
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 23 September 2022
Sec. Nutrition and Microbes
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.930883
This article is part of the Research Topic Novel Developments for Promoting Health Through Microbiota Modulation View all 16 articles
 Arun Prasath Lakshmanan1†
Arun Prasath Lakshmanan1† Alessandra Mingione2†
Alessandra Mingione2† Francesca Pivari3†
Francesca Pivari3† Elena Dogliotti4
Elena Dogliotti4 Caterina Brasacchio3
Caterina Brasacchio3 Selvasankar Murugesan5
Selvasankar Murugesan5 Daniele Cusi6,7
Daniele Cusi6,7 Monica Lazzaroni8
Monica Lazzaroni8 Laura Soldati3‡
Laura Soldati3‡ Annalisa Terranegra1*‡
Annalisa Terranegra1*‡The consumption of an optimal amount of fruits and vegetables is known to improve physical fitness and physiological body functions. Healthy eating habits, including intake of fruits and vegetables, can modify gut microbiota. This study aimed to demonstrate the effectiveness of a formulated fruit and vegetable supplement (FVS) in modulating the antioxidant capacity and the gut microbiota composition. We enrolled 30 healthy volunteer subjects, matched for age, gender, BMI, and smoking habits, and randomized them into the FVS and the placebo (PLA) groups. Among the serum vitamins, the folic acid level was significantly higher (p = 0.001) in the FVS group than in the PLA group, whereas the vitamin B2 level was significantly higher in the PLA group than in the FVS group (p = 0.028). The antioxidant capacity, measured by using the oxygen radical absorbance capacity (ORAC) method, was also slightly higher in the FVS group than in the PLA group but did not reach statistical significance. The dietary intake, assessed by 24-h recalls, did not show any significant changes after the supplementation in both the groups. The gut microbiome composition, measured by 16S rDNA sequencing, showed no difference in both alpha and beta diversities, whereas the LEfse analysis revealed a microbial shift after the treatment, with a decreased abundance of the genus Ruminococcus from the Lachnospiraceae family (p = 0.009), and the unclassified genus from the family Erysipelotrichaceae (UC36, p = 0.003) in the FVS group compared with the PLA group (confirmed by SIAMCAT analysis, AUC = 74.1%). With a minor effect, the genus Faecalibacterium and unclassified genus and family from the order Lactobacillales (UC31) were also increased in the FVS group compared with the PLA group (p = 0.0474, p = 0.0352, respectively). SCFA measurement by gas chromatography–mass spectrometry showed an increased level of 2-methylbutyrate in the FVS group compared with the PLA group (p = 0.0385). Finally, the Spearman correlation analysis showed that in the FVS group, the genus Faecalibacterium positively correlated with 2-methyl butyrate (p = 0.040). In the PLA group, none of the significant bacteria correlated with either SCFA or serum biomarkers. The network analysis confirmed the positive correlation between genus Faecalibacterium and 2-methyl butyrate. We can conclude that the FVS in healthy individuals modified the gut microbiota composition and metabolites, and it can potentially contribute to reduce the pro-inflammatory response along with the antioxidant capacity.
The reports from clinical and preclinical studies suggest that consumption of an optimal and routine intake of fruits and vegetables, which are rich in vitamins, minerals, fibers, and active molecules, is beneficial for the overall physical fitness, immune functions, and normal physiological functions of the body (1–4). Fruits and vegetables contain a high level of antioxidants, which counteract the action of free radicals. Unfortunately, the continuous exposure to free radicals, present in many harmful substances, can cause cell damage, favoring the onset of various diseases. A positive correlation between the continuous intake of fruits and vegetables and the reduced risk of vascular diseases and cancers has been established (5–7). Fruit and vegetable nutrients, such as fiber and polyphenols, are known to affect the gut microbial composition (4, 8). Gut microbiome realizes mutualistic relations with the gut, influencing the maturation of the immune system, modulating the responses to epithelial cell injury, affecting energy balance, and protecting against physiologic stress (9). According to the reports from world-renowned national and international monitoring agencies that deal with diet and nutrition, consumption of an adequate amount of fruits and vegetables could change the world map of major chronic diseases (cardiovascular disease, stroke, cancer, osteoporosis, diabetes, metabolic diseases, etc.). Nowadays, the majority of the world population is exposed to Western diets, characterized by an over-intake of saturated and omega-6 fatty acids and reduced intake of omega-3 fatty acids, fruits, vegetables, and fibers (10). A Western-like dietary pattern, together with a sedentary lifestyle, leads to several inflammatory-related disorders, such as metabolic syndrome, cardiovascular disease, and neurodegenerative diseases (11). Most of these disorders are also associated with alterations in microbiota composition in humans, especially those with reduced bacterial richness and diversity (12). In this context, the Mediterranean diet (MD) is recognized as one of the healthiest diets worldwide as it contains a high proportion of fiber, antioxidants, and polyphenols, present in vegetables, fruits, pulses, and extra-virgin olive oil, which are strongly associated with a reduced risk of developing non-communicable diseases related to Western diet and lifestyle (13).
It has been estimated that the consumption of 500–800 g/day of fruits and vegetables would avoid over 5.6 and 7.8 million premature deaths annually, a 30% reduction in the relative risk for coronary heart disease, a 28% reduction in stroke and cardiovascular diseases, and a 14% reduction in total cancer risk with 500–600 g/day of fruits and vegetables (14–16). Although antioxidant intake through whole foods, as well as low- to moderate-dose nutritional supplements, is generally considered to provide health-enhancing benefits, higher dose antioxidant supplements intake is somewhat controversial (17). A recent interventional study conducted by Ren et al. used a well-balanced combination of antioxidant nutrients, which provide an increased antioxidant defense (18). Thus, the proposed study aimed to demonstrate the effectiveness of formulated fruit and vegetable supplementation in modulating the gut microbiome composition and explore the potential mechanism that involves the gut microbiome compositional change in healthy subjects.
Healthy adult volunteers (both male and female, N = 30) aged 18–65 years were recruited during the first phase of the study at San Paolo Hospital, Milan, Italy. The study is randomized, double-blinded, and placebo-controlled, and the volunteers were randomly assigned to the treatment or placebo group, with 15 healthy subjects in each group. The randomization was conducted through open-source software.1 The flow diagram of the present study is given in the Supplementary material (Supplementary Figure 1). The subjects who were given the fruit and vegetable supplement are labeled the FVS group, and the subjects who were given placebo are labeled the PLA group. The study duration was 6 weeks, the lifestyle questionnaire was used, and the measurement of BMI and waist circumference was taken before and after the supplement/placebo treatment. The assessment of food intake was carried out by using the 24-h food recall method before and after the treatment. The blood and stool samples were collected from each participant before and after the supplement/placebo treatment. The study was approved by the Ethical Committee of Interaziendale Milan Area A, with the approval number 156/ST/2014.
The diet supplement contains a mix of fruits and vegetables, packed in stick packs to provide an equal amount of supplements to each subject, distributed by L’Angelica Istituto Erboristico, and named fruit and vegetable supplement (FVS). The placebo supplement was also packed in stick packs without the content of fruits and vegetables and named PLA (Supplementary Table 1). Each subject of both FVS and PLA groups was given two stick packs per day for a total of 6 weeks. All the subjects were recruited in the same season to avoid the effect of different seasonal foods.
The antioxidant capacity of the plasma samples was assessed by using an oxygen radical absorbance capacity (ORAC) Antioxidant Assay Kit (Zen Bio). Antioxidants present in the samples can inhibit the peroxyl radical-mediated oxidation of fluorescein formed by the breakdown of 2,2’-azobis-2-methyl-propanimidamide dihydrochloride (AAPH) at 37°C. The fluorescence signal is measured during 60 min by determining the Ex480 nm/Em520 nm ratio. The concentration of antioxidants in the plasma sample is proportional to the fluorescence intensity and is assessed by comparing the net area under the curve to that of a known antioxidant, Trolox. The ORAC of plasma samples, before and after FVS intake, was expressed as micromolar Trolox equivalents (μMTE).
Blood samples were collected before and after the start of FVS treatment. The measurement of serum levels of vitamins A, E B2, and B6 was carried out using a high-performance liquid chromatography (HPLC) system using commercial reagent kits (Chromsystems Instruments & Chemicals GmbH, Munich, Germany) according to the manufacturer’s guidelines. Vitamin K was determined using the ion-selective electrode. Serum folate was measured using the standard electrochemiluminescence assay (Roche Diagnostics, Basel, Switzerland).
The expert nutritionist collected the dietary intake of each participant, at baseline and after 6 weeks of the FVS, during a structured interview using a 24-h dietary recall to estimate subjects’ food consumption and the Scotti Bassani Photographic Atlas to better estimate food portions.2 The Diet Monitoring Solution (DMS) web platform was used to collect participants’ nutritional data, dietary micronutrients, and macronutrients (19).
Stool samples were self-collected by each participant in Norgen Stool Nucleic Acid Collection and Transport Tube (Norgen Biotek Corp., Toronto, Canada) and were stored at a –80°C freezer until further use. Bacterial DNA extraction was performed using QIAamp® Fast DNA Stool Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The quantity and quality of the extracted DNA were checked by using NanoDrop One (Thermo Fisher Scientific,Waltham, MA, United States).
The 16S rDNA library preparation and sequencing were performed according to the manufacturer’s instructions (MiSeq system, Illumina, San Diego, CA, United States), as described previously (20). In brief, the extracted genomic DNA was amplified using the primers that target the v3-v4 regions of the 16S rDNA gene. The amplified product was then cleaned up using AMPure XP magnetic beads (Beckman Coulter, Brea, CA, United States), and the product was indexed using the Nextera XT primer (Illumina, San Diego, CA, United States). Again, the product was cleaned up using magnetic beads. The size and quantity of the prepared library were estimated using an Agilent High Sensitivity Kit and a Qubit dsDNA HS Assay Kit, respectively. The pooled library and Phix control were denatured using 0.2 N NaOH as per the manufacturer’s protocol. Finally, the sample was sequenced using the MiSeq Reagent v3 (600 cycles) Kit (Illumina, San Diego, CA, United States) according to the manufacturer’s instructions. Base calling was directly carried out on MiSeq. The raw data were demultiplexed using MiSeq Reporter on Illumina MiSeq. The PEAR tool was used to merge both forward and reverse end sequences for each sample (21), and the reads with a high-quality score of 30 and above were selected using the Trimmomatic tool (22). FASTQ files were converted into FASTA files using QIIME v1.9.0 (Quantitative Insights Into Microbial Ecology) pipeline (23). Operational taxonomic units (OTUs) were obtained by aligning the sequence against the Greengenes database (gg_13_08) with a confidence threshold of 97% (24).
The analysis of short-chain fatty acid (SCFA) was performed by adaptation of the method published by Han et al. (25). In brief, the collected stool sample was homogenized using a spatula, weighed, and diluted with 50% aqueous acetonitrile (Fluka, Thermo Fisher Scientific, Waltham, MA, United States). A portion of the supernatant was taken for further analysis, along with mixed standard calibration solutions representing a range of concentrations for each fatty acid. All SCFAs from C2 to C6 along with any iso- and anteiso-methyl branched chain fatty acids were tested. The samples and standards were derivatized with 3-nitrophenylhydrazine (Sigma Aldrich, St. Louis, MO, United States) and then diluted by a factor of 10 with 10% aqueous acetonitrile. An internal standard (a mixture of SCFA derivatized as above with 13C6-3-nitrophenylhydrazine (IsoSciences, PA, United States) was added. To test if the stool matrix influenced the recovery, controls were prepared by spiking isotopically labeled straight-chain SCFA derivatized with 13C6-3-nitrophenylhydrazine to a mixture of stool sample supernatants from this study. These were analyzed along with the same mixture of isotopically labeled SCFA prepared in 50% aqueous acetonitrile, and a comparison was made. All samples were analyzed using a liquid chromatography–triple quadrupole mass spectrometer operated in negative ion scheduled MRM mode. A C18 column allowed the chromatographic separation of all derivatized SCFAs. The peak area for all chromatographic peaks was calculated and used for generating calibration curves and for calculating unknown concentrations of SCFA in stool.
We used a paired-end read merger (PEAR, v0.9.8) tool to merge both forward and reverse end sequences for each sample (21), and the reads with a high-quality score of 30 and above (≥30) were selected using the Trimmomatic (v0.36) tool (22). FASTQ files were converted into FASTA files using Quantitative Insights Into Microbial Ecology (QIIME, v1.9.0) pipeline (23). Operational taxonomic units (OTUs) were obtained by aligning the sequence against the Greengenes database (gg_13_08) with a confidence threshold of 97% (24).
Alpha diversity refers to the estimation of both richness and abundance of species in a habitat or specific area or sample. Species richness refers to the number of species present in a sample, and species abundance means the number of individuals per species. Alpha diversity was estimated using observed (species richness) and Chao1 (rare species richness), and Shannon and Simpson methods (species abundance) by using the R package (Phyloseq and ggplot2), as described previously (26). Beta diversity refers to the measurement of difference in the microbial composition between two or more groups of samples, and it was presented as a principal coordinate analysis as proposed in QIIME v1.9.0, as described previously (26).
Linear discriminant analysis effect size (LEfSe) was used to obtain the gut microbial markers for each group, and it uses the non-parametric factorial Kruskal–Wallis (KW) sum-rank test to identify features with significant differential abundance with regard to different groups, followed by linear discriminant analysis (LDA) to calculate the effect size of each differentially abundant microbial features. Features are significant if the LDA value is > 2.0 and the p-value is < 0.01 (27). Differentially abundant microbial taxa were identified also using the analysis of composition of microbiomes (ANCOM), as previously described (28).
We also performed an association analysis between microbial markers and host phenotypes using a Statistical Inference of Associations between Microbial Communities and host phenoTypes (SIAMCAT) machine learning tool, followed by “lasso” regression analysis according to the previously reported method by Wirbel et al. (29).
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis is a bioinformatics software package designed to predict the metagenome functional content from marker gene surveys and full genomes, and it was performed according to the literature review of Langille et al. (30).
We performed microbial, diet, and SCFA correlation network analysis using a MetagenoNets webtool, as described previously by Nagpal et al. (31).
Comparisons between the groups were performed by parametric (unpaired t-test) or non-parametric analyses (Mann–Whitney test) according to data distribution. Correlation analysis of microbial data with diet data and antioxidant blood parameters was performed using Spearman’s correlation method. All analyses were run on Prism Software version 9 (GraphPad, CA, United States). A value of p < 0.05 was considered statistically significant.
We enrolled 30 healthy volunteers and randomized them into two groups (15 in the FVS group and 14 in the PLA group, with the dropout of 1 subject). The two groups did not differ in gender, age, BMI, and the smoking habits (Table 1). We measured the serum levels of vitamins A, B2, B6, E, and K, and folic acid in blood of the PLA and FVS groups, and we computed the changes in the levels after the supplementation for each subject. The level of folic acid was significantly higher in the FVS group than in the PLA group (unpaired t-test, p = 0.001). Interestingly, the level of serum vitamin B2 was significantly higher in the PLA group than in the FVS group (unpaired t-test, p = 0.028). There was no significant difference in the level of other vitamins. The level of ORAC was slightly higher in the FVS group but did not reach a statistical significance (Figure 1). We assessed the dietary intake of micronutrients and macronutrients in order to determine changes in the food habits, and the study participants in both the groups did not show any significant change in their dietary intake during the treatment (Supplementary Figure 2).
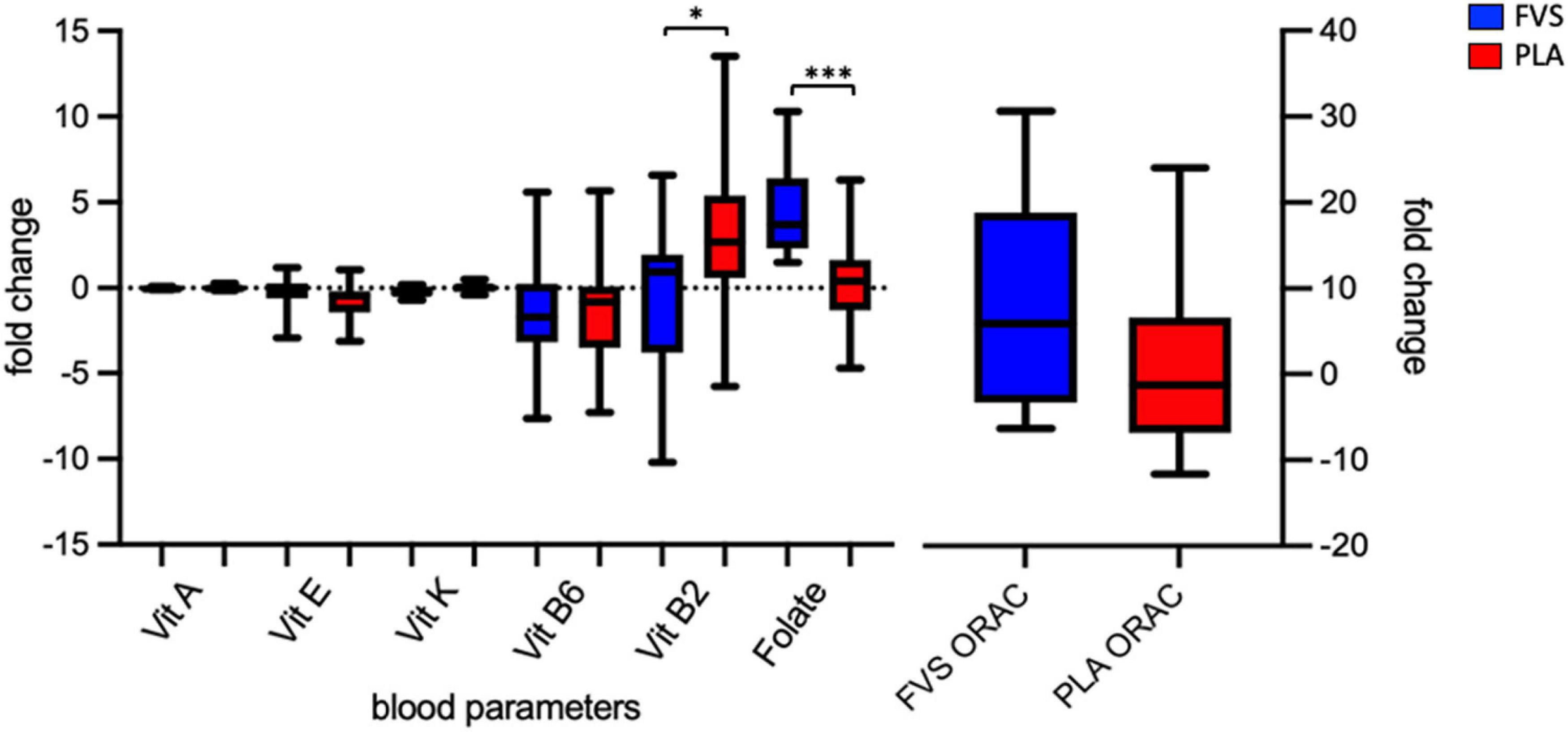
Figure 1. Serum biomarkers of the antioxidant capacity. Serum levels of vitamins A, E, K, B6, and B2 and folate, and antioxidant capacity in the FVS group (blue bars) and PLA group (red bars). The median and interquartile (IQR) ranges are showed in the box plots. FVS, N = 15, PLA, N = 14. *p < 0.05 and ***p < 0.001 when compared with the PLA group using the unpaired t-test.
To determine the effect of the FVS on gut microbial composition, we performed 16S rDNA sequencing, and the results showed that there was no significant shift in the microbial diversity, which was evident from alpha and beta diversity estimation. Bray–Curtis-based principal coordinate analysis (PCoA) was performed to visually explore the similarity and variations between the microbial composition of the samples, and it showed that there was no visual separation between the PLA and FVS groups (Figure 2A). Alpha diversity analysis calculated using observed, Chao1, Shannon, and Simpson methods showed that there was no significant difference between the FVS and PLA groups after treatment (Figure 2B and Supplementary Figure 3).
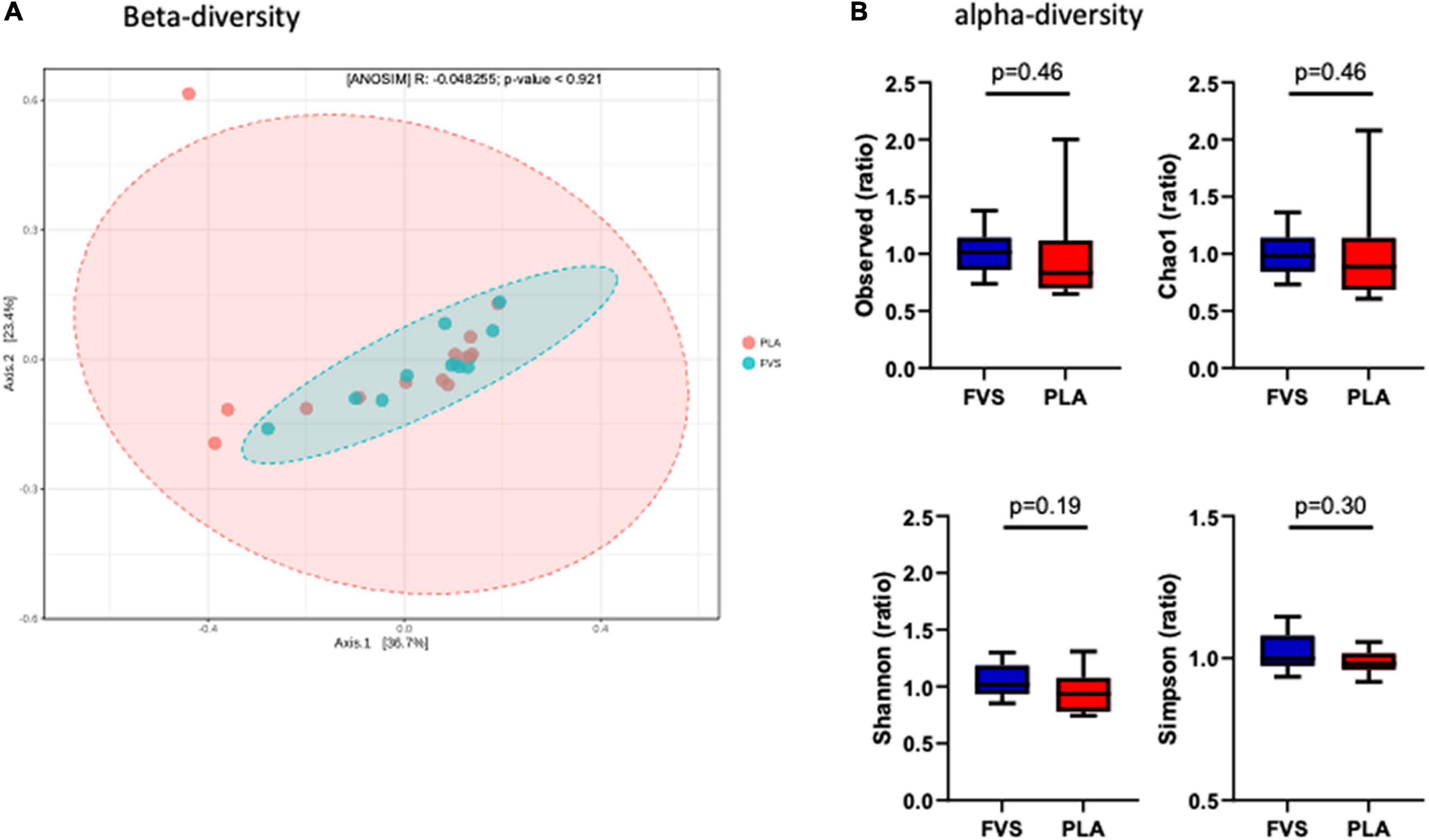
Figure 2. Gut microbial diversity in the FVS and PLA groups. (A) Beta diversity index was measured by using the Bray–Curtis method using principle coordinate analysis (PCoA) using the relative abundance of OTUs. The two variances explained by Axis.1 and Axis.2 are 36.7 and 23.4%, respectively. ANOSIM, analysis of similarity was not significant (p = 0.921). (B) Alpha diversity was measured by the four commonly used methods, such as observed, Chao1, Shannon, and Simpson methods. The box plots show interquartile (IQR) ranges with the median and whiskers. FVS, N = 15, PLA, N = 14. p < 0.05 considered statistically significant using Student’s t-test.
The analysis of the taxonomic composition revealed a shift of the microbial composition after the treatment. Applying the LefSe linear discriminant analysis, we found that the genus L-Ruminococcus (p = 0.009) and the unclassified genus from Erysipelotrichaceae family (p = 0.003) are enriched in the PLA group in comparison to the FVS group (Figure 3). The analysis of the composition of microbiomes (ANCOM) revealed that mostly L-Ruminococcus is the differential abundance bacteria between the FVS and PLA groups (Supplementary Figure 4). Also, the association analysis by using the SIAMCAT machine learning tool confirmed that L-Ruminococcus and unclassified bacteria from Erysipelotrichaceae_UC36 family are significantly associated with the PLA group, with the robustness values of 97 and 98%, respectively. Furthermore, the mean overall prediction area under the curve (AUC) and mean AUC value are 74.1% and 78.2%, respectively (Figure 4). Comparison of the relative abundance at the phyla level and the top 30 genera from the two groups did not show statistical difference, except for the genus Ruminococcus and the Erysipelotrichaceae_UC36. Furthermore, the comparison at the genus level by using Mann–Whitney test showed a slightly significant increase in the relative abundance of the genus Faecalibacterium and unclassified genus and family from the order of Lactobacillales (UC31) in the FVS group compared with the PLA group (p = 0.0474 and p = 0.0352, respectively); whereas a significant increase in the relative abundance of genus L-Ruminococcus, Lachnobacterium, and unclassified genus from the family Erysipelotrichaceae (UC36) were found in the PLA group compared with the FVS group (p = 0.0003, p = 0.0010, and p = 0.0059, respectively) (Supplementary Figures 5, 6).
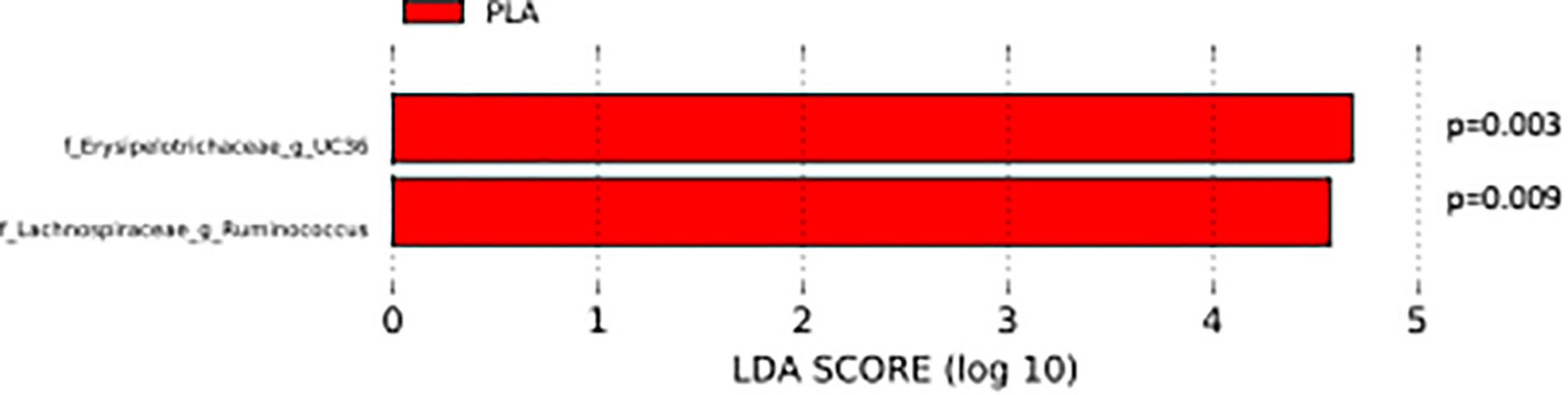
Figure 3. Gut microbial composition and microbial markers in the FVS and PLA groups. Gut microbial markers were measured by using the LEfSe analytical tool with a cutoff value of LDA > 2.0) in both the FVS and PLA groups. “g_UC” and “f_UC” represent unclassified bacteria at the genus level and family level, respectively.
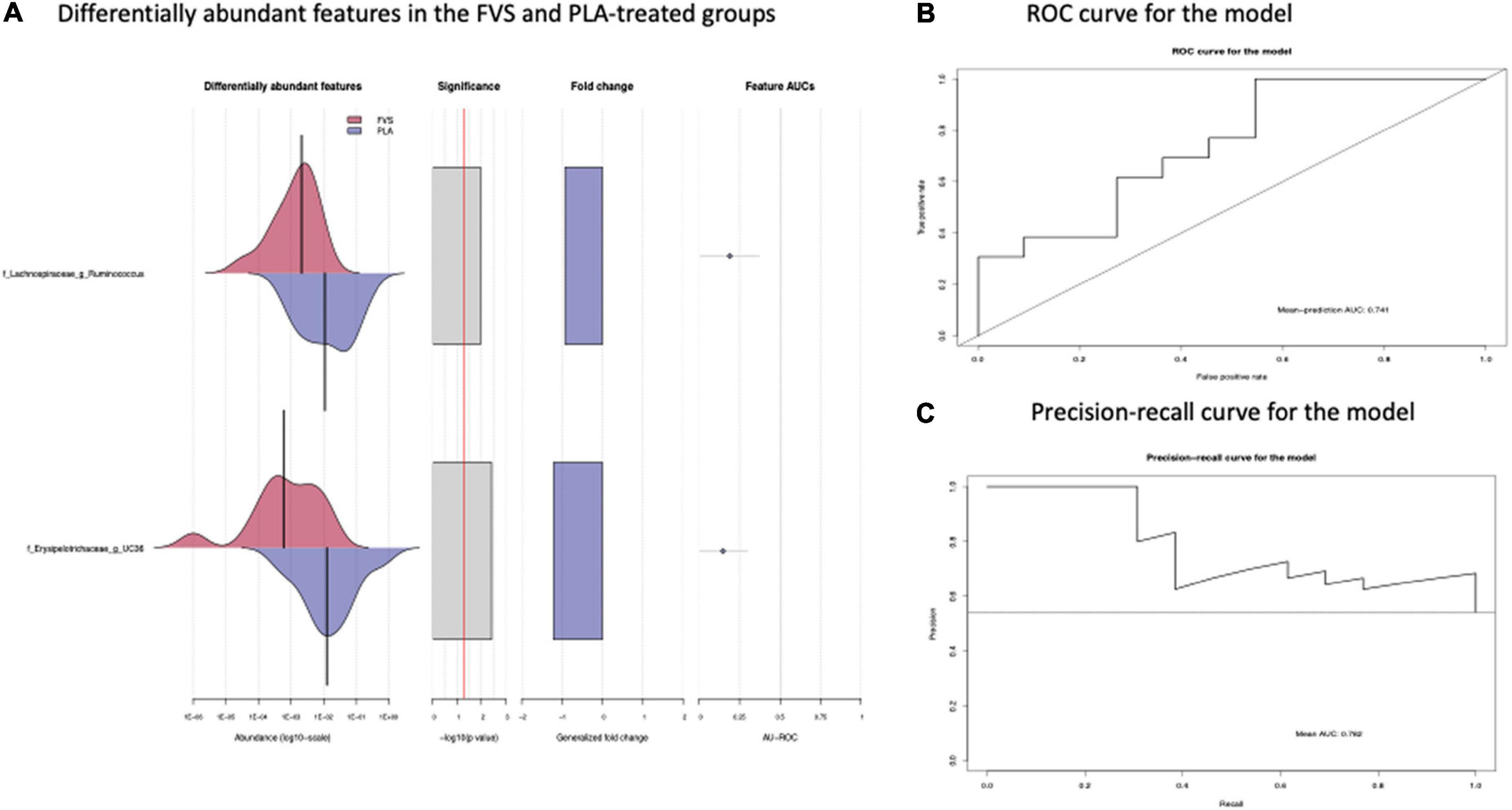
Figure 4. Validation of gut microbial markers using a machine learning tool—SIAMCAT analysis. (A) Gut microbial markers from LEfSe analysis were further validated using the SIAMCAT tool, which showed L-Ruminococcus and Erysipelotrichaceae family bacteria are significantly enriched in the PLA-treated group. (B) ROC curve for model, which displayed the cross-validation error as a receiver operating characteristic (ROC) curve with the 95% confidence interval. The area under the ROC (AUROC = 0.741) is given below the curve. The x-axis and y-axis represent false-positive and true-positive rates, respectively, for the tested markers, and (C) precision–recall curve for the model, which displays the mean AUC value of 0.782. An AUROC value of more than 0.7 is considered fairly good in terms of the discriminative ability of the test.
The network-based approach is widely used in microbiome analysis as it provides greater information about the co-occurrence nature and interaction of the microbial communities (32). Here, we observed that the genus L-Ruminococcus positively correlated with the genus Lachnobacterium (r = 0.454) and unclassified genus from the Erysipelotrichaceae family (r = 0.553) (Figure 5), indicating that the genus L-Ruminococcus co-existed with the other two bacteria, and the FVS that modifies the abundance of L-Ruminococcus could positively affect the abundance of other two bacteria.
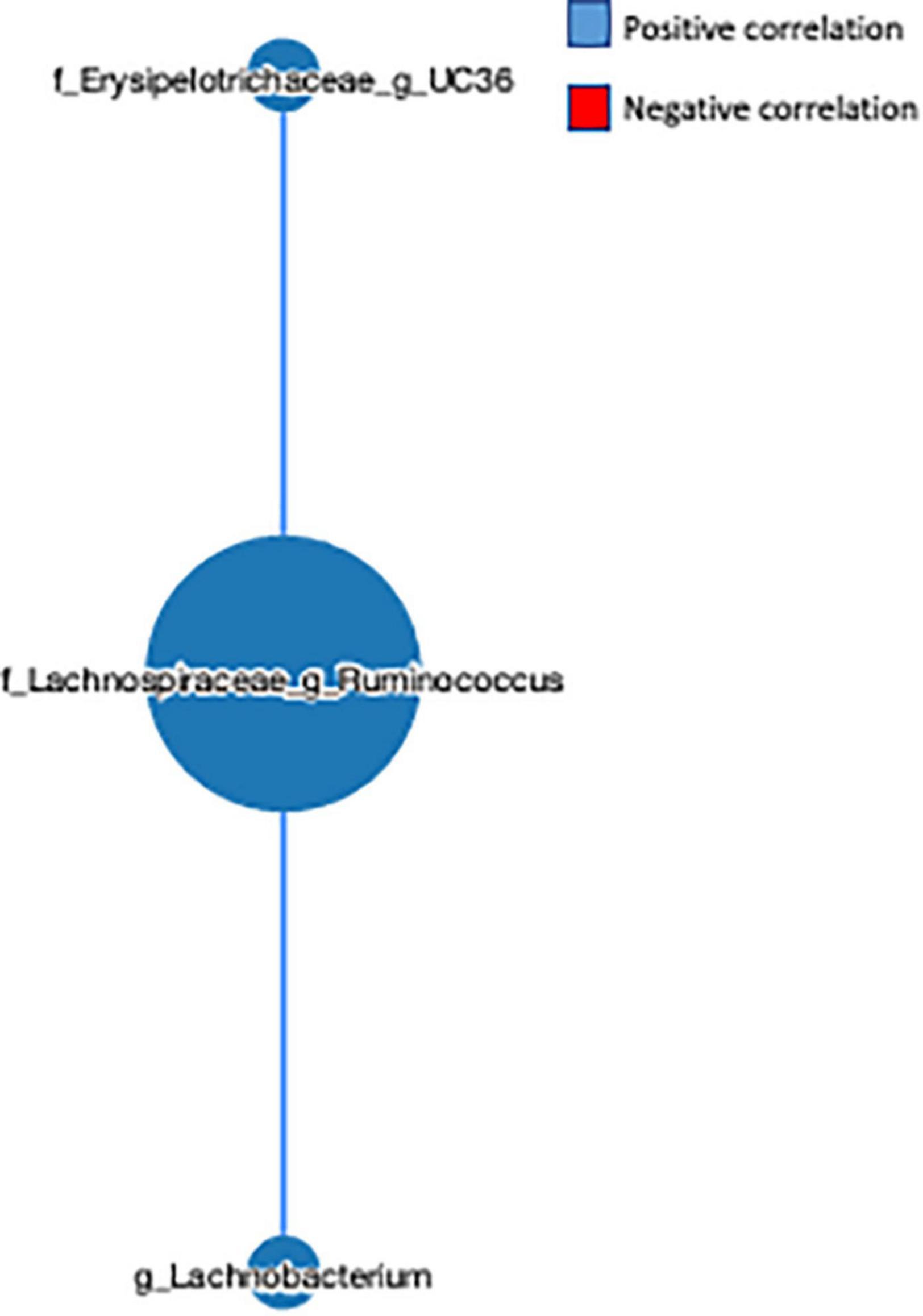
Figure 5. Microbial network analysis performed using a MetagenoNets webtool. The analysis revealed that L-Ruminococcus positively regulates the unclassified bacteria from the Erysipelotrichaceae family and the genus Lachnobacterium. Blue color indicates a positive correlation, whereas red color indicates a negative correlation.
The metabolic pathways predicted by PICRUSt revealed that the FVS group has a higher level of fructose and mannose metabolism and pentose phosphate pathways than the PLA group (Figure 6A). The measurement of various SCFAs clearly indicated that only the 2-methyl butyrate level was significantly (unpaired t-test, p = 0.0385) higher in the FVS group than in the PLA group (Figure 6B). The total amount of SCFA was higher in the FVS group but did not reach a statistical difference since the rest of the SCFAs, from acetate to hexanoate, were not significantly altered between the two groups (Figure 6C).
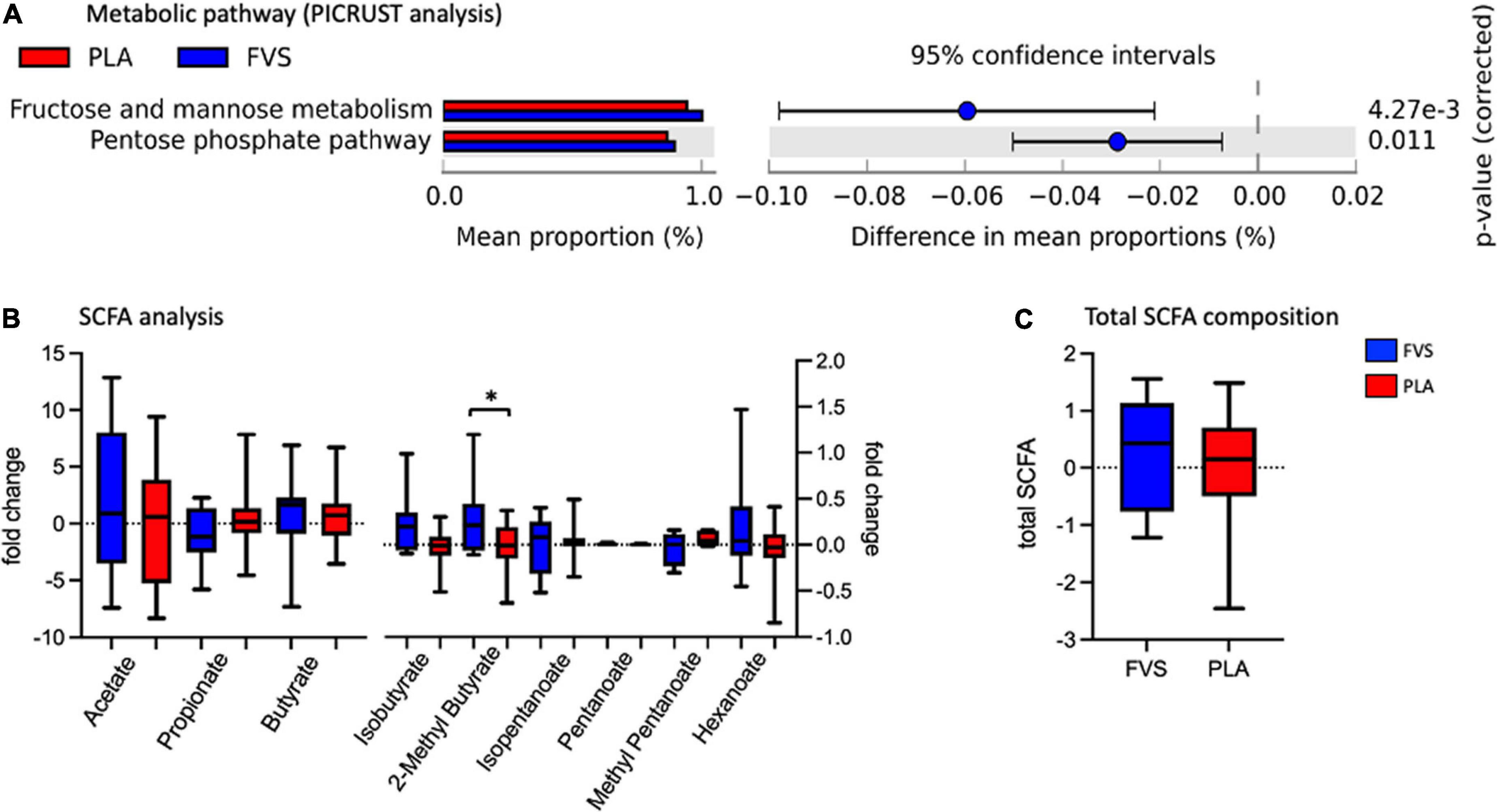
Figure 6. Predicted functional pathways and metabolite composition in the FVS and PLA groups. (A) Predicted functional pathways are obtained using the PICRUSt tool based on the bacterial abundance measured as mean proportion in the FVS and PLA groups. (B,C) SCFA composition was measured using liquid chromatography–tandem mass spectrometry. Single SCFAs are shown in panel b, and the total of the SCFA in panel c. The median and interquartile (IQR) ranges are showed in box plots. FVS, N = 15, PLA, N = 14. *p < 0.05 when compared with the PLA group using Student’s t-test.
To explore the effect of microbiota on SCFA and serum biomarkers, we performed a Spearman correlation analysis including the significant bacteria for each group, all the SCFAs, and the serum levels of vitamins and ORAC. In the FVS group, the genus Faecalibacterium positively correlated with 2-methyl butyrate (p = 0.040) and negatively correlated with the serum level of vitamin B6 (p = 0.022). 2-Methyl butyrate positively correlated with isobutyrate and isopentanoate (p = 0.006 and p = 4.175e–009, respectively), and pentanoate with hexanoate (p = 0.005). Finally, vitamin B2 negatively correlated with acetate and hexanoate (p = 0.038, p = 0.011, respectively) (Figure 7A). In the PLA group, none of the significant bacteria correlated with either SCFA or serum biomarkers. Among the SCFAs, propionate positively correlated with butyrate and pentanoate (p = 0.003 and p = 0.027, respectively), isobutyrate with 2-methyl butyrate and isopentanoate (p = 5.858e–007 and p = 6.430e–007, respectively), isopentanoate with the 2-methyl butyrate (p = 8.351e–009). Finally, vitamin K negatively correlated with vitamin E (p = 0.007), and vitamin B6 positively correlated with ORAC (p = 0.006). Intriguingly, the correlation between vitamin B2 and acetate is inverse in the FVS and PLA groups; a positive correlation was found in the PLA group (p = 0.009) and a negative correlation in the FVS group (p = 0.038) (Figure 7B). The network-based analysis of the interaction microbiota, diet, and SCFA confirmed the positive correlation of 2-methyl butyrate with the genus Faecalibacterium (Figure 8).
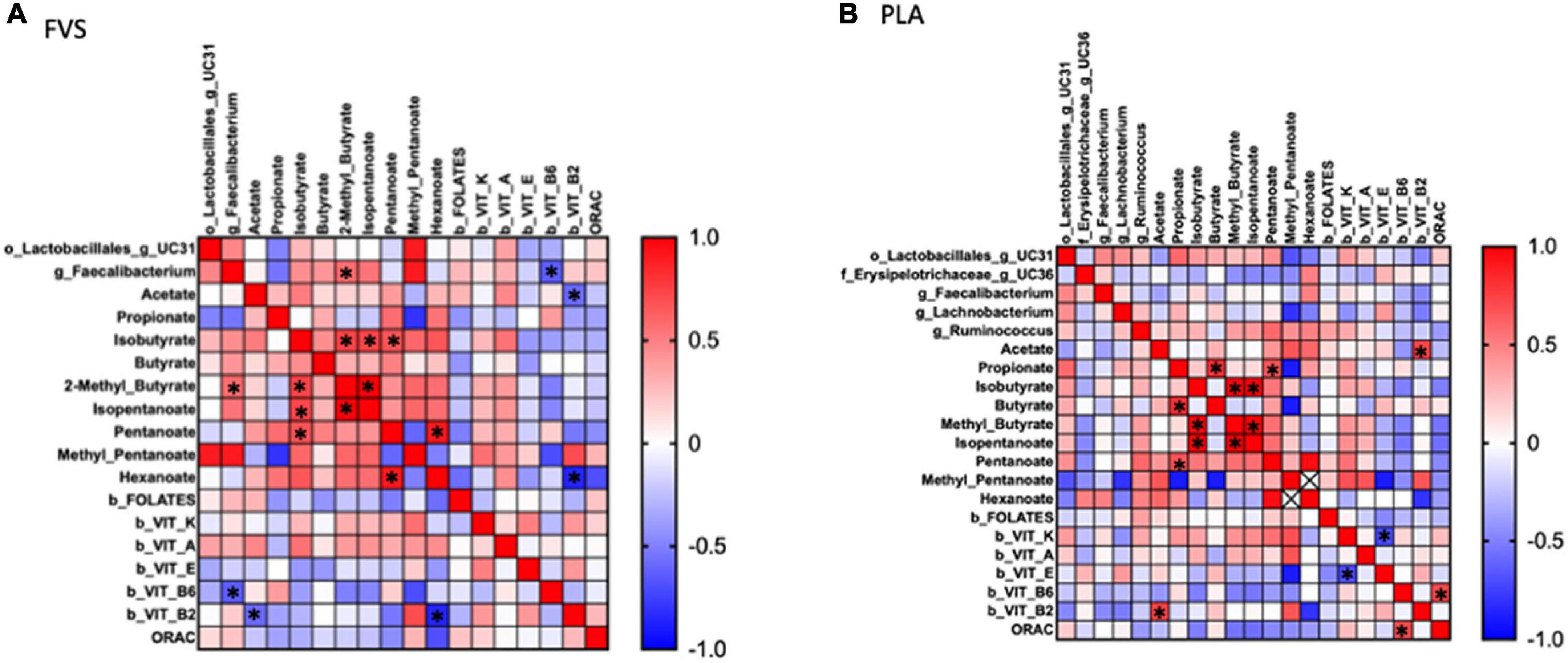
Figure 7. Correlation analysis between gut microbiome, SCFA, and serum biomarkers in the FVS and PLA groups. Spearman correlation was performed to analyze the interaction between significant microbial genus, SCFA, and serum biomarkers in both the FVS group (A) and PLA group (B). Red to blue color scale indicates a positive to a negative correlation, respectively. FVS, N = 15, PLA, N = 14. *p < 0.05 when compared with the PLA group.

Figure 8. Network analysis performed using a MetagenoNets webtool. Analysis revealed that only 2-methyl butyrate positively correlated with the genus Faecalibacterium among all significant bacteria, diet, and SCFA. Blue color indicates a positive correlation, whereas red color indicates a negative correlation.
The diet is well-known to modulate the gut microbiota composition and metabolism (33). In this study, we tested the antioxidant capacity, gut microbiota composition, and metabolic pathways in a group of healthy adult volunteers supplemented with a fruit and vegetable supplement for 6 weeks compared with a matched control group supplemented with a placebo. Typically, in the microbiome context, their health condition can be estimated by the gut microbial compositional homeostasis as the gut microbiome is proven to play a substantial role in the vicious cycle of diet-controlled pathophysiology, where the gut microbiome regulates physiology, and it is impacted by pathophysiology. Thus, any changes in the gut microbial composition clearly reflect the health or disease nature of humans. The measurement of gut microbial richness and abundance could be a vital indicator of human health. The literature review is strongly in favor of the diet as one of the factors that affect the gut microbial composition in terms of bacterial richness and abundance (34–37).
Interestingly, in this study, we observed that formulated fruit and vegetable supplementation contributes to modulate the gut microbial composition, compared with the placebo. The FVS showed a reduction in the relative abundance of genera from the Lachnospiraceae family, such as Ruminococcus and Lachnobacterium, and unclassified bacteria from the Erysipelotrichaceae family. Also, to a lesser extent, the genera Faecalibacterium and unclassified bacteria from the Lactobacillales order were affected (increased). A multiethnic study conducted by Frankenfeld et al. reported that the consumption of fruits and vegetables caused a gut microbial shift including the genera from Lachnospiraceae and Ruminococcaceae families (38). Results from different studies are controversial. The Medika study conducted by De Ioria et al. reported that the consumption of animal proteins showed a marked reduction in the relative abundance of Lachnospiraceae spp., and whether the intake of vegetable proteins, fiber, and potassium impacted the relative abundance of Lachnospiraceae spp. positively in the chronic kidney disease subjects (39). On the other hand, De Fillippis et al. demonstrated that the genus Ruminococcus from the Lachnospiraceae family (L-Ruminococcus) was positively affected by the animal-based diet and negatively by the vegetables–based diet (40). Thus, a complete vegetable and fruit-based diet or a formulated supplementation, like in our study, predominantly target the genera from the Lachnospiraceae family, especially Ruminococcus and Lachnobacterium.
The genus Ruminococcus is assigned to both Lachnospiraceae and Ruminococcaceae families, it is greatly affected by the dietary components (41), and it can play multiple biological and pathological roles, which are still not fully understood. For example, Zheng et al. conducted an interesting study in which they estimated the dietary inflammatory potential via the literature-derived index (DII) with the gut microbiota composition, and they reported that the species R. torques is associated with the pro-inflammatory diet group (42). Also, Smith-Brown et al. demonstrated that fruit intake, especially apple and pears, drastically reduced the relative abundance of R. gnavus (43). In addition, in one of our recent studies, we found that Ruminococcus callidus and other unclassified bacteria (Ruminococcaceae family) could protect from cardiovascular diseases in obese Qatari subjects, whereas species from the genus of L-Ruminococcus such as Ruminococcus gnavus and Ruminococcus torques did not involve in the protection from CVD (44). Furthermore, a study conducted by Singh et al. reported that vitamin D supplementation in the healthy Qatari subjects showed a greater increase in the abundance of Ruminococcus bromii (45). The different Ruminococcus species can probably explain the different behavior. Unfortunately, we were not able to discriminate the species in our sample. Therefore, further studies are needed to clarify the role of the Ruminococcus spp. in health and diseases.
In addition to this, we identified a decrease in an unclassified bacterium from the Erysipelotrichaceae family due to the FVS. The rationale behind this decrease might be the folic acid consumption as the blood folate level was significantly higher in the FVS-supplemented group than in the PLA group. In support of this notion, Cheng et al. demonstrated a high-methionine low-folate (HMLF) diet considerably increased the relative abundance of bacteria from the Erysipelotrichaceae family in diet-induced HHcy mice (46). In addition, folate has been demonstrated to alter the inflammatory responses via DNA methylation and DNA synthesis (47), and Moein et al. demonstrated that the serum folate level is associated with inflammatory markers and disease clinical activity, and its optimal presence would help reduce the inflammatory response in IBD patients (48). Of note, many studies reported that Erysipelotrichaceae family bacteria are abundant in colorectal and colon cancer, but the results are not consistent (49). Strikingly, Erysipelotrichaceae has been demonstrated to mediate the TNF-alpha-mediated Crohn’s disease (50). Moreover, the co-network analysis revealed that both L-Ruminococcus and unclassified bacteria from the Erysipelotrichaceae family positively regulate each other, with L-Ruminococcus having a dominant regulatory role in the Erysipelotrichaceae family bacteria. Thus, there is a strong possibility that the FVS could provide a long-term health benefit by managing the genus L-Ruminococcus abundance level.
The pro-inflammatory response is closely connected to oxidative stress as these two entities promote response of each other (51). Thus, in this study, the PLA-supplemented subjects were expected to have a low antioxidant capacity. It is a well-accepted fact that the consumption of fruits and vegetables increases the antioxidant capacity (52), and in this study, the antioxidant capacity measured by ORAC assay was higher (but not statistically significant) in the FVS-supplemented subjects than in the PLA group. Regarding the direct role of the gut microbiota in the antioxidant function, some Lactobacillus strains are known to exert beneficial effects due to their antioxidant activities. Probiotics, containing Lactobacillus strains, can produce various metabolites with antioxidant activity, such as glutathione (GSH), butyrate, and folate (53). Different mechanisms of antioxidant activity have been identified in the Lactobacillus strains: production of metabolites and antioxidant enzymes for reactive oxygen species (ROS) scavenging, upregulation of host antioxidant enzyme activities, downregulation of enzyme activities related to the production of ROS in the host, and regulation of pathways signaling related to host antioxidants and host intestinal flora (54–56). The ORAC assay is one of the best methods used to assess the antioxidant activities of different Lactobacillus strains (54). In our study, we observed an increase in plasmatic levels of folate, an increase of an unclassified genus from the order Lactobacillales and, consequently, we observed an increasing trend in the plasma antioxidant capacity of the subjects supplemented with the FVS.
The microbial metabolites, such as SCFAs, are demonstrated to be a key factor in the modulation of the inflammatory pathway, binding to specific receptors that regulate the immune function pathways (57–59). Among the measured SCFAs, we observed a nominal increase in the total SCFA levels, and a significant increase in 2-methylbutyrate positively correlated with the relative abundance of Faecalibacterium. 2-Methyl butyrate is a branched chain fatty acid derived from the intestinal metabolism of isoleucine, an essential branched amino acid (BCAA) (60). Gut microbiota is able to both produce and metabolize BCAA, and its levels are associated with insulin resistance (61), osteoporosis (62), and aging (63) via mechanisms involving the microbiota. A randomized controlled trial confirmed that the probiotic administration improves the serum amino acid concentration, including BCAA, in the subjects eating plant proteins (64). The microbiota shift that we observed in the FVS group can explain the increase in the 2-methylbutyrate level by enhancing the BCAA metabolism. Moreover, in our study, we observed an increase in the 2-methyl butyrate level and ORAC (increasing trend) in the subjects supplemented with the FVS compared with the placebo. We can speculate on a potential underlying mechanism that involves the gut microbiota and SCFA in regulating the antioxidant capacity activated by fruit and vegetable supplementation. Our hypothesis is supported by an observational study that explored the potential anti-inflammatory capacity of the SCFA in patients under hemodialysis, and it showed a negative association of 2-methyl butyrate with bone morphogenetic protein 6 (BMP-6), a biomarker of vascular calcification, and hypothesized the role of 2-methyl butyrate and other SCFAs in reducing cardiovascular risk in hemodialysis (65).
The pathway analysis showed an increase in the fructose and mannose metabolism and the pentose phosphate pathway in the subjects supplemented with the FVS compared with the placebo. Recent animal studies reported similar findings with increased fecal levels of mannose and decreased levels of fructose, among others, in rats supplemented with 1.5% of green tea polyphenols (66). The pentose phosphate pathway is also known to be affected by the microbial composition and prebiotics, as demonstrated in high-fat diet-fed mice treated with Huangjinya black tea, where a reduced lipid metabolism corresponded with many increased metabolic pathways, including the pentose phosphate pathway (67).
Our study has strengths and limitations. The strengths of this study are a randomized double-blinded study design, the supplement provided in stick packs to ensure an equal amount of nutrients to each subject, the complete set of microbial composition and metabolite analyses, the measurement of the ORAC, and finally, the exclusion of any diet effect. The main limitation of this study is the small sample size, which requires the confirmation of the findings from larger cohorts and the validation in inflammatory disease settings.
In conclusion, we propose that the FVS can provide long-term health benefits by controlling the relative abundance of bacteria such as L-Ruminococcus and unclassified bacteria from the Erysipelotrichaceae family, possibly through the reduction of pro-inflammatory response (Figure 9). Further studies are required to confirm these findings and explore potential molecular mechanisms.
The data presented in this study are deposited in the NIH NCBI repository, accession number PRJNA833767.
The study was approved by the Ethical Committee Interaziendale Milan Area A and the approval number is 156/ST/2014. The patients/participants provided their written informed consent to participate in this study.
LS and DC designed the study. ED, FP, and CB were involved in the subject recruitment process, diet data, and sample collection process. AM and ML performed blood tests. AT performed the data analysis for the diet and blood tests. AL processed stool samples and performed the gut microbiome data analysis. SM contributed to the gut microbiome data analysis. AT, AL, ML, and FP wrote the manuscript. All authors reviewed the manuscript.
We acknowledge Sidra Medicine, Doha, Qatar, and L’Angelica Istituto Erboristico, Italy, for funding the project and Fondazione Umberto Veronesi for supporting AM and FP with the post-doctoral fellowships. We also would like to acknowledge Sidra DPC core for the SCFA analysis. Open Access funding provided by the Qatar National Library.
The FVS and placebo products were provided by L’Angelica Istituto Erboristico, Italy, with the only purpose to perform the research study. L’Angelica Istituto Erboristico, Italy, did not interfere in the scope and the conduction of the research project. DC was employed by Bio4Dreams S.p.A.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.930883/full#supplementary-material
1. Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. (2013) 4:384S–92S.
2. Cobo-Cuenca AI, Garrido-Miguel M, Soriano-Cano A, Ferri-Morales A, Martínez-Vizcaíno V, Martín-Espinosa NM. Adherence to the mediterranean diet and its association with body composition and physical fitness in Spanish university students. Nutrients. (2019) 11:2830. doi: 10.3390/nu11112830
3. Eslami O, Zarei M, Shidfar F. The association of dietary patterns and cardiorespiratory fitness: a systematic review. Nutr Metab Cardiovasc Dis. (2020) 30:1442–51.
4. Zimmermann C, Wagner AE. Impact of food-derived bioactive compounds on intestinal immunity. Biomolecules. (2021) 11:1901.
5. Kamal N, Ilowefah MA, Hilles AR, Anua NA, Awin T, Alshwyeh HA, et al. Genesis and mechanism of some cancer types and an overview on the role of diet and nutrition in cancer prevention. Molecules. (2022) 27:1794.
6. Wang DD, Li Y, Bhupathiraju SN, Rosner BA, Sun Q, Giovannucci EL, et al. Fruit and vegetable intake and mortality: results from 2 prospective cohort studies of us men and women and a meta-analysis of 26 cohort studies. Circulation. (2021) 143:1642–54. doi: 10.1161/CIRCULATIONAHA.120.048996
7. Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: insights into protective effects, antioxidant potentials and mechanism(s) of action. Front Pharmacol. (2022) 13:806470. doi: 10.3389/fphar.2022.806470
8. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. (2021) 184:4137.e–53.e.
9. Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. (2019) 76:473–93.
10. Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J. (2014) 13:61. doi: 10.1186/1475-2891-13-61
11. Roses C, Cuevas-Sierra A, Quintana S, Riezu-Boj JI, Martínez JA, Milagro FI, et al. Gut microbiota bacterial species associated with mediterranean diet-related food groups in a northern spanish population. Nutrients. (2021) 13:636. doi: 10.3390/nu13020636
12. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. (2013) 500:541–6.
13. Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. (2018) 9:890. doi: 10.3389/fmicb.2018.00890
15. WHO Food based dietary guidelines in the Who European Region Nutrition. and Food Security Programme WHO Regional Office for Europe. Geneva: WHO (2003).
16. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. (2017) 46:1029–56.
17. Schmidt HH, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, et al. Antioxidants in translational medicine. Antioxid Redox Signal. (2015) 23:1130–43.
18. Ren Q, Xie H, Chen YQ, Wu CF, Li H, Lu YW, et al. Effect of micronutrient pack on micronutrient status and antioxidant capacities among institutional older adults in Shanghai, China. Asia Pac J Clin Nutr. (2019) 28:457–66. doi: 10.6133/apjcn.201909_28(3).0005
19. Conti C, Rossi E, Marceglia S, Tauro V, Rizzi F, Lazzaroni M, et al. An integrated diet monitoring solution for nutrigenomic research. Stud Health Technol Inform. (2015) 210:632–6.
20. Lakshmanan AP, Shatat IF, Zaidan S, Jacob S, Bangarusamy DK, Al-Abduljabbar S, et al. Bifidobacterium reduction is associated with high blood pressure in children with type 1 diabetes mellitus. Biomed Pharmacother. (2021) 140:111736. doi: 10.1016/j.biopha.2021.111736
21. Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate illumina paired-End reAd mergeR. Bioinformatics. (2014) 30:614–20.
22. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. (2014) 30:2114–20.
23. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. (2010) 7:335–6.
24. DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. (2006) 72:5069–72. doi: 10.1128/AEM.03006-05
25. Han J, Lin K, Sequeira C, Borchers CH. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. (2015) 854:86–94. doi: 10.1016/j.aca.2014.11.015
26. Lakshmanan AP, Kohil A, El Assadi F, Al Zaidan S, Al Abduljabbar S, Bangarusamy DK, et al. Akkermansia, a possible microbial marker for poor glycemic control in qataris children consuming Arabic diet-a pilot study on pediatric T1DM in Qatar. Nutrients. (2021) 13:836. doi: 10.3390/nu13030836
27. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. (2011) 12:R60.
28. Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis. (2015) 26:27663.
29. Wirbel J, Zych K, Essex M, Karcher N, Kartal E, Salazar G, et al. Microbiome meta-analysis and cross-disease comparison enabled by the SIAMCAT machine learning toolbox. Genome Biol. (2021) 22:93. doi: 10.1186/s13059-021-02306-1
30. Chong J, Liu P, Zhou G, Xia J. Using microbiomeanalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc. (2020) 15:799–821.
31. Nagpal S, Singh R, Yadav D, Mande SS. MetagenoNets: comprehensive inference and meta-insights for microbial correlation networks. Nucleic Acids Res. (2020) 48:W572–9. doi: 10.1093/nar/gkaa254
32. Matchado MS, Lauber M, Reitmeier S, Kacprowski T, Baumbach J, Haller D, et al. Network analysis methods for studying microbial communities: a mini review. Comput Struct Biotechnol J. (2021) 19:2687–98. doi: 10.1016/j.csbj.2021.05.001
33. Thomas CM, Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. (2010) 1:148–63. doi: 10.4161/gmic.1.3.11712
34. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. (2009) 1:6ra14. doi: 10.1126/scitranslmed.3000322
35. Singh RK, Chang HW, Yan Y, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15:73.
36. Hills RD Jr., Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. (2019) 11:1613.
37. Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. (2013) 500:585–8.
38. Frankenfeld CL, Hullar MAJ, Maskarinec G, Monroe KR, Shepherd JA, Franke AA, et al. The gut microbiome is associated with circulating dietary biomarkers of fruit and vegetable intake in a multiethnic cohort. J Acad Nutr Diet. (2022) 122:78–98. doi: 10.1016/j.jand.2021.05.023
39. Di Iorio BR, Rocchetti MT, De Angelis M, Cosola C, Marzocco S, Di Micco L, et al. Nutritional therapy modulates intestinal microbiota and reduces serum levels of total and free indoxyl sulfate and P-cresyl sulfate in chronic kidney disease (Medika Study). J Clin Med. (2019) 8:1424. doi: 10.3390/jcm8091424
40. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. (2016) 65:1812–21. doi: 10.1136/gutjnl-2015-309957
41. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. doi: 10.1038/nature12820
42. Zheng J, Hoffman KL, Chen JS, Shivappa N, Sood A, Browman GJ, et al. Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr. (2020) 124:931–42. doi: 10.1017/S0007114520001853
43. Smith-Brown P, Morrison M, Krause L, Davies PSW. Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Sci Rep. (2016) 6:32385. doi: 10.1038/srep32385
44. Lakshmanan AP, Al Zaidan S, Bangarusamy DK, Al-Shamari S, Elhag W, Terranegra A. Increased relative abundance of ruminoccocus is associated with reduced cardiovascular risk in an obese population. Front Nutr. (2022) 9:849005. doi: 10.3389/fnut.2022.849005
45. Singh P, Rawat A, Alwakeel M, Sharif E, Al Khodor S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep. (2020) 10:21641. doi: 10.1038/s41598-020-77806-4
46. Cheng CK, Wang C, Shang W, Lau CW, Luo JY, Wang L, et al. A high methionine and low folate diet alters glucose homeostasis and gut microbiome. Biochem Biophys Rep. (2021) 25:100921. doi: 10.1016/j.bbrep.2021.100921
47. Jones P, Lucock M, Scarlett CJ, Veysey M, Beckett EL. Folate and inflammation – links between folate and features of inflammatory conditions. J Nutr Intermed Metab. (2019) 18:100104.
48. Moein S, Vaghari-Tabari M, Qujeq D, Kashifard M, Shokri-Shirvani J, Hajian-Tilaki K. Association between serum folate with inflammatory markers, disease clinical activity and serum homocysteine in patients with inflammatory bowel disease. Does folate level have an effect on maintaining clinical remission? Acta Biomed. (2020) 91:e2020106. doi: 10.23750/abm.v91i4.8467
49. Kaakoush NO. Insights into the role of erysipelotrichaceae in the human host. Front Cell Infect Microbiol. (2015) 5:84.
50. Schaubeck M, Clavel T, Calasan J, Lagkouvardos I, Haange SB, Jehmlich N, et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. (2016) 65:225–37. doi: 10.1136/gutjnl-2015-309333
51. Dandekar A, Mendez R, Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol Biol. (2015) 1292:205–14. doi: 10.1007/978-1-4939-2522-3_15
52. Madej D, Granda D, Sicinska E, Kaluza J. Influence of fruit and vegetable consumption on antioxidant status and semen quality: a cross-sectional study in adult men. Front Nutr. (2021) 8:753843. doi: 10.3389/fnut.2021.753843
53. Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. (2011) 3:118–34.
54. Mu G, Gao Y, Tuo Y, Li H, Zhang Y, Qian F, et al. Assessing and comparing antioxidant activities of Lactobacilli strains by using different chemical and cellular antioxidant methods. J Dairy Sci. (2018) 101:10792–806. doi: 10.3168/jds.2018-14989
55. Ianniello RG, Ricciardi A, Parente E, Tramutola A, Reale A, Zotta T. Aeration and supplementation with heme and menaquinone affect survival to stresses and antioxidant capability of Lactobacillus casei strains. LWT Food Sci Technol. (2015) 60:817–24.
56. Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, et al. Antioxidant properties of probiotic bacteria. Nutrients. (2017) 9:521.
57. Campos-Perez W, Martinez-Lopez E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim Biophys Acta Mol Cell Biol Lipids. (2021) 1866:158900.
58. Garcia-Mazcorro JF, Lage NN, Mertens-Talcott S, Talcott S, Chew B, Dowd SE, et al. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. PeerJ. (2018) 6:e4195. doi: 10.7717/peerj.4195
59. Lymperopoulos A, Suster MS, Borges JI. Short-chain fatty acid receptors and cardiovascular function. Int J Mol Sci. (2022) 23:3303.
60. Blachier F, Mariotti F, Huneau JF, Tomé D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids. (2007) 33:547–62. doi: 10.1007/s00726-006-0477-9
61. Gojda J, Cahova M. Gut microbiota as the link between elevated BCAA serum levels and insulin resistance. Biomolecules. (2021) 11:1414. doi: 10.3390/biom11101414
62. Ling CW, Miao Z, Xiao ML, Zhou H, Jiang Z, Fu Y, et al. The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab. (2021) 106:e3852–64. doi: 10.1210/clinem/dgab492
63. Yang Y, Chen T, Zhang X, Wang X. Age-related functional changes of intestinal flora in rats. FEMS Microbiol Lett. (2021) 368:fnab051.
64. Jager R, Zaragoza J, Purpura M, Iametti S, Marengo M, Tinsley GM, et al. Probiotic administration increases amino acid absorption from plant protein: a placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob Proteins. (2020) 12:1330–9. doi: 10.1007/s12602-020-09656-5
65. Wu PH, Chiu YW, Zou HB, Hsu CC, Lee SC, Lin YT, et al. Exploring the benefit of 2-methylbutyric acid in patients undergoing hemodialysis using a cardiovascular proteomics approach. Nutrients. (2019) 11:3033. doi: 10.3390/nu11123033
66. Zhou J, Tang L, Shen CL, Wang JS. Green tea polyphenols modify gut-microbiota dependent metabolisms of energy, bile constituents and micronutrients in female Sprague-Dawley rats. J Nutr Biochem. (2018) 61:68–81. doi: 10.1016/j.jnutbio.2018.07.018
Keywords: diet supplements, gut microbiota, antioxidant capacity, fruits and vegetables, SCFA
Citation: Lakshmanan AP, Mingione A, Pivari F, Dogliotti E, Brasacchio C, Murugesan S, Cusi D, Lazzaroni M, Soldati L and Terranegra A (2022) Modulation of gut microbiota: The effects of a fruits and vegetables supplement. Front. Nutr. 9:930883. doi: 10.3389/fnut.2022.930883
Received: 28 April 2022; Accepted: 16 August 2022;
Published: 23 September 2022.
Edited by:
Carlos Gómez-Gallego, University of Eastern Finland, FinlandReviewed by:
Sergio Perez-Burillo, Public University of Navarre, SpainCopyright © 2022 Lakshmanan, Mingione, Pivari, Dogliotti, Brasacchio, Murugesan, Cusi, Lazzaroni, Soldati and Terranegra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annalisa Terranegra, YXRlcnJhbmVncmFAc2lkcmEub3Jn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.