
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 22 July 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.933557
This article is part of the Research TopicWomen in Nutritional EpidemiologyView all 10 articles
Objective: The objective of this study was to determine the association between haemoglobin level and PB.
Methods: A cross-sectional study was conducted in Khartoum, Sudan. Questionnaires on demographics and medical and obstetric factors were completed. A logistic regression analysis was performed.
Results: Of the 1,716 pregnant women, approximately two-thirds (65.7%) had anaemia (haemoglobin < 11 g/dl) and six (0.3%) had severe anaemia (haemoglobin < 8 g/dl). Of the 1,716 women, 283 (16.5%) had a PB. In multivariable logistic regression, parity (AOR = 1.15, 95% CI = 1.09–1.21, P < 0.001) was positively associated with PB. Compared to those with haemoglobin levels of 10–10.9 g/dl, pregnant women with haemoglobin levels of 8–8.9 (AOR = 0.41, 95% CI = 0.22–0.77), 9–9.9 (AOR = 0.59, 95% CI = 0.38–0.91), and 11–11.9 g/dl (AOR = 0.53, 95% CI = 0.36–0.77) were at a lower risk of PB. Women with haemoglobin levels of 12–13 g/dl were at a higher risk of PB (AOR = 1.62, 95% CI = 1.06–2.45). There was no significant association between women with haemoglobin levels < 8 g/dl and > 13 g/dl and PB.
Conclusion: This study showed different levels of association between haemoglobin levels and PB.
Preterm birth (PB) refers to the birth of a baby before 37 completed weeks of gestation or 259 days from the final day of the last menstrual period (1). PB is a worldwide health problem that affected 14.84 million births in 2014 (2). More than three-quarters (81.1%) of all PBs occur in Africa and South Asia (2). PB is a major cause of perinatal death and a significant cause of long-term consequences among the survivors (3). PB is the main direct cause of neonatal death and is associated with 50–75% of all neonatal mortality and half of all neonatal morbidity worldwide (4). PB also presents an economic burden, due to the requirement of neonatal intensive care units, and is associated with socioeconomic disadvantages and disruptive life events during pregnancy (5). Recent studies showed a high prevalence of PB in sub-Saharan African countries (6, 7). Several factors such as age, short interpregnancy interval (6, 8), being a rural resident, inadequate antenatal care (9), previous PB, multiple pregnancies, and malaria (6) are significantly associated with PB. It is of paramount importance that the factors associated with PB (especially haemoglobin) in different settings are well documented, if the goal of the WHO and United Nations 2010 of reducing mortality due to PB by 50% before 2025 is to be achieved. Risk factors for PB should be correctly identified and properly managed to reduce the incidence of PB. Maternal nutrition may impact both haemoglobin synthesis and foetal growth, development, survival, and PB (10, 11). Previous studies on the association between the haemoglobin level and PB showed inconsistent results. While some found that anaemia was associated with PB (8, 12), others showed that a high haemoglobin level carries an increased risk of PB (13, 14). Although the prevalence of PB is high in many African countries (6, 7), pertinent information on the association between haemoglobin level and PB and spontaneous PB is not adequately documented in sub-Saharan Africa, including Sudan. This study was conducted to investigate the association between the haemoglobin level and spontaneous PB in Khartoum, Sudan.
A cross-sectional study was conducted at Saad Abuelela Maternity Hospital in Khartoum, Sudan, from February to November 2020. The inclusion criteria were pregnant women with a single live baby. The exclusion criteria were post-term birth (≥ 42 weeks of gestation), unknown gestational age, women with unknown body mass index (BMI) in early pregnancy, seriously ill women, multiple births, stillbirths, and congenital malformed deliveries. After signing an informed consent form, trained medical residents conducted face-to-face interviews with the pregnant women included in the study. Questionnaires on demographics and medical and obstetric factors were filled out in the local language (Arabic). The questionnaires recorded information concerning the age, parity, education, residence, occupation, antenatal care status, history of previous miscarriages/PBs, gestational age, interpregnancy interval, haemoglobin level, and infant’s sex. Gestational age was calculated using a combination of the dates of the last menstrual period and early pregnancy ultrasound. PB refers to the birth of the baby before 37 completed weeks of gestation. Additional information was extracted from the clinical notes on pregnancy complications, such as data on hypertension, preeclampsia, or diabetes (defined as gestational or chronic). Early pregnancy (< 14 weeks) weight and height were used to calculate BMI as weight in kilograms divided by the squared height in metres. The WHO classification was used to group the women, according to their BMIs, like normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (30–34.9 kg/m2) (15). Following this, 2 mL of blood was withdrawn from every participant (before delivery) in an ethylenediaminetetraacetic acid and analysed for a complete blood count including haemoglobin, using an automated haematology analyser and following the manufacturer’s instructions (Sysmex KX-21, Japan).
The sample size was calculated considering the assumed prevalence of spontaneous PB of 13% (the ratio was 6.6:1) among all the deliveries, guided by the recent reports from Ethiopia (16). Assuming a type I error of 5% and adequate power of 80% (β = 0.2), based on the results of our previous meta-analysis (17), we assumed that 40% of the women who had a PB and 30% of women who had no PB would have anaemia, which resulted in a sample size of 1,716, considering that 10% of the women might not respond or might have incomplete data. The sample size was calculated using the OpenEpi Menu (18).
Data were entered into a computer, and SPSS for Windows was used for data analysis. Continuous data were checked for normality using the Shapiro–Wilk test. Descriptive statistical [mean (standard deviation), median (interquartile range), frequency, and percentage] were used to present the characteristics of the participants. The Mann-Whitney test was used to compare the median (interquartile range) between the two groups. A logistic regression analysis was performed with spontaneous PB as the dependent factor. The covariates (independent factors) were the sociodemographic, medical, and obstetric factors, age of women, parity, education, residence, occupation, antenatal care status, history of previous miscarriages/PB, gestational age, interpregnancy interval, BMI, haemoglobin level (before delivery), and sex of the infant as independent factors. Variables with a p-value of <0.2 were entered into the multivariable logistic regression model using the backward stepwise method (likelihood ratio). The crude odds ratio (COR), adjusted odds ratio (AOR), and 95% CI were computed to show the strength of the association. A two-sided p-value of < 0.05 was considered statistically significant.
A total of 1,716 parturient women were enrolled in the study. The median (interquartile range) of their age, parity, IPI, BMI, and haemoglobin was 27 (23–32) years, 2 (1–4), 23 (15–23) months, 26.1 (23.3–27.5) kg/m2, and 10.5 (9.8–11.4) g/dl, respectively. Of the 1,716 women, 939 (54.7%) were educated up to at least secondary level (8 years), 1,584 (92.3%) attended at least two antenatal visits, and 255 (14.9%) were obese. The details of the sociodemographic characteristics are shown in Table 1.
Of the 1,716 parturient women, 21 (1.2%) were underweight, 759 (44.2%) were of normal weight, 681 (39.7%) were overweight, and 255 (14.9%) were obese.
Approximately two-thirds (65.7%) of the women had anaemia and six (0.3%) had severe anaemia. Parturient women were classified into seven groups according to haemoglobin levels, as follows: < 8, 8–8.9, 9–9.9, 10–10.9, 11–11.9, 12–13, and > 13 g/dl. Their numbers and proportions are shown in Table 2.
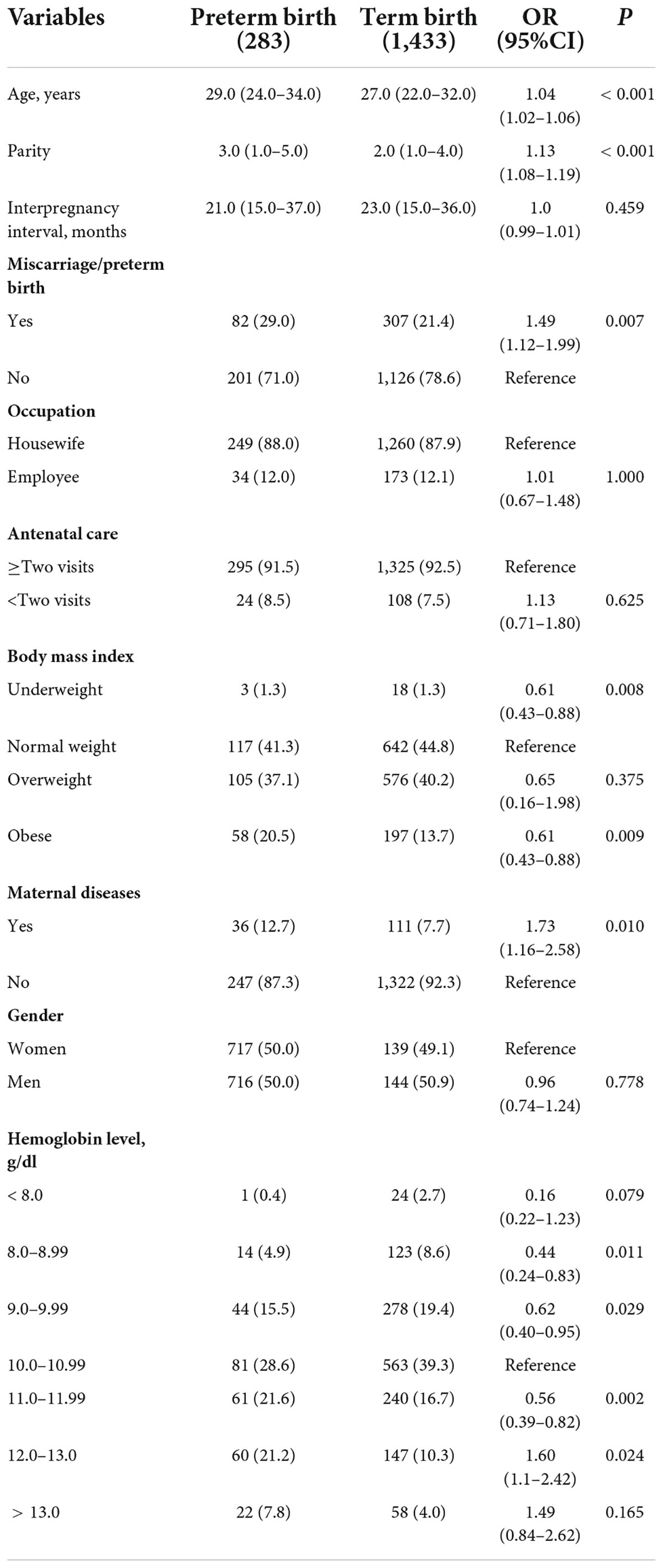
Table 2. Comparing sociodemographic and clinical variables between mothers with preterm birth and mothers with term birth in Khartoum, Sudan, 2020.
Of the 1,716 parturient women, 283 (16.5%, 95% CI = 14.7–18.2) had a PB. Compared to women who had a term birth, women who had a PB had significantly higher age, higher parity, more history of miscarriage/PB, and were more likely to be obese (see Table 2). The median (IQR) of the haemoglobin level was significantly (Mann-Whitney test) higher in women with PTB [11.3 (10–12) g/dl vs. 10.3 (9.7–11.2), P < 0.001]. Moreover, compared to women who had a term birth, women who had a PB are less likely to be anaemic [140/283 (49.5%) vs. 988/1,433 (68.9%), P < 0.001]. In multivariable logistic regression, parity (AOR = 1.15, 95% CI = 1.09–1.21, P < 0.001) was positively associated with PB. Anaemia was associated with reduced OR of PB (AOR = 0.4, 95% CI = 0.32–0.54, as seen in Table 3).
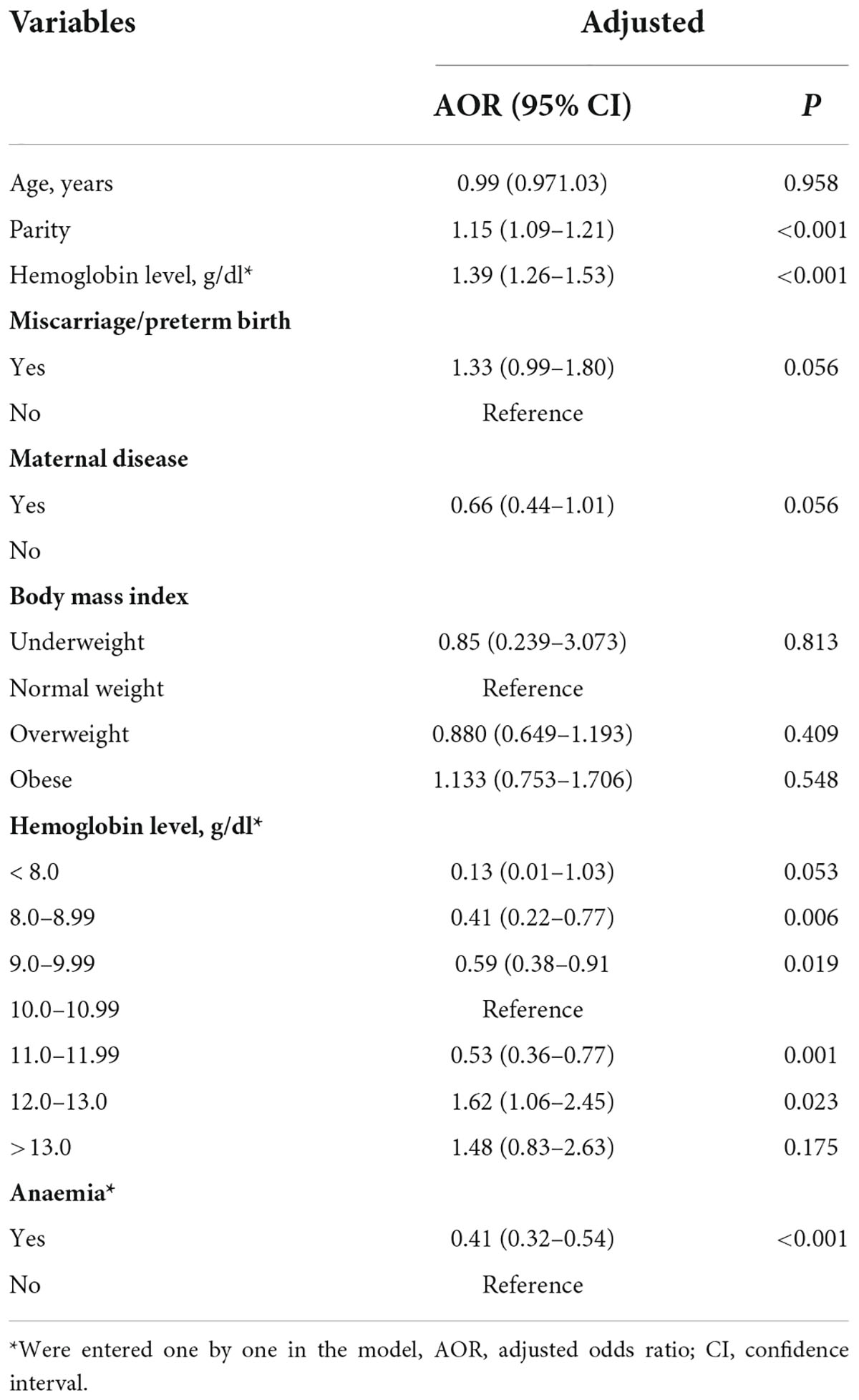
Table 3. Logistic regressions of sociodemographic and clinical variables associated with preterm birth in Khartoum, Sudan, 2020.
Compared to those with haemoglobin levels of 10–10.9 g/dl, parturient women with haemoglobin levels of 8–8.9 (AOR = 0.41, 95% CI = 0.22–0.77, P = 0.006), 9–9.9 (AOR = 0.59, 95% CI = 0.38–0.91, P = 0.019), and 11–11.9 g/dl (AOR = 0.53, 95% CI = 0.36–0.77, P = 0.002) were at lower risk of PB. Parturient women with haemoglobin levels of 12–13 g/dl were at a higher risk of PB (AOR = 1.62, 95% CI = 1.06–2.45, P = 0.23). There was no significant association between women with low haemoglobin levels < 8 g/dl and women with haemoglobin levels > 13.g/dl and PB (see Table 3 and Figure 1).
The prevalence (16.5%) of spontaneous PB in our study was found to be comparable to the prevalence of PB in other African countries, e.g., 16.9% in Ethiopia (16), 18.3% in Kenya (7), and 16.8% in Nigeria (19). However, a prevalence of 16.5% was outside the range (9.5–15.8%) reported by WHO for sub-Saharan Africa (20). The differences in the prevalence of PB could be explained by the differences in the methods (definition, inclusion criteria, and exclusion criteria) and risk factors as well as differences in social and other factors. Moreover, it was a hospital-based study that might not reflect the nature of the overall community. This might offer a plausible explanation for the high prevalence of PB in this study, especially since there is a high rate of home deliveries in Sudan (21).
In the current study, age, history of miscarriage/PB, education, ANC, BMI, duration, and IPI were not associated with PB. Similar findings were reported in other studies, which showed that IPI was not associated with PB (7). Our findings contrast with several studies showing that rural residence, short interpregnancy interval, presence of chronic illness (6), maternal age (8, 19), education, failure to attend antenatal care clinic, previous abortion (22), and previous PB were associated with PB (7).
Our results showed that, compared to those with haemoglobin levels (at the time of the delivery) of 10–10.99 g/dl, women with haemoglobin levels of 8–8.99 (AOR = 0.414), 9–9.99 (AOR = 0.59), and 11–11.99 (AOR = 0.53) g/dl were at lower risk of PB. Women with haemoglobin levels of 12–13 g/dl were at a higher risk of PB (AOR = 1.62). Previous studies refuted any association between high haemoglobin levels and PB (23, 24). In China, a high haemoglobin level in the second trimester has been shown to carry an increased risk of PB (13). Zhou et al. reported a slightly increased risk of PB associated with high haemoglobin levels (14). In Turkey, incidences of PB were significantly higher for both high and low haemoglobin levels (25). Moreover, it has been found that both low and high haemoglobin concentrations tend to be associated with an increased risk of PB, in a U-shaped pattern (26). Similarly, previous studies reported that women with high haemoglobin levels had higher risks of PB (27, 28). Interestingly, previous studies reported a U-shaped curve for the risk of PB against maternal haemoglobin concentrations (29, 30). During pregnancy, haemoglobin levels decrease due to an increase (expansion) in plasma volume. This results in a reduction in blood viscosity, which can interfere with the proper placental perfusion (31). Thus, a high haemoglobin level during pregnancy could lead to placental infarcts, poor functionality, and PB.
In Kenya (7), anaemia was not found to be associated with PB. We previously showed that the risk of PB increases significantly with anaemia (especially the severe form) (32). In a recent (2020) meta-analysis of 58 studies including a total of 134,801 women, anaemic women were found to be at higher risk of PB (8). A previous (2019) meta-analysis (of 117 studies including a total of 4,127,430 pregnancies), revealed that maternal anaemia was significantly associated with PB (12). Several mechanisms may explain the increased risk of PB in anaemia. Hypoxia and an increase in the level of norepinephrine concentrations can lead to maternal and foetal stress. This may stimulate the secretion of the corticotrophin-releasing hormone. Moreover, anaemia may also increase the risk of maternal infection, which is a known predisposing factor for PB (33). Amino acids, which can be influenced by maternal protein and quality of protein intake, play a significant role in the placenta’s function, foetus growth, and development. Low maternal protein intake can be important in reducing haemoglobin synthesis and in causing placental insufficiency. High protein intake may cause an excess of haemoglobin synthesis, leading to a relative excess of haemoglobin concentration. Moreover, plasma ammonia toxicity is potentially responsible for intrauterine growth restriction and excessive production of the metabolites of amino acids, hindering the development of the foetus (34). Also, there is accumulating evidence for changes in the maternal microbiota that might be associated with PB (35).
The study had some limitations: While there may be differential recall bias in women, haemoglobin in the first and second trimesters was not checked, serum ferritin and inflammatory biomarkers were not investigated, and several other factors and haemoglobinopathies/thalassaemia were not assessed in our cohort. These factors [e.g., malaria (6), HIV (19, 22)] and alcohol consumption (16) have been reported to be associated with PB. The prevalence of HIV and malaria is low in Khartoum. Smoking and alcohol consumption are not common in women in Sudan. Due to the sensitivity of such topics, cooperation from women may have been reduced or lost if they were asked about smoking or consuming alcohol.
Haemoglobin levels have different effects on the risk of PB; while some haemoglobin levels were associated with a lower risk of PB; other haemoglobin levels were associated with an increased risk or were not associated with PB. A large longitudinal study assessing other factors (especially inflammatory factors) is required.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee at the Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Khartoum, Sudan (reference number: 2019/09). All procedures performed in the study were in accordance with the ethical standards of the institutional and/or National Research Committee and with the 1964 Helsinki declaration and its later amendments. The patients/participants provided their written informed consent to participate in this study.
AE and NA: conceptualisation and writing—original draft, review, and editing. AE and DR: data curation. IA: formal analysis. AE: investigation. IA and OA-W: methodology. DR: project administration. All authors have read and agreed upon the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
1. Born too Soon. Linked to “Born too Soon: The Global Action Report on Preterm Birth.” Country Data and Rankings for Preterm Birth EMBARGO Until May 2nd 2012. Geneva: World Health Organization (2012).
2. Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Heal. (2019) 7:e37–46. doi: 10.1016/S2214-109X(18)30451-0
3. Mondal MNI, Hossain MK, Ali MK. Factors influencing infant and child mortality: a case study of Rajshahi district, Bangladesh. J Hum Ecol. (2009) 26:31–9.
4. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
5. Zainal H, Dahlui M, Soelar SA, Su TT. Cost of preterm birth during initial hospitalization: a care provider’s perspective. PLoS One. (2019) 14:e0211997. doi: 10.1371/journal.pone.0211997
6. Aregawi G, Assefa N, Mesfin F, Tekulu F, Adhena T, Mulugeta M, et al. Preterm births and associated factors among mothers who gave birth in Axum and Adwa Town public hospitals, Northern Ethiopia, 2018. BMC Res Notes. (2019) 12:640. doi: 10.1186/s13104-019-4650-0
7. Wagura P, Wasunna A, Laving A, Wamalwa D, Ng’ang’a P. Prevalence and factors associated with preterm birth at kenyatta national hospital. BMC Pregnancy Childbirth. (2018) 18:107. doi: 10.1186/s12884-018-1740-2
8. Laelago T, Yohannes T, Tsige G. Determinants of preterm birth among mothers who gave birth in East Africa: systematic review and meta-analysis. Ital J Pediatr. (2020) 46:10. doi: 10.1186/s13052-020-0772-1
9. Aseidu EK, Bandoh DA, Ameme DK, Nortey P, Akweongo P, Sackey SO, et al. Obstetric determinants of preterm delivery in a regional hospital. Accra, Ghana 2016. BMC Pregnancy Childbirth. (2019) 19:248. doi: 10.1186/s12884-019-2404-6
10. Abadiga M, Mosisa G, Tsegaye R, Oluma A, Abdisa E, Bekele T. Determinants of adverse birth outcomes among women delivered in public hospitals of Ethiopia, 2020. Arch Public Health. (2022) 80:12. doi: 10.1186/S13690-021-00776-0
11. Abadiga M, Wakuma B, Oluma A, Fekadu G, Hiko N, Mosisa G. Determinants of preterm birth among women delivered in public hospitals of Western Ethiopia, 2020: unmatched case-control study. PLoS One. (2021) 16:e0245825. doi: 10.1371/JOURNAL.PONE.0245825
12. Jung J, Rahman MM, Rahman MS, Swe KT, Islam MR, Rahman MO, et al. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: a systematic review and meta-analysis. Ann N Y Acad Sci. (2019) 1450:69–82. doi: 10.1111/nyas.14112
13. Zhang Y, Li Z, Li H, Jin L, Zhang Y, Zhang L, et al. Maternal haemoglobin concentration and risk of preterm birth in a Chinese population. J Obstet Gynaecol. (2018) 38:32–7. doi: 10.1080/01443615.2017.1325454
14. Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol. (1998) 148:998–1006. doi: 10.1093/oxfordjournals.aje.a009577
15. Ota E, Haruna M, Suzuki M, Anh DD, Tho H, Tam NT, et al. Maternal body mass index and gestational weight gain and their association with perinatal outcomes in Viet Nam. Bull World Heal Organ. (2011) 89:127–36. doi: 10.2471/BLT.10.077982
16. Kelkay B, Omer A, Teferi Y, Moges Y. Factors associated with singleton preterm birth in shire suhul general hospital. northern ethiopia, 2018. J Pregnancy. (2019) 2019:4629101. doi: 10.1155/2019/4629101
17. Adam I, Ibrahim Y, Elhardello O. Prevalence, types and determinants of anemia among pregnant women in Sudan: a systematic review and meta-analysis. BMC Hematol. (2018) 18:31. doi: 10.1186/s12878-018-0124-1
18. Dean AG, Sullivan KM, Soe MM. OpenEpi Menu. Available online at: http://wwww.openepi.com/Menu/OE_Menu.htm (accessed January 1, 2022). (2022)
19. Butali A, Ezeaka C, Ekhaguere O, Weathers N, Ladd J, Fajolu I, et al. Characteristics and risk factors of preterm births in a tertiary center in Lagos, Nigeria. Pan Afr Med J. (2016) 24:1. doi: 10.11604/pamj.2016.24.1.8382
20. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. (2013) 10:S2. doi: 10.1186/1742-4755-10-S1-S2
21. Abbaker AO, Salih Y, Ali AA, Imam AM, Abdulla EA, Adam I. Use of institutional delivery services in Kassala, eastern Sudan. Int J Gynecol Obstet. (2013) 123:79–80. doi: 10.1016/j.ijgo.2013.04.018
22. Mekonen DG, Yismaw AE, Nigussie TS, Ambaw WM. Proportion of preterm birth and associated factors among mothers who gave birth in debretabor town health institutions, northwest, Ethiopia 1. BMC Res Notes. (2019) 12:2. doi: 10.1186/s13104-018-4037-7
23. Ren A, Wang J, Ye RW, Li S, Liu JM, Li Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynecol Obstet. (2007) 98:124–8. doi: 10.1016/j.ijgo.2007.05.011
24. Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol. (2000) 96:741–8. doi: 10.1016/S0029-7844(00)00982-0
25. Çakmak BD, Türker ÜA, Öztaş S, Arık M, Üstünyurt E. The effect of first trimester hemoglobin levels on pregnancy outcomes. Turk J Obstet Dern Derg. (2018) 15:165–70. doi: 10.4274/tjod.87269
26. Malhotra M, Sharma JB, Batra S, Sharma S, Murthy NS, Arora R. Maternal and perinatal outcome in varying degrees of anemia. Int J Gynaecol Obstet. (2002) 79:93–100. doi: 10.1016/s0020-7292(02)00225-4
27. Gonzales GF, Steenland K, Tapia V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am J Physiol. (2009) 297:1105–10. doi: 10.1152/ajpregu.00275.2009
28. Chang S, O’Brien K, Nathanson MS, Mancini J, Witter FR. Hemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J Nutr. (2003) 133:2348–55. doi: 10.1093/jn/133.7.2348
29. Dewey KG, Oaks BM. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr. (2017) 106:1694–702S. doi: 10.3945/ajcn.117.156075
30. Zhang X, Xu Q, Yang Y, Wang L, Liu F, Li Q, et al. Preconception Hb concentration and risk of preterm birth in over 2⋅7 million Chinese women aged 20-49 years: a population-based cohort study. Br J Nutr. (2018) 120:508–16. doi: 10.1017/S0007114518001721
32. Ali AA, Rayis DA, Abdallah TM, Elbashir MI, Adam I. Severe anaemia is associated with a higher risk for preeclampsia and poor perinatal outcomes in Kassala hospital, eastern Sudan. BMC Res Notes. (2011) 4:311. doi: 10.1186/1756-0500-4-311
33. Allen L. Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. J Nutr. (2001) 131:604–15S. doi: 10.1093/JN
34. Herring CM, Bazer FW, Johnson GA, Wu G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp Biol Med. (2018) 243:525. doi: 10.1177/1535370218758275
Keywords: haemoglobin, preterm, birth, Sudan, anaemia
Citation: Elmugabil A, Alhabrdi NM, Rayis DA, Al-Wutayd O and Adam I (2022) Evaluation of the association between haemoglobin levels and preterm birth at Khartoum, Sudan: A hospital-based study. Front. Nutr. 9:933557. doi: 10.3389/fnut.2022.933557
Received: 01 May 2022; Accepted: 28 June 2022;
Published: 22 July 2022.
Edited by:
Anna Tresserra-Rimbau, University of Barcelona, SpainReviewed by:
Prema Ramachandran, Nutrition Foundation of India, IndiaCopyright © 2022 Elmugabil, Alhabrdi, Rayis, Al-Wutayd and Adam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadiah M. Alhabrdi, bi5hbGhhYnJkaUBxdS5lZHUuc2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.