- 1Taizhou Central Hospital (Taizhou University Hospital), Taizhou, China
- 2Taizhou Jiaojiang Maternal and Child Health Care Hospital, Taizhou, China
- 3Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China
- 4College of Pharmacy, Shaoyang University, Shaoyang, China
- 5Guizhou Orthopedics Hospital, Guiyang, Guizhou, China
Background: Clinical and preclinical studies suggested that certain mutagens occurring as a reaction of creatine, amino acids, and sugar during the high temperature of cooking meat are involved in the pathogenesis of human cancer. Here we conducted a systematic review and meta-analysis to examine whether meat mutagens [PhIP, MeIQx, DiMeIQx, total HCA, and B(a)P] present a risk factor for human cancer.
Methods: We searched the following databases for relevant articles published from inception to 10 Oct 2021 with no language restrictions: Pubmed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Baidu Academic, Zhejiang Digital Library. Two independent researchers screened all titles and obtained eligible texts for further screening. Independent data extraction was conducted, and meta-analysis was carried out using random-effects models to calculate the risk ratio of the meat mutagens exposure.
Results: A total of 1,786,410 participants and 70,653 cancer cases were identified. Among these, there were 12 different types of cancer at various sites, i.e., breast, bladder, colorectal, colon, rectum, prostate, lung, Non-Hodgkin lymphoma, kidney, gastric, esophagus, pancreatic, hepatocellular carcinoma. Cancer risk was significantly increased by intake of PhIP (OR = 1.13;95% CI 1.07–1.21; p < 0.001), MeIQx (OR = 1.14; 95% CI: 1.07–1.21; p < 0.001), DiMeIQx (OR = 1.07; 95% CI: 1.01–1.13; p = 0.013), total HCA (OR = 1.20; 95% CI: 1.03–1.38; p = 0.016), and cancer risk was not significantly increased by intake of B(a)P (OR = 1.04; 95% CI: 0.98–1.10; p = 0.206).
Conclusion: Meat mutagens of PhIP, MeIQx, DiMeIQx, and total HCA have a positive association with the risk of cancer.
Systematic review registration: [www.crd.york.ac.uk/prospero], identifier [CRD42022148856].
Introduction
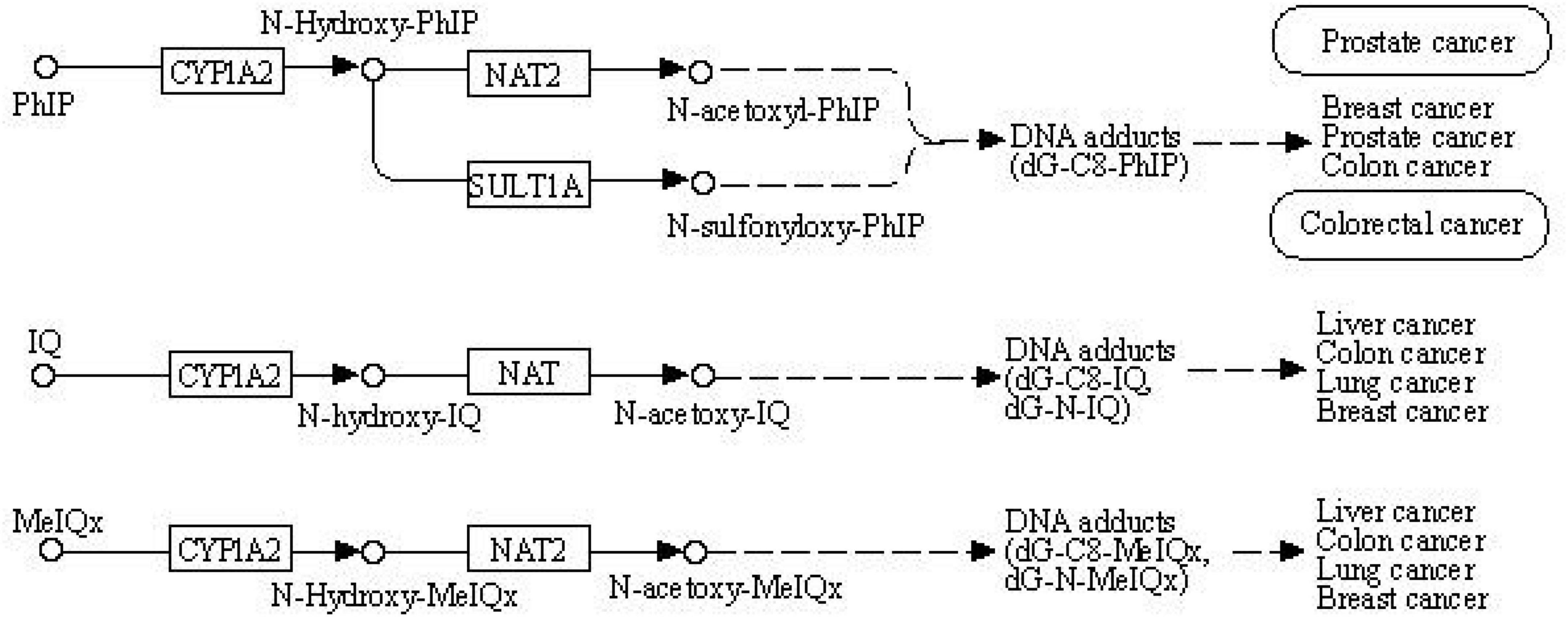
The World Cancer Research Fund and American Institute for Cancer Research provided convincing evidence on the association between red meat consumption and colorectal cancer risk (1). Processed meat has become a matter of public health concern since several epidemiological studies have indicated that high meat consumption correlates with higher rates of cancer and other chronic diseases (2–6). Animal and human studies suggested that certain mutagens occurring as a reaction of amino acids and sugar during the high temperature of cooking meat are involved in the pathogenesis of human cancer, such as heterocyclic amines (HCAs) and polycyclic aromatic amines (PAHs) (7–10). HCAs and PAHs are a group of mutagenic compounds that include 2-amino-1-methyl-6-phenylimidazo (4,5-b) pyridine (PhIP), 2-amino-3,8-dimethylimidazo (4,5-f) quinoxaline (MeIQ) 2-amino-3,4,8-trimethylimidazo (4,5-f) quinoxaline (DiMeIQx) and benzo(a)pyrene (BaP) (11–13). These mutagenic compounds have been presumed to increase the occurrence of tumors (2, 14–17). The mechanism is similar to other environmental chemical carcinogens, and metabolism enzymes metabolically activate meat mutagens (18–20). In the first step, cytochrome P450 (CYP) enables HCAs and PAHs to activate and form genotoxic electrophilic intermediates (21). In the second step, activated metabolites are detoxified by N-acetyltransferase 2 (NAT2) with N-acetylation and O-acetylation (22). This process is shown in the Kegg pathway diagram in Figure 1.
Meta-analysis can take into account a large amount of evidence (i.e., research) on a topic, which is desirable to identify a clear relationship between the variables of concern. In this study, we study the risk estimate about meat mutagens and human cancer. We conducted a systematic review and meta-analysis to shed light on the relation between meat mutagens (PhIP, MeIQx, DiMeIQx, and BaP) and different types of human cancers. The study have registered on PROSPERO. ID: CRD42022148856.
Our objectives were as follows:
(i) Conduct a meta-analysis to examine whether meat mutagens [PhIP, MeIQx, DiMeIQx, total HCA, and B(a)P] present a risk factor for human cancer.
(ii) To identify which types of human cancers are especially vulnerable to meat related mutagens.
(iii) To identify which categories of meat mutagens that warrant further in-depth evaluation according to harmfulness.
Methods
In this study, meta-analysis was according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses).
Search strategy
Exposure referred to heterocyclic amines (PhIP, MeIQx, DiMeIQx, total HCA) and benzo (a)pyrene; the outcomes of interest included all kinds of cancer. Following databases were searched from inception to 10 Oct 2021 with no language restrictions: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Baidu Schoolar, Google scholar, and Zhejiang Digital Library. We used the following search keywords [meat mutagens OR heterocyclic amin OR PhIP OR MeIQx OR DiMeIQx OR benzo(a)pyrene] and (neoplasm OR cancer OR tumor). To maximize the search for relevant articles, the reference lists of identified articles and relevant systematic reviews were examined to identify additional relevant publications.
Inclusion criteria
Articles were considered eligible if they met the following inclusion criteria: (i) study design: epidemiological study and clinical study with case-control or cohort study; (ii) object: the association between heterocyclic amine [PhIP, MeIQx, DiMeIQx, benzo(a)pyrene] intake and cancer risk; (iii) outcomes: publications presented results of relative risk [RR], odds ratio [OR], or hazard ratio (HR) for the highest vs. lowest exposure.
If more articles were produced based on the same cohort study, such as Nurses Health Study (NHS), and analyses presented utilized unique sites or exposures, the article with the largest number of cases was selected for statistical analysis. No studies were excluded due to issues related to data quality or design.
Exclusion criteria
(i) Study design with cross-sectional surveys, ecologic analyses, case reports, editorials.
(ii) Exposure comes from the working environment or smoking.
(iii) Experimental study on the animal model.
Study selection and data extraction
After the removal of duplicates, two independent researchers screened all titles and obtained eligible texts for further screening. Disagreements between researchers were solved by the third author.
The following data were extracted from the included studies; (i) basic information: first author, year of publication, study design, study region; (ii) individual data: sample size, tumor site, dietary assessment method, exposure quantification method, risk estimates [rate ratios (RR); odds ratio (OR); hazard ratio (HR); corresponding 95% confidence interval (CI)], (iii) matched or adjusted variables. We abstracted those that adjusted for multivariate or most confounding factors.
Statistical analysis
The random-effect model of the inverse variance weighting test was used to calculate OR and the corresponding 95% CI for the highest vs. the lowest level of exposures. P < 0.05 was considered statistically significant; p-values and I2 values were used for the heterogeneity test.
Subgroup analyses
We performed subgroup analyses according to cancer site, study design, and country. Forest plots were generated for PhIP, MeIQx, DiMeIQx, total HCA, and B(a)P. Subgroup analysis can be used to evaluate the potential association between exposure and influencing factors and describe sources of heterogeneity by stratifying factors.
Publication bias and sensitivity analyses
The existence of publication bias was evaluated by Begg’s test and Egger’s test, the Begg methods test was also used for funnel plot asymmetry. If the value of Begg’s Test (Pr > | z|) and Egger’s test Pr > | z| were < 0.05, the funnel plot was considered to be asymmetrical, thus suggesting the potential existence of publication bias. When potential bias was detected, a trim-and-fill analysis was further performed to assess the influence of the bias and to have the bias-corrected. We also performed a sensitivity analysis to investigate the influence of a single study on the pooled OR estimate by omitting one study in each turn. Stata12.0 software was used for all analyses.
Results
Among 1,141 publications that were initially identified from the data sources, 58 were included in the final meta-analysis. Details of the selection of publications included in the meta-analysis are shown in Supplementary Figure 1. A total of 1,786,410 participants, 70,653 cancer cases, and 12 types of cancer at various sites, i.e., breast, bladder, colorectal, colon, rectum, prostate, lung, Non-Hodgkin lymphoma, kidney, gastric, esophagus, pancreatic, and hepatocellular carcinoma were investigated. All studies used a food frequency questionnaire (FFQ) to collect dietary information, and most of the publications used the online Computerized Heterocyclic Amines Resource for Research to estimate heterocyclic amines intake from the Epidemiology of Disease (CHARRED) database.
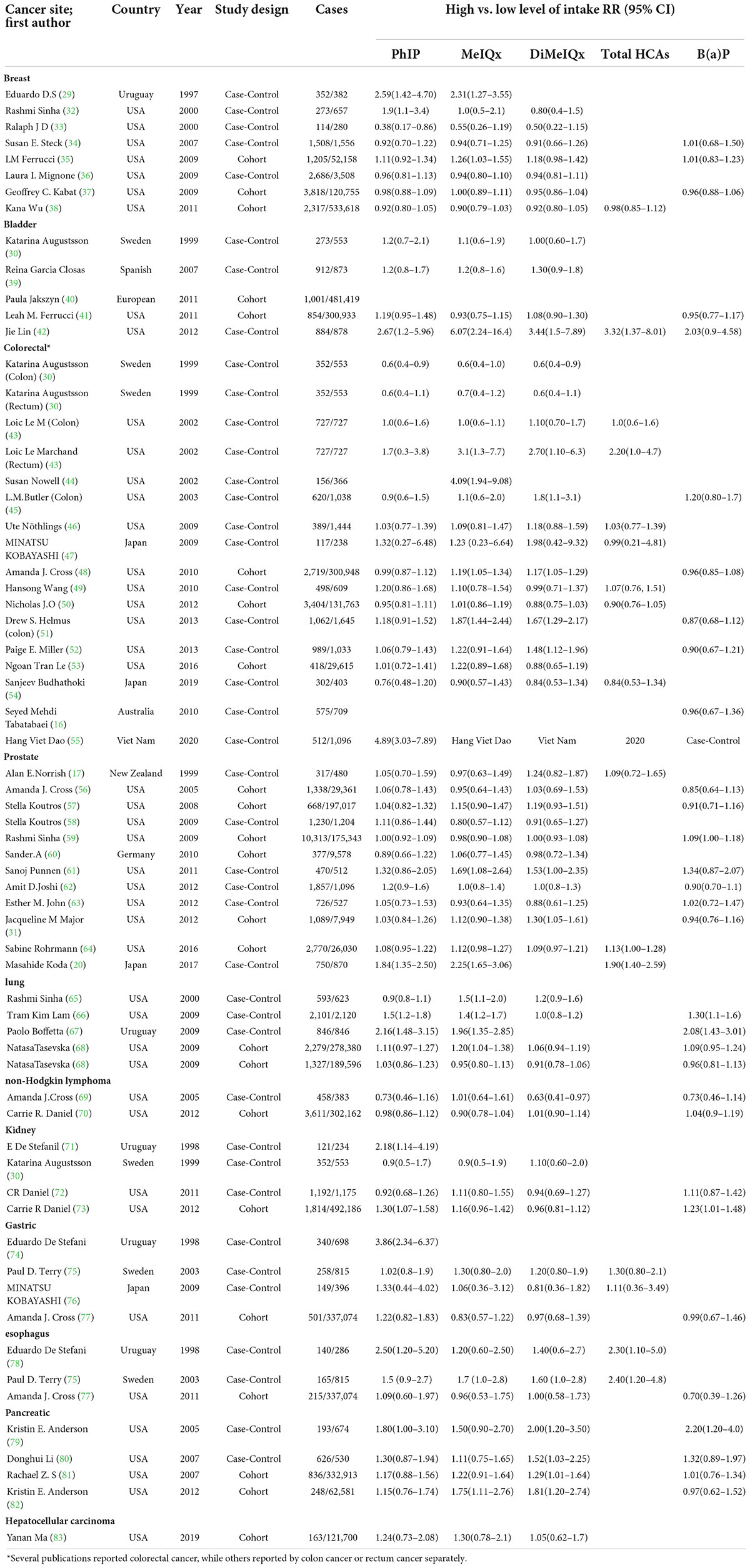
Table 1 shows the main characteristics and results of the 58 publications in this meta-analysis. As several publications reported the risk for more than one cancer site, the total number of trials included in this meta-analysis amounted to 67. When some large cohort studies had been researched more than one time with different cancer sites, only one publication had been selected to statistics the number of cases to avoid repeating counting.
PhIP
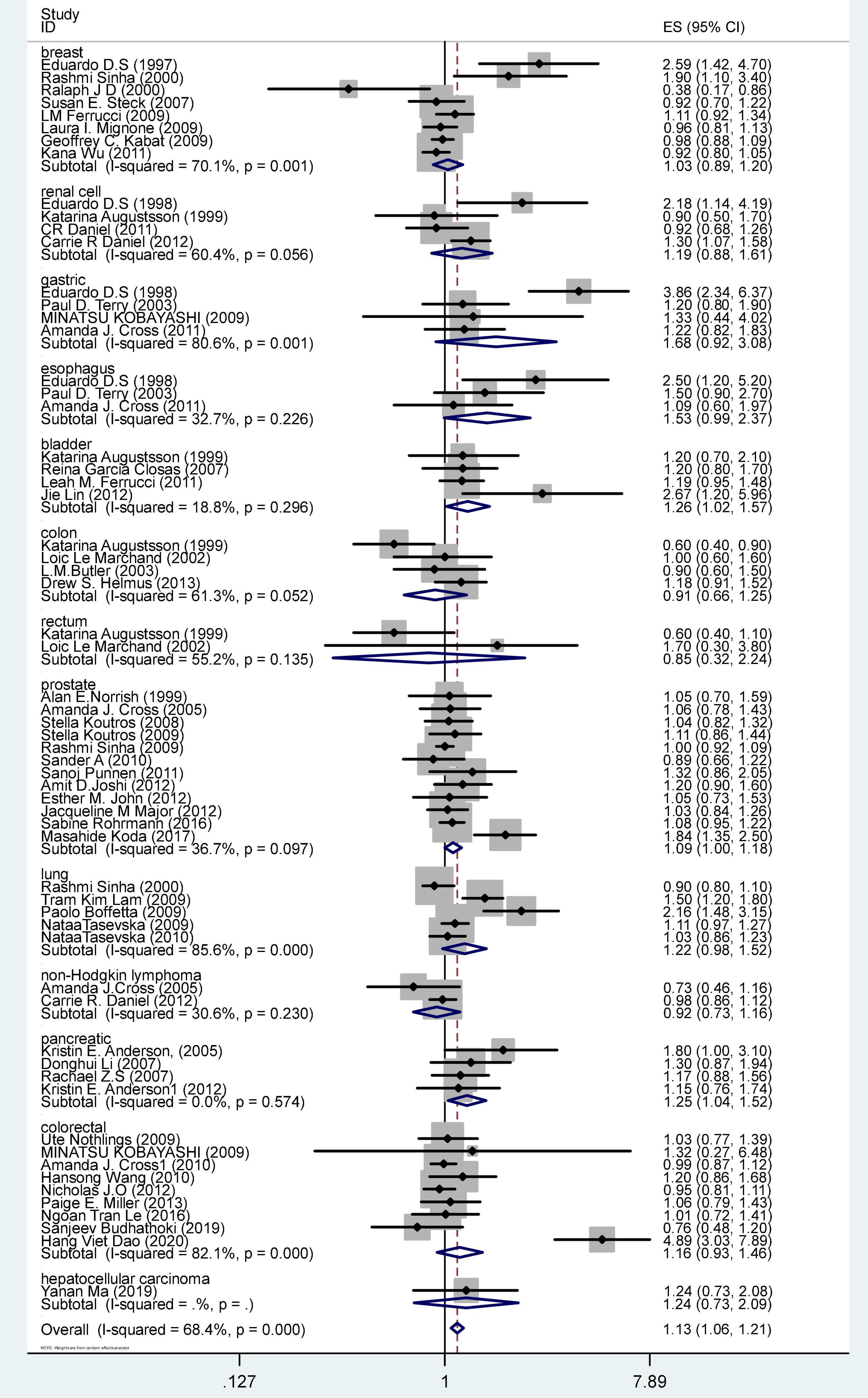
Figure 2 shows the OR and 95% CI for PhIP intake and cancer risk. The OR was 1.13 (CI 1.07–1.21), p = < 0.001. The result of the meta-analysis was a significant summary risk estimate.
MeIQx
Supplementary Figure 6 displays the pooled ORs and 95% CI for the highest vs. the lowest level of MeIQx intake according to subgroups of the cancer site. The OR was 1.14 (CI 1.07–1.21, p = 0.000). The meta-analysis revealed a significant association between MeIQx intake and carcinoma risk.
DiMeIQx
Supplementary Figure 8 displays the pooled ORs and 95% CI for the highest vs. the lowest level of DiMeIQx intake according to subgroups of the cancer site. The OR was 1.07 (CI 1.01–1.13, p = 0.013). The results indicated a weakly significant association between DiMeIQx intake and carcinoma risk.
Total heterocyclic amines
Sixteen publications evaluated the association between total HCA consumption and cancer (Supplementary Figure 10). The OR was 1.20 (CI 1.03–1.38, p = 0.016). The results revealed a statistically significant association between total HCA intake and carcinoma risk.
B(a)P
Thirty studies were included in the meta-analysis of the association between B(a)P intake and cancer risk (Supplementary Figure 12). The OR was 1.04 (CI 0.98–1.10), p = 0.206. The results revealed no statistically significant association between B(a)P intake and carcinoma risk.
Subgroups analysis
Table 2 shows the summary OR and 95% CI for high vs. low levels of PhIP, MeIQx, DiMeIQx, total HCAs, and B(a)P intake and cancer risk according to subgroups of the cancer site. First, no risk emerged for breast cancer, non-Hodgkin lymphoma, and gastric cancer. For bladder cancer, OR was 1.26 (CI 1.02–1.57) for PhIP, and 3.32 (CI 1.37–8.03) for total HCA, respectively. As for colorectal cancer, OR was 1.16 (CI 1.01–1.33) for MeIQx, and in rectum cancer, it was 2.20 (CI 1.01–4.77) for total HCAs, respectively. Concerning prostate cancer, a borderline significantly increased OR of 1.09 (CI 1.00–1.18) was found for PhIP. Lung cancer had significantly increased OR = 1.31 (CI 1.07–1.60) for MeIQx. Kidney cancer had significantly increased OR = 1.18 (CI 1.02–1.38) for B(a)P; esophagus cancer had OR = 2.35 (CI 1.4–3.93) for total HCA, while in pancreatic cancer, OR was 1.25 (CI 1.04–1.52) for PhIP, 1.31 (CI 1.08–1.59) for MeIQx, 1.50 (CI 1.24–1.82) for DiMeIQx, respectively.
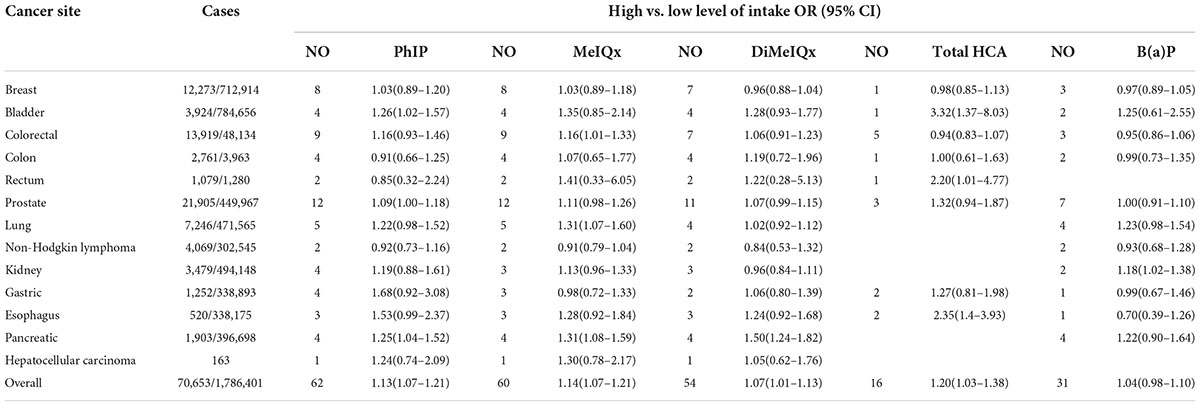
Table 2. Results of meta-analyses of epidemiological studies of Meat Mutagens intake and cancer risk.
Table 3 shows the OR estimates according to subgroups of geographic location. Nine countries were included in geographic location subgroup analyses; for America, there was a significant association between PhIP, MeIQx, DiMeIQx intake, and cancers risk; for Uruguay, a significant association was found between PhIP, total HCAs intake, and cancers risk; for Vietnam, we found a significant association between PhIP intake and cancers risk. No significant associations were observed for Sweden, Spain, Germany, Japan, New Zealand, and Australia (Supplementary Figures 2, 7, 9, 11, 13).
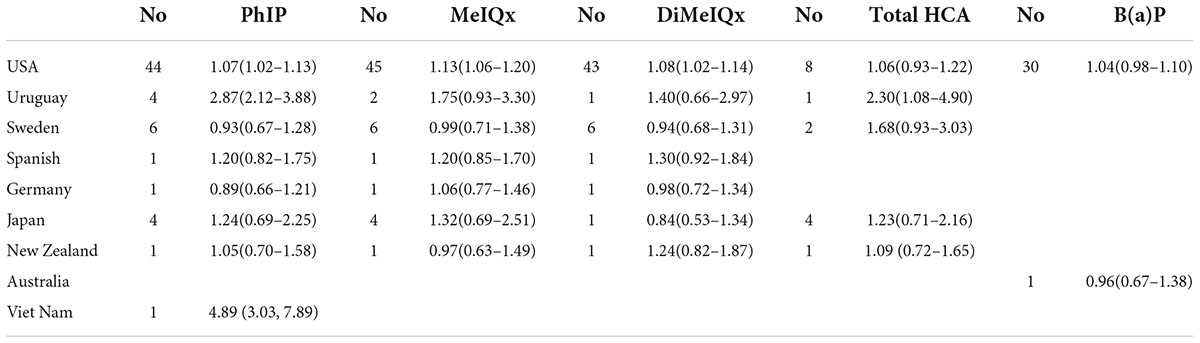
Table 3. Meta-analysis of high vs. low meat mutagens intake in relation to the risk of cancer, in subgroups of study location.
Publication bias and sensitivity analysis
Publication bias was detected in estimate of PhIP intake and cancer risk with Begg’s Test Pr > | z| = 0.013 and Egger’s test P > | t| = 0.003, the funnel plot appeared to be relatively asymmetric (Supplementary Figure 3). Trim and fill method were used to impute fourteen studies to the left of the mean; OR was 1.119 (CI: 1.045–1.198, p = 0.001), this result was the same as the original OR result, which was 1.13 (CI 1.07–1.21, p = 0.000) (Supplementary Figure 4).
Sensitivity analyses were used to investigate the influence of a single study on the pooled OR estimates by omitting one study in each turn. The obtained results suggested that the estimates of PhIP, MeIQx, DiMeIQx, total HCA, B(a)P were not substantially modified by any single study (Supplementary Figure 5).
Discussion
In this meta-analysis, we investigated the relationship between the meat mutagens [PhIP, MeIQx, DiMeIQx, benzo(a)pyrene] and all typical types of cancers.
First, our results indicated a significant association between (PhIP, MeIQx, DiMeIQx, total HCA) and total cancer risk, and no association between benzo(a)pyrene and cancer risk. This research included 1,987,798 participants, 69,874 cancer cases, and 12 types of cancer at the following sites: breast, bladder, colorectal, colon, rectum, prostate, lung, Non-Hodgkin lymphoma, kidney, gastric, esophagus, and pancreas. The result clearly showed which types of cancers are particularly vulnerable to related mutagens. Future studies are needed to identify categories of meat mutagens that warrant further in-depth evaluation according to harmfulness.
Second, we conducted meta-analyses for cancer risk and meat mutagens intake stratified by geographic location. We observe an increased risk in North America with exposure to PhIP, MeIQx, DiMeIQx; in South America, with PhIP and total HCA exposure, while no increased risk was observed in Europe, Asia, and Oceania. This difference may be related to dietary structure. As is well-known, the Mediterranean diet is preferred in Europe, and a previous meta-analysis suggested that the Mediterranean diet provides significant protection from the incidence of cancer of all types (23, 24). This protective effect is mainly due to the high consumption of olive oil and tomatoes, which have antioxidant effects on cancer cells (25). Moreover, in Japan, rice and vegetables are the mainstay of the country’s diet (26). Dietary patterns may have a dominant role, not just in the quantity of meat consumption, but also for the food factor activity as a protective factor against meat mutagens intake on risk of cancer (27, 28). More cohort data is necessary regarding meat mutagens intake and protective factors of cancer risk.
Strengths and weaknesses of this study
This systematic review and meta-analysis have several strengths. First, the present study had a large sample size and a large number of epidemiologic studies, with 63 trials being included in this meta-analysis. Second, to the best of our knowledge, this systematic review is the first that provided a comprehensive guide to the risk estimates for 12 types of cancers and meat mutagens exposure.
Furthermore, the food frequency questionnaire was used to ascertain dietary information in this meta-analysis. It is challenging to ascertain respondents’ usual exposure from an FFQ. Some early epidemiological studies included a few items of cooking methods (such as pan-frying, baking, grilling/barbequing) as surrogate measures in the food frequency questionnaire. Meat intake mutagens were calculated as follows: frequency of consumption of pan-frying meat × [(portion size) × (PhIP content for each pan-frying meat according to literature data)] (29), which was inadequate for a comprehensive assessment of meat mutagens intake. After that, some studies used color photographs to reflect the range of cooking levels for cooked meat ranging from rare to very well-done and to standardize the assessment of the preferred level of doneness in dietary surveys (30). Recently, studies attempted to use the NCI CHARRED database to estimate the amount of HCA consumption (31). Therefore, we performed subgroup analyses to investigate the preferred method for calculating meat mutagens levels in diets. We stratified the trials with the use of CHARRED and without the CHARRED database (Supplementary Figure 14); the results obtained from CHARRED database revealed slight heterogeneity (I2 = 25.8%), while the results obtained without the CHARRED database revealed large heterogeneity (I2 = 80%). Accordingly, we found that the use of the CHARRED database could effectively improve the heterogeneity from the nutritional epidemiology study.
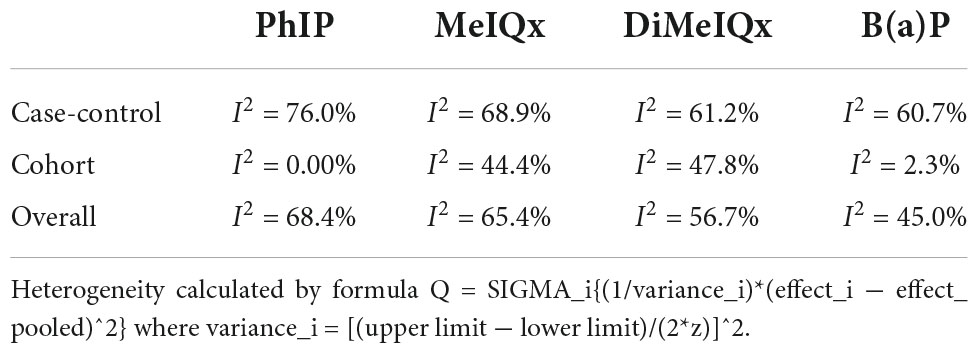
The present study also has some limitations: heterogeneity was statistically significant in case-control studies, while it was small in a cohort study (Table 4), which suggested that large heterogeneity from case-control studies contributed to the overall heterogeneity. This might be because it is difficult for cancer patients in case-control trials to retrospect their diet. It is a commonplace defect in nutritional epidemiology, and the large time span and regional span of trials may aggravate the heterogeneity. Future studies with more detailed quantitative intake may enhance the power of evidence from case-control trials.
Conclusion
The results indicated that PhIP, MeIQx, DiMeIQx, and total HCA have a positive effect on total cancer risk, while benzo(a)pyrene was not associated with an increased risk of cancer. Results support this basic tenet of prevention in public health, restricting processed meat intake is a healthy lifestyle. This meta-analysis paves the way for a prospective epidemiological study in meat intake and cancer risk.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FJ conducted the analysis, generated the figures, and wrote the manuscript. All authors designed and conducted the systematic review, contributed to edits and revisions of the manuscript, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.962688/full#supplementary-material
References
1. WCRF/AICR. Continuous Update Project Report on Colorectal Cancer. Washington, DC: WCRF/AICR (2011).
2. Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer. (2009) 61:437–46. doi: 10.1080/01635580802710741
3. Xu J, Yang XX, Wu YG, Li XY, Bai B. Meat consumption and risk of oral cavity and oropharynx cancer: A meta-analysis of observational studies. PLoS One. (2014) 9:e95048. doi: 10.1371/journal.pone.0095048
4. Saneei P, Willett W, Esmaillzadeh A. Red and processed meat consumption and risk of glioma in adults: A systematic review and meta-analysis of observational studies. J Res Med Sci. (2015) 20:602–12. doi: 10.4103/1735-1995.165970
5. Zhang S, Wang Q, He J. Intake of red and processed meat and risk of renal cell carcinoma: A meta-analysis of observational studies. Oncotarget. (2017) 8:77942–65. doi: 10.18632/oncotarget.18549
6. Abid Z, Cross AJ, Sinha R. Meat, dairy, and cancer. Am J Clin Nutr. (2014) 100:386S–93S. doi: 10.3945/ajcn.113.071597
7. Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, et al. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol. (2012) 25:1452–61. doi: 10.1021/tx300108e
8. Jamin EL, Riu A, Douki T, Debrauwer L, Cravedi JP, Zalko D, et al. Combined genotoxic effects of a polycyclic aromatic hydrocarbon (B(a)P) and an heterocyclic amine (PhIP) in relation to colorectal carcinogenesis. PLoS One. (2013) 8:e58591. doi: 10.1371/journal.pone.0058591
9. Chiang VS, Quek SY. The relationship of red meat with cancer: Effects of thermal processing and related physiological mechanisms. Crit Rev Food Sci Nutr. (2017) 57:1153–73. doi: 10.1080/10408398.2014.967833
10. Kizil M, Oz F, Besler HT. A review on the formation of carcinogenic/mutagenic heterocyclic aromatic amines. J Food Process Technol. (2011) 2:120. doi: 10.4172/2157-7110.1000120
11. Jian SH, Yeh PJ, Wang CH, Chen HC, Chen SF. Analysis of heterocyclic amines in meat products by liquid chromatography – Tandem mass spectrometry. J Food Drug Anal. (2019) 27:595–602. doi: 10.1016/j.jfda.2018.10.002
12. Turesky RJ, Goodenough AK, Ni W, McNaughton L, LeMaster DM, Holland RD, et al. Identification of 2-amino-1,7-dimethylimidazo[4,5-g]quinoxaline: An abundant mutagenic heterocyclic aromatic amine formed in cooked beef. Chem Res Toxicol. (2007) 20:520–30. doi: 10.1021/tx600317r
13. Hummel JM, Madeen EP, Siddens LK, Uesugi SL, McQuistan T, Anderson KA, et al. Pharmacokinetics of [(14)C]-Benzo[a]pyrene (BaP) in humans: Impact of Co-administration of smoked salmon and BaP dietary restriction. Food Chem Toxicol. (2018) 115:136–47. doi: 10.1016/j.fct.2018.03.003
14. Chiavarini M, Bertarelli G, Minelli L, Fabiani R. Dietary intake of meat cooking-related mutagens (HCAs) and risk of colorectal adenoma and cancer: A Systematic review and meta-analysis. Nutrients. (2017) 9:514. doi: 10.3390/nu9050514
15. Shirai T, Kato K, Futakuchi M, Takahashi S, Suzuki S, Imaida K, et al. Organ differences in the enhancing potential of 2-amino-1-methyl-6-phenylimidazo pyridine on carcinogenicity in the prostate, colon and pancreas. Mutat Res. (2002) 506:129–36. doi: 10.1016/S0027-5107(02)00159-8
16. Tabatabaei SM, Heyworth JS, Knuiman MW, Fritschi L. Dietary benzo[a]pyrene intake from meat and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. (2010) 19:3182–4. doi: 10.1158/1055-9965.EPI-10-1051
17. Norrish AE, Ferguson LR, Knize MG, Felton JS, Sharpe SJ, Jackson RT. Heterocyclic amine content of cooked meat and risk of prostate cancer. J Natl Cancer Inst. (1999) 91:2038–44. doi: 10.1093/jnci/91.23.2038
18. Hikosaka A, Asamoto M, Hokaiwado N, Kato K, Kuzutani K, Kohri K, et al. Inhibitory effects of soy isoflavones on rat prostate carcinogenesis induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis. (2004) 25:381–7. doi: 10.1093/carcin/bgh031
19. Creton SK, Zhu H, Gooderham NJ. The cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine activates the extracellular signal regulated kinase mitogen-activated protein kinase pathway. Cancer Res. (2007) 67:11455–62. doi: 10.1158/0008-5472.CAN-07-2821
20. Koda M, Iwasaki M, Yamano Y, Lu X, Katoh T. Association between NAT2, CYP1A1, and CYP1A2 genotypes, heterocyclic aromatic amines, and prostate cancer risk: A case control study in Japan. Environ Health Prev Med. (2017) 22:72. doi: 10.1186/s12199-017-0681-0
21. Suzuki H, Morris JS, Li Y, Doll MA, Hein DW, Liu J, et al. Interaction of the cytochrome P4501A2, SULT1A1 and NAT gene polymorphisms with smoking and dietary mutagen intake in modification of the risk of pancreatic cancer. Carcinogenesis. (2008) 29:1184–91. doi: 10.1093/carcin/bgn085
22. Rohrmann S, Lukas Jung SU, Linseisen J, Pfau W. Dietary intake of meat and meat-derived heterocyclic aromatic amines and their correlation with DNA adducts in female breast tissue. Mutagenesis. (2009) 24:127–32. doi: 10.1093/mutage/gen058
23. Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ. (2008) 337:a1344. doi: 10.1136/bmj.a1344
24. Capurso C, Vendemiale G. The mediterranean diet reduces the risk and mortality of the prostate cancer: A narrative review. Front Nutr. (2017) 4:38. doi: 10.3389/fnut.2017.00038
25. Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: Pooled analysis of 15 studies. Am J Clin Nutr. (2015) 102:1142–57. doi: 10.3945/ajcn.115.114306
26. Yoneoka D, Nomura S, Kurotani K, Tanaka S, Nakamura K, Uneyama H, et al. Does Japan’s national nutrient-based dietary guideline improve lifestyle-related disease outcomes? A retrospective observational cross-sectional study. PLoS One. (2019) 14:e0224042. doi: 10.1371/journal.pone.0224042
27. Murray S, Lake BG, Gray S, Edwards AJ, Springall C, Bowey EA, et al. Effect of cruciferous vegetable consumption on heterocyclic aromatic amine metabolism in man. Carcinogenesis. (2001) 22:1413–20. doi: 10.1093/carcin/22.9.1413
28. Terry P, Terry JB, Wolk A. Fruit and vegetable consumption in the prevention of cancer: An update. J Intern Med. (2001) 250:280–90. doi: 10.1046/j.1365-2796.2001.00886.x
29. De Stefani E, Ronco A, Mendilaharsu M, Guidobono M, Deneo-Pellegrini H. Meat intake, heterocyclic a case-control amines, and risk of breast cancer: Study in Uruguay. Cancer Epidemiol Biomark Prev. (1997) 6:573–81.
30. Augustsson K, Skog K, Jägerstad M, Dickman PW, Steineck G. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: A population-based study. Lancet. (1999) 353:703–7. doi: 10.1016/S0140-6736(98)06099-1
31. Major JM, Cross AJ, Watters JL, Hollenbeck AR, Graubard BI, Sinha R. Patterns of meat intake and risk of prostate cancer among African-Americans in a large prospective study. Cancer Causes Control. (2011) 22:1691–8. doi: 10.1007/s10552-011-9845-1
32. Sinha R, Gustafson DR, Kulldorff M, Wen WQ, Cerhan JR, Zheng W. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a carcinogen in hightemperature-cooked meat, and breast cancer risk. J Natl Cancer Inst. (2000) 92:1352–4. doi: 10.1093/jnci/92.16.1352
33. Delfino RJ, Sinha R, Smith C, West J, White E, Lin HJ, et al. Breast cancer, heterocyclic aromatic amines from meat and N-acetyltransferase 2 genotype. Carcinogenesis. (2000) 21:605–15. doi: 10.1093/carcin/21.4.607
34. Steck SE, Gaudet MM, Eng SM, Britton JA, Teitelbaum SL, Neugut AI, et al. Cooked meat and risk of breast cancer–lifetime versus recent dietary intake. Epidemiology. (2007) 18:373–82. doi: 10.1097/01.ede.0000259968.11151.06
35. Ferrucci LM, Cross AJ, Graubard BI, Brinton LA, McCarty CA, Ziegler RG, et al. Intake of meat, meat mutagens, and iron and the risk of breast cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Br J Cancer. (2009) 101:178–84. doi: 10.1038/sj.bjc.6605118
36. Mignone LI, Giovannucci E, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, et al. Meat consumption, heterocyclic amines, NAT2, and the risk of breast cancer. Nutr Cancer. (2009) 61:36–46. doi: 10.1080/01635580802348658
37. Kabat GC, Cross AJ, Park Y, Schatzkin A, Hollenbeck AR, Rohan TE, et al. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int J Cancer. (2009) 124:2430–5. doi: 10.1002/ijc.24203
38. Wu K, Sinha R, Holmes MD, Giovannucci E, Willett W, Cho E. Meat mutagens and breast cancer in postmenopausal women–a cohort analysis. Cancer Epidemiol Biomarkers Prev. (2010) 19:1301–10. doi: 10.1158/1055-9965.EPI-10-0002
39. Garcia-Closas R, Garcia-Closas M, Kogevinas M, Malats N, Silverman D, Serra C, et al. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer. (2007) 43:1731–40. doi: 10.1016/j.ejca.2007.05.007
40. Jakszyn P, Gonzalez CA, Lujan-Barroso L, Ros MM, Bueno-de-Mesquita HB, Roswall N, et al. Red meat, dietary nitrosamines, and heme iron and risk of bladder cancer in the European prospective investigation into cancer and nutrition (EPIC). Cancer Epidemiol Biomarkers Prev. (2011) 20:555–9. doi: 10.1158/1055-9965.EPI-10-0971
41. Ferrucci LM, Sinha R, Ward MH, Graubard BI, Hollenbeck AR, Kilfoy BA, et al. Meat and components of meat and the risk of bladder cancer in the NIH-AARP Diet and Health Study. Cancer. (2010) 116:4345–53. doi: 10.1002/cncr.25463
42. Lin J, Forman MR, Wang J, Grossman HB, Chen M, Dinney CP, et al. Intake of red meat and heterocyclic amines, metabolic pathway genes and bladder cancer risk. Int J Cancer. (2012) 131:1892–903. doi: 10.1002/ijc.27437
43. Le Marchand L, Hankin JH, Pierce LM, Sinha R, Nerurkar PV, Franke AA, et al. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutat Res. (2002) 506-507:204–15. doi: 10.1016/S0027-5107(02)00167-7
44. Nowell S, Coles B, Sinha R, MacLeod S, Luke Ratnasinghe D, Stotts C, et al. Analysis of total meat intake and exposure to individual heterocyclic amines in a case-control study of colorectal cancer: Contribution of metabolic variation to risk. Fundament Mol Mech Mutagen. (2002) 506:175–85. doi: 10.1016/S0027-5107(02)00164-1
45. Butler LM, Sinha R, Millikan RC, Martin CF, Newman B, Gammon MD, et al. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol. (2003) 157:434–45. doi: 10.1093/aje/kwf221
46. Nothlings U, Yamamoto JF, Wilkens LR, Murphy SP, Park SY, Henderson BE, et al. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. (2009) 18:2098–106. doi: 10.1158/1055-9965.EPI-08-1218
47. Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, Koizumi Y, et al. Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of colorectal cancer: A hospital-based case-control study in Japan. Scand J Gastroenterol. (2009) 44:952–9. doi: 10.1080/00365520902964721
48. Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, Park Y, et al. A large prospective study of meat consumption and colorectal cancer risk: An investigation of potential mechanisms underlying this association. Cancer Res. (2010) 70:2406–14. doi: 10.1158/0008-5472.CAN-09-3929
49. Wang H, Yamamoto JF, Caberto C, Saltzman B, Decker R, Vogt TM, et al. Genetic variation in the bioactivation pathway for polycyclic hydrocarbons and heterocyclic amines in relation to risk of colorectal neoplasia. Carcinogenesis. (2011) 32:203–9. doi: 10.1093/carcin/bgq237
50. Ollberding NJ, Wilkens LR, Henderson BE, Kolonel LN, Le Marchand L. Meat consumption, heterocyclic amines and colorectal cancer risk: The Multiethnic Cohort Study. Int J Cancer. (2012) 131:E1125–33. doi: 10.1002/ijc.27546
51. Helmus DS, Thompson CL, Zelenskiy S, Tucker TC, Li L. Red meat-derived heterocyclic amines increase risk of colon cancer: A population-based case-control study. Nutr Cancer. (2013) 65:1141–50. doi: 10.1080/01635581.2013.834945
52. Miller PE, Lazarus P, Lesko SM, Cross AJ, Sinha R, Laio J, et al. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr Cancer. (2013) 65:202–26. doi: 10.1080/01635581.2013.756534
53. Le NT, Michels FA, Song M, Zhang X, Bernstein AM, Giovannucci EL, et al. A prospective analysis of meat mutagens and colorectal cancer in the nurses’ health study and health professionals follow-up study. Environ Health Perspect. (2016) 124:1529–36. doi: 10.1289/EHP238
54. Budhathoki S, Iwasaki M, Yamaji T, Hamada GS, Miyajima NT, Zampieri JC, et al. Doneness preferences, meat and meat-derived heterocyclic amines intake, and N-acetyltransferase 2 polymorphisms: Association with colorectal adenoma in Japanese Brazilians. Eur J Cancer Prev. (2019) 29:7–14. doi: 10.1097/CEJ.0000000000000506
55. Dao HV, Nguyen TTM, Tran HH, Dang LT, Dinh MT, Le NT. A case-control study of meat mutagens and colorectal cancers in viet nam. Asian Pac J Cancer Prev. (2020) 21:2217–23. doi: 10.31557/APJCP.2020.21.8.2217
56. Cross AJ, Peters U, Kirsh VA, Andriole GL, Reding D, Hayes RB, et al. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. (2005) 65:11779–84. doi: 10.1158/0008-5472.CAN-05-2191
57. Koutros S, Cross AJ, Sandler DP, Hoppin JA, Ma X, Zheng T, et al. Meat and meat mutagens and risk of prostate cancer in the agricultural health study. Cancer Epidemiol Biomarkers Prev. (2008) 17:80–7. doi: 10.1158/1055-9965.EPI-07-0392
58. Koutros S, Berndt SI, Sinha R, Ma X, Chatterjee N, Alavanja MC, et al. Xenobiotic metabolizing gene variants, dietary heterocyclic amine intake, and risk of prostate cancer. Cancer Res. (2009) 69:1877–84. doi: 10.1158/0008-5472.CAN-08-2447
59. Sinha R, Park Y, Graubard BI, Leitzmann MF, Hollenbeck A, Schatzkin A, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol. (2009) 170:1165–77. doi: 10.1093/aje/kwp280
60. Sander A, Linseisen J, Rohrmann S. Intake of heterocyclic aromatic amines and the risk of prostate cancer in the EPIC-Heidelberg cohort. Cancer Causes Control (2010) 22:109–14. doi: 10.1007/s10552-010-9680-9
61. Punnen S, Hardin J, Cheng I, Klein EA, Witte JS. Impact of meat consumption, preparation, and mutagens on aggressive prostate cancer. PLoS One. (2011) 6:e27711. doi: 10.1371/journal.pone.0027711
62. Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, John EM, et al. Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer: Results from a multiethnic case-control study. Carcinogenesis. (2012) 33:2108–18. doi: 10.1093/carcin/bgs242
63. John EM, Stern MC, Sinha R, Koo J. Meat consumption, cooking practices, meat mutagens, and risk of prostate cancer. Nutr Cancer. (2011) 63:525–37. doi: 10.1080/01635581.2011.539311
64. Rohrmann S, Nimptsch K, Sinha R, Willett WC, Giovannucci EL, Platz EA, et al. Intake of meat mutagens and risk of prostate cancer in a cohort of U.S. health professionals. Cancer Epidemiol Biomarkers Prev. (2015) 24:1557–63. doi: 10.1158/1055-9965.EPI-15-0068-T
65. Sinha R, Kulldorff M, Swanson CA, Curtin J, Brownson RC, Alavanja MC. Dietary heterocyclic amines and the risk of lung cancer among missouri women. Cancer Res (2000) 60:3753–6.
66. Lam TK, Cross AJ, Consonni D, Randi G, Bagnardi V, Bertazzi PA, et al. Intakes of red meat, processed meat, and meat mutagens increase lung cancer risk. Cancer Res. (2009) 69:932–9. doi: 10.1158/0008-5472.CAN-08-3162
67. De Stefani E, Boffetta P, Deneo-Pellegrini H, Ronco AL, Aune D, Acosta G, et al. Meat intake, meat mutagens and risk of lung cancer in Uruguayan men. Cancer Causes Control. (2009) 20:1635–43. doi: 10.1007/s10552-009-9411-2
68. Tasevska N, Sinha R, Kipnis V, Subar AF, Leitzmann MF, Hollenbeck AR, et al. A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am J Clin Nutr. (2009) 89:1884–94. doi: 10.3945/ajcn.2008.27272
69. Cross AJ, Ward MH, Schenk M, Kulldorff M, Cozen W, Davis S, et al. Meat and meat-mutagen intake and risk of non-Hodgkin lymphoma: Results from a NCI-SEER case-control study. Carcinogenesis. (2006) 27:293–7. doi: 10.1093/carcin/bgi212
70. Daniel CR, Sinha R, Park Y, Graubard BI, Hollenbeck AR, Morton LM, et al. Meat intake is not associated with risk of non-Hodgkin lymphoma in a large prospective cohort of U.S. men and women. J Nutr. (2012) 142:1074–80. doi: 10.3945/jn.112.158113
71. De Stefani E, Fierro L, Mendilaharsu M, Ronco A, Larrinaga MT, Balbi JC, et al. Meat intake, ‘mate’ drinking and renal cell cancer in Uruguay: A case-control study. Br J Cancer. (1997) 78:1239–43. doi: 10.1038/bjc.1998.661
72. Daniel CR, Schwartz KL, Colt JS, Dong LM, Ruterbusch JJ, Purdue MP, et al. Meat-cooking mutagens and risk of renal cell carcinoma. Br J Cancer. (2011) 105:1096–104. doi: 10.1038/bjc.2011.343
73. Daniel CR, Cross AJ, Graubard BI, Park Y, Ward MH, Rothman N, et al. Large prospective investigation of meat intake, related mutagens, and risk of renal cell carcinoma. Am J Clin Nutr. (2012) 95:155–62. doi: 10.3945/ajcn.111.019364
74. De Stefani E, Boffetta P, Mendilaharsu M, Carzoglio J, Deneo-Pellegrini H. Dietary nitrosamines, heterocyclic amines, and risk of gastric cancer: A case-control study in Uruguay. Nutr Cancer. (1998) 30:158–62. doi: 10.1080/01635589809514656
75. Terry PD, Lagergren J, Wolk A, Steineck G, Nyrén O. Dietary intake of heterocyclic amines and cancers of the esophagus and gastric cardia. Cancer Epidemiol Biomark Prev. (2003) 12:940–4.
76. Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, Koizumi Y, et al. Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of stomach cancer: A hospital-based case-control study in Japan. Gastric Cancer. (2009) 12:198–205. doi: 10.1007/s10120-009-0523-x
77. Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. (2011) 106:432–42. doi: 10.1038/ajg.2010.415
78. De Stefani E, Ronco A, Mendilaharsu M, Deneo-Pellegrini H. Case-control study on the role of heterocyclic amines in the etiology of upper aerodigestive cancers in Uruguay. Nutr Cancer. (1998) 32:43–8. doi: 10.1080/01635589809514715
79. Anderson KE, Kadlubar FF, Kulldorff M, Harnack L, Gross M, Lang NP, et al. Dietary intake of heterocyclic amines and benzo(a)pyrene: Associations with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. (2005) 14:2261–5. doi: 10.1158/1055-9965.EPI-04-0514
80. Li D, Day RS, Bondy ML, Sinha R, Nguyen NT, Evans DB, et al. Dietary mutagen exposure and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. (2007) 16:655–61. doi: 10.1158/1055-9965.EPI-06-0993
81. Stolzenberg-Solomon RZ, Cross AJ, Silverman DT, Schairer C, Thompson FE, Kipnis V, et al. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev. (2007) 16:2664–75. doi: 10.1158/1055-9965.EPI-07-0378
82. Anderson KE, Mongin SJ, Sinha R, Stolzenberg-Solomon R, Gross MD, Ziegler RG, et al. Pancreatic cancer risk: Associations with meat-derived carcinogen intake in the prostate, lung, colorectal, and ovarian cancer screening trial (PLCO) cohort. Mol Carcinog. (2012) 51:128–37. doi: 10.1002/mc.20794
Keywords: meat mutagens, heterocyclic amines (HCAs), polycyclic aromatic amines (PAHs), cancer risk, meta-analysis
Citation: Reng Q, Zhu LL, Feng L, Li YJ, Zhu YX, Wang TT and Jiang F (2022) Dietary meat mutagens intake and cancer risk: A systematic review and meta-analysis. Front. Nutr. 9:962688. doi: 10.3389/fnut.2022.962688
Received: 06 June 2022; Accepted: 01 September 2022;
Published: 23 September 2022.
Edited by:
Xiaoyan Wu, Guilin Medical University, ChinaReviewed by:
Dragana Mitic-Culafic, University of Belgrade, SerbiaFatih Öz, Atatürk University, Turkey
Copyright © 2022 Reng, Zhu, Feng, Li, Zhu, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Jiang, amlhbmdmOTg0M0B0enp4eXkuY29t
 Qie Reng1
Qie Reng1 Feng Jiang
Feng Jiang