
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 12 August 2022
Sec. Nutrition, Psychology and Brain Health
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.950130
This article is part of the Research TopicNeurological, Psychological and Endocrine Markers of Eating Disorders and ObesityView all 6 articles
Obesity is a growing global health problem; it has been forecasted that over half of the global population will be obese by 2030. Obesity is complicated with many diseases, such as diabetes and cardiovascular diseases, leading to an economic impact on society. Other than diet, exposure to environmental pollutants is considered a risk factor for obesity. Exposure to perfluorooctanoic acid (PFOA) was found to impair hepatic lipid metabolism, resulting in obesity. In this study, we applied network pharmacology and systematic bioinformatics analysis, such as gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, together with molecular docking, to investigate the targets of fucoidan for treating PFOA-associated obesity through the regulation of endoplasmic reticulum stress (ERS). Our results identified ten targets of fucoidan, such as glucosylceramidase beta (GBA), glutathione-disulfide reductase (GSR), melanocortin 4 receptor (MC4R), matrix metallopeptidase (MMP)2, MMP9, nuclear factor kappa B subunit 1 (NFKB1), RELA Proto-Oncogene, NF-KB Subunit (RELA), nuclear receptor subfamily 1 group I member 2 (NR1I2), proliferation-activated receptor delta (PPARD), and cellular retinoic acid binding protein 2 (CRABP2). GO and KEGG enrichment analyses highlighted their involvement in the pathogenesis of obesity, such as lipid and fat metabolisms. More importantly, the gene cluster is responsible for obesity-associated diseases and disorders, such as insulin resistance (IR), non-alcoholic fatty liver disease, and diabetic cardiomyopathy, via the control of signaling pathways. The findings of this report provide evidence that fucoidan is a potential nutraceutical product against PFOA-associated obesity through the regulation of ERS.
Obesity is a global health problem, especially in developed countries. According to the data from World Health Organization (WHO), the number of global obese people has tripled since 1975.1 More seriously, the prevalence of obesity among children and adolescents has risen dramatically from 4 to 18% in the last 40 years. It has been foreseen that 51% of the global population will be obese by 2030 (1). Obesity increases the risk of many dysfunctional metabolism disorders, such as diabetes mellitus, adiposis hepatica, dementia, and cancer (2), leading to imposing economic impacts on our society, such as medical costs and premature mortality costs (3, 4). The pathogenesis of obesity is multifactorial, such as genetic and environmental factors, changes in substance metabolism and endocrine function, fatty hypertrophy, abnormal neuropsychiatry, living, and eating habits (5). Cumulating epidemiological and animal studies suggested the contribution of environmental pollutants as a risk factor for obesity (6, 7). It is widely reported that exposure to environmental pollutants, such as bisphenols, polychlorinated biphenyls, and perfluorocarbons, is positively correlated to the development of obesity (6). Perfluorooctanoic acid (PFOA), a family member of perfluorocarbons, has been evidenced in an increase in body mass index (BMI) and obesity risk (8). A European Youth Heart Study indicates that childhood exposure to PFOA may induce adiposity and impair glucose metabolism (9). PFOA-induced mitochondrial dysfunction may be the main cause of obesity as a result of intercellular mitochondria-dependent function loss, which is closely related to fat deposition (10, 11). In addition, endoplasmic reticulum stress (ERS) exerts pathophysiological actions in the induction of insulin resistance (IR) and the development of obesity (12). ERS mediates lipotoxicity, dyslipidemia, and IR, indicating that ERS may be a potential pharmacological target against obesity (13). Although existing therapeutic option using slimming drugs is commercially available, the medication treating obesity has proven to be mostly resistant to treatment, marked by insufficient efficacy and uncertain safety (14). Thus, the discovery of nutraceutical compounds using natural ingredients could be an alternative way of treating obesity. Fucoidan, known as fucoid polysaccharides extracted from brown alga, was found to possess nutritive value and pharmacological activities, such as anticoagulant, antitumor, antithrombotic, antiviral, and antioxidant (15). In vitro study showed that fucoidan contributes to the reduction of lipid accumulation and the regulation of glucose consumption (16). It has been reported that fucoidan has effective anti-obesity effects via modulating IR, oxidative stress (OS), and gut microbiota in vivo (17, 18). However, pharmacological mechanisms involved in fucoidan against obesity are still largely unknown. In the present report, we applied network pharmacology and systematic bioinformatics analysis to reveal the beneficial effects and pharmacological mechanisms of fucoidan against PFOA-associated obesity through the regulation of ERS.
The targets of fucoidan were identified by searching the databases, such as the Comparative Toxicogenomics Database (19), the SwissTargetPrediction database (20), the SuperPred database, and the PharmMapper database (21). The identified targets were subjected to the UniprotKB database (Swiss-Prot) for conversion to human genes (22). The ERS-related genes and PFOS-associated obese genes were obtained by searching the databases, such as the Online Mendelian Inheritance in Man (OMIM) database (23), the GeneCards database (24), and the National Center for Biotechnology Information (NCBI) database (25). The fucoidan target genes, the ERS-related genes, and the PFOS-associated obese genes were overlapped to determine the fucoidan targets against PFOA-associated obesity through the regulation of ERS. The STRING (version 11.0) tool was used to determine protein-protein interaction (PPI) of fucoidan targets against PFOA-associated obesity through the regulation of ERS (26). The Network Analyzer of Cytoscape_v3.8.2 was set under a median or maximum degree of freedom, the core targets were obtained under the upper limit of the screening range with a maximum degree value in topology data, and the lower limit was two times the median degree of freedom (27).
The overlapped fucoidan target genes, the ERS-related genes, and the PFOS-associated obese genes were subjected to ClusterProfiler and GOplot packages in R-language software for the gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses to determine the functional roles and mechanisms underlying fucoidan against PFOA-associated obesity through the regulation of ERS. The network of biological processes and the signaling pathways involved in fucoidan against PFOA-associated obesity was constructed by using the Cytoscape v3.8.2 tool (27).
Molecular docking analysis was used to assess the binding of fucoidan to its target proteins. Cytoscape was set under a median degree of freedom of 2 and a maximum degree of freedom of 3, the core protein targets were obtained at the range of median and maximum degrees of freedom. The protein structure of the core targets was obtained from the Protein Data Bank (PDB) database (28). The chemical structure of fucoidan was obtained from the PubChem database (29). The protein structure of matrix metallopeptidase (MMP)9, RELA Proto-Oncogene, NF-KB Subunit (RELA), and proliferation-activated receptor delta (PPARD) was obtained from the PDB database. Chem Bio Office 2010 software and Autodock Tools 1.5.6 were used to perform the molecular docking analysis (30). The PDB file was converted to a pdbqt file that can be recognized by the Autodock program. The docking parameter setting was assessed according to the root mean square deviation (RMSD) of the ligand molecule. In addition, the RMSD ≤ 4 Å was the threshold for the conformation of the ligand molecule. The results were displayed using PyMOL (version 2.3).
By searching the databases, a total of 363 fucoidan-associated genes, 258 PFOA-associated obese genes, and 4,293 ERS-related genes were identified (Figure 1A). When we overlapped these genes, we found 10 potential targets of fucoidan against PFOA-associated obesity through the regulation of ERS, such as glucosylceramidase beta (GBA), glutathione-disulfide reductase (GSR), melanocortin 4 receptor (MC4R), MMP2, MMP9, nuclear factor kappa B subunit 1 (NFKB1), nuclear receptor subfamily 1 group I member 2 (NR1I2), PPARD, RELA, and cellular retinoic acid binding protein 2 (CRABP2) (Figure 1A and Table 1). These targets were subjected to GO enrichment analysis to determine the functional roles of fucoidan against PFOA-associated obesity. Our results highlighted the biological processes related to biosynthesis and metabolisms of lipid and fat, such as regulation of lipid metabolic process, fatty acid oxidation, cellular lipid catabolic process, lipid modification, lipid glycosylation, cellular response to lipopolysaccharide, and regulation of lipid storage (Figure 1B). In addition, we found fucoidan’s targets in response to OS and hepatocyte growth factor and its involvement in inflammatory responses, such as the response to interleukin (IL)-6 and IL-1, the regulation of IL-12 production, the regulation of cytokine production, and leukocyte migration (Figure 1C). In addition, the fucoidan’s target was found to contribute to cell proliferation and differentiation (Figure 1D) and biosynthesis and metabolisms (Figure 1E). In the molecular function analysis, we observed the highlights of glucose- and lipid-related functions, such as glucosidase activity, glucosyltransferase activity, long-chain fatty acid binding, and fatty acid binding (Figure 1F). Taken together, our data suggested that fucoidan could target the genes that are closely associated with the development of obesity.
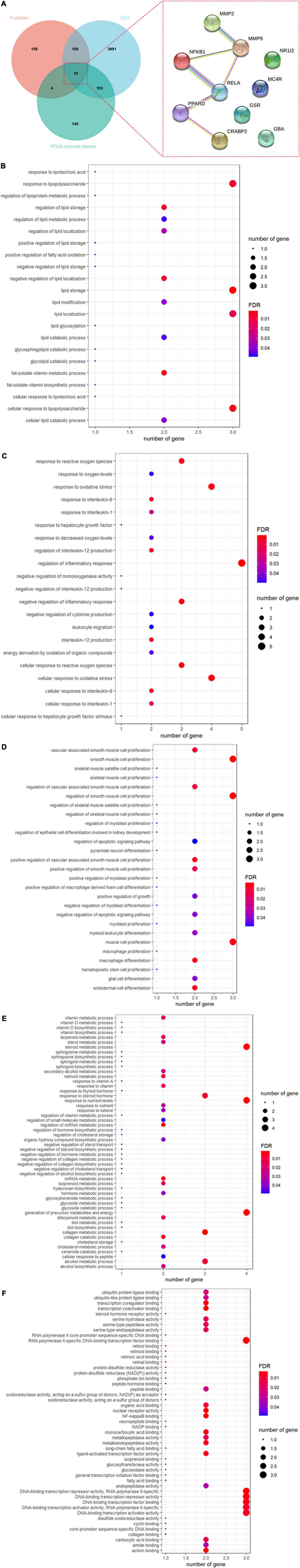
Figure 1. Identification and functional characterization of fucoidan’s targets against perfluorooctanoic acid (PFOA)-associated obesity through the regulation of endoplasmic reticulum stress. (A) A Venn diagram showed the number of shared fucoidan-, PFOA-induced obesity-, and endoplasmic reticulum stress-associated genes (left panel). The Protein–protein interaction of the ten core targets was analyzed by using STRING (right panel). Gene ontology enrichment analysis showed the involvement of fucoidan’s targets in biological processes related to (B) lipid and fat metabolisms, (C) oxidative stress and inflammatory responses, (D) cell proliferation and differentiation, (E) metabolisms and biosynthesis, and (F) molecular functions related to fat synthesis. The size of the bubble represented the number of involved genes and the color of the bubble represented the significance of the terms.
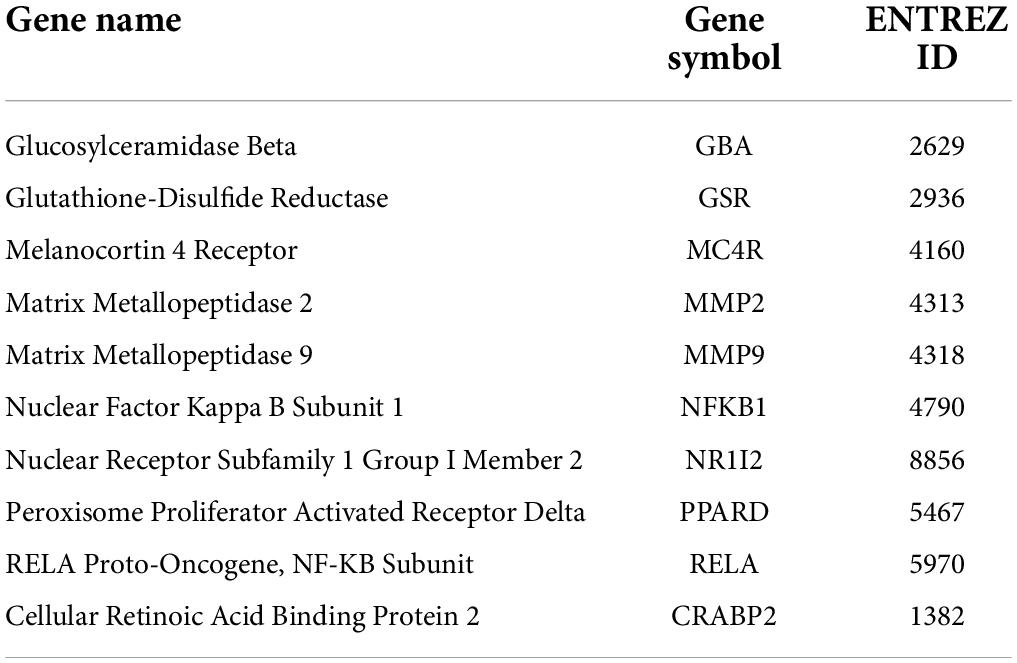
Table 1. Core targets of fucoidan against perfluorooctanoic acid (PFOA)-associated obesity via targeting endoplasmic reticulum stress.
To further determine the signaling pathways controlled by fucoidan’s targets, the KEGG pathway analysis was conducted. Our result showed that fucoidan could target the genes involved in fat metabolisms, such as the adipocytokine signaling pathway and the sphingolipid metabolism (Figure 2). In addition, many signaling pathways related to obesity, such as the Relaxin signaling pathway, the tumor necrosis factor (TNF) signaling pathway, the Prolactin signaling pathway, the NF-kappa B signaling pathway, the hypoxia-inducible factor-1 (HIF)-1 signaling pathway, the nucleotide-binding oligomerization domain (NOD)-like receptor signaling pathway, the cyclic adenosine 3’,5’-monophosphate (cAMP) signaling pathway, the Ras signaling pathway, the mitogen-activated protein kinase (MAPK) signaling pathway, the phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway, the proliferator-activated receptor (PPAR) signaling pathway, the gonadotropin-releasing hormone (GnRH) signaling pathway, and the Wnt signaling pathway, were highlighted in our result. The possible outcomes were obesity-related disorders, such as IR, non-alcoholic fatty liver disease, alcoholic liver disease, diabetic cardiomyopathy, and atherosclerosis (Figure 2). In addition, fucoidan’s targets were found to be involved in signaling pathways of immune responses, such as the IL-17 signaling pathway, the B-cell receptor signaling pathway, the Toll-like receptor signaling pathway, the T-cell receptor signaling pathway, the chemokine signaling pathway, and Th1 and Th2 cell differentiation (Figure 2). Collectively, our result highlighted the contribution of fucoidan’s targets in obesity-associated disorders.
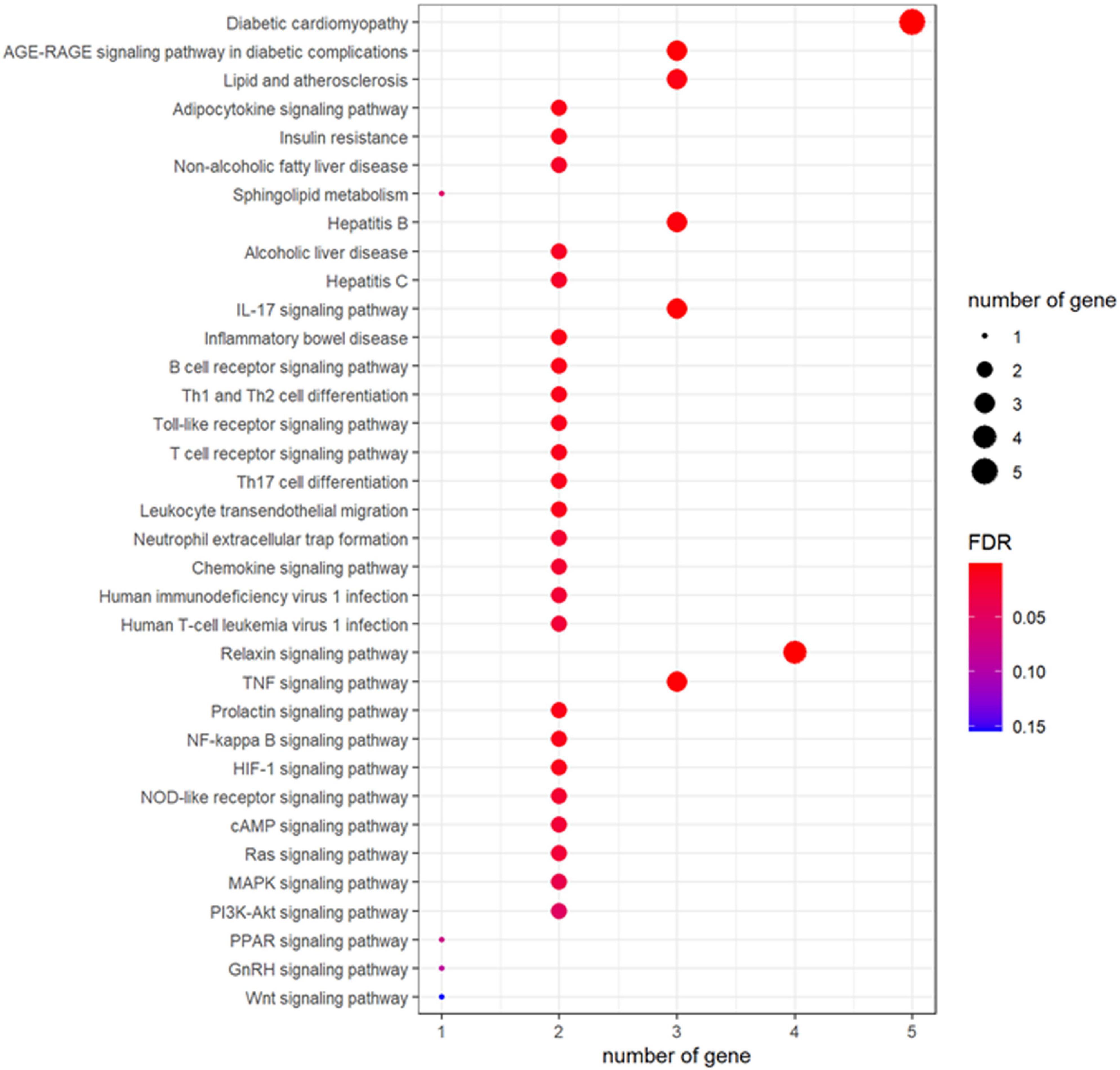
Figure 2. Fucoidan’s targets controlled the signaling pathways involved in the pathogenesis of obesity and its associated diseases. The size of the bubble represented the number of involved genes and the color of the bubble represented the significance of the terms.
In order to investigate the possible direct binding of fucoidan to its target proteins, a molecular docking analysis was performed. First, the protein targets were prioritized using the Cytoscape v3.8.2 tool. Three top-ranked core proteins, such as MMP9, RELA, and PPARD, were identified. The protein structures of MMP9 (ID: 1GKC) (31), RELA (ID: 6YQ2) (32), and PPARD (ID: 5Y7X) (33) were obtained from the PDB database. Their binding affinities with fucoidan were determined using the AutoDock Vina program. The negative value of the binding affinity represented the possible direct binding of fucoidan to its target proteins. We found the formation of hydrogen bonds between fucoidan with amino acid residues of HIS-401 (2.8 Å), HIS-405 (3.1 Å), HIS-411 (2.8 Å), GLU-402 (2.6 Å), LEU-188 (2.9 Å), and ALA-189 (2.6 Å) of MMP9 (ID: 1GKC) (Figure 3A). The binding affinity was −6.1 Kcal/mol. For the RELA (ID: 6YQ2), its amino acid residues LYS-49 (3.3 Å), LYS-122 (3.0 Å), and SER-45 (2.8 Å) were found to form hydrogen bonds with fucoidan, and the binding affinity was −5.8 Kcal/mol (Figure 3B). Similar bindings were observed between fucoidan and PPARD (ID: 5Y7X) through the amino acid residues of CYS-249 (2.2 Å) and THR-253 (2.7 Å) with −6.7 Kcal/mol (Figure 3C). Taken together, our data suggested that fucoidan potentially binds to its target proteins directly.
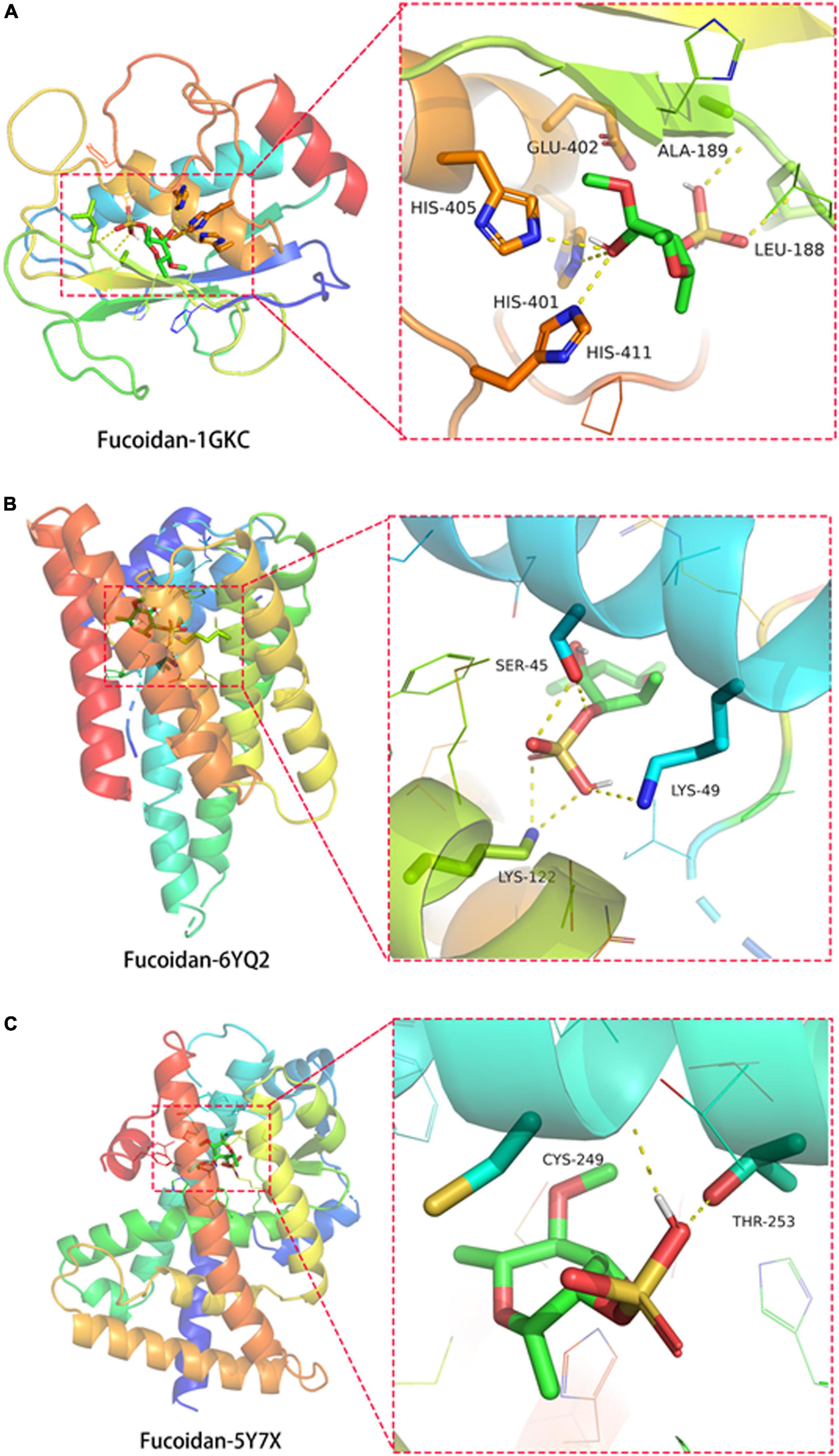
Figure 3. Direct binding of fucoidan to its target proteins matrix metallopeptidase 9 (MMP9), RELA Proto-Oncogene, NF-KB Subunit (RELA), and peroxisome proliferator-activated receptor delta (PPARD). Molecular docking showed the binding of fucoidan to (A) MMP9 (ID: 1GKC), (B) RELA (ID: 6YQ2), and (C) PPARD (ID: 5Y7X).
In this report, we applied network pharmacology and bioinformatics analysis, such as GO and KEGG enrichment analyses, and molecular docking to investigate the possible use of fucoidan as a nutraceutical product for treating PFOA-associated obesity through the regulation of ERS. First, the result of network pharmacology identified 10 potential targets, such as GBA, GSR, MC4R, MMP2, MMP9, NFKB1, RELA, NR1I2, PPARD, and CRABP2, of fucoidan against PFOA-associated obesity. Network pharmacology is a common approach to drug discovery for metabolic disorders that include obesity (34, 35). Most of the identified targets were reported to be correlated with obesity and obesity-associated diseases.
Glutathione-disulfide reductase, also known as glutathione reductase (GR) (36), is considered a marker of antioxidant defense in patients with type 2 diabetes (37). Obesity is a prevalent cause of OS and ERS through the regulation of TCA cycle activity (38, 39). A cross-sectional study conducted in Serbia suggested that GSR is one of the antioxidant defense parameters in obese students with increased cardiovascular risk (40). MC4R is reported to play a significant role in energy balance and weight control (41), and inherited MC4R variant is one of the causes of obesity. A genetics associated study showed that MC4R gene variants are common in childhood obesity in the Turkish population (41). Another study by Martinelli’s group suggested that MC4R deficiency is correlated to increased fasting insulin levels and accelerated growth phenotypes (42).
Our result also demonstrated that matrix metalloproteinase family members, MMP-2 and MMP-9, were potential targets of fucoidan against PFOA-associated obesity. A correlation study in children with obesity hypertension showed the induction of plasma levels of MMP-2 and MMP-9 in patients with obese (43). More importantly, increased MMP2 and MMP9 were found to increase the risks of cardiovascular disease and clinical hypertension in children with obesity (44, 45). NFKB signaling is an important factor for inflammatory responses in obesity (46). As the key regulators of NFKB signaling, NFKB and RELA were believed as potential targets to overcome the problem of obesity. A gene interactions study demonstrated the involvement of the NFKB signaling pathway in obesity pathogenesis (47). It is further supported by Bauman-Fortin’s group that NFKB1 genotypes were associated with BMI and waist circumference (48). In addition, our result suggested that PPARD is a target of fucoidan against PFOA-associated obesity. Although most of the studies focused on PPARα activation in response to PFOA exposure (49), there are limited reports that demonstrated the association between PPARD and PFOA (50). Therefore, this gene cluster could be a promising target of fucoidan for treating PFOA-associated obesity.
In the later part of the study, the enrichment analysis of the fucoidan’s targets highlighted their importance in different biological processes. In the analysis, we focused on the functions and pathways related to the pathogenesis of obesity and its associated diseases. Our result highlighted many lipid-related metabolisms controlled by the fucoidan’s targets. The alteration of lipid metabolism is one of the causes of obesity, because obesity was associated with increased basal lipolysis in adipose tissue and increased circulating fatty acids (51). In addition, the altered lipid metabolism was reported to be linked with many signaling pathways, such as TNF signaling (52), Wnt signaling (53), and MAPK (54), leading to worse outcomes that included T2D and non-alcoholic fatty liver disease (55). All these signaling pathways were found to be targeted by fucoidan.
Finally, we conducted molecular docking to investigate the interaction of fucoidan with its targets. Molecular docking is a tool commonly used to predict the possible interactions between two molecules and is a powerful approach for structure-based drug discovery (56). Some studies used molecular docking for the drug discovery of fucoidan. For instance, Etimad’s group conducted molecular docking to unfold the anti-inflammatory potential of fucoidan for atherosclerosis (57). A similar approach was used to understand the anti-viral, antioxidative, and anti-diabetic roles of fucoidan (58–60). In addition, our result showed a high binding affinity of fucoidan to MMP9, RELA, and PPARD through the formation of hydrogen bonds, suggesting the drug-protein interaction and the anti-obesogenic ability of fucoidan (61). However, further validation is needed to confirm this prediction.
Our results provide evidence that fucoidan is a promising nutraceutical compound for treating PFOA-associated obesity. The findings support the previous animal study that fucoidan prevents high-fat diet-induced obesity and inflammation by suppressing fat accumulation (62, 63). More importantly, our data delineate the molecular mechanism underlying the anti-obesogenic effect of fucoidan by targeting a cluster of genes involved in the pathogenesis of obesity and its related diseases. Furthermore, our results suggest the possible use of nutraceutical compound to improve the human health by reducing the toxicity of environmental pollutants. However, further preclinical study is needed to warrant the findings before the clinical use of fucoidan.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
MS, WH, and KL conceived and designed the study and prepared the manuscript. JL and CG performed the data analysis and data interpretation. JL, CG, and YW conducted the bioinformatics and statistical analyses. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China (no. 82160282).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. (2012) 42:563–70. doi: 10.1016/j.amepre.2011.10.026
2. Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. (2017) 356:j477. doi: 10.1136/bmj.j477
3. Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. (2017) 14:435. doi: 10.3390/ijerph14040435
4. Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. (2010) 3:285–95. doi: 10.2147/DMSO.S7384
5. Lee SJ, Shin SW. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. (2017) 376:1491–2. doi: 10.1056/NEJMc1701944
6. Wang Y, Hollis-Hansen K, Ren X, Qiu Y, Qu W. Do environmental pollutants increase obesity risk in humans? Obes Rev. (2016) 17:1179–97. doi: 10.1111/obr.12463
7. Mohanto NC, Ito Y, Kato S, Kamijima M. Life-time environmental chemical exposure and obesity: review of epidemiological studies using human biomonitoring methods. Front Endocrinol. (2021) 12:778737. doi: 10.3389/fendo.2021.778737
8. Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. (2012) 120:668–73. doi: 10.1289/ehp.1104034
9. Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European youth heart study. Diabetes Care. (2016) 39:1745–51. doi: 10.2337/dc16-0269
10. Jiao X, Liu N, Xu Y, Qiao H. Perfluorononanoic acid impedes mouse oocyte maturation by inducing mitochondrial dysfunction and oxidative stress. Reprod Toxicol. (2021) 104:58–67. doi: 10.1016/j.reprotox.2021.07.002
11. Brestoff JR, Wilen CB, Moley JR, Li Y, Zou W, Malvin NP, et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. (2021) 33:270–82. doi: 10.1016/j.cmet.2020.11.008
12. Tirosh A, Tuncman G, Calay ES, Rathaus M, Ron I, Tirosh A, et al. Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab. (2021) 33:319–33. doi: 10.1016/j.cmet.2020.11.009
13. Deng J, Liu S, Zou L, Xu C, Geng B, Xu G. Lipolysis response to endoplasmic reticulum stress in adipose cells. J Biol Chem. (2012) 287:6240–9. doi: 10.1074/jbc.M111.299115
14. Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. (2022) 21:201–23. doi: 10.1038/s41573-021-00337-8
15. Suprunchuk V. Ultrasonic-treated fucoidan as a promising therapeutic agent. Polim Med. (2021) 51:85–90. doi: 10.17219/pim/143961
16. Sim SY, Shin YE, Kim HK. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr Res. (2019) 65:54–62. doi: 10.1016/j.nutres.2019.02.002
17. Zhang Y, Zuo J, Yan L, Cheng Y, Li Q, Wu S, et al. Sargassum fusiforme fucoidan alleviates high-fat diet-induced obesity and insulin resistance associated with the improvement of hepatic oxidative stress and gut microbiota profile. J Agric Food Chem. (2020) 68:10626–38. doi: 10.1021/acs.jafc.0c02555
18. Xue M, Liang H, Ji X, Liu Y, Ge Y, Hou L, et al. Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways, regulation DC/Treg induced immune tolerance and improving gut microecology. Nutr Metab (Lond). (2019) 16:87. doi: 10.1186/s12986-019-0392-1
19. Davis AP, Grondin CJ, Johnson RJ, Sciaky D, King BL, et al. The comparative toxicogenomics database: update 2017. Nucleic Acids Res. (2017) 45:D972–D978.
20. Daina A, Michielin O, Zoete V. Swiss target prediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. (2019) 47:W357–W364. doi: 10.1093/nar/gkz382
21. Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. (2017) 45:W356–60. doi: 10.1093/nar/gkx374
22. The UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. (2021) 49:D1.
23. Stelzer G, Rosen R, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The genecards suite: from gene data mining to disease genome sequence analysis. Curr Protoc Bioinform. (2016) 54:1.30.1–33. doi: 10.1002/cpbi.5
24. Hamosh A, Scott AF, Amberger JS, Bocchini C, Valle D, McKusick VA. Online Mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. (2002) 33:52–5. doi: 10.1093/nar/30.1.52
25. National Center for Biotechnology Information (NCBI). U.S. National Library of Medicine 8600 Rockville Pike. Bethesda MD: National Center for Biotechnology Information (NCBI) (2022).
26. Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. (2021) 49:D605–12. doi: 10.1093/nar/gkaa1074
27. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. (2003) 13:2498–504. doi: 10.1101/gr.1239303
28. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The protein data bank. Nucleic Acids Res. (2000) 28:235–42. doi: 10.1093/nar/28.1.235
29. Wang Y, Bryant SH, Cheng T, Wang J, Gindulyte A, Shoemaker BA, et al. PubChem bioassay: 2017 update. Nucleic Acids Res. (2017) 45:D955-D963. doi: 10.1093/nar/gkw1118
30. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. (2010) 31:455–61. doi: 10.1002/jcc.21334
31. Rowsell S, Hawtin P, Minshull CA, Jepson H, Brockbank SM, Barratt DG, et al. Crystal structure of mmp9 in complex with a reverse hydroxamate inhibitor. J Mol Biol. (2002) 319:173. doi: 10.1016/S0022-2836(02)00262-0
32. Wolter M, Valenti D, Cossar PJ, Levy LM, Hristeva S, Genski T, et al. Fragment-based stabilizers of protein-protein interactions through imine-based tethering. Angew Chem Int Ed Engl. (2020) 59:21520–4. doi: 10.1002/anie.202008585
33. Bennett DJ, Carswell EL, Cooke AJ, Edwards AS, Nimz O. Design, structure activity relationships and X-Ray co-crystallography of non-steroidal LXR agonists. Curr Med Chem. (2008) 15:195–209.
34. Jang D, Jeong H, Kim CE, Leem JA. System-level mechanism of anmyungambi decoction for obesity: a network pharmacological approach. Biomolecules. (2021) 11:1881. doi: 10.3390/biom11121881
35. Zhao Z, Wang C, Jia J, Wang Z, Li L, Deng X, et al. Regulatory network of metformin on adipogenesis determined by combining high-throughput sequencing and GEO database. Adipocyte. (2022) 11:56–68. doi: 10.1080/21623945.2021.2013417
36. Yan J, Ralston MM, Meng X, Bongiovanni KD, Jones AL, Benndorf R, et al. Glutathione reductase is essential for host defense against bacterial infection. Free Radic Biol Med. (2013) 61:320–32. doi: 10.1016/j.freeradbiomed.2013.04.015
37. Gawlik K, Naskalski JW, Fedak D, Pawlica-Gosiewska D, Grudzień U, Dumnicka P, et al. Markers of antioxidant defense in patients with type 2 diabetes. Oxid Med Cell Longev. (2016) 2016:2352361. doi: 10.1155/2016/2352361
38. Skrzep-Poloczek B, Poloczek J, Chełmecka E, Dulska A, Romuk E, Idzik M, et al. The oxidative stress markers in the erythrocytes and heart muscle of obese rats: relate to a high-fat diet but not to DJOS bariatric surgery. Antioxidants (Basel). (2020) 9:183. doi: 10.3390/antiox9020183
39. Gansemer ER, McCommis KS, Martino M, King-McAlpin AQ, Potthoff MJ, Finck BN, et al. NADPH and glutathione redox link TCA cycle activity to endoplasmic reticulum homeostasis. iScience. (2020) 23:101116. doi: 10.1016/j.isci.2020.101116
40. Čolak E, Pap D, Nikolić L, Vicković S. The impact of obesity to antioxidant defense parameters in adolescents with increased cardiovascular risk. J Med Biochem. (2020) 39:346–54. doi: 10.2478/jomb-2019-0051
41. Aykut A, Özen S, Gökşen D, Ata A, Onay H, Atik T, et al. Melanocortin 4 receptor (MC4R) gene variants in children and adolescents having familial early-onset obesity: genetic and clinical characteristics. Eur J Pediatr. (2020) 179:1445–52. doi: 10.1007/s00431-020-03630-7
42. Martinelli CE, Keogh JM, Greenfield JR, Henning E, van der Klaauw AA, Blackwood A, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. (2011) 96:E181–8. doi: 10.1210/jc.2010-1369
43. Derosa G, Ferrari I, D’Angelo A, Tinelli C, Salvadeo SA, Ciccarelli L, et al. Matrix metalloproteinase-2 and −9 levels in obese patients. Endothelium. (2008) 15:219–24. doi: 10.1080/10623320802228815
44. Belo VA, Lacchini R, Miranda JA, Lanna CM, Souza-Costa DC, Tanus-Santos JE. Increased activity of MMP-2 in hypertensive obese children is associated with hypoadiponectinemia. Obesity (Silver Spring). (2015) 23:177–82. doi: 10.1002/oby.20939
45. Głowińska-Olszewska B, Urban M. Elevated matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in obese children and adolescents. Metabolism. (2007) 56:799–805. doi: 10.1016/j.metabol.2007.01.011
46. Yenmis G, Soydas T, Arkan H, Tasan E, Kanigur Sultuybek G. Genetic variation in NFKB1 gene influences liver enzyme levels in morbidly obese women. Arch Iran Med. (2018) 21:13–8.
47. Tessier F, Fontaine-Bisson B, Lefebvre JF, El-Sohemy A, Roy-Gagnon MH. Investigating gene-gene and gene-environment interactions in the association between overnutrition and obesity-related phenotypes. Front Genet. (2019) 10:151. doi: 10.3389/fgene.2019.00151
48. Bauman-Fortin J, Ma DWL, Mutch DM, Abdelmagid SA, Badawi A, El-Sohemy A, et al. The Association between plasma omega-6/omega-3 ratio and anthropometric traits differs by racial/ethnic groups and NFKB1 genotypes in healthy young adults. J Pers Med. (2019) 9:13. doi: 10.3390/jpm9010013
49. Filgo AJ, Quist EM, Hoenerhoff MJ, Brix AE, Kissling GE, Fenton SE. Perfluorooctanoic acid (PFOA)-induced liver lesions in two strains of mice following developmental exposures: PPARα is not required. Toxicol Pathol. (2015) 43:558–68. doi: 10.1177/0192623314558463
50. Kobayashi S, Sata F, Ikeda-Araki A, Miyashita C, Goudarzi H, Iwasaki Y, et al. Relationships between maternal perfluoroalkyl substance levels, polymorphisms of receptor genes, and adverse birth outcomes in the Hokkaido birth cohort study, Japan. Reprod Toxicol. (2022) 107:112–22. doi: 10.1016/j.reprotox.2021.12.004
51. Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: a review. World J Diabetes. (2010) 1:76–88. doi: 10.4239/wjd.v1.i3.76
52. Zhang XS, Zhang P, Liu YH, Xu Q, Zhang Y, Li HZ, et al. Caprylic acid improves lipid metabolism, suppresses the inflammatory response and activates the ABCA1/p-JAK2/p-STAT3 signaling pathway in C57BL/6J mice and RAW264.7 cells. Biomed Environ Sci. (2022) 35:95–106.
53. Małachowska B, Janikiewicz J, Pietrowska K, Wyka K, Madzio J, Wypyszczak K, et al. Elevated level of lysophosphatidic acid among patients with HNF1B mutations and its role in RCAD syndrome: a multiomic study. Metabolomics. (2022) 18:15. doi: 10.1007/s11306-022-01873-z
54. Adoga JO, Channa ML, Nadar A. Type-2 diabetic rat heart: the effect of kolaviron on mTOR-1, P70S60K, PKC-α, NF-kB, SOD-2, NRF-2, eNOS, AKT-1, ACE, and P38 MAPK gene expression profile. Biomed Pharmacother. (2022) 148:112736. doi: 10.1016/j.biopha.2022.112736
55. Zhou H, Zhu X, Yao Y, Su Y, Xie J, Zhu M, et al. TMEM88 modulates lipid synthesis and metabolism cytokine by regulating Wnt/β-catenin signaling pathway in non-alcoholic fatty liver disease. Front Pharmacol. (2022) 12:798735. doi: 10.3389/fphar.2021.798735
56. Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des. (2011) 7:146–57. doi: 10.2174/157340911795677602
57. Huwait E, Al-Saedi DA, Mirza Z. Anti-inflammatory potential of fucoidan for atherosclerosis: in silico and in vitro studies in THP-1 cells. Molecules. (2022) 27:3197. doi: 10.3390/molecules27103197
58. Krylova NV, Silchenko AS, Pott AB, Ermakova SP, Iunikhina OV, Rasin AB, et al. In vitro anti-orthohantavirus activity of the high-and low-molecular-weight fractions of fucoidan from the brown alga Fucus evanescens. Mar Drugs. (2021) 19:577. doi: 10.3390/md19100577
59. Mustafa S, Pawar JS, Ghosh I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int J Biol Macromol. (2021) 181:180–92. doi: 10.1016/j.ijbiomac.2021.03.110
60. S LS, Raghu C, H A A, P A. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr Polym. (2019) 209:350–5. doi: 10.1016/j.carbpol.2019.01.039
61. Wu MY, Dai DQ, Yan H. PRL-dock: protein-ligand docking based on hydrogen bond matching and probabilistic relaxation labeling. Proteins. (2012) 80:2137–53. doi: 10.1002/prot.24104
62. Kim MJ, Jeon J, Lee JS. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother Res. (2014) 28:137–43. doi: 10.1002/ptr.4965
Keywords: polysaccharides, mechanisms, obesity, immunomodulatory action, bioinformatics
Citation: Liu J, Guo C, Wang Y, Su M, Huang W and Lai KP (2022) Preclinical insights into fucoidan as a nutraceutical compound against perfluorooctanoic acid-associated obesity via targeting endoplasmic reticulum stress. Front. Nutr. 9:950130. doi: 10.3389/fnut.2022.950130
Received: 06 June 2022; Accepted: 25 July 2022;
Published: 12 August 2022.
Edited by:
Roser Granero, Universitat Autònoma de Barcelona, SpainReviewed by:
Şana Sungur, Mustafa Kemal University, TurkeyCopyright © 2022 Liu, Guo, Wang, Su, Huang and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Su, Y29sbGVnZV9zdW1pbkAxMjYuY29t; Wenjun Huang, dXB0b3duNzhAeWVhaC5uZXQ=; Keng Po Lai, Z2xtdV9rZW5ncGxhaUB5ZWFoLm5ldA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.