- 1Department of Nursing, Fourth Military Medical University, Xi’an, China
- 2School of Nursing, Yan’an University, Yan’an, China
- 3School of Nursing, Fujian University of Traditional Chinese Medicine, Fuzhou, China
Background: Stimulating food is emerging as an important modifiable factor in the development of gastrointestinal (GI) tract cancers, but the association between chili pepper consumption and the risk of GI cancers is unclear. We aimed to evaluate the direction and magnitude of the association between chili pepper consumption and the risk of GI cancers.
Methods: A literature search was performed in PubMed, Embase, and Web of Science databases from inception to 22 December 2021. Observational studies reporting the association between chili pepper consumption and the risk of gastric cancer (GC), esophageal cancer (EC), and/or colorectal cancer (CRC) in adults were eligible for inclusion. Data extraction and quality assessment were conducted independently by two reviewers for the included literature. Summary odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a random-effects model. Subgroup analyses were also performed based on the cancer type, study design, region of the study, study quality, and adjustments.
Results: A total of 11,421 studies were screened, and 14 case-control studies were included involving 5009 GI cancers among 11,310 participants. The summary OR showed that high consumption of chili pepper was positively related to the risk of GI cancers (OR = 1.64; 95% CI: 1.00–2.70). A stronger positive relationship was observed between chili pepper consumption and EC risk (OR = 2.71; 95% CI: 1.54–4.75), but there was no statistically significant association between GC and CRC risk. In analyses stratified by geographical location, a positive association was found between chili pepper consumption and the risk of GI cancers in Asian studies (OR = 2.50; 95% CI: 1.23–5.08), African studies (OR = 1.62; 95% CI: 1.04–2.52), and North American studies (OR = 2.61; 95% CI: 1.34–5.08), but an inverse association was seen in South American studies (OR = 0.50; 95% CI: 0.29–0.87) and European studies (OR = 0.30; 95% CI: 0.15–0.61).
Conclusion: This meta-analysis suggests that chili pepper is a risk factor for certain GI cancers (e.g., EC). Geographical regions influence the risk of GI cancers, especially in Asian, African, and North American populations, which require more attention during dietary guidance.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPERO/], identifier [CRD42022320670].
Introduction
Globally, gastrointestinal (GI) tract cancers are a significant cause of morbidity and mortality, of which the most prevalent are colorectal cancer (CRC), gastric cancer (GC), and esophageal cancer (EC), ranking third, fifth, and eighth in incidence, respectively, but second, fourth, and sixth in mortality in both sexes combined according to GLOBOCAN estimates for 2020 (1). Despite the availability of multiple therapeutic options such as radiation, chemotherapy, curative resection, and immunotherapy, the early signs of GI cancers are generally undetectable and identified at an advanced stage, leaving patients with limited treatment options, and a poor prognosis (2). Therefore, early identification of risk factors for GI cancers is of great significance to public health.
Diet plays a major role in the development of these diseases. Chili pepper is one of the major vegetables and spices consumed around the world (3). Chili peppers are rich in the bioactive component capsaicin (CAP), which has been reported to have diverse biological properties such as anti-obesity, anti-oxidant, and anti-inflammatory effects in vitro and vivo experiments (4–6). Increasing evidence suggests that CAP facilitates the growth and migration of esophageal squamous cell carcinoma (ESCC) and human colon cancer cells (7, 8). Because of the CAP content, the association between chili pepper consumption and the risk of GI cancers is unclear, and it is important to clarify this question from a public health perspective. Previous individual studies, however, have reported the association between chili pepper exposure and the risk of GI cancers, with controversial results. This may be explained by heterogeneity among the studies, which is attributed to differences in the methods of exposure assessment, study area, sample sizes, and adjustments. For instance, Galvan-Portillo et al. (9) conducted a study of 726 subjects in Mexico, adjusted for energy, age, sex, and education, and showed a positive association between chili pepper and GC risk, whereas Munoz et al. (10) included 191 participants in Italy and observed an inverse association between chili pepper and GC risk after adjustment for sex, age, area of residence, and education. This difference can lead to confusion among dietitians and the general public, as well as challenges in translating into dietary advice.
Previous meta-analyses focusing on the association between chili pepper consumption and GC risk have yielded conflicting findings. For example, most studies showed a positive effect on GC risk (11–13), and a meta-analysis performed by Chen et al. (14) reported a null association. However, to date, no systematic review has been published that specifically explored the relationship between GI cancer risk and chili pepper consumption. Additionally, the validity of the above meta-analyses has been questioned due to the inclusion of studies that mixed chili pepper with other foods (12, 13), used kimchi or CAP instead of chili pepper as the interesting exposure (11–13), and extracted effect estimates incorrectly (12, 14), thus an extensive systematic review and meta-analysis is needed to obtain a more accurate estimate.
Hence, we performed a systematic review and meta-analysis to evaluate the association between chili pepper consumption and the risk of GI cancers by combining all available data from eligible studies. When possible, we used meta-analysis to quantify the effects and explore the possible sources of heterogeneity among the studies.
Methods
The study was complemented following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (15). This review was registered at PROSPERO as CRD42022320670.
Search strategy
We searched PubMed, Embase, and Web of Science for studies in humans on the association between chili pepper consumption and the risk of GI cancers from inception until 22 December 2021, using (“spicy” OR “chili” OR “chilli” OR “pepper” OR “capsaicin” OR “paprika”) AND (“malignancy” OR “cancer” OR “carcinoma” OR “tumor” or “neoplasm”) as search terms. Additionally, references to relevant articles and recent reviews were manually searched to identify other eligible articles.
Selection criteria
Studies that satisfied the following criteria were included in this meta-analysis: (1) participants were adults; (2) studies were observational (cohort, case-control, or cross-sectional studies); (3) information was available on the relationship between chili pepper consumption as the exposure of interest and the risk of EC, GC, and/or CRC as the outcome of interest; and (4) studies reported available risk estimates in the form of relative risk (RR), odds ratio (OR), or hazard ratio (HR) with 95% confidence intervals (CIs). When overlapping populations were included in multiple articles, only the most recent or largest population was used to avoid duplications.
Non-English articles were excluded. Studies were excluded if they were reviews, letters, posters, meetings, or conference abstracts. We also excluded studies that included patients with precancerous lesions as an outcome of interest, mixed chili pepper with other foods (e.g., hot pepper-soybean stew) as the exposure of interest, had a sample size of fewer than 20 cases, and were conducted on children or adolescents. Additionally, studies with insufficient data were excluded. All searches were performed independently by two authors (CC and MZ), and inconsistencies were resolved through discussion.
Data extraction
Two investigators (CC and MZ) independently reviewed and performed the data extraction from all the included studies. The extracted characteristics and data were composed of the first author’s last name, publication year, country, study design (hospital case-control, population case-control, or cohort study), number of study populations and cases, mean/median age of participants, male ratio, assessment method of exposure, GI cancer type, risk estimates and corresponding 95% CI, and covariates adjusted in multivariate analysis. For studies reporting several multivariate-adjusted risk estimates, the risk estimates that were maximally adjusted for underlying confounders were the top priority for use. Any discrepancies during the data extraction process were determined through discussion with a third investigator (XZ).
Assessment of study quality
The quality of the included studies was evaluated using a modified version of the Newcastle–Ottawa Quality Assessment Scale (NOS) (16) with a nine-star scoring system. The following items were taken into consideration: selection of the study groups (up to four stars), comparability of the study groups (up to two stars), and confirmation of chili pepper exposure (up to three stars). We considered NOS scores above or equal to the median as high-quality studies (low risk of bias) and those with NOS scores below the median were regarded as low-quality (high risk of bias) (17). The results of study quality were not used as exclusion criteria.
Statistical analysis
The OR and 95% CI were identified as effect sizes to evaluate the association between chili pepper consumption and the risk of GI cancers. We used the maximally adjusted OR reported in the original research when the OR was directly available. Heterogeneity among the included studies was evaluated by I-square (I2) statistic (18). When I2 was greater than 50%, there was significant heterogeneity between studies, so a random-effects model was selected. Otherwise, a fixed-effects model was performed (19). Publication bias was evaluated through a combination of qualitative and quantitative approaches, involving funnel plots and the Egger regression test (20).
A sensitivity analysis was conducted using the leave-one-out method to determine the influence of a single study. Subgroup analyses were also performed to explore whether pooled risk estimates were affected by cancer subgroups (EC, GC, or CRC), study design (population-based or hospital-based case-control study), region of the study (Asian, African, North American, South American, or European studies), study quality (high-quality or low-quality studies), adjustment for alcohol intake (Yes or No), and adjustment for smoking (Yes or No). Statistical analyses were done using Stata 16.0 software (StataCorp LLC, College Station, TX, USA). All P-values were two-sided, with P < 0.05 considered statistically significant.
Result
Literature search
Our search strategy retrieved 11,421 studies from 3 databases, and 3,227 duplicates were excluded. A further 8,194 studies were screened based on titles and abstracts, of which 8,064 articles were excluded because they did not meet the eligibility criteria. The remaining 130 studies were identified for full-text review, and 116 studies were excluded due to 54 being non-chili exposure, 16 being non-GI cancers, 14 studies being editorial, letter, poster, meeting, and conference abstract, the data of 13 studies being unavailable, 11 studies being review, 7 studies being published in a non-English language, and 1 study reporting the same population (21) with one of the included studies in this meta-analysis. In total, 14 studies (9, 10, 22–33) were included in our final analysis, and the flow diagram of the literature search is shown in Figure 1.
Study characteristics
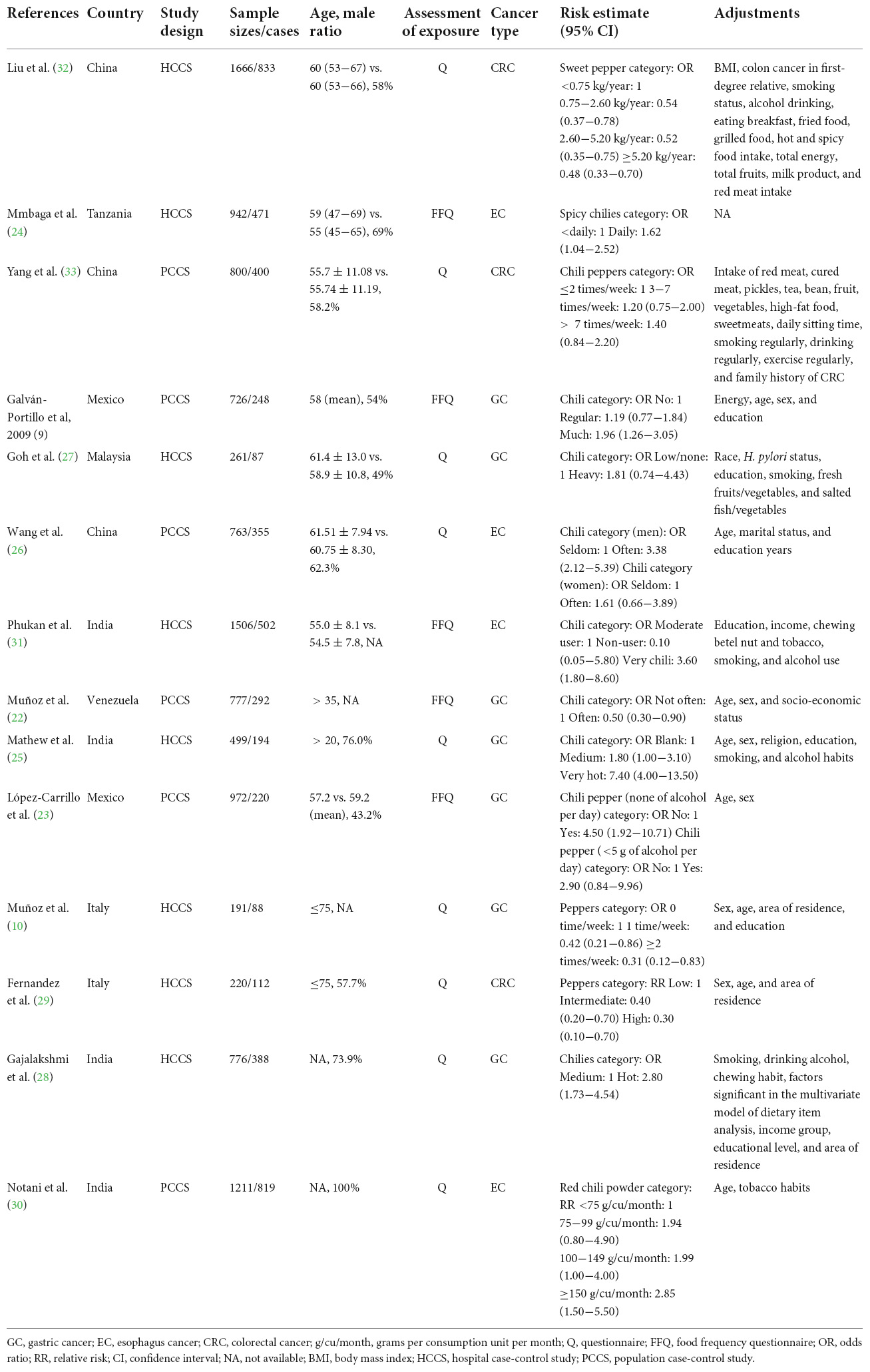
Table 1 summarizes the main characteristics of the included studies. The included studies, which were published between 1987 and 2021, included 14 case-control studies with 5009 GI cancers among 11,310 participants. The number of GI cases enrolled in these articles ranged from 87 to 833, and the number of participants ranged from 191 to 1666. Of the 14 case-control studies, eight studies were conducted in Asia (25–28, 30–33), two in Europe (10, 29), two in North America (9, 23), one in Africa (24), and one in South America (22). As to study design, most of these studies were population-based controls (9, 22, 23, 26, 30, 33), and the remaining six studies used a hospital-based case-control design (10, 24, 25, 27–29, 31, 32). Moreover, seven studies examined the association between chili pepper intake and the risk of GC (9, 10, 22, 23, 25, 27, 28), three on CRC (29, 32, 33), and four on EC (24, 26, 30, 31). In terms of the assessment methods of exposure, five studies (9, 22–24, 31) used the FFQ, while nine studies used frequency-reported questionnaires (10, 25–30, 32, 33). Almost all studies reported OR, except for two studies that reported RR (29, 30). All studies were adjusted or matched for age and sex, with only one study not adjusted for sex because all participants were male (30). Smoking (24, 25, 28, 31–33) and alcohol consumption (24, 25, 27, 28, 30–33) have been controlled in several studies.
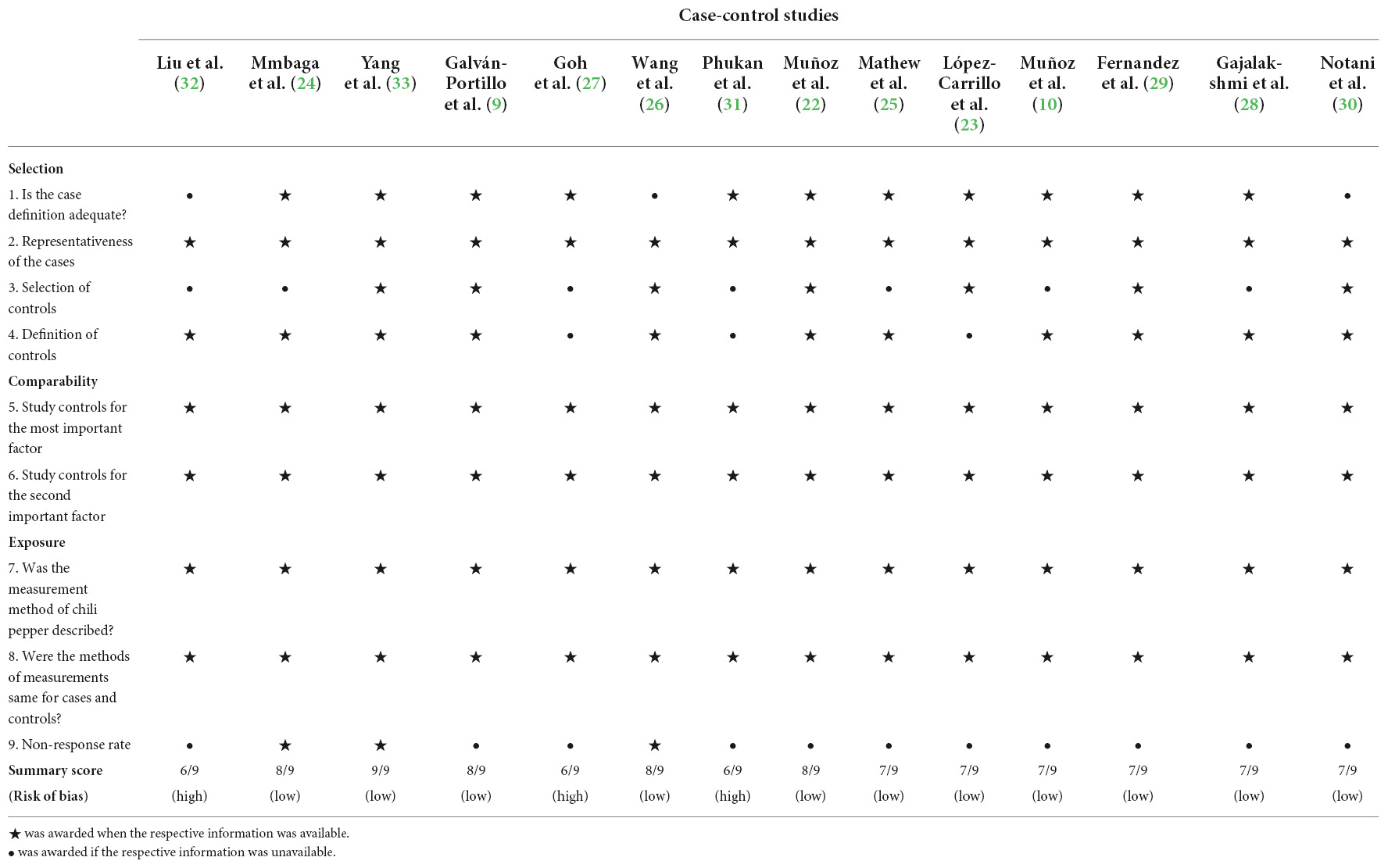
The detailed quality assessment of the included studies by the modified NOS for case-control studies is shown in Table 2. The median NOS score is 7. Eleven studies (9, 10, 21, 22, 24–26, 28–30, 33) with a NOS score of 7 or higher were evaluated as high methodological quality (low risk of bias), and three studies (27, 31, 32) with a score lower than 7 were assessed as low methodological quality (high risk of bias).
Chili pepper consumption and the risk of gastrointestinal cancers
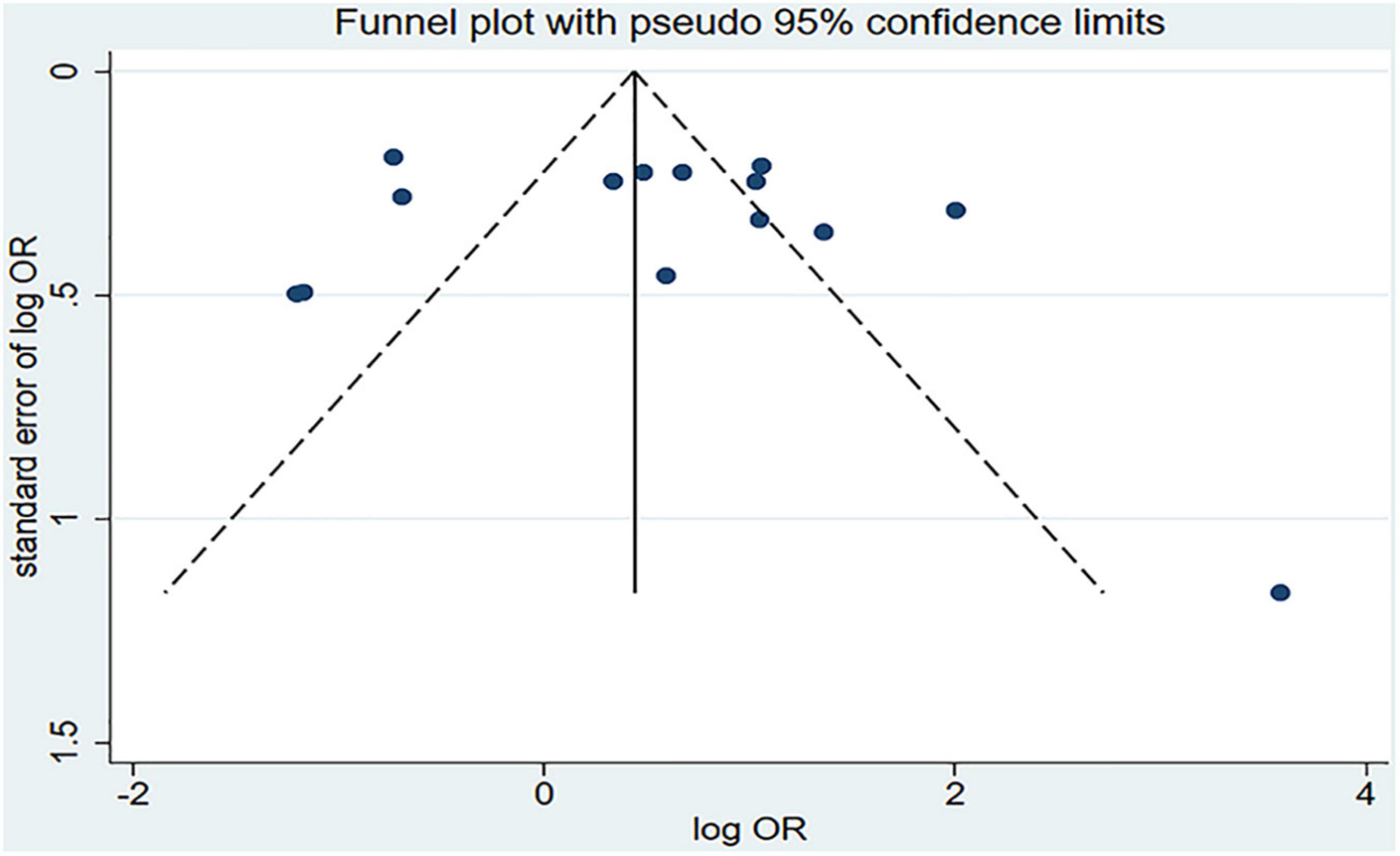
Figure 2 shows the results of the pooled analysis of the eligible studies. The association between chili pepper consumption and the risk of GI cancers was evaluated in 14 studies, consisting of 11,310 participants and 5009 cases. Pooled results showed that the highest category of chili pepper consumption was associated with an increased risk of GI cancers (OR = 1.64; 95% CI: 1.00–2.70), compared with the lowest category (or no intake). Significant heterogeneity existed across the studies (I2 = 90.3%; P < 0.001). No evidence of publication bias was found based on Egger’s test (P = 0.651) and symmetrical funnel plot (Figure 3).
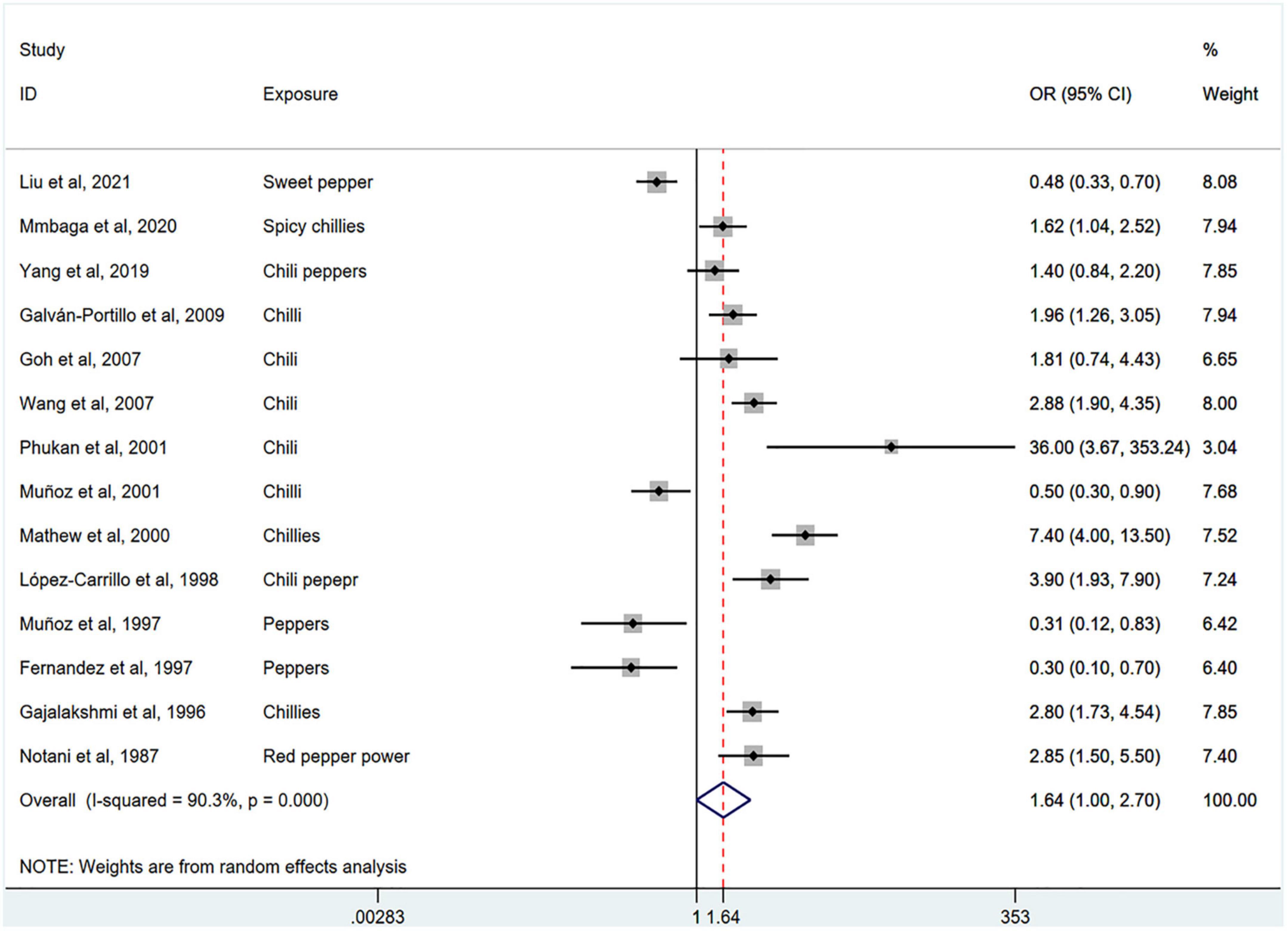
Figure 2. Pooled analysis showing associations between chili pepper consumption and the risk of GI cancers.
Subgroup analysis
Stratification by cancer type showed that higher chili pepper consumption was associated with an elevated risk of EC (OR = 2.71; 95% CI: 1.54–4.75), but not with GC (OR = 1.77; 95% CI: 0.84–3.73) and CRC risk (OR = 0.62; 95% CI: 0.26–1.47) (Figure 4). When stratified by study design (Figure 5), population-based case-control studies showed a positive association between chili pepper consumption and the risk of GI cancers (OR = 1.86; 95% CI: 1.07–3.22), whereas hospital-based case-control studies showed a null association (OR = 1.52; 95% CI: 0.65–3.52). In the subgroup analysis of the region of the study (Figure 6), chili pepper consumption obviously increased the risk of GI cancers in Asian studies (OR = 2.50; 95% CI: 1.23–5.08), North American studies (OR = 2.61; 95% CI: 1.34–5.08), and African studies (OR = 1.62; 95% CI: 1.04–2.52). However, a significantly lower risk of GI cancers was observed in South American studies (OR = 0.50; 95% CI: 0.29–0.87) and European studies (OR = 0.30; 95% CI: 0.15–0.61). We further performed subgroup analysis by study quality (Figure 7) and adjustment factors (Figures 8, 9), finding a significant positive association between the highest chili pepper consumption compared with the lowest and the risk of GI cancers was seen in high-quality studies (OR = 1.65; 95% CI: 1.02–2.69), as well as in studies that adjusted for alcohol intake (OR = 2.29; 95% CI: 1.15–4.57). However, a null association was seen between chili pepper consumption and GI cancer risk in low-quality studies (OR = 2.15; 95% CI: 0.39–11.77), and studies not adjusted for alcohol intake (OR = 1.06; 95% CI: 0.47–2.39). A non-significant association was also seen in studies either adjusted for smoking or not.
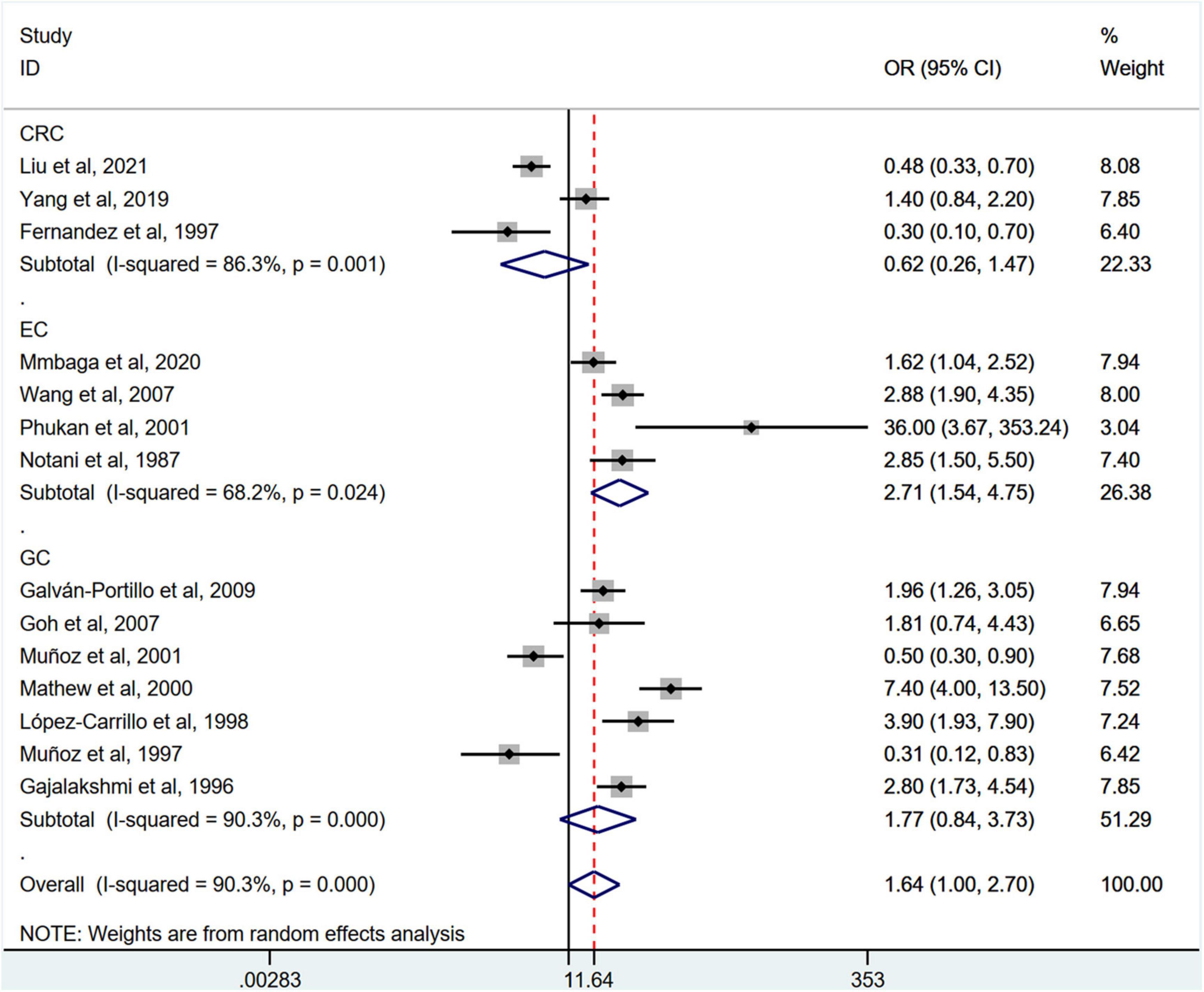
Figure 4. Subgroup analysis showing associations between chili pepper consumption and the risk of GI cancers based on the cancer type. GC, gastric cancer; EC, esophageal cancer; CRC, colorectal cancer; OR, odds ratio; CI, confidence interval.
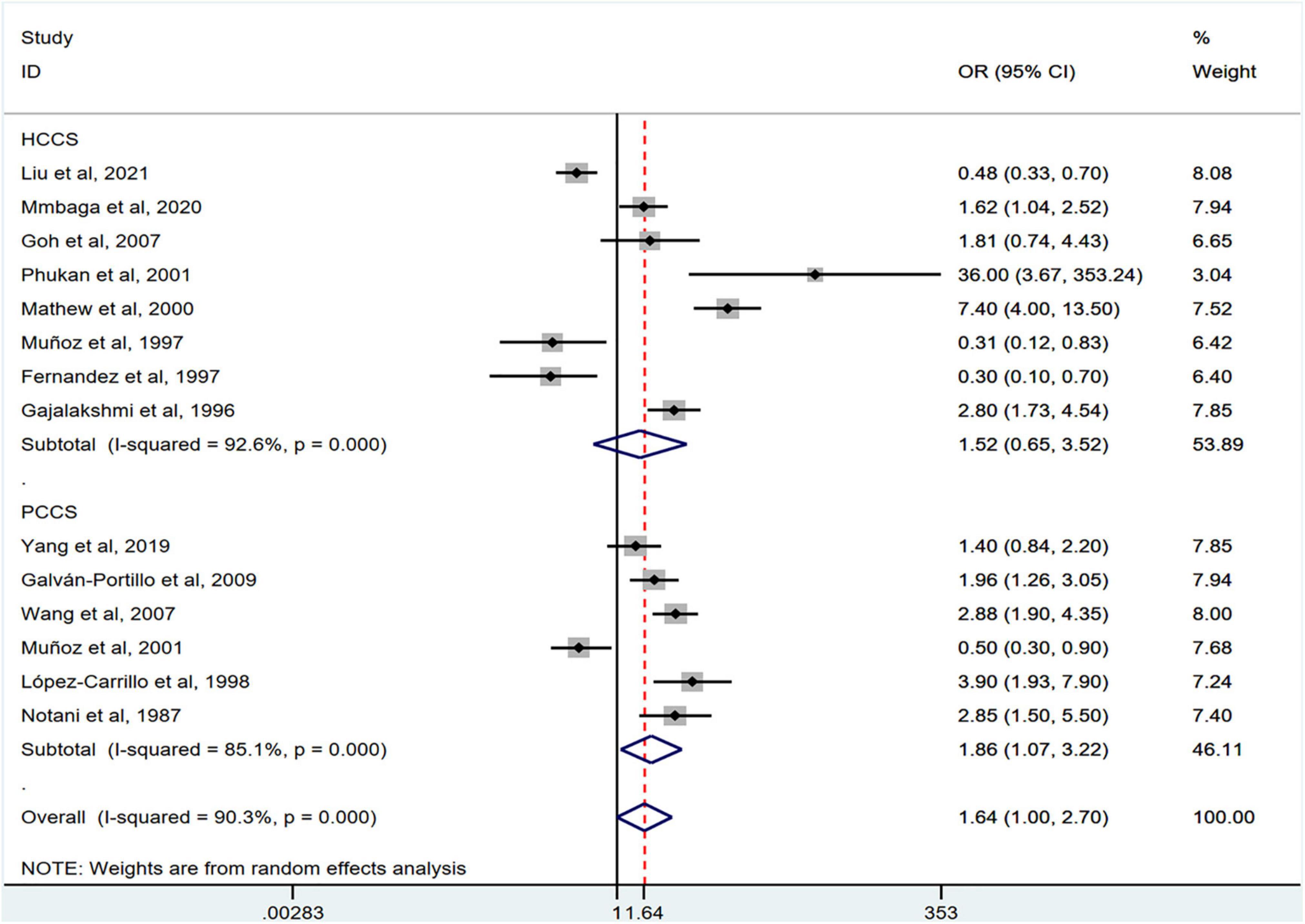
Figure 5. Subgroup analysis showing associations between chili pepper consumption and the risk of GI cancers based on the study design. HCCS, hospital case-control study; PCCS, population case-control study; OR, odds ratio; CI, confidence interval.
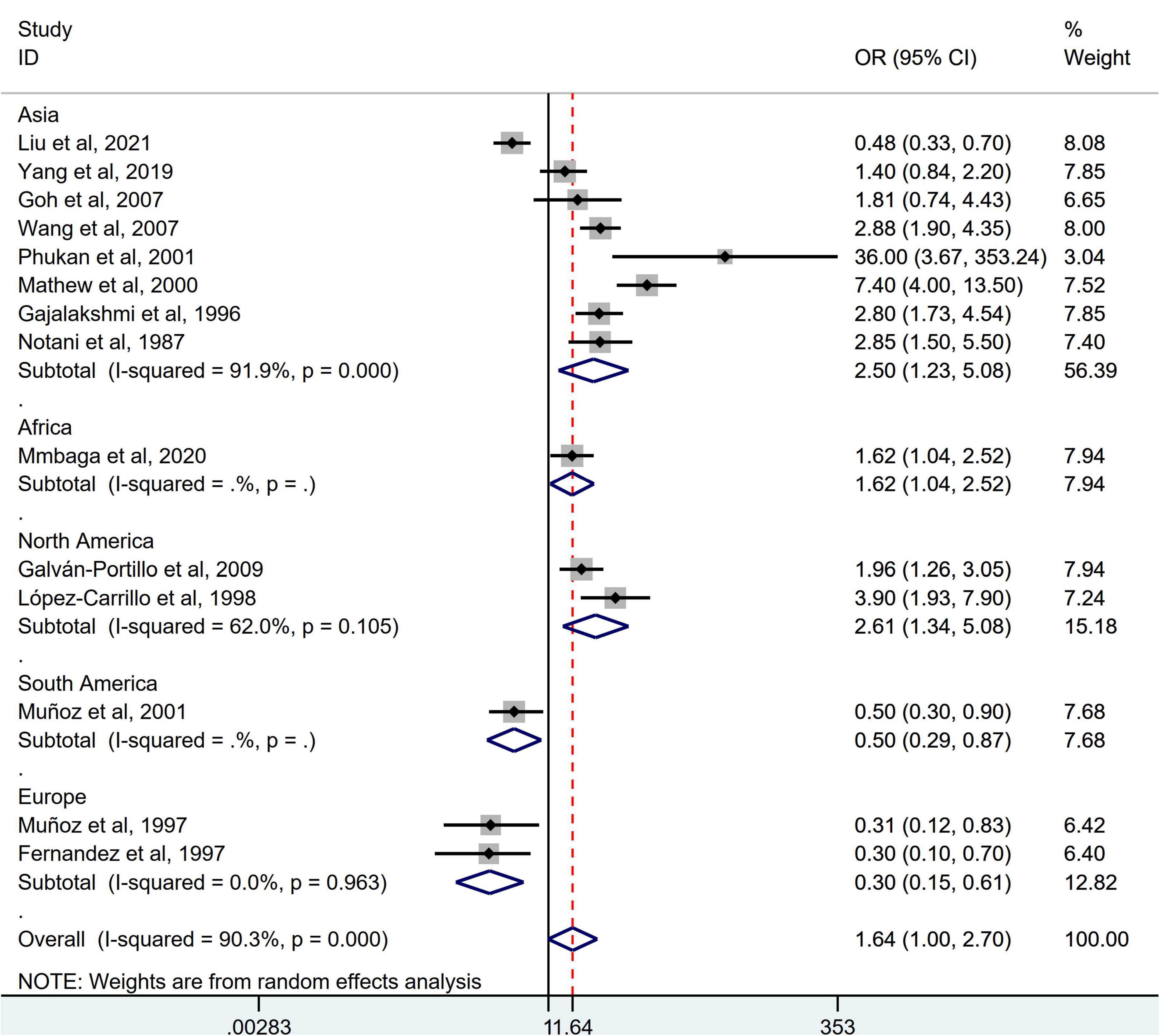
Figure 6. Subgroup analysis showing associations between chili pepper consumption and the risk of GI cancers based on the region of the study. OR, odds ratio; CI, confidence interval.
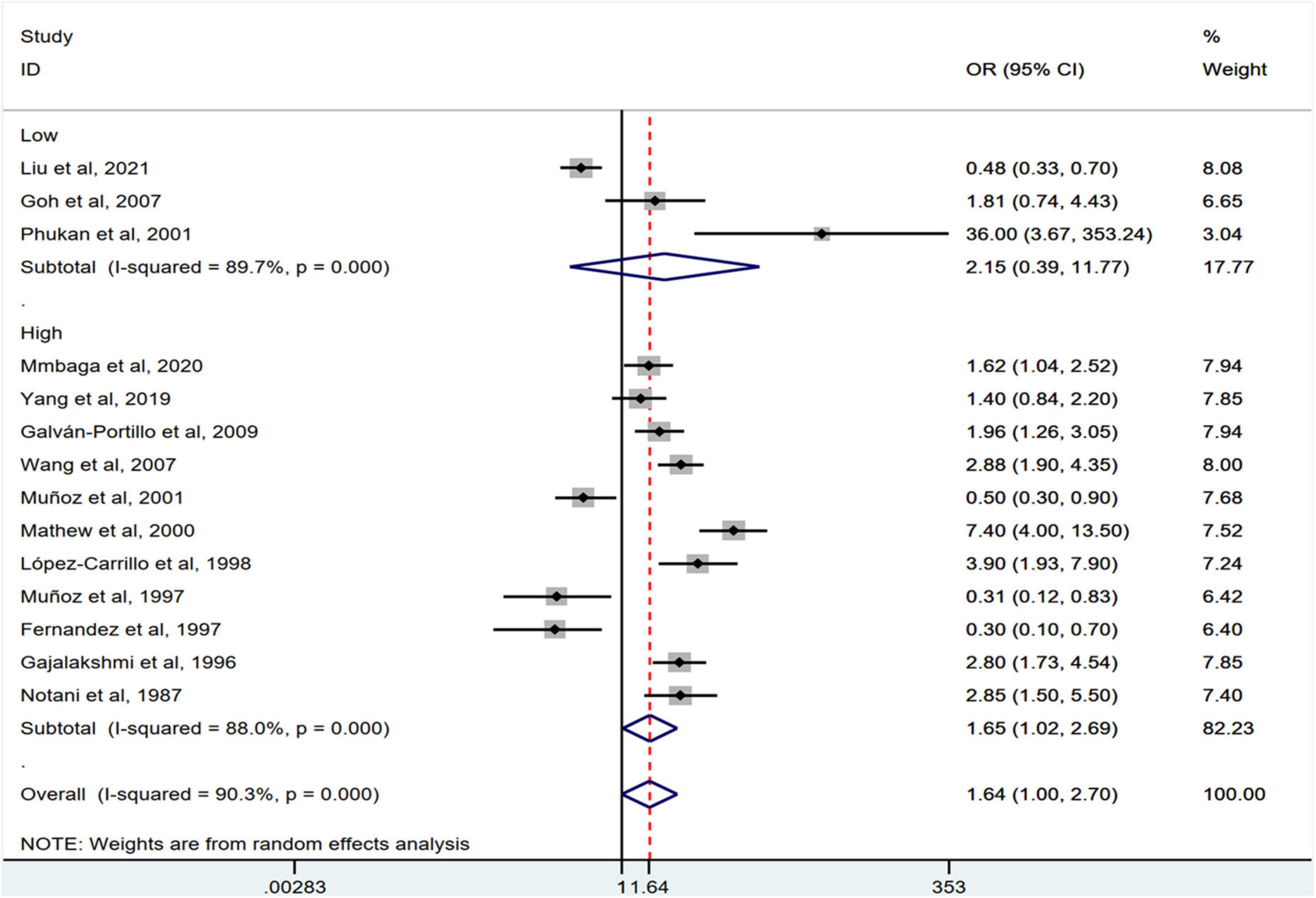
Figure 7. Subgroup analysis showing associations between chili pepper consumption and the risk of GI cancers based on the study quality. OR, odds ratio; CI, confidence interval.
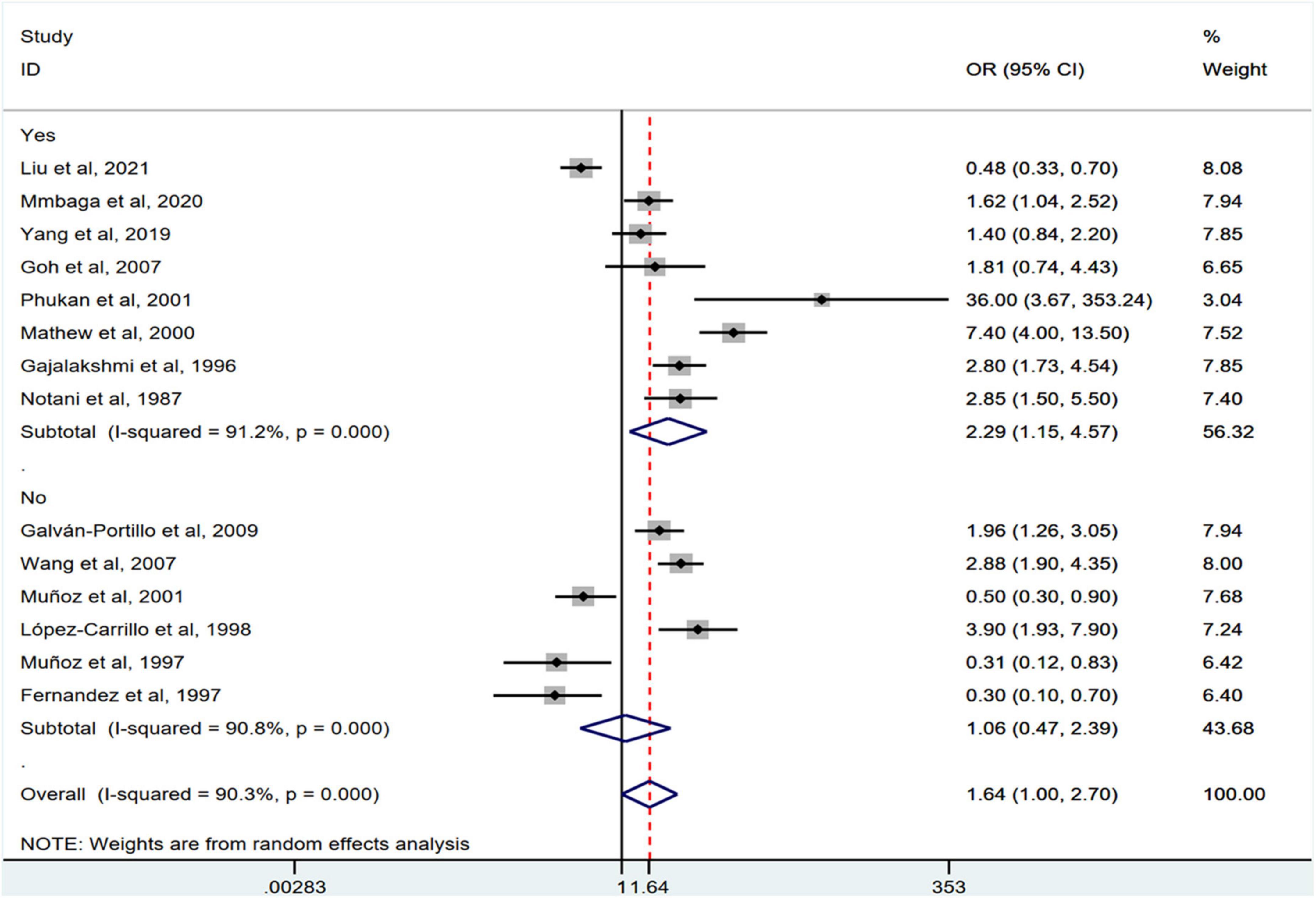
Figure 8. Subgroup analysis showing associations between chili pepper consumption and the risk of GI cancers based on the adjustment for alcohol intake.
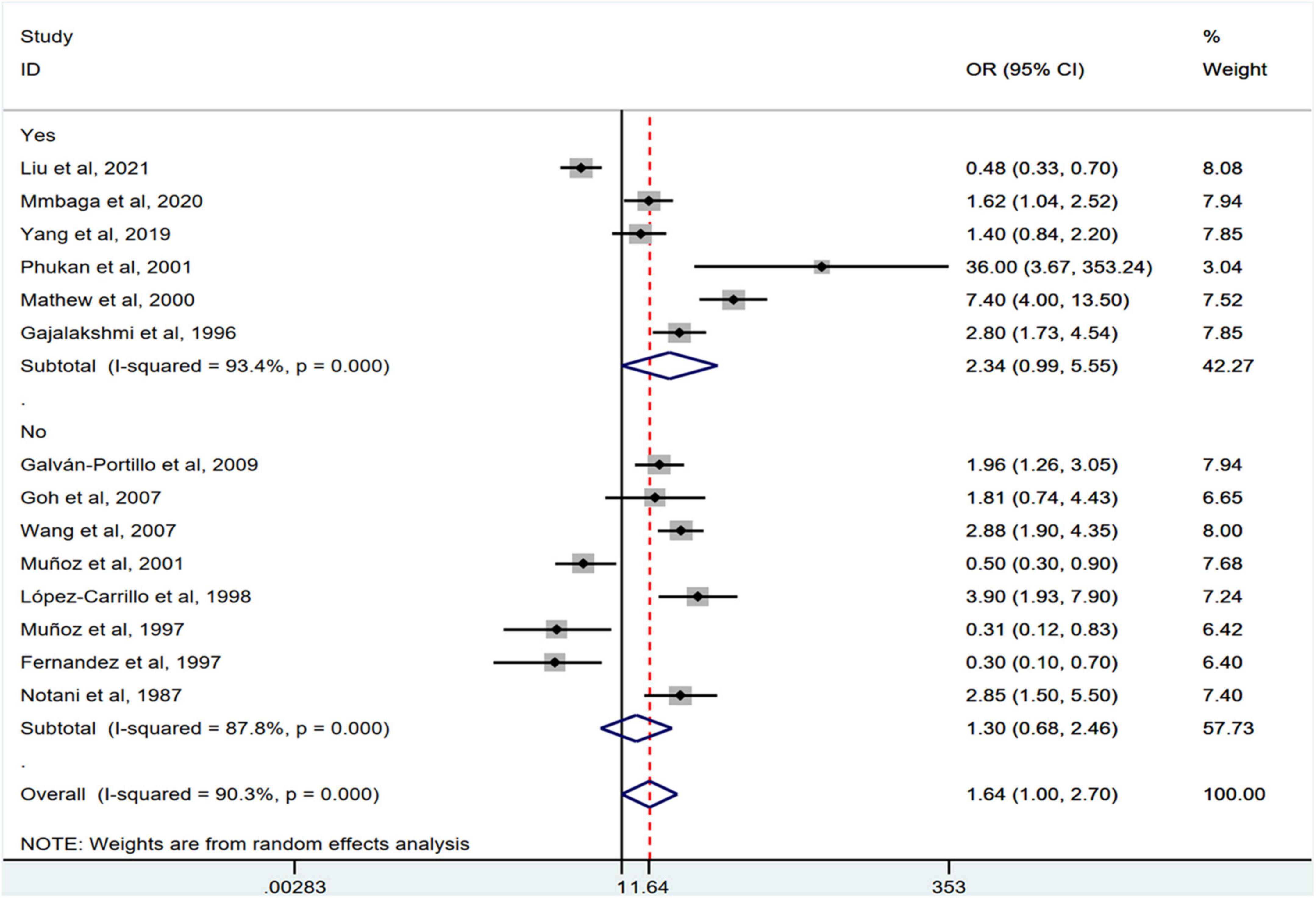
Figure 9. ‘Subgroup analysis showing associations between chili pepper consumption and the risk of GI cancers based on the adjustment for smoking.
Discussion
This systematic review and meta-analysis was designed to evaluate the association between chili pepper consumption and the risk of GI cancers. The evaluation of 14 case-control studies involving 11,310 participants found a positive association between chili pepper consumption and the risk of GI cancers. In the subgroup analysis, this correlation between high chili pepper consumption and rising GI cancer risk was applied to EC, but not to GC and CRC. Especially in Asia, Africa, and North America, chili pepper intake showed a significant positive correlation with GI cancer risk.
In this study, the intake of chili pepper was positively associated with EC risk. The same finding was also observed for the consumption of chili pepper and GI cancer risk. Given that EC is a part of all GI cancers, the observed increased association with GI cancers appears to be related to EC. Several mechanisms could explain why higher chili pepper consumption was significantly associated with an increased risk of EC, but not with GC and CRC. The different effects could be due to differences in cancer sites. Chili peppers are rich in CAP, which has an intensely pungent flavor, further leading to a sensation of tingling and burning pain by stimulating transient receptor potential vanilloid 1 (TRPV1) (34–36). The stomach and intestine share a common endodermal origin and their epithelium is renewed more rapidly than that of the esophagus (37). Therefore, the stomach and colorectum are less affected than the esophagus. In addition, the differences are associated with different signaling pathways. Studies have shown that oral intake of CAP increases NF-κB expression (38). The methyldiazonium ion is the ultimate dimethylhydrazine (DMH) oncogenic metabolite, which is responsible for the methylation of DNA bases, leading to increased proliferation of colonic epithelial cells and triggering NF-κB activation (39, 40). NF-κB can exert numerous pro-tumorigenic functions, such as stimulating cell growth and inducing cell proliferation (41). Conversely, CAP also induces the expression of NF-κB inhibitors, of which the downregulation of Smad4 plays a role in the suppression of cell growth and invasion (42). This may explain why chili pepper is not associated with GC and CRC. For EC, several studies have demonstrated the carcinogenic effects of CAP on EC. For example, Huang et al. showed that thermo-TRPVs are functionally expressed in Eca109 and TE-1 ESCC cell lines. Hyperactivation of TRPV1 and TRPV4 facilitates the growth and/or migration of ESCC (8).
Several meta-analyses have investigated the relationship between the frequency of chili pepper consumption and GI cancer risk, with controversial results. When comparing the highest with lowest categories, most meta-analyses revealed a positive association between chili pepper intake and GC risk (11–13), while Chen et al. (14) showed a null association with the risk of GC. This discrepancy between different meta-analyses may be relevant to the inaccurate inclusion of the original literature. We investigated the eligibility of the studies included in previous meta-analyses, the results of which are summarized in Table 3. The aforementioned meta-analyses included studies analyzing CAP or kimchi instead of chili pepper as exposure (11–13), chili pepper mixed with other foods as exposure (12, 13), incorrect extraction of risk estimates (12, 14), and precancerous lesions rather than GI cancers as interesting outcomes (12), which may be considered as a limitation. Moreover, in a previous meta-analysis (13), the researchers inappropriately substituted continuous variables for categorical variables (highest vs. lowest) to calculate the effect estimates. To address these limitations, we performed a systematic review and meta-analysis by solely including studies that specifically reported chili pepper consumption as the exposure and GI cancers as the outcome.
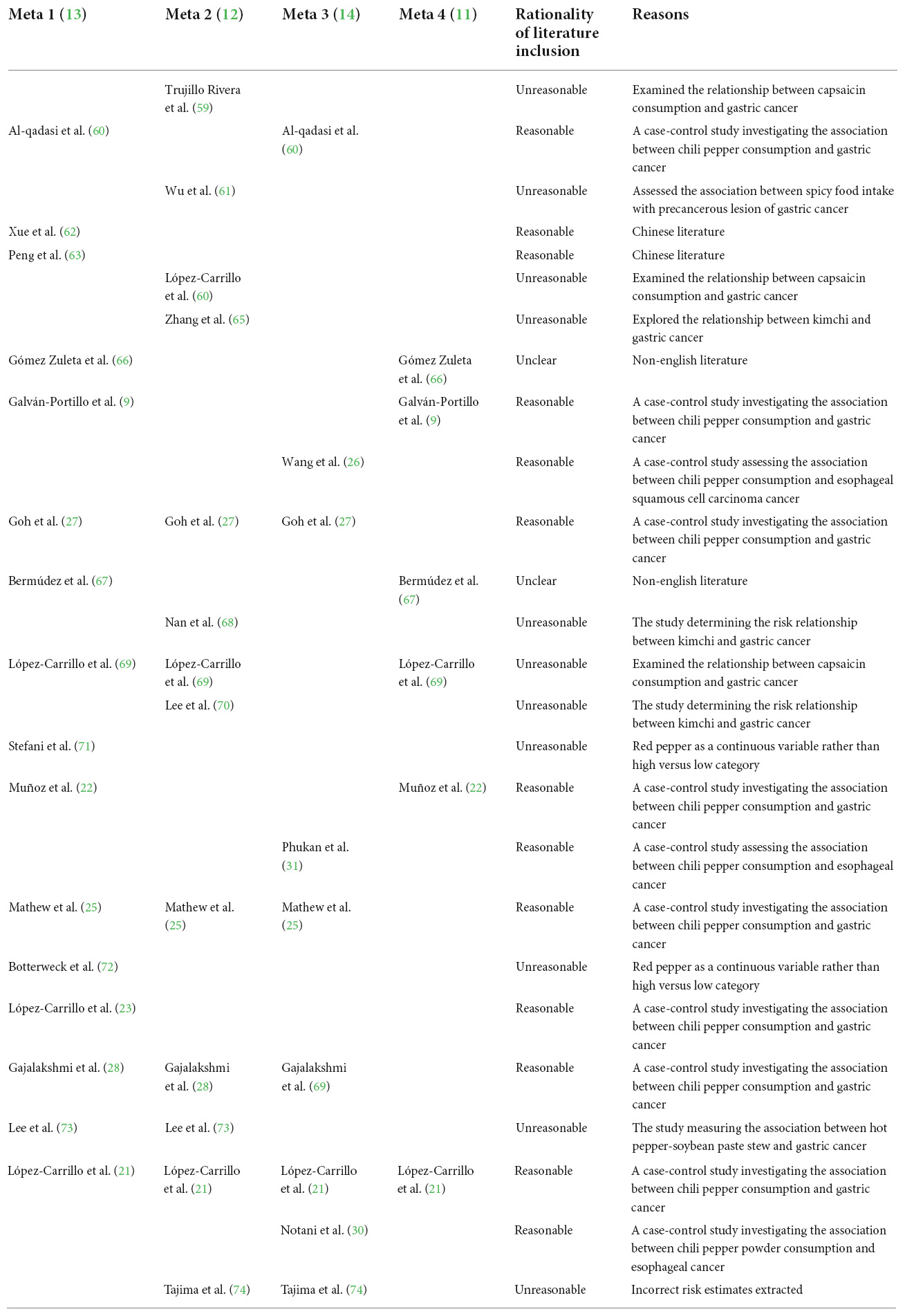
Table 3. Eligibility survey of the original included literature on the relationship between chili pepper consumption and GI cancers.
When stratified by the region of the studies, those studies conducted in Asia, North America, and Africa indicated that participants consuming the highest category of chili pepper had a greater risk of GI cancers, whereas three studies conducted in South America and Europe reported a significantly lower risk of GI cancers (10, 22, 29). One possible reason is that the number of included studies was relatively small, although an extensive search was done. Most original studies were conducted in Asian countries (25–28, 30–33), with only two in Europe (10, 29), two in North America (9, 23), one in Africa (24), and one in South America (22). The results should be cautiously interpreted. Moreover, most original studies reporting a lower risk of GI cancers were conducted in Europe. The evidence has shown that the estimated daily mean CAP intake in Europe was approximately 1.5 mg, which was less than the consumption level in Asia (e.g., Thailand) and North America (e.g., Mexico) (25–200 mg/person/day CAP) (43, 44). Therefore, the results of the highest than lowest or no chili pepper intake in studies conducted in Europe were more likely to obtain a protective effect, whereas the opposite effect was found in studies from Asia, North America, etc. Carcinogenicity or anticancer differences in chili pepper may depend on the dose. Further confirmation is needed to determine whether there is a U-shaped curve relationship, suggesting that a low dose of chili pepper intake might reduce GI cancer risk while a high dose intake might not.
In addition, subgroup analysis revealed that studies adjusted for alcohol consumption in the final model examining chili pepper intake and the risk of GI cancers had a positive association. Meanwhile, a seemingly stronger association between chili pepper consumption and the risk of GI cancers was observed in studies with adjustment for smoking than in those without such adjustment. The small number of original studies focusing on adjustment for smoking (n = 8) or alcohol consumption (n = 6) may be a possible reason. Another explanation is that numerous studies have found alcohol consumption or smoking to be related to a higher risk of GI cancers (45–48). However, there is currently no consensus on whether GI cancer risk is strongly associated with alcohol and smoking, because the evidence for heterogeneity by sex, age, cancer site, age at initiation, clinical stage of cancer, cancer grade, alcohol or smoking intensity, and duration is mixed (49–52). The mechanisms underlying the effects of alcohol consumption and smoking on GI cancers have not been comprehensively elucidated. These factors may affect the accuracy of the analysis. Regarding the study design, a significant association was found in the population-based case-control studies between chili pepper consumption and the risk of GI cancers but not in hospital-based case-control studies. The lack of representativeness may account for this difference.
Although a series of prespecified subgroup analyses were conducted, some heterogeneity generally persisted and could not be reduced. There were some other reasons for the heterogeneity among the included studies. First, the types of chili peppers consumed in different regions may have contributed to the heterogeneity among the results. However, few of the included studies reported specific types of chili peppers, except for two studies, in which the types of chili peppers were reported as sweet pepper (32) and red pepper powder (30), respectively. Second, stratified analysis of Helicobacter pylori (H. pylori) infection status was limited as data on H. pylori infection were only provided in one original study (27). H. pylori infection is a major risk factor for GI cancers. Experimental studies have suggested that combined H. pylori infection and CAP contribute to gastric inflammation and lead to GC with 50% incidence by regulating the expression of interleukin-6 (IL-6) and IFN-γ (53). On the other hand, chili pepper consumption may affect the H. pylori infection rate (54). Thus, H. pylori infection may act as a mediator and confound the association between chili pepper consumption and cancer risk.
Certain limitations of this study should be acknowledged. First, given the observational nature of the included studies, it is possible that the associations we found reflected residual confounding. Although a large number of potential confounders, such as cancer type, study design, and region of the study, were adjusted for in most studies, we cannot exclude that some other dietary biologically active components may partly or wholly affect the association. Second, recall bias associated with the assessment methods of chili pepper exposure should be considered because FFQ or frequency-reported questionnaires are subject to measurement errors, which can attenuate or overestimate the observed association (55). Additional limitations related to different cooking and processing methods for chili pepper. Several studies have examined various cooking methods (roasting, boiling, steaming, and stir-frying), cooking time, and temperature of chili pepper affect their phytonutrient content (56–58). However, the studies we included did not investigate the effect of chili pepper preparation methods, which prevented us from further exploring the sources of heterogeneity. Third, the dose–response analysis could not be conducted due to the insufficient number of available studies. Finally, only studies published in English were included, which may lead to the exclusion of related studies in other languages.
Conclusion
Our results suggest that chili pepper consumption is associated with an increased risk of certain GI cancers. An increased EC risk was observed when high levels of chili pepper were ingested. However, no significant association was found between chili pepper consumption and the risk of GC and CRC. More prospective cohort studies are necessary to clarify the dose-response effect of chili pepper on the risk of GI cancers.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CC and MZ designed the study, performed the literature search, extracted data, conducted the statistical analysis, and drafted the manuscript. CC, XZ, and HL performed data screening, interpreted the data, and performed the writing review of the manuscript. All authors contributed to the articles and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.935865/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
3. Jarret RL, Barboza GE, Da Costa Batista FR, Berke T, Chou Y, Hulse-Kemp A, et al. Capsicum—an abbreviated compendium. J Am Soc Hortic Sci. (2019) 144:3–22.
4. Srinivasan K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr. (2016) 56:1488–500. doi: 10.1080/10408398.2013.772090
5. Li R, Lan Y, Chen C, Cao Y, Huang Q, Ho CT, et al. Anti-obesity effects of capsaicin and the underlying mechanisms: a review. Food Funct. (2020) 11:7356–70. doi: 10.1039/d0fo01467b
6. Ghiasi Z, Esmaeli F, Aghajani M, Ghazi-Khansari M, Faramarzi MA, Amani A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: an in vivo study. Int J Pharm. (2019) 559:341–7. doi: 10.1016/j.ijpharm.2019.01.043
7. Liu NC, Hsieh PF, Hsieh MK, Zeng ZM, Cheng HL, Liao JW, et al. Capsaicin-mediated tNOX (ENOX2) up-regulation enhances cell proliferation and migration in vitro and in vivo. J Agric Food Chem. (2012) 60:2758–65. doi: 10.1021/jf204869w
8. Huang R, Wang F, Yang Y, Ma W, Lin Z, Cheng N, et al. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. Febs Open Bio. (2019) 9:206–25. doi: 10.1002/2211-5463.12570
9. Galván-Portillo MV, Cantoral A, Oñate-Ocaña LF, Chen J, Herrera-Goepfert R, Torres-Sanchez L, et al. Gastric cancer in relation to the intake of nutrients involved in one-carbon metabolism among MTHFR 677 TT carriers. Eur J Nutr. (2009) 48:269–76. doi: 10.1007/s00394-009-0010-5
10. Muñoz SE, Ferraroni M, La Vecchia C, Decarli A. Gastric cancer risk factors in subjects with family history. Cancer Epidemiol Biomarkers Prev. (1997) 6:137–40.
11. Bonequi P, Meneses-González F, Correa P, Rabkin CS, Camargo MC. Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control. (2013) 24:217–31. doi: 10.1007/s10552-012-0110-z
12. Du Y, Lv Y, Zha W, Hong X, Luo Q. Chili consumption and risk of gastric cancer: a meta-analysis. Nutr Cancer. (2021) 73:45–54. doi: 10.1080/01635581.2020.1733625
13. Luo L, Yan J, Wang X, Sun Z. The correlation between chili pepper consumption and gastric cancer risk: a meta-analysis. Asia Pac J Clin Nutr. (2021) 30:130–9. doi: 10.6133/apjcn.202103_30(1).0016
14. Chen YH, Zou XN, Zheng TZ, Zhou Q, Qiu H, Chen YL, et al. High spicy food intake and risk of cancer: a meta-analysis of case-control studies. Chin Med J. (2017) 130:2241–50. doi: 10.4103/0366-6999.213968
15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
16. Islam MA, Alam F, Wong KK. Comorbid association of antiphospholipid antibodies and migraine: a systematic review and meta-analysis. Autoimmun Rev. (2017) 16:512–22. doi: 10.1016/j.autrev.2017.03.005
17. Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev. (2016) 15:22–37. doi: 10.1016/j.autrev.2015.10.002
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
20. Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
21. López-Carrillo L, Hernández AM, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol. (1994) 139:263–71. doi: 10.1093/oxfordjournals.aje.a116993
22. Muñoz N, Plummer M, Vivas J, Moreno V, De Sanjosé S, Lopez G, et al. A case-control study of gastric cancer in Venezuela. Int J Cancer. (2001) 93:417–23. doi: 10.1002/ijc.1333
23. López-Carrillo L, López-Cervantes M, Ramírez-Espitia A, Rueda C, Fernández-Ortega C, Orozco-Rivadeneyra S. Alcohol consumption and gastric cancer in Mexico. Cad Saude Publica. (1998) 14(Suppl 3.):25–32. doi: 10.1590/s0102-311x1998000700004
24. Mmbaga EJ, Mushi BP, Deardorff K, Mgisha W, Akoko LO, Paciorek A, et al. A Case-Control study to evaluate environmental and lifestyle risk factors for esophageal cancer in tanzania. Cancer Epidemiol Biomark Prev. (2021) 30:305–16. doi: 10.1158/1055-9965.EPI-20-0660
25. Mathew A, Gangadharan P, Varghese C, Nair MK. Diet and stomach cancer: a case-control study in South India. Eur J Cancer Prev. (2000) 9:89–97. doi: 10.1097/00008469-200004000-00004
26. Wang JM, Xu B, Rao JY, Shen HB, Xue HC, Jiang QW. Diet habits, alcohol drinking, tobacco smoking, green tea drinking, and the risk of esophageal squamous cell carcinoma in the Chinese population. Eur J Gastroenterol Hepatol. (2007) 19:171–6. doi: 10.1097/MEG.0b013e32800ff77a
27. Goh KL, Cheah PL, Md N, Quek KF, Parasakthi N. Ethnicity and H. Pylori as risk factors for gastric cancer in Malaysia: a prospective case control study. Am J Gastroenterol. (2007) 102:40–5. doi: 10.1111/j.1572-0241.2006.00885.x
28. Gajalakshmi CK, Shanta V. Lifestyle and risk of stomach cancer: a hospital-based case-control study. Int J Epidemiol. (1996) 25:1146–53. doi: 10.1093/ije/25.6.1146
29. Fernandez E, La Vecchia C, D’Avanzo B, Negri E, Franceschi S. Risk factors for colorectal cancer in subjects with family history of the disease. Br J Cancer. (1997) 75:1381–4. doi: 10.1038/bjc.1997.234
30. Notani PN, Jayant K. Role of diet in upper aerodigestive tract cancers. Nutr Cancer. (1987) 10:103–13. doi: 10.1080/01635588709513945
31. Phukan RK, Chetia CK, Ali MS, Mahanta J. Role of dietary habits in the development of esophageal cancer in Assam, the north-eastern region of India. Nutr Cancer. (2001) 39:204–9. doi: 10.1207/S15327914nc392_7
32. Liu Y, Li S, Jiang L, Zhang Y, Li Z, Shi J. Solanaceous vegetables and colorectal cancer risk: a hospital-based matched case-control study in Northeast China. Front Nutr. (2021) 8:688897. doi: 10.3389/fnut.2021.688897
33. Yang Y, Zhang J, Weiss NS, Guo L, Zhang L, Jiang Y, et al. The consumption of chili peppers and the risk of colorectal cancer: a matched case-control study. World J Surg Oncol. (2019) 17:71–7. doi: 10.1186/s12957-019-1615-7
34. Lu M, Cao Y, Ho CT, Huang Q. Development of organogel-derived capsaicin nanoemulsion with improved bioaccessibility and reduced gastric mucosa irritation. J Agric Food Chem. (2016) 64:4735–41. doi: 10.1021/acs.jafc.6b01095
35. Lu M, Cao Y, Ho CT, Huang Q. The enhanced anti-obesity effect and reduced gastric mucosa irritation of capsaicin-loaded nanoemulsions. Food Funct. (2017) 8:1803–9. doi: 10.1039/c7fo00173h
36. Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. (2002) 108:421–30. doi: 10.1016/s0092-8674(02)00637-2
37. Wang L, Moore DC, Huang J, Wang Y, Zhao H, D-H YJ, et al. SHP2 regulates the development of intestinal epithelium by modifying OSTERIX(+) crypt stem cell self-renewal and proliferation. Faseb J. (2021) 35:e21106. doi: 10.1096/fj.202001091R
38. Yang MH, Jung SH, Sethi G, Ahn KS. Pleiotropic pharmacological actions of capsazepine, a synthetic analogue of capsaicin, against various cancers and inflammatory diseases. Molecules. (2019) 24:995. doi: 10.3390/molecules24050995
39. Perše M, Cerar A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J Biomed Biotechnol. (2011) 2011:473964. doi: 10.1155/2011/473964
40. Tanwar L, Vaish V, Sanyal SN. Chemoprevention of 1,2-dimethylhydrazine-induced colon carcinogenesis by a non-steroidal anti-inflammatory drug, etoricoxib, in rats: inhibition of nuclear factor kappaB. Asian Pac J Cancer Prev. (2009) 10:1141–6.
41. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. (2013) 12:86. doi: 10.1186/1476-4598-12-86
42. Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. (2017) 17:79–92. doi: 10.1038/nrc.2016.126
43. Kwon Y. Estimation of dietary capsaicinoid exposure in Korea and assessment of its health effects. Nutrients. (2021) 13:2461–76. doi: 10.3390/nu13072461
44. Scientific Committee on food. Opinion of the scientific committee on food on capsaicin. (2002). Available online at: https://www.semanticscholar.org/paper/Opinion-of-the-Scientific-Committee-on-Food-on/ae75389416978d3a657779a9a9279932bfb2b4d1 (accessed February 26, 2002).
45. Deng W, Jin L, Zhuo H, Vasiliou V, Zhang Y. Alcohol consumption and risk of stomach cancer: a meta-analysis. Chem Biol Interact. (2021) 336:109365–73. doi: 10.1016/j.cbi.2021.109365
46. Zhou X, Wang L, Xiao J, Sun J, Yu L, Zhang H, et al. Alcohol consumption, DNA methylation and colorectal cancer risk: results from pooled cohort studies and mendelian randomization analysis. Int J Cancer. (2022) 151:33945–56. doi: 10.1002/ijc.33945
47. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American college of gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. (2009) 104:739–50. doi: 10.1038/ajg.2009.104
48. Oze I, Matsuo K, Ito H, Wakai K, Nagata C, Mizoue T, et al. Cigarette smoking and esophageal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. (2012) 42:63–73. doi: 10.1093/jjco/hyr170
49. Huang YM, Wei PL, Ho CH, Yeh CC. Cigarette smoking associated with colorectal cancer survival: a nationwide, population-based cohort study. J Clin Med. (2022) 11:913–25. doi: 10.3390/jcm11040913
50. Yaegashi Y, Onoda T, Morioka S, Hashimoto T, Takeshita T, Sakata K, et al. Joint effects of smoking and alcohol drinking on esophageal cancer mortality in Japanese men: findings from the Japan collaborative cohort study. Asian Pac J Cancer Prev. (2014) 15:1023–9. doi: 10.7314/apjcp.2014.15.2.1023
51. Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. (2011) 22:1958–72. doi: 10.1093/annonc/mdq653
52. Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. (2004) 140:603–13. doi: 10.7326/0003-4819-140-8-200404200-00007
53. Aziz F, Xin M, Gao Y, Chakroborty A, Khan I, Monts J, et al. Induction and prevention of gastric cancer with combined helicobacter pylori and capsaicin administration and DFMO treatment, respectively. Cancers. (2020) 12:816–34. doi: 10.3390/cancers12040816
54. Monno R, De Laurentiis V, Trerotoli P, Roselli AM, Ierardi E, Portincasa P. Helicobacter pylori infection: association with dietary habits and socioeconomic conditions. Clin Res Hepatol Gastroenterol. (2019) 43:603–7. doi: 10.1016/j.clinre.2018.10.002
55. Archer E, Marlow ML, Lavie CJ. Controversy and debate: memory-based methods paper 1: the fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J Clin Epidemiol. (2018) 104:113–24. doi: 10.1016/j.jclinepi.2018.08.003
56. Hamed M, Kalita D, Bartolo ME, Jayanty SS. Capsaicinoids, polyphenols and antioxidant activities of capsicum annuum: comparative study of the effect of ripening stage and cooking methods. Antioxidants. (2019) 8:364–82. doi: 10.3390/antiox8090364
57. Hwang IG, Shin YJ, Lee S, Lee J, Yoo SM. Effects of different cooking methods on the antioxidant properties of red pepper (Capsicum annuum L.). Prev Nutr Food Sci. (2012) 17:286–92. doi: 10.3746/pnf.2012.17.4.286
58. Chuah AM, Lee Y, Yamaguchi T, Takamura H, Yin L, Matoba T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. (2008) 111:20–8. doi: 10.1016/j.foodchem.2008.03.022
59. Trujillo Rivera A, Sampieri CL, Morales RJ, Montero H, Acosta MH, Cruz RN, et al. Risk factors associated with gastric cancer in Mexico: education, breakfast and chili. Rev Esp Enferm Dig. (2018) 110:372–9. doi: 10.17235/reed.2018.5042/2017
60. Al-Qadasi FA, Shah SA, Ghazi HF. Tobacco chewing and risk of gastric cancer: a case-control study in Yemen. East Mediterr Health J. (2017) 22:719–26. doi: 10.26719/2016.22.10.719
61. Wu Y, Fan Y, Jiang Y, Wang Y, Liu H, Wei M. Analysis of risk factors associated with precancerous lesion of gastric cancer in patients from Eastern China: a comparative study. J Cancer Res Ther. (2013) 9:205–9. doi: 10.4103/0973-1482.113351
62. Xue GP, Pan XF, Li SG, Zhao Y, Chang H, Zhao ZM, et al. Association between lifestyle factors and behaviors and risk of gastric cancer, Sichuan province. Modern Prevent Med. (2015) 42:1257–60.
63. Peng H, Huang F, Zhang YY, Yuan H, Zhuang Q. A casecontrol study on risk factors of stomach cancer in Jiading district. Chin J Prev Contr Chron Dis. (2012) 06:668–71. doi: 10.16386/j.cjpccd.issn.1004-6194.2012.06.045
64. López-Carrillo L, Camargo MC, Schneider BG, Sicinschi LA, Hernández-Ramírez RU, Correa P, et al. Capsaicin consumption, helicobacter pylori CagA status and IL1B-31C>T genotypes: a host and environment interaction in gastric cancer. Food Chem Toxicol. (2012) 50:2118–22. doi: 10.1016/j.fct.2012.02.043
65. Zhang YW, Eom SY, Kim YD, Song YJ, Yun HY, Park JS, et al. Effects of dietary factors and the NAT2 acetylator status on gastric cancer in Koreans. Int J Cancer. (2009) 125:139–45. doi: 10.1002/ijc.24328
66. Gómez Zuleta M, Otero-Regino W, Ruiz-Lobo X. Risk factors for gastric cancer in Colombian patients. Revista Colombiana de Gastroenterologia. (2009) 24:134–43.
67. Bermúdez C, Jesús I, Gamarra G. Blood group a and gastric cancer risk in the hospital universitario de santander (Bucaramanga, Colombia). Acta Médica Colombiana. (2006) 31:400–10.
68. Nan HM, Park JW, Song YJ, Yun HY, Park JS, Hyun T, et al. Kimchi and soybean pastes are risk factors of gastric cancer. World J Gastroenterol. (2005) 11:3175–81. doi: 10.3748/wjg.v11.i21.3175
69. López-Carrillo L, López-Cervantes M, Robles-Díaz G, Ramírez-Espitia A, Mohar-Betancourt A, Meneses-García A, et al. Capsaicin consumption, helicobacter pylori positivity and gastric cancer in Mexico. Int J Cancer. (2003) 106:277–82. doi: 10.1002/ijc.11195
70. Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. (2003) 13:162–8. doi: 10.2188/jea.13.162
71. De Stefani E, Correa P, Boffetta P, Ronco A, Brennan P, Deneo-Pellegrini H, et al. Plant foods and risk of gastric cancer: a case-control study in Uruguay. Eur J Cancer Prev. (2001) 10:357–64. doi: 10.1097/00008469-200108000-00009
72. Botterweck AA, van den Brandt PA, Goldbohm RA. A prospective cohort study on vegetable and fruit consumption and stomach cancer risk in the Netherlands. Am J Epidemiol. (1998) 148:842–53. doi: 10.1093/oxfordjournals.aje.a009709
73. Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. (1995) 24:33–41. doi: 10.1093/ije/24.1.33
Keywords: chili pepper, gastrointestinal tract cancer, systematic review, meta-analysis, risk
Citation: Chen C, Zhang M, Zheng X and Lang H (2022) Association between chili pepper consumption and risk of gastrointestinal-tract cancers: A meta-analysis. Front. Nutr. 9:935865. doi: 10.3389/fnut.2022.935865
Received: 04 May 2022; Accepted: 10 October 2022;
Published: 03 November 2022.
Edited by:
Sue K. Park, Seoul National University, South KoreaReviewed by:
Yunshan Wang, Shandong University, ChinaHuanjie Li, Cheeloo College of Medicine, Shandong University, China
Copyright © 2022 Chen, Zhang, Zheng and Lang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjuan Lang, bGFuZ2hqQGZtbXUuZWR1LmNu
 Changchang Chen
Changchang Chen Man Zhang
Man Zhang Xutong Zheng
Xutong Zheng Hongjuan Lang
Hongjuan Lang