- 1Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 3Department of Cardiovascular Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: Omega-3 and omega-6 may be protective factors for cholelithiasis. However, this relationship has not yet been demonstrated clearly. Therefore, we attempted to identify these causal relationships.
Materials and methods: The omega-3/6 fatty acid discovery dataset was obtained from UK Biobank and contained 114,999 individuals. The validation set was derived from an independent genome-wide association study (GWAS) and contained 13,544 individuals. The cholelithiasis dataset was derived from FinnGen and contained 19,023 cases and 195,144 controls. The inverse variance weighting (IVW) method was used as the main method of analysis in this study. Multiple methods of analysis were also used in the repeated methods, including the MR-Egger, weighted median, MR-pleiotropic residual sum (MR-PRESSO), outliers, and maximum likelihood methods. In addition, we used multiple sensitivity analyses to identify the potential pleiotropy.
Result: In the discovery stage, the results of the random effect IVW analysis showed that higher omega-3 levels were correlated inversely with the risk of cholelithiasis (β = –0.22, 95% CI [–0.32 to –0.12], P = 1.49 × 10–5). When the replication analysis was performed using another set of instrumental variables (IVs), the causal relationship between omega-3 fatty acids and cholelithiasis remained stable (β = –0.42, 95% CI [–0.66 to –0.18], P = 5.49 × 10–4), except for the results obtained using the MR-Egger method, which were not significant. The results of the IVW approach showed that each SD increase in omega-6 levels was associated negatively with the risk of cholelithiasis, both in the discovery (β = –0.21, 95% CI [–0.35 to –0.06], P = 4.37 × 10–3) and the validation phases (β = –0.21, 95% CI [–0.40 to –0.02], P = 3.44 × 10–2).
Conclusion: The results of our MR study suggest that omega-3/6 is associated with cholelithiasis risk. Attention to the risk of cholelithiasis in individuals with low serum omega-3/6 levels is necessary.
Introduction
Cholelithiasis is an increasingly common hepatobiliary disease. Approximately 10–20% of adults have had cholelithiasis (1, 2). In addition to biliary malignancy, cholelithiasis is associated strongly with small intestinal, prostate, and kidney cancers (3). This is a public health concern on which greater emphasis should be placed.
In general, cholelithiasis can be classified as cholesterol and pigment gallstones according to the composition, with cholesterol gallstones accounting for approximately 80–90% of all the gallstones in most western countries (1, 4). Hepatic cholesterol hypersecretion, supersaturated bile juice, and gallbladder hypomotility contribute to the pathophysiology of cholesterol gallstones. These factors work collaboratively and cause the failure of biliary cholesterol solubility homeostasis, which subsequently results in cholesterol crystallization in bile juice and eventually biliary stone formation (1).
Among the polyunsaturated fatty acids (PUFAs), omega-3 (ω-3) and omega-6 (ω-6) are the two main families that have been shown to be relevant to human health (5, 6). In animal studies, it has been confirmed that high intake of PUFAs can decrease the risk of cholelithiasis by reducing the cholesterol saturation index (CSI) and suppressing the production of gallbladder mucin which is regarded as a trigger for gallstone formation (7, 8). Furthermore, it was reported that PUFAs combined with ursodeoxycholic acid can dissolve cholesterol stones in mice (9). However, the beneficial effects of PUFAs in humans remain debatable. While a prospective cohort study linked high intakes to a reduced prevalence of cholelithiasis in men (4), an epidemiologic study demonstrated that PUFA intake had no effect on cholelithiasis development (10).
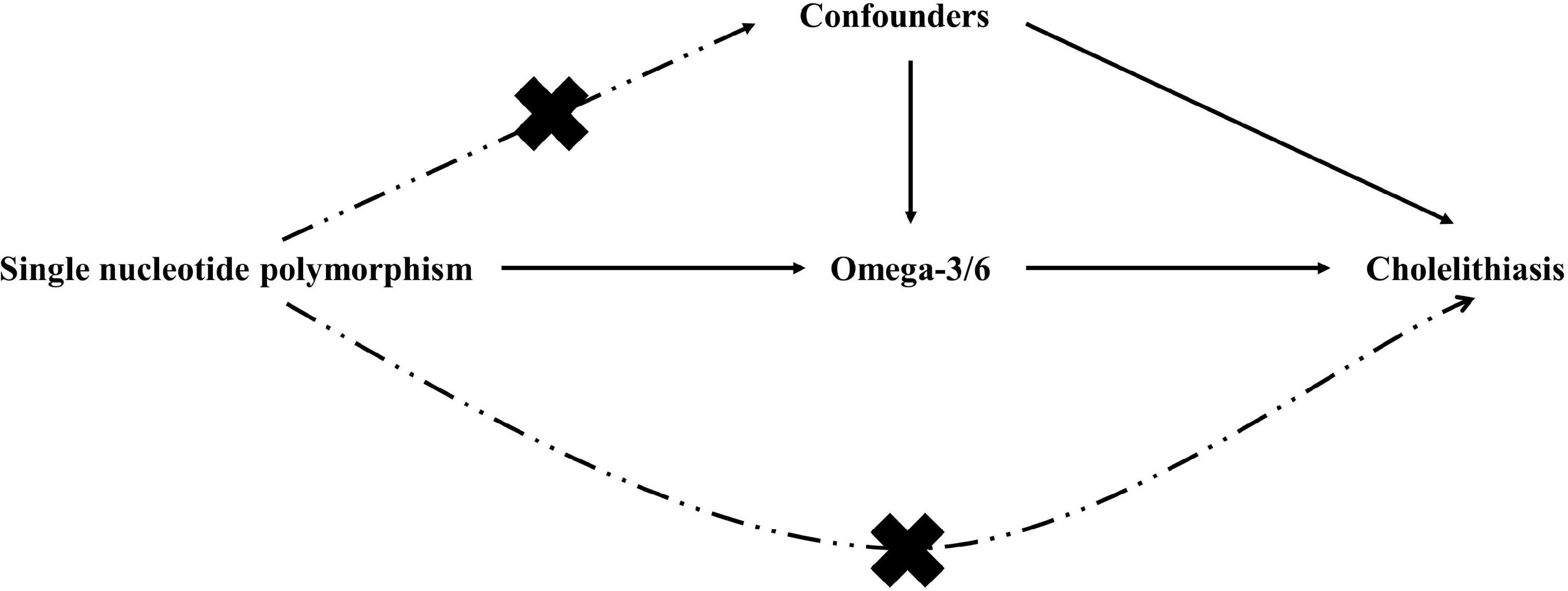
Therefore, it is necessary to understand the causal relationship between PUFAs and cholelithiasis. Mendelian randomisation (MR) is an emerging epidemiological method that uses genetic variation as an instrumental variable (IV) to assess the causal association between exposure and outcome (11). Genetic variation is passed randomly to offspring during meiosis, and thus, its estimates of causal effects are consistent with the time order in which they should be. More importantly, the use of MR minimizes the interference of confounding variables between exposure and outcome by avoiding confounding factors to the greatest extent possible (12). Therefore, to examine the potential causal relationship between PUFAs and cholelithiasis, we performed an MR analysis of two samples using summary-level genome-wide association study (GWAS) data and validated them using additional datasets.
Materials and methods
Study design
Similar to most MR analyses, our study rested on the following three assumptions: genetic variation is linked strongly to exposure, genetic variants should not be considered confounders, and genetic variants should be related to outcomes only via exposure (13). We used two exposure datasets from different sources for the analysis: the discovery and validation sets. Figures 1, 2 show the overview of the study’s design. Ethical review approval and informed consent were obtained for the original study.
Selection of instrumental variables
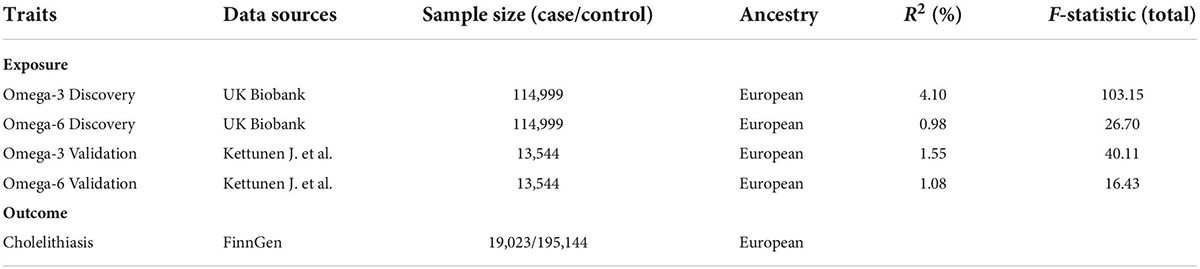
The genetic instrumental variables for ω-3/6 were derived from the UK Biobank (UKB) and included 114,999 participants (Table 1). The data were adjusted for age, age squared, and sex. Since this GWAS study included more participants and analyzed more single nucleotide polymorphisms (SNPs), it was used as the discovery set. We screened for SNPs with genome-wide significance (P < 5 × 10–8). Subsequently, to ensure that the SNPs were valid and independent, we removed the linkage disequilibrium (LD) between the SNPs at r2 < 0.001. Furthermore, the secondary phenotype of each SNP was retrieved to ensure that it was not associated with cholelithiasis. The F-statistic was performed to assess the strength of the IVs. The threshold of the F statistic > 10 indicated a relatively strong estimated effect of IVs (14).
Another group of ω-3/6 IVs was derived from a GWAS containing 13,544 European participants (15). The screening criteria were the same as those described previously. This set of IVs was used as the validation set.
Outcome data source
GWAS summary statistics for gallstone disease were obtained from the FinnGen Consortium1 (Table 1). This was a large cohort study analyzing more than 16,000,000 SNPs, adjusted for sex, age, and genotyping batches. The definition of cholelithiasis in this study was based on the K80 type in ICD-10, and strict SNP inclusion criteria (MAF > 1%) were used. Including 19,023 cholelithiasis cases and 195,144 controls were included in this study.
Statistical analysis
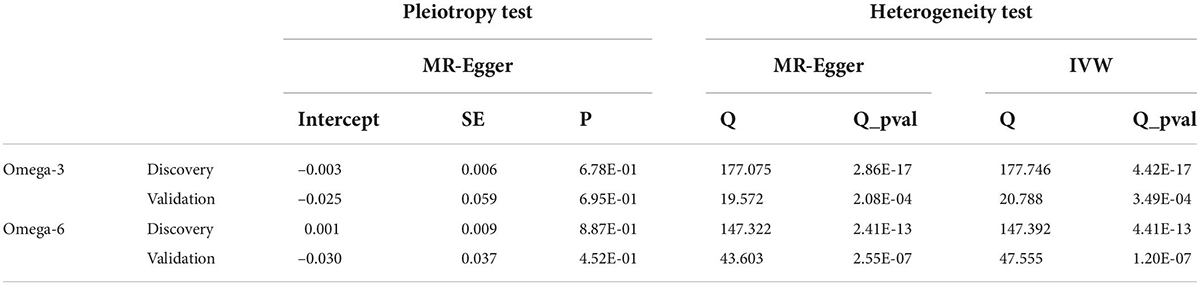
The random-effects model inverse variance weighting method was used as the main method of analysis in this study (16). Multiple analysis methods have also been used for repeated analysis, including the MR-Egger (17), weighted median (18), MR-pleiotropic residual sum and outliers (19) and maximum likelihood methods (20). Each approach employs different hypothetical models to assess causal effects, which are then used to check the robustness of the results. The MR-Egger provides calculation after adjusting for pleiotropy (17). Median weighting allowed estimation of causal effects when 50% of SNPs were invalid (18). The median weighting method allows for the estimation of causal effects when 50% of the SNPs are invalid (18). The MR-PRESSO method detects and corrects outliers, providing MR calculation that are robust in terms of heterogeneity after removing the identified outliers (19).
An MR analysis is often confounded by horizontal pleiotropy, which can lead to biased results. Therefore, we used multiple sensitivity analyses to identify potential pleiotropy. First, Cochran’s Q statistic was used to assess heterogeneity among the SNPs. Cochran’s Q-derived P < 0.05 was considered an indicator of heterogeneity in the IVs, at which point the multiplicative random effects IVW method was considered the gold standard (17). Second, an intercept test of the MR-Egger method was performed to measure horizontal multiplicity (17). Third, a leave-one-out analysis was performed to assess whether the association was driven by a single SNP (17).
The correlations with a P-value < 0.05 were considered to be statistically significant. All the analyses were performed using R software (version 4.1.2). The MR analyses were performed using the R packages “TwoSampleMR” and “MendelianRandomization.”
Results
The instrumental variable strength analysis showed that the general F statistic was greater than the empirical threshold of 10 (Table 1), indicating that a weak instrumental bias was unlikely to affect the estimation of the causal effects. Using PhenoScanner 2, we did not find any IVs of ω-3/6 that were associated with potential confounding factors.
Genetic liability to omega-3 with cholelithiasis
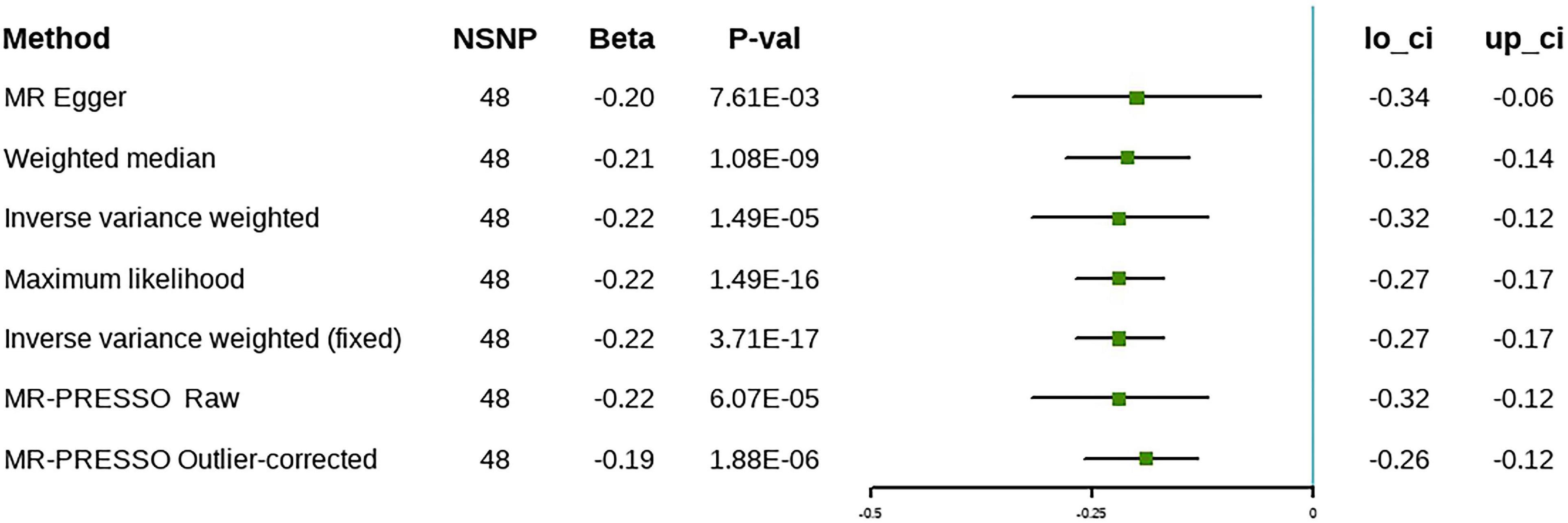
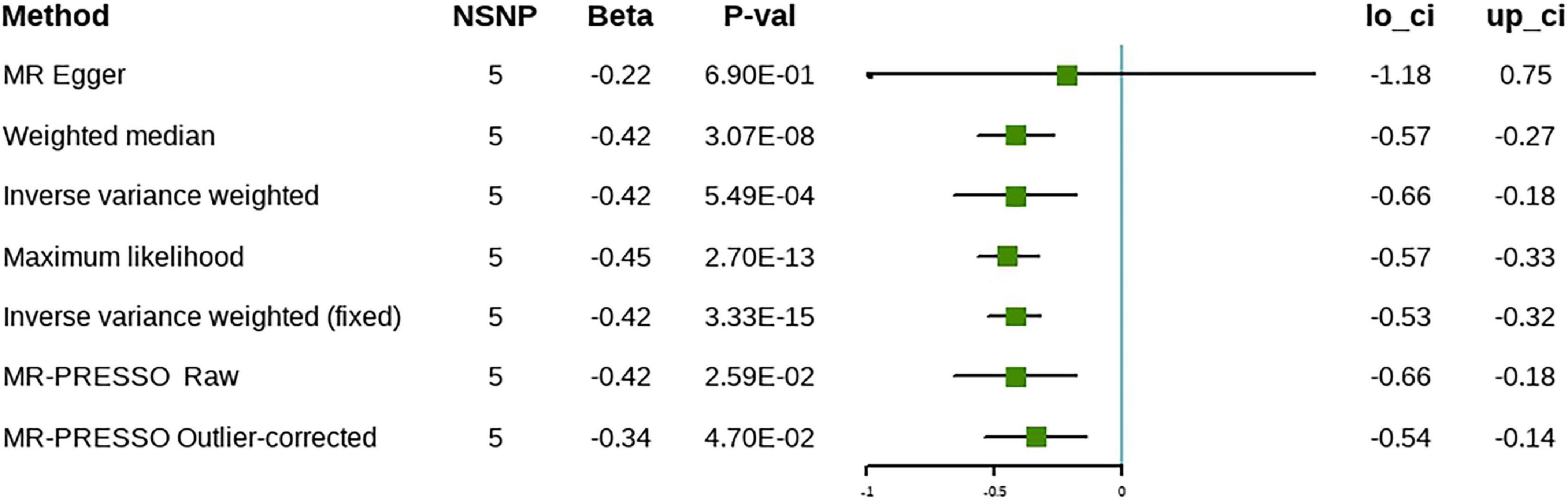
In the discovery stage, the random effect IVW analysis showed that higher ω-3 levels were correlated inversely with the risk of cholelithiasis (β = –0.22, 95% CI [–0.32 to –0.12], P = 1.49 × 10–5) (Figure 3). The Cochran’s Q statistic suggested heterogeneity; therefore, we adopted the results of the random-effect IVW analysis. The MR-Egger intercept method revealed no evidence of horizontal pleiotropy (Table 2). The remaining analyses showed that the significant results were not driven by any single SNP (Supplementary Figure 3). The MR-Egger, weighted median, and maximum likelihood methods produced the same results as the IVW method (Figure 3). The MR-PRESSO method detected outliers; however, the results did not change after the outliers were removed, which further demonstrated the reliability of our results. Forest plots and funnel plots are presented in Supplementary Figures 2, 3. Detailed information on the SNPs involved is in Supplementary Table 1.
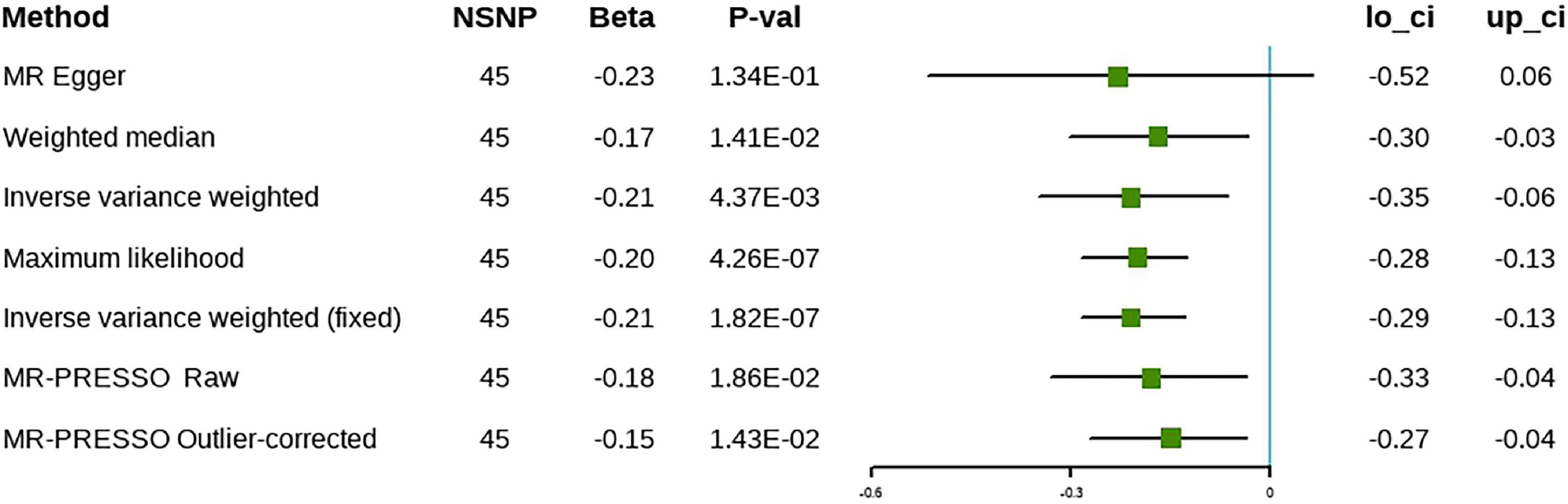
When the replication analysis was performed using another set of IVs, the causal relationship between ω-3 and cholelithiasis remained stable (β = –0.42, 95% CI [–0.66 to –0.18], P = 5.49 × 10–4), except for the findings that were obtained using the MR-Egger method, which were not significant (Figure 4). Further sensitivity analyses showed no evidence of horizontal pleiotropy despite the heterogeneity of the IVs. The results of the leave-one-out analysis were consistent with the discovery phase and the results were not caused by any single SNP (Supplementary Figure 3). The results of the MR-PRESSO method remained significant after removal of the outliers. Forest plots and funnel plots are presented in Supplementary Figures 2, 3. Detailed information on the SNPs involved is in Supplementary Table 2.
Genetic liability to omega-6 with cholelithiasis
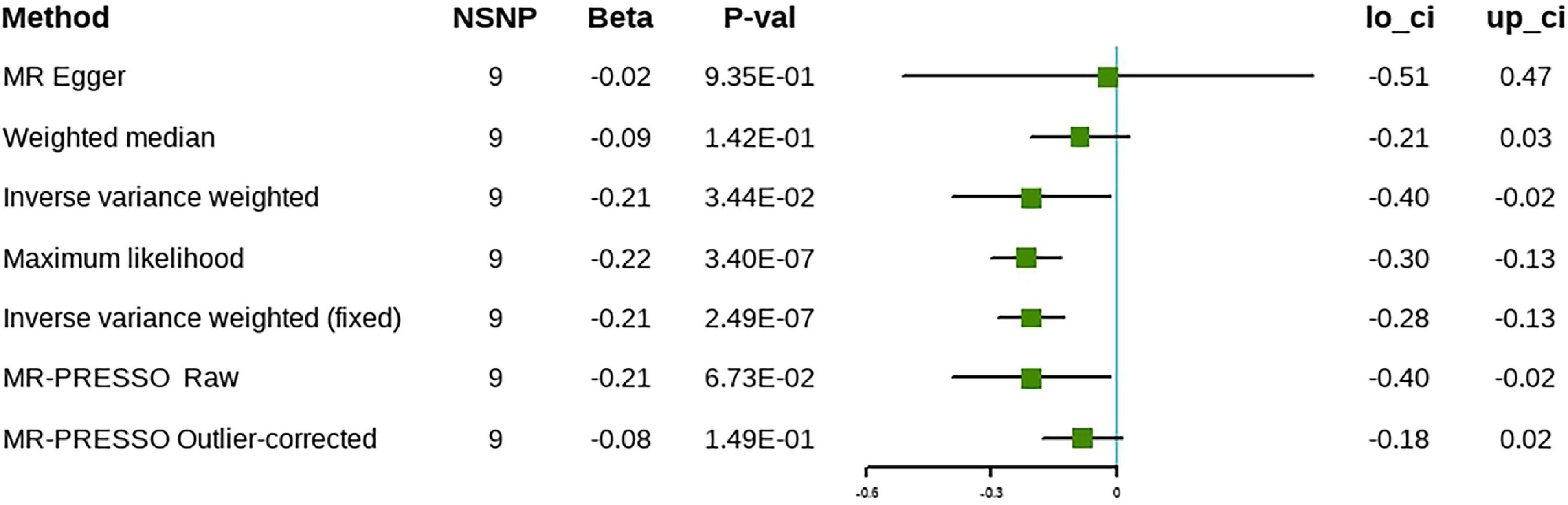
The results of the IVW approach showed that each SD increase in ω-6 was associated negatively with the risk of cholelithiasis, both in the discovery (β = –0.21, 95% CI [–0.35 to –0.06], P = 4.37 × 10–3) and in the validation phases (β = –0.21, 95% CI [–0.40 to –0.02], P = 3.44 × 10–2) (Figures 5, 6). The sensitivity analysis showed no evidence of horizontal pleiotropy, although heterogeneity was observed among the IVs (Table 2). Furthermore, the ω-6 association with cholelithiasis was not driven by any SNP (Supplementary Figure 3). In the discovery phase, the results of the MR-PRESSO method were consistent with the original values, after the removal of the outliers. However, the results were inconsistent after removing the outliers during the validation phase. One possible explanation is the heterogeneity of the IVs. However, since the validation set contained fewer IVs, the excessive elimination of SNPs would result in a loss of statistical power. Forest plots and funnel plots are presented in Supplementary Figures 2, 3. Detailed information on the SNPs involved is in Supplementary Tables 3, 4.
Discussion
In this study, both the discovery phase and the validation phase suggest that higher serum omega-3/6 fatty acid concentrations may be associated with a lower risk of cholelithiasis. At the same time, sensitivity analysis found that horizontal pleiotropy did not significantly interfere with the results of this study.
Epidemiological or clinical studies on the relationship between PUFA consumption and the risk of cholelithiasis have been conflicting and sparse. In a prospective cohort study, the high consumption of PUFAs was correlated inversely with the risk of cholelithiasis in men (4). This was supported by a cross-sectional study which indicated that high consumption of PUFAs played a protective role in cholelithiasis (21). However, PUFA intake did not seem to be associated with cholelithiasis in a observational study that was conducted in Argentina (22). Furthermore, the intake of PUFAs in patients with gallstones was higher in a retrospective study that was conducted in Spain (23). These results may have been due to the use of small sample sizes or the lack of long-term dietary information.
Our results may be explained by several possible underlying mechanisms. It has been reported previously that ω-3 PUFA supplementation may modify the composition of biliary phosphatidylcholine (24). This change may stabilize the cholesterol-phospholipid vesicles, which play a significant role in preventing cholesterol nucleation and gallstone formation (25). The reason for this change may be the fact that supplementation with ω-3 PUFA down-regulates the expression of canalicular transporters ABC, which has a major role in cholesterol secretion. Second, the antinucleating effect of ω-3 PUFA may also be explained by the arachidonic acid (AA) hypothesis. According to a study on the African Green Monkey, a high intake of ω-3 PUFA may reduce the percentage of AA in biliary phospholipids (26). In addition, the presence of AA in biliary phospholipids causes the hypersecretion of gallbladder mucins which has been considered to trigger the formation of gallstones by serving as a nidus of gallstones (27, 28). Besides, a high intake of ω-3 PUFA can also decrease mucins secretion by reducing expression of mucin gene expression such as Muc2, Muc5ac, Muc5b. Third, previous studies have demonstrated that dietary supplementation with ω-3 PUFA promoted the secretion of hepatic phospholipids by reducing the hydrophobicity of phospholipids (26, 29). Enhanced hepatic phospholipid secretion may increase the bile phospholipid concentration and reduce CSI. Finally, the effects of ω-3 PUFA may also be explained by increased insulin sensitivity. Metabolic studies have suggested that an increased intake of ω-3 PUFA may improve insulin sensitivity by changing the fatty acid composition of the adipocyte plasma membrane (30, 31). In addition, there is an increased synthesis of cholesterol and hypersecretion of biliary cholesterol in patients with insulin resistance (32–34). Previous studies have also speculated that insulin resistance may participate in the pathogenesis of cholelithiasis by promoting the release of proinflammatory cytokines that are related to gallbladder inflammation (35, 36).
Recent evidence has shown that ω-6 PUFA is inversely associated with type 2 diabetes mellitus (T2DM) (37). T2DM has been proved to be a high-risk factor for cholelithiasis. Furthermore, ω-6 PUFA may significantly decrease triglycerides and increase high-density lipoprotein (HDL) cholesterol levels (38). High triglyceride and low HDL cholesterol levels are established risk factors for cholelithiasis. In addition, ω-6 PUFA can promote the production and secretion of bile acids by inducing the synthesis of cholesterol 7α-hydroxylase (39). This may be related to the reduced expression of sterol 27-hydroxylase. Therefore, this suggested that ω-6 PUFA may also reduce CSI and prevent cholelithiasis.
Although laparoscopic cholecystectomy has become the most common minimally surgical procedure performed worldwide, it may be suboptimal in the long term (9, 40). As a surgical procedure, laparoscopic cholecystectomy is inevitably associated with surgical complications and even patient death (41, 42). In addition, cholelithiasis is considered to be one of the highest medical burdens among digestive diseases. In the future, more attention should be paid to preventing cholelithiasis. Our study may promote a paradigm shift from the diagnosis and treatment of gallstones to prevention. Patients with a strong susceptibility to gallstones may benefit from preventive PUFA supplementation.
Our study had several strengths. Firstly, for the first time, the causal association between omega fatty acids and cholelithiasis was explored using MR analysis; secondly, this study consisted of two parts: discovery and validation, which made the results more reliable, and there was no overlap in the population between different data sets. Thirdly, a series of replicate and sensitivity analyses were applied to improve the credibility of our results.
Our study also had several limitations. First, the participants involved in this study were of European origin; therefore, this result should be interpreted with caution in other populations. Second, there was heterogeneity among the IVs used in this study; however, the absence of pleiotropy in the MR-Egger test suggested balanced pleiotropy, which was unlikely to bias the results. Third, although we used several approaches to remove confounders and minimize the possibility of bias, the potential pleiotropy could not be removed completely. However, the sensitivity analyses suggested that horizontal pleiotropy was unlikely to have an impact on our results.
Conclusion
In summary, our findings indicate that individuals with lower omega-3/6 fatty acid levels have a higher risk of cholelithiasis. Given the greater disease burden of cholelithiasis, ω-3/6 fatty acid supplementation may be a promising adjunct treatment modality. Standardized randomized controlled trials should be designed to further explore the benefits of PUFAs in cholelithiasis.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QS and WX designed the study and drafted the article. QS, NG, and WX conducted the data acquisition and performed the data analysis and manuscript revision. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81970552).
Acknowledgments
We thank all the participants and researchers for their participation in this MR study. European Bioinformatics Institute GWAS Catalog provided summary data for the analyses.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JJ declared a shared parent affiliation with the authors to the handling editor at the time of the review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.964805/full#supplementary-material
Footnotes
References
1. Lammert F, Gurusamy K, Ko CW, Miquel JF, Mendez-Sanchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Primers. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
2. Wirth J, Joshi AD, Song M, Lee DH, Tabung FK, Fung TT, et al. A healthy lifestyle pattern and the risk of symptomatic gallstone disease: results from 2 prospective cohort studies. Am J Clin Nutr. (2020) 112:586–94. doi: 10.1093/ajcn/nqaa154
3. Tavani A, Rosato V, Di Palma F, Bosetti C, Talamini R, Dal Maso L, et al. History of cholelithiasis and cancer risk in a network of case-control studies. Ann Oncol. (2012) 23:2173–8. doi: 10.1093/annonc/mdr581
4. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Int Med. (2004) 141:514–22. doi: 10.7326/0003-4819-141-7-200410050-00007
5. Downie LE, Ng SM, Lindsley KB, Akpek EK. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev. (2019) 12:Cd011016. doi: 10.1002/14651858.CD011016.pub2
6. Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. (2019) 366:l4697. doi: 10.1136/bmj.l4697
7. Kim JK, Cho SM, Kang SH, Kim E, Yi H, Yun ES, et al. N-3 polyunsaturated fatty acid attenuates cholesterol gallstones by suppressing mucin production with a high cholesterol diet in mice. J Gastroenterol Hepatol. (2012) 27:1745–51. doi: 10.1111/j.1440-1746.2012.07227.x
8. Cohen BI, Mosbach EH, Ayyad N, Miki S, McSherry CK. Dietary fat and fatty acids modulate cholesterol cholelithiasis in the hamster. Lipids. (1992) 27:526–32. doi: 10.1007/bf02536135
9. Jang SI, Fang S, Kim KP, Ko Y, Kim H, Oh J, et al. combination treatment with N-3 polyunsaturated fatty acids and ursodeoxycholic acid dissolves cholesterol gallstones in mice. Sci Rep. (2019) 9:12740. doi: 10.1038/s41598-019-49095-z
10. Misciagna G, Centonze S, Leoci C, Guerra V, Cisternino AM, Ceo R, et al. Diet, physical activity, and gallstones–a population-based, case-control study in southern Italy. Am J Clin Nutr. (1999) 69:120–6. doi: 10.1093/ajcn/69.1.120
11. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
12. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The Mr-base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
13. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
14. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
15. Kettunen J, Demirkan A, Würtz P, Draisma HH, Haller T, Rawal R, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. (2016) 7:11122. doi: 10.1038/ncomms11122
16. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
17. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
18. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
19. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
20. Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-tree: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. (2015) 32:268–74. doi: 10.1093/molbev/msu300
21. Gilat T, Horwitz C, Halpern Z, Bar Itzhak A, Feldman C. Gallstones and diet in Tel Aviv and gaza. Am J Clin Nutr. (1985) 41:336–42. doi: 10.1093/ajcn/41.2.336
22. Compagnucci AB, Perroud HA, Batalles SM, Villavicencio R, Brasca A, Berli D, et al. A nested case-control study on dietary fat consumption and the risk for gallstone disease. J Hum Nutr Diet. (2016) 29:338–44. doi: 10.1111/jhn.12332
23. Ortega RM, Fernandez-Azuela M, Encinas-Sotillos A, Andres P, Lopez-Sobaler AM. Differences in diet and food habits between patients with gallstones and controls. J Am Coll Nutr. (1997) 16:88–95. doi: 10.1080/07315724.1997.10718655
24. Peled Y, Gilat T. Effect of dietary phospholipids and their constituents on bile composition in rats and hamsters. Hepatology. (1994) 19:708–13. doi: 10.1002/hep.1840190324
25. Ayyad N, Cohen BI, Ohshima A, Mosbach EH. Prevention of cholesterol cholelithiasis by dietary unsaturated fats in hormone-treated female hamsters. Lipids. (1996) 31:721–7. doi: 10.1007/bf02522888
26. Scobey MW, Johnson FL, Parks JS, Rudel LL. Dietary fish oil effects on biliary lipid secretion and cholesterol gallstone formation in the African green monkey. Hepatology. (1991) 14(4 Pt 1):679–84. doi: 10.1016/0270-9139(91)90057-3
27. LaMont JT, Smith BF, Moore JR. Role of gallbladder mucin in pathophysiology of gallstones. Hepatology. (1984) 4(5 Suppl.):51s–6s. doi: 10.1002/hep.1840040809
28. Wang DQ, Cohen DE, Lammert F, Carey MC. No Pathophysiologic relationship of soluble biliary proteins to cholesterol crystallization in human bile. J Lipid Res. (1999) 40:415–25.
29. LaMorte WW, O’Leary DP, Booker ML, Scott TE. Increased dietary fat content accelerates cholesterol gallstone formation in the cholesterol-fed prairie dog. Hepatology. (1993) 18:1498–503.
30. Rivellese AA, De Natale C, Lilli SC. Type of dietary fat and insulin resistance. Ann N Y Acad Sci. (2002) 967:329–35. doi: 10.1111/j.1749-6632.2002.tb04288.x
31. Clandinin MT, Cheema S, Field CJ, Baracos VE. Dietary lipids influence insulin action. Ann N Y Acad Sci. (1993) 683:151–63. doi: 10.1111/j.1749-6632.1993.tb35701.x
32. Bennion LJ, Grundy SM. Risk factors for the development of cholelithiasis in man (first of two parts). N Engl J Med. (1978) 299:1161–7. doi: 10.1056/nejm197811232992104
33. Bennion LJ, Grundy SM. Risk factors for the development of cholelithiasis in man (second of two parts). N Engl J Med. (1978) 299:1221–7. doi: 10.1056/nejm197811302992205
34. Attili AF, Capocaccia R, Carulli N, Festi D, Roda E, Barbara L, et al. Factors associated with gallstone disease in the micol experience. Multicenter Italian study on epidemiology of cholelithiasis. Hepatology. (1997) 26:809–18. doi: 10.1002/hep.510260401
35. Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. (2002) 5:551–9. doi: 10.1097/00075197-200209000-00015
36. Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diabetes Rep. (2005) 5:70–5. doi: 10.1007/s11892-005-0071-7
37. Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, et al. Association of plasma phospholipid N-3 and N-6 polyunsaturated fatty acids with type 2 diabetes: the epic-interact case-cohort study. PLoS Med. (2016) 13:e1002094. doi: 10.1371/journal.pmed.1002094
38. Vanhala M, Saltevo J, Soininen P, Kautiainen H, Kangas AJ, Ala-Korpela M, et al. Serum omega-6 polyunsaturated fatty acids and the metabolic syndrome: a longitudinal population-based cohort study. Am J Epidemiol. (2012) 176:253–60. doi: 10.1093/aje/kwr504
39. Sato M, Yoshida S, Nagao K, Imaizumi K. Superiority of dietary safflower oil over olive oil in lowering serum cholesterol and increasing hepatic mRnas for the LDL receptor and cholesterol 7alpha-hydroxylase in exogenously hypercholesterolemic (exHC) rats. Biosci Biotechnol Biochem. (2000) 64:1111–7. doi: 10.1271/bbb.64.1111
40. Kao LS, Ball CG, Chaudhury PK, for Members of the Evidence Based Reviews in Surgery Group. Evidence-based reviews in surgery: early cholecystectomy for cholecystitis. Ann Surg. (2018) 268:940–2. doi: 10.1097/SLA.0000000000002867
41. Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. (1993) 165:9–14. doi: 10.1016/s0002-9610(05)80397-6
Keywords: cholelithiasis, polyunsaturated acids, omega-3 (ω-3) and omega-6 (ω-6) fatty acids, mendelian randomisation, causal relationship
Citation: Sun Q, Gao N and Xia W (2022) Association between omega-3/6 fatty acids and cholelithiasis: A mendelian randomization study. Front. Nutr. 9:964805. doi: 10.3389/fnut.2022.964805
Received: 09 June 2022; Accepted: 07 September 2022;
Published: 23 September 2022.
Edited by:
Li Cai, Sun Yat-sen University, ChinaReviewed by:
Xiaoqin Luo, Xi’an Jiaotong University, ChinaJingjing Jiao, Zhejiang University, China
Copyright © 2022 Sun, Gao and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiliang Xia, eGlhd2VpbGlhbmdAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Qi Sun
Qi Sun Ning Gao
Ning Gao Weiliang Xia1,2*
Weiliang Xia1,2*