- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
- 5Beijing Translational Engineering Center for 3D Printer in Clinical Neuroscience, Beijing, China
- 6Department of Neurosurgery, Beijing Hospital, Beijing, China
- 7Savaid Medical School, University of Chinese Academy of Sciences, Beijing, China
Background: Circulating choline pathway nutrients play a critical role in first stroke and recurrent stroke. However, there is limited information available on the effects of choline pathway nutrients on the risk of moyamoya disease (MMD) and its subtypes. We investigated the association between circulating choline and betaine and the incident risk of MMD and its subtypes.
Methods: The case-control study enrolled 385 patients with MMD [i.e., 110 transient ischemic attack (TIA)-type MMD, 157 infarction-type MMD, and 118 hemorrhagic-type MMD] and 89 matched healthy controls.
Results: Serum choline and betaine were inversely related to the risk of MMD and its subtypes. The risk of MMD was decreased with each increment in choline level [per 1 μmol increase: odds ratio (OR), 0.756; 95% CI, 0.678–0.843] and betaine level (per 1 μmol increase: OR, 0.952; 95% CI, 0.932–0.972), respectively. When choline and betaine were assessed as quartiles, compared with the lowest quartile of serum choline and betaine levels, those in the highest quartile had a significantly decreased risk of MMD (choline, Q4 vs. Q1: OR, 0.023; 95% CI, 0.005–0.118; betaine, Q4 vs. Q1: OR, 0.058; 95% CI, 0.018–0.184).
Conclusions: Serum choline and betaine were associated with the decreased risk of MMD and its subtypes.
Introduction
Moyamoya disease (MMD) is a rare cerebrovascular disorder, which is characterized by progressive stenosis/occlusion of the internal carotid arteries and their proximal branches, with the formation of collateral abnormal vascular network formation at the basal part of the brain (1). Although MMD is a rare cerebrovascular disorder, it is the main cause of stroke in children and adolescents in East Asian populations (2). There are two well-established MMD phenotypes: one is the ischemic type and the other is the hemorrhagic type (3).
The etiology of MMD is currently unknown and immune, inflammation, genetic, and other factors may have all contributed to the development and progression of MMD (2, 4, 5). A strong association has been found between RNF213 p.R4810K variant and MMD. Approximately 90% of Japanese patients, 79% of Korean patients, and 23% of Chinese patients showed this variant (6–8). Obviously, the incidence of p.R4810K variant was much lower in China than in other East Asian countries. Therefore, in addition to genetic factors, there are other factors contributing to MMD in China. In our recent case-control study, we found that several traditional modifiable risk factors, such as albumin (ALB), homocysteine (Hcy), body mass index (BMI), and high-density lipoprotein (HDL), were correlated with the risk of MMD (9). Nevertheless, the traditional risk factors cannot explain all of the MMD risk factors. Identification of novel risk factors is of urgent necessity, particularly modifiable risk factors.
In recent years, choline pathway metabolites have been of extensive interest and play an important role in many physiological and pathological processes of cardiovascular and cerebrovascular diseases (10–12). Choline is an essential nutrient with diverse biological functions, such as cell membrane integrity, cholinergic neurotransmission, lipid transport, and one-carbon metabolism (13). Betaine is a product of an irreversible oxidation reaction of choline, and it participates as a methyl donor in choline metabolism, which increases the remethylation of Hcy to methionine and influences DNA and histone methylation (14). Abnormal choline metabolism was implicated in the development and progression of processes of cardiovascular and cerebrovascular diseases. The current findings raise questions regarding the role of choline pathway metabolites in MMD risk. Therefore, we conducted this prospective study to investigate the association between serum choline and betaine and the risk of MMD and its subtypes.
Methods
In this study, we recruited prospectively consecutive adult patients with MMD aged 18 years or older at the Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University from September 1, 2020 to December 31, 2021. All participants provided written informed consent. The protocol of this study was approved by the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University.
Study participants
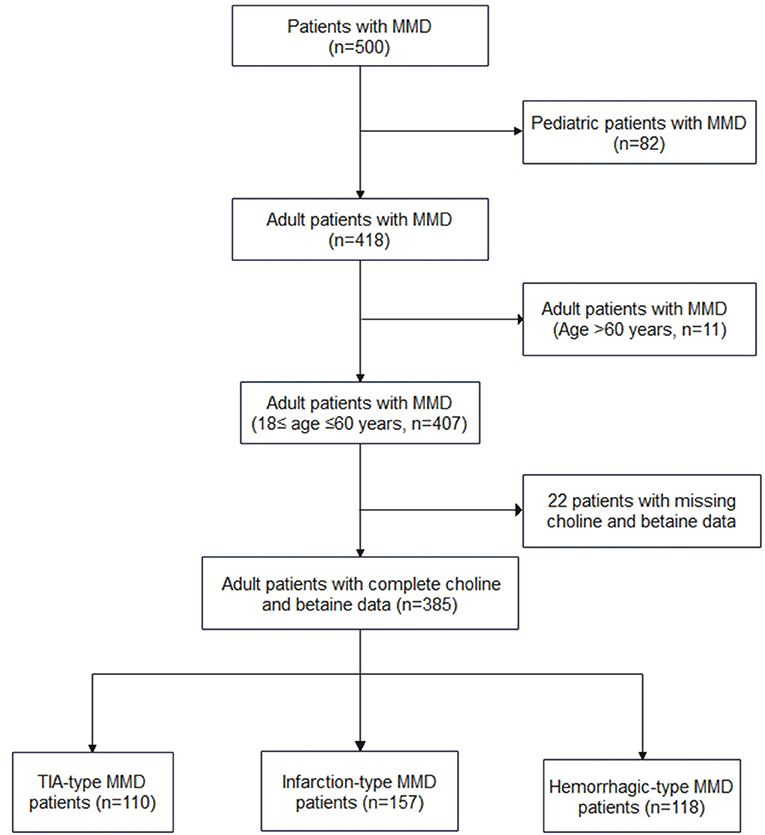
All patients with MMD were diagnosed using digital subtraction angiography according to the Japanese guidelines published in 2012 (15), and both unilateral and bilateral MMDs were enrolled. From September 1, 2020 to December 31, 2021, 500 patients (that included 418 adult patients) with MMD received their treatment at our center and 385 adult patients with complete choline and betaine measurements were enrolled in the study (Figure 1). Among the 385 adult patients, 110 cases were of transient ischemic attack (TIA)-type MMD, 157 cases were of infarction-type MMD, and 118 cases were of hemorrhagic-type MMD. In the control group, age-matched healthy individuals who came for routine checkups were recruited. Based on interviews with these individuals and their families, none of them had MMD or heart disease.
Baseline data collection
All subject data were collected by trained research coordinators via questionnaires and independent chart reviews, which included age, sex, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, medical history (hypertension, diabetes, hypercholesterolemia, cigarette smoking, and alcohol consumption). Fasting blood was used to determine white blood cell count (WBC), lymphocyte (LY) count, neutrophil count, platelet count, glucose, ALB, creatinine, uric acid, triglyceride, total cholesterol, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, Hcy, and estimated glomerular filtration rate (eGFR) by using an automatic clinical analyzer. RNF213 p.R4810K variant was detected. The primers were designed as follows: RNF213-4810F (rs112735431): 5′-GCCCTCCATTTCTAGCACAC-3′; and RNF213-4810R: 5′-AGCTGTGGCGAAAGCTTCTA-3′. Within 24 h of patients' admission to the hospital, fasting blood samples were drawn into serum separation tubes and ethylenediaminetetraacetic acid (EDTA) anticoagulation blood collection tubes and then all the blood samples were stored at −80°C in the Central Laboratory of Beijing Tiantan Hospital, Capital Medical University until testing was performed. Liquid chromatography-mass spectrometry was used to measure serum levels of free choline and betaine. Laboratory technicians who measured serum-free choline and betaine were blinded to baseline characteristics.
Statistical analysis
Baseline characteristics were presented and compared between cases and controls. Continuous variables are expressed as the means with SD. Group comparisons were carried out using Student's t-tests, Mann-Whitney U tests, or chi-squared tests, as appropriate. The generalized linear regression analysis was used to test for trends across the choline and betaine for continuous variables, and the Cochran-Armitage trend χ2-test or Mantel-Haenszel test was applied for categorical variables, as appropriate. We performed three logistic regression models to identify the independent risk factors of MMD and its subtypes: the crude model was an unadjusted logistic regression model; model 1 was adjusted for age, sex, heart rate, SBP, DBP, and BMI; model 2: model 1 was further adjusted for WBC count, LY count, neutrophil count, platelet count, ALB, creatinine uric acid, triglyceride, total cholesterol, HDL cholesterol, LDL cholesterol, apolipoprotein A (ApoA), apolipoprotein B (ApoB), Hcy, and eGFR. Statistical analysis was performed using SPSS software (version 20.0; IBM Corporation, Armonk, NY, USA) and R version 4.1.2. p < 0.05 was considered statistically significant.
Results
Study participants and characteristics
This analysis included 385 MMD cases (110 cases of TIA-type MMD, 157 cases of infarction-type MMD, and 118 cases of hemorrhagic-type MMD) and 89 matched controls with complete choline and betaine measurements. A total of 192 (40.5%) men and 282 (59.5%) women were included, and the median age was 41 years [interquartile range (IQR), 33–49 years]. The median plasma choline and betaine concentrations were 11.40 μmol/L (IQR, 8.70–13.30 μmol/L) and 33.70 μmol/L (IQR, 25.50–42.30 μmol/L), respectively.
Baseline population characteristics of MMD and controls are presented in Table 1. Patients with MMD had more risk factors for stroke than the healthy controls. MMD and its subtypes had higher levels of SBP, triglyceride, and ApoA and a higher prevalence of hypertension, diabetes mellitus, hypercholesterolemia, cigarette smoking, alcohol consumption, and evaluated Hcy (p < 0.05 for all). In addition, patients with MMD had lower levels of HDL cholesterol, choline, and betaine (p < 0.05 for all).
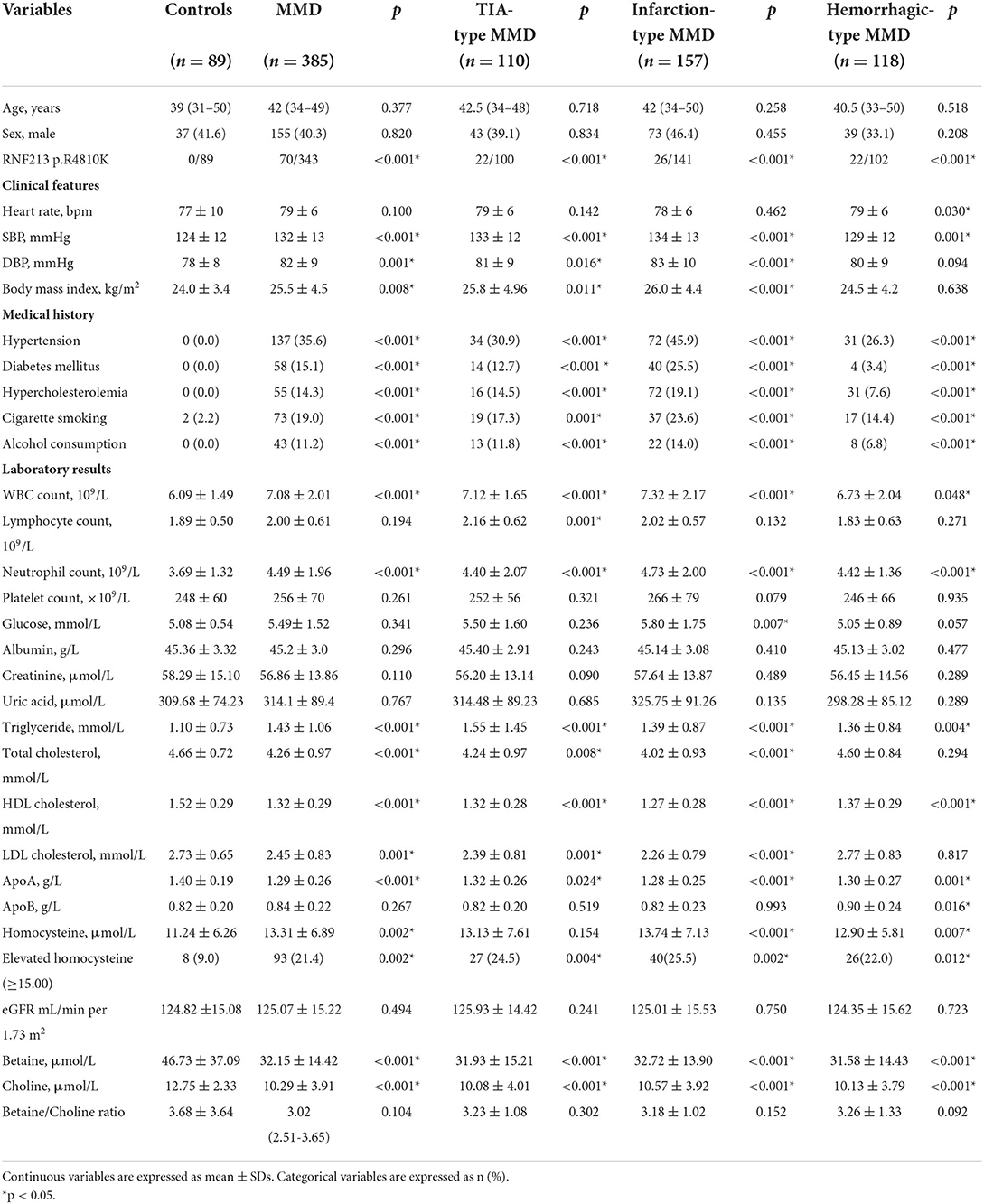
Table 1. Clinical and laboratory characteristics in patients with moyamoya disease (MMD) and healthy controls.
Clinical characteristics of the patients with MMD according to the choline and betaine quartiles are shown in Table 2. Patients with higher choline levels tended to be men and older; had higher levels of glucose and betaine. Participants with higher betaine levels tended to be men and older, had higher creatinine and choline levels, and lower levels of triglyceride, total cholesterol, LDL cholesterol, HDL cholesterol, ApoA, ApoB, and eGFR. The correlation between betaine, choline, and other risk factors was done by Pearson's correlation coefficient test. There is a significant positive association between betaine and choline or total cholesterol (Figure 2).
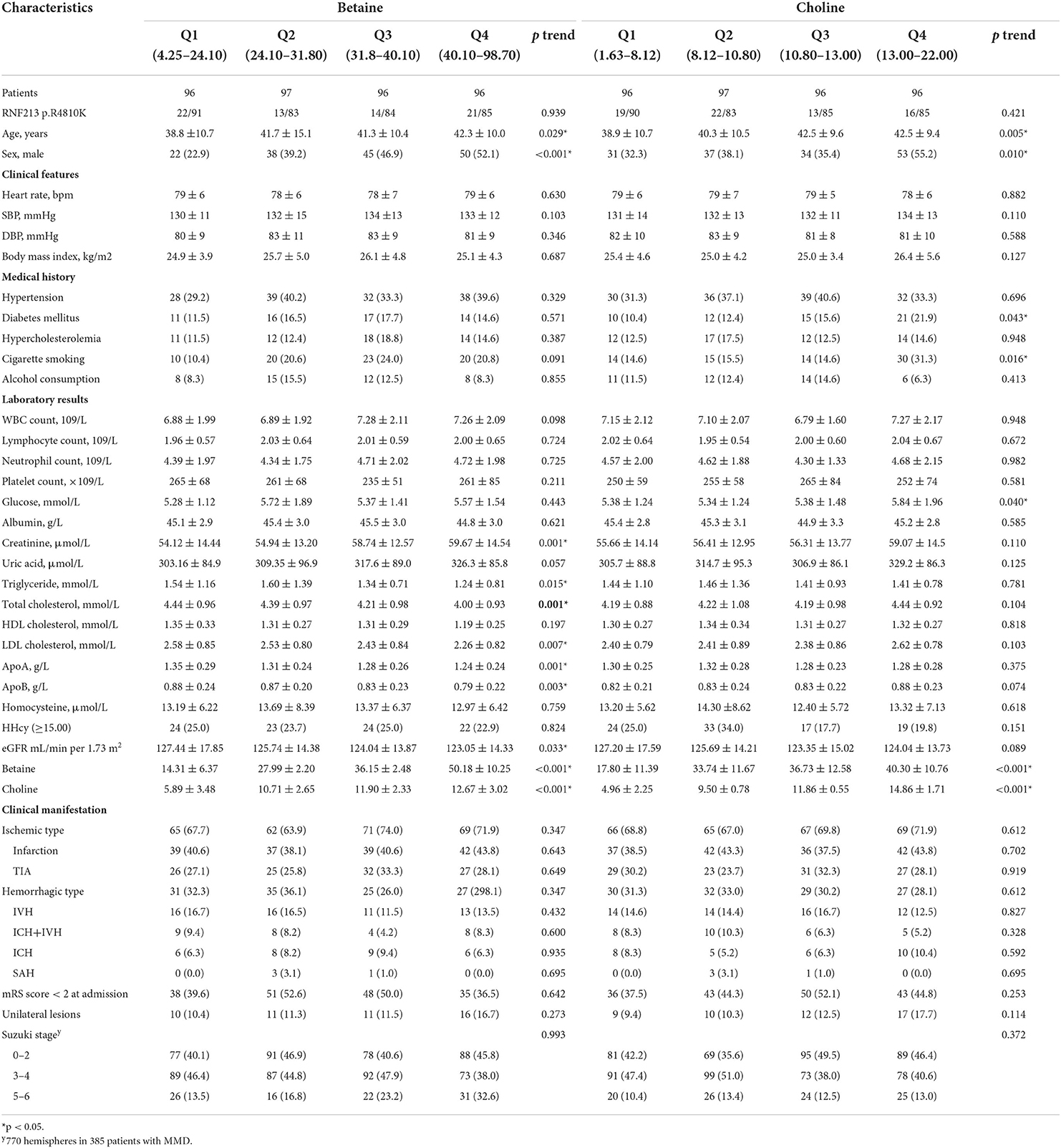
Table 2. Baseline characteristics of patients with moyamoya disease (MMD) according to quartiles of serum choline pathway nutrients.
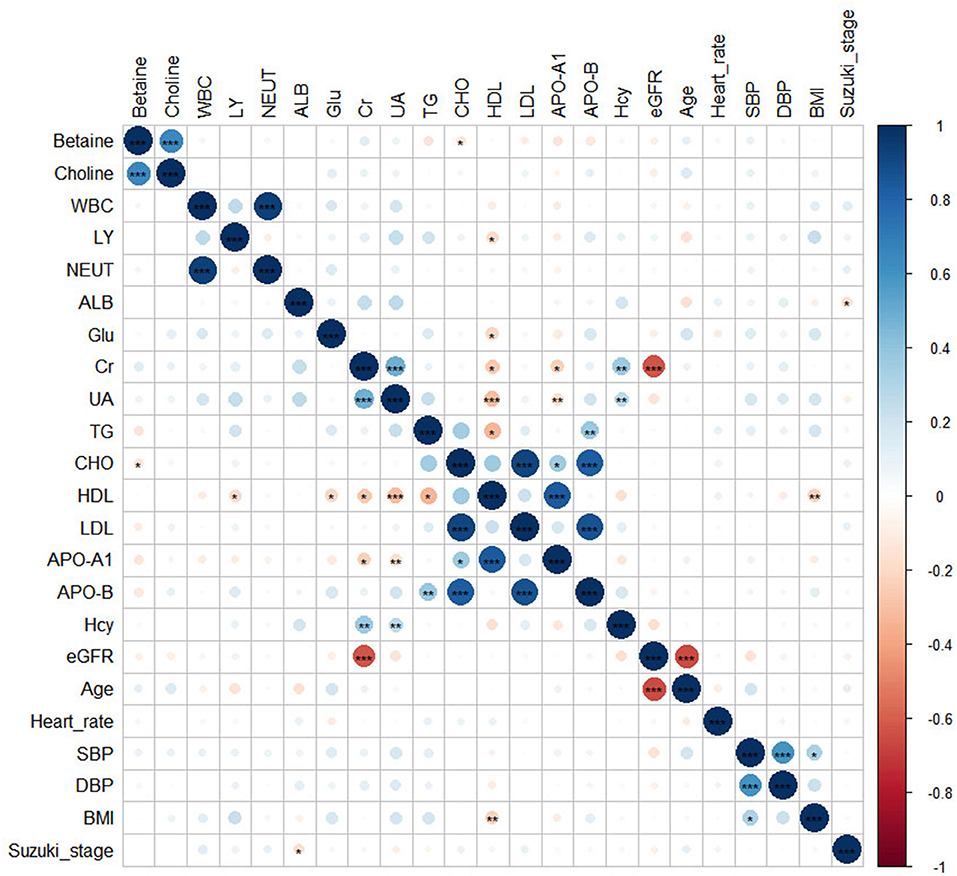
Figure 2. The heatmap showed the correlations between betaine, choline, and other risk factors. Significance: ***p < 0.001; **p < 0.01; *p < 0.05.
Choline pathway metabolites and risk of MMD
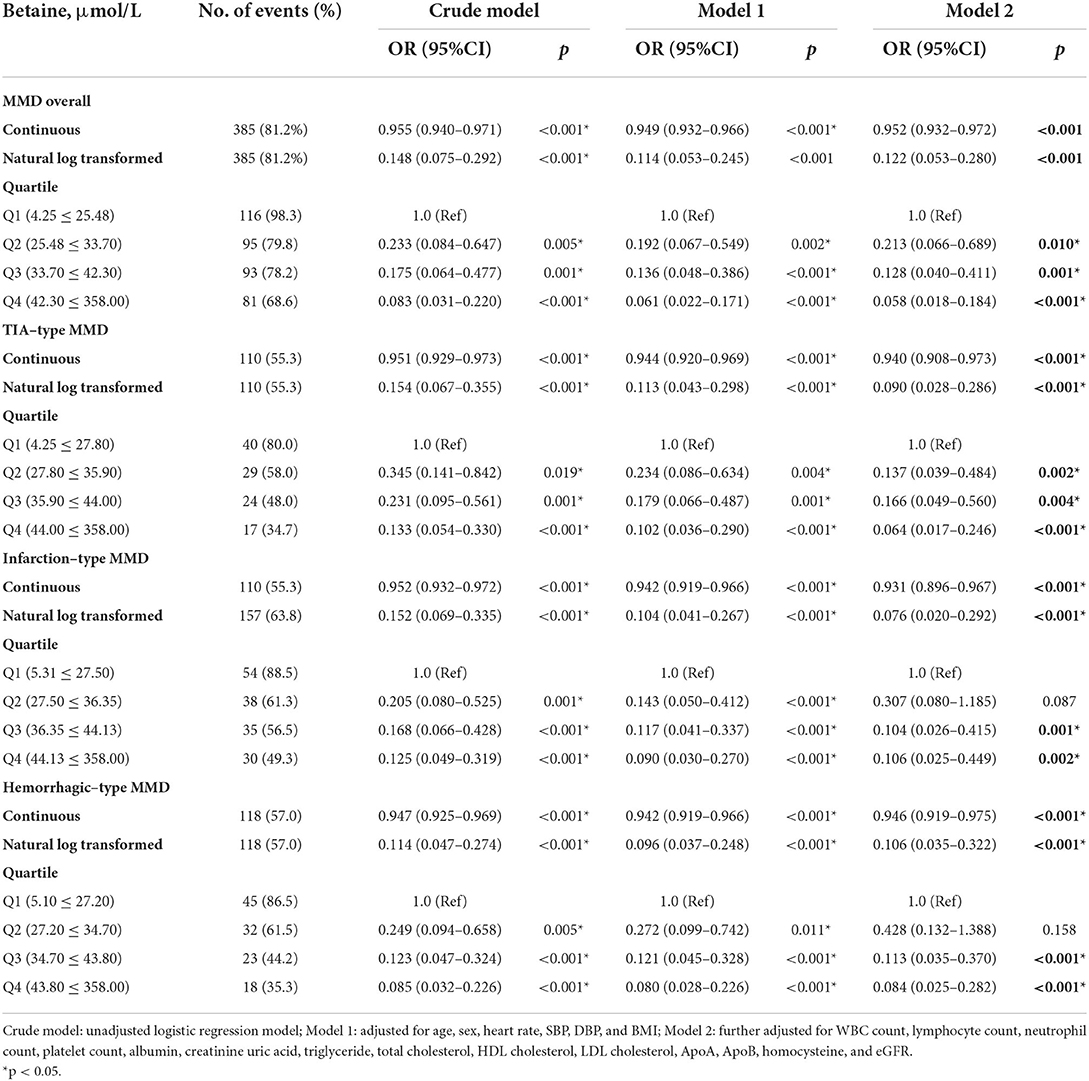
The associations of serum choline and betaine with the risks of MMD are presented in Tables 3, 4. Both serum choline and betaine were inversely associated with the risk of MMD. Overall, after fully adjusting for age, sex, heart rate, SBP, DBP, BMI, WBC count, LY count, neutrophil count, platelet count, ALB, creatinine uric acid, triglyceride, total cholesterol, HDL cholesterol, LDL cholesterol, ApoA, ApoB, Hcy, and eGFR, the risk of MMD was decreased with each increment in choline level [per 1 μmol increase: odds ratio (OR), 0.756; 95% CI, 0.678–0.843] and betaine level (per 1 μmol increase: OR, 0.952; 95% CI, 0.932–0.972), respectively. When choline and betaine were assessed as quartiles, compared with the lowest quartile of serum choline and betaine levels, those in the highest quartile had a significantly decreased risk of MMD (choline, Q4 vs. Q1: OR, 0.023; 95% CI, 0.005–0.118; betaine, Q4 vs. Q1: OR, 0.058; 95% CI, 0.018–0.184).
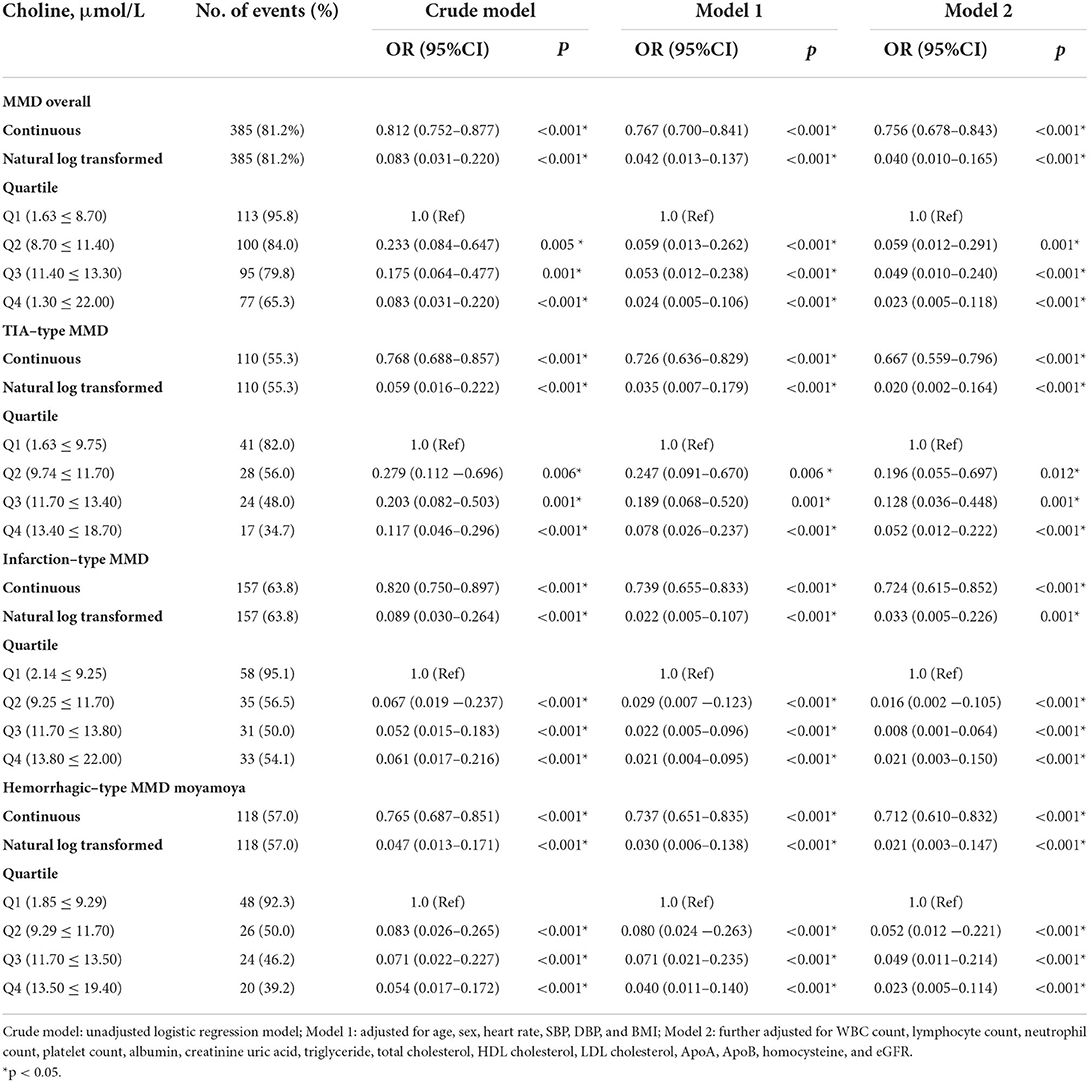
Table 3. The association between baseline choline levels and the risk of moyamoya disease (MMD) and its subtypes.
Choline pathway metabolites and risk of MMD subtypes
Consistently, the risk of TIA-type MMD was decreased with each increment in choline level (per 1 μmol increase: OR, 0.667; 95% CI, 0.559–0.796) and betaine level (per 1 μmol increase: OR, 0.940; 95% CI, 0.908–0.973), respectively. When choline and betaine were assessed as quartiles, compared with the lowest quartile of serum choline and betaine levels, those in the highest quartile had a significantly decreased risk of TIA-type MMD (choline, Q4 vs. Q1: OR, 0.052; 95% CI, 0.012–0.222); betaine, Q4 vs. Q1: OR, 0.064; 95% CI, 0.017–0.246).
The risk of infarction-type MMD was decreased with each increment in choline level (per 1 μmol increase: OR, 0.756; 95% CI, 0.678–0.843) and betaine level (per 1 μmol increase: OR, 0.952; 95% CI, 0.932–0.972), respectively. When choline and betaine were assessed as quartiles, compared with the lowest quartile of serum choline and betaine levels, those in the highest quartile had a significantly decreased risk of MMD (choline, Q4 vs. Q1: OR, 0.021; 95% CI, 0.003–0.150; betaine, Q4 vs. Q1: OR, 0.106; 95% CI, 0.025–0.449).
The risk of hemorrhagic-type MMD was decreased with each increment in choline level (per 1 μmol increase: OR, 0.712; 95% CI, 0.610–0.832) and betaine level (per 1 μmol increase: OR, 0.946; 95% CI, 0.919–0.975), respectively. When choline and betaine were assessed as quartiles, compared with the lowest quartile of serum choline and betaine levels, those in the highest quartile had a significantly decreased risk of MMD (choline, Q4 vs. Q1: OR, 0.023; 95% CI, 0.005–0.114; betaine, Q4 vs. Q1: OR, 0.084; 95% CI, 0.025–0.282).
Discussion
In this case-control study, we found that serum choline and betaine levels were inversely associated with the risk of MMD. After stratified by MMD subtypes, consistently, choline and betaine levels were also related to the risk of TIA-type, infarction-type, and hemorrhagic-type MMD. Therefore, our findings suggest that metabolites from choline pathways may play a crucial role in the risk of MMD and its subtypes.
Choline is a dietary component essential for normal functions of cell membranes, cholinergic neurotransmission, transmembrane signaling, lipid transport, and one-carbon metabolism (13, 16). Dietary deficiency of choline results in fatty liver disease, hemorrhagic kidney necrosis, muscle damage, and organ dysfunction (17). In the present study, we found that serum choline levels were inversely associated with the risk of MMD and its subtypes. Though it is unclear how choline contributes to MMD, several possible explanations can be proposed. First, existing evidence indicates that low dietary intake of choline and betaine may change epigenetic patterns and regulate gene expression via epigenetic modifications (18). One recent study reveals that DNA methylation was involved in the pathogenesis of MMD (19), the other study suggested that DNA methylation status at the sortilin 1 promoter CpG site may be a potential biomarker for MMD (20). Second, choline and betaine are important sources of 1-carbon units (11, 13, 16), when choline stores are inadequate, methylation of Hcy to methionine is decreased resulting in increased Hcy, and increased Hcy has been related to greater risk of MMD (9). Additionally, choline deficiency increases the activity of acetylcholinesterase (21), and serum cholinesterase activities reflect the intensity of the neuroinflammatory response in patients with stroke (22). Moreover, choline and betaine are metabolites of the choline pathway associated with decreased cardiovascular risks and recurrent strokes (23, 24).
Betaine is an oxidation product of choline, which is linked to folate and serves as a methyl donor in one-carbon metabolism (12, 14, 25). A low concentration of betaine is associated with a number of stroke risk factors, such as insulin resistance, diabetes mellitus, lipid metabolism, and atherogenic dyslipidemia (24, 26–28). In the current study, we observed an inverse relationship between betaine and risk of MMD and its subtypes. For these inverse associations, several possible pathophysiologic pathways have been proposed. First, betaine converts Hcy to methionine and dimethylglycine, which may reduce Hcy-induced oxidative stress, inflammation, apoptosis, autophagy, and endothelial dysfunction (14, 29–31). Second, betaine inhibits nuclear factor-κB activity, and nod-like receptor protein 3 (NLRP3) inflammasome activation has anti-inflammatory effects (14). Additionally, experimental studies have shown that betaine could reverse platelet aggregation in vivo and in vitro and protect against coagulation events (32).
The optimal treatment for MMD still remains controversial (33, 34). Currently, revascularization surgery is a routine treatment for MMD, but surgical bypass will not reverse the MMD process, and the major goal of the revascularization surgery is to reduce the risk of recurrent stroke and improve neurological functions (34). Meanwhile, the appropriate medical treatment, before or after surgical revascularization, is almost completely ignored by scientific reports. In the present study, choline and betaine levels were also related to the risk of MMD and its subtypes, which may suggest choline and betaine supplementation might be a novel medical strategy in patients with MMD. In addition, previous studies indicated that one-carbon metabolism supplementation has some benefits on stroke outcome, which may increase neuroplasticity and recovery after stroke (14, 35, 36). Recent research finds that methylenetetrahydrofolate reductase gene (MTHFR) and transcobalamin II (TCN2), which regulate Hcy metabolism, were novel susceptibility genes for MMD (37). In addition, betaine plays a major role in determining total Hcy levels, particularly in case of folate deficiency and MTHFR mutations (38). Furthermore, an observational study found that the high intake of choline and betaine was inversely associated with inflammation (39). These results highlight the need for prospective studies that choline and betaine supplementation in patients with MMD. As a further note, it has been found that choline and betaine intakes were not related to cardiovascular disease mortality risk (40, 41), which indicates choline and betaine supplementation in patients with MMD should be taken with caution and much further investigation. There were several limitations of this study. First, all of the patients in the present study were selected from one single neurosurgery center with heterogeneous study populations and risk of bias. Second, this study included only Chinese adults with MMD, so the results cannot be generalized to pediatrics or other ethnic groups. Third, data on dietary intakes of choline and betaine, phosphocholine, sphingomyelin, or other precursors were not collected. Therefore, further investigation of the association between choline and betaine intakes and MMD was limited. Fourth, as a result of the study design, we were unable to demonstrate that choline pathway biomarkers are causally linked with MMD and its subtypes, even after considering many potential confounders. To understand the causality of these risk factors, treatment and long-term follow-up are required.
Conclusions
Serum choline and betaine were associated with decreased risk of MMD and its subtypes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PG, DZ, and JZ: conception and design. YZhao, YZhai, and JW: acquisition of data. PG and YZhai: analysis and interpretation of data. PG: drafting the article. QZ, XY, RW, YZhan, and DZ: technical supports. JZ: study supervision. All authors critically revising the article and approved the final version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFC2500502), Beijing Municipal Organization Department Talents Project (2015000021469G219), the National Natural Science Foundation of China (81701137), and Beijing Municipal Administration of Hospitals' Mission Plan (SML20150501).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. (2008) 7:1056–66. doi: 10.1016/S1474-4422(08)70240-0
2. Kim JS. Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke. (2016) 18:2–11. doi: 10.5853/jos.2015.01627
3. Liu XJ, Zhang D, Wang S, Zhao YL, Teo M, Wang R, et al. Clinical features and long-term outcomes of moyamoya disease: a single-center experience with 528 cases in China. J Neurosurg. (2015) 122:392–9. doi: 10.3171/2014.10.JNS132369
4. Fujimura M, Sonobe S, Nishijima Y, Niizuma K, Sakata H, Kure S, et al. Genetics and biomarkers of moyamoya disease: significance of RNF213 as a susceptibility gene. J Stroke. (2014) 16:65–72. doi: 10.5853/jos.2014.16.2.65
5. Bang OY, Fujimura M, Kim SK. The pathophysiology of moyamoya disease: an update. J Stroke. (2016) 18:12–20. doi: 10.5853/jos.2015.01760
6. Kim EH, Yum MS, Ra YS, Park JB, Ahn JS, Kim GH, et al. Importance of RNF213 polymorphism on clinical features and long-term outcome in moyamoya disease. J Neurosurg. (2016) 124:1221–7. doi: 10.3171/2015.4.JNS142900
7. Zhang Q, Liu Y, Zhang D, Wang R, Zhang Y, Wang S, et al. RNF213 as the major susceptibility gene for Chinese patients with moyamoya disease and its clinical relevance. J Neurosurg. (2017) 126:1106–13. doi: 10.3171/2016.2.JNS152173
8. Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, et al. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. (2012) 78:803–10. doi: 10.1212/WNL.0b013e318249f71f
9. Ge P, Zhang Q, Ye X, Liu X, Deng X, Wang J, et al. Modifiable risk factors associated with moyamoya disease: a case-control study. Stroke. (2020) 51:2472–9. doi: 10.1161/STROKEAHA.120.030027
10. Zhong C, Lu Z, Che B, Qian S, Zheng X, Wang A, et al. Choline pathway nutrients and metabolites and cognitive impairment after acute ischemic stroke. Stroke. (2021) 52:887–95. doi: 10.1161/STROKEAHA.120.031903
11. Yahn GB, Leoncio J, Jadavji NM. The role of dietary supplements that modulate one-carbon metabolism on stroke outcome. Curr Opin Clin Nutr Metab Care. (2021) 24:303–7. doi: 10.1097/MCO.0000000000000743
12. Rosas-Rodríguez JA, Valenzuela-Soto EM. The glycine betaine role in neurodegenerative, cardiovascular, hepatic, and renal diseases: insights into disease and dysfunction networks. Life Sci. (2021) 285:119943. doi: 10.1016/j.lfs.2021.119943
13. Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. (2006) 26:229–50. doi: 10.1146/annurev.nutr.26.061505.111156
14. Zhao G, He F, Wu C, Li P, Li N, Deng J, et al. Betaine in inflammation: mechanistic aspects and applications. Front Immunol. (2018) 9:1070. doi: 10.3389/fimmu.2018.01070
15. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir. (2012) 52:245–66. doi: 10.2176/nmc.52.245
16. Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. (2017) 9:815. doi: 10.3390/nu9080815
17. Yang JJ, Lipworth LP, Shu XO, Blot WJ, Xiang YB, Steinwandel MD, et al. Associations of choline-related nutrients with cardiometabolic and all-cause mortality: results from 3 prospective cohort studies of blacks, whites, and Chinese. Am J Clin Nutr. (2020) 111:644–56. doi: 10.1093/ajcn/nqz318
18. Friso S, Udali S, De Santis D, Choi SW. One-carbon metabolism and epigenetics. Mol Aspects Med. (2017) 54:28–36. doi: 10.1016/j.mam.2016.11.007
19. He S, Ye X, Duan R, Zhao Y, Wei Y, Wang Y, et al. Epigenome-wide association study reveals differential methylation sites and association of gene expression regulation with ischemic moyamoya disease in adults. Oxid Med Cell Longev. (2022) 2022:7192060. doi: 10.1155/2022/7192060
20. Sung HY, Lee JY, Park AK, Moon YJ, Jo I, Park EM, et al. Aberrant promoter hypomethylation of sortilin 1: a moyamoya disease biomarker. J Stroke. (2018) 20:350–61. doi: 10.5853/jos.2018.00962
21. Vučević DB, Cerović IB, Mladenović DR, Vesković MN, Stevanović I, Jorgačević BZ, et al. Methionine-choline deprivation alters liver and brain acetylcholinesterase activity in C57BL6 mice. Gen Physiol Biophys. (2016) 35:363–70. doi: 10.4149/gpb_2015052
22. Ben Assayag E, Shenhar-Tsarfaty S, Ofek K, Soreq L, Bova I, Shopin L, et al. Serum cholinesterase activities distinguish between stroke patients and controls and predict 12-month mortality. Mol Med. (2010) 16:278–86. doi: 10.2119/molmed.2010.00015
23. Millard HR, Musani SK, Dibaba DT, Talegawkar SA, Taylor HA, Tucker KL, et al. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: the Jackson Heart Study. Eur J Nutr. (2018) 57:51–60. doi: 10.1007/s00394-016-1296-8
24. Zhong C, Miao M, Che B, Du J, Wang A, Peng H, et al. Plasma choline and betaine and risks of cardiovascular events and recurrent stroke after ischemic stroke. Am J Clin Nutr. (2021) 114:1351–9. doi: 10.1093/ajcn/nqab199
25. Xie L, Zhao BX, Luo J, Li Y, Zhu F, Li GF, et al. A U-shaped association between serum betaine and incident risk of first ischemic stroke in hypertensive patients. Clin Nutr. (2020) 39:2517–24. doi: 10.1016/j.clnu.2019.11.011
26. Pan XF, Yang JJ, Shu XO, Moore SC, Palmer ND, Guasch-Ferré M, et al. Associations of circulating choline and its related metabolites with cardiometabolic biomarkers: an international pooled analysis. Am J Clin Nutr. (2021) 114:893–906. doi: 10.1093/ajcn/nqab152
27. Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, et al. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients. (2018) 10:131. doi: 10.3390/nu10020131
28. Cholewa JM, Guimarães-Ferreira L, Zanchi NE. Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids. (2014) 46:1785–93. doi: 10.1007/s00726-014-1748-5
29. Smith AD, Refsum H. Homocysteine - from disease biomarker to disease prevention. J Intern Med. (2021) 290:826–54. doi: 10.1111/joim.13279
30. Arumugam MK, Paal MC, Donohue TM Jr., Ganesan M, Osna NA, Kharbanda KK. Beneficial effects of betaine: a comprehensive review. Biology. (2021) 10:456. doi: 10.3390/biology10060456
31. Veskovic M, Mladenovic D, Milenkovic M, Tosic J, Borozan S, Gopcevic K, et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur J Pharmacol. (2019) 848:39–48. doi: 10.1016/j.ejphar.2019.01.043
32. Nemmar A, Yuvaraju P, Beegam S, Ali BH. Betaine (N,N,N-trimethylglycine) averts photochemically-induced thrombosis in pial microvessels in vivo and platelet aggregation in vitro. Exp Biol Med. (2015) 240:955–60. doi: 10.1177/1535370214564749
33. Deng X, Ge P, Wang S, Zhang D, Zhang Y, Wang R, et al. Treatment of moyamoya disease. Neurosurgery. (2018) 65(CN_suppl_1):62–5. doi: 10.1093/neuros/nyy114
34. Acker G, Fekonja L, Vajkoczy P. Surgical management of moyamoya disease. Stroke. (2018) 49:476–82. doi: 10.1161/STROKEAHA.117.018563
35. Li Q, Qu M, Wang N, Wang L, Fan G, Yang C. Betaine protects rats against ischemia/reperfusion injury-induced brain damage. J Neurophysiol. (2022) 127:444–51. doi: 10.1152/jn.00400.2021
36. Jadavji NM, Emmerson JT, MacFarlane AJ, Willmore WG, Smith PD. B-vitamin and choline supplementation increases neuroplasticity and recovery after stroke. Neurobiol Dis. (2017) 103:89–100. doi: 10.1016/j.nbd.2017.04.001
37. Duan L, Wei L, Tian Y, Zhang Z, Hu P, Wei Q, et al. Novel susceptibility loci for moyamoya disease revealed by a genome-wide association study. Stroke. (2018) 49:11–8. doi: 10.1161/STROKEAHA.117.017430
38. Jadavji NM, Mosnier H, Kelly E, Lawrence K, Cruickshank S, Stacey S, et al. One-carbon metabolism supplementation improves outcome after stroke in aged male MTHFR-deficient mice. Neurobiol Dis. (2019) 132:104613. doi: 10.1016/j.nbd.2019.104613
39. Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. (2008) 87:424–30. doi: 10.1093/ajcn/87.2.424
40. Nagata C, Wada K, Tamura T, Konishi K, Kawachi T, Tsuji M, et al. Choline and betaine intakes are not associated with cardiovascular disease mortality risk in Japanese men and women. J Nutr. (2015) 145:1787–92. doi: 10.3945/jn.114.209296
Keywords: moyamoya, choline, betaine, risk factors, subtypes
Citation: Ge P, Zhao Y, Zhai Y, Zhang Q, Ye X, Wang J, Wang R, Zhang Y, Zhang D and Zhao J (2022) Circulating choline pathway nutrients and risk of moyamoya disease. Front. Nutr. 9:953426. doi: 10.3389/fnut.2022.953426
Received: 27 May 2022; Accepted: 13 July 2022;
Published: 01 August 2022.
Edited by:
Nafisa M. Jadavji, Midwestern University, United StatesReviewed by:
Xinyin Jiang, Brooklyn College (CUNY), United StatesMilena Veskovic, Faculty of Medicine, University of Belgrade, Serbia
Copyright © 2022 Ge, Zhao, Zhai, Zhang, Ye, Wang, Wang, Zhang, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Zhang, emhhbmdkb25nMDY2MEBhbGl5dW4uY29t; Jizong Zhao, emhhb2p6MjA1QDE2My5jb20=
 Peicong Ge
Peicong Ge Yaobo Zhao
Yaobo Zhao Yuanren Zhai1,2,3,4,5
Yuanren Zhai1,2,3,4,5 Qian Zhang
Qian Zhang Rong Wang
Rong Wang Yan Zhang
Yan Zhang Dong Zhang
Dong Zhang Jizong Zhao
Jizong Zhao