
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 10 November 2022
Sec. Nutrition and Food Science Technology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.968930
This article is part of the Research Topic Bioaccessibility of Food-Derived Bioactive Components and Their Health-Promoting Mechanisms View all 8 articles
Tongxie Yaofang (TXYF), a Traditional Chinese Medicine (TCM) with four components as follows: Rhizoma Atractylodis Macrocephalae (baizhu), Radix Paeoniae Alba (baishao), Pericarpium Citri Reticulatae (chenpi) and Radix Saposhnikovia Divaricata (fangfeng), benefits irritable bowel syndrome (IBS). Nonetheless, proofs of this formula ameliorating D-IBS and T2DM are required. This research aimed at investigating the efficacy of TXYF in treating inflammation in rats with D-IBS and T2DM using animal models. In this study, gavage with high-fat diet, fasciculation, and senna was given to develop rat models with target diseases. To determine intestinal inflammations, major inflammatory factors, and intestinal permeability proteins, H&E staining, ELISA, and immunohistochemistry methods were employed, respectively. This study also utilized Western blot to discover potential inflammatory targets. Results of this research illustrates that TXYF treatment reduced the level of TNF-α, IL-1β, and IL-6, and raised the IL-10 concentration in liver-depressed spleende ficient rats with D-IBS and T2DM, indicating controlled inflammatory reactions. Staining analysis also showed improved disease states of animal models. Furthermore, efficient rebounds of claudin-1, an intestinal permeability-associated protein, were detected. Moreover, TXYF may treat D-IBS and T2DM in rats via the rage pathway.
Irritable bowel syndrome (IBS) is a digestive illness marked by recurrent stomach pain or discomfort, as well as changes in bowel habits and stool patterns. IBS is a worldwide problem, with prevalence rates ranging from 5 to 15% in Western countries (1), and 5–10% in Asia (2, 3). According to an assessment by Peking Union Medical College Hospital, IBS is one of the most common ailments in urban China. Meanwhile, patients with IBS account for 34.3% in outpatient gastrointestinal clinic appointments (4). Among all types of IBS, irritable bowel syndrome with diarrhea (D-IBS) that can cause long-term symptoms and significant impacts on both physical and mental health, is difficult to be cured and may easily recur. However, the etiology of D-IBS remains unclear.
Diabetes mellitus is a metabolic disorder marked by elevated blood sugar levels, whereas chronic hyperglycemia can cause vision problems, cardiovascular and renal organ damage, and even organ failure. Type 2 diabetes mellitus (T2DM) with risk factors as listed: age, gender, obesity, dyslipidemia, genetic abnormalities, and pre-diabetes (such as IFG and IGT), takes about 90% of all diabetes. According to the International Diabetes Federation, 380 million people in the world will have diabetes by 2025 (5). Besides, diabetic complications are growingly common in T2DM patients and may have substantial influences on their lives (6).
Nevertheless, the prevalence of pre-diabetes was considerably higher in IBS patients than in control groups, indicating IBS as a risk factor for diabetes. In a previous random controlled study on 201 D-IBS patients and 220 healthy volunteers at Yangzhou University Affiliated Hospital, the incidence of diabetes was significantly higher in the IBS group, especially in patients with a longer disease course, and IBS may be related to diabetes (7). Hence, detecting risk factors of diabetes in time is critical for its prevention.
Intestinal mucosa immunity is an important system in intestinal mechanism, and its disequilibration may lead to chronic inflammation and be connected with D-IBS. Although most patients with D-IBS show no pathology change in routine histological examinations, a quantitative histological study and electron microscopic ultrastructural analysis have detected the enlarged space among epithelial cells and cytoskeletal cohesion in the intestinal mucosa of D-IBS patients (8), which may result from aberrant synthesis of tight junction protein 1 (claudin-1) that plays a significant role in regulating the osmotic pressure of intestinal mucosa. Reduced claudin-1 level in intestinal mucosa may increase intestinal osmolality (9), vice versa (10), as low-grade inflammation may cause bacterial or protease-induced breakdown (11). Meanwhile, morphological changes of intestinal mucosa that frequently accompanied by changes in immune cells and intestinal endocrine cells, may alter its permeability (12–14), which could allow more lipopolysaccharides and other inflammatory factors entering into the circulatory system and improve the absorption of endotoxins in intestines, resulting in chronic systemic inflammatory response, insulin resistance, and even diabetes (15).
Inflammatory factors are important components in immunological and inflammatory responses. Interleukin (IL) 10 is the first discovered anti-infection immunomodulatory factor in mice by inhibiting IL-1, IL-2, IL-3, IL-12, chemokines, TNF-α, and Th1 cytokines from macrophages (16). Nonetheless, the expression of IL-10 can be aberrant in patients with autoimmune diseases (17, 18), including D-IBS that may lower the IL-10 level in plasma (19, 20). Besides, high serum HbA1c expression in patients with T2DM may cause a decrease of IL-10, therefore high IL-10 grade may be related to hyperglycemia (21). Furthermore, reduced IL-10 production can interfere the down-regulate of inflammatory response in intestinal mucosa in patients with D-IBS and T2DM, which may lead to chronic low-grade inflammation. Furthermore, inflammatory agents and chemokines can impair the function of cellular carriers, influence intestinal permeability, and exacerbate the inflammatory response (11).
TXYF was initially recorded by Zhu in Dan-Xi-Xin-Fa as “in treating unpleasant diarrhea, prepare 3 taels of Rhizoma Atractylodis Macrocephalae (baizhu), 2 taels of stir-baked Radix Paeoniae Alba (baishao), 2.5 taels of dried Pericarpium Citri Reticulatae (chenpi) and 1 tael of Radix Saposhnikovia Divaricata (fangfeng).” According to traditional Chinese medicine (TCM), this formula can treat diarrhea with pain that results from liver-depression and spleen-deficiency by balancing and relieving liver and spleen, and dispelling dampness, including acute enteritis, chronic colitis, and IBS. TXYF on patients with D-IBS illustrated efficacy to a certain extent in some studies (22). Plants like barley are rich in bioactive phytochemicals that may exhibit an anti-obesity effect (23). Meanwhile, it may also decrease the blood glucose level by inhibiting glucosidase and dipeptidyl peptidase-IV (DPP-IV), which can effectively degrade glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide (GIP) that stimulating the production of insulin (24). As a result, TXYF has the potential to be employed in the future to prevent and control obesity-related disorders.
Hence, it’s reasonable to explore the efficacy and its mechanism of TXYF in treating D-IBS and T2DM with rat models.
Rhizoma Atractylodis Macrocephalae (baizhu), Radix Paeoniae Alba (baishao), Pericarpium Citri Reticulatae (chenpi) and Radix Saposhnikovia Divaricata (fangfeng) (Huahong Pharmaceutical Company, China) in this study were identified by Prof. Ouyang Zhen, School of Pharmacy, Jiangsu University.
According to Chinese Pharmacopoeia, 6 g of baizhu, 4 g of baishao, 3 g of chenpi, and 2 g of fangfeng were soaked in 50 g of distilled water for 3 h then boiled. Afterward, the formulation was separated from the filtrate, and concentrated to 0.4 g/mL. The solution was stored at 4°C and diluted with water when use.
Twenty male SD rats and 20 female SD rats weighing 190 ± 10 g (Jiangsu University, China) were fed with a 45% high-fat and high-sugar diet after 3 days of rest for 6–7 weeks, then received intraperitoneal injection of streptozotocin solution (S6089) at the dose of 35 mg/kg. Rats with fasting blood glucose greater than 11.1 mmol/L were considered as successful models of type 2 diabetes. Afterward, long-term restraint and 2 mL of 1 g/mL Folium Sennae decoction gavage two times a day were applied on T2DM rats. Animal models were restrained with wide scotch tape on their shoulder, forelimbs and chest, and restricted from scratching the head and face for 1 h, whereas other activities were allowed.
Modeled rats were divided into five groups as listed: model group, three TXYF groups of low (5 mL/kg), medium (10 mL/kg), and high dosage (15 mL/kg), respectively, and Trimebutine Maleate (TMT) as positive control group, 6 rats in each. A control group that received Weifuan, a control drug, was also established. All rats were treated for 14 days.
The study was approved by the Ethics Committee of Jiangsu University. Approval code is UJS-lACUC-2021070103.
The model was evaluated by the changes in body weight, fecal water content, and blood glucose of rats in each group, before, and after treatment, respectively.
The histopathology of colons was observed by HE method. Fresh tissues were fixed for 18 h in 10% formaldehyde and dehydrated with ethanol solutions, then embedded in paraffin. Four microgram of continuous sections were dewaxed after baking at 58°C for 18 h, then hydrated with all levels of ethanol solutions, stained with hematoxylin for 5 min, fractionated in 1% hydrochloric acid alcohol for 30 s, colored with ammonia, and sealed with resin after ethanol dehydration. All sections were examined and photoed under a microscope for analysis.
After accurately weighing the animal tissue 9 times the volume of its weight of normal saline, was added to mechanically homogenize a 10% solution under ice-water bath conditions. Next, the homogenate was centrifuged for 10 min at 2,500–3,000 rpm. TNF-α, IL1-β, IL-6, and IL-10 in the supernatant were measured according to the instruction ELISA kit (Thermo Fisher Scientific Invitrogen, USA) of TNF-α, IL1-β, IL-6, and IL-10 to determine the content in the samples.
The slices were sequentially placed in xylene I for 15 min, xylene II for 15 min, xylene III for 15 min, anhydrous ethanol I for 5 min, anhydrous ethanol II for 5 min, 85% alcohol for 5 min, 75% alcohol for 5 min, respectively, and washed with distilled water. Afterward, tissue sections were placed in a repair box filled with citric acid antigen retrieval buffer (pH 6.0) and retrieved in a microwave oven at medium heat for 8 min for boiling, then incubation for 8 min, and medium-low heat for 7 min. After natural cooling, the slides were placed in PBS (pH 7.4) and rinsed for 5 min on a destaining shaker for 3 times. Next, prepared sections were incubated in 3% hydrogen peroxide solution, for 25 min in the dark at room temperature then rinsed for 5 min on a destaining shaker for 3 times. In the histochemical circle, 3% BSA or rabbit serum was added to cover the tissue evenly and seal it at room temperature for 30 min. After gently shaken off the blocking solution, slices were dropped with the primary antibody prepared in a certain proportion of PBS and incubate in a wet box at 4°C overnight, then. The slides were washed in PBS (pH 7.4) for 5 min on a destaining shaker for 3 times. Next, when the section was slightly dried, it was dropped a secondary antibody (HRP-labeled) corresponding to the primary antibody was added to cover the tissue, then incubated at room temperature for 50 min. Afterward, slides were washed in PBS (pH 7.4) for 5 min on a destaining shaker for 3 times. And then, freshly prepared DAB solution was applied to develop color under the microscope. After the HE re-staining for about 3 min and washed with running water, the slide was put into solutions in the order of 75% alcohol for 5 min, 85% alcohol for 5 min, anhydrous ethanol I for 5 min, anhydrous ethanol II for 5 min, and xylene I for 5 min, respectively, for dehydration and transparency. Finally, sections were mounted with neutral gum, then examined and photoed with a microscope.
Cross-sectional targets were collated and imported to the metascape database1 with GO and KEGG analysis to screen out the biological process and related pathways of TXYF in treating D-IBS and T2DM under the condition of P < 0.01.
After washing the tissue with PBS to remove blood stains, it was cut into small pieces and homogenized, then applied protein lysis buffer and centrifuged at 12,000 rpm for 10 min. The supernatant was collected. Next, protein solutions were balanced with BCA reagents and added 5X protein loading buffer at the ratio of 4:1, then boiled until denaturation. Afterward, the protein concentration was measured with a BCA protein detection kit, and an equal volume of protein was loaded on 12.5% SDS-PAGE. Whereafter, the surface were blocked with 5% non-fat milk for an hour and incubated with RAGE antibody (GB11278) for 2 h, respectively. Finally, the membranes were rinsed 3 times and incubated with specific secondary antibodies for 1 h, respectively. Visualization of protein signals was detected with an ECL system (Millipore Corporation, Billerica, USA) using equal amounts of BeyoECL A and BeyoECL B. the levels of SOD (Cat# KTB1030) and MDA (Cat # KTB1050) in serum were tested following the instructions (Abbkine). All the SOD and MDA enzyme concentrations were expressed in international units.
Data were expressed as average ± SD. Comparisons between the two groups were determined by a Student’s t-test or by One-Way ANOVA (GraphPad software, San Diego CA, USA). A p-value less than 0.05 was considered statistically significant.
As showed in Figure 1, the body weight of rats in disease groups was statistically reduced than that in the control group. After the TXYF treatment, their body weight significantly increased. Besides, rats in TXYF groups and the positive control group had significantly lower fecal water content and blood glucose than those in the control group. These results suggest TXYF treatments significantly reversed the weight loss and diarrhea symptoms caused by D-IBS of rats ameliorated their blood glucose.
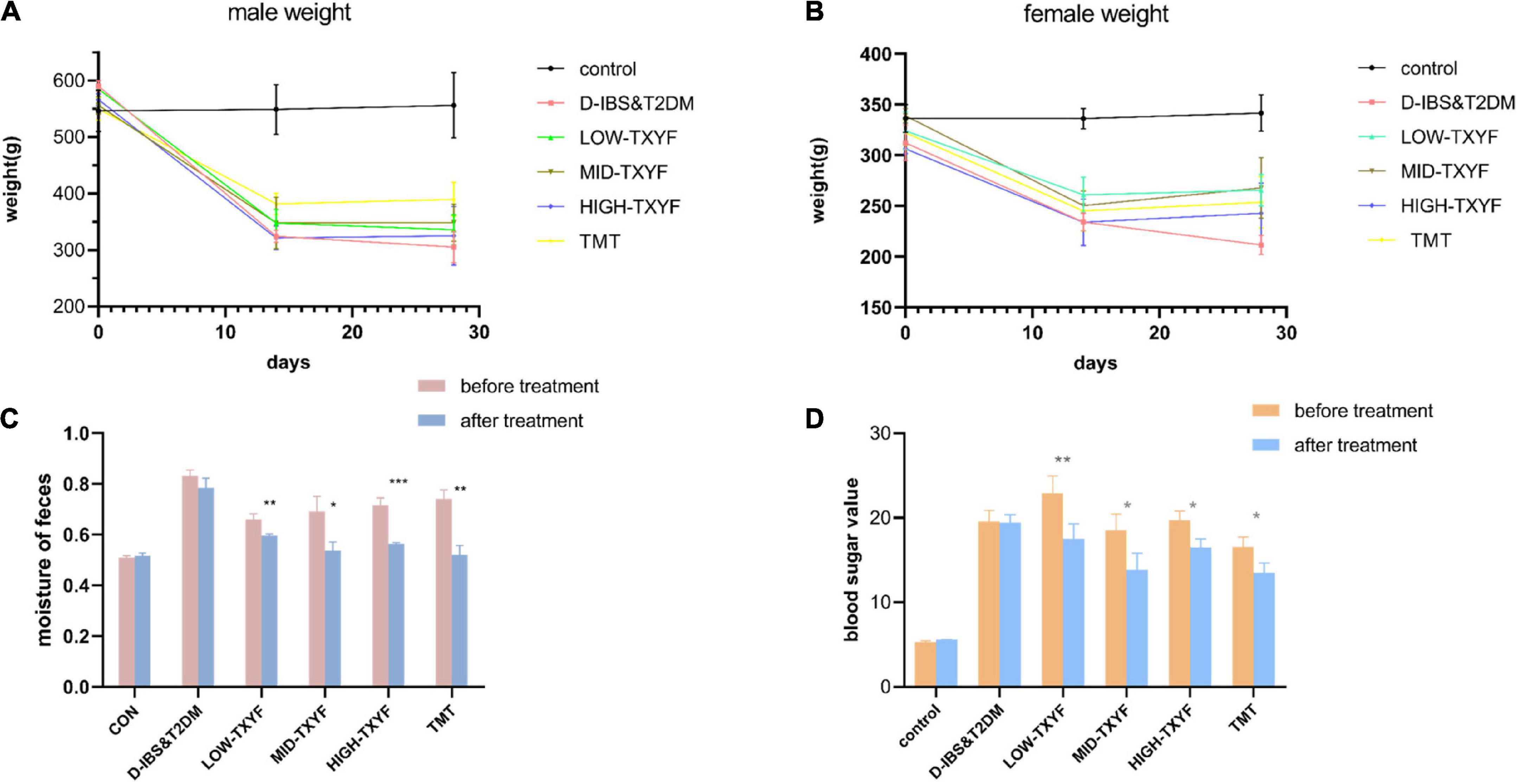
Figure 1. Effect of TXYF on characterization in male D-IBS combined with T2DM rats. Effect of TXYF on weights in male rats (A); effect of TXYF on weights in female rats (B); water content of rat feces (C); blood glucose values in rats (D); data are represented as mean ± S.D. (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001 vs. D-IBS&T2DM group.
As showed in Figure 2, edema, a small amount of inflammatory cell infiltration and hyperplasia in connective tissues were observed in the colon of rats with D-IBS and T2DM. TXYF treatments significantly ameliorated these symptoms, controlled the inflammation and recruited the structure of the colon.
Levels of TNF-, IL-1, IL-6, and IL-10 in the tissues in Figure 3 were determined by ELISA. Among them, the expression of TNF-α in disease groups was significantly higher than that in the normal group (P < 0.0001). After TXYF administrations, the TNF-α level decreased significantly (P < 0.001, Figure 3A). Concentrations of IL-1β and IL-6 in colonic tissues were also quantified by ELISA with consistent results (Figures 3B,C). Besides, the expression of IL-10 was higher in disease groups than that in the control group (P < 0.0001, Figure 3D). Hence, TXYF may have helped reducing the chronic inflammation of rats with D-IBS and T2DM induced by Folium Sennae.
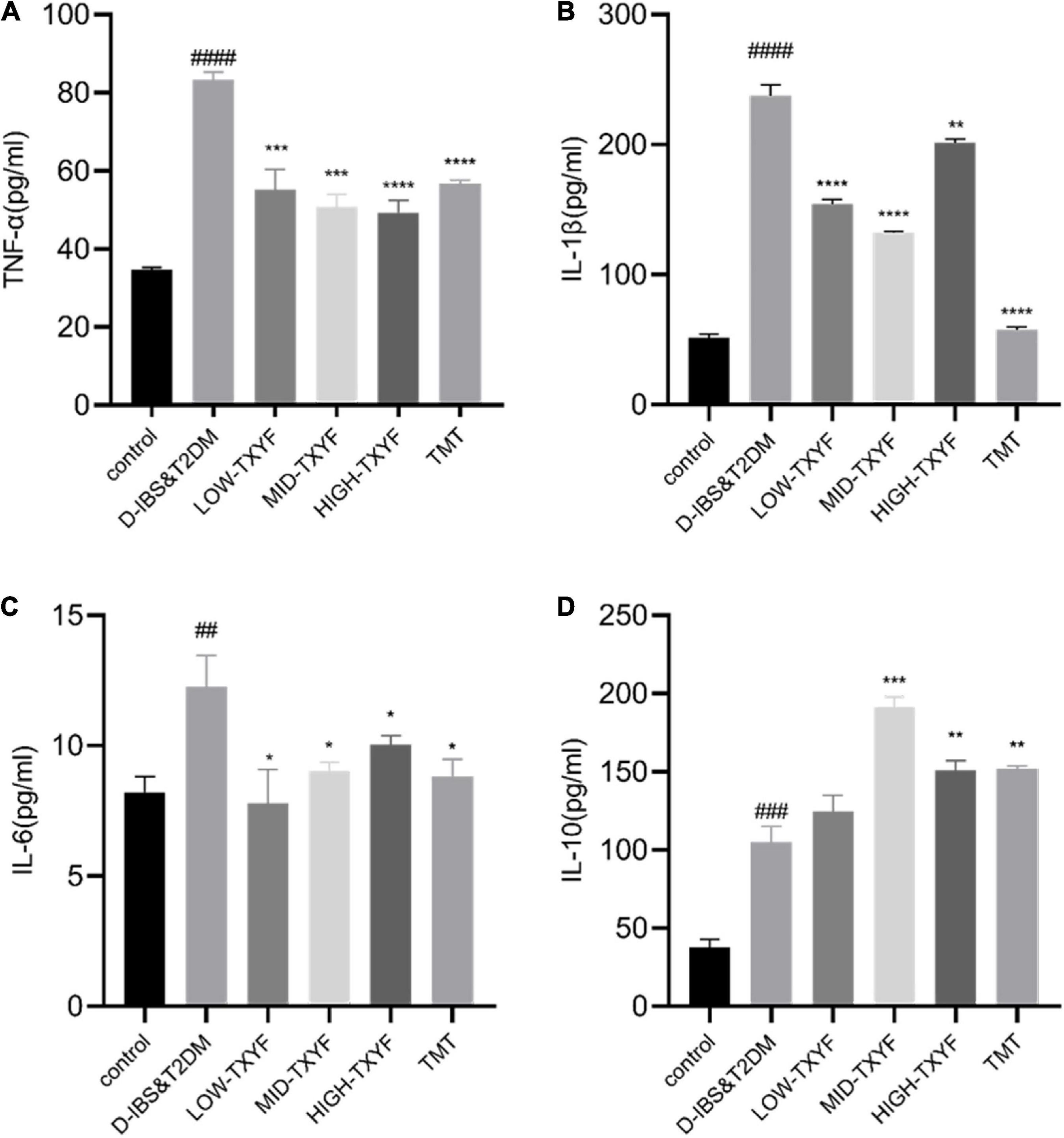
Figure 3. Effects of TXYF on TNF-α, IL-1β, and IL-6 and IL-10 in colonic tissue in rats with D-IBS&T2DM. The expression levels of TNF-α (A), IL-1β (B), IL-6 (C) and IL-10 (D) in colonic tissue were determined using ELISA (n = 6). Values are presented as means ± SD (n = 3). ##P < 0.01, ###P < 0.001, and ####P < 0.0001 and ##P < 0.01 compared with the control group; *P < 0.05 and **P < 0.01 compared with the D-IBS&T2DM group.
Claudin-1 levels were measured by immunohistochemistry methods (Figure 4). The expression of claudin-1 in colonic tissues was lower in disease groups than that in the control group. After drug administrations in each TXYF group and positive control drug, TXYF increase the claudin-1 level and effectively ameliorated the change in intestinal permeability induced by Folium Sennae in rats with D-IBS and T2DM.
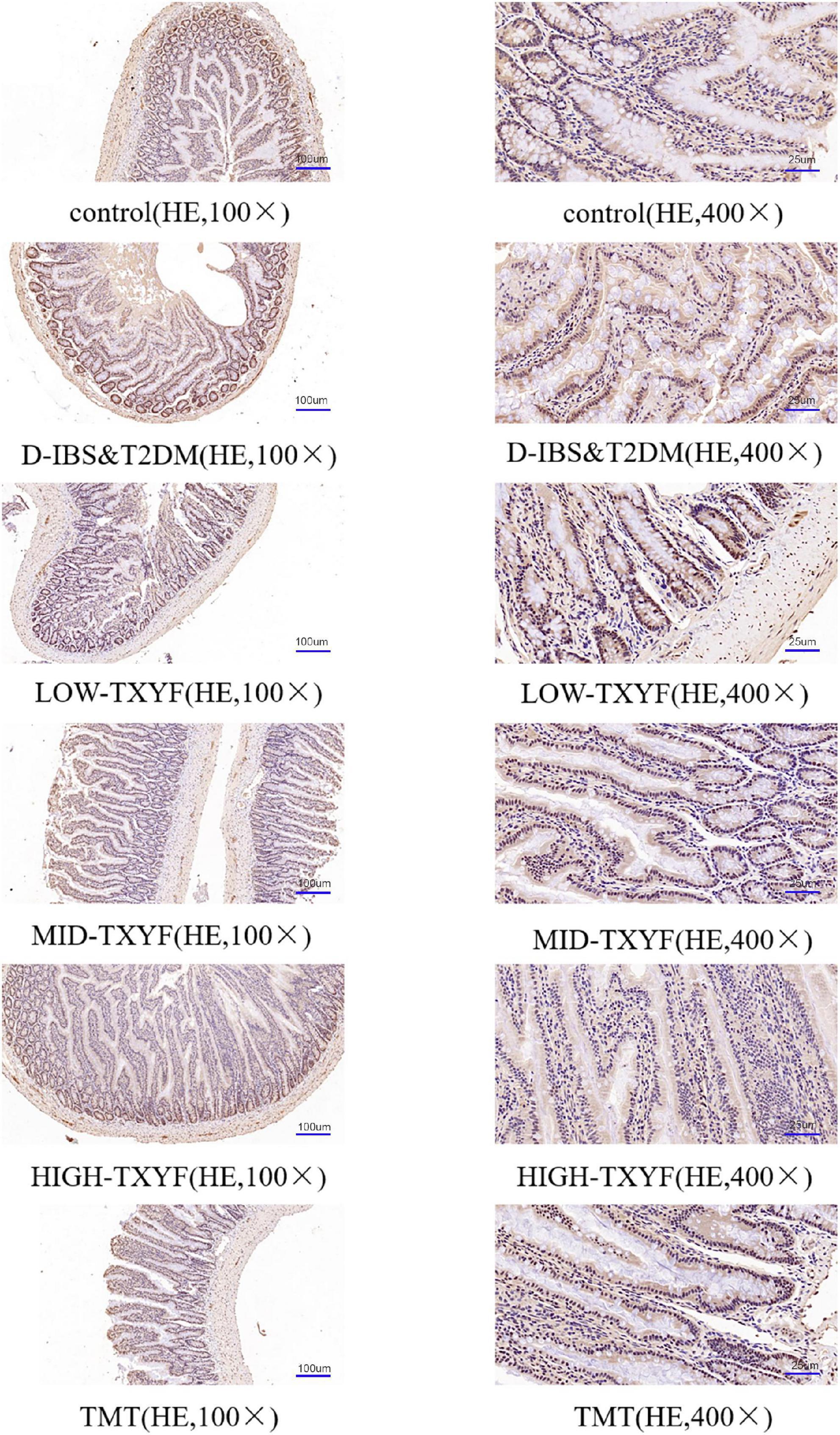
Figure 4. Representative images of claudin-1 in IHC sections (100×, 400×) from colon tissue (n = 3).
As showed in Figure 5A, active ingredients of TXYF effects 65 overlapped targets of D-IBS and T2DM. Afterward, analyzed by GO and KEGG enrichment, the RAGE signal pathway has the highest association with its mechanism (Figures 5B,C).
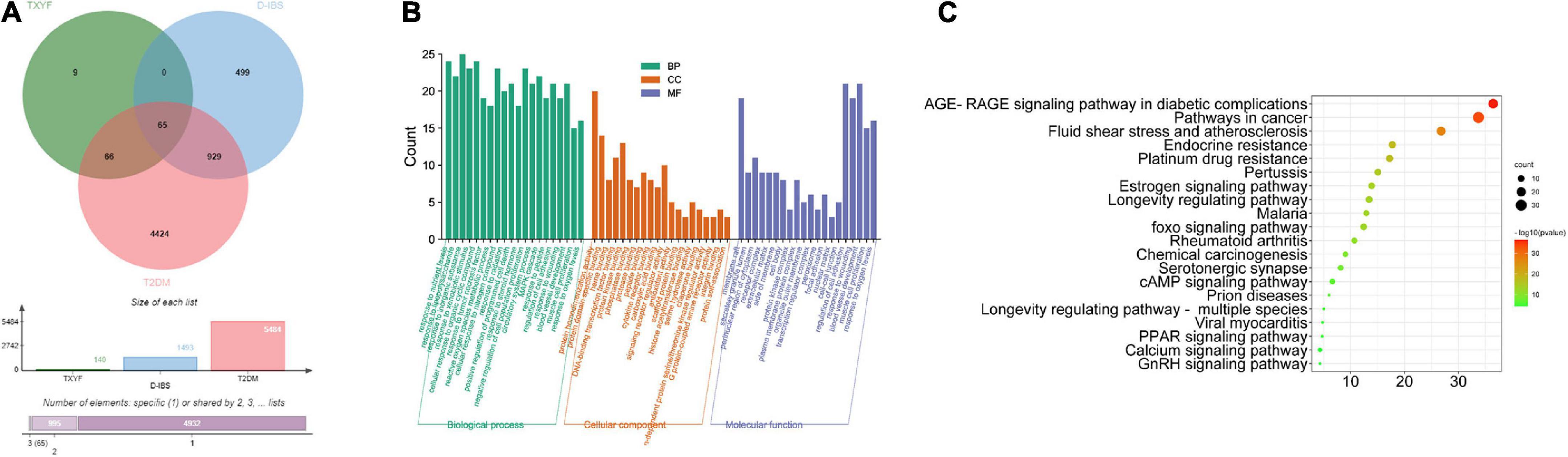
Figure 5. Common targets of TYXF, D-IBS and T2DM (A); list of relevant pathways obtained by GO enrichment and KEGG enrichment (B,C).
The expression level of RAGE proteins in intestinal mucosa of rats with D-IBS and T2DM in each disease group is shown in Figure 6. The level of RAGE on disease groups was significantly lower than that in the control group (P < 0.01). Compared to the model group, RAGE protein expressions in all TXYF groups and the positive control group were significantly higher. As showed in Figures 6C,D, the expression of SOD in disease groups was statistically reduced than that in the control group (P < 0.0001). After the TXYF treatment, the SOD level increased. Besides, the expression of MDA in disease groups was significantly higher than that in the normal group (P < 0.001). After TXYF administrations, the MDA level decreased significantly. TXYF may alleviate inflammation by regulating RAGE-induced oxidative stress.
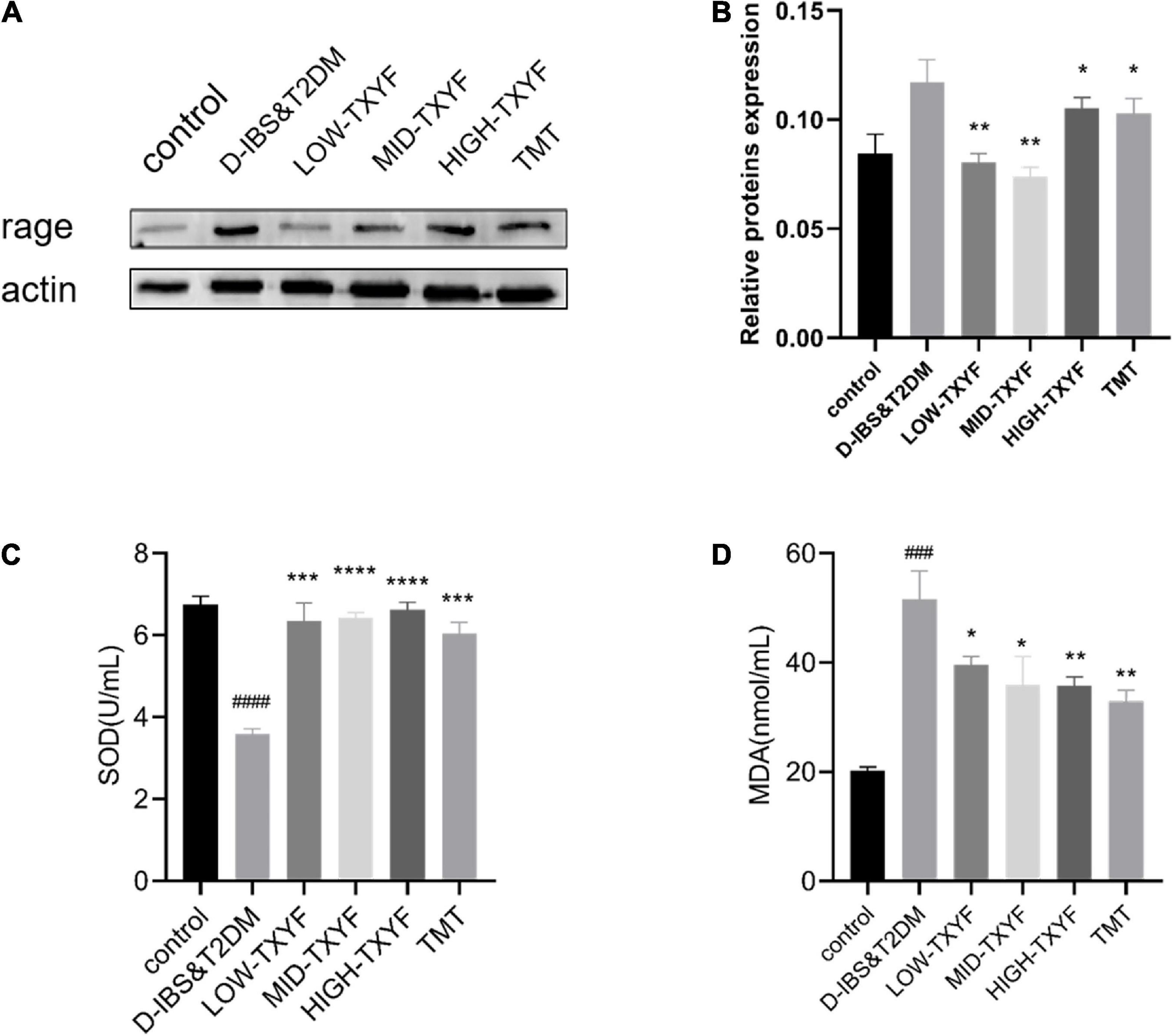
Figure 6. Protein expression level of RAGE was measured using western blotting (n = 3) (A,B). Effects of TXYF on SOD and MDA in serum in rats with D-IBS&T2DM. Values are presented as means ± SD (n = 3), ###P < 0.001 and ####P < 0.0001 compared with the control group; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with the D-IBS&T2DM group (C,D).
This study confirmed the therapeutical effect of TXYF in reducing intestinal inflammation in liver-depression and spleen-deficiency rats with D-IBS and T2DM, and ameliorating the expression of TNF-α, IL-1β, IL-6, and IL-10 in the colon. We also find that TXYF may influence inflammatory factors via the RAGE pathway.
There is mounting evidence that IBS and mental health issues are inextricably linked, with dysregulation of gastrointestinal tissues and disruption of gut flora diversity leading to disruption of the gut-central nervous connection and triggering a variety of mental health issues, according to research that has shown that there is growing evidence of this connection (25). 5-hydroxytryptamine (5-HT) plays an important role in intestinal motility, secretion, visceral hypersensitivity and inflammation, and the imbalance of this neurotransmitter and its transporters may lead to IBS (26). Alterations in the intestinal flora caused by probiotics have also been found to have an effect on the expression of associated factors and signaling pathway proteins in the intestinal tissues, this was discovered through research (27). Probiotics have been shown to have an effect on the microflora of the gut, however, the mechanism underlying this effect has not been studied. To sum up, IBS inflammation is the result of a combination of neurological, brain-gut axis, and intestinal flora mediations, all of which merit additional research.
TXYF, originally recorded by Zhu Zhenheng in Danxi Xinfa in Yuan Dynasty, is commonly employed in treating diarrhea, including gastrointestinal discomfort and mental issues as its complications, caused by liver-depression and spleen-deficiency. In modern medicine, TXYF is most frequently prescribed for patients with IBS and chronic enteritis. Pericarpium Citri Reticulatae (chenpi) in TXYF may reduce inflammation by acting on certain signaling pathways, such as PI3K/AKT (28). In a study of Radix Paeoniae Alba (baishao) and Rhizoma Atractylodis Macrocephalae (baizhu), these TCMs may ameliorate the expression of inflammatory factors (29). In addition, a number of research have come to the conclusion that ketones have a high capacity for scavenging free radicals. This is because of the strong reactivity of the hydroxyl groups, which can provide hydrogen and electrons to radicals in order to stabilize them. Zhou et al. discovered that Atractylodis macrocephala had the ability to inhibit NF-κB and MAPK, which resulted in a reduction in the inflammatory expression of murine macrophages (30). In addition, a number of research have come to the conclusion that ketones have a high capacity for scavenging free radicals. This is because of the strong reactivity of the hydroxyl groups, which can provide hydrogen and electrons to radicals in order to stabilize them (31). TXYF is rich in ketones, and it hasn’t been found out yet if it has any effect on pathways related to oxidation.
RAGE is a therapeutic target for inflammatory bowel disease (IBD) as it has been demonstrated that RAGE receptors are linked with the activation of immune system in inflammatory responses and mice lacking RAGE are less susceptible to colonic inflammations, as well as RAGE causes inflammation by inducing oxidative stress and its antagonists could effectively threat IBD mice (32) IBD presents an opportunity for RAGE to be used as a therapeutic target.
According to the findings of a number of research projects, the interaction between RAGE receptors and ligands creates a chronic inflammatory pathway that is involved in the mechanism of diabetic complications. Taking all of these factors into account leads one to speculate that blocking RAGE could be an effective therapeutic approach for a variety of diabetic complications (33). Therefore, the rage signaling pathway is expected to be an effective target for the treatment of D-IBS combined with T2DM disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Jiangsu University (UJS IACUC).
WX: conceptualization, methodology, data curation, and writing—original draft. ZZ: methodology, data curation, and writing—original draft. YL: data curation and validation. ML: data curation and investigation. JL: software and validation. WT: writing—original draft, methodology, funding acquisition, and writing—review and editing. All authors agreed to be accountable for all aspects of work ensuring integrity and accuracy.
This study was funded by the National Natural Science Foundation of China (81904166).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Altomare A, Di Rosa C, Imperia E, Emerenziani S, Cicala M, Guarino MPL. Diarrhea predominant-irritable bowel syndrome (IBS-D): effects of different nutritional patterns on intestinal dysbiosis and symptoms. Nutrients. (2021) 13:1506. doi: 10.3390/nu13051506
2. Wang H, Zhou G, Luo L, Crusius JBA, Yuan A, Kou J, et al. Serological screening for celiac disease in adult Chinese patients with diarrhea predominant irritable bowel syndrome. Medicine (Baltimore). (2015) 94:e1779. doi: 10.1097/MD.0000000000001779
3. Devanarayana NM, Rajindrajith S, Pathmeswaran A, Abegunasekara C, Gunawardena NK, Benninga MA. Epidemiology of irritable bowel syndrome in children and adolescents in Asia. J Pediatr Gastroenterol Nutr. (2015) 60:792–8. doi: 10.1097/MPG.0000000000000714
4. Wei X, Chen M, Wang J. The epidemiology of irritable bowel syndrome and functional constipation of Guangzhou residents. Zhonghua Nei Ke Za Zhi. (2001) 40:517–20.
5. Gao HX, Regier EE, Close KL. International diabetes federation world diabetes congress 2015. J Diabetes. (2016) 8:300–2. doi: 10.1111/1753-0407.12377
6. Gulcan E, Taser F, Toker A, Korkmaz U, Alcelik A. Increased frequency of prediabetes in patients with irritable bowel syndrome. Am J Med Sci. (2009) 338:116–9. doi: 10.1097/MAJ.0b013e31819f7587
7. Tao W-H. Correlation between irritable bowel syndrome and diabetes. World Chin J Dig. (2016) 24:1770–4. doi: 10.11569/wcjd.v24.i11.1770
8. Martinez C, Lobo B, Pigrau M, Ramos L, Gonzalez-Castro AM, Alonso C, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. (2013) 62:1160–8. doi: 10.1136/gutjnl-2012-30209
9. Bertiaux-Vandaele N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. (2011) 106:2165–73. doi: 10.1038/ajg.2011.257
10. Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. (1999) 78:849–55. doi: 10.1016/s0171-9335(99)80086-7
11. Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. (2014) 14:189. doi: 10.1186/s12876-014-0189-7
12. Coeffier M, Dechelotte P, Ducrotte P. Intestinal permeability in patients with diarrhea-predominant irritable bowel syndrome: is there a place for glutamine supplementation? Gastroenterology. (2015) 148:1079–80. doi: 10.1053/j.gastro.2015.02.057
13. Mujagic Z, Ludidi S, Keszthelyi D, Hesselink MA, Kruimel JW, Lenaerts K, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther. (2014) 40:288–97. doi: 10.1111/apt.12829
14. Shulman RJ, Jarrett ME, Cain KC, Broussard EK, Heitkemper MM. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol. (2014) 49:1467–76. doi: 10.1007/s00535-013-0919-6
15. Hirabara SM, Gorjao R, Vinolo MA, Rodrigues AC, Nachbar RT, Curi R. Molecular targets related to inflammation and insulin resistance and potential interventions. J Biomed Biotechnol. (2012) 2012:379024. doi: 10.1155/2012/379024
16. Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. (2008) 226:219–33. doi: 10.1111/j.1600-065X.2008.00711.x
17. Wang H, Shi P, Zuo L, Dong J, Zhao J, Liu Q, et al. Dietary non-digestible polysaccharides ameliorate intestinal epithelial barrier dysfunction in IL-10 knockout mice. J Crohns Colitis. (2016) 10:1076–86. doi: 10.1093/ecco-jcc/jjw065
18. Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J Neuroimmunol. (2016) 292:126–36. doi: 10.1016/j.jneuroim.2016.01.015
19. Hua MC, Lai MW, Kuo ML, Yao TC, Huang JL, Chen SM. Decreased interleukin-10 secretion by peripheral blood mononuclear cells in children with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. (2011) 52:376–81. doi: 10.1097/MPG.0b013e3181fd9816
20. Schmulson M, Pulido-London D, Rodriguez O, Morales-Rochlin N, Martinez-Garcia R, Gutierrez-Ruiz MC, et al. Lower serum IL-10 is an independent predictor of IBS among volunteers in Mexico. Am J Gastroenterol. (2012) 107:747–53. doi: 10.1038/ajg.2011.484
21. Acharya AB, Thakur S, Muddapur MV. Evaluation of serum interleukin-10 levels as a predictor of glycemic alteration in chronic periodontitis and type 2 diabetes mellitus. J Indian Soc Periodontol. (2015) 19:388–92. doi: 10.4103/0972-124X.150876
22. Wang SS, Wang XR, Yang RY, Xu Y, Li MY. [Efficacy and mechanism of acupuncture combined with tongxieyaofang for diarrhea-type irritable bowel syndrome of liver depression and spleen deficiency]. Zhongguo Zhen Jiu. (2020) 40:605–9. doi: 10.13703/j.0255-2930.20190818-k0004
23. Zhang J, Deng H, Bai J, Zhou X, Zhao Y, Zhu Y, et al. Health-promoting properties of barley: a review of nutrient and nutraceutical composition, functionality, bioprocessing, and health benefits. Crit Rev Food Sci Nutr. (2022) doi: 10.1080/10408398.2021.1972926
24. Li M, Bao X, Zhang X, Ren H, Cai S, Hu X, et al. Exploring the phytochemicals and inhibitory effects against α-glucosidase and dipeptidyl peptidase-IV in Chinese pickled chili pepper: insights into mechanisms by molecular docking analysis. LWT. (2022) 162:113467. doi: 10.1016/j.lwt.2022.113467
25. Aziz MNM, Kumar J, Muhammad Nawawi KN, Raja Ali RA, Mokhtar NM. Irritable bowel syndrome, depression, and neurodegeneration: a bidirectional communication from gut to Brain. Nutrients. (2021) 13:3061. doi: 10.3390/nu13093061
26. Vahora IS, Tsouklidis N, Kumar R, Soni R, Khan S. How serotonin level fluctuation affects the effectiveness of treatment in irritable bowel syndrome. Cureus. (2020) 12:e9871. doi: 10.7759/cureus.9871
27. Vincenzi A, Goettert MI, Volken de Souza CF. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-alpha) signaling and gene expression. Cytokine Growth Factor Rev. (2021) 57:27–38. doi: 10.1016/j.cytogfr.2020.10.004
28. Yang WL, Chen SY, Ho CY, Yen GC. Citrus flavonoids suppress IL-5 and ROS through distinct pathways in PMA/ionomycin-induced EL-4 cells. Food Funct. (2020) 11:824–33. doi: 10.1039/c9fo02815c
29. Cai H, Xu Y, Xie L, Duan Y, Zhou J, Liu J, et al. Investigation on spectrum-effect correlation between constituents absorbed into blood and bioactivities of baizhu shaoyao San before and after processing on ulcerative colitis rats by UHPLC/Q-TOF-MS/MS coupled with gray correlation analysis. Molecules. (2019) 24:940. doi: 10.3390/molecules24050940
30. Zhou Y, Tao H, Wang A, Zhong Z, Wu X, Wang M, et al. Chinese herb pair paeoniae radix alba and atractylodis macrocephalae rhizoma suppresses LPS-induced inflammatory response through inhibiting MAPK and NF-kappaB pathway. Chin Med. (2019) 14:2. doi: 10.1186/s13020-019-0224-2
31. Shang Z, Li M, Zhang W, Cai S, Hu X, Yi J. Analysis of phenolic compounds in pickled chayote and their effects on antioxidant activities and cell protection. Food Res Int. (2022) 157:111325. doi: 10.1016/j.foodres.2022.111325
32. Body-Malapel M, Djouina M, Waxin C, Langlois A, Gower-Rousseau C, Zerbib P, et al. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for inflammatory bowel diseases. Mucosal Immunol. (2019) 12:468–78. doi: 10.1038/s41385-018-0119-z
Keywords: TXYF, inflammation, D-IBS, T2DM, RAGE
Citation: Xu W, Zhang Z, Lu Y, Li M, Li J and Tao W (2022) Traditional Chinese medicine Tongxie Yaofang treating irritable bowel syndrome with diarrhea and type 2 diabetes mellitus in rats with liver-depression and spleen-deficiency: A preliminary study. Front. Nutr. 9:968930. doi: 10.3389/fnut.2022.968930
Received: 14 June 2022; Accepted: 10 October 2022;
Published: 10 November 2022.
Edited by:
Tian Ren, Shaanxi Normal University, ChinaReviewed by:
Zhi-ping Wang, Guangdong Pharmaceutical University, ChinaCopyright © 2022 Xu, Zhang, Lu, Li, Li and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhua Tao, dGFvd2hAdWpzLmVkdS5jb24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.