
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 30 September 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.937352
This article is part of the Research TopicWomen in Clinical NutritionView all 8 articles
Background: The association between serum total cholesterol (TC) and bone mineral density (BMD) is still controversial. We aimed to evaluate the association of serum TC with BMD in general US adult women.
Methods: A cross-sectional study consisting of 7,092 (age range 20–85) participants from the National Health and Nutrition Examination Survey (NHANES) database was conducted. Weighted multivariate linear regression analyses were performed to evaluate association between serum TC and lumbar spine BMD. In addition, subgroup and interaction analysis were used in this study.
Results: The serum TC was negatively correlated with lumbar spine BMD after adjusting for confounders. Subgroup analysis found that the strongest negative association mainly exists in women aged over 45 years with body mass index (BMI) <24.9 kg/m2, and this association is not significant in other groups.
Conclusions: This study found that serum TC exhibit an inverse association with lumbar spine BMD in Us women aged over 45 years. The measurement of serum TC may provide information for predicting poor bone health outcomes in these women.
Osteoporosis is a high incidence disease characterized by decreased bone mass, microarchitectural degeneration, and fragility fractures (1). Osteoporosis causes ~1.5 million fractures each year in the United States, the great majority of which occur in postmenopausal women (2). In 1997, the medical expenditures for osteoporotic fractures in the United States were $14 billion, and the cost is expected to reach nearly $ 50 billion by 2040 (3). Bone mineral density (BMD) is an effective indicator for assessing osteoporosis, and low BMD is associated with high fracture risk (4). Therefore, recognizing the risk factors for low BMD is critical for the predicting and prevention of osteoporosis.
There is growing evidence of a strong relationship between BMD and cardiovascular diseases and the metabolic syndrome, with the serum lipid profile possibly playing a key role in this interaction (5–9). As a result, a growing number of studies have examined the relationship between BMD and serum lipids. In a cross-sectional study, Sun C et al. reported that serum total cholesterol (TC) had a large negative impact on BMD in US population (10). Another study from Denmark observed that TC showed significant negative correlation with BMD at the lumbar spine and distal forearm, but not at the hip (11). Also, Trimpou et al. reported that serum TC is an independent risk factor for osteoporotic fracture, and high TC level is a long-term cause of osteoporotic fractures (12). However, a cross-sectional analysis in 136 Caucasian found that higher level of TC was positively associated with BMD of various skeletal sites (13). In addition, several studies have detected no association of serum TC levels with BMD (14–17).
The results of these studies show that the association between serum TC and BMD is still uncertain. Therefore, it's worthwhile to investigate the association between serum TC and BMD to determine whether serum TC can be used to predict the likelihood of osteoporosis or osteopenia. This study aimed to investigate the association between serum TC and lumbar spine BMD in US adult women using data from the National Health and Nutrition Examination Survey (NHANES) database.
The NHANES database is a population-based national survey that provided information on the nutrition and health of the American population. The NHANES database is available publicly at www.cdc.gov/nchs/nhanes. Data from 1999 to 2006 in NHANES were combined in our study. Of the 41,474 participants, there were 10,701 women aged 20–85 years, 2,943 had missing data on TC or BMD, and 666 had a cancer diagnosis. After applying these exclusion criteria, 7,092 participants were included in the final analysis (Figure 1).
The independent variable in this study was serum TC. The dependent variable was lumbar spine BMD measured by the dual-energy x-ray (DEXA) scans. The following variables were included in final analysis as covariates: age, race, physical activity, education, body mass index (BMI), triglycerides, HDL cholesterol, serum phosphorus, serum calcium, serum potassium, alkaline phosphatase, LDL cholesterol, phosphorus intake, and protein intake. The examination parts related to clinical and laboratory evaluations were all carried out by well-trained medical experts. The detailed acquisition process and measuring method of each variable are available at www.cdc.gov/nchs/nhanes.
All analyses used weights from the NHANES examination sample adjusted for non-response, non-coverage, and unequal probabilities of selection. In the descriptive analysis, continuous variables were reported as mean ± standard deviation; categorical variables were reported as percentages. Weighted multivariate linear regression models were performed to evaluate the association between serum TC and lumbar spine BMD. Interaction and stratified analyses by age and BMI were performed. P < 0.05 (two-sided) was considered statistically significant. Modeling was performed with the EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and statistical software packages R (http://www.R-project.org, The R Foundation).
A total of 7,092 women aged 20–85 years were included in this study. The weighted population characteristics of participants by serum TC quartiles (Q1: 82–174 mg/dL; Q2: 175–199 mg/dL; Q3: 200–228 mg/dL; Q4: 229–419 mg/dL) were shown in Table 1. Among different groups of serum TC (quartiles, Q1–Q4), age, race, physical activity, education, BMI, triglycerides, HDL cholesterol, serum phosphorus, serum calcium, serum potassium, alkaline phosphatase, LDL cholesterol, phosphorus intake, protein intake, and lumbar spine BMD are all significantly different. Participants in the top quartile of serum TC were more likely to be older, whites, have lower values of lumbar spine BMD and higher triglycerides, LDL cholesterol, and alkaline phosphatase. In addition, the distribution of serum TC is presented in Figure 2.
Figure 2. Distribution histogram of serum TC. (A) Among all participants; (B) Among participants aged <45; (C) Among participants aged ≥45. TC, total cholesterol.
The results of univariate analysis were shown in Table 2. The results of univariate analysis showed that age, race, triglycerides, HDL cholesterol, serum phosphorus, serum calcium, serum potassium, alkaline phosphatase, and LDL cholesterol were negatively correlated with higher lumbar spine BMD. We also found that education levels, physical activity, protein intake, phosphorus intake, and BMI were correlated with higher lumbar spine BMD.
Three weighted multivariate linear regression models were constructed (Table 3). In the unadjusted model, serum TC was negatively correlated with lumbar spine BMD [β = −0.0006, 95% CI: (– 0.0007, – 0.0005)]. After adjusting for confounding factors, this negative association remained in Model 2 [β = – 0.0003, 95% CI: (– 0.0004, – 0.0002)] and Model 3 [β= – 0.0167, 95% CI: (– 0.0334, – 0.0001)]. After converting serum TC from a continuous variable to a categorical variable (quartiles), individuals in the highest quartile had a 0.0135 g/cm2 lower lumbar spine BMD than those in the lowest quartile of serum TC.
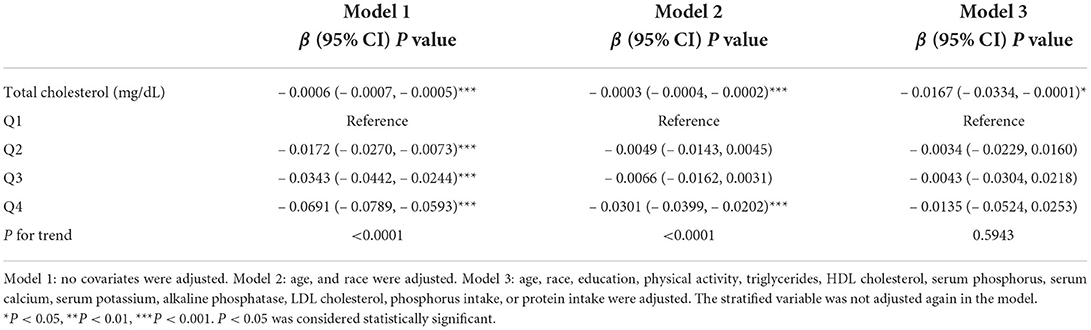
Table 3. Association of serum TC with lumbar spine bone mineral density in 7,092 female participants aged 20–85 years.
On subgroup analysis (Table 4), we observed the association between serum TC and lumbar spine BMD stratified by demographic variables. When stratified by age, a significant negative association existed in participants aged ≧45 years [– 0.0286 (– 0.0528, – 0.0045)]. BMI was converted into categorical variable using 24.9 and 29.9 kg/m2 as cut points. When stratified by BMI, serum TC was negatively correlated with lumbar spine BMD in participants with BMI <24.9 kg/m2 [– 0.0280 (– 0.0568, – 0.0008)]. Interaction analyses revealed the association between serum TC levels and lumbar spine BMD was modified by age and BMI (Table 4). The association between TC and lumbar spine BMD was stronger among participants aged over 45 years (β = – 0.0286, Pint = 0.0049), among participants with BMI <24.9 kg/m2 (β = – 0.0280, Pint = 0.0055).
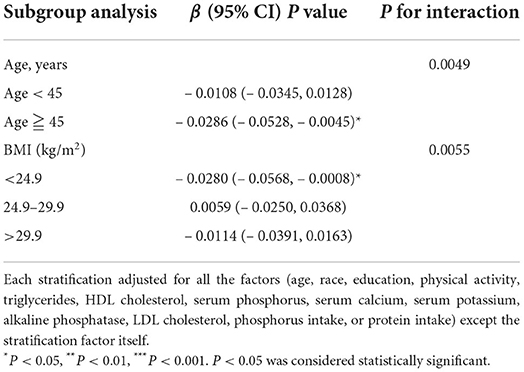
Table 4. Subgroup analysis of serum TC with lumbar spine bone mineral density, stratified by age and body mass index.
In the present cross-sectional study, we used the representative samples of the NHANES 1999–2006 to analyze the association between serum TC and lumbar spine BMD in US adult women. We found that serum TC were negatively correlated with lumbar spine BMD, especially in women aged over 45 years with BMI <24.9 kg/m2.
Osteoporosis is a metabolic disease, resulting in a progressive reduction in bone strength and enhanced bone fragility with susceptibility to fractures (18). Decreased BMD is an important diagnostic criterion for osteoporosis. The World Health Organization (WHO) defines osteoporosis as a BMD that lies 2.5 SDs below the mean maximum BMD (19). In recent years, there is growing epidemiological and biological evidence supports correlation between osteoporosis and cardiovascular disease (20, 21). Lipid metabolism is involved in the progress of these two disease (20). Among lipid profiles, serum TC is a derivative of cyclopentane dihydrophenanthrene, which is an important participant in the metabolism of tissue cells in the body. Studies show that cholesterol and its metabolites inhibit osteoblastic differentiation in bone cells (22). Moreover, a recent meta-analysis including 33 studies reported that statins increase BMD at the total hip and lumbar spine and decreased the risk of fractures (23). Collectively, these observations seem to suggest that serum TC might serve as a possible biomarker for predicting osteoporosis.
Clinical investigations of the association between serum TC and BMD in adults are controversial. In a cross-sectional study conducted in the USA, researchers discovered a negative correlation between serum TC level and left arm BMD, left leg BMD, and total BMD (10). A Camargo cohort study from Spanish found that serum TC was positively correlated to BMD at lumbar spine and hip (24). Brownbill et al. reported a positive correlation between high level of TC and BMD in 136 Caucasian postmenopausal women (13). Other studies from US also obtained different results regarding the association between serum TC and BMD (14, 25). The population in this study was a representative and large sample of US women aged 20–85 years. Our findings were similar with previous studies (10, 26, 27). The results indicate that serum TC was negatively correlated with lumbar spine BMD in US adult women.
Obesity has been demonstrated to be associated with abnormal lipid metabolism (28). BMI is the most widely used standard for measuring general obesity (29). Some studies reported that BMI was independently related to BMD (30, 31). Another cross-sectional study reported a negative association between waist circumference and lumbar BMD in premenopausal and postmenopausal women with BMI < 25 kg/m2, and middle aged men with BMI ≥30 kg/m2 (32). Similarly, we converted BMI into a categorical variable using 24.9 and 29.9 kg/m2 as cut points. When stratified by BMI, serum TC was negatively correlated with lumbar spine BMD in participants with BMI <24.9 kg/m2. Further prospective intervention studies are need to confirm this association.
Age was an important factor in this study that we could not ignore. Osteoporosis is much more common in women than in men. Approximately, one in three women and one in five men over the age of 50 have osteoporosis (33). In women, rapid bone loss occurs in the early postmenopausal period and lasts for 5–10 years after menopause, suggesting that estrogen loss is an important etiologic factor for osteoporosis in women (34). Several studies have shown a significant increase in serum TC in early menopause (35). The findings of these researches indicated that age is closely related to BMD and lipid metabolism. In subgroup analysis, we found that a higher serum TC level was associated with lower lumbar spine BMD in women aged over 45 years. Therefore, these present results further illustrated the association between lipid metabolism and BMD.
The strength of this study is that the NHANES database contains representative samples of the multi-ethnic population. In addition, the large sample size allows us to better conduct subgroup analyses. However, several limitations needed to be acknowledged in our study. First, the nature of the cross-sectional design makes it difficult to determine the causal relationship between serum TC and lumbar spine BMD. Therefore, further prospective studies are needed to confirm the relationship between serum TC and lumbar spine BMD. Second, the lumbar spine, the hip and the femoral neck are the most commonly used regions for evaluation of BMD. However, BMD of the hip and the femoral neck were not available in most participants and we only examined the association between serum TC and lumbar spine BMD. Finally, there may be other incomplete or unmeasured confounding variables that could alter the results we observed.
In summary, this cross-sectional study discovered a negative association between serum TC and lumbar spine BMD, especially in women aged over 45 years with BMI <24.9 kg/m2. It is suggested that the measurement of serum TC may provide information for predicting poor bone health outcomes in these women.
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes.
The studies involving human participants were reviewed and approved by board of the National Center for Health Statistics. All the participants provided their written informed consent to participate in this study.
WF and PP wrote the article. FX collected the data. WH, QW, and MH designed the study. All authors contributed toward data analysis, drafting and critically revising the paper, and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the project of the National Natural Science Foundation of China (grant numbers 82274544, 81873327, 82004392, and 81573996), the Double First-class Discipline Construction Project of Guangzhou University of Chinese Medicine (grant number Z2015002), the Major Project of Double First-class and High-level University Discipline Collaborative Innovation Team of Guangzhou University of Chinese Medicine (grant number 2021XK05), the Cultivated Project of Double First-class and High-level University Discipline Collaborative Innovation Team of Guangzhou University of Chinese Medicine (grant numbers 2021XK41 and 2021XK46), and the Foundation of Guangdong Educational Committee for Youth Scientists (grant number 2019KQNCX017).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fuggle NR, Curtis EM, Ward KA, Harvey NC, Dennison EM, Cooper C. Fracture prediction, imaging and screening in osteoporosis. Nat Rev Endocrinol. (2019) 15:535–47. doi: 10.1038/s41574-019-0220-8
2. Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. (2016) 374:254–62. doi: 10.1056/NEJMcp1513724
3. Miller PD. Management of osteoporosis. Dis Mon. (1999) 45:21–54. doi: 10.1016/S0011-5029(99)90010-X
4. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. (2014) 142:155–70. doi: 10.1016/j.jsbmb.2013.09.008
5. Chen GD, Ding D, Tian HY, Zhu YY, Cao WT, Wang C, et al. Adherence to the 2006 American heart association's diet and lifestyle recommendations for cardiovascular disease risk reduction is associated with bone mineral density in older Chinese. Osteoporos Int. (2017) 28:1295–303. doi: 10.1007/s00198-016-3857-3
6. Barzilay JI, Buzkova P, Cauley JA, Robbins JA, Fink HA, Mukamal KJ. The associations of subclinical atherosclerotic cardiovascular disease with hip fracture risk and bone mineral density in elderly adults. Osteoporos Int. (2018) 29:2219–30. doi: 10.1007/s00198-018-4611-9
7. Fohtung RB, Brown DL, Koh WJ, Bartz TM, Carbone LD, Civitelli R, et al. Bone mineral density and risk of heart failure in older adults: the cardiovascular health study. J Am Heart Assoc. (2017) 6:e004344. doi: 10.1161/JAHA.116.004344
8. Nóbrega da Silva V, Goldberg TB, Mosca LN, Bisi Rizzo Ada C, Teixeira Ados S, Corrente JE. Metabolic syndrome reduces bone mineral density in overweight adolescents. Bone. (2014) 66:1–7. doi: 10.1016/j.bone.2014.05.011
9. da Silva VN, Fiorelli LN, da Silva CC, Kurokawa CS, Goldberg TB. Do metabolic syndrome and its components have an impact on bone mineral density in adolescents? Nutr Metab. (2017) 14:1. doi: 10.1186/s12986-016-0156-0
10. Sun C, Zhu B, Zhu S, Zhang L, Du X, Tan X. Risk factors analysis of bone mineral density based on lasso and quantile regression in America during 2015-2018. Int J Environ Res Public Health. (2021) 19:355. doi: 10.3390/ijerph19010355
11. Tankó LB, Bagger YZ, Nielsen SB. Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone. (2003) 32:8–14. doi: 10.1016/S8756-3282(02)00918-3
12. Trimpou P, Odén A, Simonsson T, Wilhelmsen L, Landin-Wilhelmsen K. High serum total cholesterol is a long-term cause of osteoporotic fracture. Osteoporos Int. (2011) 22:1615–20. doi: 10.1007/s00198-010-1367-2
13. Brownbill RA, Ilich JZ. Lipid profile and bone paradox: higher serum lipids are associated with higher bone mineral density in postmenopausal women. J Womens Health. (2006) 15:261–70. doi: 10.1089/jwh.2006.15.261
14. Samelson EJ, Cupples LA, Hannan MT, Wilson PW, Williams SA, Vaccarino V, et al. Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham osteoporosis study. Bone. (2004) 34:557–61. doi: 10.1016/j.bone.2003.11.024
15. Kopiczko A, Łopuszańska-Dawid M, Gryko K. Bone mineral density in young adults: the influence of vitamin D status, biochemical indicators, physical activity and body composition. Arch Osteoporos. (2020) 15:45. doi: 10.1007/s11657-020-0684-0
16. Go JH, Song YM, Park JH, Park JY, Choi YH. Association between serum cholesterol level and bone mineral density at lumbar spine and femur neck in postmenopausal Korean women. Korean J Fam Med. (2012) 33:166–73. doi: 10.4082/kjfm.2012.33.3.166
17. Wu LY, Yang TC, Kuo SW, Hsiao CF, Hung YJ, Hsieh CH, et al. Correlation between bone mineral density and plasma lipids in Taiwan. Endocr Res. (2003) 29:317–25. doi: 10.1081/ERC-120025039
18. Eastell R, O'Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. (2016) 2:16069. doi: 10.1038/nrdp.2016.69
19. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. (1994) 843:1–129.
20. Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab. (2008) 5:19–34.
21. den Uyl D, Nurmohamed MT, van Tuyl LH, Raterman HG, Lems WF. (Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Res Ther. (2011) 13:R5. doi: 10.1186/ar3224
22. Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol. (2000) 20:2346–8. doi: 10.1161/01.ATV.20.11.2346
23. An T, Hao J, Sun S, Li R, Yang M, Cheng G, et al. Efficacy of statins for osteoporosis: a systematic review and meta-analysis. Osteoporos Int. (2017) 28:47–57. doi: 10.1007/s00198-016-3844-8
24. Hernández JL, Olmos JM, Ramos C, et al. Serum lipids and bone metabolism in Spanish men: the Camargo cohort study. Endocr J. (2010) 57:51–60. doi: 10.1507/endocrj.K09E-228
25. Solomon DH, Avorn J, Canning CF, Wang PS. Lipid levels and bone mineral density. Am J Med. (2005) 118:1414. doi: 10.1016/j.amjmed.2005.07.031
26. Makovey J, Chen JS, Hayward C, Williams FM, Sambrook PN. Association between serum cholesterol and bone mineral density. Bone. (2009) 44:208–13. doi: 10.1016/j.bone.2008.09.020
27. Anagnostis P, Florentin M, Livadas S, Lambrinoudaki I, Goulis DG. Bone health in patients with dyslipidemias: an underestimated aspect. Int J Mol Sci. (2022) 23:1639. doi: 10.3390/ijms23031639
28. Abeyratne T, Perera R, Fernando S. Obesity and cardiovascular risk among Sri Lankan adolescents: association of adipokines with anthropometric indices of obesity and lipid profile. Nutrition. (2020) 78:110942. doi: 10.1016/j.nut.2020.110942
29. Smith KB, Smith MS. Obesity statistics. Prim Care. (2016) 43:121–35, ix. doi: 10.1016/j.pop.2015.10.001
30. Ma M, Feng Z, Liu X, Jia G, Geng B, Xia Y. The saturation effect of body mass index on bone mineral density for people over 50 years old: a cross-sectional study of the US population. Front Nutr. (2021) 8:763677. doi: 10.3389/fnut.2021.763677
31. Je M, Kim H, Kim Y, A. Structural equation modelling approach to determine factors of bone mineral density in Korean women. Int J Environ Res Public Health. (2021) 18:11658. doi: 10.3390/ijerph182111658
32. Hua Y, Fang J, Yao X, Zhu Z. Can waist circumference be a predictor of bone mineral density independent of BMI in middle-aged adults? Endocr Connect. (2021) 10:1307–14. doi: 10.1530/EC-21-0352
33. Grgurevic A, Gledovic Z, Vujasinovic-Stupar N. Factors associated with postmenopausal osteoporosis: a case-control study of Belgrade women. Women Health. (2010) 50:475–90. doi: 10.1080/03630242.2010.507656
34. Riggs BL. Endocrine causes of age-related bone loss and osteoporosis. Novartis Found Symp. (2002) 242:247–59. doi: 10.1002/0470846542.ch15
Keywords: total cholesterol, bone mineral density, osteoporosis, NHANES, cross-sectional study
Citation: Fang W, Peng P, Xiao F, He W, Wei Q and He M (2022) A negative association between total cholesterol and bone mineral density in US adult women. Front. Nutr. 9:937352. doi: 10.3389/fnut.2022.937352
Received: 10 May 2022; Accepted: 15 September 2022;
Published: 30 September 2022.
Edited by:
Christelle Guillet, University of Auvergne, FranceReviewed by:
Sook Yee Lim, UCSI University, MalaysiaCopyright © 2022 Fang, Peng, Xiao, He, Wei and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiushi Wei, d2VpcWl1c2hpMTk2N0BnenVjbS5lZHUuY24=; Mincong He, bWluLWNvbmcuaGVAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.