- 1Noncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran
- 2Department of Nutrition, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- 3National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Department of Public Health, School of Health, Larestan University of Medical Sciences, Larestan, Iran
- 5Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
- 6Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran
Background/objectives: There are limited data on the association between dairy products consumption and nonalcoholic fatty liver disease (NAFLD). This study was conducted to evaluate the association between total intake of different dairy products and fatty liver index (FLI), a marker of subclinical fatty liver.
Methods: A total of 7,540 adults were included in this population-based cohort study. Dairy products consumption was evaluated by a validated interview questionnaire for food intake frequency. The FLI was calculated using the standard formula. Liver enzyme levels, lipid profiles, glycemic profiles and demographic characteristics were recorded for all participants. Univariate and multiple logistic regression models were used to respectively assess the mean percentage difference of mean FLI and odds ratios (ORs) for subclinical NAFLD across quantiles of dairy consumption.
Results: The mean age of all participants was 48.81 ± 9.631 years. FLI measurements for men and women were 26.71 ± 23.39 and 39.99 ± 26.64 respectively, which was significantly higher in women (P < 0.05). Multiple logistic regression analysis demonstrated that the amount of milk consumption was an independent preventive predictor of FLI (OR = 0.96; 95% CI: 0.94–0.99), conversely, it did not predict higher levels of liver enzymes. In term of cheese intake, participants in the third tertile of cheese intake had significantly lower FLI than lower tertiles (P = 0.01). However, there wasn't any significant association between cheese intake and the odds of FLI in the multivariate model (P > 0.05). We didn't find any significant association between yogurt consumption and NAFLD indicators (P > 0.05).
Conclusion: Higher milk consumption was inversely associated with FLI. However, there wasn't any significant association between other types of dairy products and NAFLD indicators.
Introduction
Nonalcoholic Fatty Liver Disease (NAFLD) is a clinical histopathological condition in which triglyceride accumulates in the liver cells of patients with little or no history of alcohol consumption (1). This disease usually happens when fat accumulation reaches 5–10% of the liver's weight. NAFLD has a wide histological spectrum ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and more severe complications like cirrhosis or liver fibrosis (2, 3).
The total Prevalence of NAFLD based on a Meta-analytic study from 22 countries, has been reported as 25.24 % (4). In Asia, the number of patients with NAFLD is gradually increasing (5) due to lifestyle changes (high-fat diet, low physical activity, central obesity, and type 2 diabetes mellitus), which is comparable to Western countries (6).
NAFLD can lead to cirrhosis, cardiovascular disease, type 2 diabetes mellitus, and chronic kidney disease if left untreated (7).
Today, various studies have shown that improving the microbial profile of the intestine plays an important role in the prevention and treatment of NAFLD by reducing oxidative stress and inflammation, as well as decreasing triglycerides (TG) accumulation in the liver (8–12). As a result, the chance of hepatic steatosis will decrease. Dairy products are widely included in daily diet due to their high nutrition contents of protein, fat, minerals, and vitamins (13). Currently, it is recommended to limit the consumption of dairy products due to high levels of saturated fat and cholesterol, in order for reducing the risk of cardiovascular disease. However, a cross-sectional analysis of the Oslo Health Study has shown that increasing the frequency of consumption of some dairy products, such as cheese, is positively associated with improved serum concentrations of high-density lipoprotein (HDL) and is inversely related to serum triglyceride levels (14). Moreover, the consumption of some dairy products, especially those fortified with probiotics, appears to reduce the level of low-density lipoprotein (LDL) cholesterol in human intervention studies (15).
This study aims to focus on the relevance of dairy products consumption with NAFLD and fatty liver index in a large-population cohort in Fasa, Iran.
Patients and methods
Study population
In a population-based cohort, at least 10,000 people within the age range of 35–70 years old from Sheshdeh, the suburb of Fasa city and its 24 satellite villages were recruited. A detailed demographic, socioeconomic, anthropometric, nutrition, and medical history was obtained for each individual besides limited physical examinations and determination of physical activity and sleep patterns supplemented by body composition and electrocardiographic records (16). Participants older than 35 years with one of the following conditions were included in the study: alanine aminotransferase level >30 U/L, Gamma-glutamyltransferase >35 U/L.
Individuals with evidence of alcohol consumption of more than 20 grams per day, hepatitis, drug-induced liver disease, Wilson's disease, hereditary hemochromatosis, encephalopathy, or variceal bleeding were excluded from the study. Routine laboratory assessments were done, and a comprehensive biobank was compiled for future biological investigations. All data were stored online using a dedicated software.
Measurement of clinical parameters and biochemical analysis
Fatty liver index (FLI) is a standard predictor of hepatic steatosis severity. The following formula was designed and validated by Bogoni et al. for calculating FLI using laboratory and anthropometric data, including gamma glutamyl transferase concentration, triglyceride, Body mass index and waist circumference (17).
FLI = (e 0.953 × loge (TG) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × WC – 15.745) / (1 + e 0.953 × loge (TG) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × WC – 15.745) × 100
To calculate the amount of dairy intake, a validated food frequency questionnaire (FFQ) was used. Different dairy products consumption was recorded separately according to the amount of daily, weekly, monthly and annual intake units. The questionnaire included 100 items with a specified serving size, 7 response frequency categories from “almost never eat” to “twice or more per day” for foods, and 8 response frequency categories from “almost never drink” to “Four or more times a day” for beverages. Total daily energy and nutrient intake are calculated based on the latest available table of Iranian food composition. Frequency of consumption of dairy products (milk, yogurt, cheese, doogh, curd and cream and any other dairy products) in the last month was assessed using 7 categories of answers as follows: Almost never, less than once a week, once a week, 2–3 times a week, 4–6 times a week, once a day and twice a day. Detailed information about the design, foods included and the validity of this questionnaire has been reported elsewhere (18). Previous validation studies of this FFQ designed specifically for Iranian adults revealed good correlation between dietary intakes assessed by a similar FFQ and multiple days of 24-h dietary recalls (18, 19).
Statistical analysis
The characteristics of the participants across this study were showed in quartiles of FLI. Continuous variables were presented as mean ± SD and categorical variables as frequency and percentages (%). Data were analyzed using SPSS (version 18; SPSS Inc., Chicago, IL, USA) by running the student's t-test and one way ANOVA, and chi-squared tests. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated from univariate and multiple logistic regression for being classified with an undesirable level of FLI, ALT, AST and GGT and increasing quartile groups (Q1–Q4) for intake of dairy products. Multiple logistic regression models using backward elimination method were applied, adjusted for age, energy, physical activity, BMI, vitamin D levels, selenium levels, smoking status, hypertension, hypercholesterolemia, and diabetes mellitus, and for each relevant food items. Statistical tests were two-sided and P < 0.05 was considered statistically significant.
Results
Characteristics of study participants by sex and age
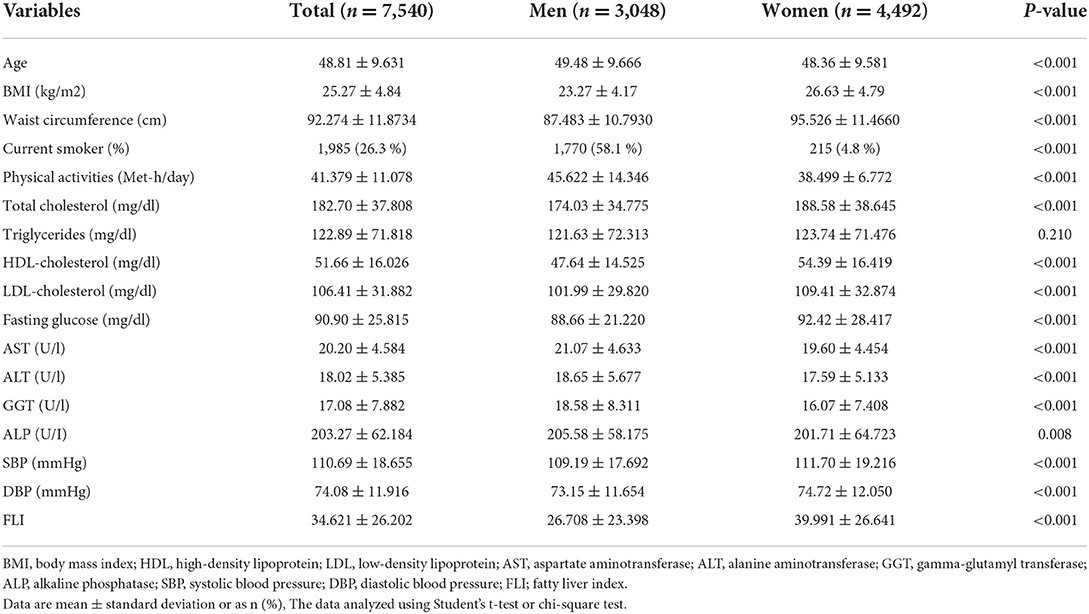
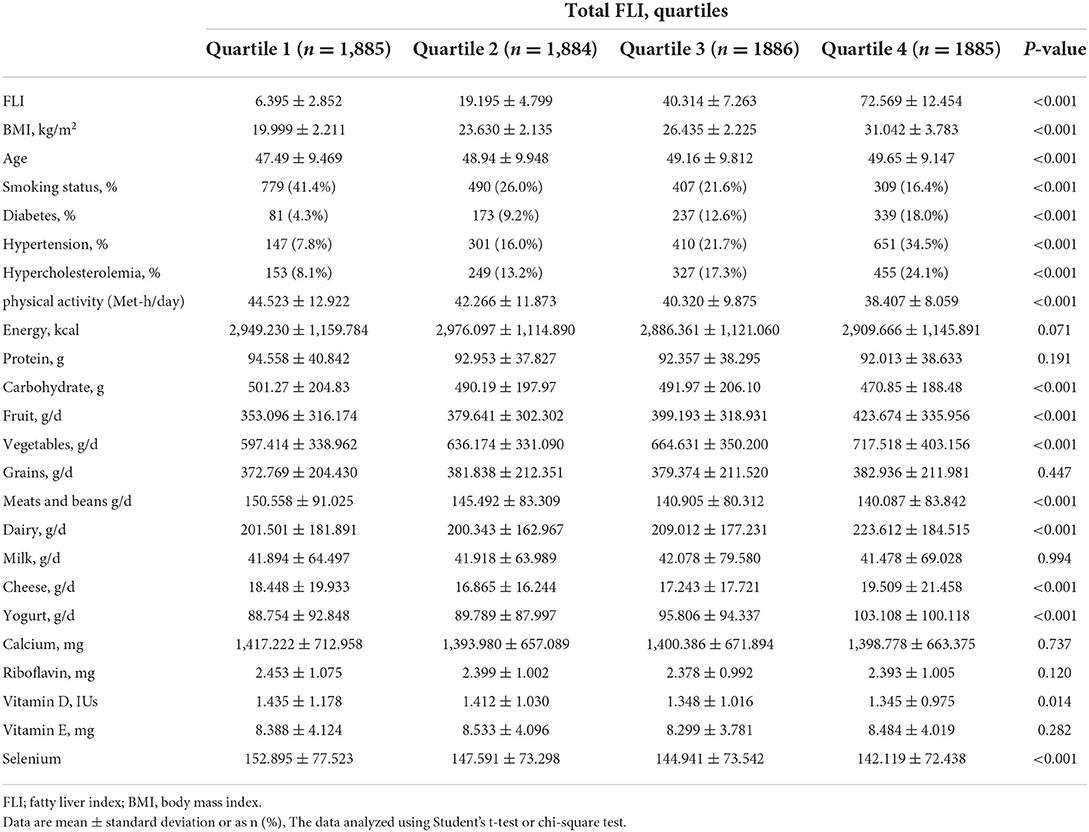
Characteristics of the study participants are presented in the Table 1. Data from 7,540 participants (59% female) were included in the final analysis. The mean age of all participants in this study was 48.81 ± 9.631 years. BMI was significantly higher in females as compared with males (26.63 ± 4.79 kg/m2 vs. 23.27 ± 4.17 kg/m2; P < 0.001). FLI was higher in females than males (39.991 ± 26.64 vs. 26.708 ± 23.39; P < 0.05). Also, serum levels of HDL, TC, LDL, FBS, SBP and DBP were significantly higher in females than males (P < 0.001). Dietary intakes of study participants across quartile of FLI score are presented in Table 2. A greater FLI score was significantly associated with the higher intake of carbohydrates, fruits, vegetables, meats, beans, dairy, vitamin D and selenium (P < 0.05).
Association of fatty liver index with dairy products
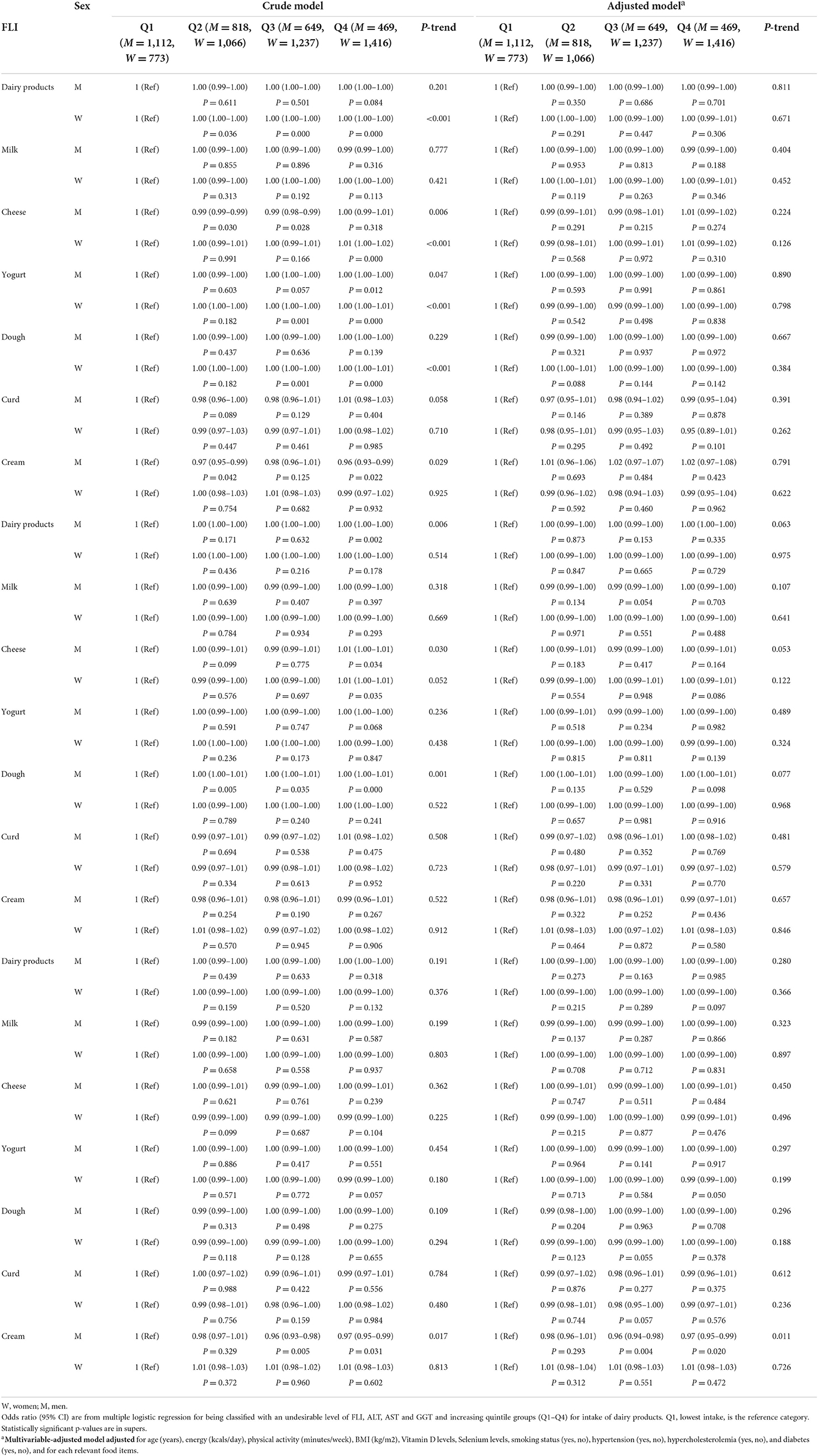
Crude and multiple-adjusted models for mean intake of dairy intake across quartiles of FLI are shown in Table 3. After controlling for potential confounders, participants in the highest quartile of FLI didn't have higher odds for dairy products intake [(OR = 1.00; 95% CI: 0.99, 1.00; P = 0.084) for males and (OR = 1.00; 95% CI: 0.99, 1.01; P = 0.306) for females]. Also, both females and males in the highest quartile of FLI didn't have higher odds for consumption of milk, yogurt, cheese, doogh, curd and cream (P > 0.05). We also examined the association between ALT and AST quartiles and odds of dairy consumption. The results showed that individuals in the highest ALT quartile did not have odds of more dairy products consumption. Participants in the highest quartile of AST had higher odds only for cream intake (OR= 0.97; 95% CI: 0.95, 0.99; Ptrend = 0.011). There wasn't any significant association between other dairy products and ALT and AST quartiles (P > 0.05).
The results of univariate and multiple analyses for determining independent association between fatty liver index and dairy consumption are shown in the Table 4. The mean FLI in the tertile 3 milk intake was 34.08 (95% CI: 32.99, 35.17) that wasn't significantly different from tertile 1 (P = 0.171). Mean liver enzymes did not differ significantly in different milk consumption tertiles (P = 0.08 for AST, P = 0.174 for ALT, and P = 0.491 for GGT).
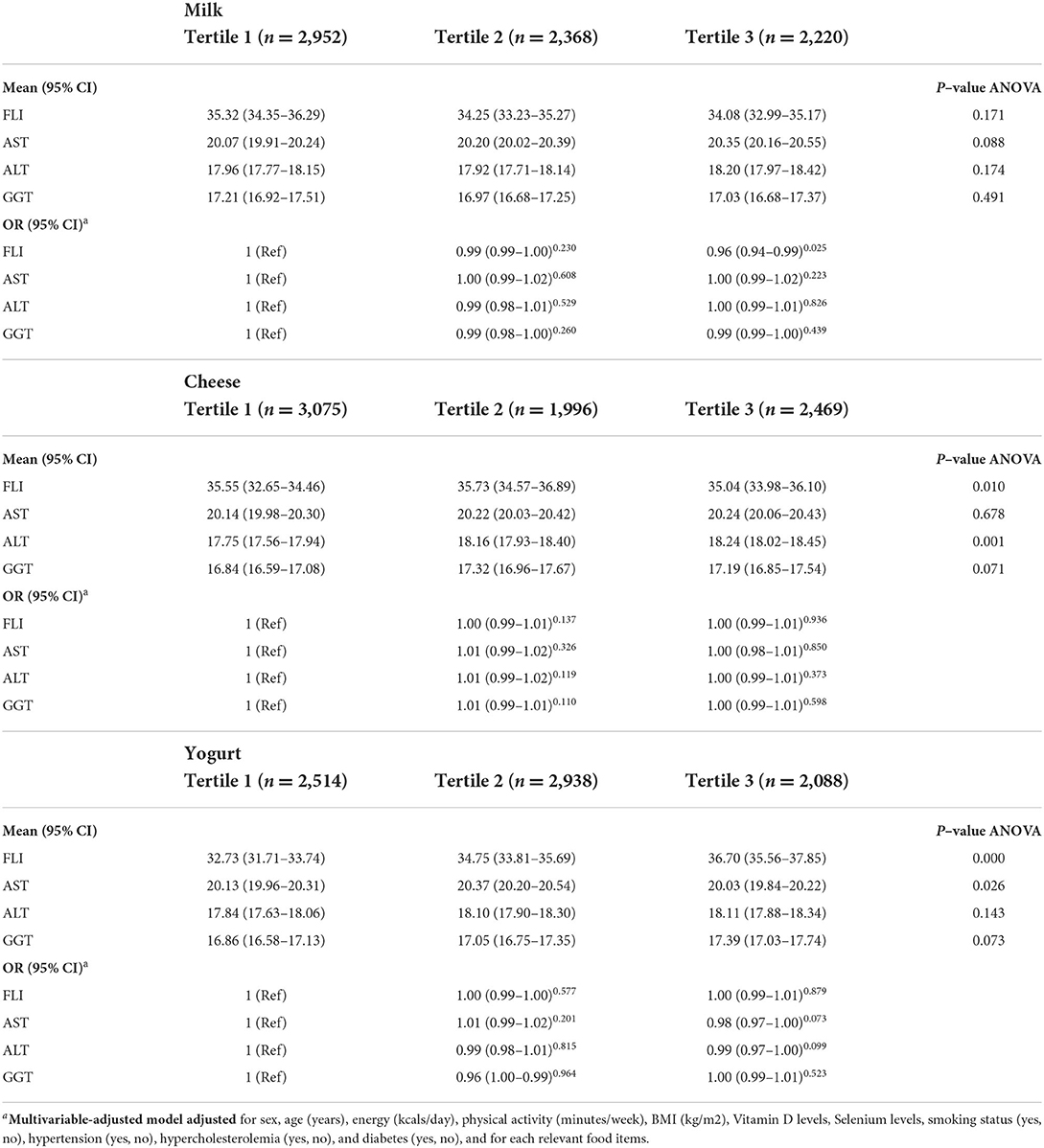
Table 4. Univariate and multiple analyses to determine the independent association between fatty liver index and dairy consumption.
The association between the amount of dairy consumption and severity of fatty liver disease is shown in Table 4. When controlled for confounding factors, multiple logistic regression analysis demonstrated that the amount of milk consumption was the independent preventive predictor of FLI (OR = 0.96; 95% CI: 0.94, 0.99; P = 0.025), but was not the predictors of higher levels of AST, ALT and GGT (P > 0.05). For the cheese intake, participants in the tertile 3 had significantly lower FLI than the lowest tertile 35.04 (95% CI: 33.98, 36.10; P = 0.01). Individuals in the highest tertile of cheese intake had higher levels of ALT than lowest tertile 18.24 mg/dl (95% CI: 18.02, 18.45; P = 0.001). However, there wasn't any significant association between cheese intake and odds of fatty liver index (P > 0.05).
Participants in the highest tertile of yogurt intake had significantly higher FLI scores 36.70 (95% CI: 35.56, 37.85; P < 0.001) and AST concentration 20.03 mg/dl (95% CI: 19.84, 20.22; P = 0.026). We did not find a significant association between yogurt consumption and fatty liver index or biomarkers (P > 0.05).
Discussion
The results of this study clearly indicate that participants with higher FLI score consume more dairy, cheese and yogurt compared to the subjects with lower FLI. Moreover, there is no strong correlation between dairy consumption and fatty liver indicators and only milk consumption is a preventive indicator of FLI. Also, in our study, the consumption of dairy products in women had more protective effects against NAFLD than in men.
The impacts of dairy products on the amounts of liver-derived lipoproteins have been widely studied and discussed previously (20, 21). Several studies have looked at dietary factors, including dairy intake as the key interventional variable on NAFLD-related parameters in NAFLD patients. In some observational studies, researchers have reported an inverse association between low-fat dairy intake and the severity of NAFLD. Ferolla et al. in a cross-sectional study of 96 patients with NAFLD observed an inverse association between low fat dairy intake and NAFLD severity (22). Similar findings were found in another study (23). Conversely, it has been shown that some dietary regiments which contain adequate amounts of dairy products such as DASH (Dietary Approaches to Stop Hypertension) can have a positive effect on reducing the symptoms of NAFLD. Razavi Zade et al. conducted a clinical trial with 3 daily servings with low dairy products to evaluate the effects of DASH diet on the NAFLD severity and showed adherence to DASH eating pattern leads to a significant reduction in serum levels of ALT, AST, and insulin resistance (24). However, part of the positive effects of DASH diet on fatty liver can be attributed to high amounts of fibers and phytochemicals in the DASH diet (25).
In the present study, except for milk and FLI correlation, we did not observe any correlations between dairy products and fatty liver biomarkers. Contrary to our findings, in a 6-week randomized controlled trial, Dugan et al. found a significant reduction in ALT, AST, hepatic steatosis index, and mRNA expression of IL-6 and IL-1β in peripheral blood mononuclear cells (PBMCs), following the consumption of 3 daily servings of low-fat dairy (296 mL 1% milk, 170 g non-fat yogurt, 56.7 g 2% cheese) as compared with isocaloric carbohydrate-based control foods (26). A recent meta-analysis was done on the association between food groups and the risk of NAFLD which included three cross sectional studies and one case control study. The cross-sectional studies didn't find a significant correlation between dairy consumption and the likelihood of NAFLD. However, the case–control study showed a positive association between dairy product consumption and the possibility of NAFLD (27).
The diversity in the type of dairy products and their fat percentages may partly account for the difference observed in the results. Most previous studies that have reported significant changes on fatty liver related indicators following dairy consumption have examined the effect of low-fat dairy products, while in our study participants consumed both high-fat and low-fat dairy products. In fact, the diet of Iranian people especially those in rural areas is mostly based on high consumption of high-fat dairy products (28). Esmaillzadeh et al. studied 486 healthy Iranian women aged 40–60 years and found an inverse association between low fat dairy intake and C-reactive protein, IL-6 and soluble vascular cell adhesion molecule (29). Moreover, they reported an increase in serum amyloid A and soluble vascular cell adhesion molecule-1 among women with high fat dairy consumption (30). High-fat dairy products contain high levels of saturated fatty acids which play a key role in dyslipidemia, insulin resistance and inflammation, that altogether are major risk factors for NAFLD (31).
In contrast with our findings, Zhang et al. in a cross-sectional study of 24,389 adults found that participants with yogurt consumption more than 4 times per weeks had lower likelihood for NAFLD (32). Other similar studies have shown a preventative role for yogurt consumption in the development of other chronic diseases such as cardiovascular disease and type 2 diabetes (29, 33). Most studies that have shown the positive effect of yogurt consumption on liver enzymes have used a variety of probiotic yogurts (9, 34).
In our study, participants in the higher FLI quartile consumed significantly more yogurt than subjects in the lower quartile of FLI. The present study was a cross-sectional study and due to the nature of cross-sectional studies it is not possible to identify the causal relationship between two variables. However, it seems that this difference might be attributed to the fact that people who are at risk of NAFLD increase their yogurt consumption to get more probiotics because of the positive role of bacteria in yogurt against NAFLD, especially probiotic yogurt. The positive effect of yogurt on fatty liver has been attributed to the presence of various probiotic compounds in yogurt. Animal studies have shown that some bacterial strains, such as Lactobacillus, have the ability to reduce the inflammation caused by high levels of lipopolysaccharide (LPS) and subsequent hepatic toll-like receptor 4 (TLR4) activation in NAFLD patients (35). Additionally, part of the beneficial effects of dairy products, especially low-fat dairy products, might be due to high amounts of nutrients, especially magnesium, potassium, protein and calcium in dairy products, which in turn can increase the whole-body fat oxidation (36, 37).
In the present study, we conducted sex-specific analyses which showed that the consumption of dairy products in women had stronger protective effects against NAFLD than in men. While the exact mechanisms for this difference are unclear, several studies have reported a higher risk of NALFD in men and post-menopausal women than in pre-menopausal women (38, 39). Interestingly, experimental results suggest a protective role for estradiol in hepatic injuries by suppressing lipid accumulation and liver fibrosis (40). The presence of estrone (E1) and 17β-estradiol (E2) in raw whole cow's milk has been demonstrated (41). Dairy products have been estimated to account for up to 60% of estrogens in a German diet (42). Some studies have shown that the estrogen in dairy products may increase the concentration of estrogen in the serum (43).
The results of some studies contradicted with the findings of our study. Lee et al. in a study of 5171 adults showed higher dairy protein intake was significantly and inversely associated with the risk of incident NAFLD in men and women aged ≥50 years (44). They suggested that replacing macronutrients equivalent to carbohydrate intake with dairy protein intake may have contributed to lowering the risk of developing NAFLD (32, 44). It has also been suggested that some of the beneficial effects of dairy products in preventing NAFLD are related to the whey protein found in dairy products. Several human and animal studies have shown that whey protein or dairy products reduce weight and fat mass (45–47). Other mechanisms have been suggested for the beneficial effects of dairy products against NAFLD, some of which are related to insulin resistance. The population-based prospective Coronary Artery Risk Development in Young Adults study found an inverse association between frequency of dairy intake and development of insulin resistance syndrome (48). Also, a meta-analysis study showed that the consumption of dairy products, especially low-fat dairy products had a beneficial effect on Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), waist circumference, and body weight (49).
This study has several important advantages including the large sample size, and extensive information on lifestyle and dietary factors, which allowed us to control for many potential confounders. In this study, we adjusted the results for several variables, especially BMI which is a crude measure of body fat and is associated with NAFLD and may negatively affect the accuracy of the results (32). On the other hand, the present study was not free of limitations. Due to the structure of the questionnaires used, we were not able to examine low-fat and high-fat dairy products separately. Also, because of the nature of questionnaires filled in interviews, there is always possibility of bias which can affect the accuracy of the results. Another limitation of the current study was the presence of some potential confounding factors such as diabetes, hyperlipidemia, and high blood pressure and their variability between FLI quartile. However, in the adjusted models, we adjusted the effects of these confounding factors. Finally, because of the difficulties in budget management and the high sample size, we were not able to assess the liver through imaging studies. Evaluation of hepatic steatosis and fibrosis could increase the accuracy of the results in future studies.
Conclusion
In summary, this population-based cohort study didn't show any strong association between dairy products consumption and NAFLD indicators. However, a modest inverse correlation was observed between milk consumption and FLI. Therefore, based on the results of this study, consumption of appropriate amounts of milk, especially low-fat types (at least one unit of milk more than 5–6 times a week) can play a positive role in preventing NAFLD. The results of the present study seem to be in line with the existing recommendations for consumption of a healthy dietary pattern to prevent NAFLD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocol was approved by the Research Council and the Ethics Committee of Fasa University of Medical Sciences (IR.FUMS.REC.1400.085). The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZK, MF, RH, and RT make substantial contributions to conception, design, acquisition of data, analysis and interpretation of data, and drafting of this study. ZK, MR, AA, and MK participate in drafting the article or revising it. MF and RH gives final approval of the version to be submitted and any revised version. MF and RT are the guarantors of this work. All authors have read and approved the manuscript.
Funding
This study was supported by the Deputy of Research and Technology of Fasa University of Medical Sciences, Fasa, Iran (No. 400067).
Acknowledgments
The authors thank all the study participants for their valuable contributions. This paper was derived from a thesis for fulfillment of obtaining a MD degree.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NAFLD, Nonalcoholic fatty liver disease; FLI, Fatty liver index; OR, Odds ratio; FFQ, Food frequency questionnaire; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; SBP, systolic blood pressure; DBP, diastolic blood pressure; ANOVA, One-way Analysis of Variance.
References
1. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, Mccullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. (1999) 116:1413–9. doi: 10.1016/S0016-5085(99)70506-8
2. Organization WH. Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization (2014).
3. Rezende AC, Souza LG, Jardim TV, Perillo NB, Araújo YCL, De Souza SG, et al. Is waist-to-height ratio the best predictive indicator of hypertension incidence? A cohort study. BMC Public Health. (2018) 18:1–11. doi: 10.1186/s12889-018-5177-3
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
5. Wong S-W, Chan W-K. Epidemiology of non-alcoholic fatty liver disease in Asia. Indian J Gastroenterol. (2020) 39:1–8. doi: 10.1007/s12664-020-01018-x
6. Ashtari S, Pourhoseingholi MA, Zali MR. Non-alcohol fatty liver disease in Asia: Prevention and planning. World J Hepatol. (2015) 7:1788. doi: 10.4254/wjh.v7.i13.1788
7. Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. (2020) 111:154170. doi: 10.1016/j.metabol.2020.154170
8. Kim D-H, Kim H, Jeong D, Kang I-B, Chon J-W, Kim H-S, et al. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: targeted and untargeted community analysis with correlation of biomarkers. J Nutr Biochem. (2017) 44:35–43. doi: 10.1016/j.jnutbio.2017.02.014
9. Bakhshimoghaddam F, Shateri K, Sina M, Hashemian M, Alizadeh M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr. (2018) 148:1276–84. doi: 10.1093/jn/nxy088
10. Chen Y, Feng R, Yang X, Dai J, Huang M, Ji X, et al. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. (2019) 109:1611–9. doi: 10.1093/ajcn/nqy358
11. Kong CY, Li ZM, Han B, Zhang ZY, Chen HL, Zhang SL, et al. Diet consisting of balanced yogurt, fruit, and vegetables modifies the gut microbiota and protects mice against nonalcoholic fatty liver disease. Mol Nutr Food Res. (2019) 63:1900249. doi: 10.1002/mnfr.201900249
12. Egresi A, Drexler D, Hagymási K, Blázovics A, Jakab Z, Kocsis I, et al. The potential role of organic and conventional yoghurt consumption in the treatment of non-alcoholic fatty liver disease. Orv Hetil. (2020) 161:1466–74. doi: 10.1556/650.2020.31839
13. Colín-Ramírez E, Rivera-Mancía S, Infante-Vázquez O, Cartas-Rosado R, Vargas-Barrón J, Madero M, et al. Protocol for a prospective longitudinal study of risk factors for hypertension incidence in a Mexico City population: the Tlalpan 2020 cohort. BMJ Open. (2017) 7:e016773. doi: 10.1136/bmjopen-2017-016773
14. Høstmark AT, Tomten SE. The Oslo Health Study: cheese intake was negatively associated with the metabolic syndrome. J Am Coll Nutr. (2011) 30:182–90. doi: 10.1080/07315724.2011.10719959
15. Guo Z, Liu X, Zhang Q, Shen Z, Tian F, Zhang H, et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutr Metabol Cardiovasc Dis. (2011) 21:844–50. doi: 10.1016/j.numecd.2011.04.008
16. Farjam M, Bahrami H, Bahramali E, Jamshidi J, Askari A, Zakeri H, et al. A cohort study protocol to analyze the predisposing factors to common chronic non-communicable diseases in rural areas: Fasa Cohort Study. BMC Public Health. (2016) 16:1–8. doi: 10.1186/s12889-016-3760-z
17. Krauss RM, Winston M, Fletcher BJ, Grundy SM. Obesity: impact on cardiovascular disease. Circulation. (1998) 98:1472–6. doi: 10.1161/01.CIR.98.14.1472
18. Keshteli AH, Esmaillzadeh A, Rajaie S, Askari G, Feinle-Bisset C, Adibi P. A dish-based semi-quantitative food frequency questionnaire for assessment of dietary intakes in epidemiologic studies in Iran: design and development. Int J Prev Med. (2014) 5:29. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3915470/
19. Malekshah A, Kimiagar M, Saadatian-Elahi M, Pourshams A, Nouraie M, Goglani G, et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: pilot phase of Golestan cohort study of esophageal cancer. Eur J Clin Nutr. (2006) 60:971–7. doi: 10.1038/sj.ejcn.1602407
20. Huth PJ, Park KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr. (2012) 3:266–85. doi: 10.3945/an.112.002030
21. Astrup A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr 99 1235s-1242s. (2014). doi: 10.3945/ajcn.113.073015
22. Ferolla SM, Ferrari TC, Lima ML, Reis TO, Tavares WC Jr, Couto OF, et al. Dietary patterns in Brazilian patients with nonalcoholic fatty liver disease: a cross-sectional study. Clinics. (2013) 68:11–7. doi: 10.6061/clinics/2013(01)OA03
23. Lei S, Liu ZW, Yun L, Cai G, Zhang H, Song LJ, et al. The prevalence of nonalcoholic fatty liver disease and its association with lifestyle/dietary habits among university faculty and staff in Chengdu. Biomed Environ Sci. (2012) 25:383–91. doi: 10.3967/0895-3988.2012.04.002
24. Razavi Zade M, Telkabadi MH, Bahmani F, Salehi B, Farshbaf S, Asemi Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int. (2016) 36:563–71. doi: 10.1111/liv.12990
25. Dreher ML. Dietary patterns, foods, nutrients and phytochemicals in non-alcoholic fatty liver disease. In: Dietary Patterns and Whole Plant Foods in Aging and Disease. New York, NY: Springer (2018). p. 291–311.
26. Dugan CE, Aguilar D, Park YK, Lee JY, Fernandez ML. Dairy consumption lowers systemic inflammation and liver enzymes in typically low-dairy consumers with clinical characteristics of metabolic syndrome. J Am Coll Nutr. (2016) 35:255–61. doi: 10.1080/07315724.2015.1022637
27. He K, Li Y, Guo X, Zhong L, Tang S. Food groups and the likelihood of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Br J Nutr. (2020) 124:1–13. doi: 10.1017/S0007114520000914
28. Salehi-Sahlabadi A, Sadat S, Beigrezaei S, Pourmasomi M, Feizi A, Ghiasvand R, et al. Dietary patterns and risk of non-alcoholic fatty liver disease. BMC Gastroenterol. (2021) 21:1–12. doi: 10.1186/s12876-021-01612-z
29. Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, et al. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. (2014) 12:215. doi: 10.1186/s12916-014-0215-1
30. Esmaillzadeh A, Azadbakht L. Dairy consumption and circulating levels of inflammatory markers among Iranian women. Public Health Nutr. (2010) 13:1395–402. doi: 10.1017/S1368980009992126
31. Fan JG, Cao HX. Role of diet and nutritional management in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2013) 28:81–7. doi: 10.1111/jgh.12244
32. Zhang S, Fu J, Zhang Q, Liu L, Lu M, Meng G, et al. Association between habitual yogurt consumption and newly diagnosed non-alcoholic fatty liver disease. Eur J Clin Nutr. (2020) 74:491–9. doi: 10.1038/s41430-019-0497-7
33. Buendia JR, Li Y, Hu FB, Cabral HJ, Bradlee ML, Quatromoni PA, et al. Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am J Hypertens. (2018) 31:557–65. doi: 10.1093/ajh/hpx220
34. Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. (2014) 97:7386–93. doi: 10.3168/jds.2014-8500
35. Xue L, He J, Gao N, Lu X, Li M, Wu X, et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. (2017) 7:1–13. doi: 10.1038/srep45176
36. Melanson E, Sharp T, Schneider J, Donahoo W, Grunwald G, Hill J. Relation between calcium intake and fat oxidation in adult humans. Int J Obes. (2003) 27:196–203. doi: 10.1038/sj.ijo.802202
37. Zemel MB. Proposed role of calcium and dairy food components in weight management and metabolic health. Phys Sportsmed. (2009) 37:29–39. doi: 10.3810/psm.2009.06.1707
38. Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. (2014) 59:1406–14. doi: 10.1002/hep.26761
39. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. (2019) 70:1457–69. doi: 10.1002/hep.30626
40. Lee C, Kim J, Jung Y. Potential therapeutic application of estrogen in gender disparity of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Cells. (2019) 8:1259. doi: 10.3390/cells8101259
41. Abshirini M, Siassi F, Koohdani F, Qorbani M, Golpour-Hamedani S, Khosravi S, et al. Association between dairy consumption and menopausal symptoms: A cross-sectional study among Iranian postmenopausal women. Int Dairy J. (2020) 105:104688. doi: 10.1016/j.idairyj.2020.104688
42. Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chem. (1998) 62:7–20. doi: 10.1016/S0308-8146(97)00150-7
43. Pape-Zambito D, Roberts R, Kensinger R. Estrone and 17β-estradiol concentrations in pasteurized-homogenized milk and commercial dairy products. J Dairy Sci. (2010) 93:2533–40. doi: 10.3168/jds.2009-2947
44. Lee JH, Lee HS, Ahn SB, Kwon YJ. Dairy protein intake is inversely related to development of non-alcoholic fatty liver disease. Clin Nutr. (2021) 40:5252–60. doi: 10.1016/j.clnu.2021.08.012
45. Frestedt JL, Zenk JL, Kuskowski MA, Ward LS, Bastian ED. A whey-protein supplement increases fat loss and spares lean muscle in obese subjects: a randomized human clinical study. Nutr Metab. (2008) 5:1–7. doi: 10.1186/1743-7075-5-8
46. Eller LK, Reimer RA. Dairy protein attenuates weight gain in obese rats better than whey or casein alone. Obesity. (2010) 18:704–11. doi: 10.1038/oby.2009.300
47. Trottier SK, Macpherson RE, Knuth CM, Townsend LK, Peppler WT, Mikhaeil JS, et al. Dairy attenuates weight gain to a similar extent as exercise in rats fed a high-fat, high-sugar diet. Obesity. (2017) 25:1707–15. doi: 10.1002/oby.21941
48. Pereira MA, Jacobs DR Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA study. JAMA. (2002) 287:2081–9. doi: 10.1001/jama.287.16.2081
Keywords: nonalcoholic fatty liver diseases, dairy, yogurt, cheese, cohort
Citation: Keshavarz Z, Rahimlou M, Farjam M, Homayounfar R, Khodadost M, Abdollahi A and Tabrizi R (2022) Non-alcoholic fatty liver disease and dairy products consumption: Results from FASA Persian cohort study. Front. Nutr. 9:962834. doi: 10.3389/fnut.2022.962834
Received: 06 June 2022; Accepted: 22 August 2022;
Published: 09 September 2022.
Edited by:
Zachary Clayton, University of Colorado Boulder, United StatesReviewed by:
Mee Young Hong, San Diego State University, United StatesDevin Wahl, Colorado State University, United States
Copyright © 2022 Keshavarz, Rahimlou, Farjam, Homayounfar, Khodadost, Abdollahi and Tabrizi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reza Tabrizi, a21zcmM4OUBnbWFpbC5jb20=; Reza Homayounfar, cl9ob21heW91bmZhckB5YWhvby5jb20=
†These authors have contributed equally to this work
 Zahra Keshavarz1†
Zahra Keshavarz1† Mehran Rahimlou
Mehran Rahimlou Mojtaba Farjam
Mojtaba Farjam Reza Homayounfar
Reza Homayounfar Mahmoud Khodadost
Mahmoud Khodadost Ashkan Abdollahi
Ashkan Abdollahi Reza Tabrizi
Reza Tabrizi