
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 08 November 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.947008
This article is part of the Research TopicInsights in Clinical NutritionView all 30 articles
 Yi-Min Gu†
Yi-Min Gu† Qi-Xin Shang†
Qi-Xin Shang† Han-Lu Zhang
Han-Lu Zhang Yu-Shang Yang
Yu-Shang Yang Wen-Ping Wang
Wen-Ping Wang Yong Yuan
Yong Yuan Yang Hu
Yang Hu Guo-Wei Che*
Guo-Wei Che* Long-Qi Chen*
Long-Qi Chen*Background: This study aims to investigate the relationship between preoperative body mass index changes (ΔBMI) and prognosis in patients with esophageal squamous cell carcinoma who underwent esophagectomy.
Methods: We identified 1,883 patients with esophageal squamous cell carcinoma who underwent curative resection in our department between January 2005 and December 2013. Patients were grouped into a stable body mass index (ΔBMI = 0) group and a decreased body mass index (ΔBMI < 0) group. Risk factors for ΔBMI were assessed using logistic regression analysis. The impact of ΔBMI on survival was investigated using Kaplan–Meier curves and Cox regression. A nomogram for survival prediction was constructed and validated.
Results: The results showed that T stage (OR: 1.30, 95% CI: 1.16–1.45, P < 0.001) and N stage (OR: 1.24, 95% CI: 1.11–1.38, P < 0.001) were independent risk factors for ΔBMI. The ΔBMI < 0 group had worse overall survival than the stable body mass index group (HR: 1.25, 95% CI: 1.08–1.44, P = 0.002). When stratified by stage, ΔBMI had the greatest prognostic impact in stage I tumors (HR: 1.82, 95%: 1.05–3.15, P = 0.033). In addition, multiple comparisons showed that decreasing ΔBMI correlated with worse prognosis. The ΔBMI-based nomogram presented good predictive ability with a C-index of 0.705.
Conclusion: This study demonstrates that ΔBMI < 0 had an adverse impact on the long-term survival of patients with esophageal squamous cell carcinoma undergoing esophagectomy. These results may support further investigation of preoperative nutrition support.
Esophageal cancer is the seventh most common malignant tumor and the sixth leading cause of cancer-related deaths worldwide (1, 2). The recently published 10-year outcome of the CROSS study reported that 49% of patients had overall disease progression in the neoadjuvant chemoradiotherapy plus surgery group (3). The prognosis for patients with esophageal cancer remains unsatisfactory. Unlike many other malignancies, esophageal cancer is more likely to cause malnutrition in patients because an obstructing tumor leads to different degrees of dysphagia. The nutritional state has been shown to be related to poor prognosis in multiple cancers (4–6). Thus, a better understanding of how the nutritional state influences cancer survival may open novel therapeutic strategies to improve cancer outcomes for patients with esophageal cancer.
Body mass index is a crucial diagnostic criterion of cancer-associated weight loss and is based on body weight and height (7). Although several studies have demonstrated the effect of preoperative body mass index (BMI) on the outcomes of patients who underwent esophagectomy for esophageal cancer (8, 9), BMI alone is not a reliable indicator of survival (10, 11). A better measure than weight alone, BMI accounts for how height might influence the effects of weight on a health response. Therefore, body mass index changes (ΔBMI) may better help to estimate the correlation between nutritional state and prognosis in various populations.
The aim of this study was to investigate the relationship between preoperative ΔBMI and prognosis in patients with esophageal squamous cell carcinoma who underwent curative esophagectomy. These findings may be helpful for the development of new potential therapeutic strategies.
We conducted a retrospective review of our prospectively collected database to identify consecutive patients who underwent curative esophagectomy at West China Hospital of Sichuan University between January 2005 and December 2013. Eligible patients were previously diagnosed with esophageal cancer. Inclusion criteria included (1) esophageal squamous cell carcinoma; (2) receiving esophagectomy with or without neoadjuvant or adjuvant therapy; (3) R0 resection; and (4) at least 15 lymph nodes should be removed and assessed to achieve adequate nodal staging. Exclusion criteria included the coexistence of other malignancies. Ethics approval for this study was granted by the Ethics Committee of West China Hospital, Sichuan University (No. 2019641), and informed consent was waived.
BMI was defined as weight (kg) divided by height squared (m2). Diagnostic BMI was based on weight and height at the first visit to the outpatient clinic. BMI at 3 months before diagnosis was also recorded as the baseline BMI at the same visit, which was based on patient-reported weight and height. ΔBMI was calculated as diagnostic BMI minus baseline BMI.
Tumor staging was based on esophagoscopy, contrast-enhanced computed tomography of the neck, chest, and abdomen, endoscopic ultrasonography, bone scan, and magnetic resonance imaging of the brain. Standard surgery included minimally invasive esophagectomy or open thoracotomy. The surgical approach, whether minimally invasive or open, was not associated with completeness of resection. The extent of lymphadenectomy included two-field lymph node dissections and was conducted in most patients. Three-field lymph node dissections were performed in only a few patients who had suspicious cervical node disease. Postoperative patients with lymph node involvement were recommended to receive adjuvant therapy.
We classified tumor stage according to the 7th edition of the TNM staging system of esophageal cancer (11). We recorded tumor recurrence, mortality, and survival status. Overall survival was measured as the time from operation to death. Patients alive or lost to follow-up were censored at the date of the last follow-up. The patients were followed up every 3 months for the first 2 years and every 6 months thereafter. Follow-up information was available over 5 years postoperatively or at the date of death.
Statistical analyses were performed using SPSS Statistics (version 24, IBM, Armonk, NY) and the R programming language (version 3.6.3, Vienna, Austria). Normally distributed continuous variables are presented as the mean ± standard deviation (SD), non-normally distributed continuous variables as the median with interquartile range (IQR), and categorical variables as frequencies and percentages. Categorical variables were compared using the χ2-test, while continuous variables were analyzed by Student’s t-test. Risk factors were identified using Cox regression modeling. Survival analyzes were analyzed using the survival package, and Kaplan–Meier survival curves were plotted using the survminer package in R. We used the rsm package in R to develop the prediction model. For multiple comparisons, the P-value was adjusted using the Benjamini and Hochberg method by the fdrtool package in R. A bootstrap with 1,000 resamples was used to perform internal validation. The C-index was used to measure the prediction performance. Calibration was assessed by using the Hosmer–Lemeshow goodness-of-fit test (12). Statistical significance was set at a two-sided P-value less than 0.05.
Of 1,883 eligible patients, 1,162 patients (61.7%) were grouped into the ΔBMI = 0 group, and 721 patients (38.3%) were grouped into the ΔBMI < 0 group. No patient had an increased body mass index in the ΔBMI < 0 group. The characteristics of the ΔBMI = 0 and ΔBMI < 0 groups are summarized in Table 1. There was no significant difference in baseline BMI between the two groups (P = 0.083).
On univariate analysis, T stage (OR: 1.385, 95% CI: 1.249–1.535, P < 0.001), N stage (OR: 1.348, 95% CI: 1.216–1.495, P < 0.001), and differentiation (OR: 1.195, 95% CI: 1.027–1.390, P = 0.021) were identified as potential risk factors and included in multivariate analysis (Table 2). On multivariate analysis, our results indicated that T stage (OR: 1.296, 95% CI: 1.163–1.445, P < 0.001) and N stage (OR: 1.241, 95% CI: 1.113–1.383, P < 0.001) were independent risk factors for body mass index changes (Table 2).
The median follow-up for the entire cohort was 34.6 months (95% CI: 32.8–36.4) using the reverse Kaplan–Meier method. The median overall survival was 36 months (95% CI: 32.4–44.4) among 1,162 patients without body mass index changes and 28.8 months (95% CI: 25.2–33.6) among 721 patients with body mass index changes. There was a significant difference in overall survival between these two groups (HR: 1.25, 95% CI: 1.08–1.44, P = 0.002) (Figure 1A).
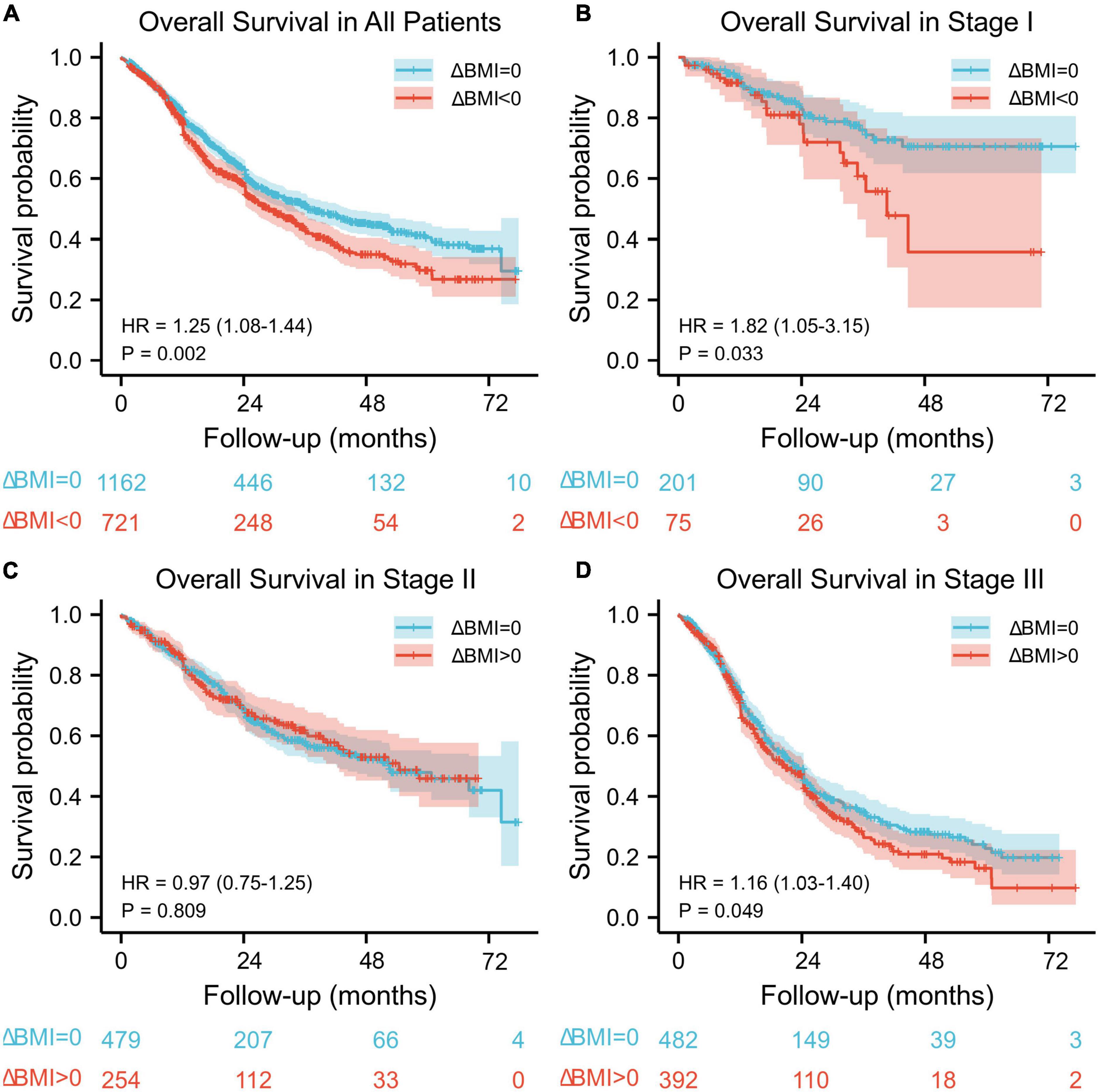
Figure 1. Kaplan–Meier overall survival curve according to preoperative body mass index changes for all patients (A) and for stage I (B), stage II (C), and stage III (D) patients.
When stratified by stage, there was a significant difference in overall survival between the ΔBMI = 0 group and ΔBMI < 0 group with stage I (HR: 1.82, 95%: 1.05–3.15, P = 0.033) and stage III (HR: 1.16, 95%: 1.03–1.40, P = 0.049) disease (Figures 1B,D), but no significant difference was found in stage II (HR: 0.97, 95%: 0.75–1.25, P = 0.809) (Figure 1C).
Based on the results from the X-tile program, the optimal cutoff points for overall survival were determined to be −1.5 and −2.3 kg/m2 (Figure 2). Accordingly, patients with 0 > ΔBMI ≥ −1.5 kg/m2 were defined as the minor ΔBMI group, −1.5 kg/m2 > ΔBMI ≥ −2.3 kg/m2 moderate ΔBMI group, and ΔBMI < −2.3 kg/m2 severe ΔBMI group. Then, we validated the cutoff points for survival stratification. The results of the Kaplan–Meier overall survival curve showed that decreasing BMI changes were associated with worse prognosis (severe ΔBMI vs. minor ΔBMI, adjusted P < 0.001; severe ΔBMI vs. moderate ΔBMI, adjusted P = 0.019; moderate ΔBMI vs. minor ΔBMI, adjusted P = 0.049) (Figure 3).
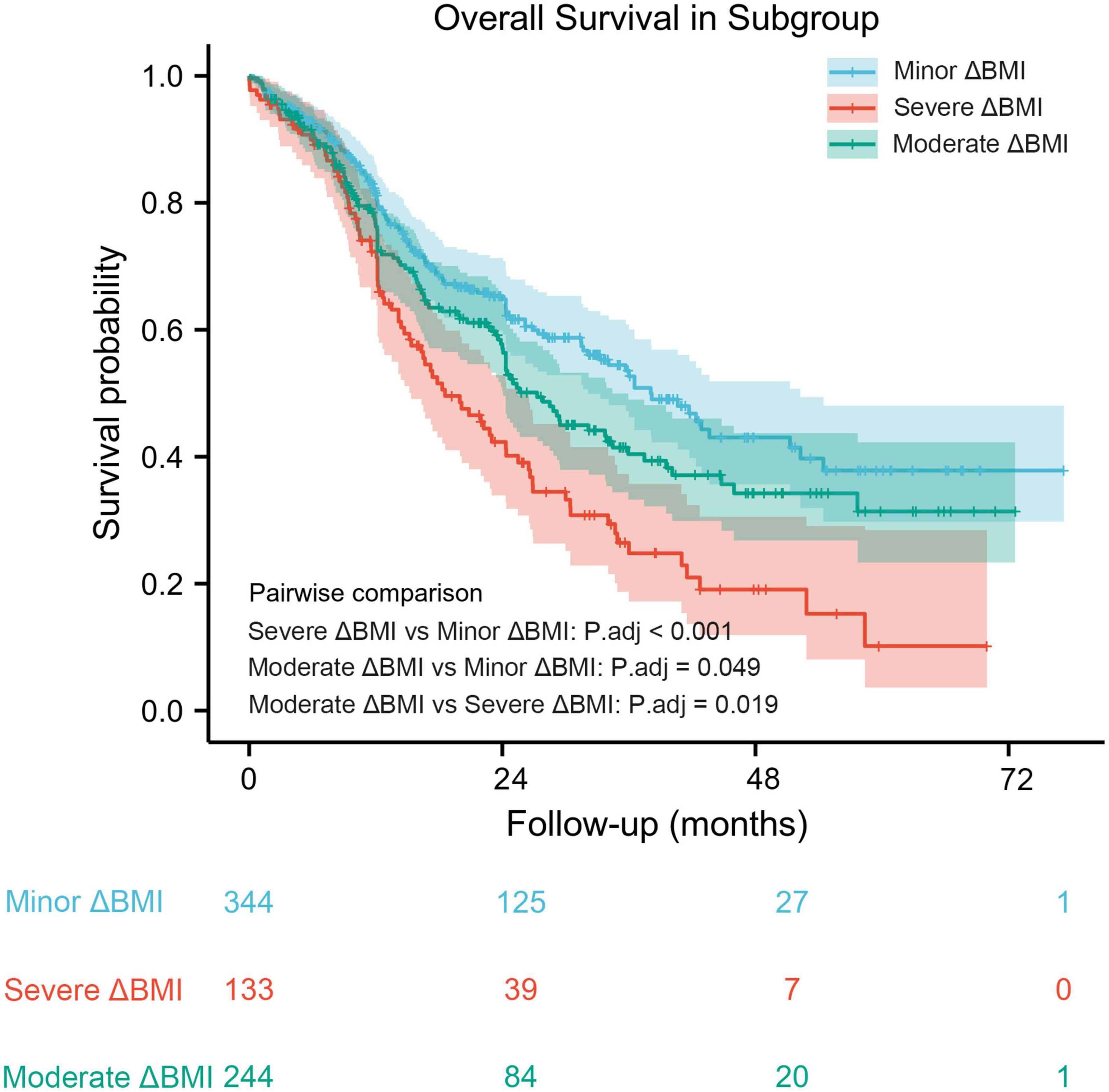
Figure 2. A preoperative body mass index changes-based nomogram for predicting prognosis after curative resection of esophageal squamous cell carcinoma.
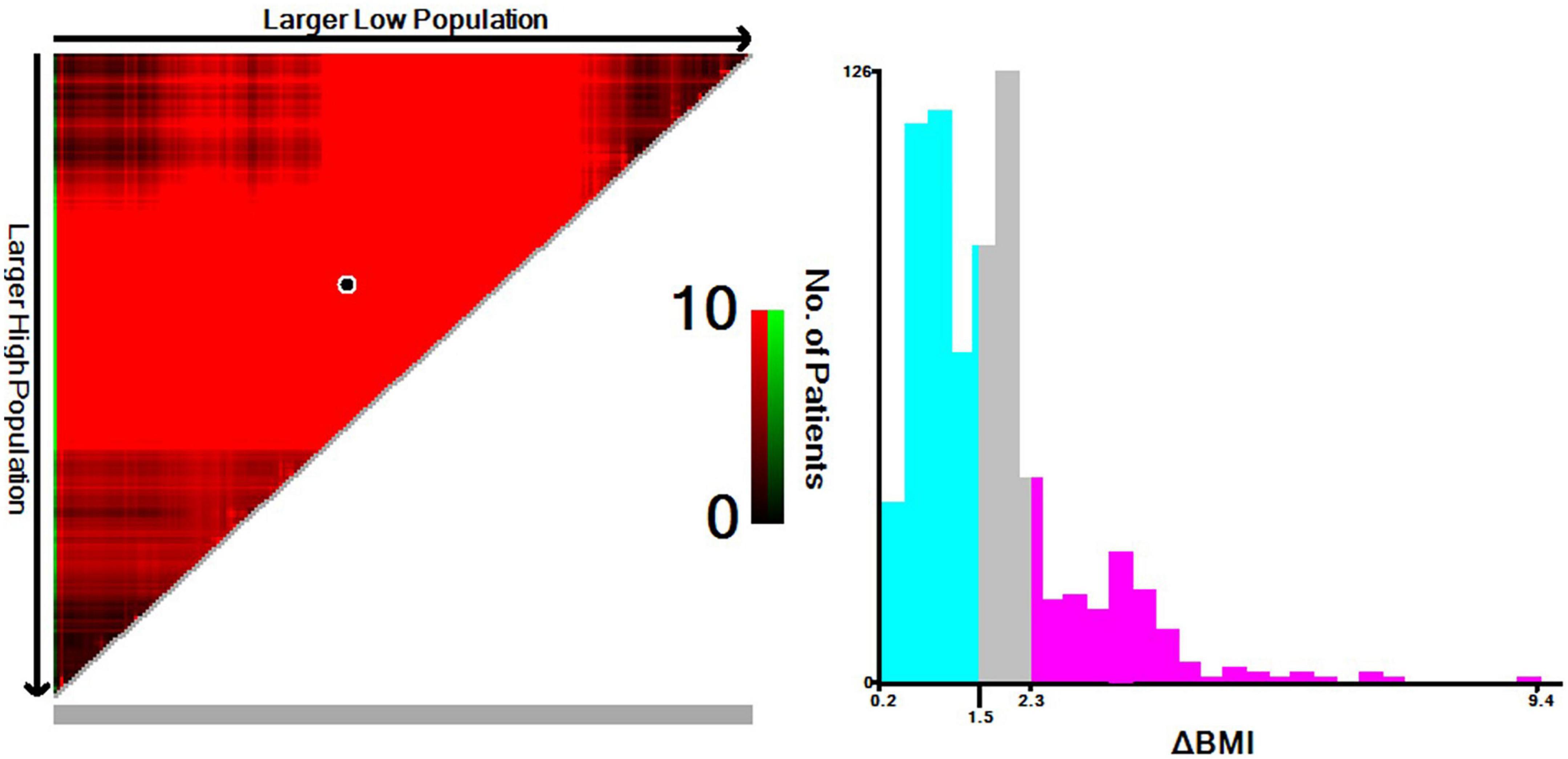
Figure 3. Kaplan–Meier overall survival curve stratified by optimal cutoff points in patients with decreased body mass index.
Variables with statistical significance in univariate analysis were entered into multivariate analysis. Multivariate Cox regression models determined that T stage (HR: 1.325, 95% CI: 1.210–1.451; P < 0.001), N stage (HR: 1.206, 95% CI: 1.114–1.306; P < 0.001), ΔBMI (HR: 1.174, 95% CI: 1.014–1.358; P = 0.032), differentiation (HR: 1.134, 95% CI: 1.001–1.284; P = 0.047), presence of lymphovascular invasion (HR: 1.415, 1.034–1.936; P = 0.03), and adjuvant therapy (HR: 0.819, 95% CI: 0.768–0.874; P < 0.001) were significant predictors of overall survival (Table 3).
From this model, we developed a ΔBMI-based nomogram to predict the prognosis for patients with esophageal squamous cell carcinoma who underwent surgery (Figure 4). The Harrell C-index for the predictive nomogram was 0.706 (95% CI, 0.693–0.721) in the training cohort, which was 0.710 by bootstrapping validation. The nomogram calibration plot indicates that the nomogram was well calibrated, with mean predicted probabilities for each subgroup close to observed probabilities (Figure 5). The Hosmer–Lemeshow goodness-of-fit test P-value for the logistic regression model was not significant, indicating a good model fit.
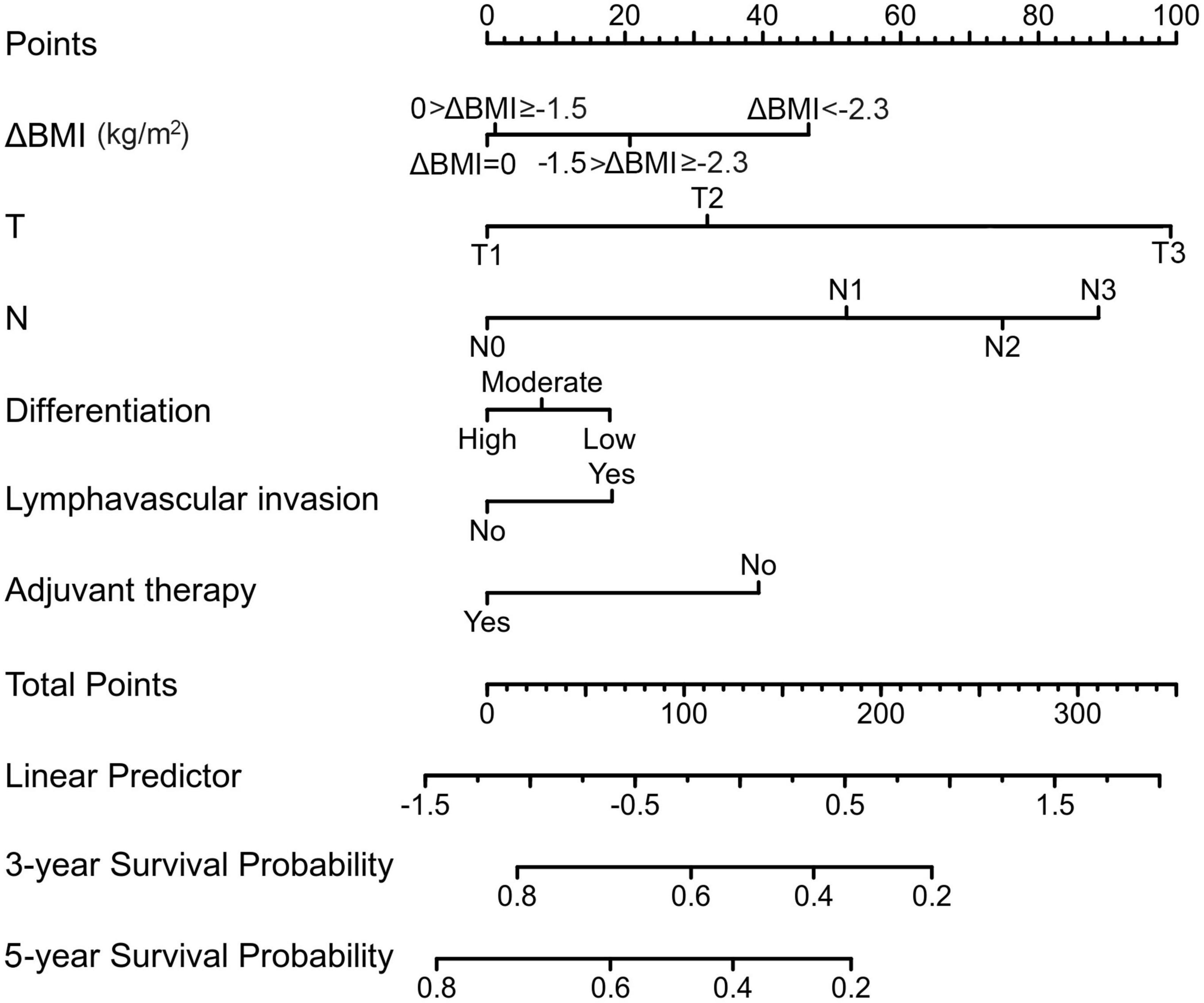
Figure 4. A preoperative body mass index changes-based nomogram for predicting prognosis after curative resection of esophageal squamous cell carcinoma.
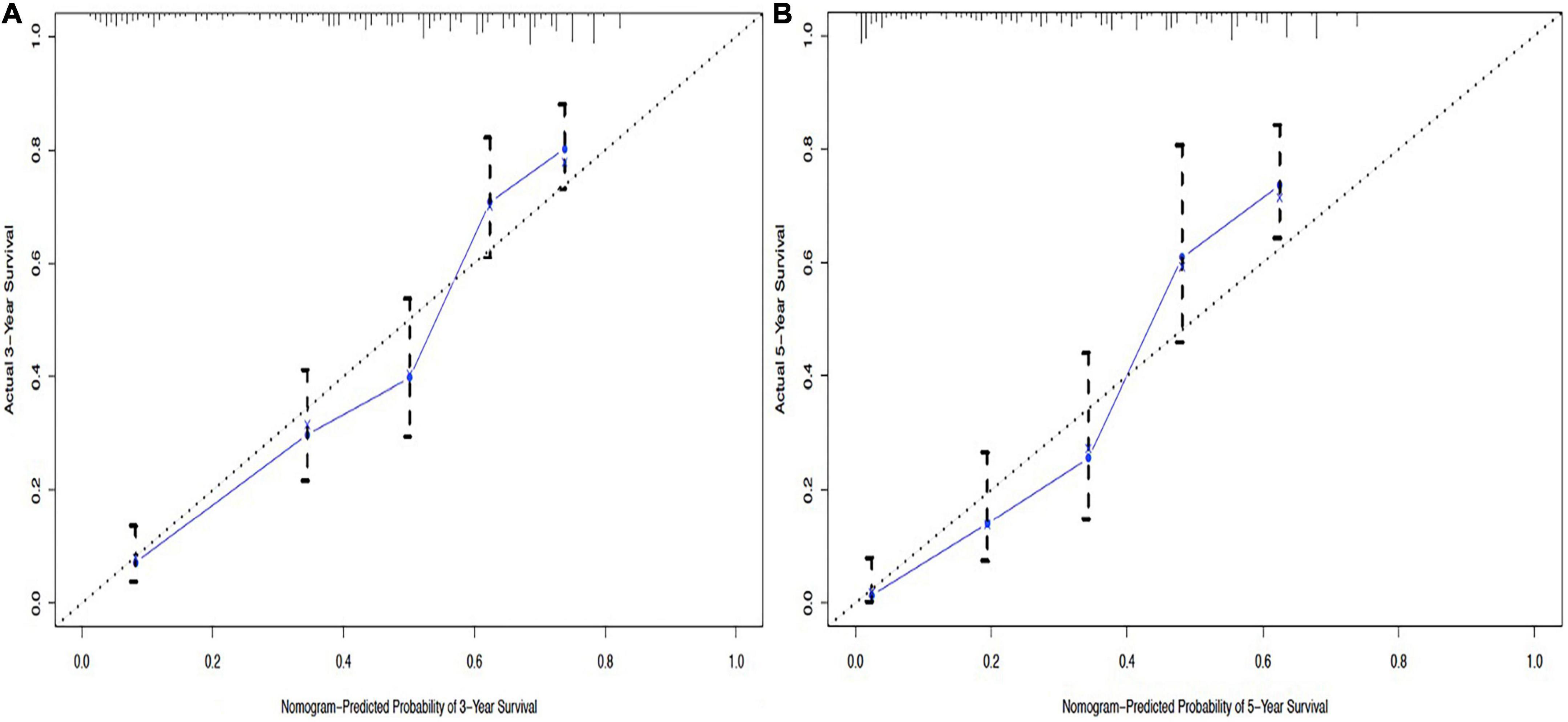
Figure 5. Calibration curves of the nomogram to predict 3-year overall survival (A) and 5-year overall survival (B).
In this large-scale, long-term follow-up, retrospective, cohort study, we found that patients with ΔBMI = 0 had a median overall survival of approximately 7 months longer than the ΔBMI < 0 group. Several studies have revealed that preoperative weight loss was associated with worse long-term prognosis (13–15). Rather than measuring weight changes alone, BMI also takes into account how height might affect an individual’s health. However, few studies have focused on the impact of body mass index changes on the survival of esophageal squamous cell carcinoma patients. Loehrer et al. (16) found that BMI gain compared with average adult BMI was associated with poor esophageal adenocarcinoma survival. However, the definition of BMI changes was quite different from the current study, and the sample size was too small (285 patients) to make robust conclusions.
In the subgroup analysis, we demonstrated that ΔBMI was a strong predictor of poor prognosis for stage I disease. In contrast, ΔBMI has less prognostic impact for stage III disease. Unexpectedly, no statistical significance in mortality was found between the two groups for patients with stage II disease. These findings may be due to the inherent poor prognosis of locally advanced disease diluting the strength of ΔBMI. These results also parallel the previous detection by Zhang et al. (14), in which preoperative weight loss correlated with worse survival in patients with early-stage esophageal cancer.
In the current study, the optimal ΔBMI cutoff points were also determined. Kaplan–Meier survival analysis showed that a larger body mass index decrease was associated with worse survival. These results might warrant further investigation of preoperative nutrition support for patients with severe body mass index changes, especially in early-stage (I–II) esophageal squamous cell carcinoma.
The potential weakness might be that the impact of neoadjuvant therapy on ΔBMI was not explored. Neoadjuvant chemoradiotherapy formed the standard treatment based on the CROSS trial (17) published in 2012 and the NEOCRTEC5010 trial (18) published in 2018. However, this long-term follow-up study screened patients from 2005 to 2013, and neoadjuvant therapy was not well established in that period. Most patients enrolled in this study received adjuvant therapy. However, we would like to emphasize that it would not affect the applicability of our main conclusion. To our knowledge, few studies have reported the relationship between ΔBMI and survival outcomes during neoadjuvant therapy in esophageal cancer, except for breast, rectal, and pancreatic cancer (19–21). The predictive value of ΔBMI during neoadjuvant therapy requires further investigation in future studies.
The strengths of this study include its large scale with long-term follow-up compared with previous literature (16, 22, 23). Moreover, we evaluated whether the relationship between body mass index changes and prognosis varies with tumor stage. In addition, we found that the optimal cutoff points of body mass index changes can be used to stratify patient survival. Nomograms have long been proposed as a tool for estimating an individual risk or prognosis based on clinical variables (24). Accordingly, a body mass index changes-based nomogram was established to facilitate individualized prediction of 3- and 5-year survival probability.
There were also some limitations in our study. First, it was retrospective and observational, with inherent flaws. Second, the study population was predominantly Chinese people. Globally, esophageal squamous cell carcinoma remains the most common histological type in Asia, while adenocarcinoma is the major histology in North America and Europe (25). There are differences in body mass index between the Western and Eastern populations. Thus, the generalizability of these data to the Western population requires further validation. Finally, although the prediction accuracy of the model was validated internally, external validation was not performed.
In conclusion, ΔBMI < 0 had an adverse impact on long-term survival in patients with esophageal squamous cell carcinoma who underwent esophagectomy. The earlier the tumor stage was, the greater the impact. In addition, decreasing ΔBMI was associated with worse prognosis. These results might warrant further investigation of preoperative nutrition support, especially in early cancer with severe ΔBMI.
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital, Sichuan University. The Ethics Committee waived the requirement of written informed consent for participation.
L-QC and Y-MG conceptualized the study, revised the manuscript, and supervised the study. Y-MG and Q-XS collected the data, drafted the manuscript, and made the figures. H-LZ, Y-SY, YY, YH, and G-WC revised the manuscript. All authors read and approved the final manuscript.
This research was supported by the National Natural Science Foundation of China (82000514) and Key Projects of Sichuan Provincial Department of Science and Technology (2021YFS0222) and did not receive any commercial interest support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39:1995–2004. doi: 10.1200/jco.20.03614
4. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. (2018) 4:798–804. doi: 10.1001/jamaoncol.2018.0137
5. Thomson CA, E Crane T, Wertheim BC, Neuhouser ML, Li W, Snetselaar LG, et al. Diet quality and survival after ovarian cancer: results from the women’s health initiative. J Natl Cancer Inst. (2014) 106:dju314. doi: 10.1093/jnci/dju314
6. Heckler M, Klaiber U, Hüttner FJ, Haller S, Hank T, Nienhüser H, et al. Prospective trial to evaluate the prognostic value of different nutritional assessment scores for survival in pancreatic ductal adenocarcinoma (NURIMAS pancreas SURVIVAL). J Cachexia Sarcopenia Muscle. (2021) 12:1940–7. doi: 10.1002/jcsm.12796
7. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. (2015) 33:90–9. doi: 10.1200/jco.2014.56.1894
8. Pan W, Sun Z, Xiang Y, Fang W. The correlation between high body mass index and survival in patients with esophageal cancer after curative esophagectomy: evidence from retrospective studies. Asia Pac J Clin Nutr. (2015) 24:480–8. doi: 10.6133/apjcn.2015.24.3.05
9. Zhang SS, Yang H, Luo KJ, Huang QY, Chen JY, Yang F, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer. (2013) 109:2894–903. doi: 10.1038/bjc.2013.666
10. Deng HY, Alai G, Li G, Luo J, Zhuo ZG, Lin YD. High BMI has no impact on the survival of Chinese patients with lower thoracic esophageal adenocarcinoma treated with curative esophagectomy: a propensity score-matched study. Dis Esophagus. (2019) 32:doy059. doi: 10.1093/dote/doy059
11. Hasegawa T, Kubo N, Ohira M, Sakurai K, Toyokawa T, Yamashita Y, et al. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. J Gastrointest Surg. (2015) 19:226–33. doi: 10.1007/s11605-014-2686-y
12. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med. (2007) 35:2052–6. doi: 10.1097/01.Ccm.0000275267.64078.B0
13. van der Schaaf MK, Tilanus HW, van Lanschot JJ, Johar AM, Lagergren P, Lagergren J, et al. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg. (2014) 147:490–5. doi: 10.1016/j.jtcvs.2013.07.072
14. Zhang S, Tan Y, Cai X, Luo K, Wu Z, Lu J. Preoperative weight loss is associated with poorer prognosis in operable esophageal cancer patients: a single-center retrospective analysis of a large cohort of Chinese patients. J Cancer. (2020) 11:1994–9. doi: 10.7150/jca.40344
15. Liu B, Cheng B, Wang C, Chen P, Cheng Y. The prognostic significance of metabolic syndrome and weight loss in esophageal squamous cell carcinoma. Sci Rep. (2018) 8:10101. doi: 10.1038/s41598-018-28268-2
16. Loehrer EA, Giovannucci EL, Betensky RA, Shafer A, Christiani DC. Prediagnostic adult body mass index change and esophageal adenocarcinoma survival. Cancer Med. (2020) 9:3613–22. doi: 10.1002/cam4.3015
17. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
18. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/jco.2018.79.1483
19. Kogawa T, Fujii T, Fouad TM, Liu DD, Harano K, Masuda H, et al. Impact of change in body mass index during neoadjuvant chemotherapy and survival among breast cancer subtypes. Breast Cancer Res Treat. (2018) 171:501–11. doi: 10.1007/s10549-018-4853-4
20. Liu H, Wei R, Li C, Zhao Z, Guan X, Yang M, et al. BMI may be a prognostic factor for local advanced rectal cancer patients treated with long-term neoadjuvant chemoradiotherapy. Cancer Manag Res. (2020) 12:10321–32. doi: 10.2147/cmar.S268928
21. Naumann P, Habermehl D, Welzel T, Debus J, Combs SE. Outcome after neoadjuvant chemoradiation and correlation with nutritional status in patients with locally advanced pancreatic cancer. Strahlenther Onkol. (2013) 189:745–52. doi: 10.1007/s00066-013-0393-3
22. Pan YP, Kuo HC, Hsu TY, Lin JY, Chou WC, Lai CH, et al. Body mass index-adjusted body weight loss grading predicts overall survival in esophageal squamous cell carcinoma patients. Nutr Cancer. (2021) 73:1130–7. doi: 10.1080/01635581.2020.1792950
23. Hynes O, Anandavadivelan P, Gossage J, Johar AM, Lagergren J, Lagergren P. The impact of pre- and post-operative weight loss and body mass index on prognosis in patients with oesophageal cancer. Eur J Surg Oncol. (2017) 43:1559–65. doi: 10.1016/j.ejso.2017.05.023
24. Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. (2002) 20:791–6. doi: 10.1200/jco.2002.20.3.791
Keywords: esophageal squamous cell carcinoma, body mass index changes, prognosis, nomogram, nutrition
Citation: Gu Y-M, Shang Q-X, Zhang H-L, Yang Y-S, Wang W-P, Yuan Y, Hu Y, Che G-W and Chen L-Q (2022) The prognostic impact of preoperative body mass index changes for patients with esophageal squamous cell carcinoma who underwent esophagectomy: A large-scale long-term follow-up cohort study. Front. Nutr. 9:947008. doi: 10.3389/fnut.2022.947008
Received: 18 May 2022; Accepted: 21 October 2022;
Published: 08 November 2022.
Edited by:
Maurizio Muscaritoli, Sapienza University of Rome, ItalyReviewed by:
Jingfeng Liu, First Affiliated Hospital of Fujian Medical University, ChinaCopyright © 2022 Gu, Shang, Zhang, Yang, Wang, Yuan, Hu, Che and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long-Qi Chen, ZHJjaGVuQHNjdS5lZHUuY24=; Guo-Wei Che, Y2hlZ3Vvd2VpeHdAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.