
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 02 August 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.931790
This article is part of the Research TopicThe Nutritional Evidence and Research on TeaView all 10 articles
Tea is a popular traditional drink and has been reported to exhibit various health-promoting effects because of its abundance of polyphenols. Among all the tea products, fermented tea accounts for the majority of tea consumption worldwide. Microbiota plays an important role in the fermentation of tea, which involves a series of reactions that modify the chemical constituents and thereby affect the flavor and bioactivities of tea. In the present review, the microorganisms involved in fermented tea and tea extracts in the recent studies were summarized and the modulation effects of microorganisms on tea in fermentation, including polyphenols composition and content, biological activities and sensory characteristics, were also critically reviewed. It is expected that the data summarized could provide some references for the development of microbial fermented tea drinks with specific nutrition and health benefits.
Tea, one of the most popular beverages in the world, is generally made from the leaves of the Camellia sinensis plant through processing techniques such as curing, rolling, heaping, and drying. Tea contains various bioactive ingredients, such as polyphenols, polysaccharides, caffeine, minerals and other active substances (1). Previous studies have indicated that tea possessed antioxidant, hypoglycemic (2), antihypertensive, lipid-lowering (3), antibacterial, anti-cancer, and anti-obesity effects, which were mainly attributed to its abundant polyphenols (4).
With the rise of fermentation engineering, researchers applied microbes to tea. Unlike green tea, black tea and other teas that have not undergone microbial fermentation, microbial fermented tea has special sensory characteristics, including bright tea infusion, unique aroma, sweet, and smooth taste with low levels of bitterness and astringency. Therefore, dark tea and kombucha, two kinds of microbial fermentation tea, are favored by consumers for their special taste and aroma (5, 6). Furthermore, tea has been fermented by microorganisms that are attracting increasing attention because of its various health benefits, including protection against hypertension and cardiovascular diseases (7, 8). At the same time, the content of its active ingredients will change (9–11). After fermentation, some molecules in tea that are not easy to be absorbed change from combined state to free state, which is conducive to the absorption of nutrients in tea and the exertion of its efficacy. Microbial fermentation is the key factor responsible for the formation of sensory attributes and the chemical components of tea.
The changes of chemical constituents and bioactivities of tea during microbial fermentation have been revealed in recent years, which were related to the bioconversion of active components by microorganisms. The purpose of this paper is to review the types of microbiota, changes of polyphenols content and composition, biological activity and sensory evaluation in tea after microbial fermentation, according to comprehensively understand the characteristics and current situation of microbial fermented tea, and look forward to its development prospects and research trends, which may provide a theoretical basis for the in-depth study of tea fermented by microorganisms.
According to the type of microbiota, the microbial fermented tea can be divided into four types, including bacterial fermented tea, mold fermented tea, yeast fermented tea and edible and medicinal fungi fermented tea, as shown in Figure 1. After fermentation, the antibacterial activity and antioxidant activity of tea were enhanced due to the increase of phenolic substances. The reduction of caffeine after fermentation makes the tea taste better. In addition, after fermentation, the variety of aromatic substances increases, giving the tea a new aroma. As well as changes in the types and content of tea pigments, giving the tea soup has a clear color. Tea are fermented by microorganisms, a variety of active substances produced by microbial metabolism increased, thereby improving the overall quality of the tea.
In recent years, the research on using bacteria to ferment tea is mainly focused on kombucha. During the fermentation process of kombucha, changes in sugar and acid content give the tea a new taste, the production of aromatic substances increases the aroma of the tea, and changes in phenolic substances increase the antioxidant capacity of the tea.
Zhao et al. reported that lactic acid bacteria fermentation may be an effective method to improve the bioavailability of phenols and protect cells from oxidative stress (12). Furthermore, kombucha is fermented by acetic acid bacteria and yeast, during which the content of beneficial ingredients such as vitamin C and glucuronic acid increases (13). Simultaneously kombucha has significant changes in antioxidant potential, pH, acetic acid, alcohol and sugar contents, and beneficial ingredients such as organic acids, minerals and vitamins, amino acids and polyphenols can be produced in the fermentation process (14).
On account of tea polyphenols in tea have no inhibitory effect on mold, and some metabolites produced by mold will produce a series of reactions in tea, including degradation, oxidation, methylation, etc., which can improve the quality of tea. Therefore, when tea is fermented with mold, some researchers selected Streptomyces, Aspergillus niger, and Mucor to conduct fermentation studies (15).
The results showed that compared with fresh tea, the polyphenol content of tea fermented by Streptomyces bacillaris strain R9 and Streptomyces cinereus strain Y11 strains was higher, and the total phenol content was 32.9 ± 0.1 mg/mL and 31.9 ± 0.1 mg/mL, respectively, after 42 days of fermentation (16). In another set of experiments, the contents of polyphenols and purine alkaloids in solid state fermentation system of Aspergillus niger and Aspergillus fumigatu were experimentally studied, which provided a reliable basis for dynamic data description and metabolic pathway of tea polyphenols in fermented pu-erh tea (17). Similarly, when molds and yeasts were used in the fermentation of black and green teas, molds fermentation increased caffeine content, which are related to the methylation process and the increase of the premise substance of caffeine, while yeasts fermentation decreased caffeine content. It was found that Aspergillus niger had the best fermentation performance of the three molds in this study (18).
In addition, Eurotium cristatum was also used for tea fermentation in recent years. The bacteria secrete amylase and oxidase, which can catalyze the transformation of protein and starch in tea to monosaccharides, catalyze the oxidation of polyphenol compounds, and transform them into substances beneficial to human body, so as to improve and optimize tea taste and other characteristics. When the extract of raw dark tea was fermented by using Eurotium cristatum, the dry weight of mycelia increased by about 10 times after the fermentation, the mass concentrations of tea polyphenols, total protein and water extract were decreased, the concentrations of total flavonoids, free amino acids and theabrownin were increased, and 12 kinds of aromatic components were increased, most of which were esters and alcohols (19).
Using yeast to ferment tea can not only improve the activity of tea fermentation, but also the complex biochemical reactions in the fermentation process will produce ethanol, acids and esters and other flavor substances to improve the sensory quality of the microbial fermentation of tea.
Studies have shown that the fermentation of black tea by Dabaryomyces hansenii results in the reduction of caffeine and a large amount of tannins, and improves its nutritional and medicinal value, so the ingestion of fermented tea is more advantageous than black tea (20). Additionally, when yeast strains were isolated from pu-erh tea and fermented raw tea samples of pu-erh tea, the study found that after yeast fermentation, the contents of tea polyphenols, theaflavins and catechins in tea were increased, while the contents of amino acids, caffeine, flavonoids, thearubigins and theabrownin were decreased. The contents of amino acids, catechins and caffeine affect the taste of tea, and the content of tea pigment determines the color of tea soup. The decrease of theanine content improved the bitter taste of tea, while the increase of theaflavins improved the color, aroma and taste of tea. Therefore, yeast has a great influence on the quality formation of pu-erh tea (21).
With the in-depth study of tea and modulation effects of microorganisms on tea beverage in fermentation process, many researchers also introduced edible and medicinal fungi for tea fermentation. With the fermentation of edible and medicinal fungi, the tea fermented by microorganisms goes through biochemical reaction to obtain aroma substances such as esters and alcohols. There were also changes in substances such as polyphenols and proteins. Improved the stale taste, sour taste and astringency of tea, and gave tea a new aroma and taste.
Rigling et al. used Poria cocos to adjust the smell of green tea. The study found that after immersion and fermentation for 17 h, due to the formation of methyl anthranilate, linalool, 2-phenylethanol and geraniol, Poria cocos changed the unique smell of green tea into jasmine flower and slightly citrus flavor. Meanwhile, the antioxidant activity of green tea is retained (22).
In addition, after the fermentation of Jinxuan oolong tea with medicinal mushrooms Grifola frondosa and Tianzhi (new variants of Ganoderma lucidum), the contents of polysaccharide, free amino acid and protein of the fermented tea were significantly increased, and the taste of the fermented tea was fresher and mellow. The contents of tea polyphenols, caffeine and water extract in the fermented products were significantly reduced, which reduced the turbidity of tea juice, reduced the bitterness, and gave it sweet taste and aroma (23).
Tea polyphenols are the main components that determine the color, aroma, taste and efficacy of tea. They are classified as flavonoid (flavonols, flavanols, flavones, flavanones, isoflavones, and anthocyanins) and non-flavonoid molecules (phenolic acids, hydroxycinnamic acids, lignans, stilbenes, and tannins) (24). Tea polyphenols have anti-inflammatory, antiviral, antibacterial, hypolipidemic, hypoglycemic, weight loss and other effects. After microbial fermentation, the content of polyphenols changes in the tea (25–28).
In recent years, researches on flavonoids polyphenols in tea mainly focus on flavanols, flavonols, and flavones. This section will review the changes of flavonoid content of tea before and after microbial fermentation.
Catechins are synthesized from sugars through shikimic acid pathway through the action of a series of enzymes to form benzene ring compounds. Catechins are typical flavanols, accounting for about 18 to 36% of the dry weight of tea (29). As shown in Figure 2, there are four important structures of catechins, namely (-)-epigallocatechin-3-gallate (EGCG), (-)-epicatechin-3-gallate (ECG), (-)-Epicallocatechin (EGC) and (-)-Epicatechin (EC). In the process of tea fermentation, the content of catechin varies with the degree of tea fermentation, fermentation time, fermentation temperature and other factors (30). In another study, Qin et al. using the method of quantitative analysis and high performance liquid chromatography to study the change of tea polyphenols content of pu-erh tea in solid-state fermentation system, it was found that the content of ester-catechins increased slightly at the initial stage of fermentation, and then the ester-catechins gradually degraded to produce catechin and gallic acid. In the initial stage (0 to 8 h), the content of EGCG increased slightly, from 23.392 and 23.431 mg/g to 24.983 and 24.897 mg/g (17). According to another set of study, used the dominant strain of qingzhuan brick tea to conduct solid fermentation of qingzhuan brick tea, and after 6 days of fermentation, the catechin content decreased by 27.6% under the action of Aspergillus fumigatus M1 (25). The content of catechins in most teas decreases during fermentation, possibly due to the breakdown of catechins into substances such as theaflavins during fermentation (31).
The main flavonols and flavones in tea include kaempferol, quercetin, myricetin, and apigenin. Wang et al. identified flavonoid glycoside (quercetin-3,4'-O-di-β-glucoside, quercetin 3-O-galactosyl rutin, myricetin 3-galactoside, luteolin 6-C-glucoside, vitexin (apigenin-8-C-glucoside), kathinol 7-O-glucoside) in green tea extracts fermented by Lactiplantibacillus plantarum 299 V significantly were decreased, which may be related to the absorption and utilization of flavonoids by lactic acid bacteria cell wall (32). Seven kinds of tea fungis were used to ferment the sun-dried green tea, which promoted to the accumulation of kahenol and myricetin. It was found that the antioxidant activity of tea increased after fermentation, and the positive correction of gallic acid and kamanol to the antioxidant activity of fermented tea was observed (33). In addition, Ma et al. found that Aspergillus palladium PT-3 and Aspergillus sesamae PT-4, two tea fungis, could promote the biosynthesis of various flavonoids such as neferol, quercetin, and myricetin in the metabolic process of phenolic compounds, thus increasing the content of flavonoids in tea during fermentation (34). Furthermore, other researchers have used bacterial strains to ferment black tea to make drinks. For example, when Starmerella davenportii strain Do18 was used to fermented black tea extract, the research results showed that the flavonoid content of fermented tea drinks was higher, and the total flavonoid content of fermented drinks was significantly higher than that of unfermented samples, and reached the highest level after 36 h of fermentation (35).
Moreover, changes in acids in the fermentation environment also lead to the release of bound flavonoids (36–38). However, in the study of inoculated fermented tea, the flavonoid content of fermented tea was higher than that of unfermented tea.
Phenolic acids in tea are secondary metabolites of aromatic substances and non-flavonoids, which have many biological characteristics. The phenolic acids in tea mainly include gallic acid, chlorogenic acid, salicylic acid, vanillic acid and so on. During the fermentation of tea, the content of phenolic acid will vary with the degree of fermentation (39, 40).
Gallic acid is one of the important active ingredients in tea. Fermentation of tea will affect the content of gallic acid in tea. When studying the fermentation of loose tea by Eurotium cristatum at different temperatures, it was found that appropriately increasing the fermentation temperature was beneficial to increase the content of gallic acid in fu brick tea (41).
Liu et al. used Aspergillus Niger to ferment tea. It was found that tannase was involved in the metabolism of gallic acid during the fermentation of pu-erh tea, which increased the content of gallic acid during the fermentation (42). However, Gallic acid showed a decreasing trend in the early fermentation stage of dark tea fermented by M. coronoid (43). In addition, in the fermentation process of black tea extract, with the increase of fermentation time, it is not conducive to the accumulation of phenolic acids such as gallic acid and cinnamic acid (44). In the fermentation process of tea, the change of phenolic acid content should be analyzed in combination with the specific situation (45).
Tea has biological activities such as antibacterial, antioxidant, hypoglycemic, weight loss, anticancer and so on (46–48). With the fermentation of tea, its active function will change. The antibacterial, anti-oxidant, hypoglycemic, anti-lipid, anti-inflammatory, anti-toxin and anti-cancer activities of microbial fermented tea can be applied to the food, medicine and cosmetics industry and have a good development prospect (49–52). In this section, the antibacterial, antioxidant, hypoglycemic, lipid-lowering and other activities of tea fermented by microorganism would be elaborated.
With the deepening of fermentation, the metabolites of some fungi in tea have the function of inhibiting intestinal microorganisms (53, 54). Comparing the inhibitory effects of black tea extracts before fermentation and black tea extracts after fermentation on Escherichia coli, the research results showed that among the three different concentrations of non-fermented black tea extracts, only the tea extract at a concentration of 25 mg/mL can inhibit Escherichia coli. The fermented black tea extracts at concentrations of 5, 10, and 25 mg/mL can significantly inhibit the growth of Escherichia coli. Figure 3 shows the damage of 25 mg/ml fermented black tea extract to Escherichia coli (55). When studying unfermented black tea extracts and fermented black tea extracts, it was found that as the fermentation time increased, the antibacterial effect of black tea extracts increased. The results showed that under the condition of PH 7.0, black tea extracts had no inhibitory effect on S. typhimurium when it was not fermented; when fermented for 14 days, the inhibition of S. typhimurium by fermented tea could reach 30–35 mm in diameter; Unfermented black tea extracts has no inhibitory effect on Escherichia coli, and after 14 days of fermentation, the diameter of the inhibitory zone of fermented tea on Escherichia coli can reach 30–35 mm; when fermented for 0–4 days, fermented tea has no inhibitory effect on Candida albicans. When fermented for 4–14 days, the inhibition halo diameter of fermented tea on Candida albicans reaches 10–15 mm (56). Studies have found that the fermented fu brick tea contains a class of triterpenoids with 6-hydroxy-7-one function. The antimicrobial activity of compound enteric pathogenic Escherichia coli, Escherichia coli, Staphylococcus aureus, Shigella dysenteriae, and Salmonella typhi was evaluated by plate diffusion. The test results show that the compound has weak antibacterial activity against enteropathogenic Escherichia coli (EPEC) and Salmonella typhi. The microbial fermented tea has a certain inhibitory effect on harmful bacteria, and the inhibitory ability may be related to the fermentation time, pH and other factors of the microorganisms (57).
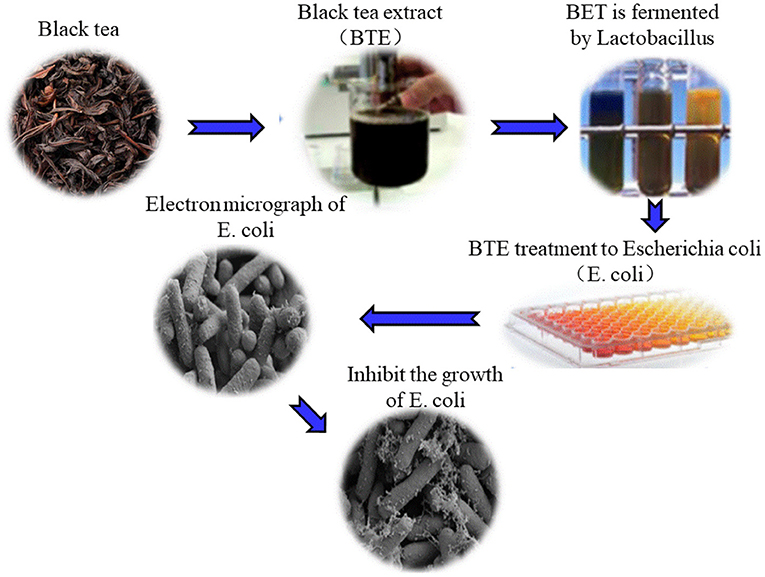
Figure 3. The inhibitory effect of fermented black tea extract on Escherichia coli. The cell membrane and cell division of Escherichia coli were damaged by 25 mg/ml fermented tea extract.
In addition, black tea extracts fermented by acetic acid bacteria and yeast showed obvious inhibitory effects on Staphylococcus aureus ATCC6538 (S. aureus) and E. coli ATCC11229 (E. coli) (58), which suggest that fermented tea might be a potential source of preservatives. The antimicrobial activity of fu-brick tea after fermentation by bursa corundum is obviously improved compared with that without fermentation. When the concentration of fermented tea extract was 5 mg/mL or less, the growth of intestinal pathogenic bacteria Shigella and Staphylococcus aureus could be reduced by 50%, and the minimum inhibitory concentration of fermented tea extract against Staphylococcus aureus was 0.625 mg/mL (59). After microbial fermentation, the antibacterial activity of tea was improved, and its antibacterial effect on Escherichia coli, Staphylococcus aureus, Salmonella and other pathogenic bacteria had improved significantly.
During the fermentation of tea, the antioxidant capacity of tea will also change with the changes in content of secondary metabolites (12, 60, 61). A single strain isolated from pu-erh tea was used to inoculate fresh tea to study its fermentation effect. The results of the study showed that fresh tea was inoculated with these strains, and the antioxidant capacity was significantly enhanced after 42 days of fermentation. Among them, the polyphenol content of tea inoculated with Streptomyces bacillus strain R9 was 3.3 mg/100 g, and the scavenging ability of DPPH free radicals reached 92%, the total polyphenol content was the highest, and the antioxidant capacity was the strongest (11). Fermentation of fu brick tea using Eurotium cristatum has found that fermented tea at 28 and 37°C has strong antioxidant capacity (26). Compared with unfermented tea, fermented tea has better antioxidant properties, and fermentation temperature has a significant impact on the antioxidant properties of fermented tea. Furthermore, using kombucha to ferment black tea, and study the changes in its antioxidant activity with fermentation time, the results of the study found that as the fermentation time increases, the antioxidant activity of fermented tea increases. After 8 days of fermentation, the antioxidant activity of fermented tea can reach up to 89.69% (62). And the DPPH scavenging ability of green tea reached 94.38% after fermentation of GABA-producing lactic acid bacteria for 5 days (63).
Combined with the above research, when studying the changes of antioxidant activity of microbial fermented tea, we can improve the antioxidant activity of tea through microbial fermentation, so as to provide a theoretical basis for the research and development of antioxidant functional food and the food industry.
In recent years, many researchers have used animal experiments to study the biological activity of tea and found that after microbial fermentation, it is demonstrated that the activity of tea to reduce blood glucose has been improved (64–66).
The regulation effect of fermented tea on glucose metabolism is mainly related to the metabolites of fermented tea. For example, the relative contents of 10 kinds of polyphenol metabolites (4 kinds of fatty acids, 1 kind of artemisylline derivative, 3 kinds of lysophosphatidylcholine and 2 kinds of triterpenoids) increased while the relative contents of the other 5 kinds of polyphenol metabolites decreased after the fermentation of dark tea by Cystis canopetiformis. These metabolites are related to the hypoglycemic activity of fermented tea (67). It was found that the kombucha has obvious therapeutic effect to diabetic rats after fermentation. The findings from the histopathological analyses revealed that those of the alloxan-induced diabetic rats showed clear atrophy of β-Cells. The pancreas of the diabetic rats that were treated with fermented black tea, on the other hand, noted to undergo a marked amelioration. Mainly because after fermentation of tea extract on plasma and alpha amylase and lipase activity in pancreas have better inhibitory effect, at the same time improve the pancreas of diabetic mice structure, have better inhibitory effect on blood sugar levels rise, as shown in Figure 4 (68). The green tea extracts fermented by acetic acid bacteria, yeast and lactic acid bacteria can improve the intestinal flora of mice. The improvement of intestinal flora reduces the damage of intestinal barrier, thus reducing lipopolysaccharide replacement and inhibiting the occurrence of insulin resistance in vivo. In addition, increasing the number of SCFAs-producing bacteria can increase the number of SCFAs, improve the function of islet β cells, and reduce blood glucose by promoting the secretion of gastrointestinal hormones (69).
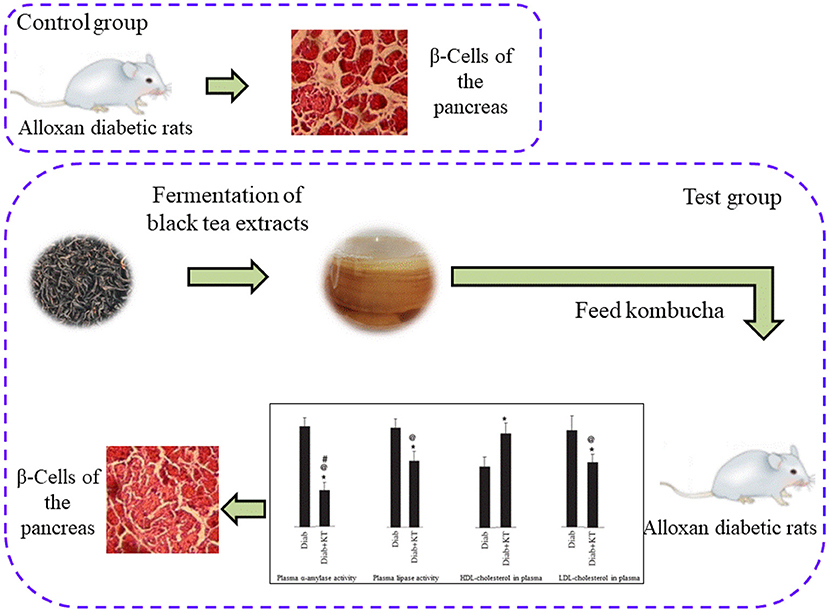
Figure 4. Effect of fermented black tea extract on β-cells of the Pancreas in Alloxan diabetic rats.
At present, research on the lipid-lowering activity of microbial fermented tea is mainly focused on lowering blood lipids and weight loss (70, 71). For example, the fu brick tea was fermented by Eurotium cristatum, and the water extract of the fermented fu brick tea was fed to high-fat zebrafish. The results showed that when the concentration was 500 μg/mL, the lipid level of the zebrafish was compared with the control group, it decreased by 51.49%. The water extract of fermented Eurotium cristatum showed effective lipid-lowering activity on high-fat zebrafish (72). In addition, Lactobacillus paracasei subsp was used to fermentation Houttuynia cordata leaf tea and green tea, and the anti-obesity activity of fermented tea was studied through in vivo and in vitro experiments. Wang et al. demonstrated that the fermented tea contained epigallocatechin gallate, epigallocatechin, and chlorogenic acid, which inhibit lipogenesis in mature 3T3-L1 adipocytes by stimulating adipose decompose (73). Qin et al. fermented Anhua black tea with Monascus and Cystaphylococcus coronatum, resulting in many active substances in the fermented products, such as lovastatin, which had certain lipids lowering effects (74). Therefore, long-term drinking of microbial fermentation tea has a good role in medical care.
The tea was fermented by microorganisms has antibacterial, antioxidant, hypoglycemic, lipid-lowering, anti-inflammatory and anti-cancer activities (75, 76). Zhang et al. found that lovastatin produced by monascus fermentation of pu-erh tea can induce neutrophil apoptosis by phosphorylation of ERK/AKT and reduce neutrophil recruitment to inflammatory sites, thereby reducing inflammation in zebrafish (77). In addition, Assam tea after fungal fermentation, compared with unfermented tea, microbial biological conversion of phenol makes total polyphenol, total tannin content in the fermented tea enhancement and enrichment of condensed tannins, thus as a target, the function of the bioactive ingredients in anti-inflammation mediated diseases such as cancer and cardiovascular disease found in the corresponding application (78). Moreover, Villarreal-soto et al. studied the bioactivity of extracts from black tea fermented by kombucha bacteria. After 21 days of fermentation, the anti-inflammatory bioactivity was enhanced, with IC50 values up to 9.0 μg/mL (79). Li et al. used Nigrospora sphaerica HCH285 to ferment the sun-dried green tea, so as to develop a kind of melanospora fermented tea. With the increase of fermentation time, after 45 days of fermentation, the content of bostrycin in the fermented tea reached 3.18 g/kg, and the content of bostrycin was the highest in the whole fermentation process. Bostrycin has good anticancer activity, so the anticancer activity of green tea is enhanced in the fermentation process (80). Tea are fermented by microorganisms, most of the biological activity of tea will be enhanced.
Not only there is a change in the content of tea polyphenols and bioactive functional, the sensory properties of tea changed after fermentation (81, 82). For example, the change of tea taste and the color and brightness of tea juice are mainly caused by the change of amino acid content in the fermented tea and the formation of theaflavins and thearubigen (83, 84).
Compared with unfermented tea, the taste of tea is enhanced by microbial fermentation. This is because, after tea fermentation, the protein in tea is decomposed into free amino acids, increasing the flavor of tea. At the same time, catechins were degraded and the bitter taste of tea beverage was reduced. Besides, sugar content increased and pH value decreased, making fermented tea beverage taste sour and sweet.
Laphet, a fermented tea from Myanmar, has a bitter taste in the raw tea without fermentation, but the bitter taste of the tea is reduced after fermentation (85). Nishioka et al. used Lactobacillus to ferment Awaban tea and found that the free amino acids of fermented tea contained large amounts of theine and glutamate, which enhanced the umami taste of tea (86). At the same time, the quality of Awaban tea is improved by some other ingredients produced by lactic acid bacteria. In addition, Zhao et al. used tea bacteria to discover the extract of pu-erh tea and studied the flavor of tea in the fermentation process. It was found that the total amount of free amino acids increased slightly with the increase of fermentation time, and these free amino acids, as the most important aromatic precursors of fermented tea, could significantly improve the flavor quality of fermented tea. At the same time, due to the presence of acetobacter, the PH of fermentation liquid is reduced, tea polyphenols are oxidized, catechins are degraded, and bitterness is reduced, and fermented tea is endowed with sweet and sour taste (87). Moreover, it was found that the sensory properties of black brick tea were changed during fermentation by liquid chromatography-mass spectrometry. Based on liquid chromatography-mass spectrometry of metabonomics analysis revealed that microbial fermentation of the tea samples and microbial fermentation before samples have significant differences, a total of 102 compounds were identified as the key to lead to metabolic changes and green tea processing metabolites, catechins content decreased significantly, and form new phenolic acids and catechins derivatives. The sensory quality of the green brick tea is mainly formed in the process of microbial fermentation, which greatly reduced the astringency and bitterness of raw tea and produced its characteristic woody and stale aroma as well as mellow taste (88). When the dark tea was fermented by Aspergillus Niger, the bitterness and astringency of tea were reduced significantly due to the catechin content was decreased in the fermentation process. In addition, bitterness and umami taste were also changed due to amino acids were changed in tea (89). After the fermentation of bacteria, the pH of black tea decreases at the initial stage of fermentation, during which sucrose is hydrolyzed and lactic acid content increases, and tea is endowed with a sour and sweet taste (5).
Tea has been fermented by microorganisms. Aroma substances in tea are produced, including alcohols and aldehydes. Besides enzymatic and non-enzymatic reactions occur in tea, and the hydrolysis of some substances, such as glycoside hydrolysis, has a great influence on the formation of tea aroma (90–92).
For example, in the post-fermentation process of dark tea, ketones are formed. These ketones are very important flavor compounds with special floral and woody odors. Therefore, the fermented dark tea has a special fragrance (93). In the fermentation process of pu-erh tea extract, the aroma of tea becomes weaker under the action of microorganisms, while the compositions of fermented tea such as camalool, phenylacetaldehyde, heptanaldehyde and 2, 4-dimethylbenzaldehyde are produced, which makes the tea fermentation liquid have the fruit flavor (60). In addition, the study used four non-Saccharomyces cerevisiae strains to ferment green tea slurry, and the proper fermentation of these four strains changed the characteristics of aroma compounds, so that the aroma of fermented green tea changed. In the microbial fermentation process of tea, the aroma composition and aroma will change benignly, which makes the sensory sense of tea more abundant (94). After the fermentation of green tea with four kinds of mixed bacteria, a fruity ethyl ester was produced, which increases the content of aroma compounds such as methyl salicylate, geraniol and 2-phenylethanol, giving tea a special aroma (32). Furthermore, the presence of D-limonene (27.71%) and β-cinnamene (11.55%) in fu brick tea was fermented by Eurotium cristatum gives the tea a fruity and special aroma compared with the unfermented green tea (72). Besides, Cyberlindnera aturnus var. mrakii NCYC 2251 was used to ferment green tea pulp. With the extension of fermentation time, glycosylated aroma substances such as methyl salicylate, benzyl alcohol and 2-phenylethanol increased (95).
After the tea was fermented, the taste and aroma would be changed. What's more, the color of tea will also be changed. Under the action of microbial fermentation, the content and type of tea pigment in tea change, and then change the color of tea soup. Therefore, the color of tea beverage will become bright, and the tea soup will be improved after microbial fermentation.
For example, the brown pigment content affects the color of black tea. After fermentation with Aspergillus fumigata M1, the brown pigment content of pu-erh tea extracts increases by 110.6%, which improves the color of pu-erh tea (25). Kim et al. conducted sensory evaluation on Monascus Pilosus fermented green tea and found that the brightness evaluation of fermented tea soup was as high as 4.26, significantly higher than that of unfermented tea. Therefore, microbial fermentation of tea can improve the color of tea soup and the overall quality of tea (96), as shown in Figure 5. After fermentation, the color of the tea soup mostly become translucent and the overall quality will be improved.
In the process of microbial fermentation of tea, the content of catechins and flavonoids in tea mostly showed a decreasing trend, mainly due to the oxidation or degradation of catechins and flavonoids into gallic acid during the fermentation process. The biological activity of unfermented tea and microbial fermented tea is also different. After the tea is fermented, its antibacterial and antioxidant capacity would be enhanced. In addition, microbial fermented tea also has anti-inflammatory and weight loss effects. Long-term drinking of microbial fermented tea has good medical and health care effects. Compared with unfermented tea, microbial fermented tea has improved sensory performance, reduced bitterness and astringency, stronger aroma, and brighter tea juice. At present, researchers have not done much research on the fermentation of tea with inoculated microorganisms. In the future, the fermentation of tea with inoculated microorganisms may have a good development prospect. At present, there are many researches on fermented tea in the field of food, such as kombucha, which uses mixed strains to ferment different kinds of tea. Tea fermented by microorganisms has a good prospect in functional food research and development because of its antioxidant, hypoglycemic and lipid lowering properties. The antibacterial properties of fermented tea have a good application prospect in food preservation. In recent years, with the development of fermentation engineering, bostrycin and lovastatin obtained by microbial fermentation of tea have anti-cancer and anti-inflammatory effects, and are expected to be applied in medical clinical research. This article will provide a theoretical basis for future researchers to explore more the functional activities of microbial fermented tea, and provide a certain scientific basis for the research and development of tea in the field of functional health products, food and medicine.
TH: conceptualization, project administration, writing—original draft, and writing—review and editing. SS: investigation and writing—original draft. QM: conceptualization and writing—review and editing. All authors contributed to the article and approved the submitted version.
This research was financially supported by the National Natural Science Foundation of China (21702156), Hubei Provincial Natural Science Foundation of China (2017CFB200), Graduate Innovative Fund of Wuhan Institute of Technology (CX2021452), and the Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (R2019YJ-YB1003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dickmann M, Schneider R, Armando S, Seehusen K, Hager P. Analysis of the role of acidity and tea substrate on the inhibition of α-amylase by Kombucha. J Food Nutr Res-Slov. (2017) 1:1–5. doi: 10.30881/jnfrt.00001
2. Shahbazi H, Hadi HG, Mohammad-Taghi G, Eskandari MH, Movahedi M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of kombucha. Food Sci Nutr. (2018) 6:2568–77. doi: 10.1002/fsn3.873
3. Xia Y, Tan DH, Akbary R, Kong J, Seviour R, Kong YH. Aqueous raw and ripe Pu-erh tea extracts alleviate obesity and alter cecal microbiota composition and function in diet-induced obese rats. Appl Microbiol Biot. (2019) 103:1823–35. doi: 10.1007/s00253-018-09581-2
4. Wu SC, Yen GC, Wang BS, Chiu CK, Yen WJ, Chang LW, et al. Antimutagenic and antimicrobialactivities of pu-erh tea. LWT. (2007) 40:506e512. doi: 10.1016/j.lwt.2005.11.008
5. Cvetkovic D, Ranitovic A, Savic D, Jokovic N, Tomic A, Pezo L, et al. Survival of wild strains of Lactobacilli during Kombucha fermentation and their contribution to functional characteristics of beverage. Pol J Food Nutr Sci. (2019) 69:407–415. doi: 10.31883/pjfns/112276
6. Xiao Y, Zhong K, Bai JR, Wu YP, Gao H. Insight into effects of isolated Eurotium cristatum from Pingwu Fuzhuan brick tea on the fermentation process and quality characteristics of Fuzhuan brick tea. J Sci Food Agr. (2020) 100:3598–607. doi: 10.1002/jsfa.10353
7. Kangwan N, Kongkarnka S, Boonkerd N, Unban K, Shetty K, Khanongnuch C. Protective effect of probiotics isolated from traditional fermented tea leaves (Miang) from northern Thailand and role of Synbiotics in ameliorating experimental ulcerative colitis in mice. Nutrients. (2022) 14:227. doi: 10.3390/nu14010227
8. Zuo AR, Dong HH, Yu YY, Shu QL, Zheng LX, Yu XY, et al. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin Med. (2018) 13:51. doi: 10.1186/s13020-018-0206-9
9. Tong T, Liu YJ, Kang JH, Zhang CM, Kang SG. Antioxidant activity and main chemical components of a novel fermented tea. Molecules. (2019) 24:2917. doi: 10.3390/molecules24162917
10. Ma Y, Ling TJ, Su XQ, Jiang B, Nian B, Chen LJ, et al. Integrated proteomics and metabolomics analysis of tea leaves fermented by Aspergillus niger, Aspergillus tamarii and Aspergillus fumigatus. Food Chem. (2020) 334:127560. doi: 10.1016/j.foodchem.2020.127560
11. Nishioka H, Mizuno T, Iwahashi H, Horie M. Changes in lactic acid bacteria and components of Awa-bancha by anaerobic fermentation. Biosci Biotech Bioch. (2020) 84:1921–35. doi: 10.1080/09168451.2020.1771677
12. Zhao DY, Shah NP. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J Funct Foods. (2016) 20:182–94. doi: 10.1016/j.jff.2015.10.033
13. Neffe-Skocinska K, Sionek B, Scibisz I, Kolozyn-Krajewska D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. Cyta J Food. (2017) 15:601–7. doi: 10.1080/19476337.2017.1321588
14. Jakubczyk K, Kaldunska J, Kochman J, Janda K. Chemical profile and antioxidant activity of the Kombucha beverage derived from White, Green, Black and Red Tea. Antioxidants. (2020) 9:447. doi: 10.3390/antiox9050447
15. Li ZY, Feng CX, Luo XG, Yao HL, Zhang DC, Zhang TC. Revealing the influence of microbiota on the quality of Pu-erh tea during fermentation process by shotgun metagenomic and metabolomic analysis. Food Microbiol. (2018) 76:405–15. doi: 10.1016/j.fm.2018.07.001
16. Jeng KC, Chen CS, Fang YP, Hou RCW, Chen YS. Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. J Agric Food Chem. (2007) 55:8787–92. doi: 10.1021/jf071629p
17. Qin JH, Li N, Tu PF, Ma ZZ, Zhang L. Change in tea polyphenol and purine alkaloid composition during solid-state fungal fermentation of postfermented tea. J Agric Food Chem. (2012) 60:1213–7. doi: 10.1021/jf204844g
18. Wang XG, Wan XC, Hu SX, Pan CY. Study on the increase mechanism of the caffeine content during the fermentation of tea with microorganisms. Food Chem. (2008) 107:1086–91. doi: 10.1016/j.foodchem.2007.09.023
19. Zou MM, Dong QH, Huang Y, Su EZ. Submerged liquid fermentation of raw dark tea by Eurotium cristatum. Chin J Biotechnol Eng (2019) 17:409–17. doi: 10.3969/j.issn.1672-3678.2019.04.012
20. Pasha C, Reddy G. Nutritional and medicinal improvement of black tea by yeast fermentation. Food Chem. (2005) 89:449–53. doi: 10.1016/j.foodchem.2004.02.054
21. Li XL, Chen HH, Zhang JL, Wang B, Zhou HJ. The effects of a Saccharomycetes strain on the main functional ingredients of Pu-er tea. Food Res Dev. (2017) 38:167–72. doi: 10.3969/j.issn.1005-6521.2017.21.033
22. Rigling M, Liu ZB, Hofele M, Prozmann J, Zhang C, Ni L, et al. Aroma and catechin profile and in vitro antioxidant activity of green tea infusion as affected by submerged fermentation with Wolfiporia cocos (Fu Ling). Food Chem. (2021) 361:130065. doi: 10.1016/j.foodchem.2021.130065
23. Bai WF, Guo XY, Ma LQ, Guo LQ, Lin JF. Chemical composition and sensory evaluation of fermented tea with medicinal mushrooms. Indian J Microbiol. (2013) 53:70–76. doi: 10.1007/s12088-012-0345-0
24. Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and human health: the role of bioavailability. Nutrients. (2021) 13:273. doi: 10.3390/nu13010273
25. Xu Q, Sun M, Ning JM, Fang SM, Ye ZL, Chen JH, et al. The core role of Bacillus subtilis and Aspergillus fumigatus in pile-fermentation processing of Qingzhuan Brick tea. Indian J Microbiol. (2019) 59:288–94. doi: 10.1007/s12088-019-00802-4
26. Li YJ, Zhang S, Sun YM. Measurement of catechin and gallic acid in tea wine with HPLC. Saudi J Biol Sci. (2020) 27:214–21. doi: 10.1016/j.sjbs.2019.08.011
27. Wang J, Zhang JW, Chen Y, Yu L, Teng JW, Xia N, et al. The relationship between microbial dynamics and dominant chemical components during Liupao tea processing. Food Biosci. (2021) 43:10135. doi: 10.1016/j.fbio.2021.101315
28. Tran T, Romanet R, Roullier-Gall C, Verdier F, Martin A, Schmitt-Kopplin P, et al. Non-Targeted metabolomic analysis of the Kombucha production process. Metabolites. (2022) 12:160. doi: 10.3390/metabo12020160
29. Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. (2007) 81:519e33. doi: 10.1016/j.lfs.2007.06.011
30. Li J, Xu R, Zong L, Brake J, Cheng L, Wu J, et al. Dynamic evolution and correlation between metabolites and microorganisms during manufacturing process and storage of Fu Brick Tea. Metabolites. (2021) 11:703. doi: 10.3390/metabo11100703
31. Jayabalan R, Marimuthu S, Swaminathan K. Changes in content of organic acids and tea polyphenols during Kombucha tea fermentation. Food Chem. (2007) 102:392–8. doi: 10.1016/j.foodchem.2006.05.032
32. Wang R, Sun JC, Lassabliere B, Yu B, Liu SQ. UPLC-Q-TOF-MS based metabolomics and chemometric analyses for greentea fermented with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. Curr Res Food Sci. (2022) 5:471–8. doi: 10.1016/j.crfs.2022.02.012
33. Wang ZH, Zheng CQ, Ma CQ, Ma BS, Wang JC, Zhou BX, et al. Comparative analysis of chemical constituents and antioxidant activity in tea-leaves microbial fermentation of seven tea-derived fungi from ripened Pu-erh tea. LWT. (2021) 142:111006. doi: 10.1016/j.lwt.2021.111006
34. Ma CQ, Li XH, Xia T. Comparison of characteristic components in tea-leaves fermented by Aspergillus pallidofulvus PT-3, Aspergillus sesamicola PT-4 and Penicillium manginii PT-5 using LC-MS metabolomics and HPLC analysis. Food Chem. (2021) 350:129228. doi: 10.1016/j.foodchem.2021.129228
35. Tu CH, Hu WX, Tang SJ, Meng L, Huang ZH, Xu X, et al. Isolation and identification of Starmerella davenportii strain Do18 and its application in Black tea beverage fermentation. Food Sci Hum Well. (2020) 9:355–62. doi: 10.1016/j.fshw.2020.04.010
36. Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process. (2003) 42:129–33. doi: 10.1016/S0255-2701(02)00037-5
37. Chakravorty S, Bhattacharya S, Chatzinotas A, Chakraborty W, Gachhui R. Kombucha tea fermentation: microbial and biochemical dynamics. Int J Food Microbiol. (2016) 220:63–72. doi: 10.1016/j.ijfoodmicro.2015.12.015
38. Dueñas M, Fernández D, Hernández T, Estrella I, Muñoz R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J Sci Food Agr. (2005) 85:297–304. doi: 10.1002/jsfa.1924
39. An TT, Chen MX, Zu ZQ, Chen Q, Lu HQ, Yue PX, et al. Untargeted and targeted metabolomics reveal changes in the chemical constituents of instant Dark tea during liquid-state fermentation by Eurotium cristatum. Food Res int. (2021) 148:110623. doi: 10.1016/j.foodres.2021.110623
40. Somsong P, Santivarangkna C, Tiyayon P, Hsieh CM, Srichamnong W. Assessing polyphenol components and antioxidant activity during fermented Assam tea ball processing. Sustainability. (2020) 12:5853. doi: 10.3390/su12145853
41. Yao YN, Wu MY, Huang YJ, Li CK, Pan X, Zhu W, et al. Appropriately raising fermentation temperature beneficial to the increase of antioxidant activity and gallic acid content in Eurotium cristatum-fermented loose tea. LWT. (2017) 82:248–54. doi: 10.1016/j.lwt.2017.04.032
42. Liu ML, Xie HF, Ma Y, Li HY, Li CP, Chen LJ, et al. High performance liquid chromatography and metabolomics analysis of Tannase metabolism of gallic acid and gallates in tea leaves. Agri Food Chem. (2020) 68:4946–54. doi: 10.1021/acs.jafc.0c00513
43. Xiao Y, He C, Chen YL, Ho CT, Wu X, Huang YX, et al. UPLC-QQQ-MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of Dark tea. Food Chem. (2022) 378:131999. doi: 10.1016/j.foodchem.2021.131999
44. de Noronha MC, Cardoso RR, Dos Santos D'Almeida CT, Vieira do Carmo MA, Azevedo L, Maltarollo VG, et al. Black tea Kombucha: physicochemical, microbiological and comprehensive phenolic profile changes during fermentation, and antimalarial activity. Food Chem. (2022) 384:132515. doi: 10.1016/j.foodchem.2022.132515
45. Tanaka T, Miyata Y, Tamaya K, Kusano R, Matsuo Y, Tamaru S, et al. Increase of theaflavin gallates and thearubigins by acceleration of catechin oxidation in a new fermented tea product obtained by the tea-rolling processing of loquat (Eriobotrya japonica) and Green tea leaves. J Agric Food Chem. (2009) 57:5816–22. doi: 10.1021/jf900963p
46. Ruiz RB, Hernández PS. Cancer chemoprevention by dietary phytochemicals: epidemiological evidence. Maturitas. (2016) 94:13–19. doi: 10.1016/j.maturitas.2016.08.004
47. Chen XQ, Du Y, Wu L, Xie JC, Chen XL, Hu BB, et al. Effects of tea-polysaccharide conjugates and metal ions on precipitate formation by epigalloca-techin gallate and caffeine, the key components of green tea infusion. J Agric Food Chem. (2019) 67:3744–51. doi: 10.1021/acs.jafc.8b06681
48. Dhatwalia SK, Kumar M, Dhawan D. Role of EGCG in containing the progression of lung tumorigenesis-A multistage targeting approach. Nutr Cancer. (2018) 70:334–49. doi: 10.1080/01635581.2018.1445762
49. Ziemlewska A, Niziol-Lukaszewska Z, Bujak T, Zagorska-Dziok M, Wojciak M, Sowa I. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha yerba mate extracts. Sci Rep. (2021) 11:18792. doi: 10.1038/s41598-021-98191-6
50. La Torre C, Fazio A, Caputo P, Plastina P, Caroleo MC, Cannataro R, et al. Effects of long-term storage on radical scavenging properties and phenolic content of Kombucha from Black Tea. Molecules. (2021) 26:5474. doi: 10.3390/molecules26185474
51. Cho D, Jeong HW, Kim JK, Kim AY, Hong YD, Lee JH, et al. Gallocatechin gallate-containing fermented Green tea extract ameliorates obesity and hypertriglyceridemia through the modulation of lipid metabolism in adipocytes and myocytes. J Med Food. (2019) 22:779–88. doi: 10.1089/jmf.2018.4327
52. Neffe-Skocinska K, Jaworska D, Kolozyn-Krajewska D, Dolatowski Z, Jachacz-Jowko L. The effect of LAB as probiotic starter culture and Green tea extract addition on dry fermented pork loins quality. Biomed Res Int. (2015) 2015:452757. doi: 10.1155/2015/452757
53. Mo HZ, Zhu Y, Chen ZM. Microbial fermented tea-A potential source of natural food preservatives. Trends Food Sci Tech. (2008) 19:124–130. doi: 10.1016/j.tifs.2007.10.001
54. Kridsada U, Nuttapong K, Kalidas S, Chartchai K. Nutritional biotransformation in traditional fermented tea (Miang) from north Thailand and its impact on antioxidant and antimicrobial activities. J Food Sci Technol. (2019) 56:2687–99. doi: 10.1007/s13197-019-03758-x
55. Yang KD, Duley ML, Zhu JJ. Metabolomics study reveals enhanced inhibition and metabolic dysregulation in Escherichia coli induced by Lactobacillus acidophilus-fermented Black tea extract. J Agr Food Chem. (2018) 66:1386–93. doi: 10.1021/acs.jafc.7b04752
56. Sreeramulu G, Zhu Y, Knol W. Kombucha fermentation and its antimicrobial activity. J Agr Food Chem. (2000) 48:2589–94. doi: 10.1021/jf991333m
57. Ling TJ, Wan XC, Ling WW, Zhang ZZ, Xia T, Li DX, et al. New triterpenoids and other constituents from a special microbial-fermented tea-Fuzhuan Brick tea. J Agr Food Chem. (2010) 58:4945–50. doi: 10.1021/jf9043524
58. Al-Mohammadi A-R, Ismaiel AA, Ibrahim RA, Moustafa AH, Abou Zeid A, Enan G. Chemical constitution and antimicrobial activity of Kombucha fermented beverage. Molecules. (2021) 26:5026. doi: 10.3390/molecules26165026
59. Keller AC, Weir TL, Broeckling CD, Ryan EP. Antibacterial activity and phytochemical profile of fermented Camellia sinensis (fuzhuan tea). Food Res Int. (2013) 53:945–9. doi: 10.1016/j.foodres.2013.04.023
60. Muhialdin BJ, Osman FA, Muhamad R, Sapawi CWNSCW, Anzian A, Voon WWY, Hussin ASM. Effects of sugar sources and fermentation time on the properties of tea fungus (Kombucha) beverage. Int Food Res J. (2019) 26:481–7.
61. Kridsada U, Nuttapong K, Thanawat P, Chalermpong S, Kalidas S, Chartchai K. Microbial community dynamics during the non-filamentous fungi growth-based fermentation process of Miang, a traditional fermented tea of north thailand and their product characterizations. Front Microbiol. (2020) 11:1515. doi: 10.3389/fmicb.2020.01515
62. Ahmed RF, Hikal MS, Abou-Taleb KA. Biological, chemical and antioxidant activities of different types Kombucha. Ann Agric Sci. (2020) 65:35–41. doi: 10.1016/j.aoas.2020.04.001
63. Jin YH, Hong JH, Lee J-H, Yoon H, Pawluk AM, Yun SJ, et al. Lactic acid fermented Green tea with Levilactobacillus brevis capable of producingγ-aminobutyric acid. Fermentation. (2021) 7:110. doi: 10.3390/fermentation7030110
64. Yang CS, Wang H, Sheridan ZP. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. J Food Drug Anal. (2018) 26:1–13. doi: 10.1016/j.jfda.2017.10.010
65. Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu Rev Nutr. (2013) 33:161–81. doi: 10.1146/annurev-nutr-071811-150717
66. Yang CS, Zhang JS, Zhang L, Huang JB, Wang YJ. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol Nutr Food Res. (2016) 60:160–74. doi: 10.1002/mnfr.201500428
67. Xiang XL, Su C, Shi QX, Wu JN, Zeng ZX, Zhang LJ, et al. Potential hypoglycemic metabolites in dark tea fermented by Eurotium cristatum based on UPLC-QTOF-MS/MS combining global metabolomic and spectrum-effect relationship analyses. Food Funct. (2021) 12:7546–56. doi: 10.1039/D1FO00836F
68. Ahmed A, Khaled H, Dhouha E, Madiha BA, Khaoula H, Bassem J, et al. Hypoglycemic and antilipidemicproperties of Kombucha tea in alloxan-induced diabetic rats. BMC Complem Altern M. (2012) 12:63. doi: 10.1186/1472-6882-12-63
69. Xu S Wang Y, Wang J, Geng W. Kombucha reduces hyperglycemia in type 2 diabetes of mice by regulating gut microbiota and its metabolites. Foods. (2022) 11:754. doi: 10.3390/foods11050754
70. Deng XJ, Hou Y, Zhou HJ, Li YL, Xue ZQ, Xue XT, et al. Hypolipidemic, anti-inflammatory, and anti-atherosclerotic effects of tea before and after microbial fermentation. Food Sci Nutr. (2021) 9:1160–70. doi: 10.1002/fsn3.2096
71. Yoo A, Kim MJ, Ahn J, Jung CH, Seo HD, Ly SY, et al. Fuzhuan brick tea extract prevents diet-induced obesity via stimulation of fat browning in mice. Food Chem. (2022) 377:132006. doi: 10.1016/j.foodchem.2021.132006
72. Xiao Y, Zhong K, Bai JR, Wu YP, Zhang JQ, Gao H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (MF800948) and its hypolipidemic activity in a zebrafish model. LWT. (2020) 117:108629. doi: 10.1016/j.lwt.2019.108629
73. Wang LC, Pan TM, Tsai TY. Lactic acid bacteria -fermented product of Green tea and Houttuynia cordata leaves exerts anti-adipogenic and anti-obesity effects. J Food Drug Anal. (2018) 26:973–84. doi: 10.1016/j.jfda.2017.11.009
74. Qin Y, Yuan ZJ, Yang FY, Yu YG. Development of a new type of Anhua black tea and its application: black tea wine. J Food Process Prese. (2021) e158162. doi: 10.1111/jfpp.15862
75. Yang F, Feng B, Niu YJ, Hu CY, Meng YH. Fu instant tea ameliorates fatty liver by improving microbiota dysbiosis and elevating short-chain fatty acids in the intestine of mice fed a high-fat diet. Food Biosci. (2021) 42:101207. doi: 10.1016/j.fbio.2021.101207
76. Zhao X, Song JL, Kim JD, Lee JS, Park KY. Fermented Pu-erh Tea increases in vitro anticancer activities in HT-29 cells and has antiangiogenetic effects on HUVECs. J Environ Pathol Tox. (2013) 32:275–88. doi: 10.1615/JEnvironPatholToxicolOncol.2013007074
77. Zhang H, Sang ST, Xu HM, Piao LH, Liu XD. Lovastatin suppresses bacterial therapy-induced neutrophil recruitment to the tumor by promoting neutrophil apoptosis. J Funct Foods. (2021) 86:104693. doi: 10.1016/j.jff.2021.104693
78. Abdullahi AD, Kodchasee P, Unban K, Pattananandecha T, Saenjum C, Kanpiengjai A, et al. Comparison of phenolic contents and scavenging activities of miang extracts derived from filamentous and non-filamentous fungi-based fermentation processes. Antioxidants. (2021) 10:1144. doi: 10.3390/antiox10071144
79. Villarreal-Soto SA, Beaufort S, Bouajila J, Souchard JP, Renard T, Ronan S, et al. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in Kombucha tea extracts. Process Biochem. (2019) 83:44–54. doi: 10.1016/j.procbio.2019.05.004
80. Li JH, Shi L, Xu SY, Gu SY, Wen X, Xu DY, et al. Optimal fermentation time for Nigrospora-fermented tea rich in bostrycin. J food Agri. (2021) 101:2483–90. doi: 10.1002/jsfa.10874
81. Horie M, Tada A, Kanamoto N, Tamai T, Fukuda N, Sugino S, et al. Evaluation of lactic acid bacteria and component change during fermentation of Ishizuchi-kurocha. J Food Process Pres. (2019) 43:e14186. doi: 10.1111/jfpp.14186
82. Yan K, Yan LF, Meng LN, Cai HB, Duan AL, Wang L, et al. Comprehensive analysis of bacterial community structure and diversity in Sichuan Dark tea (Camellia sinensis). Front Microbiol. (2021) 12:735618. doi: 10.3389/fmicb.2021.735618
83. Zhang CY, Guo JF, Zhang ZX, Tian SH, Liu ZtH, Shen CW. Biochemical components and fungal community dynamics during the flowering process of Moringa-Fu brick tea, a novel microbially fermented blended tea. LWT. (2021) 140:110822. doi: 10.1016/j.lwt.2020.110822
84. Zhang L, Cao QQ, Granato D, Xu YQ, Ho CT. Association between chemistry and taste of tea: a review. Trends Food Sci Tech. (2020) 101:139–9. doi: 10.1016/j.tifs.2020.05.015
85. Han T, Aye KN. The legend of laphet: A Myanmar fermented tea leaf. J Ethn Food. (2015) 2:173–8. doi: 10.1016/j.jef.2015.11.003
86. Nishioka H, Ohno T, Iwahashi H, Horie M. Diversity of Lactic acid bacteria involved in the fermentation of Awa-bancha. Microbes Environ. (2021) 36:ME21029. doi: 10.1264/jsme2.ME21029
87. Zhao ZJ, Sui YC, Wu HW, Zhou CB, Hu XC, Zhang J. Flavour chemical dynamics during fermentation of Kombucha tea. Emir J Food Agric. (2018) 30:732–41. doi: 10.9755/ejfa.2018.v30.i9.1794
88. Cheng LZ, Yang QQ, Chen ZY, Zhang JR, Chen Q, Wang YF, et al. Distinct changes of metabolic profile and sensory quality during Qingzhuan tea processing revealed by LC-MS-Based metabolomics. J Agric Food Chem. (2020) 68:4955–65. doi: 10.1021/acs.jafc.0c00581
89. Li M, Xiao Y, Zhong K, Wu Y, Gao H. Delving into the biotransformation charakcteristics and mechanism of steamed Green Tea fermented by Aspergillus niger PW-2 based on metabolomic and proteomic approaches. Foods. (2022) 11:865. doi: 10.3390/foods11060865
90. Lv SD, Wu YS, Wei JF, Lian M, Wang C, Gao X M, et al. Application of gas chromatography-mass spectrometry and chemometrics methods for assessing volatile profiles of Pu-erh tea with different processing methods and ageing years. RSC Adv. (2015) 5:87806–17. doi: 10.1039/C5RA15381F
91. Wang DM, Kurasawa E, Yamaguchi Y, Kubota K, Kobayashi A. Analysis of glycosidically bound aroma precursors in tea leaves. 2. Changes in glycoside contents and glycosidase activities in tea leaves during the black tea manufacturing process. J Agric Food Chem. (2001) 49:1900–3. doi: 10.1021/jf001077+
92. Miyata Y, Tanaka T, Noda M, Tamaya K, Matsui T, Nishizono S, et al. Characteristics of aroma compounds in mixed fermented tea containing Green Tea and Loquat Leaves. J Jpn Soc Food Sci. (2010) 57:171–4. doi: 10.3136/nskkk.57.171
93. Cao LT, Guo XM, Liu GJ, Song YL, Ho CT, Hou R, et al. A comparative analysis for the volatile compounds of various Chinese dark teas using combinatory metabolomics and fungal solid-state fermentation. J Food Drug Anal. (2018) 26:112–23. doi: 10.1016/j.jfda.2016.11.020
94. Wang R, Sun J C, Lassabliere B, Yu B, Liu SQ. Fermentation characteristics of four non-Saccharomyces yeasts in Green tea slurry. Food Microbiol. (2020) 92:103609. doi: 10.1016/j.fm.2020.103609
95. Wang R, Sun JC, Lassabliere B, Yu B, Liu SQ. 13-Glucosidase activity of Cyberlindnera (Williopsis) saturnus var. mrakii NCYC 2251 and its fermentation effect on Green tea aroma compounds. LWT. (2021) 151:112184. doi: 10.1016/j.lwt.2021.112184
Keywords: tea beverage, microbial fermentation, tea polyphenols, biological activity, sensory characteristics
Citation: Hu T, Shi S and Ma Q (2022) Modulation effects of microorganisms on tea in fermentation. Front. Nutr. 9:931790. doi: 10.3389/fnut.2022.931790
Received: 29 April 2022; Accepted: 08 July 2022;
Published: 02 August 2022.
Edited by:
Minhao Xie, Nanjing University of Finance and Economics, ChinaReviewed by:
Ioanna Mantzourani, Democritus University of Thrace, GreeceCopyright © 2022 Hu, Shi and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Ma, cWlubWFfZ2Fhc0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.