
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 29 September 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.967996
This article is part of the Research Topic Diet Behavior and Heart Health View all 21 articles
Introduction: People with sleep disorders are under disrupted biological rhythms. Whether changing the timing of specific food consumption contributes to decreasing cardiovascular and all-cause risk is unknown.
Methods: A total of 8,005 participants with sleep disorders were selected from the U.S. National Health and Nutrition Examination Survey (NHANES) from 2005 to 2014. Cox proportional hazards regression models were used to analyze the relationship between the consumption time of foods and cardiovascular disease (CVD) and all-cause death. Moreover, equivalent food substitution models were carried out to evaluate the alterations in the risk of CVD mortality for the changed food intake time.
Results: After adjusting for multiple confounders, participants who consume red and orange vegetables, starchy vegetables, and fermented dairy in the morning (hazard ratio (HR)red and orange vegetables = 0.45, 95% CI: 0.26–0.81; HRstarchy vegetables = 0.47, 95% CI: 0.25–0.88; HRfermented dairy = 0.57, 95% CI: 0.36–0.89) and milk and eggs in the evening contribute to reducing the likelihood of death from CVD (HRmilk = 0.65, 95% CI: 0.43–0.96; HReggs = 0.72, 95% CI: 0.53–0.98). Iso-calorically switching 0.1 serving of starchy vegetable and fermented dairy and milk intake from one period to another does significantly reduce the mortality risk of CVD.
Conclusion: Higher intake of red and orange vegetables, starchy vegetables, and fermented dairy in the morning and milk and eggs in the evening confers a lower risk of CVD among individuals with sleep disorders.
Sleep disorders have become a new public health burden worldwide. Epidemiological surveys show that people with insufficient sleep alone account for one-third of the total adults in the USA (1, 2). Sleep disorder has been shown to be associated with multiple chronic diseases (3, 4), among which cardiovascular disease (CVD), as the leading cause of death worldwide, accounts for about one-third of global deaths (5, 6). The mechanism between sleep disorders and CVD could be explained by the relationships between sleep, circadian disruption, and metabolic physiology (7), which means that the disordered circadian rhythm of inflammation, oxidative stress, and sympathetic activity induced by sleep disorders further contribute to endothelial dysfunction and metabolic disturbances (8, 9). However, the circadian rhythm disorder caused by sleep disorders could be improved by accepted modifiable behaviors, among which adjusting the dietary factor is the most economical and convenient way (10–13).
However, most studies related to dietary interventions focus on the quantity or quality of dietary factors (14, 15). As an emerging field of nutritional research, chrono-nutrition aims to emphasize the importance of dietary intake time for health (16). It is well known that circadian rhythms could regulate a variety of physiological activities, such as food intake, which in turn feedback regulates nutrient absorption, distribution, metabolism, and excretion by driving peripheral circadian clocks (16). In short, the concept of “chrono-nutrition” is mainly derived from the abovementioned coordination between food intake time and the circadian body rhythm, which is broadly covers in three parts: (1) the distribution of energy intake in a day; (2) the frequency of food intake per day; and (3) the timing of food intake (17). Several studies published recently successively demonstrated the close association between the fields of chrono-nutrition and health (18–21). Circadian rhythms have a strong association between sleep and food intake time, but few studies investigated whether and how the timing of food consumption affects CVD in people with sleep disturbances. Therefore, our study was conducted to examine the association between the intake time of different foods and the mortality of all-cause and CVD among individuals with sleep disorders, aiming to provide practical intervention strategies for the prevention and treatment of CVD.
The National Health and Nutrition Examination Survey (NHANES) is a stratified, multistage study conducted by the U.S. National Center for Health Statistics (NCHS). The researcher used professional interviews and examinations to collect nationally representative data of American civilians. Adults with a sleep duration of less than 6 h, self-reporting sleep trouble, or diagnosed with a sleep disorder by a doctor-were classified as having a sleep disorder (22, 23). After excluding the participants who had missed dietary intake and/or mortality variables, a total of 8,005 participants with sleep disorders (3,636 men and 4,370 women) were selected. The NHANES data obtained the approval of the NCHS Institutional Review Board and the informed consent of the participants and could be available through https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
The dietary food intake of each participant was assessed over 2 non-consecutive days through 24-h diet recalls. Dietary foods and energy intake were estimated based on the guidelines of the U.S. Department of Agriculture’s Dietary Research Food and Nutrition Database. According to the user guide of the MPED2.0 of the U.S. Department of Agriculture Survey Foods, 15 different food groups were selected and used for further analysis. A sleep questionnaire was used to assess the sleep status of each participant. Based on the time point of food intake, these meals are classified as breakfast, lunch, dinner, and snacks.
The food groups included in this study were mainly whole grains, refined grains, dark green vegetables, red and orange vegetables, starchy vegetables, total fruit, milk, fermented dairy, eggs, red meat, poultry, cured meat, seafood, soybean products, and legumes. The exposure variables were set as food intakes in the morning, afternoon, and evening.
The main outcome variable was the mortality of CVD and all causes, which was determined by the National Death Index (NDI). As a highly reliable resource, NDI is widely used for death identification. The International Classification of Diseases 10th Revision (ICD-10) is used to determine disease-specific death, among which ICD-10 codes, such as I00–I09, I11, I13, I20–I51, or I60–I69 are defined as CVD mortality. A total of 658 deaths, including 189 deaths due to CVD, were used for further analysis.
Potential covariates are as follows: age (years), sex (men/women), race/ethnicity (non-Hispanic white/non-Hispanic black/Mexican American/other), annual household income level (less than $20,000, between $20,000 and $45,000, between $45,000 and $75,000, between $75,000 and $100,000, or over $100,000), educational level (below 9th grade, between 9th and 11th grade, finish high school, finish GED or equivalent, finish college or Associate in Arts degree, or finish college graduate or above), regular exercise (yes/no), cigarette and alcohol use (yes/no), body mass index (BMI) (kg/m2), disease history of diabetes, hypertension, and dyslipidemia (yes/no), covered by health insurance (yes/no), total energy intake (kcal/day), total fat intake (g/day), total carbohydrate intake (g/day), total protein intake (g/day), whole grains (ounce equivalents), refined grains (ounce equivalents), dark green vegetables (cup equivalents), red and orange vegetables (cup equivalents), starchy vegetables (cup equivalents), fruit (cup equivalents), milk (cup equivalents), fermented dairy (cup equivalents), eggs (ounce equivalents), red meat (ounce equivalents), poultry (ounce equivalents), cured meat (ounce equivalents), seafood (ounce equivalents), soybean products (ounce equivalents), legumes (ounce equivalents), and the Alternative Healthy Eating Index (AHEI).
The intake of food groups in the morning, afternoon, and evening were divided into two (whether or not to eat) or three (base on the distribution) parts according to their distribution. For social demographics, lifestyle and eating behavior, and anthropometric indicators, categorical variables were represented by percentages, and continuous variables were represented by median (P25, P75). The baseline characteristics were compared by the Mann–Whitney U-test and the Chi-square test. R 4.0.2 was used to conduct all statistical analyses, and a two-sided p-value < 0.05 was considered to be statistically significant. The median was used to replace missing values for covariates with less than 5% missing values.
Cox proportional hazards models were established to evaluate the relationship between food intake across a day and CVD and all-cause mortality. Confounding factors were adjusted in all models, such as age, gender, race, BMI, drinking, smoking, exercise, income, education, total energy intake, total fat intake, total carbohydrate intake, total protein intake, AHEI, health insurance coverage, disease history of diabetes, hypertension, and dyslipidemia. In addition, the total intake of every food group in a 24-h period was adjusted in the model.
An equivalent food substitution model was carried out to evaluate the alterations in the risk of CVD mortality for the changed food intake time. A substitution analysis is mainly conducted by converting food intake time points and keeping the total energy and other food intake constant.
In set 1, sensitivity analyses were performed to assess the relationship between total red and orange vegetable, starchy vegetable, milk, fermented dairy, and egg intake in a whole day and CVD and all-cause mortality to check whether the intake time could provide more information. In set 2, sensitivity analyses were performed among individuals with sound sleep to evaluate the impact of sleep status on the results. In set 3, AHEI was additionally adjusted to evaluate the impact of dietary quality on the results. In set 4, a sensitivity analysis was conducted to evaluate whether sex, diabetes, hypertension, and dyslipidemia could affect the relationship between food intake time and CVD and all-cause risk.
The demographic and nutrition characteristics on the basis of the disease status of CVD mortality are presented in Table 1. Compared with other participants, the participants who died due to CVD were more likely to be men, less likely to be non-Hispanic white, had a higher age, the prevalence of dyslipidemia, hypertension, and diabetes, and a lower percentage of health insurance coverage, exercise, education, income, total energy, fat, protein, carbohydrate intake, and AHEI (P < 0.05).
The association of red and orange vegetable, starchy vegetable, milk, fermented dairy, and egg intake consumed in the morning and evening with all-cause and CVD mortality is presented in Table 2 among participants with sleep disorders. In addition, the association of other dietary foods consumed in the morning and evening with CVD and all-cause mortality is presented in Supplementary Table 1. The results indicated that participants who eat red and orange vegetables, starchy vegetables, and fermented dairy in the morning had lower CVD mortality, compared with participants who skip red and orange vegetables, starchy vegetables, and fermented dairy in the morning, as indicated by hazard ratio (HR) and 95% CI (HRred and orange vegetables = 0.46, 95% CI: 0.26–0.82; HRstarchy vegetables = 0.47, 95% CI: 0.25–0.88; and HRfermented dairy = 0.57, 95% CI: 0.36–0.90). Furthermore, compared with participants in the highest quintile of milk intake in the evening, those in the lowest quintile were more likely to die due to CVD, as indicated by HR and 95% CI (HRmilk = 0.65, 95% CI: 0.43–0.96). Similarly, the consumption of eggs in the evening also confers a lower risk of CVD (HReggs = 0.72, 95% CI: 0.53–0.98). Moreover, participants who consumed red and orange vegetables in the morning and fermented dairy in the evening had a lower association with all-cause mortality (HRred and orange vegetables = 0.75, 95% CI: 0.58–0.97; and HRfermented dairy = 0.77, 95% CI: 0.64–0.92).

Table 2. Multivariate adjusted hazard ratios (HRs) of the dietary red and orange vegetables, starchy vegetables, milk, fermented dairy, and eggs intake in the morning and the evening and CVD-mortality and all-cause mortality among participants with sleep disorders.
Figure 1 shows that the mortality risk of CVD in the predicted equivalent substitution models through switching food intake from one period to another among participants with sleep disorders. Figure 1 shows that the HR of CVD mortality decreased by 3% (HR = 0.97, 95% CI: 0.94–0.99) in models with 0.1 serving of starchy vegetables in the evening being equivalently switched to morning. Similarly, the results indicated that HRs for CVD decreased by 12 or 9% (HR = 0.90, 95% CI: 0.83–0.97; and HR = 0.92, 95% CI: 0.85–0.99) in models with 0.1 serving of fermented dairy in the afternoon or evening being equivalently switched to the morning. In addition, it can be concluded that HRs for CVD decreased by 4% (HR = 0.97, 95% CI: 0.94–0.99) in models with 0.1 serving of milk intake in the afternoon being equivalently switched to the evening.
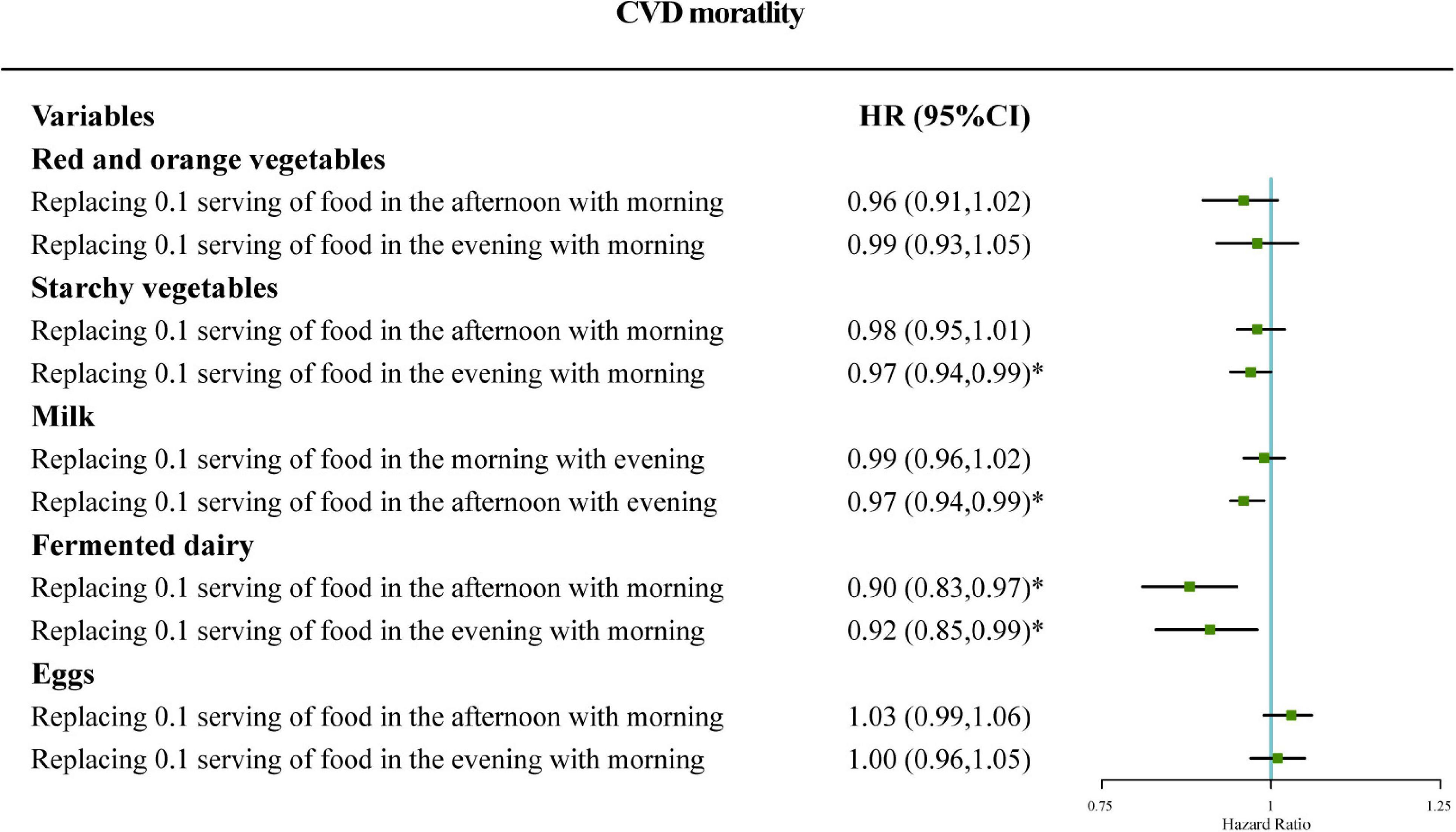
Figure 1. Adjusted hazard ratio (HR) for cardiovascular disease (CVD) mortality: iso-caloric substitution of red and orange vegetables, starchy vegetables, and fermented dairy consumed in the afternoon or the evening to morning and iso-caloric substitution of milk and eggs in the morning or the afternoon to the evening. Adjustments included age, gender, race, BMI, drinking, smoking, exercise, income, education, total energy intake, total fat intake, total carbohydrate intake, total protein intake, AHEI, health insurance coverage, disease history of diabetes, disease history of hypertension, disease history of dyslipidemia, and total intake of specific food group in the 24 h period. Case/N, number of case participants/total; Q, quarter; AHEI, Alternative Healthy Eating Index.
The result of the first sensitivity analysis showed that the total intake of red and orange vegetables, starchy vegetables, milk, fermented dairy, and eggs was not associated with CVD and all-cause mortality, which suggested that the analysis of the dietary intake time could provide more information than the total daily food intake only (Supplementary Table 2). The second set of the sensitivity analysis demonstrated that the association between intake of fermented dairy in the morning and milk and eggs in the evening with CVD mortality disappeared among participants with normal sleep (Supplementary Table 3), which means that the intake time of the above foods may play a role in reducing CVD death by improving the adverse effects of sleep or sleep deprivation among participants with sleep disorders. The third set of the sensitivity analysis indicated that, after additionally adjusting dietary quality, the relationship between the consumption time of the above foods and CVD was still significant (Supplementary Table 4). The fourth set of the sensitivity analysis showed that sex, diabetes, hypertension, and dyslipidemia could not affect the relationship between the consumption time of the above food items and CVD (Supplementary Tables 5–7).
Our study showed that people with sleep disorders who have red and orange vegetables, starchy vegetables, and fermented dairy consumption in the morning and milk and eggs in the evening seem to have decreased CVD mortality. In addition, while keeping food quality and quantity constant, switching 0.1 serving of milk intake from afternoon to morning, switching 0.1 serving of fermented dairy intake from afternoon or evening to morning, or switching 0.1 serving of starchy vegetables intake from evening to morning confer a lower risk of CVD.
Most studies focused on investigating the association between quantity and quality of different foods or dietary patterns and CVD (14, 15). However, as we all know, this was the first study to explore the association of the consumption time of dietary foods with CVD mortality among individuals with sleep disorders. Abundant studies illustrated that sleep disorders could activate inflammatory gene expression and the production of proinflammatory cytokines in the morning by increasing sympathetic nerve activity at midnight, which gradually decreases under normal circumstances, and exacerbate impairment on vascular reactivity in the morning (24–27). Based on the above evidence, attenuating the inflammatory response may be a way for reducing adverse effects caused by sleep disorders in the morning. As a type of food rich in anti-inflammatory phytochemicals, such as lycopene and carotenoids, red orange vegetables have been found to reduce oxidative stress and inflammation by influencing inflammatory markers and their downstream targets (28–30). This indicated that the benefits of a higher intake of red orange vegetables in the morning may be explained by the anti-inflammatory effects of phytochemicals, which suggests that we should pay more attention to increase its intake in the morning rather than other foods in the case of sleep disorder.
In addition to attenuating inflammatory effects, the interaction of sleep disorders and metabolic disorders is a basis of CVD (31, 32), which should be paid more attention. First, sleep disorders have been shown to increase the total amount and time of energy intake (33), which manifests as a decrease in energy intake at breakfast and an increase in energy intake at dinner, with fat and carbohydrate accounting for a large proportion of energy source at dinner (34, 35). However, evidence indicated that carbohydrate metabolism have an internal biological rhythm, which is highest in the morning and gradually decreases in the evening (36). It means that the right time of carbohydrates consumption should be in the morning rather than in the evening, which is the same as our result. In addition, another study demonstrated that higher energy intake at breakfast with lower intake at dinner have beneficial effects on metabolic control (37). Similarly, several random control trails showed that the carbohydrate-rich breakfast and fermented dairy could decrease food intake after lunch or later by increasing satiety and decreasing hunger rating (38–40), which supports the beneficial effect of high intakes of starchy vegetables and fermented dairy in the morning. The mechanism of the benefit of eating eggs in the evening in our results is still opaque. As a food source of high-quality protein, eggs have been shown to increase satiety and decrease food intake later (41). In addition, consuming eggs in the evening has been proven to improve glycometabolism, compared with other high-carbohydrate protein-matched sources (42). It demonstrated that eggs may be a suitable energy source in the evening and may help to improve metabolic disorders by reducing excessive energy intake in the evening.
Studies showed that most hormones regulate metabolism and energy balance through own circadian rhythms (43). Circadian rhythm disturbance caused by a sleep disorder has a great impact on the role of hormones. First of all, under normal circumstances, insulin sensitivity, and insulin secretion will decrease at night and increase in the morning (44). Therefore, people with insulin rhythm disturbances caused by sleep disorders should adapt to their normal rhythmic activity at an appropriate time, which is consistent with our result that starchy vegetables should be eaten in the morning (45). In addition, the nocturnal secretion of the appetite-related hormone leptin is reduced due to sleep disorders, which leads to an increase in total energy intake to energy imbalance (46). Consistent with the above statement, the energy imbalance is mainly manifested in the increased intake of high-carbohydrate and high-fat foods at night (35). However, as a decomposition hormone, cortisol itself has a low secretion level at night (44), and the dual effects of increased nighttime energy intake and decreased catabolism lead to increased susceptibility to metabolic disturbances. Therefore, it is recommended to consume some satiety foods, such as eggs at night to restrict energy intake. Melatonin, an important hormone that maintains the biological clock and regulates body rhythms (47), is also affected by sleep disorders, resulting in decreased secretion at night (44). Therefore, drinking milk rich in tryptophan at night helps to provide precursors for the synthesis of melatonin and improves sleep.
In addition to attenuating inflammatory effects and metabolic disorders, improving sleep may be another way to affect cardiovascular health. Our results found that the intake of milk and eggs in the evening helps to reduce CVD mortality. As the precursor of serotonin and melatonin, milk-rich tryptophan has been shown to play a major role in sleep/wake and cortical activity and contributes to promoting sleep (48–50). These studies provided the potential mechanism and evidence for the association of higher milk consumption in the evening and lower mortality of CVD in this study. Moreover, taking egg protein hydrolyzate before sleep also has been shown to improve sleep quality (51, 52), which may be another reason to reduce adverse effects of sleep disorder after eating eggs in the evening.
Moreover, the association between foods consumption time and CVD mortality was improved but not statistically protective after switching red and orange vegetable and egg intake time points, which may also be explained by lower energy intake in the morning caused by the excessive single red and orange vegetable intake and the high energy intake in the evening caused by excessive egg intake in the food substitution analysis. Therefore, it is suggested that we should consider the dietary quality, quantity, and time of food intake in all direction so as to avoid the nutritional imbalance caused by excessive attention to one part, which results in adverse consequences.
Based on our results above, we put forward the following suggestions for people with sleep disorders on the basis of keeping energy distribution balanced and reasonable in all three meals. Carbohydrates are best eaten in the forenoon, and in the selection of high-carbohydrate foods types, it is highly recommended to choose high-fiber-carbohydrate foods, such as starchy vegetables rather than high-energy carbohydrate-type foods, such as refined grains. High fiber-carbohydrate foods combined with red and orange vegetables rich in lycopene and carotenoids for breakfast provide energy in a nutritionally balanced manner and are more beneficial to combat the increased levels of oxidative stress and inflammation caused by sleep disorders. Fermented dairy products should be selected for forenoon snacks, which not only have higher nutritional value but also have a satiety effect to reduce subsequent energy intake. For dinner, a moderate amount of high-quality protein meals, such as eggs, should be used to replenish energy and increase satiety after meals. While reducing the adverse effects of excessive energy intake on sleep, a moderate intake of tryptophan-rich milk can also help improve sleep.
The advantages and limitations of our study are presented in the following. First, it is the first time to explore the relationship between the consumption time of foods and CVD mortality among individuals with sleep disorders. Then, consistent results were observed after adjusting lots of important confounding factors and conducting multiple sensitivity analyses in the process of research. Besides, the NHANES provides the nationally representative data in the USA, such as dietary intake and lifestyle factors. In addition, our research has certain limitations. First, the dietary data were only obtained through the 24-h diet recall on 2 days within 2 weeks, which cannot reflect long-term exposure. Second, the status of sleep condition was assessed by a single sleep disorder questionnaire, which is highly subjective and less accurate, compared with a real-time sleep monitoring equipment. In addition, other factors related to sleep disorders that might not be aware of or measured can also bring about bias.
There are some important implications in our findings. People with sleep disorders are under a disrupted biological rhythm, which indicates that ill-rested people should pay more attention to eating habits due to their worse health condition. Accumulating evidence has suggested that food consumption time is no less important than quantity and quality for maintaining health. Based on the above results, we believe that the specific food consumption time play an important role in changing the risk of CVD death among individuals with sleep disorders. Therefore, adjusting the intake time of different food groups is of importance in the prevention and treatment of CVD and should be integrated into the nutritional recommendation.
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
The studies involving human participants were reviewed and approved by the NCHS Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
YL, TH, and WW designed the research and had primary responsibility for final manuscript. JZ and YZ performed all statistical analyses and wrote the manuscript. LL, XW, and XX conducted the data review. All authors contributed to conduct literature research and providing corresponding suggestion, and read and approved the final manuscript.
This research was supported by funds from the National Natural Science Foundation of China (82030100 to YL).
We thank the contributions from participants and staff of the National Health and Nutrition Examination Survey 2005–2014.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.967996/full#supplementary-material
1. Zee PC, Badr MS, Kushida C, Mullington JM, Pack AI, Parthasarathy S, et al. Strategic opportunities in sleep and circadian research: report of the joint task force of the sleep research society and American academy of sleep medicine. Sleep. (2014). 37:219–27. doi: 10.5665/sleep.3384
2. Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults–United States, 2014. MMWR Morb Mortal Wkly Rep. (2016) 65:137–41. doi: 10.15585/mmwr.mm6506a1
3. Wallace DM, Ramos AR, Rundek T. Sleep disorders and stroke. Int J Stroke. (2012) 7:231–42. doi: 10.1111/j.1747-4949.2011.00760.x
4. Silvani A. Sleep disorders, nocturnal blood pressure, and cardiovascular risk: a translational perspective. Auton Neurosci. (2019) 218:31–42. doi: 10.1016/j.autneu.2019.02.006
5. DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1603–58. doi: 10.1016/S0140-6736(16)31460-X
6. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1459–544. doi: 10.1016/S0140-6736(16)31012-1
7. McHill AW, Wright KP Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes Rev. (2017) 18(Suppl. 1):15–24. doi: 10.1111/obr.12503
8. Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U.S.A. (2013) 110:E1132–41. doi: 10.1073/pnas.1217154110
9. Chellappa SL, Vujovic N, Williams JS, Scheer F. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. (2019) 30:767–79. doi: 10.1016/j.tem.2019.07.008
10. Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. (2012) 485:62–8. doi: 10.1038/nature11030
11. Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. (1989) 244:1328–33. doi: 10.1126/science.2734611
12. Barger LK, Wright KP Jr, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am J Physiol Regul Integr Comp Physiol. (2004) 286:R1077–84. doi: 10.1152/ajpregu.00397.2003
13. Gill S, Panda SA. Smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. (2015) 22:789–98. doi: 10.1016/j.cmet.2015.09.005
14. Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, et al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. (2019) 321:1081–95. doi: 10.1001/jama.2019.1572
15. Petersen KS, Kris-Etherton PM. Diet quality assessment and the relationship between diet quality and cardiovascular disease risk. Nutrients. (2021) 13:4305. doi: 10.3390/nu13124305
16. Almoosawi S, Vingeliene S, Karagounis LG, Pot GK. Chrono-nutrition: a review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc Nutr Soc. (2016) 75:487–500. doi: 10.1017/S0029665116000306
17. Almoosawi S, Vingeliene S, Gachon F, Voortman T, Palla L, Johnston JD, et al. Chronotype: implications for epidemiologic studies on chrono-nutrition and cardiometabolic health. Adv Nutr. (2019) 10:30–42. doi: 10.1093/advances/nmy070
18. Morgan LM, Shi JW, Hampton SM, Frost G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br J Nutr. (2012) 108:1286–91. doi: 10.1017/S0007114511006507
19. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. (2018) 27:1212–21.e13. doi: 10.1016/j.cmet.2018.04.010
20. Han T, Gao J, Wang L, Li C, Qi L, Sun C, et al. The association of energy and macronutrient intake at dinner versus breakfast with disease-specific and all-cause mortality among people with diabetes: the U.S. national health and nutrition examination survey, 2003-2014. Diabetes Care. (2020) 43:1442–8. doi: 10.2337/dc19-2289
21. Wei W, Jiang W, Huang J, Xu J, Wang X, Jiang X, et al. Association of meal and snack patterns with mortality of all-cause, cardiovascular disease, and cancer: the us national health and nutrition examination survey, 2003 to 2014. J Am Heart Assoc. (2021) 10:e020254. doi: 10.1161/JAHA.120.020254
22. Li W, Ruan W, Peng Y, Lu Z, Wang D. Associations of socioeconomic status and sleep disorder with depression among US adults. J Affect Disord. (2021) 295:21–7. doi: 10.1016/j.jad.2021.08.009
23. Inoue K, Semba E, Yamakawa T, Terauchi Y. Associations of impaired glucose tolerance and sleep disorders with mortality among the US general population. BMJ Open Diabetes Res Care. (2021) 9:e002047. doi: 10.1136/bmjdrc-2020-002047
24. Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. (2019) 99:1325–80. doi: 10.1152/physrev.00010.2018
25. Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Souza SB, Irigoyen MC, et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol. (2012) 113:232–6. doi: 10.1152/japplphysiol.01604.2011
26. Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. (2006) 166:1756–62. doi: 10.1001/archinte.166.16.1756
27. Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. (2008) 64:538–40. doi: 10.1016/j.biopsych.2008.05.004
28. Maiani G, Caston MJ, Catasta G, Toti E, Cambrodon IG, Bysted A, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. (2009) 53(Suppl. 2):S194–218. doi: 10.1002/mnfr.200800053
29. Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. (2014) 34:907–29. doi: 10.1016/j.nutres.2014.07.010
30. Saini RK, Rengasamy KRR, Mahomoodally FM, Keum YS. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: an update on epidemiological and mechanistic perspectives. Pharmacol Res. (2020) 155:104730. doi: 10.1016/j.phrs.2020.104730
31. Ribeiro DC, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J Endocrinol. (1998) 158:305–10. doi: 10.1677/joe.0.1580305
32. Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. (2014) 24:90–9. doi: 10.1016/j.tcb.2013.07.002
33. Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovas JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. (2015) 6:648–59. doi: 10.3945/an.115.008623
34. Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. (2013) 36:981–90. doi: 10.5665/sleep.2792
35. Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U.S.A. (2013) 110:5695–700. doi: 10.1073/pnas.1216951110
36. Service FJ, Hall LD, Westland RE, O’Brien PC, Go VL, Haymond MW, et al. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia. (1983) 25:316–21. doi: 10.1007/BF00253193
37. Fuse Y, Hirao A, Kuroda H, Otsuka M, Tahara Y, Shibata S. Differential roles of breakfast only (one meal per day) and a bigger breakfast with a small dinner (two meals per day) in mice fed a high-fat diet with regard to induced obesity and lipid metabolism. J Circadian Rhythms. (2012) 10:4. doi: 10.1186/1740-3391-10-4
38. Chowdhury EA, Richardson JD, Tsintzas K, Thompson D, Betts JA. Carbohydrate-rich breakfast attenuates glycaemic, insulinaemic and ghrelin response to ad libitum lunch relative to morning fasting in lean adults. Br J Nutr. (2015) 114:98–107. doi: 10.1017/S0007114515001506
39. Dougkas A, Minihane AM, Givens DI, Reynolds CK, Yaqoob P. Differential effects of dairy snacks on appetite, but not overall energy intake. Br J Nutr. (2012) 108:2274–85. doi: 10.1017/S0007114512000323
40. Hull S, Re R, Tiihonen K, Viscione L, Wickham M. Consuming polydextrose in a mid-morning snack increases acute satiety measurements and reduces subsequent energy intake at lunch in healthy human subjects. Appetite. (2012) 59:706–12. doi: 10.1016/j.appet.2012.08.004
41. Lee JJ, Brett NR, Chang JT, de Zepetnek JOT, Bellissimo N. Effects of white potatoes consumed with eggs on satiety, food intake, and glycemic response in children and adolescents. J Am Coll Nutr. (2020) 39:147–54. doi: 10.1080/07315724.2019.1620659
42. Abbie E, Francois ME, Chang CR, Barry JC, Little JP. A low-carbohydrate protein-rich bedtime snack to control fasting and nocturnal glucose in type 2 diabetes: a randomized trial. Clin Nutr. (2020) 39:3601–6. doi: 10.1016/j.clnu.2020.03.008
43. Serin Y, Acar Tek N. Effect of circadian rhythm on metabolic processes and the regulation of energy balance. Ann Nutr Metab. (2019) 74:322–30. doi: 10.1159/000500071
44. Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, et al. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes. (1984) 33:1150–3. doi: 10.2337/diab.33.12.1150
45. Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. (2019) 15:75–89. doi: 10.1038/s41574-018-0122-1
46. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. (2004) 1:e62. doi: 10.1371/journal.pmed.0010062
47. Cipolla-Neto J, Amaral FGD. Melatonin as a hormone: new physiological and clinical insights. Endocr Rev. (2018) 39:990–1028. doi: 10.1210/er.2018-00084
48. Nongonierma AB, FitzGerald RJ. Milk proteins as a source of tryptophan-containing bioactive peptides. Food Funct. (2015) 6:2115–27. doi: 10.1039/c5fo00407a
49. Valtonen M, Niskanen L, Kangas AP, Koskinen T. Effect of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord J Psychiatry. (2005) 59:217–21. doi: 10.1080/08039480510023034
50. Kaluzna-Czaplinska J, Gatarek P, Chirumbolo S, Chartrand MS, Bjorklund G. How important is tryptophan in human health? Crit Rev Food Sci Nutr. (2019) 59:72–88. doi: 10.1080/10408398.2017.1357534
51. Mohajeri MH, Wittwer J, Vargas K, Hogan E, Holmes A, Rogers PJ, et al. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br J Nutr. (2015) 113:350–65. doi: 10.1017/S0007114514003754
Keywords: sleep disorder, chrono-nutrition, cardiovascular diseases, mortality, NHANES
Citation: Zhang J, Zhang Y, Liu L, Wang X, Xu X, Li Y, Han T and Wei W (2022) Associations between the timing of different foods’ consumption with cardiovascular disease and all-cause mortality among adults with sleep disorders. Front. Nutr. 9:967996. doi: 10.3389/fnut.2022.967996
Received: 13 June 2022; Accepted: 12 September 2022;
Published: 29 September 2022.
Edited by:
Hongtao Tie, The First Affiliated Hospital of Chongqing Medical University, ChinaReviewed by:
Quanman Li, Zhengzhou University, ChinaCopyright © 2022 Zhang, Zhang, Liu, Wang, Xu, Li, Han and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Li, bGl5aW5nX2hlbGVuQDE2My5jb20=; Tianshu Han, c25vd2NhbGVuZGFyQDEyNi5jb20=; Wei Wei, d2Vpd2VpYnViYmxlMTk5NEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.