
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 20 September 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.942155
This article is part of the Research TopicDietary Intake, Eating Behavior and Health OutcomesView all 36 articles
Background: Observational studies have suggested processed and red meat may increase the risk of cancer. However, the causal effects and direction between them were still unclear. We conducted two-sample Mendelian randomization (MR) analysis to evaluate the causal effect of processed meat and red meat on the risk of nine common types of cancer, namely, lung, ovarian, endometrial, breast, kidney, gastric, prostate, skin, and oropharyngeal cancer.
Methods: Genome-wide association studies (GWAS) for processed meat and red meat (pork, beef, and mutton) were obtained from the UK Biobank. GWAS of types of cancer in this study were extracted from the genetic consortia and the FinnGen consortium. The inverse variance weighted (IVW) was carried out as the main method for two-sample MR analysis. Sensitivity analyses were used to assess the robustness of the results.
Results: Genetically predicted processed meat intake was causally associated with increased risk of lung cancer (OR [odds ratio] = 1.923, 95% CI = 1.084–3.409, P = 0.025). There is no convincing evidence for the associations between genetically determined processed meat, red meat, and the risk of other cancers we studied.
Conclusion: Our results suggested that intake of processed meat may increase the risk of lung cancer. These findings provided no evidence to support that consumption of processed and red meat has a large effect on the risk of other cancers we studied. Further research is needed to clarify the results.
Cancer is the main cause of morbidity and mortality in the world. According to the research of the International Agency for Research on Cancer (IARC), it was estimated that there were approximately 20 million new cancer cases and nearly 10 million cancer deaths globally in 2020, which had become the main health burden of all countries (1). Previous studies found that dietary factors were associated with cancer risk, especially red and processed meat intake may be an important risk factor for many types of cancer (2).
Red meat (pork, beef, mutton, etc.) is an important source of protein, vitamins, amino acids, minerals, and other nutrients (3). Processed meat refers to improving the taste of meat or increasing the shelf life through several processes such as salting, curing, fermentation, and smoking (4). In recent decades, the consumption of meat has been increasing all over the world. However, it has been reported that high consumption of red and processed meat may increase the risk of cancer (5).
Myoglobin, hemoglobin, and cytochrome which have high levels in red meat were transformed into denatured protein hemes, hemichromes, and hemochromes during cooking and other processing. Oxidative reactions catalyzed by hemoglobin and iron can damage various components of biological systems, such as lipids, proteins, nucleic acids, and other substances. Free radical damage caused by oxidative stress can lead to cancer (6). The IARC working group has shown that consumption of red meat may increase the chance of colorectal cancer, pancreatic cancer, and prostate cancer, while consuming processed meat may increase the possibility of colorectal cancer and gastric cancer (7).
According to previously published systematic reviews and meta-analyses, red and processed meat consumption may lead to an increased risk of cancer (8–11). However, there are still many studies showing that the consumption of processed and red meat may not be linked to higher cancer risk (12–16). Observational studies evaluating the relationship between processed meat, red meat, and the risk of cancers have reported inconsistent results, most likely due to sampling size limitations. Furthermore, observational epidemiological studies are susceptible to confounding and reverse causation (17). Whether there is a causal relationship between the intake of processed or red meat and cancer remains unclear (18). Compared with the observational studies, randomized controlled trials (RCTs) on the consumption of red meat and processed meat could potentially help establish the causal relationship (19). A recent RCT on this topic showed that processed meat intake was not associated with the risk of cancers, and red meat intake could increase the risk of breast cancer (20). However, it is worth noting that volunteers included in the study had more health-conscious behaviors and higher educational levels compared to the general population, which will inevitably bias the results. In addition, the number of cases of cancer at specific sites is relatively small. Therefore, the extrapolation of these results still needs to be cautious.
Mendelian randomization (MR) is a research method used in epidemiology in recent years, mainly through genetic variation to infer the causal relationship between exposure and disease outcomes based on single nucleotide polymorphisms (SNPs) (21). In MR, causal inference of exposure-outcome associations can be improved by using phenotype-related genetic variants as instrumental variables for exposure. Genetic variation follows the rules that alleles segregate randomly from parent to offspring and are determined at conception by genetic variation, so it is not easy to be disturbed by population confounding factors in traditional observational research (22). In addition, the genotypes are not affected by disease phenotypes, so inverse correlation bias can also be avoided (23). Currently, MR has been applied to studies on the causal relationship between dietary habits such as vegetable intake and cancer (24, 25). For example, Chen Jin et al. conducted a two-sample MR analysis to explore the relationship between the causal relationship between dried fruit intake and the risk of cancers. Studies have shown that the consumption of dried fruit may have a protective effect against cancer. It is suggested that health education and reasonable adjustment of dietary ratios may contribute to the primary prevention of cancer (26). There is also a high-quality MR study on the association between processed meat and the risk of cancer. Qi Feng et al. performed both observational analyses with UK Biobank and genetic analysis with MR to explore the effect of processed meat intake on the risk of colorectal cancer. The results showed that heavy consumption of processed meat independently increases the risk of colorectal cancer, and processed meat intake reduction may be an effective strategy for preventing colorectal cancer (27).
In our study, we performed a two-sample MR analysis to assess the potential causal relationship between processed red meat intake and the risk of cancers from the GWASs and UK Biobank that were publicly available.
We used data from published studies or GWAS summaries that were openly available. Since no primary data were used in this study, ethical approval was not required. All the studies included were permitted by their academic ethics review committees, and each participant signed written informed consent.
Dietary exposures (processed meat, pork, beef, and mutton) were obtained from the UK BioBank cohort with 461,981, 460,162, 461,053, and 460,006 individuals of European ancestry, respectively. To minimize the effects of linkage disequilibrium (LD), single nucleotide polymorphisms (SNP) that passed the generally accepted genome-wide significance threshold (P < 5 × 10−8, R2>0.001 within a 10,000 kb window) for exposures were chosen as instrumental variables (Figure 1A). The detailed information on these independent, genome-wide SNPs was shown in Supplementary material 1. F statistics and proportion of variance explained (PVE) were computed to test whether a weak instrument bias was present.
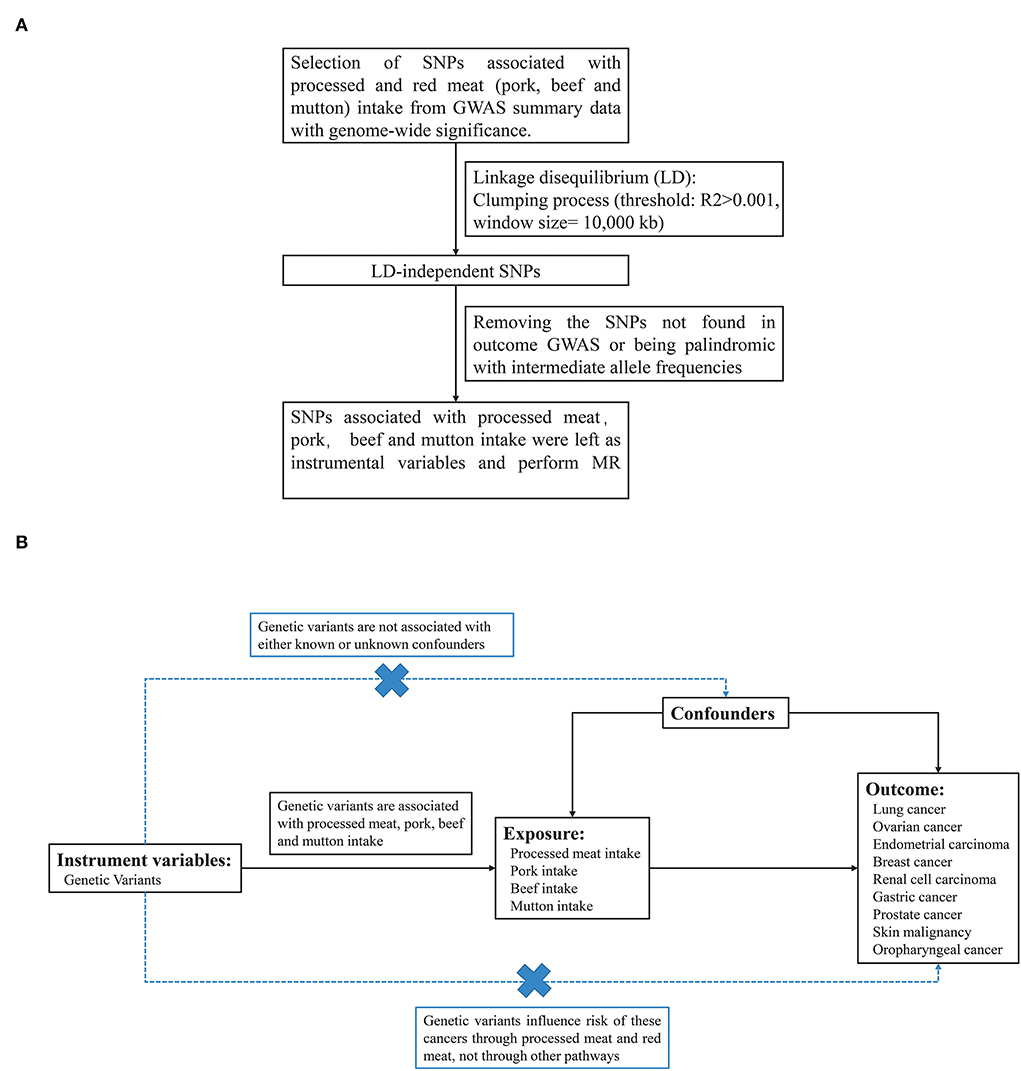
Figure 1. (A) The design of MR analysis in our study. (B) The flow chart about the process of screening for SNPs associated with exposure.
We use large-scale GWAS data for nine types of cancer as outcome factors. Breast cancer data were obtained from GWAS meta-analysis from Breast Cancer Association Consortium (BCAC) studies involving people of European ancestry (46,785 cases and 42,892 controls). Data for prostate cancer was derived from a genome-wide association analysis of 79,148 patients and 61,106 controls of European ancestry by the Prostate Cancer Association Group to Investigate Cancer-Associated Alterations (PRACTICAL) in the Genome Consortium. For lung cancer, we used data from the International Lung Cancer Consortium, consisting of 11,348 cases and 15,861 controls of European descent. GWAS data for ovarian cancer were acquired from the Ovarian Cancer Alliance Consortium, which included 25,509 patients of European ancestry. Genome-wide association analysis results for gastric cancer, renal cell carcinoma, and skin malignancy were all derived from European ancestry data in FinnGen Biobank analysis (Table 1). Our study only utilized the results of published GWAS and did not involve individual-level data. All exposure and outcome summary data were downloaded from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/).
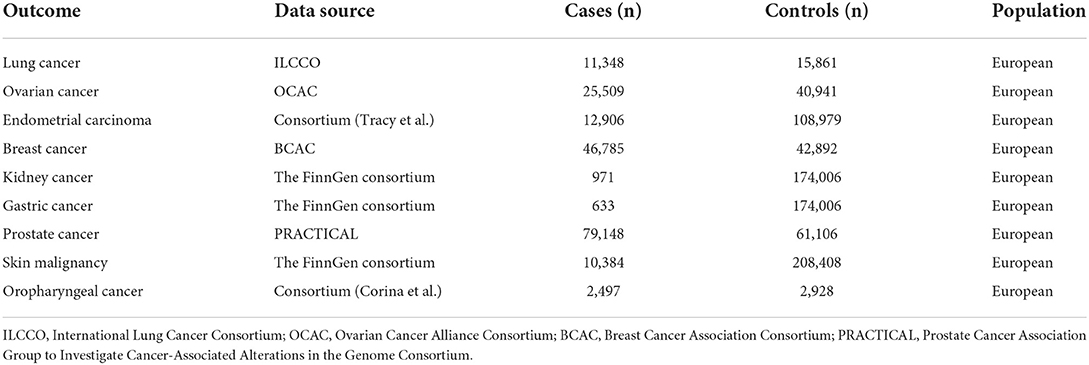
Table 1. Number of cancer cases and controls in the Mendelian randomization study on the association of processed meat and red meat intake with risk of site-specific cancer.
The MR analysis was carried out using the TwoSampleMR R package and the “MR-PRESSO” R package (version 0.4.13, http://github.com/MRCIEU/TwoSampleMR). All of our studies were based on a two-sample MR framework, which obtained SNP-exposure (processed meat intake, pork intake, beef intake, and mutton intake) associations and SNP-outcome (lung cancer, ovarian cancer, endometrial carcinoma, breast cancer, renal cell carcinoma, gastric cancer, prostate cancer, skin malignancy, and oropharyngeal cancer) associations from different cohorts to estimate the causal effects of exposure on the outcome. In total, six MR methods were used to estimate the effect of genetically predicted exposure on cancers namely the main analysis method inverse variance weighted (IVW), and other five additional analysis methods, Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO), maximum likelihood, MR Egger, weighted median, and penalized weighted media. For the IVW method, we used a random-effects model when the results were heterogeneous, and a fixed-effects model was used when there was no heterogeneity. The maximum likelihood method was performed by estimating the causal effects of the effect of SNPs on exposure and outcome by direct maximization of the likelihood (28). The MR-PRESSO method was used to detect outlier variables in IVW analysis by comparing the actual distance of the genetic variants to the expected distance from the regression, assuming the absence of horizontal pleiotropy and evaluating the causal estimates after removing outliers (29). The MR–Egger approach utilizes InSIDE to perform a weighted linear regression of exposure results but is susceptible to IVs (30). In addition, the weighted median method can significantly improve the detection ability of causal effects and reduce type I errors (31).
To test for heterogeneity, MR Egger and IVW were carried out. The SNP-exposure association and the SNP-outcome association estimates were involved in MR Egger. Using the slope of the weighted regression line, we estimated the causal effect of exposure on the outcome, independent of horizontal pleiotropy. An estimate of the causal effect of exposure on outcome was provided by the slope of the weighted regression line and was not affected by horizontal pleiotropy. In the MR-Egger test, the intercept assesses the mean pleiotropy of genetic variation, with values greater or less than zero indicating possible bias in IVW estimates. The sensitivity of the results was analyzed using the leave-one-out method. The SNPs were sequentially removed one at a time to examine whether the individual SNPs with potentially large horizontal pleiotropic effects could affect MR estimate.
We used the summary GWAS data from UK Biobank for each processed meat, pork, beef, and mutton as exposures and risk for 9 types of cancer as the outcome in different studies (Figure 1B). Two-sample MR analysis was performed to explore the causal relationship between processed/red meat and cancer. Supplementary material 1 showed the SNP information of four exposures (intake of processed meat, pork, beef and mutton), consisting of the name, chromosome location, genes, function, effect allele (EA), other alleles, and effect allele frequency (EAF). We calculated the F statistic for instrumental variable selection and F statistics were >10, which indicated that we have effectively avoided the bias caused by weak instrumental variables (Supplementary material 1) (32).
The inverse variance weighted (random effect and fixed effect), maximum likelihood, MR Egger, weighted median, and penalized weighted media were used to estimate causal associations between genetically predicted processed/red meat and the risk of 9 types of cancer. It showed that processed meat was associated with an increased risk odds of lung cancer (IVW: OR = 1.923, 95% CI = 1.084–3.409, P = 0.025) (Figure 2). A higher processed meat intake was not associated with the risk of ovarian cancer, endometrial carcinoma, breast cancer, renal cell carcinoma, gastric cancer, prostate cancer, skin malignancy, and oropharyngeal cancer. Consumption of red meat did not significantly increase the risk of cancer (Supplementary material 2).
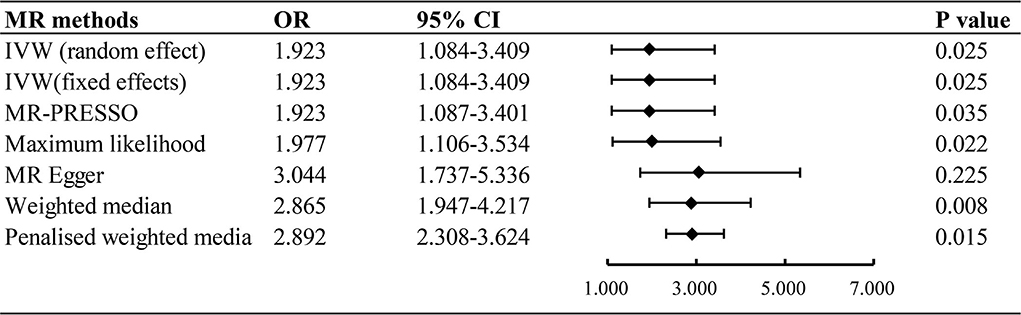
Figure 2. Associations of genetically predicted processed meat intake with risk of lung cancer. OR, odds ratio; CI, confidence interval.
The horizontal pleiotropy between SNPs and outcomes was assessed by MR-Egger regression, which showed no evidence of horizontal pleiotropy (Supplementary material 4). The funnel plots showed a symmetric pattern of effect size variation around the point estimates, indicating no apparent horizontal pleiotropy (Supplementary material 3). The results of the leave-one-out sensitivity analyses demonstrated that no potentially influential SNPs drive the causal link and the stability of our conclusion (Supplementary material 3).
In this study, a two-sample MR analysis was performed using the instrumental variables of large-scale GWAS to assess the causal relationship between processed/red meat and cancers using genetic data from populations of European descent. In our MR analysis, genetic predisposition to processed meat consumption was associated with a higher risk of lung cancer, with an OR of 1.923 [95% CI, 1.084–3.409; P = 0.025]. Results from a two-sample MR analysis suggested that processed meat consumption was not associated with the risk of ovarian cancer, endometrial carcinoma, breast cancer, renal cell carcinoma, gastric cancer, prostate cancer, skin malignancy, and oropharyngeal cancer. In this study, no strong evidence was found to support associations between red meat intake and the risk of types of cancer.
There was growing evidence that high levels of red meat intake, and processed meat consumption were linked to an increased risk of types of cancer (33, 34). A large observational study involving more than 470,000 people with a follow-up of 11.4 years showed a reduced risk of colorectal cancer and breast cancer in people who consumed less red meat (35). World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) also advised that limiting red meat intake and avoiding consumption of processed meat may modestly reduce the risk of cancer (36). Giuseppe et al. (37) compiled 24 meta-analyses of the association of red meat and 39 processed meat consumption with the risk of cancer published between 2005 and 2015. The results indicated an increased risk of cancer in subjects consuming large amounts of red and processed meat. It is possible that high-temperature cooked meats can produce N-nitroso compounds (NOCs). Heterocyclic amines formed in meat smoking can become carcinogens after metabolic activation (38). Heterocyclic aromatic amines (HAAs) can be derived from high-temperature cooked red meat (39). The rich heme in red meat can catalyze the production of NOC and lipid peroxidation products (LPO). These carcinogens combine with DNA to form DNA adducts, which interfere with DNA replication and repair, and cause gene mutations during cell division, inducing the occurrence of cancer (40). The 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP), a heterocyclic amine widely present in processed and red meat, induced cancer through cytochrome P450-mediated DNA damage and metabolic activation of mutagens (41, 42).
In MR analysis, it failed to detect significant associations of genetic predisposition to processed/ red meat with most of the cancers studied (P > 0.05). This was consistent with the conclusions of some guidelines and observational studies (43–47). A meta-analysis of 6 million participants in 56 cohort studies also found evidence of low quality that with the reduction of unprocessed red meat, the total cancer mortality would decrease. An intake reduction of three servings of processed meat per week was not associated with a lower incidence of cancer of the mouth, stomach, small bowel, liver, pancreas, endometrial, or prostate. Although studies have shown that reducing the intake of processed meat can reduce the risk of esophageal, colorectal, and breast cancer, the certainty of the evidence was very low due to the observation design and inaccuracy. In addition, there was low-certainty evidence that reducing the intake of red meat was associated with a very small overall reduction in cancer mortality (48). According to the Nutritional Recommendations (NutriRECS) Consortium's report, there was a low and very low quality that meat consumption could lead to potential adverse health outcomes. The probability of esophageal cancer, colorectal cancer, and breast cancer caused by high consumption of processed meat was not significantly different from that of low consumption (49). A definitive causal relationship requires more in-depth mechanism studies and RCT studies in the future.
The Mendelian randomization can avoid bias from unmeasured confounding and avoid bias from reverse causation and offer some protection against biases that can be conceptualized as reverse causation (50, 51). Our study tried to avoid some problems of confounding factors and reverse causality, but there were still some limitations. First, this is a pooled analysis of individual studies, due to the lack of original data, we could not conduct a patient-level analysis. Second, like all MR studies, horizontal pleiotropy, as a common issue, is difficult to avoid. Although some MR methods such as the leave-one-out method and MR-Egger were used to test, which indicated our results were not affected by pleiotropy, the possibility of bias could not be ruled out. Third, our results suggested a potential causal relationship between processed/red meat and types of cancer, the analysis presented here does not provide evidence for specific mechanisms of tumorigenesis. Fourth, wide CIs was observed under the MR-Egger method in MR analyses, which may hint at low potency. However, the MR-Egger method is often underpowered in studies and other Mendelian analyses were qualitatively consistent with the primary analysis of the inverse-variance weighted method. The last but not least, although using a single European population to investigate the causal relationship between processed/red meat and cancer can minimize population stratification bias, it might not be generalizable to other populations.
In conclusion, there is an obvious positive causal relationship between the genetically predicted processed meat and lung cancer. We did not find a causal relationship between processed, red meat, and other studied cancers. Observational studies had previously suggested an association between processed/red meat and cancer. Although traditional epidemiological studies can help us preliminarily understand the correlation between meat consumption and cancer, traditional epidemiological studies are influenced by confounding factors, such as social and demographic components. In addition, unrecognized bias may lead to inaccurate results. Further MR studies may be needed to assess the relationship between meat consumption and important risk factors for cancer.
All summary statistics based on association data are available free of charge. The data of processed meat intake (ID: ukb-b-6324), pork intake (ID: ukb-b-5640), beef intake (ID: ukb-b-2862), mutton intake (ID: ukb-b-14179), lung cancer (ID: ieu-a-966), prostate cancer (ID: ieu-b-85), oral cavity and pharyngeal cancer (ID: ieu-b-89), breast cancer (ID: ieu-a-1130), ovarian cancer (ID: ieu-a-1120), endometrial cancer (ID: ebi-a-GCST006464), skin malignancy (ID: finn-b-C3_SKIN), kidney cancer (ID: finn-b-C3_KIDNEY_NOTRENALPELVIS) and gastric cancer (ID:finn-b-C3_STOMACH_EXALLC) can be obtained from https://gwas.mrcieu.ac.uk/.
XS and DX contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by KW, TS, and AL. The first draft of the manuscript was written by KW and LL. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
This work was supported by the Foundation of Science and Technology, Department of Sichuan Province (2020YJ0485).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.942155/full#supplementary-material
Supplementary material 1. Instrumental SNPs from processed meat and red meat (pork, beef, and mutton) GWASs.
Supplementary material 2. MR estimates from each method of the causal effect of exposure on cancers.
Supplementary material 3. Leave-one-out analysis, funnel plot, and MR effect size for processed meat, pork, beef, and mutton intake on cancer.
Supplementary material 4. Heterogeneity and level pleiotropy test in the Mendelian randomization analyses.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Godfray HCJ, Aveyard P, Garnett T, Hall JW, Key TJ, Lorimer J, et al. Meat consumption, health, and the environment. Science (New York, NY). (2018) 361. doi: 10.1126/science.aam5324
3. Wolk A. Potential health hazards of eating red meat. J Intern Med. (2017) 281:106–22. doi: 10.1111/joim.12543
4. Ferro A, Rosato V, Rota M, Costa AR, Morais S, Pelucchi C, et al. Meat intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project. Int J Cancer. (2020) 147:45–55. doi: 10.1002/ijc.32707
5. Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. (2015) 16:1599–600. doi: 10.1016/S1470-2045(15)00444-1
6. Mrkonjic M, Chappell E, Pethe VV, Manno M, Daftary D, Greenwood CM, et al. Association of apolipoprotein E polymorphisms and dietary factors in colorectal cancer. Br J Cancer. (2009) 100:1966–74. doi: 10.1038/sj.bjc.6605097
7. Humans IWGotEoCRt. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat. Lyon (FR): International Agency for Research on Cancer © International Agency for Research on Cancer, 2018. (2018). Available online at: cHVibGljYXRpb25zQGlhcmMuZnI=.
8. Qu X, Ben Q, Jiang Y. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Intern Med. (2013) 23:762–70.e1. doi: 10.1016/j.annepidem.2013.09.003
9. Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. (2021) 36:937–51. doi: 10.1007/s10654-021-00741-9
10. Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: a meta-analysis. J Natl Cancer Inst. (2006) 98:1078–87. doi: 10.1093/jnci/djj301
11. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. (2008) 67:253–6. doi: 10.1017/S002966510800712X
12. Battaglia Richi E, Baumer B, Conrad B, Darioli R, Schmid A, Keller U. Health risks associated with meat consumption: a review of epidemiological studies. Int J Vitam Nutr Res. (2015) 85:70–8. doi: 10.1024/0300-9831/a000224
13. Turner ND, Lloyd SK. Association between red meat consumption and colon cancer: A systematic review of experimental results. Exp Biol Med (Maywood, NJ). (2017) 242:813–39. doi: 10.1177/1535370217693117
14. Zhao Z, Yin Z, Zhao Q. Red and processed meat consumption and gastric cancer risk: a systematic review and meta-analysis. Oncotarget. (2017) 8:30563–75. doi: 10.18632/oncotarget.15699
15. Yen H, Li WQ, Dhana A, Li T, Qureshi A, Cho E. Red meat and processed meat intake and risk for cutaneous melanoma in white women and men: Two prospective cohort studies. J Am Acad Dermatol. (2018).79:252–7.e6. doi: 10.1016/j.jaad.2018.04.036
16. Luo J, Yang Y, Liu J, Lu K, Tang Z, Liu P, et al. Systematic review with meta-analysis: meat consumption and the risk of hepatocellular carcinoma. Aliment Pharmacol Ther. (2014) 39:913–22. doi: 10.1111/apt.12678
17. Venkatesh SS, Ferreira T, Benonisdottir S, Rahmioglu N, Becker CM, Granne I, et al. Obesity and risk of female reproductive conditions: a Mendelian randomisation study. PLoS Med. (2022) 19:e1003679. doi: 10.1371/journal.pmed.1003679
18. Händel MN, Rohde JF, Jacobsen R, Heitmann BL. Processed meat consumption and the risk of cancer: a critical evaluation of the constraints of current evidence from epidemiological studies. Nutrients. (2021) 13. doi: 10.3390/nu13103601
19. Lee CF, Ho JWC, Fong DYT, Macfarlane DJ, Cerin E, Lee AM, et al. Dietary and physical activity interventions for colorectal cancer survivors: a randomized controlled trial. Scientific Rep. (2018) 8:5731. doi: 10.1038/s41598-018-24042-6
20. Diallo A, Deschasaux M, Latino-Martel P, Hercberg S, Galan P, Fassier P, et al. Red and processed meat intake and cancer risk: Results from the prospective NutriNet-Santé cohort study. Int J Cancer. (2018) 142:230–7. doi: 10.1002/ijc.31046
21. Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
22. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
23. Tian D, Zhang L, Zhuang Z, Huang T, Fan D. A Mendelian randomization analysis of the relationship between cardioembolic risk factors and ischemic stroke. Sci Rep. (2021) 11:14583. doi: 10.1038/s41598-021-93979-y
24. Cornelis MC, Munafo MR. Mendelian randomization studies of coffee and caffeine consumption. Nutrients. (2018) 10. doi: 10.3390/nu10101343
25. Sacerdote C, Guarrera S, Smith GD, Grioni S, Krogh V, Masala G, et al. Lactase persistence and bitter taste response: instrumental variables and mendelian randomization in epidemiologic studies of dietary factors and cancer risk. Am J Epidemiol. (2007) 166:576–81. doi: 10.1093/aje/kwm113
26. Jin C, Li R, Deng T, Lin Z, Li H, Yang Y, et al. Association between dried fruit intake and pan-cancers incidence risk: a two-sample Mendelian randomization study. Front Nutr. (2022) 9:899137. doi: 10.3389/fnut.2022.899137
27. Feng Q, Wong SH, Zheng J, Yang Q, Sung JJ, Tsoi KK. Intake of processed meat, but not sodium, is associated with risk of colorectal cancer: Evidence from a large prospective cohort and two-sample Mendelian randomization. Clin Nutr (Edinburgh, Scotland). (2021) 40:4551–9. doi: 10.1016/j.clnu.2021.05.036
28. Ying K, Zhai R, Pyrkov TV, Shindyapina AV, Mariotti M, Fedichev PO, et al. Genetic and phenotypic analysis of the causal relationship between aging and COVID-19. Commun Med. (2021) 1:35. doi: 10.1038/s43856-021-00033-z
29. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
30. Luo Q, Hu Y, Chen X, Luo Y, Chen J, Wang H. Effects of Gut Microbiota and Metabolites on Heart Failure and Its Risk Factors: A Two-Sample Mendelian Randomization Study. Front Nutr.. (2022) 9:899746. doi: 10.3389/fnut.2022.899746
31. Ye Z, Zheng J. Verification of the Role of ADAMTS13 in the cardiovascular disease using two-sample mendelian randomization. Front Genet. (2021) 12:660989. doi: 10.3389/fgene.2021.660989
32. Zheng C, He MH, Huang JR, He Y. Causal relationships between social isolation and osteoarthritis: a mendelian randomization study in european population. Int J Gen Med. (2021) 14:6777–86. doi: 10.2147/IJGM.S331864
33. Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R, et al. prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. (2007) 4:e325. doi: 10.1371/journal.pmed.0040325
34. Johnson IT. The cancer risk related to meat and meat products. Br Med Bull. (2017) 121:73–81. doi: 10.1093/bmb/ldw051
35. Watling CZ, Schmidt JA, Dunneram Y, Tong TYN, Kelly RK, Knuppel A, et al. Risk of cancer in regular and low meat-eaters, fish-eaters, and vegetarians: a prospective analysis of UK Biobank participants. BMC Med. (2022) 20:73. doi: 10.1186/s12916-022-02256-w
36. Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. (2020) 150:663–71. doi: 10.1093/jn/nxz268
37. Lippi G, Mattiuzzi C, Cervellin G. Meat consumption and cancer risk: a critical review of published meta-analyses. Crit Rev Oncol Hematol. (2016) 97:1–14. doi: 10.1016/j.critrevonc.2015.11.008
38. Khan N, Afaq F, Mukhtar H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett. (2010) 293:133–43. doi: 10.1016/j.canlet.2009.12.013
39. Zeng L, Ruan M, Liu J, Wilde P, Naumova EN, Mozaffarian D, et al. Trends in processed meat, unprocessed red meat, poultry, and fish consumption in the United States, 1999-2016. J Acad Nutr Diet. (2019) 119:1085–98.e12. doi: 10.1016/j.jand.2019.04.004
40. Turesky RJ. Mechanistic evidence for red meat and processed meat intake and cancer risk: a follow-up on the international agency for research on cancer evaluation of 2015. Chimia. (2018) 72:718–24. doi: 10.2533/chimia.2018.718
41. Malik DE, David RM, Gooderham NJ. Ethanol potentiates the genotoxicity of the food-derived mammary carcinogen PhIP in human estrogen receptor-positive mammary cells: mechanistic support for lifestyle factors (cooked red meat and ethanol) associated with mammary cancer. Arch Toxicol. (2018) 92:1639–55. doi: 10.1007/s00204-018-2160-9
42. Toden S, Belobrajdic DP, Bird AR, Topping DL, Conlon MA. Effects of dietary beef and chicken with and without high amylose maize starch on blood malondialdehyde, interleukins, IGF-I, insulin, leptin, MMP-2, and TIMP-2 concentrations in rats. Nutr Cancer. (2010) 62:454–65. doi: 10.1080/01635580903532382
43. Fedirko V, Trichopolou A, Bamia C, Duarte-Salles T, Trepo E, Aleksandrova K, et al. Consumption of fish and meats and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Oncol. (2013) 24:2166–73. doi: 10.1093/annonc/mdt168
44. Genkinger JM, Friberg E, Goldbohm RA, Wolk A. Long-term dietary heme iron and red meat intake in relation to endometrial cancer risk. Am J Clin Nutr. (2012) 96:848–54. doi: 10.3945/ajcn.112.039537
45. González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. (2006) 98:345–54. doi: 10.1093/jnci/djj071
46. Wallin A, Orsini N, Wolk A. Red and processed meat consumption and risk of ovarian cancer: a dose-response meta-analysis of prospective studies. Br J Cancer. (2011) 104:1196–201. doi: 10.1038/bjc.2011.49
47. Bylsma LC, Alexander DD. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr J. (2015) 14:125. doi: 10.1186/s12937-015-0111-3
48. Guyatt GH, Vernooij RWM, El Dib R, Zhang Y, et al. Reduction of red and processed meat intake and cancer mortality and incidence: a systematic review and meta-analysis of cohort studies. Ann Intern Med. (2019) 171:711–20. doi: 10.7326/M19-0699
49. Johnston BC, Zeraatkar D, Han MA, Vernooij RWM, Valli C, El Dib R, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) consortium. Ann Intern Med. (2019) 171:756–64. doi: 10.7326/M19-1621
50. Burgess S, Swanson SA, Labrecque JA. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur J Epidemiol. (2021) 36:253–7. doi: 10.1007/s10654-021-00726-8
Keywords: processed meat, red meat, cancer, Mendelian randomization, genome-wide association studies
Citation: Wu K, Liu L, Shu T, Li A, Xia D and Sun X (2022) The relationship between processed meat, red meat, and risk of types of cancer: A Mendelian randomization study. Front. Nutr. 9:942155. doi: 10.3389/fnut.2022.942155
Received: 01 June 2022; Accepted: 17 August 2022;
Published: 20 September 2022.
Edited by:
Ouyang Chen, Duke University, United StatesReviewed by:
Hongtao Lu, Second Military Medical University, ChinaCopyright © 2022 Wu, Liu, Shu, Li, Xia and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demeng Xia, ZGVtZW5neGlhQDE2My5jb20=; Xiaobin Sun, eGJzdW4xMTk3QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.