
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 18 August 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.944451
This article is part of the Research Topic Fruits, Vegetables and Herbs: Medicinal Chemistry, Metabolic and Health Effects View all 10 articles
Objective: Previous studies on the association of cruciferous vegetables intake with bladder cancer risk have reported inconsistent results. We performed the present meta-analysis to summarize evidence on this association and to quantify the potential dose-response relation based on all available cohort studies.
Methods: A comprehensive literature search of relevant articles up to March 2022 was performed in PubMed and EMBASE. The summary risk estimates with 95% confidence intervals for the highest vs. the lowest intake of cruciferous vegetables were calculated. Dose-response meta-analysis was also performed for studies reporting categorical risk estimates for at least three quantitative levels of cruciferous vegetables intake.
Results: We found that the highest cruciferous vegetables intake was not significantly associated with a lower risk of bladder cancer, compared with the lowest cruciferous vegetables intake category (RR = 0.92, 95% CI 0.80–1.06). Linear dose-response meta-analysis indicated that the pooled RRs for 10 g/day or 1 servings/week increment of cruciferous vegetables intake was not significantly associated with a reduced risk of bladder cancer (P = 0.106 and P = 0.147, respectively). There was no evidence of significant publication bias either with Begg’s test (P = 0.386) or Egger’s test (P = 0.253).
Conclusion: The results of this study did not support the hypothesis that dietary cruciferous vegetables intake was associated with a lower risk of bladder cancer. Further large prospective cohort studies are warranted to confirm our preliminary findings.
Bladder cancer is a common disease, which ranks ninth in cancer incidence and is the 13th leading cause of cancer death among men and women worldwide (1). An estimated 573,278 new cases and 212,536 deaths from bladder cancer occurred in 2020 (2). Bladder cancer is classified as muscle-invasive bladder cancer (MIBC) and non-muscle-invasive bladder cancer (NMIBC) based on depth of tumor invasion. 75% of bladder cancers are non-muscle invasive (Tis, Ta, T1) (3, 4). Smoking is the most established risk factor for bladder cancer, among other risk factors including occupational exposure to arylamines and schistosomal infection (5). Emerging evidence indicates a significant influence of dietary factors [e.g., dairy product (6) and processed meat (7)] and dietary patterns [e.g., Western diet and Mediterranean diet (8)] on the risk of bladder cancer.
Cruciferous vegetables (e.g., broccoli, cauliflower, and cabbage) intake has been associated with multiple health outcomes (9). Epidemiologic studies investigating the association between cruciferous vegetables intake and bladder cancer risk, however, have yielded inconsistent results. Several case-control studies (10, 11), as well as a cohort study (12), reported a significant inverse association between cruciferous vegetables intake and bladder cancer risk. Nevertheless, many other studies found no association (13–15), including an international pooled study (15). Therefore, the aim of the present meta-analysis is to summarize the evidence on the association between cruciferous vegetables intake and bladder cancer risk based on all available cohort studies.
A comprehensive literature search of relevant articles was performed in the PubMed and EMBASE databases from their inception through March 2022 with the following search algorithm: (diet or nutrition or vegetable or cruciferous or broccoli or cauliflower or cabbage) and (bladder neoplasm or bladder cancer) and (cohort or prospective or nested case-control). The cited references from retrieved articles and reviews were also checked for additional relevant studies. No language restriction was applied. This systematic review and meta-analysis was planned, performed, and reported according to the standards of quality for reporting meta-analyses (16, 17).
Studies included in this meta-analysis met all of the following criteria: (i) the exposure of interest was the cruciferous vegetables intake; (ii) the outcome of interest was bladder cancer incidence; (iii) the study design was prospective or cohort; and (iv) the risk estimates with their corresponding 95% confidence intervals (CIs) were reported or data were provided to calculate them. We excluded reviews/meta-analyses, editorials, correspondences, case reports, and non-human studies. Studies of other exposures and diseases were also removed. If multiple publications based on overlapping population were retrieved, the most informative one was included.
Two authors (PY and YL) independently extracted the data using a predefined extraction form. The following information was extracted from each study: the first author’s name, year of publication, study region, study name, study population or source, sample size (number of cases and participants), participants’ age and sex, follow-up time, method of diet assessment, method of outcome assessment, and adjusted confounders in the data analysis.
The same two authors (PY and YL) independently completed the quality assessment using the Newcastle-Ottawa Scale (NOS).1 NOS is an eight-item instrument and awards a maximum of nine points to each study. A higher score indicates better methodological quality. Any disagreements were resolved by consensus and discussion.
The summary RRs and 95% CIs were estimated using a DerSimonian and Laird random effects model (18). Summary risk estimates were estimated by comparing the two extreme categories of the cruciferous vegetables intake related to bladder cancer risk.
Dose-response meta-analysis was performed using the method proposed by Greenland (19) and Orsini (20). Briefly, this method required that studies reported categorical risk estimates for at least three quantitative levels of cruciferous vegetables intake and the number of cases and person-years in each exposure category. The median/mean value or the midpoint of each category was regarded as the corresponding exposure dose. For upper, open-ended exposure categories, we assumed the width of the interval to be the same as the closest neighboring category. The lowest category of exposure was treated as the reference group. In addition, we tested a potential non-linear dose-response relationship between the cruciferous vegetables intake and bladder cancer risk by modeling the cruciferous vegetables intake using restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles of the distribution (21). A P-value for non-linearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to 0.
The heterogeneity across studies was assessed by the Q statistic and the I2 score (22). The Q statistic was used to determine the presence of heterogeneity with a significance level set at P ≤ 0.10. The value of I2 was used to calculate the proportion of variation (I2 < 25% low heterogeneity; I2 = 25–50% moderate heterogeneity; I2 > 50% high heterogeneity). The subgroup analyses were performed based on study region, study year, gender, number of cases, and number of participants. A sensitivity analysis was performed by repeating the meta-analysis after exclusion of each included study in turn. Potential publication bias was assessed by Begg’s test (rank correlation method) (23) and Egger’s test (linear regression method) (24). All of the statistical analyses were performed using STATA 11.0 (StataCorp, College Station, TX). A 2-sided P-value < 0.05 was considered significant unless stated otherwise.
The detailed process of the literature search and selection has been presented in a flow diagram (Figure 1). A total of seven prospective studies (12–15, 25–27) met the inclusion criteria for this meta-analysis evaluating the association between cruciferous vegetables intake and bladder cancer risk. Park et al.’ study (13) reported the results separately by gender and thus was regarded as two independent cohorts. These cohorts were from the following regions: America (n = 5), Europe (n = 2), and International (n = 1). A total of 1,503,016 participants with 13,669 cases were included in this study. These studies were published from 1999 to 2021. Information on cruciferous vegetables consumption was obtained by self-reports or interviews with food-frequency questionnaires (FFQs). The outcome was collected from cancer registry, health insurance records or medical records. The study quality as assessed by the NOS was generally high. Only one study Larsson et al. (25) study had a score of 7, with all the other studies having a score of 8 (Supplementary Table 1). The detailed information of the studies at baseline are shown in Table 1.
The multivariable-adjusted RRs of the highest vs. the lowest categories of the cruciferous vegetables intake in each study and for the combination of all of the studies are shown in Figure 2. The highest cruciferous vegetables intake was not significantly associated with a lower risk of bladder cancer, compared with the lowest category (RR = 0.92, 95% CI 0.80–1.06).
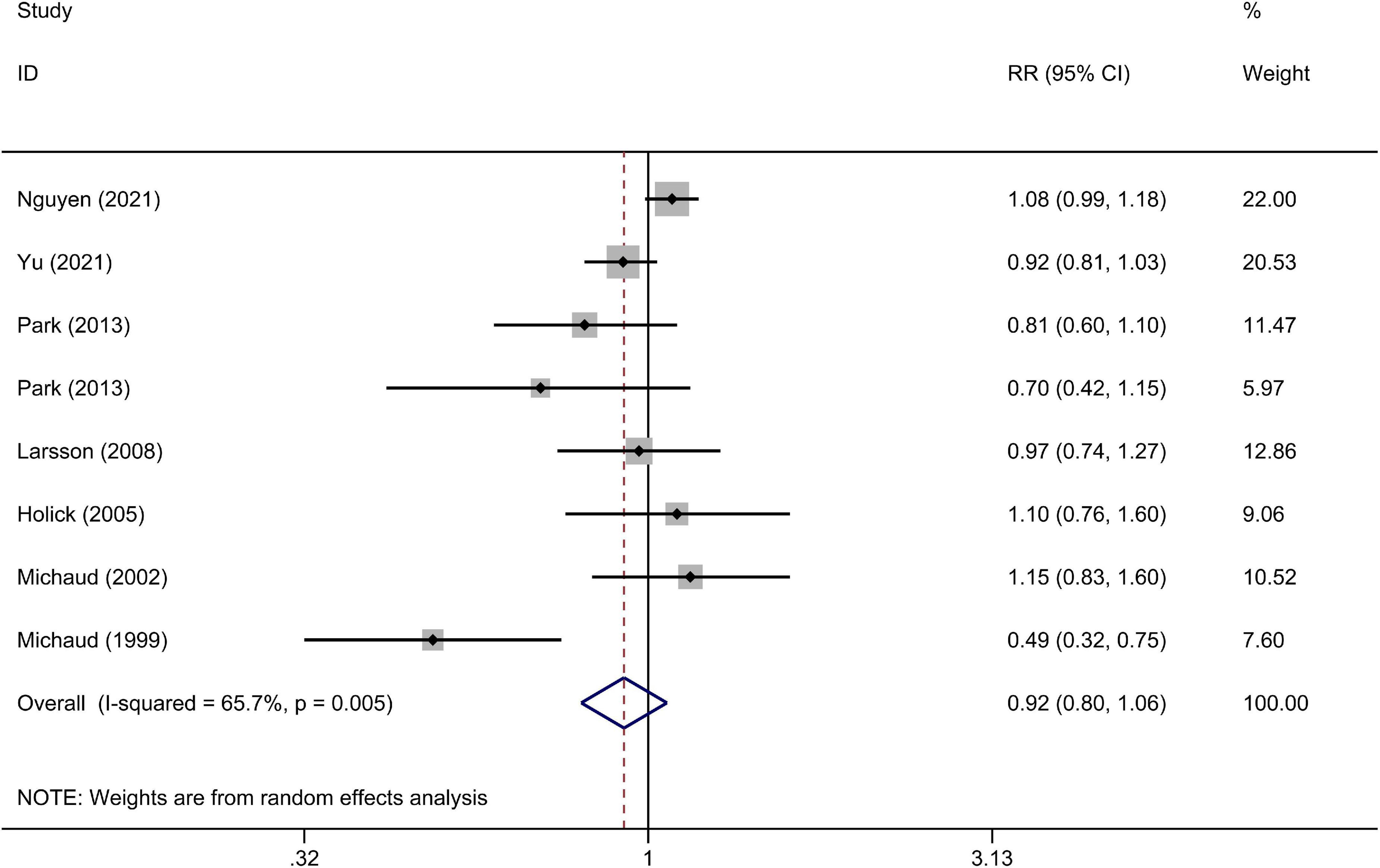
Figure 2. A forest plot of pooled relative risks (RRs) for cruciferous vegetables intake and bladder cancer risk (the highest category compared with the lowest category).
Two units, i.e., grams/day and servings/week, were used for cruciferous vegetables intake in the included studies and thus the dose-response analysis was performed separately. There was no evidence of a non-linear relationship between cruciferous vegetables intake and bladder cancer risk (P = 0.169 and P = 0.708 for non-linearity, respectively). Linear dose-response meta-analysis indicated that neither 10 g/day or 1 servings/week increment of cruciferous vegetables intake was significantly associated with a reduced risk of bladder cancer (Figure 3, P = 0.106 and P = 0.147, respectively).
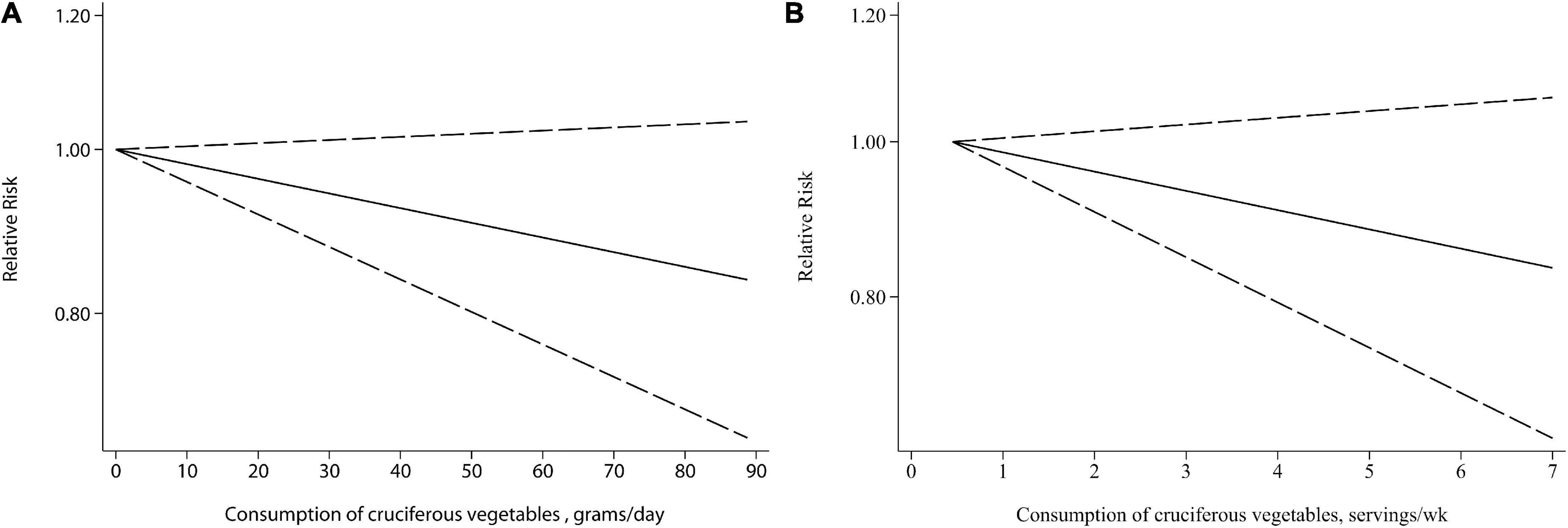
Figure 3. Relative risks (RRs) and the corresponding 95% confidence intervals (CIs) for the dose-response relationship between cruciferous vegetables intake and bladder cancer risk. The solid line and dash line represent the estimated RRs and their 95% CIs. (A) Grams per day; (B) servings/week.
There was some statistically significant heterogeneity among the studies, either assessed by Q statistic (P = 0.005) or I2 index (I2 = 65.7%). In the stratified analyses, no significant association was observed in any pre-specified subgroups (Table 2).
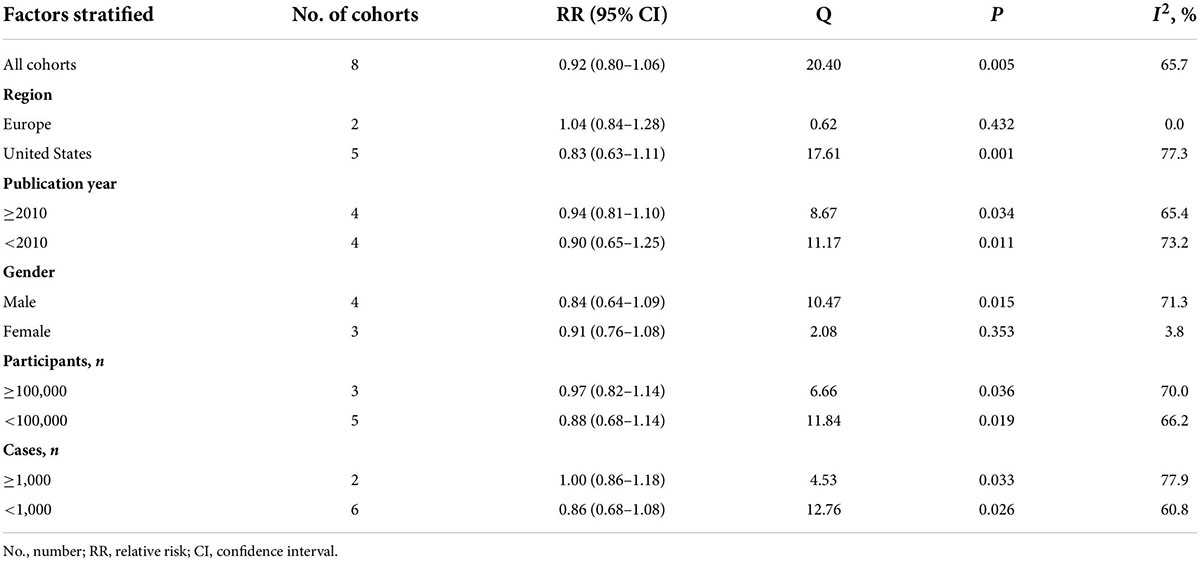
Table 2. Subgroup analyses for the relationship between consumption of cruciferous vegetables and the risk of bladder cancer.
In the sensitivity analysis, the impact of each study on the pooled RR was evaluated by repeating the meta-analysis after omitting one study at a time. As a result, exclusion of any single study did not substantially alter the pooled RR (Figure 4). There was no evidence of significant publication bias with Begg’s test (Figure 5, P = 0.386) or with Egger’s test (P = 0.253).
This meta-analysis of prospective studies, including approximately 1,500,000 participants, showed that cruciferous vegetables intake was not significantly associated with the risk of bladder cancer, with consistent findings from the dose-response analysis and subgroup analysis.
A previous meta-analysis, published in 2013 by Liu et al. (28), exploring the association between cruciferous vegetables intake and bladder cancer risk was based on five cohort and five case-control studies. In the analysis of highest vs. lowest levels, cruciferous vegetables intake was significantly associated with a lower bladder cancer risk (RR 0.80, 95% CI 0.69–0.92). However, in a subgroup analysis of cohort studies the significant association did not hold, with a RR of 0.86 (95% CI, 0.61–1.11) which was pretty similar with our findings. Besides, the earlier meta-analysis did not examine the dose-response relationship and the sample size was relatively limited. By contrast, our meta-analysis included the recent studies, which further increased the sample size and improved the statistical power. In addition, only prospective studies were included in our study, which avoided the select or recall bias from case-control studies.
Cruciferous vegetable intake has been associated with multiple health outcomes. Recently, Li et al. (9) performed an umbrella review of 41 systematic reviews and meta-analyses of 303 observational studies. It revealed that cruciferous vegetable intake might have beneficial effects on several outcomes, including gastric cancer, lung cancer, endometrial cancer, and all-cause mortality. Similarly, another umbrella review of meta-analyses and systematic reviews reported that consumption of cruciferous vegetable was associated with a reduced risk of death from any cause, cancers, and depression (29). The inverse association between cruciferous vegetable intake and mortality was also supported by a large prospective cohort study with a median follow-up of 16.9 years. HR (95% CIs) for all-cause mortality in the highest compared to the lowest quintile was 0.86 (0.80–0.93) for men (P = 0.0002 for trend) and 0.89 (0.81–0.98) for women (P = 0.03 for trend) (30).
Previously several mechanisms have been proposed to explain the potential relationship between dietary cruciferous vegetables intake and bladder cancer risk. Sulforaphane, an isothiocyanate, presents naturally in cruciferous vegetables and acts as a chemopreventive agent (31). Sulforaphane plays an important role in ROS (reactive oxygen species) and ROS-related pathways, which are associated with the initiation and progression of bladder cancer (32). He et al. (33) found that the inhibitory effect of Sulforaphane on bladder cancer cells also depends on GSH (glutathione, r-glutamyl cysteingl + glycine) depletion induced by Nrf2 translocation. Xia et al. (34) reported that Sulforaphane suppressed non-muscle-invasive bladder cancer cells proliferation through inhibition of HIF-1α-mediated glycolysis in hypoxia. Benzyl isothiocyanate, another isothiocyanate presented in cruciferous vegetables, also has been reported to prevent bladder cancer progression by suppressing IGF1R, FGFR3, and mTOR (35). Abbaoui et al. (36) proposed that cruciferous vegetable isothiocyanates, including sulforaphane (SFN) and erucin (ECN), may suppress bladder carcinogenesis via epigenetic modulation of gene expression associated with histone H1 phosphorylation. Although various isothiocyanates from cruciferous vegetables have been proved to exert a promising anticancer effect from a substantial amount of scientific research, our study, as well as many previous epidemiological studies, did not support that cruciferous vegetable intake was associated with the bladder cancer risk.
Our meta-analysis has several strengths. First, the present study had large sample size and statistical power and only prospective studies were included. Second, the methodological quality of the included studies was generally high as assessed by NOS. Third, both categorical analysis and dose-response analysis were performed with consistent results, indicating that the findings were robust and sound. However, several limitations should also be noted. First, the number of eligible studies was still limited and no Asian studies were available. Second, although no significant publication bias was detected as assessed either by Begg’s test or Egger’s test, some publication bias may still exist as small studies with null results were less likely to be published. Third, the methods of cruciferous vegetables assessment and the cut-off points used differed across the included studies, which might distort the pooled results. Finally, a significant heterogeneity was observed among the included studies, which may weaken the robustness of the conclusion.
In summary, the results of the current study did not support the hypothesis that dietary cruciferous vegetables intake was associated with a lower risk of bladder cancer. Further large prospective cohort studies are still warranted to confirm our preliminary findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
PY and YL contributed to the conception or design of the work and drafted the manuscript. PY, LY, and YL contributed to the acquisition, analysis, and interpretation of data for the work. YL critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.944451/full#supplementary-material
1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. (2017). 71:96–108. doi: 10.1016/j.eururo.2016.06.010
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. (2013) 63:234–41. doi: 10.1016/j.eururo.2012.07.033
4. Matulewicz RS, Steinberg GD. Non-muscle-invasive bladder cancer: overview and contemporary treatment landscape of neoadjuvant chemoablative therapies. Rev Urol. (2020) 22:43–51.
5. Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. (2017) 119:371–80. doi: 10.1111/bju.13760
6. Bermejo LM, López-Plaza B, Santurino C, Cavero-Redondo I, Gómez-Candela C. Milk and dairy product consumption and bladder cancer risk: a systematic review and meta-analysis of observational studies. Adv Nutr. (2019) 10(Suppl_2):S224–38. doi: 10.1093/advances/nmy119
7. Crippa A, Larsson SC, Discacciati A, Wolk A, Orsini N. Red and processed meat consumption and risk of bladder cancer: a dose-response meta-analysis of epidemiological studies. Eur J Nutr. (2018) 57:689–701. doi: 10.1007/s00394-016-1356-0
8. Dianatinasab M, Forozani E, Akbari A, Azmi N, Bastam D, Fararouei M, et al. Dietary patterns and risk of bladder cancer: a systematic review and meta-analysis. BMC Public Health. (2022) 22:73. doi: 10.1186/s12889-022-12516-2
9. Li YZ, Yang ZY, Gong TT, Liu YS, Liu FH, Wen ZY, et al. Cruciferous vegetable consumption and multiple health outcomes: an umbrella review of 41 systematic reviews and meta-analyses of 303 observational studies. Food Funct. (2022) 13:4247–59. doi: 10.1039/d1fo03094a
10. Lin J, Kamat A, Gu J, Chen M, Dinney CP, Forman MR, et al. Dietary intake of vegetables and fruits and the modification effects of GSTM1 and NAT2 genotypes on bladder cancer risk. Cancer Epidemiol Biomarkers Prev. (2009) 18:2090–7. doi: 10.1158/1055-9965.epi-08-1174
11. Castelao JE, Yuan JM, Gago-Dominguez M, Skipper PL, Tannenbaum SR, Chan KK, et al. Carotenoids/vitamin C and smoking-related bladder cancer. Int J Cancer. (2004) 110:417–23. doi: 10.1002/ijc.20104
12. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. (1999) 91:605–13. doi: 10.1093/jnci/91.7.605
13. Park SY, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, Kolonel LN. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. (2013) 143:1283–92. doi: 10.3945/jn.113.174920
14. Nguyen TP, Zhang CA, Sonn GA, Eisenberg ML, Brooks JD. Consumption of cruciferous vegetables and the risk of bladder cancer in a prospective US cohort: data from the NIH-AARP diet and health study. Am J Clin Exp Urol. (2021) 9:229–38.
15. Yu EY, Wesselius A, Mehrkanoon S, Goosens M, Brinkman M, van den Brandt P, et al. Vegetable intake and the risk of bladder cancer in the BLadder Cancer Epidemiology and Nutritional Determinants (BLEND) international study. BMC Med. (2021) 19:56. doi: 10.1186/s12916-021-01931-8
16. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. (2005) 93:387–91. doi: 10.1038/sj.bjc.6602678
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
19. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
20. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. doi: 10.1093/aje/kwr265
21. Harrell FE Jr., Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. (1988) 80:1198–202.
22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
23. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34.
25. Larsson SC, Andersson SO, Johansson JE, Wolk A. Fruit and vegetable consumption and risk of bladder cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. (2008) 17:2519–22. doi: 10.1158/1055-9965.epi-08-0407
26. Holick CN, De Vivo I, Feskanich D, Giovannucci E, Stampfer M, Michaud DS. Intake of fruits and vegetables, carotenoids, folate, and vitamins A, C, E and risk of bladder cancer among women (United States). Cancer Causes Control. (2005) 16:1135–45. doi: 10.1007/s10552-005-0337-z
27. Michaud DS, Pietinen P, Taylor PR, Virtanen M, Virtamo J, Albanes D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. Br J Cancer. (2002) 87:960–5. doi: 10.1038/sj.bjc.6600604
28. Liu B, Mao Q, Lin Y, Zhou F, Xie L. The association of cruciferous vegetables intake and risk of bladder cancer: a meta-analysis. World J Urol. (2013) 31:127–33. doi: 10.1007/s00345-012-0850-0
29. Li N, Wu X, Zhuang W, Wu C, Rao Z, Du L, et al. Cruciferous vegetable and isothiocyanate intake and multiple health outcomes. Food Chem. (2022) 375:131816. doi: 10.1016/j.foodchem.2021.131816
30. Mori N, Shimazu T, Charvat H, Mutoh M, Sawada N, Iwasaki M, et al. Cruciferous vegetable intake and mortality in middle-aged adults: a prospective cohort study. Clin Nutr. (2019) 38:631–43. doi: 10.1016/j.clnu.2018.04.012
31. Mastuo T, Miyata Y, Yuno T, Mukae Y, Otsubo A, Mitsunari K, et al. Molecular mechanisms of the anti-cancer effects of isothiocyanates from cruciferous vegetables in bladder cancer. Molecules. (2020) 25:575. doi: 10.3390/molecules25030575
32. Xie H, Chun FK, Rutz J, Blaheta RA. Sulforaphane impact on reactive oxygen species (ROS) in bladder Carcinoma. Int J Mol Sci. (2021) 22:5938. doi: 10.3390/ijms22115938
33. He C, Buongiorno LP, Wang W, Tang JCY, Miceli N, Taviano MF, et al. The inhibitory effect of sulforaphane on bladder cancer cell depends on GSH depletion-induced by Nrf2 translocation. Molecules. (2021) 26:4919. doi: 10.3390/molecules26164919
34. Xia Y, Kang TW, Jung YD, Zhang C, Lian S. Sulforaphane inhibits nonmuscle invasive bladder cancer cells proliferation through suppression of HIF-1α-mediated glycolysis in hypoxia. J Agric Food Chem. (2019) 67:7844–54. doi: 10.1021/acs.jafc.9b03027
35. Tsai TF, Chen PC, Lin YC, Chou KY, Chen HE, Ho CY, et al. Benzyl isothiocyanate promotes miR-99a expression through ERK/AP-1-dependent pathway in bladder cancer cells. Environ Toxicol. (2020) 35:47–54. doi: 10.1002/tox.22841
Keywords: cruciferous vegetables, bladder cancer, meta-analysis, cohort, risk
Citation: Yu P, Yu L and Lu Y (2022) Dietary consumption of cruciferous vegetables and bladder cancer risk: A systematic review and meta-analysis. Front. Nutr. 9:944451. doi: 10.3389/fnut.2022.944451
Received: 23 May 2022; Accepted: 01 August 2022;
Published: 18 August 2022.
Edited by:
Maria Beatriz Prior Pinto Oliveira, University of Porto, PortugalReviewed by:
Thelma Machado, Fluminense Federal University, BrazilCopyright © 2022 Yu, Yu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengkui Yu, c3p5cGs0MDgwQDE2My5jb20=; Yi Lu, MTMzNjI1NTA4NThAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.