
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 12 July 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.930316
This article is part of the Research Topic Nutrition in Prevention and Management of Non-alcoholic Fatty Liver Disease View all 8 articles
 Xiaofei Luo1,2†
Xiaofei Luo1,2† Ying Li1†
Ying Li1† Yi Zhou2
Yi Zhou2 Chun Zhang2
Chun Zhang2 Lijun Li2
Lijun Li2 Yating Luo2
Yating Luo2 Jiangang Wang1
Jiangang Wang1 Yinglong Duan3*
Yinglong Duan3* Jianfei Xie3*
Jianfei Xie3*Objectives: Given the significance of dietary factors in the development of non-alcoholic fatty liver disease (NAFLD). We conducted a cross-sectional study to investigate the association of NAFLD with salt intake and dietary diversity in a medical examination population aged 18–59 years.
Methods: Data from two Chinese health management centers were utilized between January 2017 and December 2019. The general information, laboratory tests, lifestyle habits, and diet of the participants were all evaluated. Based on alcohol consumption and abdominal ultrasound results, a total of 23,867 participants were divided into the NAFLD (n = 7,753) and control (n = 16,114) groups. Salt intake and dietary diversity were calculated separately for study participants using the spot urine method and dietary diversity scores (DDS). The multilevel logistic model and subgroup analysis were used to analyze the relationship between salt intake, dietary diversity, and NAFLD.
Results: We found that the prevalence of NAFLD was 32.48%. Salt intake was associated with increased NAFLD (Q2 vs. Q1: OR = 1.201, 95% CI 1.094-1.317, P < 0.001; Q3 vs. Q1: OR = 1.442, 95% CI 1.316-1.580, P < 0.001; Q4 vs. Q1: OR = 1.604, 95% CI 1.465-1.757, P < 0.001), whereas sufficient dietary diversity was a protective factor for NAFLD (Sufficient DDS vs. Insufficient DDS: OR: 0.706, 95% CI 0.517-0.965, P < 0.05). The effects of salt intake and dietary diversity on NAFLD were equally stable in the subgroup analysis.
Conclusions: We can conclude that NAFLD is highly prevalent in medical examination adults aged 18-59 years in China. Furthermore, the risk of salt intake for NAFLD and the protective effect of dietary diversity on NAFLD should be taken into account in the management of NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is a metabolic syndrome characterized by abnormal accumulation of fatty material in the liver (1). NAFLD has become the leading cause of chronic liver disease worldwide, accounting for ~25% of the prevalence (2). More than 30% of the population in the United States is affected by NAFLD (3). The growth rate of NAFLD in China is expected to reach its highest point in 2030 (4). NAFLD is associated with adverse outcomes of liver diseases and chronic conditions such as cardiovascular disease and diabetes, making it significant global health and economic burden (5). The pathogenesis of NAFLD is complex, and metabolic disorder syndromes such as obesity, hypertension, and dyslipidemia are the main causative agents of NAFLD (3). Increasing evidence also suggests that dietary intake plays a vital role in developing NAFLD (6, 7).
High salt intake is a major dietary risk factor, resulting in ~1.8 million deaths worldwide in 2019 (8). A large-scale study of the global impact of diet on health noted that the daily salt intake of the Chinese population exceeds that of other countries and regions (9). Evidence has shown that reducing salt intake can prevent and control obesity and chronic conditions such as hypertension and cardiovascular disease (10). As a result, it is essential to focus on salt intake in the Chinese chronic disease population and analyze its association. Current studies on salt intake are usually conducted by self-perception reports and 24-h urine sodium measurements. Self-perception is less reliable, while 24-h urine sodium measurement is practically difficult to perform and is more suitable for application in small sample sizes (11). Casual spot urine estimation of 24-h urine sodium is the more commonly used method for estimating the level of salt intake in the population (12). Sodium is an essential nutrient for the body, but in asymptomatic adults, higher dietary sodium intake as measured by 24-h urine sodium can increase the development of NAFLD by contributing to obesity (13). Shen et al. (14) found that perceived high salt intake was also associated with a higher risk of NAFLD. However, no studies have directly explored casual spot urine estimates of salt intake and NAFLD in the Chinese population aged 18–59 years.
In addition, since most nutrients may interact with each other in the diet, it is not enough to consider the association of a single nutrient or food with NAFLD (15). Several previous studies have examined various dietary patterns and the risk of NAFLD (16–19). However, little has been reported on the relationship between dietary diversity and NAFLD. Dietary diversity is an essential indicator for evaluating the dietary quality index (20). Being based on food groups is more helpful in predicting nutritional adequacy than individual food indicators, although food groups vary considerably across studies (21). It was proposed that dietary diversity was positively correlated with micronutrient adequacy (22). Besides, a study conducted in Iran showed that higher dietary diversity was associated with higher quality dietary intake (23). It may be thought that subjects with higher quality diets may follow a healthier diet and therefore may be negatively associated with NAFLD. Currently, there is limited evidence, small sample sizes, and inconsistent findings regarding the relationship between dietary diversity and NAFLD, and no studies on dietary diversity and the risk of NAFLD in Chinese populations have been identified.
Salt intake and dietary diversity, as single nutrient and overall dietary quality evaluation indicators, respectively, may be combined to better evaluate the role of dietary factors on NAFLD. Therefore, this study intends to investigate the relationship between salt intake, dietary diversity, and NAFLD in Chinese adults aged 18–59 years by analyzing data from medical examination centers and providing reference evidence for further development of dietary management guidelines for NAFLD.
Data for this cross-sectional survey were obtained from two general tertiary care medical examination centers in China. A total of 49,420 adults underwent health examinations from 1 January 2017 to 31 December 2019. Inclusion criteria were (1) age between 18 and 59 years and (2) completion of all general information, clinical and laboratory tests, lifestyle habits and dietary surveys, and abdominal ultrasonography. Exclusion criteria included (1) use of hypoglycemic and hypolipidemic drugs; (2) significant abnormal liver function and other diseases affecting liver fat content, hematologic diseases, kidney disease, and those with existing related chronic diseases; and (3) history of excessive alcohol consumption (alcohol equivalent ethanol consumption ≥210 g/week for men and ≥140 g/week for women) (24). All included participants were divided into the NAFLD group and the control group by abdominal ultrasound results. After data cleaning, 23,867 participants were finally enrolled, and the detailed participant selection process is shown in Figure 1. Ethical approval for the study was obtained from the relevant hospitals (No. 2020-S587), and all participants voluntarily signed a written informed consent form.
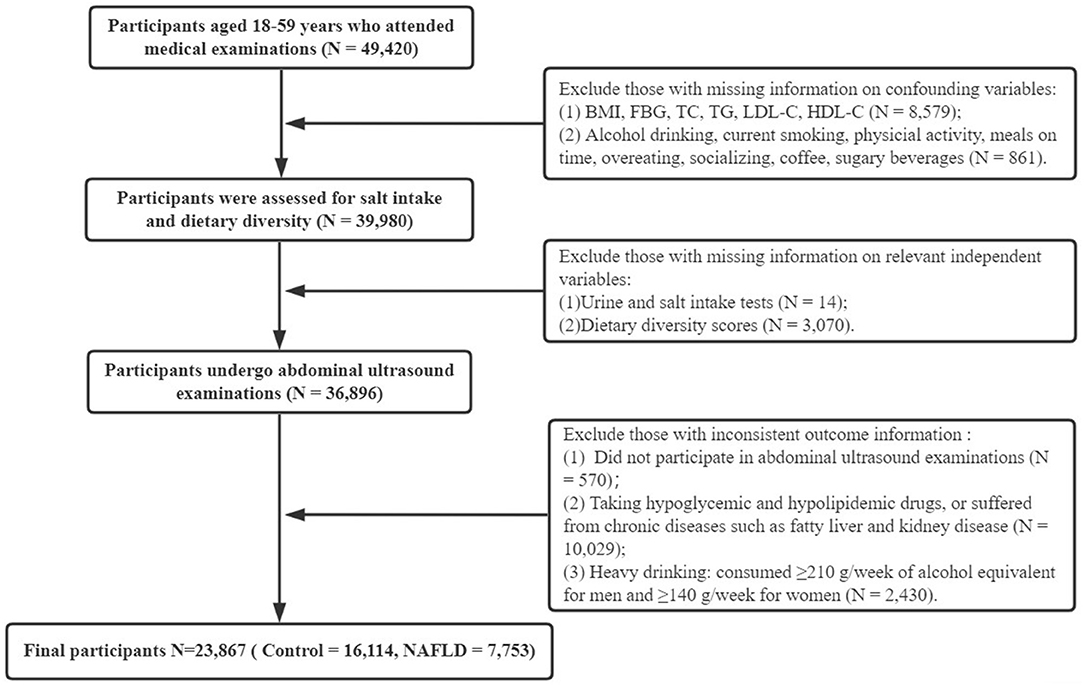
Figure 1. Flowchart of the study. BMI, body mass index; FBG, fasting blood glucose; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease.
General characteristics were collected, including age and sex. Age was divided into the youth group (18–44 years) and the middle-aged group (45–59 years) according to the World Health Organization's classification of the population.
Clinical and laboratory tests were examined by uniformly trained professionals using nationally certified instruments after an overnight fast, including height and weight and blood tests. Body mass index (BMI) was calculated as follows: weight (kg)/height2 (m2). BMI was classified according to the Chinese health industry standards into lean, BMI < 18.5 kg/m2; normal, BMI between 18.5–23.9 kg/m2; overweight, 24.0–27.9 kg/m2; and obese, BMI ≥ 28 kg/m2 (25). Blood indicators measured participants' fasting blood glucose (FBG), total cholesterol (TC), triacylglycerol (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Hyperglycemia was defined as FBG ≥ 7.0 mmol/L (26). Dyslipidemia was considered as meeting one of the conditions, TC ≥ 5.2 mmol/L, TG ≥ 1.7 mmol/L, LDL-C ≥ 3.4 mmol/L, or HDL-C < 1.0 mmol/L (27).
The lifestyle habits were collected through self-report by participants. The questionnaire consisted of four components: alcohol drinking, current smoking, physical activity, and dietary habits. Alcohol drinking was defined as those who consumed <210 g/week of alcohol equivalent for men and <140 g/week for women (24). Never smoked and quit smoking were classified as non-smoking. Physical activity assessed whether individuals exercised in their leisure time and was defined as “yes” ≥3 times/week for ≥30 mins each time (28). In addition, five common related risky diet habits were investigated: meals on time, overeating, socializing, coffee, and sugary beverages, with a “yes” or “no” response for each section.
The salt intake of participants was estimated by the spot urine sodium method. It is convenient, operational, and currently recognized as one of the reliable indicators of salt intake (11). Spot urine samples were collected from the participants, and urine creatinine, sodium, and potassium concentrations were measured using the ion electrode method. Then, the 24-h urine sodium excretion was estimated using Tanaka's (29) formula to infer the salt intake.
The dietary diversity was captured by the dietary diversity score (DDS), which has been well-validated and widely used in the Chinese population (30, 31). The DDS was developed according to the WHO and the Chinese Dietary Guidelines and the Balanced Diet Pagoda and assessed the consumption of nine food groups: grains, vegetables, fruits, livestock meat, fish and shrimp, eggs, milk and dairy products, beans, and oils and fats (32, 33). The calculation of DDS was based on participants' recall of the number of food groups consumed in the past three days, with one point for each food group consumed, without counting the frequency or quantity of intake. The scores ranged from 0 to 9 and could be divided into three levels (30): insufficient (score of 0–3), moderate (score of 4–6), and sufficient (score of 7–9).
The ultrasound diagnostic criteria for NAFLD were referenced from the Chinese Guidelines for the Treatment of NAFLD (2018 Updated Version) (24). First, participants who already had abnormal liver function or disease affecting liver fat content were excluded, as well as those who consumed alcohol ≥210 g/week for men and ≥140 g/week for women. Next, qualified physicians performed fasting abdominal ultrasound examinations on the examinee and issued a report. The findings of the report were signed by the responsible physician and entered into the system. Depending on the reported results, participants were divided into two groups: NAFLD patients and controls.
Statistics and analysis were conducted using SPSS version 25.0. Exploratory analysis was used to test normality for continuous variables. Descriptive statistics were expressed as medians [interquartile range (IQR)] or frequencies (percentages). The chi-square tests and Mann–Whitney tests were used to determine whether the participants' characteristics, clinical laboratory tests, and diet differed among patients with NAFLD. The direct correlation between salt intake, dietary diversity, and NAFLD was tested by the Mann–Whitney test, chi-square test, and Kruskal–Wallis test. Multilevel binary logistic regression analysis assessed the relationship between salt intake, dietary diversity, and NAFLD by constructing four models controlling sequentially for confounding variables. The crude model was the unadjusted model; Model 1 added age and sex; Model 2 added clinical and laboratory results on BMI, hyperglycemia, and dyslipidemia to Model 1; and Model 3 was a complete model with lifestyle habits including alcohol drinking, current smoking, physical activity, meals on time, overeating, socializing, coffee, and sugary beverages added to Model 2. Subgroup analysis was then performed by age, sex, BMI, hyperglycemia, dyslipidemia, alcohol drinking, current smoking, physical exercise, socializing, sugary beverages, and salt intake/DDS to further explore the relationship between salt intake, dietary diversity, and NAFLD. The odds ratio (OR) and 95% confidence interval (95% CI) were calculated, and P < 0.05 was considered significant for all analyses.
Of the 23,867 adults aged 18–59 years included in this study, 7,753 participants had NAFLD, with an incidence of 32.48% (see Table 1). The majority of participants were assessed at the medical examination center 1 (91.4%). The age of participants had a median and IQR of [43 (35, 50)]; 56.0% of participants were aged 18–44 years; 45.6% were female. Overweight or obese individuals made up 47.1% of participants, and more than half of participants had abnormal lipids (56.4%), while a mere 3.4% were hyperglycemia. Concerning lifestyle, most participants did not drink alcohol (72.9%), did not smoke (77.7%), did not overeat (93.6%), and did not socialize (80.3%). Physical exercise, meals on time, and non-sugary beverages accounted for the majority of participants, at 66.5%, 65.5%, and 51.7%, respectively. Only 27.5% of the participants drank coffee.
The results of the univariate analysis of NAFLD are also shown in Table 1. There was no statistically significant difference between participants from different medical examination centers and having NAFLD (P = 0.502). In terms of general characteristics, participants with NAFLD were more likely to be men, older, and 45–59 years old (middle age) compared to those without NAFLD (P < 0.001). The clinical and laboratory results indicated that participants who were overweight or obese, hyperglycemia, and dyslipidemia were at higher risk for NAFLD (P < 0.001). Participants who did not have NAFLD had lower TC [4.8 (4.3, 5.4) mmol/L], TG [1.1 (0.8, 1.6) mmol/L], LDL-C [2.8 (2.3, 3.3) mmol/L], and higher HDL-C [1.4 (1.2, 1.6) mmol/L]. Among lifestyles, participants with NAFLD reported much higher alcohol drinking, current smoking, overeating, socializing, sugary beverages, and less physical activity (P < 0.001). In addition, the association of meals on time and coffee with the finding of NAFLD was also statistically significant (P < 0.05).
As shown in Table 2, the median and IQR of the urine creatinine, urine sodium, urine potassium, and urine sodium-potassium ratios were [11,288 (4,767, 14,594)], [116.3 (68.3, 158.6)], [48.7 (27.5, 71.1)], and [2.3 (1.6, 3.3)], respectively. The analysis results showed that participants with NAFLD had significantly higher urine creatinine, sodium, potassium, sodium-potassium ratio, and salt intake than those without NAFLD (P < 0.001).
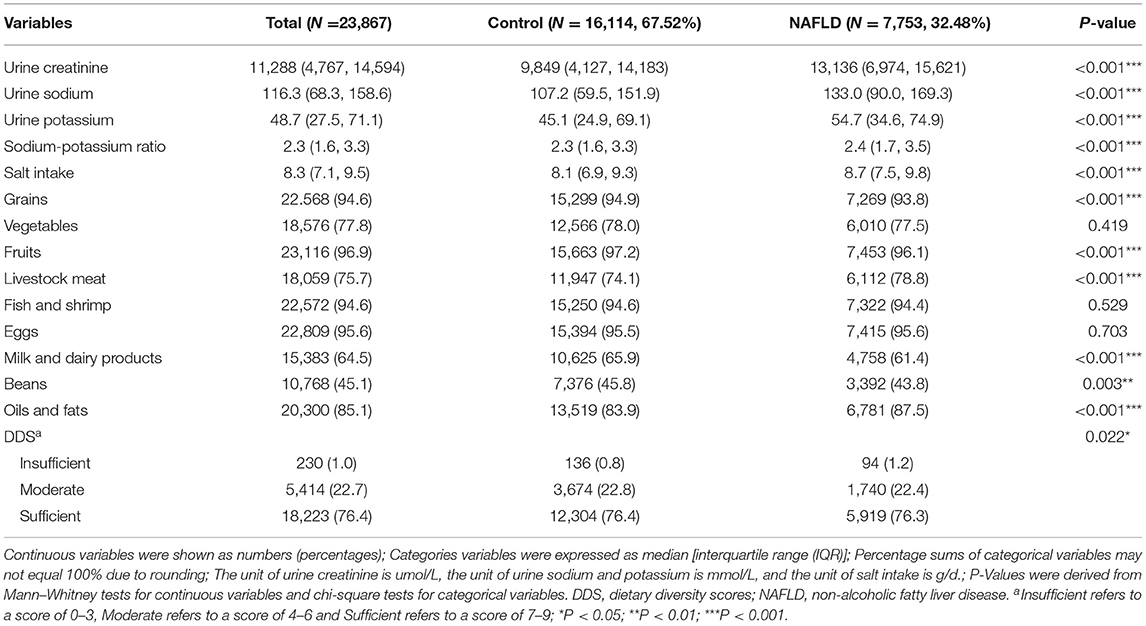
Table 2. Distribution of salt intake and dietary diversity among participants with and without NAFLD.
The food group most consumed among participants was fruit (96.9%), followed by eggs (95.6%), fish and shrimp (94.6%), and grains (94.6%), with the least consumed being beans (45.1%). Participants with NAFLD consumed fewer grains (93.8%), fruits (96.1%), milk and dairy products (61.4%), and beans (43.8%) and consumed more livestock meat (78.8%), oils and fats (87.5%). This suggests that the former may reduce the risk of NAFLD, while the latter may be considered a risk factor for NAFLD (P < 0.01).
Regarding the main variables (see Table 2; Figure 2), the median and IQR of salt intake was [8.3 (7.1, 9.5)], and dietary diversity was achieved by 76.4% of the participants. The correlation between high salt intake in patients with NAFLD was significant (P < 0.001). A greater proportion of patients with NAFLD had an insufficient DDS, and fewer had a moderate and sufficient DDS than the control group (P = 0.022). Furthermore, the median salt intake in the insufficient, moderate, and sufficient groups was 8.19, 8.23, and 8.30, respectively. The Kruskal–Wallis test indicated a significant direct correlation between dietary diversity and salt intake (P = 0.013).
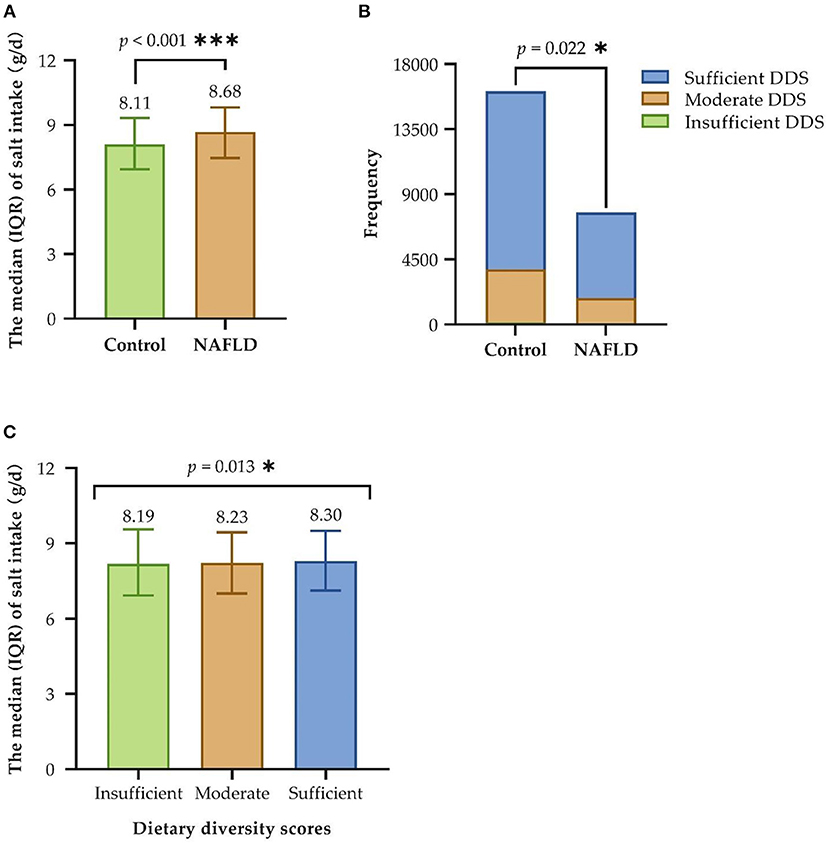
Figure 2. Correlation between salt intake, dietary diversity, and NAFLD. (A) Univariate analysis of salt intake and NAFLD; (B) Univariate analysis of dietary diversity and NAFLD; (C) Univariate analysis of salt intake and dietary diversity. IQR, interquartile range; DDS, dietary diversity scores; NAFLD, non-alcoholic fatty liver disease; Insufficient refers to a score of 0–3, Moderate refers to a score of 4–6 and Sufficient refers to a score of 7–9; *P < 0.05; ***P < 0.001.
The multilevel binary logistic regression model was constructed with salt intake and dietary diversity as independent variables and NAFLD as the outcome variable (see Table 3). Both the unadjusted and all adjusted models indicated that salt intake was a risk factor for NAFLD, and the likelihood of developing NAFLD increased with high quartiles of salt intake (P < 0.001). Compared to the lowest quartile group, the odds ratio for NAFLD decreased with higher salt intake after adjusting for confounders. The odds ratio [95% confidence interval] for the fourth quartile of salt intake in the crude model and the full Model 3 was 2.071 [1.915–2.240] and 1.604 [1.465–1.757], respectively.
Additionally, the crude and adjusted models showed that dietary diversity was a protective factor for NAFLD (P < 0.05). An increase in DDS with more sufficient dietary diversity reduced the risk of NAFLD. Both moderate DDS and sufficient DDS versus insufficient DDS in the crude model (0.681 [0.519–0.893], P < 0.01; 0.683 [0.523–0.893], P < 0.01) and Model 1 (0.698 [0.527–0.926], P < 0.01; 0.686 [0.520–0.905], P < 0.01) after adjusting for age and sex showed significant odds ratios. Model 2 (0.705 [0.517–0.962], P < 0.05) and Model 3 (0.706 [0.517–0.965], P < 0.05) after continuing to adjust for BMI, hyperglycemia, dyslipidemia, alcohol drinking, current smoking, physical exercise, meals on time, overeating, socializing, coffee, and sugary beverages had a significant odds ratio to NAFLD for sufficient DDS over insufficient DDS.
To further test the stability of the results, subgroup analyses were performed by age, sex, BMI, hyperglycemia, dyslipidemia, alcohol drinking, current smoking, physical exercise, socializing, and sugary beverages. An additional subgroup of DDS was added for salt intake and NAFLD, as well as a subgroup of salt intake for dietary diversity with NAFLD. Salt intake was presented as an independent risk factor for NAFLD in all subgroups after adjusted models (P < 0.05, see Figure 3A). Dietary diversity (see Figure 3B) was a protective factor for NAFLD in the female group, the overweight group, the current non-smoking group, the non-physical exercise group, the non-socializing group, and the non-sugary beverages group (P < 0.05).
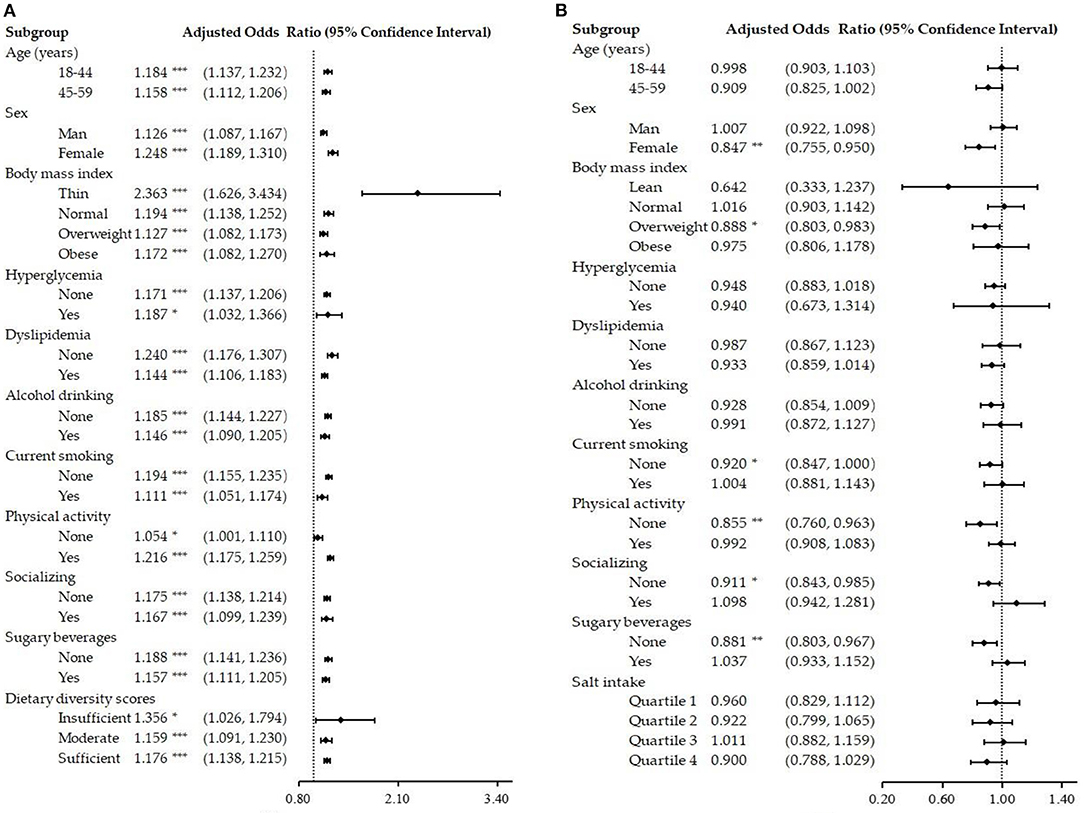
Figure 3. Subgroup analysis of the salt intake, dietary diversity, and NAFLD. (A) Association of salt intake with NAFLD after adjusting for other confounding variables; (B) Association of dietary diversity with NAFLD after adjusting for other confounding variables. NAFLD, non-alcoholic fatty liver disease; Insufficient refers to a score of 0–3, Moderate refers to a score of 4–6, and Sufficient refers to a score of 7–9; *P < 0.05; **P < 0.01; ***P < 0.001.
This study was conducted to understand the relationship between salt intake, dietary diversity, and NAFLD in Chinese adult medical examination adults aged 18–59 years. The results showed that the prevalence of NAFLD among Chinese medical examination adults aged 18–59 years was 32.48%, which was significantly higher than that of a meta-analysis of 256,367 cases on the prevalence of NAFLD in China (20%) (34). In addition, we found that salt intake increased the risk of NAFLD, whereas dietary diversity was a protective factor for NAFLD, and the results were equally stable in the subgroup analysis.
NAFLD is typically a “lifestyle disease,” and the role of modifiable dietary factors in NAFLD is of increasing concern. The imbalance of nutrient intake can easily lead to overweight and obesity, increased blood glucose and lipid levels, and other health risks related to NAFLD (35). Studies have pointed out that dietary therapy can control the absorption of basal state free fatty acids, control postprandial hyperlipidemia, reduce insulin resistance, promote the metabolism and transport of lipoproteins, increase the number of antioxidants in the body, and adjust the dietary structure and balance (36). By preventing or eliminating the causes of fatty liver through reasonable and healthy dietary nutrition, the steatosis of early hepatocytes can be reversed while reducing the patient's symptoms (37). After the development of liver lesions in patients with NAFLD, even with tighter control of risk factors such as BMI, hyperglycemia, and dyslipidemia, only 17.8% of patients eventually reverse their liver lesions to normal (38). Therefore, early screening for dietary factors that can affect NAFLD and targeted prevention is essential to reduce the risk of developing NAFLD.
High salt intake is an essential dietary risk factor for disease burden in the population (39) and has a positive association with obesity, blood pressure, and cardiovascular disease (10, 40). Therefore, it may be associated with the development of NAFLD by the same pathway. Our study found the median and IQR of salt intake in adults aged 18–59 years to be (8.3 [7.1, 9.5]) g/d. This is well above the WHO and FAO recommended daily salt intake of <6 g/d (41). It is also higher than the Norwegian adult population study (8.05 g/d) (42). The results of logistic regression analysis further suggested that salt intake was a risk factor for NAFLD. After controlling for significant confounders in univariate analysis, the results still indicated that increased salt intake increased the risk of NAFLD. Although the mechanisms linking salt intake to NAFLD are unclear, researchers have suggested that insulin resistance may be a metabolic intermediate explaining the relationship between high salt intake and NAFLD (43). A study in rats showed that high salt intake enhances insulin sensitivity in adipocytes, improves glucose uptake and insulin-induced glucose metabolism, and promotes adipocyte hypertrophy (44). In addition, high salt intake activates the aldose reductase-fructokinase pathway in the liver and hypothalamus, leading to obesity, insulin resistance, and NAFLD (45). The significant role of obesity in the development of NAFLD may also be a potential mechanism for the association between salt intake and NAFLD. A high salt diet was found to be independently associated with elevated leptin levels in humans (46), and there was a direct positive correlation between salt intake and BMI or body fat percentage (13). Another study pointed out that high salt can also play a role in hepatic inflammation and fibrosis by modulating the renin-angiotensin system, leading to the progression of NAFLD (47). This provides the supporting basis for the present study. Several previous studies have also found an association between salt and NAFLD. However, the estimation of salt intake is often performed by dietary surveys or 24-h urine sodium assessment rather than the direct calculation of salt intake (11). Spot urine estimation of 24-h urine sodium excretion is considered a simple, rapid, and generalizable method for estimating the level of salt intake in a population (12). This study is the first to analyze the relationship between salt intake and NAFLD by examining spot urine in a large sample of the Chinese medical examination population.
In terms of dietary diversity, participants with NAFLD in this study consumed fewer grains, fruits, milk and dairy products, beans, and more livestock meat, and oils and fats. However, diets that increase the intake of vegetables and fruits, beans, fish and yogurt, and whole grains and reduce foods with meat, salt, and trans fats benefit the organism (48). The Mediterranean diet, characterized by this diet, was associated with lower levels of systemic inflammation and a reduced incidence of NAFLD (49). It is recommended to increase the consumption of grains, fruits, milk and dairy products, and beans and reduce the intake of fats and livestock meats. Several other dietary patterns have been associated with NAFLD. Healthy dietary patterns and Dietary Approaches to Stop Hypertension can play a key role in the prevention and control of NAFLD (6, 16). In a study of Chinese adults, the “Animal Food” dietary pattern of food consumption was associated with an increased risk of NAFLD, and the “Grains-Vegetables” dietary pattern was related to a reduced risk of NAFLD (17). However, it has also been found that a vegetable-rich diet was not associated with NAFLD (18), and the present study also did not find an association between vegetables and NAFLD. In centrally obese adolescents, a Western dietary pattern characterized by consumption of red meat, refined grains, processed meats, and high-sugar beverages may increase the risk of NAFLD (50). A Greek study reported that the fast food-type dietary pattern was independently associated with higher odds of NAFLD due to high levels of C-reactive protein and unsaturated fatty acids (19). A Lebanese study noted that the high fruit diet group was also a major potential risk factor for NAFLD, while the traditional diet consisting of vegetables and legumes was negatively associated with the odds of NAFLD (51). It can be learned that different dietary patterns have different effects on NAFLD, and the evidence focusing on the overall quality of the diet and NAFLD is still weak.
The present study also suggested that dietary diversity, as a protective factor for NAFLD, decreased the risk of NAFLD development. Sufficient DDS was associated with a reduced risk of NAFLD compared to insufficient DDS. Some trial evidence suggested that greater food diversity affected the composition of the gut flora, thereby further improving immune function and health status (52). Previous studies have found that increased dietary diversity reduces the incidence of diseases closely related to NAFLD such as overweight, metabolic syndrome, and cardiovascular disease (31, 53, 54). In addition, higher DDS was related to healthier eating habits and better metabolic profiles. Increased dietary diversity leads to more opportunities to choose foods that are negatively associated with NAFLD. Ebrahimi et al. (55) noted that dietary diversity was correlated with increased vitamin C, calcium, and fiber, all of which were negatively associated with liver disease. Furthermore, dietary diversity may increase the intake of various micronutrients, dietary fiber, and some healthful phytochemicals, which are positively associated with dietary balance (56, 57). A wide variety of foods from the same and different food groups can provide vitamins, minerals, and other micronutrients necessary for the health of the body, thereby improving dietary patterns and optimizing the structure of the diet. The more established “second strike” theory of NAFLD points to the importance of oxidative stress in the development of NAFLD (58). Dietary diversity may increase the adequate intake of antioxidants in the diet (59) and may be associated with NAFLD. Notably, another study found that DDS increase was not significantly associated with NAFLD (15). This difference in results may be due to the inclusion of food groups with different assessment methods, different populations assessed, and smaller samples for dietary diversity in their included studies. Furthermore, dietary diversity is not focused on increasing the number of nutrients but rather on promoting diversity and balance among nutrients. It should be clarified that the dietary diversity of an individual does not indicate complete nutritional health. Dietary diversity can ensure the intake of all types of nutrients, but if a particular nutrient is consumed too much or not enough, it is an unreasonable dietary structure, affecting nutritional health.
This study also showed that NAFLD was related to higher age, considering an association with increased metabolic decrease (38). Men were also prone to NAFLD because they were more socially active in their careers, and many had drinking and smoking habits (35). Consistent with previous studies, NAFLD was linked to overweight/obesity, hyperglycemia, and dyslipidemia, with higher BMI, glucose, and lipids in the NAFLD group than in the control group (35, 58). Alcohol drinking and current smoking were also associated with NAFLD. Evidence has shown that patients with NAFLD should avoid alcohol consumption (60), and passive smoking increases NAFLD risk by ~1.38-fold (61). Conversely, physical activity can improve the metabolic function of all bodies, which helps to control BMI and improve insulin resistance, therefore reducing the incidence of NAFLD (36). In addition, socializing and drinking sugary beverages can also affect NAFLD. Additional socializing can lead to excess nutrients being stored in the liver and the formation of a fatty liver. Sugary beverages can increase the metabolic burden so may be a key driver of NAFLD (62). Interestingly, previous studies suggested a protective effect of meals on time and coffee consumption on NAFLD (63). However, this study found no association between meals on time and coffee consumption and NAFLD in the adjusted regression model. This may be related to other factors, such as different subjects consuming coffee with different types, amounts, and food frequencies. Moreover, the present study found that salt intake was associated with dietary diversity. This has not been described in previous studies. Even without considering the amount and frequency of food intake, a greater variety of food intake can affect salt intake levels. Increased dietary diversity is accompanied by the intake of more categories of foods, which implies that people with sufficient dietary diversity have an increased probability of consuming salt-rich foods. This suggests that in addition to managing single nutrients such as sodium, it is equally important to consider whether the overall dietary intake is balanced for NAFLD (15).
To exclude the effect of these factors on NAFLD, we performed further subgroup analysis that illustrated the stability of the results of this study. The results indicated that salt intake remained a risk factor for NAFLD in all subgroups. This suggests that salt intake must be reduced in the management of patients with NAFLD. The protective effect of dietary diversity on NAFLD was more pronounced in the female, overweight, non-current smoking, non-physical activity, non-socializing, and non-sugary beverage groups. This may be due to the fact that dietary diversity is also influenced by multiple factors. It also reinforces the recommendation that the impact of dietary diversity on the health of different populations of patients with NAFLD should be analyzed on a case-by-case basis.
To our knowledge, this study was the first to examine the relationship between salt intake, dietary diversity, and NAFLD in Chinese adults aged 18–59 years. We obtained the results using data from a large sample of Chinese medical examinations. All data were collected by professionals and quality-controlled to ensure the reliability of the information. We adjusted for multiple confounders in our analysis to build models and performed subgroup analysis to make our results more stable. In addition, our study subjects were the medical examination population, and clinical health care professionals will provide post-test health promotion education and dietary and exercise instructions to these individuals.
This study still had several limitations. First, this was a cross-sectional study, and the results did not reflect causality and the prediction of NAFLD progression, which needs to be further demonstrated in future cohort studies and interventional studies. Second, selection bias needs to be considered, and the population selected for this study was a healthy physical examination population, which may have higher health awareness than other populations. Third, this study evaluated whether the participants had NAFLD and did not assess its severity, which needs to be further explored in future studies. Fourth, the type of food intake in this study was self-reported, it might exist over-reporting and under-reporting situations, thus may causing bias to the results. Besides, the indicator of dietary diversity in this study assessed the variety of foods and did not address the daily energy intake of participants which might be a confounding factor. Finally, we used spot urine to estimate salt intake, and its assessment accuracy may be less than the gold standard.
The prevalence of NAFLD was 32.48% among adults aged 18–59 years in the two Chinese physical examination centers in this study. Salt intake was associated with dietary diversity and NAFLD. Salt intake was an independent risk factor for NAFLD, while a sufficient DDS was a protective factor for NAFLD, and the results were equally stable in the subgroup analysis. It is important to change adverse lifestyle habits and pay attention to the control of salt intake and the balance of dietary diversity in the management of high-risk people to reduce the risk of NAFLD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Third Xiangya Hospital of Central South University (No. 2020-S587). The patients/participants provided their written informed consent to participate in this study.
XL and YLi: conceptualization, formal analysis, data curation, and methodology. XL, YZ, CZ, LL, and YLu: software, validation, and visualization. YLi and JW: investigation. YLi, JW, and JX: resources. XL: writing—original draft preparation. XL, YD, and JX: writing—review and editing. JW, YD, and JX: supervision and project administration. YD and JX: funding acquisition. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University (No. YX202006) and the Special Funding for the Construction of Innovative Provinces in Hunan (No. 2020SK2091).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to all participants for their voluntary participation and to all the members of our research team.
1. Anstee QM, Reeves HL, Kotsiliti E, Govaere O. Heikenwalder, M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. (2019) 16:411–28. doi: 10.1038/s41575-019-0145-7
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L. Wymer, M. Global epidemiology of non-alcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
3. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
4. Fan JG, Kim SU, Wong VWS. New trends on obesity and NAFLD in Asia. J Hepatol. (2017) 67:862–73. doi: 10.1016/j.jhep.2017.06.003
5. Arab JP, Arrese M. Trauner, M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol Mech Dis. (2018) 13:321–50. doi: 10.1146/annurev-pathol-020117-043617
6. Hekmatdoost A, Shamsipour A, Meibodi M, Gheibizadeh N, Eslamparast T, Poustchi H. Adherence to the dietary approaches to stop hypertension (dash) and risk of nonalcoholic fatty liver disease. Int J Food Sci Nutr. (2016) 67:1024–29. doi: 10.1080/09637486.2016.1210101
7. Tajima R, Kimura T, Enomoto A, Yanoshita K, Saito A, Kobayashi S, et al. Association between rice, bread, and noodle intake and the prevalence of non-alcoholic fatty liver disease in japanese middle-aged men and women. Clin Nutr. (2017) 36:1601–08. doi: 10.1016/j.clnu.2016.09.034
8. Collaborators GBD, Ärnlöv J. Global Burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
9. Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, et al. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 393:1958–72. doi: 10.1016/S0140-6736(19)30041-8
10. He FJ, Tan M, Ma Y. MacGregor, GA. Salt reduction to prevent hypertension and cardiovascular disease: jacc state-of-the-art review. J Am Coll Cardiol. (2020) 75:632–47. doi: 10.1016/j.jacc.2019.11.055
11. Peng YG, Li W, Wang Y, Chen H, Bo J, Wang XY et al. Validation and assessment of three methods to estimate 24-h urinary sodium excretion from spot urine samples in chinese adults. Plos One. (2016) 11:e0149655. doi: 10.1371/journal.pone.0149655
12. Yang P, Chen Z, Zhu X, Cao X, Wang Y, Li Y. Frequency of eating away from home is associated with salt intake: a cross-sectional study of 18,242 population. J Clin Cardiol. (2020) 36:270–75. doi: 10.13201/j.issn.1001-1439.2020.03.017
13. Choi Y, Lee JE, Chang Y, Kim MK, Sung E, Shin H, et al. Dietary sodium and potassium intake in relation to non-alcoholic fatty liver disease. Br J Nutr. (2016) 116:1447–56. doi: 10.1017/S0007114516003391
14. Shen X, Jin C, Wu Y, Zhang Y, Wang X, Huang W, et al. Prospective study of perceived dietary salt intake and the risk of non-alcoholic fatty liver disease. J Hum Nutr Diet. (2019) 32:802–09. doi: 10.1111/jhn.12674
15. Hashemi Kani A, Alavian SM, Esmaillzadeh A, Adibi P. Azadbakht L. Dietary quality indices and biochemical parameters among patients with non alcoholic fatty liver disease (NAFLD). Hepat Mon. (2013) 13:e10943. doi: 10.5812/hepatmon.10943
16. Adriano LS, Sampaio HAC, Arruda SPM, Portela CL, de Melo MLP, Carioca AAF, et al. Healthy dietary pattern is inversely associated with non-alcoholic fatty liver disease in elderly. Br J Nutr. (2016) 115:2189–95. doi: 10.1017/S0007114516001410
17. Yang CQ, Shu L, Wang S, Wang JJ, Zhou Y, Xuan YJ, et al. Dietary patterns modulate the risk of non-alcoholic fatty liver disease in chinese adults. Nutrients. (2015) 7:4778–91. doi: 10.3390/nu7064778
18. Zhang S, Gu Y, Bian S, Górska MJ, Zhang Q, Liu L, et al. Dietary patterns and risk of non-alcoholic fatty liver disease in adults: a prospective cohort study. Clin Nutr Edinb Scotl. (2021) 40:5373–82. doi: 10.1016/j.clnu.2021.08.021
19. Kalafati IP, Borsa D, Dimitriou M, Revenas K, Kokkinos A, Dedoussis GV. Dietary patterns and non-alcoholic fatty liver disease in a greek case-control study. Nutr Burbank Los Angel Cty Calif. (2019) 61:105–10. doi: 10.1016/j.nut.2018.10.032
20. Vandevijvere S, De Vriese S, Huybrechts I, Moreau M, Van Oyen H. Overall and within-food group diversity are associated with dietary quality in Belgium. Public Health Nutr. (2010) 13:1965–73. doi: 10.1017/S1368980010001606
21. Zhao W, Yu K, Tan S, Zheng Y, Zhao A, Wang P, et al. Dietary diversity scores: an indicator of micronutrient inadequacy instead of obesity for chinese children. BMC Public Health. (2017) 17:440. doi: 10.1186/s12889-017-4381-x
22. Mallard SR, Houghton LA, Filteau S, Chisenga M, Siame J, Kasonka L, et al. Micronutrient adequacy and dietary diversity exert positive and distinct effects on linear growth in urban Zambian infants. J Nutr. (2016) 146:2093–101. doi: 10.3945/jn.116.233890
23. Azadbakht L, Esmaillzadeh A. Dietary energy density is favorably associated with dietary diversity score among female university students in Isfahan. Nutrition. (2012) 28:991–95. doi: 10.1016/j.nut.2011.12.017
24. National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Chin J Hepatol. (2018) 26:195–203. doi: 10.3760/cma.j.issn.1007-3418.2018.03.008
25. Cooperative Meta-Analysis Group of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in chinese adult population. Chin J Epidemiol. (2002) 23:5–10. doi: 10.3760/j.issn:0254-6450.2002.01.003
26. Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Int J Endocrinol Metab. (2021) 41:482–48. doi: 10.3760/cma.j.cn121383-20210825-08063
27. Zhu J, Gao R, Zhao S, Lu G, Zhao D, Li J. Guidelines for the prevention and treatment of dyslipidemia in adults in China (2016 Revised Edition). Chin Circ J. (2016) 31:937–53. doi: 10.3969/j.issn.1000-3614.2016.10.001
28. Healthy China Initiative Promotion Committee. Healthy China Initiative (2019-2030): general requirements, major initiatives and main indicators. Chin Circ J. (2019) 34:846–58. doi: 10.3969/j.issn.1000-3614.2019.09.003
29. Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, et al. A Simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. (2002) 16:97–103. doi: 10.1038/sj.jhh.1001307
30. Jin Y. Study on Associations of Dietary Diversity with Nutrients Adequacy and Nutrition Related Chronic Disease in Chinese Adults. PhD, Chinese Center for Disease Control and Prevention: Beijing. (2009).
31. Wang Y, Li L, Li Y, Liu M, Gan G, Zhou Y, et al. The impact of dietary diversity, lifestyle, and blood lipids on carotid atherosclerosis: a cross-sectional study. Nutrients. (2022) 14:815. doi: 10.3390/nu14040815
32. World Health Organization. “Preparation and use of food-based dietary guidelines: report of a joint FAO/WHO consultation,” in Preparation and Use of Food-Based Dietary Guidelines: Report of a Joint FAO/WHO Consultation; (1998), pp. vi–108.
34. Li Z, Xue J, Chen P, Chen L, Yan S, Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of china: a meta-analysis of published studies. J Gastroenterol Hepatol. (2014) 29:42–51. doi: 10.1111/jgh.12428
35. Hu XY Li Y, Li LQ, Zheng Y, Lv JH, Huang SC, et al. Risk factors and biomarkers of non-alcoholic fatty liver disease: an observational cross-sectional population survey. BMJ Open. (2018) 8:e019974. doi: 10.1136/bmjopen-2017-019974
36. Lu L, Zeng M. Treatment of nonalcoholic fatty liver disease: behavior modification, diet therapy and exercise programs. Chin J Hepatol. (2005) 13:138. doi: 10.3760/cma.j.issn.1007-3418.2005.12.030
37. Li L, Gong L, Zhang Y, Jiang Y, Gu Y, Zhou J. Relationship between dietary patterns and nonalcoholic fatty liver disease among karamay adults. Chin J Health Manag. (2013) 7:312–16. doi: 10.3760/cma.j.issn.1674-0815.2013.05.007
38. Lu ZY, Shao Z, Li YL, Wulasihan M, Chen XH. Prevalence of and risk factors for non-alcoholic fatty liver disease in a chinese population: an 8-year follow-up study. World J Gastroenterol. (2016) 22:3663. doi: 10.3748/wjg.v22.i13.3663
39. Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1345–422. doi: 10.1016/S0140-6736(17)32366-8
40. Zhou L, Stamler J, Chan Q, Van Horn L, Daviglus ML, Dyer AR, et al. Salt intake and prevalence of overweight/obesity in Japan, China, the United Kingdom, and the United States: the INTERMAP study. Am J Clin Nutr. (2019) 110:34–40. doi: 10.1093/ajcn/nqz067
41. World Health Organization. Diet, nutrition and the prevention of chronic diseases. report of a joint WHO/FAO expert consultation. WHO Tech. Rep. Ser. (2003) 916:34–8. Available online at: https://apps.who.int/iris/handle/10665/42665
42. Chen SL, Dahl C, Meyer HE, Madar AA. Estimation of salt intake assessed by 24-h urinary sodium excretion among somali adults in Oslo, Norway. Nutrients. (2018) 10:900. doi: 10.3390/nu10070900
43. van den Berg EH, Gruppen EG, Blokzijl H, Bakker SJL, Dullaart RPF. Higher sodium intake assessed by 24 h urinary sodium excretion is associated with non-alcoholic fatty liver disease: The PREVEND cohort study. J Clin Med. (2019) 8:2157. doi: 10.3390/jcm8122157
44. Fonseca-Alaniz MH, Takada J, Andreotti S, De Campos TBF, Campaña AB, Borges-Silva CN, et al. High sodium intake enhances insulin-stimulated glucose uptake in rat epididymal adipose tissue. Obesity. (2008) 16:1186–92. doi: 10.1038/oby.2008.69
45. Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci. (2018) 115:3138–43. doi: 10.1073/pnas.1713837115
46. Zhu H, Pollock NK, Kotak I, Gutin B, Wang X, Bhagatwala J, et al. Dietary sodium, adiposity, and inflammation in healthy adolescents. Pediatrics. (2014) 133:e635–42. doi: 10.1542/peds.2013-1794
47. Emamat H, Farhadnejad H, Movahedian M, Tangestani H, Mirmiran P, Hekmatdoost A. Dietary sodium intake in relation to non-alcoholic fatty liver disease risk: a case-control study. Nutr Food Sci. (2020) 51:541–50. doi: 10.1108/NFS-05-2020-0183
48. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. (2016) 133:187–25. doi: 10.1161/CIRCULATIONAHA.115.018585
49. Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, et al. Adherence to the mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. (2014) 33:678–83. doi: 10.1016/j.clnu.2013.08.014
50. Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O'Sullivan TA, Ayonrinde OT, et al. The western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. (2013) 108:778–85. doi: 10.1038/ajg.2013.95
51. Fakhoury-Sayegh N, Younes H, Heraoui GNHA, Sayegh R. Nutritional profile and dietary patterns of lebanese non-alcoholic fatty liver disease patients: a case-control study. Nutrients. (2017) 9:1245. doi: 10.3390/nu9111245
52. Claesson MJ, Jeffery IB, Conde S, Power SE, O'connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. (2012) 488:178–84. doi: 10.1038/nature11319
53. Vadiveloo M, Sacks FM, Champagne CM, Bray GA. Mattei, J. Greater healthful dietary variety is associated with greater 2-year changes in weight and adiposity in the preventing overweight using novel dietary strategies (POUNDS Lost). Trial J Nutr. (2016) 146:1552–59. doi: 10.3945/jn.115.224683
54. Farhangi MA, Jahangiry L. Dietary diversity score is associated with cardiovascular risk factors and serum adiponectin concentrations in patients with metabolic syndrome. BMC Cardiovasc Disord. (2018) 18:68. doi: 10.1186/s12872-018-0807-3
55. Ebrahimi Mousavi S, Dehghanseresht N, Dashti F, Khazaei Y, Salamat S, Asbaghi O, et al. The association between dietary diversity score and odds of nonalcoholic fatty liver disease: a case-control study. Eur J Gastroenterol Hepatol. (2022) 34:678–85. doi: 10.1097/MEG.0000000000002344
56. Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J Hypertens. (2007) 25:2361–9. doi: 10.1097/HJH.0b013e3282efc214
57. Bernstein MA, Tucker KL, Ryan ND, O'Neill EF, Clements KM, Nelson ME, et al. Higher dietary variety is associated with better nutritional status in frail elderly people. J Am Diet. Assoc. (2002) 102:1096–04. doi: 10.1016/S0002-8223(02)90246-4
58. Cichoz-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases World J Gastroenterol. WJG. (2014) 20:8082. doi: 10.3748/wjg.v20.i25.8082
59. Braun LR, Feldpausch MN, Czerwonka N, Weiss J, Branch K, Lee H, et al. Effects of pitavastatin on insulin sensitivity and liver fat: a randomized clinical trial. J Clin Endocrinol Metab. (2018) 103:4176–86. doi: 10.1210/jc.2018-01446
60. Seitz HK, Mueller S, Hellerbrand C, Liangpunsakul S. Effect of chronic alcohol consumption on the development and progression of non-alcoholic fatty liver disease (NAFLD) Hepatobiliary. Surg Nutr. (2015) 4:147–51. doi: 10.3978/j.issn.2304-3881.2014.12.01
61. Akhavan Rezayat A, Dadgar Moghadam M, Ghasemi Nour M, Shirazinia M, Ghodsi H, Rouhbakhsh Zahmatkesh MR, et al. Association between smoking and non-alcoholic fatty liver disease: a systematic review and meta-analysis. SAGE Open Med. (2018) 6:2050312117745223. doi: 10.1177/2050312117745223
62. Chhimwal J, Patial V, Padwad Y. Beverages and non-alcoholic fatty liver disease (NAFLD): think before you drink. Clin Nutr. (2021) 40:2508–19. doi: 10.1016/j.clnu.2021.04.011
Keywords: non-alcoholic fatty liver disease, salt intake, dietary diversity, adults, medical examinations
Citation: Luo X, Li Y, Zhou Y, Zhang C, Li L, Luo Y, Wang J, Duan Y and Xie J (2022) Association of Non-alcoholic Fatty Liver Disease With Salt Intake and Dietary Diversity in Chinese Medical Examination Adults Aged 18–59 Years: A Cross-Sectional Study. Front. Nutr. 9:930316. doi: 10.3389/fnut.2022.930316
Received: 27 April 2022; Accepted: 10 June 2022;
Published: 12 July 2022.
Edited by:
Nenad Naumovski, University of Canberra, AustraliaReviewed by:
Zahra Yari, National Nutrition and Food Technology Research Institute, IranCopyright © 2022 Luo, Li, Zhou, Zhang, Li, Luo, Wang, Duan and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinglong Duan, eWluZ2xvbmdkdWFuQG91dGxvb2suY29t; Jianfei Xie, eGllamlhbmZlaUBjc3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.