
443. Messina M, Messina VL, Chan P. Soyfoods, hyperuricemia and gout: a review of the epidemiologic and clinical data. Asia Pacific J Clin Nutr. (2011) 20:347–58.
444. Kaneko K, Aoyagi Y, Fukuuchi T, Inazawa K, Yamaoka N. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biol Pharm Bull. (2014) 37:709–21. doi: 10.1248/bpb.b13-00967
445. Yamanaka H. Japanese Society of Gout and Nucleic Acid Metabolism. Japanese guideline for the management of hyperurticemia and gout. Nucleosides Nucleotides Nucleic Acids. (2011) 30:1018–29. doi: 10.1080/15257770.2011.596496
446. Beyl RN Jr., Hughes L, Morgan S. Update on importance of diet in gout. Am J Med. (2016) 129:1153–8. doi: 10.1016/j.amjmed.2016.06.040
447. Becerra-Tomas N, Mena-Sanchez G, Diaz-Lopez A, Martinez-Gonzalez MA, Babio N, Corella D, et al. Cross-sectional association between non-soy legume consumption, serum uric acid and hyperuricemia: the PREDIMED-Plus study. Eur J Nutr. (2020) 59:2195–206. doi: 10.1007/s00394-019-02070-w
448. Clifford AJ, Riumallo JA, Young VR, Scrimshaw AS. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J Nutr. (1976) 106:428–34. doi: 10.1093/jn/106.3.428
449. Zgaga L, Theodoratou E, Kyle J, Farrington SM, Agakov F, Tenesa A, et al. The association of dietary intake of purine-rich vegetables, sugar-sweetened beverages and dairy with plasma urate, in a cross-sectional study. PloS ONE. (2012) 7:e38123. doi: 10.1371/journal.pone.0038123
450. Yamakita J, Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Higashino K. Effect of Tofu (bean curd) ingestion and on uric acid metabolism in healthy and gouty subjects. Adv Exp Med Biol. (1998) 431:839–42. doi: 10.1007/978-1-4615-5381-6_161
451. Garrel DR, Verdy M, PetitClerc C, Martin C, Brule D, Hamet P. Milk- and soy-protein ingestion: acute effect on serum uric acid concentration. Am J Clin Nutr. (1991) 53:665–9. doi: 10.1093/ajcn/53.3.665
452. Dalbeth N, Wong S, Gamble GD, Horne A, Mason B, Pool B, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis. (2010) 69:1677–82. doi: 10.1136/ard.2009.124230
453. Brule D, Sarwar G, Savoie L. Changes in serum and urinary uric acid levels in normal human subjects fed purine-rich foods containing different amounts of adenine and hypoxanthine. J Am Coll Nutr. (1992) 11:353–8. doi: 10.1080/07315724.1992.10718238
454. Zhang M, Gao Y, Wang X, Liu W, Zhang Y, Huang G. Comparison of the effect of high fruit and soybean products diet and standard diet interventions on serum uric acid in asymptomatic hyperuricemia adults: an open randomized controlled trial. Int J Food Sci Nutr. (2016) 67:335–43. doi: 10.3109/09637486.2016.1153608
455. Liu ZM, Ho CS, Chen YM, Woo J. Can soy intake affect serum uric acid level? Pooled analysis from two 6-month randomized controlled trials among Chinese postmenopausal women with prediabetes or prehypertension. Eur J Nutr. (2015) 54:51–8. doi: 10.1007/s00394-014-0684-1
456. Liu ZM, Ho SC, Chen YM, Ho YP. The effects of isoflavones combined with soy protein on lipid profiles, C-reactive protein and cardiovascular risk among postmenopausal Chinese women. Nutr Metab Cardiovasc Dis. (2012) 22:712–9. doi: 10.1016/j.numecd.2010.11.002
457. Zhang M, Lin L, Liu H. Acute effect of soy and soy products on serum uric acid concentration among healthy Chinese men. Asia Pacific J Clin Nutr. (2018) 27:1239–42.
458. Zhang Y, Chen C, Choi H, Chaisson C, Hunter D, Niu J, et al. Purine-rich foods intake and recurrent gout attacks. Ann Rheum Dis. (2012) 71:1448–53. doi: 10.1136/annrheumdis-2011-201215
459. Li R, Yu K, Li C. Dietary factors and risk of gout and hyperuricemia: a meta-analysis and systematic review. Asia Pacific J Clin Nutr. (2018) 27:1344–56.
460. Villegas R, Xiang YB, Elasy T, Xu WH, Cai H, Cai Q, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men's Health Study. Nutr Metab Cardiovasc Dis. (2012) 22:409–16. doi: 10.1016/j.numecd.2010.07.012
461. Teng GG, Pan A, Yuan JM, Koh WP. Food sources of protein and risk of incident gout in the Singapore Chinese Health Study. Arthritis Rheumatol. (2015) 67:1933–42. doi: 10.1002/art.39115
462. Chiu THT, Liu CH, Chang CC, Lin MN, Lin CL. Vegetarian diet and risk of gout in two separate prospective cohort studies. Clin Nutr. (2020) 39:837–44. doi: 10.1016/j.clnu.2019.03.016
463. Hui M, Carr A, Cameron S, Davenport G, Doherty M, Forrester H, et al. The british society for rheumatology guideline for the management of gout. Rheumatology. (2017) 56:1246. doi: 10.1093/rheumatology/kex250
464. Mohammadifard N, Sajjadi F, Haghighatdoost F. Effects of soy consumption on metabolic parameters in patients with metabolic syndrome: a systematic review and meta-analysis. EXCLI journal. (2021) 20:665–85.
465. Baranska A, Blaszczuk A, Polz-Dacewicz M, Kanadys W, Malm M, Janiszewska M, et al. Effects of soy isoflavones on glycemic control and lipid profile in patients with Type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2021) 13:1886. doi: 10.3390/nu13061886
466. Asbaghi O, Ashtary-Larky D, Mousa A, Kelishadi MR, Moosavian SP. The effects of soy products on cardiovascular risk factors in patients with Type 2 diabetes: a systematic review and meta-analysis of clinical trials. Adv Nutr. (2021) 13:455–73. doi: 10.1093/advances/nmab121
467. Yan Z, Zhang X, Li C, Jiao S, Dong W. Association between consumption of soy and risk of cardiovascular disease: a meta-analysis of observational studies. Eur J Prev Cardiol. (2017) 24:735–47. doi: 10.1177/2047487316686441
468. Chen Z, Prosperi M, Bird VY. Prevalence of kidney stones in the USA: THE National Health and Nutrition Evaluation Survey. J Clinical Urol. (2019) 12:296–302. doi: 10.1177/2051415818813820
469. Daudon M, Donsimoni R, Hennequin C, Fellahi S, Le Moel G, Paris M, et al. Sex- and age-related composition of 10 617 calculi analyzed by infrared spectroscopy. Urol Res. (1995) 23:319–26. doi: 10.1007/BF00300021
470. Ferraro PM, Bargagli M. Dietetic and lifestyle recommendations for stone formers. Arch Esp Urol. (2021) 74:112–22.
471. Han H, Segal AM, Seifter JL, Dwyer JT. Nutritional management of kidney stones (nephrolithiasis). Clin Nutr Res. (2015) 4:137–52. doi: 10.7762/cnr.2015.4.3.137
472. Worcester EM, Coe FL. Clinical practice. Calcium kidney stones. N Engl J Med. (2010) 363:954–63. doi: 10.1056/NEJMcp1001011
473. Moyad MA. Calcium oxalate kidney stones: another reason to encourage moderate calcium intakes and other dietary changes. Urol Nurs. (2003) 23:310–3.
474. Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. (2001) 59:270–6. doi: 10.1046/j.1523-1755.2001.00488.x
475. Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol. (2007) 18:2198–204. doi: 10.1681/ASN.2007020219
476. Massey LK, Roman-Smith H, Sutton RA. Effect of dietary oxalate and calcium on urinary oxalate and risk of formation of calcium oxalate kidney stones. J Am Diet Assoc. (1993) 93:901–6. doi: 10.1016/0002-8223(93)91530-4
477. Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clin J Am Soc Nephrol. (2008) 3:1453–60. doi: 10.2215/CJN.01410308
478. Holmes RP, Assimos DG. The impact of dietary oxalate on kidney stone formation. Urol Res. (2004) 32:311–6. doi: 10.1007/s00240-004-0437-3
479. Chai W, Liebman M, Kynast-Gales S, Massey L. Oxalate absorption and endogenous oxalate synthesis from ascorbate in calcium oxalate stone formers and non-stone formers. Am J Kidney Dis. (2004) 44:1060–9. doi: 10.1053/j.ajkd.2004.08.028
480. Goldfarb S. The role of diet in the pathogenesis and therapy of nephrolithiasis. Endocrinol Metab Clin North Am. (1990) 19:805–20. doi: 10.1016/S0889-8529(18)30294-9
481. Liebman M, Chai W. Effect of dietary calcium on urinary oxalate excretion after oxalate loads. Am J Clin Nutr. (1997) 65:1453–9. doi: 10.1093/ajcn/65.5.1453
482. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. (1993) 328:833–8. doi: 10.1056/NEJM199303253281203
483. Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: nurses' health study ii. Arch Intern Med. (2004) 164:885–91. doi: 10.1001/archinte.164.8.885
484. Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. (1997) 126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001
485. Taylor EN, Curhan GC. Dietary calcium from dairy and nondairy sources, and risk of symptomatic kidney stones. J Urol. (2013) 190:1255–9. doi: 10.1016/j.juro.2013.03.074
486. Hess B, Jost C, Zipperle L, Takkinen R, Jaeger P. High-calcium intake abolishes hyperoxaluria and reduces urinary crystallization during a 20-fold normal oxalate load in humans. Nephrol Dial Transplant. (1998) 13:2241–7. doi: 10.1093/ndt/13.9.2241
487. Al-Wahsh IA, Horner HT, Palmer RG, Reddy MB, Massey LK. Oxalate and phytate of soy foods. J Agric Food Chem. (2005) 53:5670–4. doi: 10.1021/jf0506378
488. Ellis D, Lieb J. Hyperoxaluria and genitourinary disorders in children ingesting almond milk products. J Pediatr. (2015) 167:1155–8. doi: 10.1016/j.jpeds.2015.08.029
489. Massey LK. Food oxalate: factors affecting measurement, biological variation, and bioavailability. J Am Diet Assoc. (2007) 107:1191–4. doi: 10.1016/j.jada.2007.04.007
490. Grases F, March JG, Prieto RM, Simonet BM, Costa-Bauza A, Garcia-Raja A, et al. Urinary phytate in calcium oxalate stone formers and healthy people–dietary effects on phytate excretion. Scand J Urol Nephrol. (2000) 34:162–4. doi: 10.1080/003655900750016526
491. Grases F, Simonet BM, March JG, Prieto RM. Inositol hexakisphosphate in urine: the relationship between oral intake and urinary excretion. BJU Int. (2000) 85:138–42. doi: 10.1046/j.1464-410x.2000.00324.x
492. Grases F, Garcia-Gonzalez R, Torres JJ, Llobera A. Effects of phytic acid on renal stone formation in rats. Scand J Urol Nephrol. (1998) 32:261–5. doi: 10.1080/003655998750015412
493. Fakier S, Rodgers A. Exploring the potential relationship between phytate ingestion, urinary phytate excretion, and renal stone risk in a unique human model: no hard evidence in support of phytate as a stone inhibitor. J Ren Nutr. (2020) 30:396–403. doi: 10.1053/j.jrn.2019.10.006
494. Massey LK, Grentz LM, Horner HT, Palmer RG. Soybean and soyfood consumption increase oxalate excretion. Topics Clin Nutr. (2002) 17:49–59. doi: 10.1097/00008486-200203000-00009
495. Boyce JA, Assa'a A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. Nutrition. (2011) 27:253–67. doi: 10.1016/j.jada.2010.10.033
496. Zarkadas M, Scott WF, Salminen J, Pong AH. Common allergenic foods and their labelling in Canada - a review. Can J Allergy Clin Immunol. (1999) 4:118–41.
497. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. (2011) 127:594–602. doi: 10.1016/j.jaci.2010.11.044
498. Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. (2018) 142:e20181235. doi: 10.1542/peds.2018-1235
499. Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. (2019) 2:e185630. doi: 10.1001/jamanetworkopen.2018.5630
500. Awazuhara H, Kawai H, Maruchi N. Major allergens in soybean and clinical significance of IgG4 antibodies investigated by IgE- and IgG4-immunoblotting with sera from soybean-sensitive patients. Clin Exp Allergy. (1997) 27:325–32. doi: 10.1111/j.1365-2222.1997.tb00711.x
501. Wilson S, Blaschek K, de Mejia E. Allergenic proteins in soybean: processing and reduction of P34 allergenicity. Nutr Rev. (2005) 63:47–58. doi: 10.1111/j.1753-4887.2005.tb00121.x
502. Taylor SL, Houben GF, Blom WM, Westerhout J, Remington BC, Crevel RWR, et al. The population threshold for soy as an allergenic food - why did the reference dose decrease in VITAL 3.0? Trend Food Sci Technol. (2021) 112:99–1–8. doi: 10.1016/j.tifs.2021.03.036
503. Verhoeckx KCM, Vissers YM, Baumert JL, Faludi R, Feys M, Flanagan S, et al. Food processing and allergenicity. Food Chem Toxicol. (2015) 80:223–40. doi: 10.1016/j.fct.2015.03.005
504. Holzhauser T, Wackermann O, Ballmer-Weber BK, Bindslev-Jensen C, Scibilia J, Perono-Garoffo L, et al. Soybean (Glycine max) allergy in Europe: gly m 5 (beta-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J Allergy Clin Immunol. (2009) 123:452–8. doi: 10.1016/j.jaci.2008.09.034
505. L'Hocine L, Boye JI. Allergenicity of soybean: new developments in identification of allergenic proteins, cross-reactivities and hypoallergenization technologies. Crit Rev Food Sci Nutr. (2007) 47:127–43. doi: 10.1080/10408390600626487
506. Hefle SL, Nordlee JA, Taylor SL. Allergenic foods. Crit Rev Food Sci Nutr. (1996) 36:S69–89. doi: 10.1080/10408399609527760
507. Food Allergen Labeling and Consumer Protection Act of 2004. (FALCPA). Public Law 108-282, Title II.
508. Gendel SM. Comparison of international food allergen labeling regulations. Regul Toxicol Pharmacol. (2012) 63:279–85. doi: 10.1016/j.yrtph.2012.04.007
509. H.R.1202 — 117th Congress (2021-2022), Food Allergy Safety, Treatment, Education, and Research Act of 2021 or the FASTER Act of 2021. Available online at: https://www.congress.gov/bill/117th-congress/house-bill/1202 (accessed May 2, 2022).
510. Lavine E, Ben-Shoshan M. Anaphylaxis to hidden pea protein: A Canadian pediatric case series. J Allergy Clin Immunol Pract. (2019) 7:2070–1. doi: 10.1016/j.jaip.2019.02.010
511. Lyons SA, Burney PGJ, Ballmer-Weber BK, Fernandez-Rivas M, Barreales L, Clausen M, et al. Food allergy in adults: substantial variation in prevalence and causative foods across europe. J Allergy Clin Immunol Pract. (2019) 7:1920–8 e11. doi: 10.1016/j.jaip.2019.02.044
512. Food and Agriculture Organization of the United Nations World Health Organization. Summary Report of the ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens. Part 1: Review and Validation of Codex Priority Allergen List Through Risk Assessment. Virtual Meeting, 30 November – 11 December, 2020, 28 January, 2021, 8 February, 2021 (2021).
513. McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. (2013) 132:1216–9 e5. doi: 10.1016/j.jaci.2013.07.018
514. Verrill L, Bruns R, Luccioli S. Prevalence of self-reported food allergy in U.S. adults: 2001, 2006, and 2010. Allergy Asthma Proc. (2015) 36:458–67. doi: 10.2500/aap.2015.36.3895
515. Soller L, Ben-Shoshan M, Harrington DW, Fragapane J, Joseph L, St Pierre Y, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. (2012) 130:986–8. doi: 10.1016/j.jaci.2012.06.029
516. Savage JH, Kaeding AJ, Matsui EC, Wood RA. The natural history of soy allergy. J Allergy Clin Immunol. (2010) 125:683–6. doi: 10.1016/j.jaci.2009.12.994
517. Crevel RW, Kerkhoff MA, Koning MM. Allergenicity of refined vegetable oils. Food Chem Toxicol. (2000) 38:385–93. doi: 10.1016/S0278-6915(99)00158-1
518. Errahali Y, Morisset M, Moneret-Vautrin DA, Kanny G, Metche M, Nicolas JP, et al. Allergen in soy oils. Allergy. (2002) 57:648–9. doi: 10.1034/j.1398-9995.2002.23672.x
519. Moneret-Vautrin DA, Morisset M, Flabbee J, Kanny G, Kirch F, Parisot L. Unusual soy oil allergy. Allergy. (2002) 57:266–7. doi: 10.1034/j.1398-9995.2002.1n3528.x
520. Renaud C, Cardiet C, Dupont C. Allergy to soy lecithin in a child. J Pediatr Gastroenterol Nutr. (1996) 22:328–9. doi: 10.1097/00005176-199604000-00019
521. Rigby NM, Sancho AI, Salt LJ, Foxall R, Taylor S, Raczynski A, et al. Quantification and partial characterization of the residual protein in fully and partially refined commercial soybean oils. J Agric Food Chem. (2011) 59:1752–9. doi: 10.1021/jf103560h
522. Martin-Hernandez C, Benet S, Obert L. Determination of proteins in refined and nonrefined oils. J Agric Food Chem. (2008) 56:4348–51. doi: 10.1021/jf7036888
523. Bush RK, Taylor SL, Nordlee JA, Busse WW. Soybean oil is not allergenic to soybean-sensitive individuals. J Allergy Clin Immunol. (1985) 76:242–5. doi: 10.1016/0091-6749(85)90709-2
524. Palm M, Moneret-Vautrin DA, Kanny G, Denery-Papini S, Fremont S. Food allergy to egg and soy lecithins. Allergy. (1999) 54:1116–7. doi: 10.1034/j.1398-9995.1999.00305.x
525. Paschke A, Zunker K, Wigotzki M, Steinhart H. Determination of the IgE-binding activity of soy lecithin and refined and non-refined soybean oils. J Chromatogr B Biomed Sci Appl. (2001) 756:249–54. doi: 10.1016/S0378-4347(01)00085-8
526. Martin-Hernandez C, Benet S, Marvin-Guy LF. Characterization and quantification of proteins in lecithins. J Agric Food Chem. (2005) 53:8607–13. doi: 10.1021/jf0510687
527. USDA. Economic Research Service Using Data From USDA, National Agricultural Statistics Service, Fats & Oils: Oilseed Crushings and Peanut Stocks and Processors and USDA, Foreign Agricultural Service, Global Agricultural Trade System.
528. Parcell J, Kojima Y, Roach A, Cain W. Global edible vegetable oil market trends. Biomed J Sci Tech Res. (2018) 2:1–10. doi: 10.26717/BJSTR.2018.02.000680
529. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. (2011) 93:950–62. doi: 10.3945/ajcn.110.006643
530. Abdelghany AM, Zhang S, Azam M, Shaibu AS, Feng Y, Qi J, et al. Natural variation in fatty acid composition of diverse world soybean germplasms grown in China. Agronomy. (2020) 10:24. doi: 10.3390/agronomy10010024
531. Naghshi S, Aune D, Beyene J, Mobarak S, Asadi M, Sadeghi O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies. BMJ. (2021) 375:n2213. doi: 10.1136/bmj.n2213
532. Simopoulos AP, Leaf A, Salem N. Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. (1999) 43:127–30. doi: 10.1159/000012777
533. Simopoulos AP, DiNicolantonio JJ. The importance of a balanced omega-6 to omega-3 ratio in the prevention and management of obesity. Open Heart. (2016) 3:e000385. doi: 10.1136/openhrt-2015-000385
534. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation. Food and Nutrition Paper 91. Food and Agriculture Organization of the United Nations, Rome (2010).
535. de Deckere EA, Korver O, Verschuren PM, Katan MB. Health aspects of fish and n-3 polyunsaturated fatty acids from plant and marine origin. Eur J Clin Nutr. (1998) 52:749–53. doi: 10.1038/sj.ejcn.1600641
536. Wang C, Chung M, Lichtenstein A, Balk E, Kupelnick B, DeVine D, et al. Effects of Omega-3 Fatty Acids on Cardiovascular Disease. Evidence Report/Technology Assessment No. 94 (Prepared by Tufts-New England Medical Center Evidence-based Practice Center, under Contract No. 290-02-0022). AHRQ Publication No. 04-E009-2. Rockville, MD: Agency for Healthcare Research and Quality (2004).
537. Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press (2005).
538. Stanley JC, Elsom RL, Calder PC, Griffin BA, Harris WS, Jebb SA, et al. UK Food Standards Agency Workshop Report: the effects of the dietary n-6:n-3 fatty acid ratio on cardiovascular health. Br J Nutr. (2007) 98:1305–10. doi: 10.1017/S000711450784284X
539. Kris-Etherton PM, Innis S, Ammerican Dietetic A, Dietitians Dietitians of C. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. (2007) 107:1599–611. doi: 10.1016/j.jada.2007.07.024
540. Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. (2009) 119:902–7. doi: 10.1161/CIRCULATIONAHA.108.191627
541. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. (2010) 8:1–107. doi: 10.2903/j.efsa.2010.1461
542. EAT FOR HEALTH. Australian Dietary Guidelines Providing the Scientific Evidence for Healthier Australian Diets. Available online at: https://www.eatforhealth.gov.au/sites/default/files/content/n55_australian_dietary_guidelines.pdf (2013).
543. Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. (2012) 112:1029–41. doi: 10.1016/j.jand.2012.03.029
544. Su H, Liu R, Chang M, Huang J, Wang X. Dietary linoleic acid intake and blood inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Food Funct. (2017) 8:3091–103. doi: 10.1039/C7FO00433H
545. Karupaiah T, Chuah KA, Chinna K, Matsuoka R, Masuda Y, Sundram K, et al. Comparing effects of soybean oil- and palm olein-based mayonnaise consumption on the plasma lipid and lipoprotein profiles in human subjects: a double-blind randomized controlled trial with cross-over design. Lipids Health Dis. (2016) 15:131. doi: 10.1186/s12944-016-0301-9
546. Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. (2002) 43:445–52. doi: 10.1016/S0022-2275(20)30151-6
547. Assuncao ML, Ferreira HS, dos Santos AF, Cabral CR Jr., Florencio TM. Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids. (2009) 44:593–601. doi: 10.1007/s11745-009-3306-6
548. Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr. (2006) 84:497–504. doi: 10.1093/ajcn/84.3.497
549. Baer DJ, Henderson T, Gebauer SK. Consumption of high-oleic soybean oil improves lipid and lipoprotein profile in humans compared to a palm oil blend: a randomized controlled trial. Lipids. (2021) 6:313–25. doi: 10.1002/lipd.12298
550. Papageorgiou N, Tousoulis D, Psaltopoulou T, Giolis A, Antoniades C, Tsiamis E, et al. Divergent anti-inflammatory effects of different oil acute consumption on healthy individuals. Eur J Clin Nutr. (2011) 65:514–9. doi: 10.1038/ejcn.2011.8
551. Rozati M, Barnett J, Wu D, Handelman G, Saltzman E, Wilson T, et al. Cardio-metabolic and immunological impacts of extra virgin olive oil consumption in overweight and obese older adults: a randomized controlled trial. Nutr Metab. (2015) 12:28. doi: 10.1186/s12986-015-0022-5
552. Richaud E, Audouin L, Fayolle B, Verdu J, Matisova-Rychla L, Rychly J. Rate constants of oxidation of unsaturated fatty esters studied by chemiluminescence. Chem Phys Lipids. (2012) 165:753–9. doi: 10.1016/j.chemphyslip.2012.09.002
553. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. (1989) 320:915–24. doi: 10.1056/NEJM198904063201407
554. Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. (1977) 46:897–930. doi: 10.1146/annurev.bi.46.070177.004341
555. Koenig W, Karakas M, Zierer A, Herder C, Baumert J, Meisinger C, et al. Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study. Clin Chem. (2011) 57:1196–200. doi: 10.1373/clinchem.2011.165134
556. Wu T, Willett WC, Rifai N, Shai I, Manson JE, Rimm EB. Is plasma oxidized low-density lipoprotein, measured with the widely used antibody 4E6, an independent predictor of coronary heart disease among U.S. men and women? J Am Coll Cardiol. (2006) 48:973–9. doi: 10.1016/j.jacc.2006.03.057
557. Mata P, Alonso R, Lopez-Farre A, Ordovas JM, Lahoz C, Garces C, et al. Effect of dietary fat saturation on LDL oxidation and monocyte adhesion to human endothelial cells in vitro. Arterioscler Thromb Vasc Biol. (1996) 16:1347–55. doi: 10.1161/01.ATV.16.11.1347
558. Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, et al. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr. (2002) 56:72–81. doi: 10.1038/sj.ejcn.1601288
559. Reaven PD, Grasse BJ, Tribble DL. Effects of linoleate-enriched and oleate-enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans. Arterioscler Thromb. (1994) 14:557–66. doi: 10.1161/01.ATV.14.4.557
560. Esterbauer H, Dieber-Rotheneder M, Striegl G, Waeg G. Role of vitamin E in preventing the oxidation of low-density lipoprotein. Am J Clin Nutr. (1991) 53:314S−21S. doi: 10.1093/ajcn/53.1.314S
561. Kiokias S, Proestos C, Oreopoulou V. Effect of natural food antioxidants against LDL and DNA oxidative changes. Antioxidants. (2018) 7:133. doi: 10.20944/preprints201809.0422.v1
562. Costa ESLM, Pereira de Melo ML, Faro Reis FV, Monteiro MC, Dos Santos SM, Quadros Gomes BA, et al. Comparison of the effects of Brazil nut oil and soybean oil on the cardiometabolic parameters of patients with metabolic syndrome: a randomized trial. Nutrients. (2019) 12:46. doi: 10.3390/nu12010046
563. Utarwuthipong T, Komindr S, Pakpeankitvatana V, Songchitsomboon S, Thongmuang N. Small dense low-density lipoprotein concentration and oxidative susceptibility changes after consumption of soybean oil, rice bran oil, palm oil and mixed rice bran/palm oil in hypercholesterolaemic women. J Int Med Res. (2009) 37:96–104. doi: 10.1177/147323000903700111
564. Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. (2015) 66:1538–48. doi: 10.1016/j.jacc.2015.07.055
565. Mousavi SM, Jalilpiran Y, Karimi E, Aune D, Larijani B, Mozaffarian D, et al. Dietary intake of linoleic acid, its concentrations, and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetes Care. (2021) 44:2173–81. doi: 10.2337/dc21-0438
566. U.S. Department of Health Human Services and U.S. Department of Agriculture, 2015 – 2020. Dietary Guidelines for Americans. 8th ed (2015). Available online at: http://health.gov/dietaryguidelines/2015/guidelines/.
567. de Pablo P, Romaguera D, Fisk HL, Calder PC, Quirke AM, Cartwright AJ, et al. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case-control study. Ann Rheum Dis. (2018) 77:981–7. doi: 10.1136/annrheumdis-2017-212274
568. He Y, Li Y, Yang X, Hemler EC, Fang Y, Zhao L, et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982-2012: a cross-sectional population-based study. Lancet Diabetes Endocrinol. (2019) 7:540–8. doi: 10.1016/S2213-8587(19)30152-4
569. Takeuchi M, Horikawa C, Hatta M, Takeda Y, Nedachi R, Ikeda I, et al. Secular trends in dietary intake over a 20-year period in people with Type 2 Diabetes in Japan: a comparative study of two nationwide registries; Japan Diabetes Complications Study (JDCS) and Japan Diabetes Clinical Data Management Study (JDDM). Nutrients. (2021) 13:3428. doi: 10.3390/nu13103428
570. Zhao W, Hasegawa K, Chen J. The use of food-frequency questionnaires for various purposes in China. Public Health Nutr. (2002) 5:829–33. doi: 10.1079/PHN2002374
571. Lee SA, Wen W, Xiang YB, Barnes S, Liu D, Cai Q, et al. Assessment of dietary isoflavone intake among middle-aged Chinese men. J Nutr. (2007) 137:1011–6. doi: 10.1093/jn/137.4.1011
572. Yang G, Shu XO, Jin F, Zhang X, Li HL, Li Q, et al. Longitudinal study of soy food intake and blood pressure among middle-aged and elderly Chinese women. Am J Clin Nutr. (2005) 81:1012–7. doi: 10.1093/ajcn/81.5.1012
573. Shu XO, Li H, Yang G, Gao J, Cai H, Takata Y, et al. Cohort profile: the Shanghai Men's Health Study. Int J Epidemiol. (2015) 44:810–8. doi: 10.1093/ije/dyv013
574. Ueshima H, Miura K, Okuda N. NIPPON DATA80/90 nutrition study: appendix tables. J Epidemiol. (2010) 20:S587–96. doi: 10.2188/jea.JE20101002
575. Berryman CE, Lieberman HR, Fulgoni VL 3rd, Pasiakos SM. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: analysis of the National Health and Nutrition Examination Survey, 2001-2014. Am J Clin Nutr. (2018) 108:405–13. doi: 10.1093/ajcn/nqy088
576. Akhter M, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Dietary soy and isoflavone intake and risk of colorectal cancer in the Japan public health center-based prospective study. Cancer Epidemiol Biomark Prev. (2008) 17:2128–35. doi: 10.1158/1055-9965.EPI-08-0182
Keywords: soyfoods, counseling, patients, isoflavones, review, protein, recommendations, clients
Citation: Messina M, Duncan A, Messina V, Lynch H, Kiel J and Erdman JW Jr (2022) The health effects of soy: A reference guide for health professionals. Front. Nutr. 9:970364. doi: 10.3389/fnut.2022.970364
Received: 15 June 2022; Accepted: 25 July 2022;
Published: 11 August 2022.
Edited by:
Vijaya Juturu, LONZA Capsules and Health Ingredients, United StatesReviewed by:
Anna H. Wu, University of Southern California, United StatesCopyright © 2022 Messina, Duncan, Messina, Lynch, Kiel and Erdman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Messina, bWFya2pvaG5tZXNzaW5hQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.