
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 15 August 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.963471
This article is part of the Research Topic Nutrition and Metabolic Aging View all 11 articles
 Zhaoqing Li1†
Zhaoqing Li1† Haoyuan Lei1†
Haoyuan Lei1† Hong Jiang1†
Hong Jiang1† Yahui Fan1
Yahui Fan1 Jia Shi1
Jia Shi1 Chao Li1
Chao Li1 Fangyao Chen1
Fangyao Chen1 Baibing Mi1
Baibing Mi1 Mao Ma2*
Mao Ma2* Jing Lin1*
Jing Lin1* Le Ma1,3*
Le Ma1,3*Background and aims: Evidence regarding associations of circulating saturated fatty acids (SFAs) with chronic diseases is mixed. The objective of this study was to determine the associations between total or individual SFA biomarkers and the risk of cardiometabolic diseases.
Methods: Four electronic databases were searched from inception to March 2022. Three investigators independently assessed for inclusion and extracted data. Random-effects or fixed-effects models was used to estimate the pooled relative risks (RRs) and corresponding 95% confidence intervals (CIs) for the association of total or individual SFA biomarkers, including even-chain SFAs (e.g., 14:0, myristic acid; 16:0, palmitic acid; 18:0, stearic acid), odd-chain SFAs (e.g., 15:0, pentadecanoic acid; 17:0, margaric acid) and very-long-chain SFAs (VLCSFAs; e.g., 20:0, arachidic acid; 22:0, behenic acid; 24:0, lignoceric acid), with risk of incident type 2 diabetes (T2D), cardiovascular disease [CVD; coronary heart disease (CHD) inclusive of stroke], CHD and stroke.
Results: A total of 49 prospective studies reported in 45 articles were included. Higher concentration of circulating total SFAs was associated with an increasing risk of cardiometabolic diseases, the risk increased significantly by 50% for CVD (95%CI:1.31–1.71), 63% for CHD (95%CI:1.38–1.94), 38% for stroke (95%CI:1.05–1.82), respectively. Similarly, levels of even-chain SFAs were positively associated with higher risk of chronic diseases, with RRs ranging from 1.15 to 1.43. In contrast, the risk of cardiometabolic diseases was reduced with increasing odd-chain SFA levels, with RRs ranging from 0.62 to 0.91. A higher level of VLCSFAs corresponded to 19% reduction in CVD. Further dose-response analysis indicated that each 50% increment in percentage of total SFAs in circulating was associated with an 8% higher risk of T2D (RR: 1.08, 95%CI: 1.02–1.14) and trends toward higher risk of CVD (RR: 1.15, 95%CI: 0.98–1.34). Inverse linear relationships were observed between 17:0 biomarker and T2D or CVD risk.
Conclusion: Our findings support the current recommendations of reducing intake of saturated fat as part of healthy dietary patterns. Further studies are needed to confirm our findings on these SFAs in relation to cardiometabolic outcomes and to elucidate underlying mechanisms.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022329182], identifier [CRD42022329182].
Limiting intake of saturated fatty acids (SFAs) has been considered as a key component to reduce the risk of chronic disorders for decades (1). The 2020 Dietary Guidelines for Americans recommended that SFAs comprise below 10% of total daily energy intake starting at age 2 years (2). The American Heart Association/American College of Cardiology guidelines have also set a target to decrease SFA intake to 5–6% of calories (3). In vitro and animal studies have demonstrated that SFAs can accelerate the atherosclerotic process, induce cellular inflammation and influence the insulin sensitivity (4, 5). Results from a meta-analysis of randomized controlled trials conducted by the American Heart Association demonstrated that replacing SFAs with unsaturated fats, especially polyunsaturated fats, will lower the incidence of cardiovascular disease (CVD) by approximately 30% (6). Therefore, it is important to accurately evaluate the effects of SFAs on health for the control of cardiometabolic diseases.
Associations of dietary SFAs with the incidence of major cardiometabolic diseases have been reported by some large prospective cohort studies, and findings of these studies were inconsistent (7–9). Interpretation has been complicated by the imprecision of dietary questionnaires, measurement errors, different study designs, variation in population characteristics, as well as bioavailability of these fatty acids (10–12). To reduce these limitations, biomarkers which are free of misclassification, reporting bias and other measurement errors can be used to elucidate associations between the intake of SFAs and disease risk (13, 14). An increasing number of prospective studies have been performed to investigate the associations between circulating total SFAs and risk of metabolic and cardiovascular event; however, the results were conflicting (15–17). In addition, previous studies mostly focused on the coronary heart disease (CHD), evidence regarding associations of circulating SFA with other cardiometabolic disease [such as type 2 diabetes (T2D) and stroke] is still needed to be explored. Furthermore, increasing evidence suggested that individual SFA tend to have different biological functions (18). Previous meta-analyses in 2014 reported that even-chain SFAs were positively associated with coronary risk (19); however, null or inverse association was observed for other subtypes of SFAs such as odd-chain SFAs and very-long-chain SFAs (VLCSFAs) in recent studies (20, 21). Therefore, the impact of individual SFA on cardiometabolic disease needs to be further studied and established.
To fill this knowledge gap, a dose-response meta-analysis of data from prospective studies was conducted to evaluate the associations between the circulating even-chain SFAs, odd-chain SFAs, VLCSFAs as well as the total SFAs and the incident of T2D, CVD, CHD, and stroke.
This study was registered in PROSPERO (CRD42022329182) and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
A comprehensive literature search of Web of Science, PubMed, EMBASE, and Cochrane Library were conducted to identify relevant published articles from inception to March 2022. The following terms were used: “fatty acids” or “saturated fatty acid” or “saturated fatty acids” or “lauric acid” or “myristic acid” or “pentadecanoic acid” or “palmitic acid” or “margaric acid” or “stearic acid” or “arachidic acid” or “heneicosylic acid” or “behenic acid” or “tricosylic acid” or “lignoceric acid,” AND “type 2 diabetes” or “diabetes mellitus” or “impaired glucose” or “impaired fasting insulin” or “cardiovascular disease” or “coronary heart disease” or “heart disease” or “ischemic heart disease” or “coronary artery disease” or “myocardial infarction” or “stroke” or “ischemic stroke” or “haemorrhagic stroke,” AND “blood” or “marker” or “biomarker” or “serum” or “plasma” or “whole blood” or “adipose tissue” or “circulating” or “erythrocytes” or “red blood cell” or “cholesterol esters,” AND “prospective” or “nested case-control” or “cohort” or “case-cohort” or “follow-up” or “longitudinal.” The search was restricted to human studies without any language restriction. Additionally, reference lists of retrieved articles, review articles, and meta-analyses were manually scanned to identify any other relevant studies. Corresponding authors of eligible articles were contacted for any further relevant work, published or unpublished, to reduce risk of publication bias.
Studies were included in the meta-analysis if they satisfied the following criteria: (1) the study design was prospective (cohort, nested case-control, and case-cohort) with a follow-up more than 1 year; (2) the exposures of interest were total or individual SFA biomarkers [even-chain SFAs (e.g., 14:0, myristic acid; 16:0, palmitic acid; 18:0, stearic acid), odd-chain SFAs (e.g., 15:0, pentadecanoic acid; 17:0, margaric acid) and VLCSFAs (e.g., 20:0, arachidic acid; 22:0, behenic acid; 24:0, lignoceric acid)] measured in any type of tissue [circulating blood (whole blood, serum, plasma, and erythrocyte fraction) or adipose tissue]; (3) the endpoints of interest included incident T2D, CVD, CHD, and stroke; (4) the adjusted relative risks (RRs), odds ratios, or hazard ratios with corresponding 95% confidence intervals (CIs) or standard errors were presented or could be calculated. Whenever reports referred to identical outcomes from the same population, only those with the highest number of cases or the longest follow-up times were retained to avoid data duplication. Potentially eligible studies were assessed independently by three investigators (ZQL, HYL, and HJ), with discrepancies resolved by discussion until consensus was reached.
Data extraction were conducted independently by 3 investigators (ZQL, HYL, and HJ). A standardized data collection form was applied to extract the following baseline characteristics from each study: first author name, publication year, study location, study name, study design, follow-up duration, participant characteristics (age, proportion of men, and number of participants), SFA subtype, biological sample type (plasma, serum, erythrocyte membrane, and adipose tissue), assessment method [gas chromatography (GC), gas-liquid chromatography (GLC), and nuclear magnetic resonance-based profiling (NMR)], outcomes of interest (type and number of cases), covariates adjusted for in the multivariable model, and the risk estimate with 95%CIs for all categories of each biomarkers. When studies reported multiple results based on different numbers of covariates included in statistical adjustments, the results that adjusted for the most number of variables were extracted.
Study quality was performed by the same authors according to the validated Newcastle-Ottawa Scale (NOS) (22), which awards 0–9 points and incorporates information on selection (range 0–4 points), comparability (range 0–2 points), and outcome assessment (range 0–3 points). We considered NOS scores of 0–3, 4–6, and 7–9 as low, medium, and high quality, respectively. Any discrepancy in data extraction or quality assessments between investigators were discussed or resolved by a senior author (LM) until a joint consensus was reached.
In this meta-analysis, the RRs were used as the common measures of associations across studies, and odds ratios and hazard ratios were considered approximations of RRs. As different studies might report different exposure categories, methods previously described were used to derive estimates of associations corresponding to the comparison between the top and bottom third of SFAs distributions for the meta-analysis (19). The strategy was to harmonize different comparison groups used in individual studies, such as quartiles, quintiles, or other categorizations, or per standard deviation (SD) change. In brief, for studies that provided RRs per SD change of SFAs, we applied a factor of 2.18 to the log RR to derive the RRs comparing extreme thirds, assuming a normal distribution. Similarly, the factor of 2.54 or 2.80 was applied to convert estimates for comparing extreme quartiles or quintiles, respectively. The standard error of the transformed log RRs was calculated after applying the same factors (23). When studies used multiple measures as biomarker, the overall risk estimate was based on different duration of intake reflection according to the following list: adipose tissue, erythrocyte phospholipids, plasma phospholipids, total plasma or serum, and cholesterol esters. Studies that separately reported results by sex without presenting overall estimates were pooled to derive a single effect size for the study. Assessment of heterogeneity between the studies was based on Q test and I2 statistic (24). A Cochran’s Q P < 0.10 and I2 value > 50% was considered to indicate significant heterogeneity and the random-effects model was used. Otherwise, fixed-effects model was performed. Meta-regression was conducted to examine sources of heterogeneity and the influence of potential residual confounding factors, such as study design [prospective cohort study (PC), prospective case-cohort study (PCC), or nested case-control study (NCC)], geographical location (the United States, Europe, or Asia), assessment method (GC, GLC, or NMR), biomarker type (plasma, serum, erythrocyte membrane, or adipose tissue), number of cases (< 300 or ≥ 300), as well as study quality (moderate or high). Dose-response analyses were conducted based on the method proposed by Greenland and Longnecker (25), studies that reported RRs with 95%CIs for at least three exposure categories were included. When studies reported only the total number of cases or total person-years and the exposure was defined in quantile, the distribution of cases or person-years was calculated by dividing the total number by the number of quantiles. For the studies that did not present the median or mean doses of SFAs, we chose the midpoint of each category as the alternative. When the highest category did not have an upper bound, the length of the open-ended interval was assumed to be the same as that of the adjacent interval. When the lowest category was open-ended, the lower bound was set to zero. Sensitivity analyses were conducted by omitting one study in each turn to assess the impact of individual studies on the overall estimated risk. Potential publication bias was explored by using visual appreciation of funnel plots, Begg’s rank correlation and Egger’s weighted regression tests. The influence of a potential publication bias on findings was explored by using the Duval and Tweedie trim and fill procedure (26). All analyses were performed using Stata, version 12.0 (Stata Corporation, College Station, TX, United States). All P-values reported are two-sided and a P-value < 0.05 was considered statistically significant, except where otherwise specified.
A total of 14,919 potentially relevant citations were identified by search strategy, of which 2,935 duplicates were initially excluded. After screening titles and abstracts, 11,808 records were further removed, leaving 176 articles retrieved for full-text review. Finally, a total of 49 studies reported in 45 articles were included for the present meta-analysis (Figure 1) (15–17, 20, 21, 27–66).
The main characteristics of the included prospective studies are listed in Supplementary Table 1. The 49 identified studies comprised of 21 prospective cohort studies, three case-cohort studies, and 25 nested case-control studies. The number of participants in each included study varied from 188 to 95,854, with a total of 220,590 participants and 46,355 cases (21,252 T2D cases, 14,613 CVD cases, 7,314 CHD cases, and 3,176 stroke cases). 24 studies were conducted in the United States, 15 in Europe, and 10 in Asia. The duration of follow-up in prospective studies ranged from 1.5 to 30.7 years. The average age of participants ranged from 48.9 to 79.2 years. 32 studies comprised both men and women as participants, nine studies included men only and eight included women only. Concentrations of circulating SFAs were quantified by GC (n = 28), GLC (n = 18), NMR (n = 2), and GC-tandem mass spectrometry (n = 1). 48 studies measured SFA biomarkers in circulating blood (21 in plasma, 15 in erythrocyte membrane, and 12 in serum) and one in adipose tissue. Most studies were adjusted for age (n = 47) and smoking (n = 46), and alcohol drinking (n = 40), physical activity (n = 39) and body mass index (n = 38) were also controlled for in many studies. The results of the study quality assessment showed that 44 studies were graded as high quality, others were graded as moderate quality (Supplementary Table 2).
Twenty-two studies were assessed to estimate risk between the concentrations of SFA biomarkers and T2D, including 166,745 participants and 21,252 T2D events (17, 20, 27–44). The pooled estimate indicated that a higher level of total SFAs was associated with a non-significant higher risk of T2D (RR: 1.14, 95%CI: 0.83–1.56, P = 0.43; Pheterogeneity < 0.001; Figure 2 and Supplementary Figure 1). For even-chain SFAs, compared with participants in the lowest tertile, those in the highest tertile of 16:0 level had a 28% significantly higher T2D risk (RR: 1.28, 95%CI: 1.07–1.52, P = 0.01; Pheterogeneity < 0.001; Supplementary Figure 2). Similar association was also found between other two even-chain SFAs and CVD risk, with the RR of 1.17 (95%CI: 1.01–1.36, P = 0.03; Pheterogeneity = 0.004; Supplementary Figure 3) for 14:0 and 1.25 (95%CI: 1.04–1.49, P = 0.01; Pheterogeneity < 0.001; Supplementary Figure 4) for 18:0, respectively. By contrast, when comparing the extreme tertiles of odd-chain SFA level, the risk in T2D decreased significantly by 24% for 15:0 (RR: 0.76, 95%CI: 0.63–0.91, P = 0.003; Pheterogeneity = 0.002; Supplementary Figure 5), 38% for 17:0 (RR: 0.62, 95%CI: 0.44–0.87, P = 0.01; Pheterogeneity < 0.001; Supplementary Figure 6). Higher VLCSFAs status was associated with a non-significant lower risk of T2D [RR was 0.79 for 20:0 (95%CI: 0.62–1.00, P = 0.06; Pheterogeneity < 0.001; Supplementary Figure 7), 0.83 for 22:0 (95%CI: 0.65–1.07, P = 0.16; Pheterogeneity < 0.001; Supplementary Figure 8), 0.90 for 24:0 (95%CI: 0.70–1.16, P = 0.42; Pheterogeneity = 0.001; Supplementary Figure 9)]. The association between circulating SFAs and T2D risk was not significantly modified by study type, sex, geographical location, number of cases, assessment method, biomarker type and study quality (Supplementary Figures 10–12). The result of dose-response analysis found that each 50% increment in percentage of total SFAs in circulating corresponded to an 8% (RR: 1.08, 95%CI: 1.02–1.14, P = 0.01; Plinearity = 0.84) higher risk of T2D. For every 50% increase in percentage of odd-chain SFAs concentration in total SFAs, the risk of T2D was reduced by 8% for 15:0 (RR: 0.92, 95%CI: 0.83–1.02, P = 0.11; Plinearity = 0.25), 16% for 17:0 (RR: 0.84, 95%CI: 0.76–0.92, P < 0.001; Plinearity = 0.12), respectively.
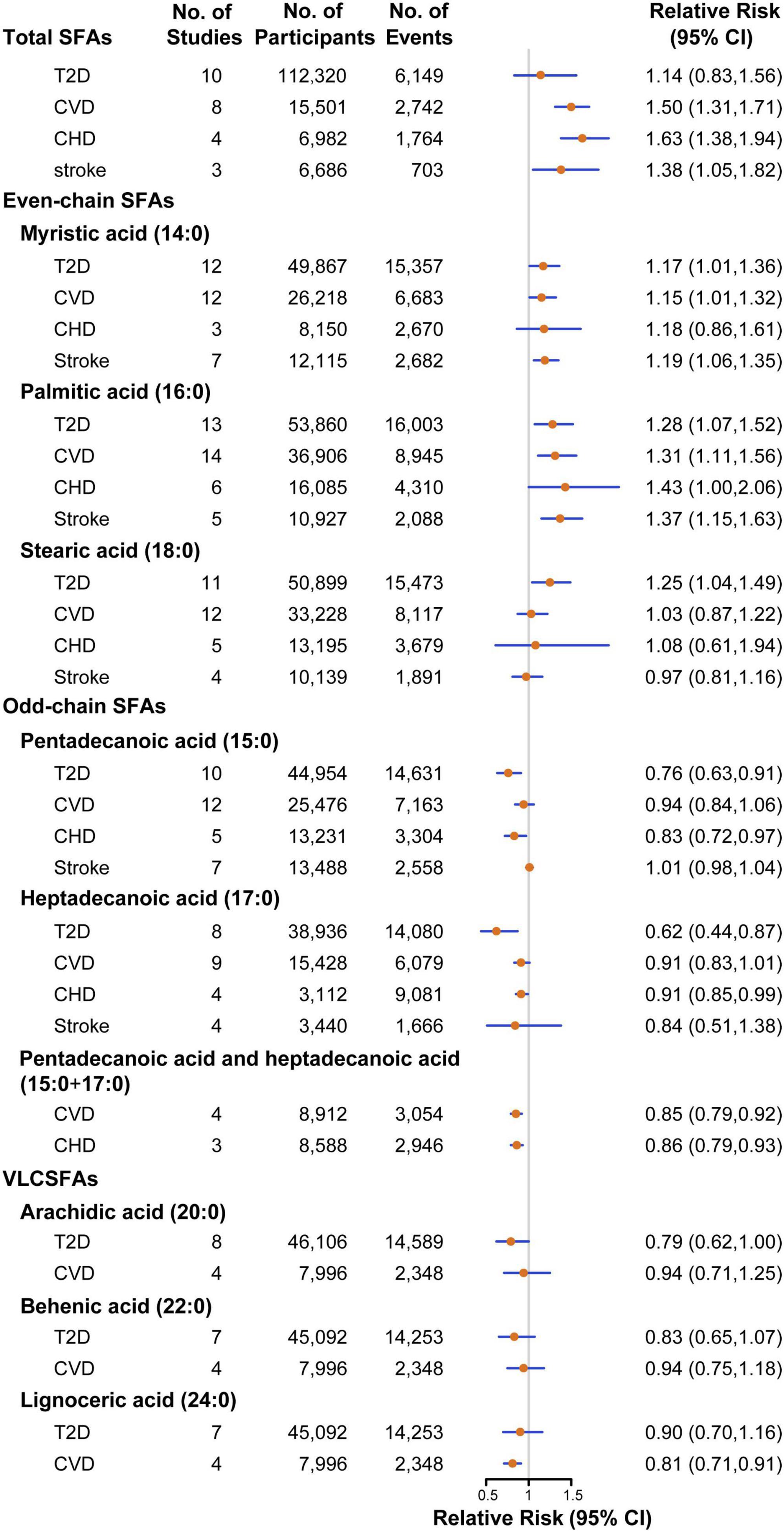
Figure 2. Pooled relative risks of T2D, CVD, CHD, stroke comparing the highest with the lowest tertile of SFA biomarkers. CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; SFAs, saturated fatty acids; T2D, type 2 diabetes; VLCSFAs, very-long-chain SFAs.
Association between circulating SFAs and risk of CVD were investigated in 27 studies, with 53,845 participants and 14,613 CVD events (15, 16, 21, 45–66). Participants in the highest category of circulating total SFAs had a 50% higher risk with CVD compared with those in the lowest category (RR: 1.50, 95%CI: 1.31–1.71, P < 0.001; Pheterogeneity = 0.56; Supplementary Figure 13). For even-chain SFAs, the pooled results indicated that higher 16:0 status was associated with a 31% increased risk of CVD (RR: 1.31, 95%CI: 1.11–1.56, P = 0.002; Pheterogeneity < 0.001; Supplementary Figure 14). Similarly, higher levels of 14:0 was also associated with a 15% higher risk of CVD (RR: 1.15, 95%CI: 1.01–1.32, P = 0.03; Pheterogeneity < 0.001; Supplementary Figure 15). For odd-chain SFAs, higher levels of the sum of 15:0 and 17:0 had a 15% lower risk of CVD (RR: 0.85, 95%CI: 0.79–0.92, P < 0.001; Pheterogeneity = 0.15; Supplementary Figure 16). For VLCSFAs, in comparison with the lowest category, the highest category of 24:0 was associated with a 19% (RR: 0.81, 95%CI: 0.71–0.91, P = 0.001; Pheterogeneity = 0.45; Supplementary Figure 17) reduced risk of CVD. 15:0, 17:0, 18:0, 20:0, and 22:0 biomarkers were not significantly associated with incidence of CVD (Supplementary Figures 18–22). In stratified analyses, the association between 16:0 status and CVD risk was significantly stronger among NCC studies (RR: 1.86, 95%CI: 1.32–2.62) compared with PC studies (Supplementary Figures 10–12). In the dose-response analysis, a non-significant linear association was noted between total SFAs biomarker and CVD risk, with the RR of 1.15 (95%CI: 0.98–1.34, P = 0.09; Plinearity = 0.96) for each 50% increment in percentage of total SFAs in circulating. For every 50% increment in percentage of 17:0 and 24:0 concentrations of total SFAs, the RR of CVD decreased by 18% for 17:0 (RR:0.82, 95%CI: 0.70–0.97, P = 0.02; Plinearity = 0.84), 10% for 24:0 (RR:0.90, 95%CI: 0.82–0.98, P = 0.02; Plinearity = 0.14), respectively.
A total of 14 studies with 7,314 cases in 26,772 participants were included to investigate the relationship between circulating SFAs and CHD risk (15, 16, 49–59). The overall effect estimates of CHD comparing the top to bottom categories were 1.63 for total SFAs (95%CI: 1.38–1.94, P < 0.001; Pheterogeneity = 0.45; Supplementary Figure 23). For even-chain SFAs, the pooled result indicated that a higher level of 16:0 was associated with a higher risk of CHD (RR: 1.43, 95%CI: 1.00–2.06, P = 0.05; Pheterogeneity = 0.003; Supplementary Figure 24). The levels of odd-chain SFAs was associated with a significantly lower CHD risk by 17% for 15:0 (RR: 0.83, 95%CI: 0.72–0.97, P = 0.02; Pheterogeneity = 0.21; Supplementary Figure 25), 9% for 17:0 (RR: 0.91, 95%CI: 0.85–0.99, P = 0.03; Pheterogeneity = 0.22; Supplementary Figure 26) and 14% for the sum of 15:0 and 17:0 (RR: 0.86, 95%CI: 0.79–0.93, P < 0.001; Pheterogeneity = 0.23; Supplementary Figure 27), respectively. No significant associations were observed between 14:0, 18:0 concentrations and incidence of CHD (Supplementary Figures 28, 29). In stratified analyses, no evidence of heterogeneity was detected between subgroups. Due to the limited number of included studies, evaluation for dose-response analysis between SFA biomarkers and risk of CHD was not performed.
The association between circulating SFAs with stroke risk were investigated in nine studies, comprising 16,589 participants and 3,176 stroke events (49, 60–66). The estimated RR of stroke for the comparison of extreme categories was 1.38 for total SFAs (95%CI: 1.05–1.82, P = 0.02; Pheterogeneity = 0.76; Supplementary Figure 30). For even-chain SFAs, both 16:0 and 14:0 biomarkers showed positive associations with stroke risk, with the pooled effect estimates of 1.37 (95%CI: 1.15–1.63, P < 0.001; Pheterogeneity = 0.11; Supplementary Figure 31) and 1.19 (95%CI: 1.06–1.35, P = 0.004; Pheterogeneity = 0.10; Supplementary Figure 32), respectively. No significant associations were detected for circulating 15:0, 17:0 or 18:0 and stroke risk (Supplementary Figures 33–35). No evidence of heterogeneity was detected within any of the subgroups. Dose-response analysis between circulating SFAs and stroke risk was limited by a lack of studies.
Sensitivity analysis showed that no single study substantially changed the statistical significance or direction of the combined RR for any of the outcomes. Visual inspection of the funnel plot did not reveal substantial asymmetry (Supplementary Figures 36–67). Similarly, the results of the Egger’s tests and Begg’s tests indicated that no evidence of publication bias was found for associations between circulating SFAs and incidence of these cardiometabolic diseases (P > 0.05 for both tests).
In the present meta-analysis, higher concentration of circulating total SFAs was associated with an increasing risk of CVD, CHD, and stroke, rather than T2D. Compared with the adverse effect of even-chain SFAs, odd-chain SFAs and VLCSFAs showed a significant inverse association with these cardiometabolic diseases. Our study suggested that individual circulating SFAs may not be equally associated with the risk of chronic diseases, which raises the possibility of generating public health recommendations and nutritional guidelines of SFAs in these cardiometabolic diseases prevention.
Previous studies have found that higher dietary intake of SFAs was associated with an increasing risk of chronic health condition (67). Data from prospective cohort studies also showed that dietary replacement of SFAs with polyunsaturated fat or whole grain carbohydrates might significantly reduce the incidence of cardiometabolic disease (8). Nevertheless, investigations into the effects of dietary SFA consumption on chronic disease in humans have been limited by challenges in accurately assessing saturated fat intake from dietary questionnaires, due to errors in recall and wide variations in SFA content of otherwise similar foods. In this setting, measurements of circulating fatty acids provide a more valid and objective biomarker of dietary fat. Up to date, the associations between circulating saturated fats and the risk of vascular disease still remain controversial. A meta-analysis conducted in 2013 have found essentially null associations between circulating SFAs and coronary risk (19), while a recent matched case-control study among 2,428 postmenopausal women in the Women’s Health Initiative Observational Study showed that 1 mol% increase in plasma phospholipid SFAs was associated with a 20% higher CHD risk (59). Similarly, among 7,354 participants from the European Prospective Investigation into Cancer study who were free of CHD at baseline, the risk of developing CHD almost doubled in participants with highest level of circulating SFAs, when compared with those with lowest level (68). Consistent with these recent findings, the present studies suggested that circulating total SFAs was associated with an increased risk of cardiovascular events. Meanwhile, this result was also supported by subgroups analysis of even-chain SFAs which increment in circulating myristic acid, palmitic acid and stearic acid corresponded to the increased risk of these diseases. As the major components of SFAs, 14:0, 16:0, and 18:0 contribute more than 90% of the fatty acid composition of plasma (69). The strength of the positive associations with cardiometabolic risk were similar for this fatty acid subtype and total SFAs, indicating that the detrimental effects of SFAs may mainly be attributed to even-chain SFAs. The results of the present study also indicated that the positive association with cardiometabolic diseases was more pronounced for high palmitic acid level, as compared with other even-chain SFAs (such as: myristic acid, and stearic acid). In a study of 6,379 postmenopausal women followed up for 10 years, participants in the highest category of circulating palmitic acid level experienced a 24% higher T2D risk compared with those in the lowest category, whereas a weak or no association was found for other even-chain SFAs (39). Similarly, the results from the Cardiovascular Health Study also showed an increased risk of cardiometabolic diseases with increasing circulating concertation of palmitic acid, rather than stearic acid (38). Therefore, our observations, together with evidence from previous studies, indicated that decreasing even-chain SFA level, especially palmitic acid, confers benefits for the prevention of cardiometabolic diseases.
The potential mechanisms through which circulating SFAs especially even-chain SFAs increase the risk of chronic disorders have been proposed, including producing inflammation, inducing lipoprotein disorders, and activating endoplasmic reticulum (ER) stress (70–73). Systemic inflammation is recognized as an important contributor to insulin resistance and atherosclerosis, all of which drives the development of metabolic diseases and CVD (72, 74). In previous in vitro experiments, Pal et al. found that even-chain SFAs could induce proinflammatory cytokine expression in adipocytes through the FetA and TLR4 pathway, resulting in insulin resistance (75). Animal experiments have also suggested that SFAs like 16:0 could activate the NLRP3 inflammasome in macrophages by an AMPK-autophagy-ROS signaling pathway (76). Along with the activating of proinflammatory responses, SFAs was thought to impair the LDL-receptor activity and consequently increase the concentration of LDL-cholesterol (LDL-C), an apolipoprotein B-containing lipoprotein that can become trapped in the artery wall and ultimately implicated in the generation of atherosclerosis (77, 78). The adverse effect of palmitic acid on lipoprotein metabolism might explain part of the stronger association between palmitic acid and risk of cardiometabolic diseases. A previous animal study suggested that increasing the absolute amount of dietary 16:0 could increase LDL-cholesterol (LDL-C) more than other even-chain SFAs, through specific modulation of the expression of the LDL receptor and apolipoprotein B genes (79). Clinical trials also showed that serum total cholesterol, apo A-I concentrations and plasma cholesteryl ester transfer protein activity significantly increased in the palmitic acid diet as compared with the stearic acid diet (80, 81). Meanwhile, emerging evidence has suggested that palmitic acid but not myristic or stearic acid exerts adverse effects on ER function by stimulating stress signaling XBP1 and ATF6 (82), which may play a pivotal role in arresting the cell cycle progression in islet (83) and initiating a positive feedback loop in production of even-chain SFAs via de novo lipogenesis (84), ultimately contributes to the occurrence of metabolic diseases.
Compared with the deleterious effects of even-chain SFAs, the present results suggested that a higher level of odd-chain SFAs was associated with a lower risk of chronic conditions. Data from Northern Sweden Health and Disease Study suggested that each percent increase of the proportion of this fatty acid species was significantly associated with a risk reduction of a first myocardial infarction (54). The Västerbotten Intervention Programme has also reported that for participants who were free from diabetes at baseline, per SD increase in circulating 15:0 and 17:0 confer relative reductions in T2D incidence of 29 and 46%, respectively (30). The protection against dysregulated lipid metabolism and low-grade inflammation have been proposed to underlie the beneficial role of odd-chain SFAs because the disorder of fat homeostasis and the expression of inflammatory proteins trigger atherosclerosis and other metabolic syndrome (85). In the European Prospective Investigation into Cancer and Nutrition-InterAct study, inverse associations have been observed between plasma phospholipid 15:0 and 17:0 and lipid markers, such as total cholesterol, triglycerides, apolipoprotein A-1, apolipoprotein B. This study also suggested that increasing odd-chain SFAs was accompanied by a decreased C-reactive protein, per 1-SD increment of the sum of 15:0 and 17:0 was associated with a 10% decrement of this inflammatory marker (86). Additionally, emerging evidence has indicated that this subclass of fatty acids may provide protection against incident T2D by ameliorating the degree of insulin resistance and β-cell dysfunction. In a multicenter study analyzing different race groups in America without diabetes at baseline, Santaren et al. found that serum concentrations of pentadecanoic acid was positively associated with insulin sensitivity and β cell function (Disposition Index), suggesting that this fatty acid species may play a role in T2D prevention (87). Moreover, as the primary source of odd-chain SFAs, similar association was also found in dairy products (53, 88–93). Two recent meta-analyses of randomized controlled trials reported significant reduction in cardiovascular events with dairy foods intake (94, 95). Despite the possible interactions among these two odd-numbered FAs and multiple bioactive substances in dairy products are largely unknown, the previous studies for dairy consumption still lend support to accumulating evidences of an inverse association between odd-chain SFAs and chronic disease.
For VLCSFAs, the present study of an inverse association between circulating VLCSFAs and CVD risk was in line with the results from previous epidemiological studies (20, 96). While diet is unlikely the main source of these SFAs in the human body (97), the VLCSFAs can be synthesized endogenously (98, 99) and serve as essential components of sphingolipids that is the key constituents of membranes in the human body (100, 101). Previous studies have demonstrated a potential association between sphingolipid metabolism and early markers of the latter chronic disease condition such as adiponectin. In a rat model treated with dietary sphingolipids, a 45-day VLCSFAs supplementation significantly improved adiponectin signaling and consequently increased insulin sensitivity (102). Moreover, as the elongation products of long chain SFAs, this consistent association has also been observed between these fatty acids and plasmalogen (103), an ether lipid which may function as cellular antioxidants and scavenge a variety of reactive oxygen species (104).
Some limitations of this study should be mentioned. Firstly, individual blood SFAs were quantitatively measured once and changes of these SFAs over time might lead to potential random measurement error caused by within-person variation. However, among the Nurses’ Health Study and Nurses’ Health Study II participants, reasonable validity and reproducibility of concentration of SFAs such as 15:0, 16:0, and 24:0 have been demonstrated with intraclass correlation coefficients, indicating a single measurement of fatty acid biomarker levels can reliably represent long-term levels over time (105). Secondly, participants with high SFAs consumption tended to have more unhealthy dietary habits and engage in less physical activity (8) which associated with higher cardiometabolic risk (106). Despite all included studies have adjusted for potential confounding factors, we cannot fully exclude the impact of residual or unmeasured confounding on the observed associations. Because of the prospective design of the current study, misclassification caused by potential confounders such as lifestyle was independent of the outcome ascertainment and was therefore more likely to be non-differential, which would tend to attenuate true associations toward the null. However, the positive association between 16:0 status and CVD risk appeared to be more evident among NCC studies compared with PC studies. This may be attributed to their inherent limitations as the NCC studies are more prone to be influenced by survival bias due to all the cases and controls are enrolled at the same time, which may lead to an overestimation of the magnitude of the association (107). Thirdly, different subtypes of SFAs may have possible additive or synergistic effects that results in the development of cardiometabolic diseases. Further additional studies are warranted to assess the potential interaction between levels of different SFA types and cardiovascular disease. Finally, publication bias should be ascertained. Although no significant publication bias was detected, the potential bias could not be completely ruled out.
Our meta-analysis of existing prospective studies indicated that a higher concentration of total SFAs and even-chain SFAs was associated with an increasing risk of cardiometabolic diseases, which supported the current recommendations of reducing intake of saturated fat as part of healthy dietary patterns. In addition, our finding of protective effects of odd-chain SFAs has potentially important clinical implications for preventing cardiometabolic diseases. Further studies are apparently needed to confirm our findings on these SFAs in relation to cardiometabolic outcomes and to elucidate underlying mechanisms.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
LM, JL, and MM generated the idea for the study, formulated an analytical plan, and supervised the study. ZL, HL, and HJ searched for relevant studies, conducted quality assessment, and extracted the data. ZL and HJ performed data analysis. ZL and HL wrote the manuscript and all other authors revised the manuscript. All authors approved the final manuscript for submission and acquired, analyzed, or interpreted the data.
This research was funded by the National Natural Science Foundation of China (NSFC-81473059, NSFC-81973025, NSFC-81202198, and NSFC-81803263), the Natural Science Foundation of Shaanxi Province of China (2017JM8041), the Fundamental Research Funds for the Central Universities (qngz2016004 and xzy032019008), and the Nutrition Research Foundation Fund of the Chinese Nutrition Society-DSM Special Research Foundation (CNSDSM 2016-041). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.963471/full#supplementary-material
1. Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. (2020) 5:CD011737. doi: 10.1002/14651858.CD011737.pub2
2. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020-2025. 9th ed. (2020). Available online at: https://health.gov/our-work/food-nutrition/current-dietary-guidelines (accessed December 1, 2020).
3. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Miller HN, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. (2015) 131:E326. doi: 10.1161/01.cir.0000437740.48606.d1
4. Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu Rev Nutr. (2015) 35:517–43. doi: 10.1161/01.cir.0000437740.48606.d1
5. Hunnicutt JW, Hardy RW, Williford J, McDonald JM. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes. (1994) 43:540–5. doi: 10.1146/annurev-nutr-071714-034449
6. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. (2017) 136:e1–23. doi: 10.1161/CIR.0000000000000510
7. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. (2015) 351:h3978. doi: 10.1136/bmj.h3978
8. Zong G, Li Y, Wanders AJ, Alssema M, Zock PL, Willett WC, et al. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ. (2016) 355:i5796. doi: 10.1136/bmj.i5796
9. Jiao J, Liu G, Shin HJ, Hu FB, Rimm EB, Rexrode KM, et al. Dietary fats and mortality among patients with type 2 diabetes: analysis in two population based cohort studies. BMJ. (2019) 366:l4009. doi: 10.1136/bmj.l4009
10. Shim J-S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. (2014) 36:e2014009. doi: 10.4178/epih/e2014009
11. Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. (1995) 24:389–98. doi: 10.1093/ije/24.2.389
12. Hebert JR, Ma YS, Clemow L, Ockene IS, Saperia G, Stanek EJ, et al. Gender differences in social desirability and social approval bias in dietary self-report. Am J Epidemiol. (1997) 146:1046–55. doi: 10.1093/oxfordjournals.aje.a009233
13. Arab L. Biomarkers of fat and fatty acid intake. J Nutr. (2003) 133:925S–32S. doi: 10.1093/jn/133.3.925S
14. Cantwell MM. Assessment of individual fatty acid intake. Proc Nutr Soc. (2000) 59:187–91. doi: 10.1017/s0029665100000203
15. Clarke R, Shipley M, Armitage J, Collins R, Harris W. Plasma phospholipid fatty acids and CHD in older men: Whitehall study of London civil servants. Br J Nutr. (2009) 102:279–84. doi: 10.1017/S0007114508143562
16. Chei C-L, Yamagishi K, Kitamura A, Kiyama M, Sankai T, Okada T, et al. Serum fatty acid and risk of coronary artery disease-Circulatory Risk in Communities Study (CIRCS). Circ J. (2018) 82:3013–20. doi: 10.1253/circj.CJ-18-0240
17. Bragg F, Kartsonaki C, Guo Y, Holmes M, Du H, Yu C, et al. Circulating metabolites and the development of type 2 diabetes in Chinese adults. Diabetes Care. (2022) 45:477–80. doi: 10.2337/dc21-1415
18. Flock MR, Kris-Etherton PM. Diverse physiological effects of long-chain saturated fatty acids: implications for cardiovascular disease. Curr Opin Clin Nutr Metab Care. (2013) 16:133–40. doi: 10.1097/MCO.0b013e328359e6ac
19. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. (2014) 160:398–406. doi: 10.7326/m13-1788
20. Ardisson Korat AV, Malik VS, Furtado JD, Sacks F, Rosner B, Rexrode KM, et al. Circulating very-long-chain SFA concentrations are inversely associated with incident type 2 diabetes in US men and women. J Nutr. (2020) 150:340–9. doi: 10.1093/jn/nxz240
21. de Oliveira OMC, Lemaitre RN, Song X, King IB, Siscovick DS, Mozaffarian D. Serial measures of circulating biomarkers of dairy fat and total and cause-specific mortality in older adults: the Cardiovascular Health Study. Am J Clin Nutr. (2018) 108:476–84. doi: 10.1093/ajcn/nqy117
22. Wells GA, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. (2020). Available online at: http://www.ohri.caprograms/clinical_epidemiology/oxford.asp (accessed April 21, 2020).
23. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease – meta-analyses of prospective studies. JAMA. (1998) 279:1477–82. doi: 10.1001/jama.279.18.1477
24. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
26. Duval S, Tweedie RA. non parametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. (2000) 95:89–98. doi: 10.1080/01621459.2000.10473905
27. Laaksonen DE, Lakka TA, Lakka HM, Nyyssonen K, Rissanen T, Niskanen LK, et al. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med. (2002) 19:456–64. doi: 10.1046/j.1464-5491.2002.00707.x
28. Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Am J Clin Nutr. (2003) 78:91–8. doi: 10.1093/ajcn/78.1.91
29. Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, Gibson RA, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. (2007) 86:189–97. doi: 10.1093/ajcn/86.1.189
30. Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, et al. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. (2008) 18:503–10. doi: 10.1016/j.numecd.2007.04.005
31. Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med. (2010) 153:790–9. doi: 10.7326/0003-4819-153-12-201012210-00005
32. Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw K-T, Wareham NJ, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. (2010) 92:1214–22. doi: 10.3945/ajcn.2010.29182
33. Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EHJM, Döring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam study. Am J Clin Nutr. (2011) 93:127–42. doi: 10.3945/ajcn.110.005447
34. Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, et al. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. (2013) 97:854–61. doi: 10.3945/ajcn.112.045468
35. Zong G, Zhu J, Sun L, Ye X, Lu L, Jin Q, et al. Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am J Clin Nutr. (2013) 98:319–26. doi: 10.3945/ajcn.113.061218
36. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. (2014) 2:810–8. doi: 10.1016/S2213-8587(14)70146-9
37. Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the cardiovascular health study. Am J Clin Nutr. (2015) 101:1047–54. doi: 10.3945/ajcn.114.101857
38. Ma W, Wu JHY, Wang Q, Lemaitre RN, Mukamal KJ, Djoussé L, et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the cardiovascular health study. Am J Clin Nutr. (2015) 101:153–63. doi: 10.3945/ajcn.114.092601
39. Harris WS, Luo J, Pottala JV, Margolis KL, Espeland MA, Robinson JG. Red blood cell fatty acids and incident diabetes mellitus in the women’s health initiative memory study. PLoS One. (2016) 11:e0147894. doi: 10.1371/journal.pone.0147894
40. Yakoob MY, Shi P, Willett WC, Rexrode KM, Campos H, Orav EJ, et al. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation. (2016) 133:1645–54. doi: 10.1161/CIRCULATIONAHA.115.018410
41. Akter S, Kurotani K, Sato M, Hayashi T, Kuwahara K, Matsushita Y, et al. High serum phospholipid dihomo-γ-linoleic acid concentration and low Δ5-desaturase activity are associated with increased risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. J Nutr. (2017) 147:1558–66. doi: 10.3945/jn.117.248997
42. Lin J, Dong H, Chen G, Chen Z, Dong X, Zheng J, et al. Erythrocyte saturated fatty acids and incident type 2 diabetes in Chinese men and women: a prospective cohort study. Nutrients. (2018) 10:1393. doi: 10.3390/nu10101393
43. Lu Y, Wang Y, Zou L, Liang X, Ong CN, Tavintharan S, et al. Serum lipids in association with type 2 diabetes risk and prevalence in a Chinese population. J Clin Endocrinol Metab. (2018) 103:671–80. doi: 10.1210/jc.2017-02176
44. Zhuang P, Liu X, Li Y, Li H, Zhang L, Wan X, et al. Circulating fatty acids and genetic predisposition to type 2 diabetes: gene-nutrient interaction analysis. Diabetes Care. (2022) 45:564–75. doi: 10.2337/dc21-2048
45. Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr. (2008) 88:203–9. doi: 10.1093/ajcn/88.1.203
46. Woodward M, Tunstall-Pedoe H, Batty GD, Tavendale R, Hu FB, Czernichow S. The prognostic value of adipose tissue fatty acids for incident cardiovascular disease: results from 3944 subjects in the Scottish Heart Health Extended Cohort Study. Eur Heart J. (2011) 32:1416–23. doi: 10.1093/eurheartj/ehr036
47. Chien K-L, Lin H-J, Hsu H-C, Chen P-C, Su T-C, Chen M-F, et al. Comparison of predictive performance of various fatty acids for the risk of cardiovascular disease events and all-cause deaths in a community-based cohort. Atherosclerosis. (2013) 230:140–7. doi: 10.1016/j.atherosclerosis.2013.06.015
48. Fretts AM, Mozaffarian D, Siscovick DS, King IB, McKnight B, Psaty BM, et al. Associations of plasma phospholipid SFAs with total and cause-specific mortality in older adults differ according to SFA chain length. J Nutr. (2016) 146:298–305. doi: 10.3945/jn.115.222117
49. Trieu K, Bhat S, Dai Z, Leander K, Gigante B, Qian F, et al. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: a cohort study, systematic review, and meta-analysis. PLoS Med. (2021) 18:e1003763. doi: 10.1371/journal.pmed.1003763
50. Simon JA, Hodgkins ML, Browner WS, Neuhaus JM, Bernert JT, Hulley SB. Serum fatty acids and the risk of coronary heart disease. Am J Epidemiol. (1995) 142:469–76. doi: 10.1093/oxfordjournals.aje.a117662
51. Wang L, Folsom AR, Eckfeldt JH, The ASI. Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nutr Metab Cardiovasc Dis. (2003) 13:256–66. doi: 10.1016/s0939-4753(03)80029-7
52. Warensjö E, Jansson J-H, Berglund L, Boman K, Ahrén B, Weinehall L, et al. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction. A prospective case-control study. Br J Nutr. (2004) 91:635–42. doi: 10.1079/BJN20041080
53. Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. (2007) 86:929–37. doi: 10.1093/ajcn/86.4.929
54. Warensjö E, Jansson J-H, Cederholm T, Boman K, Eliasson M, Hallmans G, et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr. (2010) 92:194–202. doi: 10.3945/ajcn.2009.29054
55. Wu JHY, Lemaitre RN, Imamura F, King IB, Song X, Spiegelman D, et al. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: the cardiovascular health study. Am J Clin Nutr. (2011) 94:431–8. doi: 10.3945/ajcn.111.012054
56. Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med. (2012) 9:e1001255. doi: 10.1371/journal.pmed.1001255
57. Malik VS, Chiuve SE, Campos H, Rimm EB, Mozaffarian D, Hu FB, et al. Circulating very-long-chain saturated fatty acids and incident coronary heart disease in US men and women. Circulation. (2015) 132:260–8. doi: 10.1161/CIRCULATIONAHA.114.014911
58. Sun Y, Koh HWL, Choi H, Koh W-P, Yuan J-M, Newman JW, et al. Plasma fatty acids, oxylipins, and risk of myocardial infarction: the Singapore Chinese Health Study. J Lipid Res. (2016) 57:1300–7. doi: 10.1194/jlr.P066423
59. Liu Q, Matthan NR, Manson JE, Howard BV, Tinker LF, Neuhouser ML, et al. Plasma phospholipid fatty acids and coronary heart disease risk: a matched case-control study within the Women’s Health Initiative Observational Study. Nutrients. (2019) 11:1672. doi: 10.3390/nu11071672
60. Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, et al. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. (2002) 33:2086–93. doi: 10.1161/01.str.0000023890.25066.50
61. Wiberg B, Sundström J, Aìrnlöv J, Tereìnt A, Vessby B, Zethelius B, et al. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men. Stroke. (2006) 37:2898–903. doi: 10.1161/01.STR.0000249056.24657.8b
62. Warensjo E, Smedman A, Stegmayr B, Hallmans G, Weinehall L, Vessby B, et al. Stroke and plasma markers of milk fat intake–a prospective nested case-control study. Nutr J. (2009) 8:21. doi: 10.1186/1475-2891-8-21
63. Yaemsiri S, Sen S, Tinker LF, Robinson WR, Evans RW, Rosamond W, et al. Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke. (2013) 44:2710–7. doi: 10.1161/STROKEAHA.111.000834
64. Yamagishi K, Folsom AR, Steffen LM Aric Study Investigators. Plasma fatty acid composition and incident ischemic stroke in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Cerebrovasc Dis. (2013) 36:38–46. doi: 10.1159/000351205
65. Yakoob MY, Shi P, Hu FB, Campos H, Rexrode KM, Orav EJ, et al. Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am J Clin Nutr. (2014) 100:1437–47. doi: 10.3945/ajcn.114.083097
66. Huang NK, Biggs ML, Matthan NR, Djousse L, Longstreth WT, Mukamal KJ, et al. Serum nonesterified fatty acids and incident stroke: the CHS. J Am Heart Assoc. (2021) 10:e022725. doi: 10.1161/JAHA.121.022725
67. Mann JI. Diet and risk of coronary heart disease and type 2 diabetes. Lancet. (2002) 360:783–9. doi: 10.1016/s0140-6736(02)09901-4
68. Katan MB, Khaw K-T, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med. (2012) 9:e1001255. doi: 10.1371/journal.pmed.1001255
69. Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. (2005) 162:373–81. doi: 10.1093/aje/kwi213
70. Dias JA, Wirfält E, Drake I, Gullberg B, Hedblad B, Persson M, et al. A high quality diet is associated with reduced systemic inflammation in middle-aged individuals. Atherosclerosis. (2015) 238:38–44. doi: 10.1016/j.atherosclerosis.2014.11.006
71. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. (2012) 32:2045–51. doi: 10.1161/atvbaha.108.179705
72. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes. (2003) 52:1799–805. doi: 10.2337/diabetes.52.7.1799
73. Vallim T, Salter AM. Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. (2010) 82:211–8. doi: 10.1016/j.plefa.2010.02.016
75. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. (2012) 18:1279–85. doi: 10.1038/nm.2851
76. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. (2011) 12:408–15. doi: 10.1038/ni.2022
77. Ference BA, Kastelein JJP, Catapano AL. Update on lipids and lipoproteins-reply. JAMA. (2021) 325:400–1.
78. Jialal I, Devaraj S. Update on lipids and lipoproteins. JAMA. (2021) 325:400. doi: 10.1001/jama.2020.22621
79. Salter AM, Mangiapane EH, Bennett AJ, Bruce JS, Billett MA, Anderton KL, et al. The effect of different dietary fatty acids on lipoprotein metabolism: concentration-dependent effects of diets enriched in oleic, myristic, palmitic and stearic acids. Br J Nutr. (1998) 79:195–202. doi: 10.1079/bjn19980031
80. Snook JT, Park S, Williams G, Tsai Y-H, Lee N. Effect of synthetic triglycerides of myristic, palmitic, and stearic acid on serum lipoprotein metabolism. Eur J Clin Nutr. (1999) 53:597–605. doi: 10.1038/sj.ejcn.1600815
81. Schwab US, Maliranta HM, Sarkkinen ES, Savolainen MJ, Kesäniemi YA, Uusitupa MI. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metabolism. (1996) 45:143–9. doi: 10.1016/s0026-0495(96)90044-x
82. Cnop M, Abdulkarim B, Bottu G, Cunha DA, Igoillo-Esteve M, Masini M, et al. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes. (2014) 63:1978–93. doi: 10.2337/db13-1383
83. Babour A, Bicknell AA, Tourtellotte J, Niwa MA. surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell. (2010) 142:256–69. doi: 10.1016/j.cell.2010.06.006
84. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. (2018) 98:2133–223. doi: 10.1152/physrev.00063.2017
85. Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, et al. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab. (2016) 23:165–78. doi: 10.1016/j.cmet.2015.10.003
86. Zheng JS, Sharp SJ, Imamura F, Koulman A, Schulze MB, Ye Z, et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med. (2017) 15:203. doi: 10.1186/s12916-017-0968-4
87. Santaren ID, Watkins SM, Liese AD, Wagenknecht LE, Rewers MJ, Haffner SM, et al. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr. (2014) 100:1532–40. doi: 10.3945/ajcn.114.092544
88. Lankinen M, Schwab U. Biomarkers of dairy fat. Am J Clin Nutr. (2015) 101:1101–2. doi: 10.3945/ajcn.114.104554
89. Mitri J, Tomah S, Furtado J, Tasabehji MW, Hamdy O. Plasma free fatty acids and metabolic effect in type 2 diabetes, an ancillary study from a randomized clinical trial. Nutrients. (2021) 13:1145. doi: 10.3390/nu13041145
90. Abdullah MM, Cyr A, Lepine MC, Labonte ME, Couture P, Jones PJ, et al. Recommended dairy product intake modulates circulating fatty acid profile in healthy adults: a multi-centre cross-over study. Br J Nutr. (2015) 113:435–44. doi: 10.1017/S0007114514003894
91. Pfeuffer M, Jaudszus A. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv Nutr. (2016) 7:730–4. doi: 10.3945/an.115.011387
92. Golley RK, Hendrie GA. Evaluation of the relative concentration of serum fatty acids C14:0, C15:0 and C17:0 as markers of children’s dairy fat intake. Ann Nutr Metab. (2014) 65:310–6. doi: 10.1159/000368325
93. Brevik A, Veierød MB, Drevon CA, Andersen LF. Evaluation of the odd fatty acids 15:0 and 17:0 in serum and adipose tissue as markers of intake of milk and dairy fat. Eur J Clin Nutr. (2005) 59:1417–22. doi: 10.1038/sj.ejcn.1602256
94. Zhang K, Chen X, Zhang L, Deng Z. Fermented dairy foods intake and risk of cardiovascular diseases: a meta-analysis of cohort studies. Crit Rev Food Sci Nutr. (2020) 60:1189–94. doi: 10.1080/10408398.2018.1564019
95. Companys J, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Solà R, Pedret A, et al. Fermented dairy products, probiotic supplementation, and cardiometabolic diseases: a systematic review and meta-analysis. Adv Nutr. (2020) 11:834–63. doi: 10.1093/advances/nmaa030
96. Gallo S, Egeland G, Meltzer S, Legault L, Kubow S. Plasma fatty acids and desaturase activity are associated with circulating adiponectin in healthy adolescent girls. J Clin Endocrinol Metab. (2010) 95:2410–7. doi: 10.1210/jc.2009-1975
97. Us Department of Agriculture, Agricultural Research Service. Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28 (Slightly revised). (2016). Available online at: http://www.ars.usda.gov/nea/bhnrc/mafcl (accessed May 1, 2016)
98. Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem. (2012) 152:387–95. doi: 10.1093/jb/mvs105
99. Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. (2006) 45:237–49. doi: 10.1016/j.plipres.2006.01.004
100. Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. (2014) 510:58–67. doi: 10.1038/nature13475
101. Merrill AH Jr. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. (2002) 277:25843–6. doi: 10.1074/jbc.R200009200
102. Yunoki K, Renaguli M, Kinoshita M, Matsuyama H, Mawatari S, Fujino T, et al. Dietary sphingolipids ameliorate disorders of lipid metabolism in Zucker fatty rats. J Agric Food Chem. (2010) 58:7030–5. doi: 10.1021/jf100722f
103. Yamazaki Y, Kondo K, Maeba R, Nishimukai M, Nezu T, Hara H. The Proportion of nervonic acid in serum lipids is associated with serum plasmalogen levels and metabolic syndrome. J Oleo Sci. (2014) 63:527–37. doi: 10.5650/jos.ess13226
104. Dean JM, Lodhi IJ. Structural and functional roles of ether lipids. Protein Cell. (2018) 9:196–206. doi: 10.1007/s13238-017-0423-5
105. Kotsopoulos J, Tworoger SS, Campos H, Chung F-L, Clevenger CV, Franke AA, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. (2010) 19:938–46. doi: 10.1158/1055-9965.EPI-09-1318
106. Zhang YB, Chen C, Pan XF, Guo J, Li Y, Franco OH, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. (2021) 373:n604. doi: 10.1136/bmj.n604
Keywords: saturated fatty acid biomarker, cardiometabolic disease, type 2 diabetes, coronary heart disease, stroke, cardiovascular disease, meta-analysis
Citation: Li Z, Lei H, Jiang H, Fan Y, Shi J, Li C, Chen F, Mi B, Ma M, Lin J and Ma L (2022) Saturated fatty acid biomarkers and risk of cardiometabolic diseases: A meta-analysis of prospective studies. Front. Nutr. 9:963471. doi: 10.3389/fnut.2022.963471
Received: 15 June 2022; Accepted: 26 July 2022;
Published: 15 August 2022.
Edited by:
Devin Wahl, Colorado State University, United StatesReviewed by:
Marta Gonzalez-Freire, Balearic Islands Health Research Institute (IdISBa), SpainCopyright © 2022 Li, Lei, Jiang, Fan, Shi, Li, Chen, Mi, Ma, Lin and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Ma, bWFsZUBtYWlsLnhqdHUuZWR1LmNu; Jing Lin, bGluamluZzAxMjdAeGp0dS5lZHUuY24=; Mao Ma, bWFtYW8yMDA3QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.