
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 30 August 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.931599
This article is part of the Research TopicInsights in Clinical NutritionView all 30 articles
 Xuepeng Zhang1,2,3†
Xuepeng Zhang1,2,3† Lifan Zhang4†
Lifan Zhang4† Canzheng Wei2,5†
Canzheng Wei2,5† Liwei Feng1†
Liwei Feng1† Juqin Yang6
Juqin Yang6 Geng Zhang2
Geng Zhang2 Guoyan Lu7
Guoyan Lu7 Xiying Gui8
Xiying Gui8 Yue Zhou2
Yue Zhou2 Kaiying Yang1
Kaiying Yang1 Jiangyuan Zhou1
Jiangyuan Zhou1 Xinle Zhou9
Xinle Zhou9 Ruoran Wang2
Ruoran Wang2 Siyuan Chen2*
Siyuan Chen2* Yi Ji1*
Yi Ji1*Introduction: The detailed association between albumin levels and mortality has not been studied in critically ill children. The aim of this study was to reveal an association between albumin levels in detail and mortality in critically ill children.
Materials and methods: We retrospectively collected data from children admitted to four pediatric intensive care units (PICUs) in China between January 2015 and October 2020. Restricted cubic spline curves based on logistic regression models were generated to evaluate the detailed associations between serum albumin levels and PICU mortality. Threshold effect analysis was performed using two piecewise regression models.
Results: The study included 9,123 children. The overall mortality was 5.3%. The detailed association between serum albumin levels and the risk of mortality followed a U-shape. The risk of mortality decreased with increasing serum albumin levels (OR = 0.919; 95% CI: 0.886, 0.954) in children with serum albumin levels < 43.2 g/L and increased with increasing serum albumin levels (OR = 1.174; 95% CI: 1.044, 1.316) in children with serum albumin levels ≥ 43.2 g/L.
Conclusion: There was a U-shaped association between serum albumin levels and mortality in critically ill children in the PICU.
A serum albumin level of less than 35 g/L is considered hypoalbuminemia, which is frequent in hospitalized patients, especially critically ill patients. It has been reported that more than 60% of pediatric patients have hypoalbuminemia in intensive care units. Many studies have revealed that hypoalbuminemia is associated with poor clinical outcomes. However, the detailed association between albumin levels and outcomes has not been well elucidated. In this study, we found a U-shaped association between serum albumin levels and mortality in critically ill children in the pediatric intensive care unit (PICU). Low albumin levels and high albumin levels were associated with mortality. The findings in this study suggest that maintaining the optimal serum albumin levels is important in critically ill patients in the PICU.
Serum albumin is the main contributor to intravascular colloid osmotic pressure. The normal range of serum albumin concentrations in healthy subjects is approximately 35–50 g/L. A serum albumin level of less than 35 g/L is considered hypoalbuminemia, which is frequent in hospitalized patients, especially critically ill patients. It has been reported that more than 60% of pediatric patients and nearly 50% of adult patients have hypoalbuminemia in intensive care units (ICUs) (1, 2). Albumin is frequently administered in the ICU to combat low albumin production. However, many albumin prescriptions are thought to be inappropriate, which may result in albumin administration being less cost-effective (3, 4). Determining the detailed association between albumin levels and outcomes could be clinically meaningful. On the one hand, it is impossible to maintain a serum albumin level above 35 g/L throughout the ICU stay. More importantly, this information could help intensivists appropriately administer albumin and reduce albumin abuse.
Serum albumin levels have long been recognized as an indicator of outcomes in hospitalized patients (5–8). A large number of studies have revealed that hypoalbuminemia is associated with adverse outcomes, including increased mortality, readmission, ICU admission, and prolonged hospital stay (1, 2, 8–10). However, the detailed association between albumin levels and outcomes has not been well elucidated. In this study, we aimed to quantify the association between serum albumin levels and mortality in critically ill children in the pediatric intensive care unit (PICU) in detail.
This was a retrospective study of critically ill children admitted to four PICUs in three tertiary referral hospitals in China. None of the four PICUs were a neonatal intensive care unit (NICU) and only received very few neonates. All children admitted to the PICUs between January 2015 and October 2020 were included in this study except those discharged from the PICU with uncertain outcomes, those without documented albumin values during the ICU stay, and those older than 18 years. The study was approved by the Ethics Committee of the Central Processing Center (West China Hospital of Sichuan University) (20201113-202022402017). The requirement for informed consent was waived due to the retrospective nature of the study and because the study did not divulge the patients’ private information.
The patients’ demographics, clinical characteristic data, laboratory values, vital signs upon admission to the ICU, including respiratory rate, blood pressure, heart rate and oxygen saturation, and PICU outcome were extracted from the Electronic Medical Record Systems of the hospitals. Albumin levels on admission, the lowest albumin level during the PICU stay, and the duration with an albumin level below the normal range were collected for analysis. The duration with an albumin level below the normal range was defined as the total number of days with an albumin level below 35 g/L during the PICU stay. It was 0 days for patients with no days with an albumin level below 35 g/L and 1 day for patients with a single day with albumin levels below 35 g/L. For patients with more than 1 day with an albumin level below 35 g/L, the duration was calculated by summing the number of days with an albumin level below 35 g/L. If there were days with albumin levels higher than 35 g/L between two albumin levels below 35 g/L, we separately calculated the durations and then added them together. Data management was performed by PostgreSQL version 11.11 (PostgreSQL Global Development Group).
The detailed associations between serum albumin levels and PICU mortality were evaluated with restricted cubic spline curves based on logistic regression models with adjustment for other covariates, including Pediatric Risk of Mortality (PRISM) score, age, race, sex, admission from emergency department, cardiac surgery, oxygen partial pressure (PO2), partial pressure of carbon dioxide (PCO2), shock index, respiratory rate (RR), bilirubin, estimated glomerular filtration rate (eGFR) being calculated according to Schwartz equation (11), mechanical ventilation, inotropes, and acute kidney injury (AKI) development (The baseline creatinine levels were the creatinine values on admission). The number of knots was set as 4 (0.05, 0.35, 0.65, 0.95) because 4 knots not only provide sufficient fit of the model but are a good compromise between flexibility and overfitting (12). Stratified analyses also used the same number of knots for comparison of overall and stratified analyses. The albumin levels not associated with mortality were defined as the albumin concentrations at which the 95% confidence interval (CI) of the odds ratio (OR) for mortality included a value of 1.0 in the cubic spline curves. Threshold effect analysis was performed using two piecewise regression models.
Continuous variables with normal distributions are presented as the means ± standard deviations (SDs). Non-normally distributed variables were described as medians [interquartile ranges (IQRs)]. Categorical variables were expressed as counts (percentages). P < 0.05 was considered statistically significant. All statistical analyses were performed in R version 3.6.1.
A total of 9,671 pediatric patients were admitted to the PICU during the study period. Five hundred forty-eight patients were excluded for the following reasons: 235 patients had no albumin values during the ICU stay; 131 children had unknown outcomes; and 192 patients were older than 18 years. Finally, 9,123 patients were included in the analysis. Table 1 lists the characteristics of the patients by albumin centile subgroups according to the knots. In the overall population, the median age of the pediatric population was 1.0 (IQR, 0.0–4.0) years. The ratio of males was 54.7%. The mean albumin level was 38.2 ± 5.5 g/L. The most frequent reasons for PICU admission were respiratory disorders, followed by cardiovascular and digestive disorders. The median length of ICU stay was 3.0 (IQR, 1.0–6.0) days. The overall hospital mortality rate was 5.3%. The mortality of children with the lowest albumin level was 18.5%, which was the highest among the five categories.
The association between serum albumin levels and the risk of mortality was U-shaped (Figure 1). Low and high albumin levels were both associated with the risk of mortality. In the unadjusted cubic spline, albumin levels between 35.9 g/L (OR = 1.101; 95% CI: 0.998–1.215) and 43.2 g/L (OR = 1.139; 95% CI: 0.993–41.306) were not associated with mortality (Figure 1A). In multivariable adjusted analyses, the association between serum albumin levels and the risk of mortality still followed a U-shape (Figure 1B). Albumin levels between 35.8 g/L (OR = 1.196; 95% CI: 0.993–1.440) and 50.1 g/L (OR = 1.731; 95% CI: 0.999–3.0) were not associated with mortality in the adjusted cubic spline (Figure 1B). The albumin level associated with the lowest risk of mortality in multivariable analyses was 40.6 g/L. The lowest albumin levels also had a U-shaped association with the risk of mortality in the unadjusted model (Figure 2A) but not in the adjusted model (Figure 2B). In addition, the risk of mortality increased with increasing duration of albumin levels below 35 g/L (Figure 3).
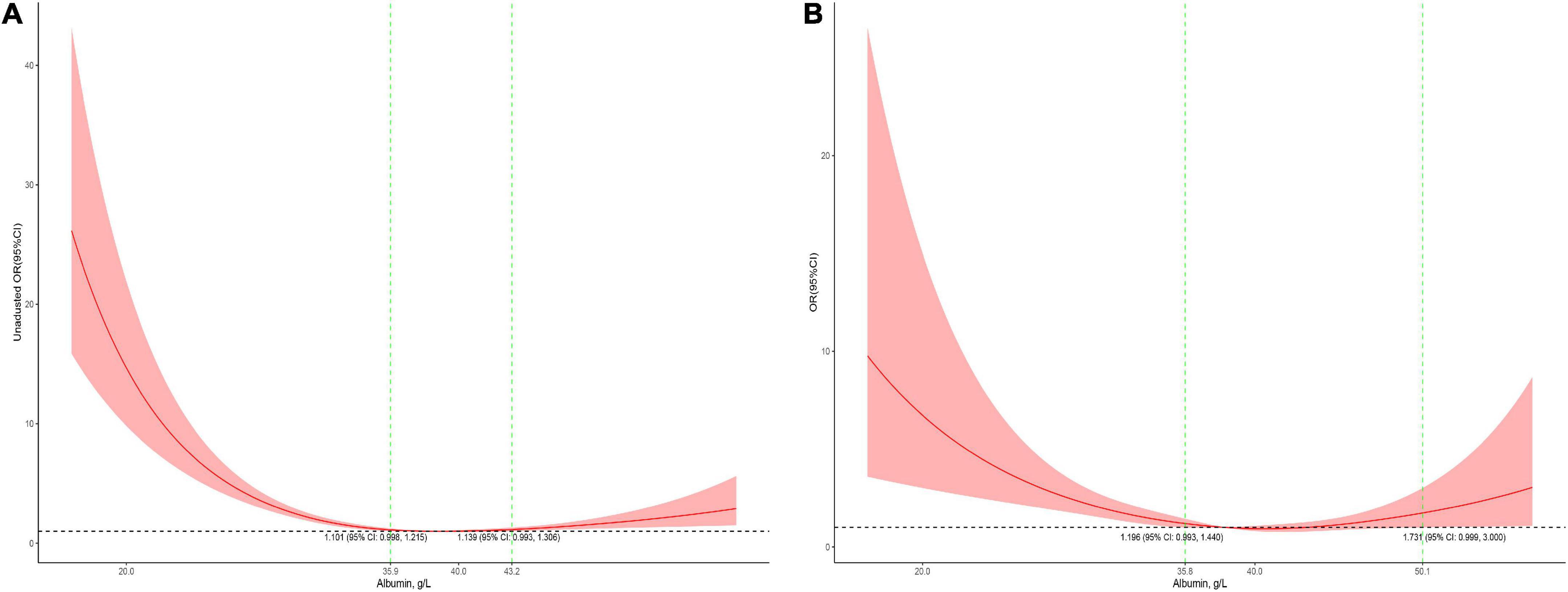
Figure 1. Association between serum albumin levels and mortality in all patients (A, the crude model; B, the model adjusted for age, race, sex, admission form emergency department, cardiac surgery, PO2, PCO2, shock index, RR, bilirubin, eGFR, mechanical ventilation, inotropes, AKI, and PRISM). Y-axis present the odds ratio, and the X-axis present albumin values. The 95% CI included an OR of 1.0 at albumin concentrations between 35.9 and 43.2 g/L in the crude model and between 35.9 and 50.1 g/L in the adjusted model.
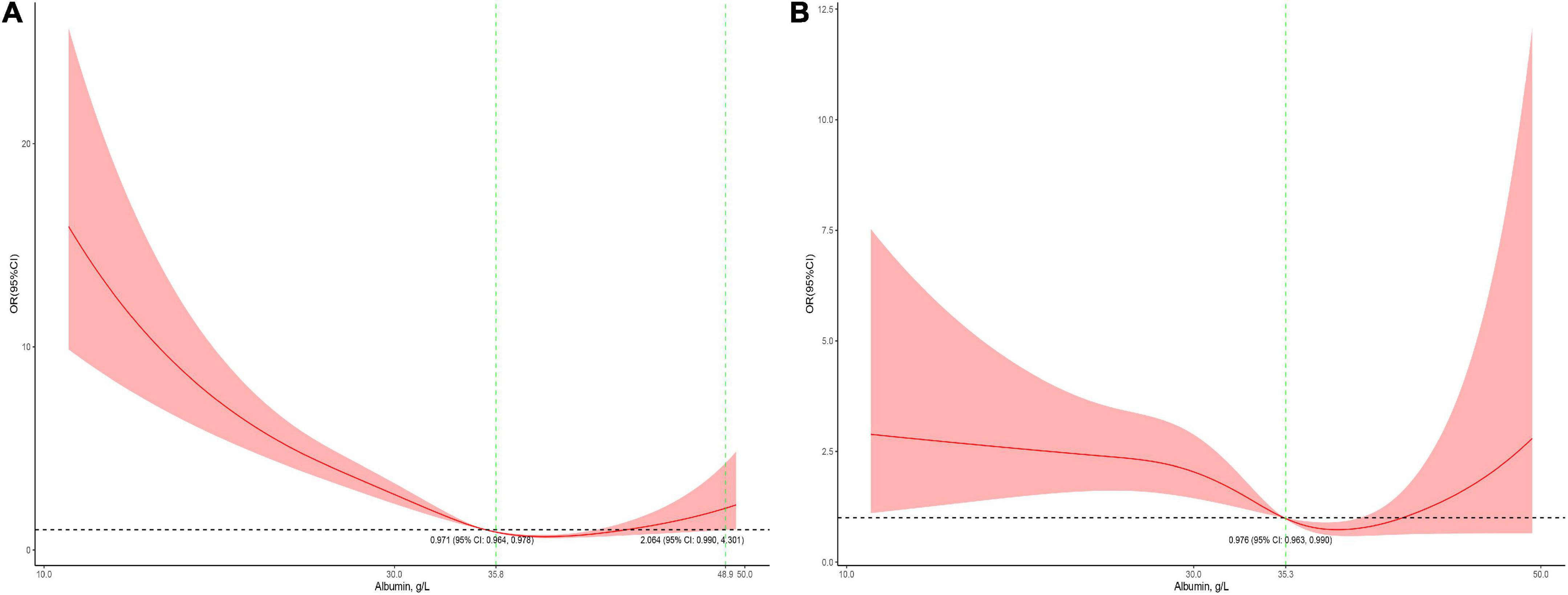
Figure 2. Association between the lowest serum albumin levels during the PICU stay and mortality in all patients (A, the crude model; B, the model adjusted for age, race, sex, admission form emergency department, cardiac surgery, PO2, PCO2, shock index, RR, bilirubin, eGFR, mechanical ventilation, inotropes, AKI, and PRISM). The 95% CI included an OR of 1.0 at albumin concentrations between 35.8 and 48.9 g/L in the crude model and > 35.3 in the adjusted model.
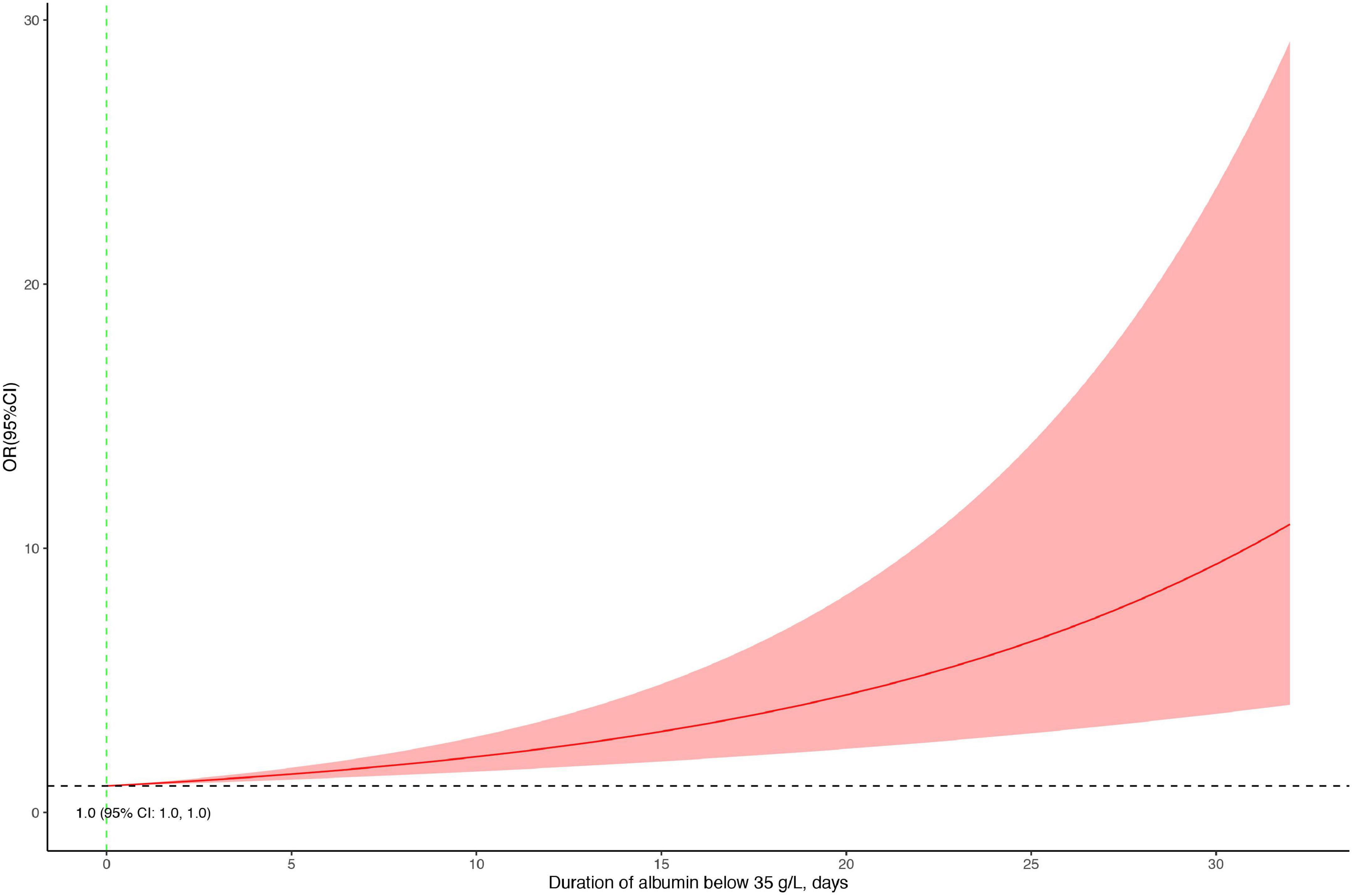
Figure 3. Association between the duration with an albumin level below 35 g/L and the risk of mortality.
In the threshold effect analysis using two piecewise regression models, the turning points were 43.2 g/L in multivariable analyses and 35.8 g/L in univariable analysis (Table 2). In multivariable analyses, the risk of mortality was significantly decreased with the increment of albumin level (OR = 0.919; 95% CI: 0.886–0.954) in children with a serum albumin level < 43.2 g/L and increased with the increment of serum albumin level (OR = 1.174; 95% CI: 1.044–1.316) in children with a serum albumin level ≥ 43.2 g/L. In the univariable analysis, albumin level also showed a negative association with the risk of mortality (OR = 0.841; 95% CI: 0.815–0.867) in children with a serum albumin level < 35.8 g/L and a positive association with risk of mortality (OR = 1.038; 95% CI: 1.038–1.069) in children with a serum albumin level ≥ 35.8 g/L.

Table 2. Threshold effect analyses of serum albumin levels on mortality using two piecewise regression models.
Serum albumin concentrations had a U-shaped association with the risk of mortality in male patients and a J-shaped association with the risk of mortality in female patients (Figure 4). In the children who received cardiac surgery, the 95% CI included an OR of 1.0 at any concentration of albumin (Figure 5A). In the patients who underwent non-cardiac surgery, the association between serum albumin and the risk of mortality followed a U-shape (Figure 5B).
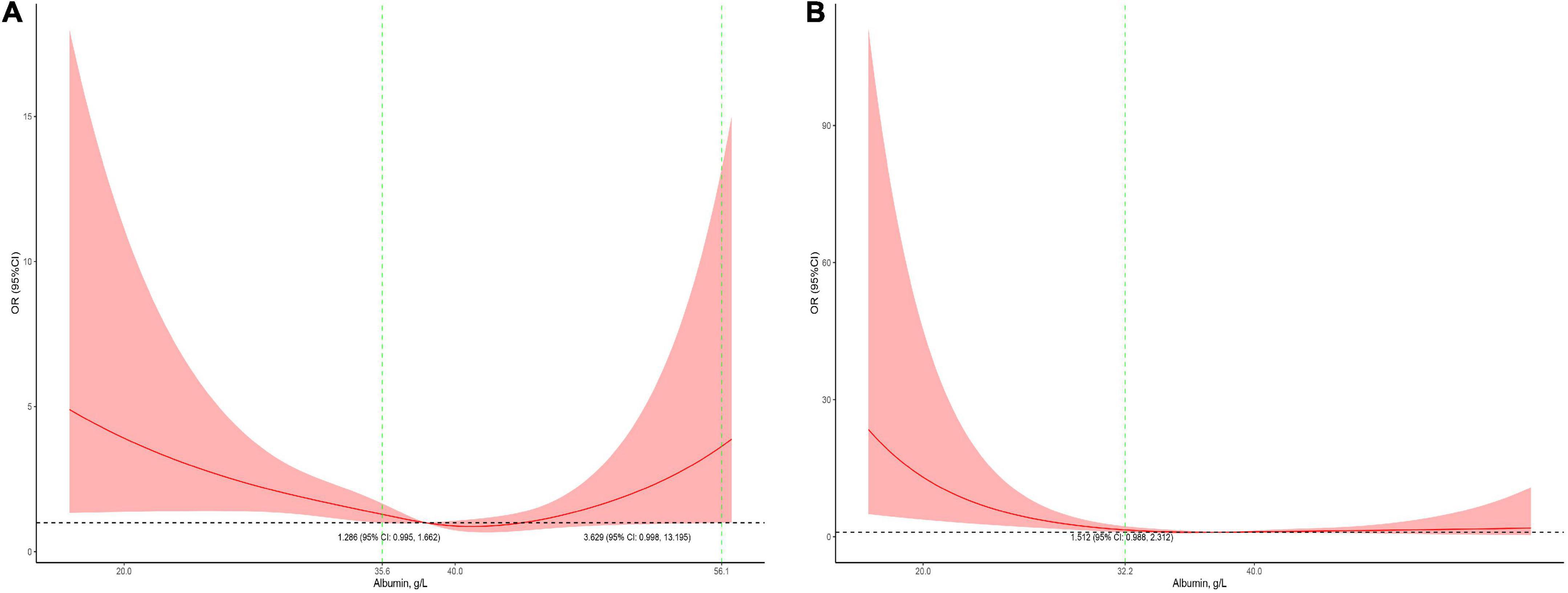
Figure 4. Stratified analysis for the association between serum albumin levels and mortality (A, subgroup analysis in male patients; B, subgroup analysis in female patients). The models were adjusted for age, race, admission form emergency department, cardiac surgery, PO2, PCO2, shock index, RR, bilirubin, eGFR, mechanical ventilation, inotropes, AKI, and PRISM.
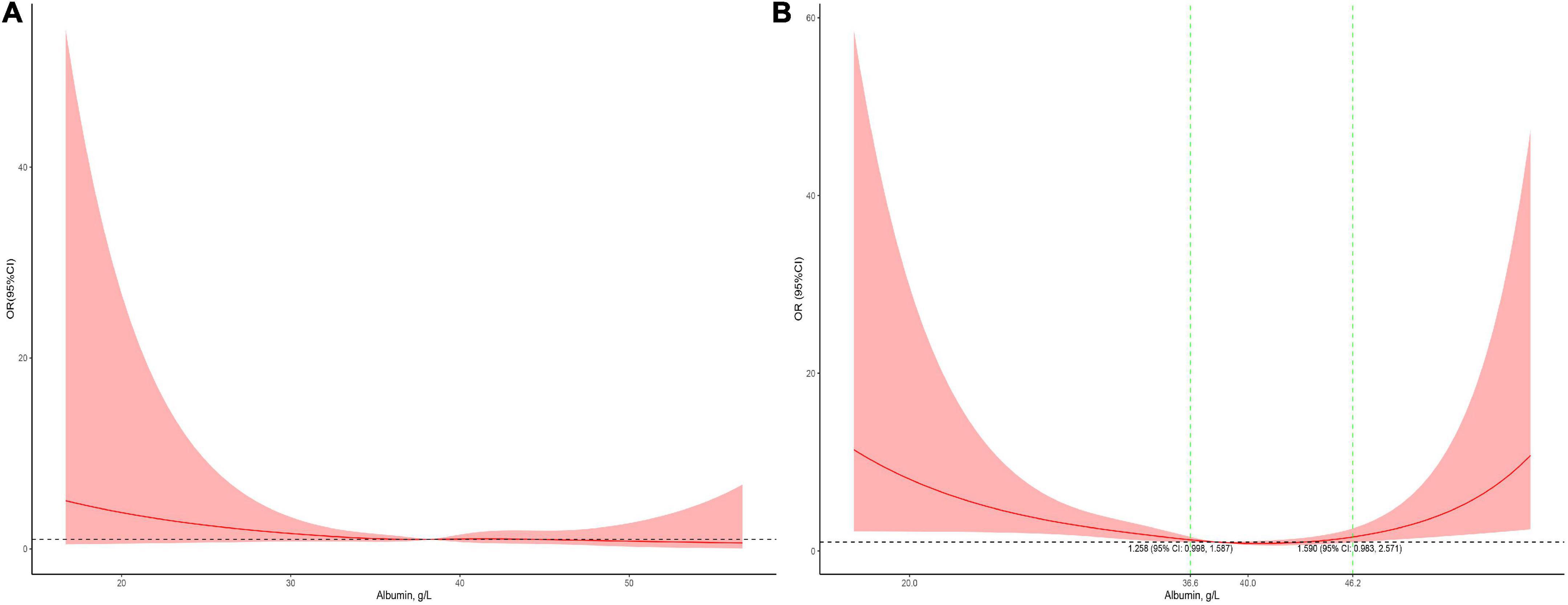
Figure 5. Stratified analysis for the association between serum albumin levels and mortality in surgical patients (A, subgroup analysis in cardiac surgery patients; B, subgroup analysis in non-cardiac surgery patients). The models were adjusted for age, race, sex, admission form emergency department, PO2, PCO2, shock index, RR, bilirubin, eGFR, mechanical ventilation, inotropes, AKI, and PRISM.
In this study of 9,123 critically ill patients admitted to the PICU, we evaluated the association in detail between serum albumin concentrations and mortality by using restricted cubic spline curves based on a logistic regression model. We found a U-shaped association between serum albumin levels and the risk of mortality in critically ill children. Low and high albumin levels were both associated with a higher risk of mortality.
Serum albumin has been found to be a predictor of clinical outcomes in the last century (7, 13). A lower serum albumin concentration is inversely related to the risk of mortality. For each 25 g/L decrement in serum albumin concentration, the risk of mortality was estimated to increase as high as 56% (13). In addition to mortality, albumin levels have also been revealed to be associated with morbidities, such as sepsis and major infections (7). In a large prospective study, a decrease in albumin levels of 25 g/L was associated with an increase in morbidity of 55%. Consistently, albumin concentrations have also been shown to be associated with poor clinical outcomes in critically ill children (2, 14). Each increase of 10 g/L in serum albumin in critically ill children is associated with a 73% reduction in the risk of 60-day mortality (hazard ratio = 0.27; 95% CI: 0.14–0.51) (2). In a recent study, albumin levels still showed a negative association with mortality in patients admitted to the PICU (OR = 0.289; 95% CI: 0.136–0.615) (14).
In the current study, as expected, we also found an association between low serum albumin levels and a higher risk of mortality in critically ill children. In patients with serum albumin levels < 43.2 g/L, each increase of 1 g/L in serum albumin was associated with an 8.1% reduction in the risk of PICU mortality. This finding was similar to that of a previous study in critically ill children, in which a 7.3% reduction in the risk of mortality was associated with an increase of 1 g/L in serum albumin (hazard ratio = 0.27; 95% CI: 0.14–0.51) (2). In critically ill adult patients, each 1 g/L increase in serum albumin also resulted in a reduction in mortality of more than 60% (OR = 0.383; 95% CI: 0.198–0.740) (15).
What was new in this study was that high serum albumin also showed an association with mortality. There was a U-shaped association between serum albumin levels and the risk of mortality in critically ill children. To the best of our knowledge, there is no evidence that a high serum albumin level is associated with mortality in critically ill children. However, there was a study revealing a U-shaped association between clinical outcomes and children with end-stage renal disease (16). In the study, serum albumin levels < 35 g/L and ≥ 45 g/L were both associated with hospitalization frequency and hospitalization days. Similar findings were also found in adult patients. In a retrospective study, by stratifying patients into groups according to serum albumin levels, the investigators revealed that the risk of hospital-acquired AKI was related to a low albumin level (≤ 24 g/L) (OR = 1.52; 95% CI: 1.18–1.94) and a high albumin level (≥ 45 g/L) (OR = 2.16; 95% CI: 1.74–2.96) (17). In a recent study among adult patients with hypertension, a U-shaped association between serum albumin levels and chronic kidney disease was also revealed by using cubic spline curves (18). In the study, albumin levels < 51.4 g/L and ≥ 51.4 showed opposite associations with outcomes in patients (OR = 0.92; 95% CI: 0.88–0.96 vs. OR = 1.06; 95% CI: 1.01–1.11). The underlying mechanism of the association between high albumin levels and poor clinical outcomes is not fully understood. One of the potential mechanisms might be dehydration, which is common in sick young children. Studies have reported that high serum albumin levels are mostly caused by dehydration (19, 20). In the current study, the patients with the highest serum albumin levels had relatively higher blood urea nitrogen (BUN), hematocrit (HCT%) and serum sodium levels as well as a higher shock index but a lower estimated glomerular filtration rate (eGFR) (Table 1), which is a sign of dehydration. However, further studies are needed to understand the potential mechanism because high albumin levels are generally regarded as normal.
In children receiving cardiac surgery, hypoalbuminemia is associated with an increased length of hospital stay and mortality (21, 22). However, in this study, the serum albumin levels did not appear to be not associated with mortality in children receiving cardiac surgery. The most likely explanation may be that the number of patients with an albumin level < 29.5 g/L was too small. Only 31 patients undergoing cardiac surgery had albumin levels < 29.5 g/L, which may be too small to reveal statistical significance. Unlike the subgroup analysis in cardiac surgical patients, serum albumin levels showed the same U-shaped association with mortality in non-cardiac surgical patients.
In the adjusted analysis in the overall population, albumin levels between 35.8 and 50.1 g/L were not associated with mortality. This range might be clinically meaningful because maintaining the optimal serum albumin level is important in critically ill children in the PICU. Male patients had an approximate albumin level range between 35.6 and 56.1 g/L. The range in non-cardiac surgical patients was 36.6–46.2 g/L, narrower than those in the overall population.
This study has several limitations. First, although we included nearly all patients with documented albumin test results in this study, incomplete data were inevitable due to the inherent limitations of retrospective studies. Second, the dataset used for analysis came from large tertiary referral centers. The study design may limit the generalizability of our results to other non-tertiary centers and may introduce referral bias because of increased disease severity. Furthermore, we failed to reveal an association between serum albumin levels and mortality in children receiving cardiac surgery. This result should be cautiously treated because of the small sample of cardiac surgery patients with low albumin levels.
In this study, we found a U-shaped association between serum albumin levels and mortality in critically ill children in the PICU. Both low and high albumin levels were associated with mortality. Serum albumin levels between 35.8 and 50.1 g/L were identified as the range not related to mortality in critically ill children. The range may suggest that maintaining optimal serum albumin levels is important in critically ill patients in the PICU.
The datasets used for the analysis in the current study are not in a public repository. However, the datasets are available from the corresponding author upon reasonable request.
The studies involving human participants were reviewed and approved by the Ethics Committee of the West China Hospital of Sichuan University. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: The requirement for informed consent was not required due to the retrospective nature of the study and containing no individual information.
XPZ, LZ, CW, SC, and YJ: concept and design. XPZ, LZ, CW, LF, GZ, KY, JZ, and RW: acquisition, analysis, and interpretation of data. XPZ, LZ, JY, and YZ: statistical analysis. LF, JY, and XG: administrative, technical, or material support. XPZ, CW, and LZ: drafting of the manuscript. SC and YJ: revision of the manuscript. All authors read and approved the final version of the manuscript.
This work was supported by the Key Project in the Science and Technology Program of Sichuan Province (grant nos. 2022YFS0233, 2022YFS0225, and 2019YFS0322), the Project of “0–1” of Sichuan University (grant no. 2022SCUH0033), and the 1⋅3⋅5 Project for Disciplines of Excellence Clinical Research Incubation Project, West China Hospital of Sichuan University (grant nos. ZYJC21060, 2020HXFH048, and 2019HXFH056).
We acknowledge the staff at the Information Technology Center of West China Hospital of Sichuan University for assistance with the data extraction and data management. We acknowledge Wenbiao Zhao (School of Statistics, Renmin University of China, Beijing, China) and Xiaochu Zhong (Imperial College, London, England) for their advice on the data analyses and interpretation. We also acknowledge Tong Qiu (West China Hospital, Sichuan University, Chengdu, China) for her help on statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. van Beek DEC, Königs MHH, Kuijpers YAM, van der Horst ICC, Scheeren TWL. Predictive value of serum albumin levels on noradrenaline and fluid requirements in the first 24 h after admission to the intensive care unit – a prospective observational study. J Crit Care. (2018) 47:99–103. doi: 10.1016/j.jcrc.2018.06.011
2. Leite HP, Rodrigues da Silva AV, de Oliveira Iglesias SB, Koch Nogueira PC. Serum albumin is an independent predictor of clinical outcomes in critically Ill children. Pediatr Crit Care. (2016) 17:e50–7. doi: 10.1097/pcc.0000000000000596
3. Caraceni P, Domenicali M, Tovoli A, Napoli L, Ricci CS, Tufoni M, et al. Clinical indications for the albumin use: still a controversial issue. Eur J Intern Med. (2013) 24:721–8. doi: 10.1016/j.ejim.2013.05.015
4. Lyu PF, Hockenberry JM, Gaydos LM, Howard DH, Buchman TG, Murphy DJ. Impact of a sequential intervention on albumin utilization in critical care. Crit Care Med. (2016) 44:1307–13. doi: 10.1097/ccm.0000000000001638
5. Reinhardt GF, Myscofski JW, Wilkens DB, Dobrin PB, Mangan JE Jr, Stannard RT. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. JPEN J Parenter Enteral Nutr. (1980) 4:357–9. doi: 10.1177/014860718000400404
6. Ferguson RP, O’Connor P, Crabtree B, Batchelor A, Mitchell J, Coppola D. Serum albumin and prealbumin as predictors of clinical outcomes of hospitalized elderly nursing home residents. J Am Geriatr Soc. (1993) 41:545–9. doi: 10.1111/j.1532-5415.1993.tb01893.x
7. Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the national VA surgical risk study. Arch Surg. (1999) 134:36–42. doi: 10.1001/archsurg.134.1.36
8. Viasus D, Garcia-Vidal C, Simonetti A, Manresa F, Dorca J, Gudiol F, et al. Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia. J Infect. (2013) 66:415–23. doi: 10.1016/j.jinf.2012.12.007
9. Herrmann FR, Safran C, Levkoff SE, Minaker KL. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch Intern Med. (1992) 152:125–30.
10. Rich MW, Keller AJ, Schechtman KB, Marshall WG Jr, Kouchoukos NT. Increased complications and prolonged hospital stay in elderly cardiac surgical patients with low serum albumin. Am J Cardiol. (1989) 63:714–8. doi: 10.1016/0002-9149(89)90257-9
11. Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. (1976) 58:259–63.
12. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Berlin: Springer (2015).
13. Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. (1997) 50:693–703. doi: 10.1016/s0895-4356(97)00015-2
14. Bekhit OE, Yousef RM, Abdelrasol HA, Mohammed MA. Serum albumin level as a predictor of outcome in patients admitted to pediatric intensive care units. Pediatr Emerg Care. (2021) 37:e855–60. doi: 10.1097/pec.0000000000002567
15. Ramesh VJ, Umamaheswara Rao GS, Kandavel T, Kumaraswamy SD, Iyyamanda UB, Chandramouli BA. Predictive model for survival among neurosurgical intensive care patients. J Neurosurg Anesthesiol. (2011) 23:183–7. doi: 10.1097/ANA.0b013e31821cb9ec
16. Okuda Y, Obi Y, Streja E, Laster M, Rhee C, Langman CB, et al. Serum albumin and hospitalization among pediatric patients with end-stage renal disease who started dialysis therapy. Pediatr Nephrol. (2019) 34:1799–809. doi: 10.1007/s00467-019-04270-2
17. Thongprayoon C, Cheungpasitporn W, Mao MA, Sakhuja A, Kashani K. U-shape association of serum albumin level and acute kidney injury risk in hospitalized patients. PLoS One. (2018) 13:e0199153. doi: 10.1371/journal.pone.0199153
18. Jiang C, Wang B, Li Y, Xie L, Zhang X, Wang J, et al. U-shaped association between serum albumin and development of chronic kidney disease in general hypertensive patients. Clin Nutr. (2020) 39:258–64. doi: 10.1016/j.clnu.2019.02.002
19. Wegman DH, Apelqvist J, Bottai M, Ekström U, García-Trabanino R, Glaser J, et al. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health. (2018) 44:16–24. doi: 10.5271/sjweh.3659
20. Tanriverdi O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: a medical oncologist’s perspective. Med Oncol. (2014) 31:282. doi: 10.1007/s12032-014-0282-3
21. Henry BM, Borasino S, Ortmann L, Figueroa M, Rahman A, Hock KM, et al. Perioperative serum albumin and its influence on clinical outcomes in neonates and infants undergoing cardiac surgery with cardiopulmonary bypass: a multi-centre retrospective study. Cardiol Young. (2019) 29:761–7. doi: 10.1017/s1047951119000738
Keywords: children, albumin, critical care, restricted cubic spline, mortality
Citation: Zhang X, Zhang L, Wei C, Feng L, Yang J, Zhang G, Lu G, Gui X, Zhou Y, Yang K, Zhou J, Zhou X, Wang R, Chen S and Ji Y (2022) U-shaped association between serum albumin and pediatric intensive care unit mortality in critically ill children. Front. Nutr. 9:931599. doi: 10.3389/fnut.2022.931599
Received: 29 April 2022; Accepted: 03 August 2022;
Published: 30 August 2022.
Edited by:
Maurizio Muscaritoli, Sapienza University of Rome, ItalyReviewed by:
Suchetha S. Rao, Manipal Academy of Higher Education, IndiaCopyright © 2022 Zhang, Zhang, Wei, Feng, Yang, Zhang, Lu, Gui, Zhou, Yang, Zhou, Zhou, Wang, Chen and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siyuan Chen, c2l5X2NoZW5AMTYzLmNvbQ==; Yi Ji, amlqaXl1YW55dWFuQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.