
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 11 November 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.930272
This article is part of the Research TopicDietary Intake, Eating Behavior and Health OutcomesView all 36 articles
Background: Although chronic low-grade inflammation has been linked to the development of erectile dysfunction (ED), the association between pro-inflammatory diets and ED is unclear. The dietary inflammation index (DII) is a novel method to quantify the inflammatory potential of a diet.
Objective: Our objective was to investigate the association between the DII and ED among US males.
Design: This cross-sectional study included 3,693 males 20–85 year of age from the National Health and Nutrition Examination Survey (NHANES) 2001–2004. Multivariable-adjusted logistic regression models were used to assess the association between the DII and ED. All analyses accounted for the complex sampling design.
Results: The mean ± SE of the DII was 0.8 ± 0.1 and 0.4 ± 0.1 among participants with and without ED, respectively. After adjusting for age, race/ethnicity, education, smoking status, physical activity, drinking status, hypertension, diabetes, cardiovascular disease, hypercholesterolemia, BMI, and eGFR, the DII score was associated with ED (odds ratio 1.12; 95% CI: 1.04–1.19). Moreover, this association was also stable in our subgroup analysis or sensitivity analyses.
Conclusion: Dietary inflammatory potential, as estimated by the DII score, is positively associated with ED among US males.
Erectile dysfunction (ED) is one of the most common types of sexual dysfunction in men (1, 2) and is predicted to affect approximately 322 million men worldwide by 2025 (3). ED has led to a poorer quality of life and reduction of economic productivity in males, as well as a substantial financial burden on society (4, 5). Some ED risk factors have been identified, including aging, history of diabetes, cardiovascular disease, chronic kidney disease, and smoking (6, 7). However, those risk factors, in most cases, are limited or unmodifiable. Thus, the identification of modifiable risk factors for ED is important.
Diet is a potential source of chronic low-grade inflammation which is related to the pathogenesis of ED (8). Previously, some dietary patterns, especially anti-inflammatory diet, have been linked to ED (9, 10). For example, Mediterranean diet is a kind of anti-inflammatory diet, and recently a long-term randomized clinical trial by Maiorino et al. has noticed a protective effect of Mediterranean diet on erectile function, as well as a reduction of C-reactive protein levels in males with newly diagnosed type 2 diabetes (T2DM) (11). It can be inferred that dietary inflammatory potential is related to the development of ED. However, the impact of the dietary inflammatory potential on ED is still unclear. The DII is a novel method re-devised by Shivappa et al. to quantify the potential inflammatory levels of our daily diet (12). Since its development, DII has been validated against many inflammatory biomarkers in different ethnics and was widely used in a large number of studies (13–19). To our knowledge, the association between the level of dietary inflammation potential and ED has not been reported before. To best understand the influence of dietary inflammation on ED and provide clues for its prevention, this cross-sectional study explored the association between DII and ED among US adults, using data from the National Health and Nutrition Examination Survey (NHANES).
The NHANES is a nationally representative survey with a stratified, multistage probability cluster sampling design in the USA. It is administered by the National Center for Health Statistics (NCHS) and can be used to assess the health or nutritional status of the non-institutionalized US population (20). Data for erectile function is available in the NHANES 2001–2004 and all data were collected following standardized protocols from the NCHS. To provide reliable estimates, we utilized the 4-year data for analyses. The study was approved by the NCHS Research Ethics Review Board and written consents were obtained from the participants before their participating. We followed the Strengthening the Reporting of Observational Studies in Epidemiology – Nutritional Epidemiology (STROBE-nut) guidelines in reporting.
Of 4116 males (≥20 years) with available information for self-reported ED in the NHANES 2001–2004, 125 were excluded due to having or having received surgery/radiation/medicine treatment for prostate cancer. Eighteen men were further excluded for taking phosphodiesterase type 5 (PDE5) inhibitors or Yohimbine (21), and 83 participants were excluded due to incompleteness of data needed to calculate the dietary inflammatory index. According to a previous study, a daily calorie intake below 800 kcal or above 5,000 kcal is thought to be implausible (22). Thus, 199 participants were excluded for this reason. The flow chart for subject selection is presented in Figure 1.
In the NHANES, erection function was assessed by a question: “How would you describe your ability to get and keep an erection adequate for satisfactory intercourse?” The following response options were provided: “always or almost always able,” “usually able,” “sometimes able,” or “never able.” This single question has been validated in a sub-sample from the Massachusetts Male Aging Study (MMAS) and was considered as a practical tool for assessing ED (23). In the present study, ED was defined as a dichotomous variable where men who responded “sometimes able” or “never able” to maintain an erection were considered as with ED and those who responded “always or almost always able” or “usually able” were considered as without ED (24, 25).
Based on the previous study, the DII score was calculated based on 27 food parameters extracted from the NHANES 2001–2004 by a 24-h dietary recall interview (12, 26). Then, the DII score was calculated by the following steps. First, the z-score for each of the food parameters for each individual was calculated based on the world average and standard deviation. Second, to control the effect of “skewing,” each z-score was converted to a centered percentile value. Third, the food parameter-specific DII score was calculated as the centered percentile value times its respective standardized overall inflammatory effect score (12). Finally, the parameter-specific DII scores were summed to get the final index for each individual. A more positive DII value indicates a more pro-inflammatory diet, and a more negative DII value indicates a more anti-inflammatory diet. Supplementary Table 1 lists the respective world average, SD, and standardized overall inflammatory effect score of the 27 food parameters used in the present study.
Potential confounders in the present study included age (27), race/ethnicity (non-Hispanic white, Mexican-American, non-Hispanic black, and others) (28), education (high school or less, some college, and college graduate or higher) (29), body mass index (BMI) (30), diabetes (31), hypertension (32), cardiovascular disease, hypercholesterolemia (33), estimated glomerular filtration rate (eGFR) (34, 35), smoking status (36), alcohol drinking status (37), and physical activity level (38). Diabetes was defined as any participant with self-reported diabetes or who had a fasting plasma glucose level of 126 mg/dl or greater or a glycated hemoglobin level of 6.5% or greater. Hypertension was defined as taking anti-hypertensive agents, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Cardiovascular disease was defined as self-reported history of one of the following conditions: coronary heart disease, myocardial infarction, congestive heart failure, and stroke. Hypercholesterolemia was defined as being told to take cholesterol-lowering medications or total cholesterol ≥240 mg/dl. The eGFR was calculated based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. In the NHANES, self-reported smoking status can be assessed by the following two survey question: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes every day, some days or not at all?” According to the National Health Interview Survey, smoking status can be categorized into three groups: never smoker (smoked less than 100 cigarettes in the lifetime, or has never smoked), ex-smoker (smoked at least 100 cigarettes in the lifetime and responded that now do not smoke), current smoker (smoked at least 100 cigarettes in the lifetime and responded that now smoke cigarettes every day or some days) (39). Serum cotinine is a biomarker of current smoking. Considering that there is no minimum duration of smoking cessation, we took serum cotinine into consideration to reduce the misclassification of current smoker into ex-smoker. Specifically, self-reported never smokers and ex-smokers who having a serum cotinine level above 10 ng/ml were corrected to be current smokers (40). Alcohol drinking status was determined by self-reporting. Those who drank at least 12 standard drinks in any one year were defined as “alcohol drinking,” otherwise they were designated as “without alcohol drinking.” Physical activity was divided into three groups based on self-reported leisure-time physical activity: inactive, moderate, and vigorous.
The sampling weights were applied in our analyses following the NHANES analytic guidelines (41). A 4-year sampling weight was calculated using the formula: Dietary day one 4-Year sample weight = 1/2 × Dietary day one 2-Year sample weight (WTDRD1). Weighted means/proportions and standard errors (SEs) were used to describe the characteristics of the participants. Continuous data were compared using the survey t-test, and categorical data were compared by the survey (Rao–Scott) χ2 test. Since the missing data was small (missing rate ranged from 0 to 3.9%) for any variable, no imputation method was used in the present study. Odds ratio (OR) and 95% confidence interval (CI) were calculated to show the association between DII and ED by using logistic regression models. Four models were conducted using the logistic regression analyses and generalized variance-inflation factors (GVIF) ≥3 indicated the presence of multicollinearity in the analysis. Model 1 was the crude model with no covariate adjusted. Model 2 was adjusted for age, race/ethnicity. Model 3 was the main model. If a covariate changed the estimates of DII and ED by more than 10% when entered into the crude model or eliminated from the complete model, it was included as a potential confounder in model 3. Therefore, model 3 was adjusted for age, race/ethnicity, education levels, smoking status, physical activity levels and hypertension. A fully adjusted model was done for model 4, which was adjusted for covariates in model 3 and drinking status, diabetes, cardiovascular disease, hypercholesterolemia, BMI, and eGFR. To further explore the potential associations, the DII score was also classified by tertiles for multivariable logistic regression analyses, and tests for trend were conducted by entering the median value of each DII tertiles as a continuous variable in the multivariable logistic regression models. Stratified and interaction analyses were performed according to age groups, race and ethnicity, hypertension, diabetes, and cardiovascular disease. Finally, two sensitivity analyses were additionally performed to assess the robustness of our findings. In the first sensitivity analysis, we excluded participants taking medicines that potentially affect erectile function, including antidepressants (42), antipsychotics (43), antihyperglycemic agents (9, 44), sex hormones and corticosteroids (45). In the second sensitivity analysis, a stricter criterion of ED was used. Only those who responded “never able to get and keep an erection adequate for satisfactory intercourse” were considered as having ED. All statistical analyses were performed with the statistical software R (The R Foundation)1. A P-value <0.05 (two-sided) was considered to indicate statistical significance. A post hoc power analysis was performed, which demonstrated that the power for the primary outcomes was sufficient (power >0.90).
Table 1 shows the weighted characteristics stratified by ED status. There were 3,693 males included in our analyses and 1,011 of them had ED. The weighted number of all participants is 3,809,255,599, and the weighted prevalence of ED is 33.7%. For all participants, the weighted mean age was 44.8 years old (SE = 0.4), and most of them were non-Hispanic whites (74.6%, SE = 2.0). The DII scores ranged from −5.15 (most anti-inflammatory) to +4.93 (most pro-inflammatory), and the mean of DII score was higher in participants with vs. without ED (0.8 vs. 0.4, P < 0.001). Participants with ED were more likely to be older and have a higher BMI, lower educational level, lower eGFR, lower physical activity level, and diabetes, hypertension, cardiovascular disease, and hypercholesterolemia.
Table 2 summarizes results from sample-weighted logistic regression analyses. The association between DII and ED was stable in different adjusted models. In the crude model (model 1), the odds ratio of DII on ED was 1.13 (95% CI, 1.08–1.19). Males in the highest DII tertiles vs. those the lowest DII tertiles were at a higher risk of ED [OR 1.64 (95% CI, 1.30–2.08)]. In the main model (model 3) adjusted for age, race and ethnicity, education levels, hypertension, smoking status, and physical activity levels, the odds ratio was 1.11 (95% CI, 1.05–1.18). The odds ratios were 1.19 (95% CI, 0.85–1.66) and 1.47 (95% CI, 1.12–1.94) for DII tertiles 2 and 3, respectively (p for trend = 0.01). Furthermore, this association was stable in the fully adjusted model and the trend was robust.
Figure 2 shows the results of subgroup analysis. The DII score was associated ED among those aged 20 to 40 years (OR, 1.33; 95% CI, 1.05–1.68), and those with (OR, 1.10; 95% CI, 1.00–1.21) and without hypertension (OR, 1.13; 95% CI, 1.01–1.26), without diabetes (OR, 1.13; 95% CI, 1.04–1.22). No significant interaction was detected in the interaction analysis. Results of sensitivity analyses are presented in Table 3. After excluding participants taking medicines that potentially affect erectile function, the odds ratio was 1.11 (95% CI, 1.03–1.20) after adjusting for age, race and ethnicity, education levels, hypertension, smoking status, and physical activity levels. After re-defining ED to self-reported “never able” to maintain an erection, the odds ratio was 1.16 (95% CI, 1.06–1.27) in the adjusted model.
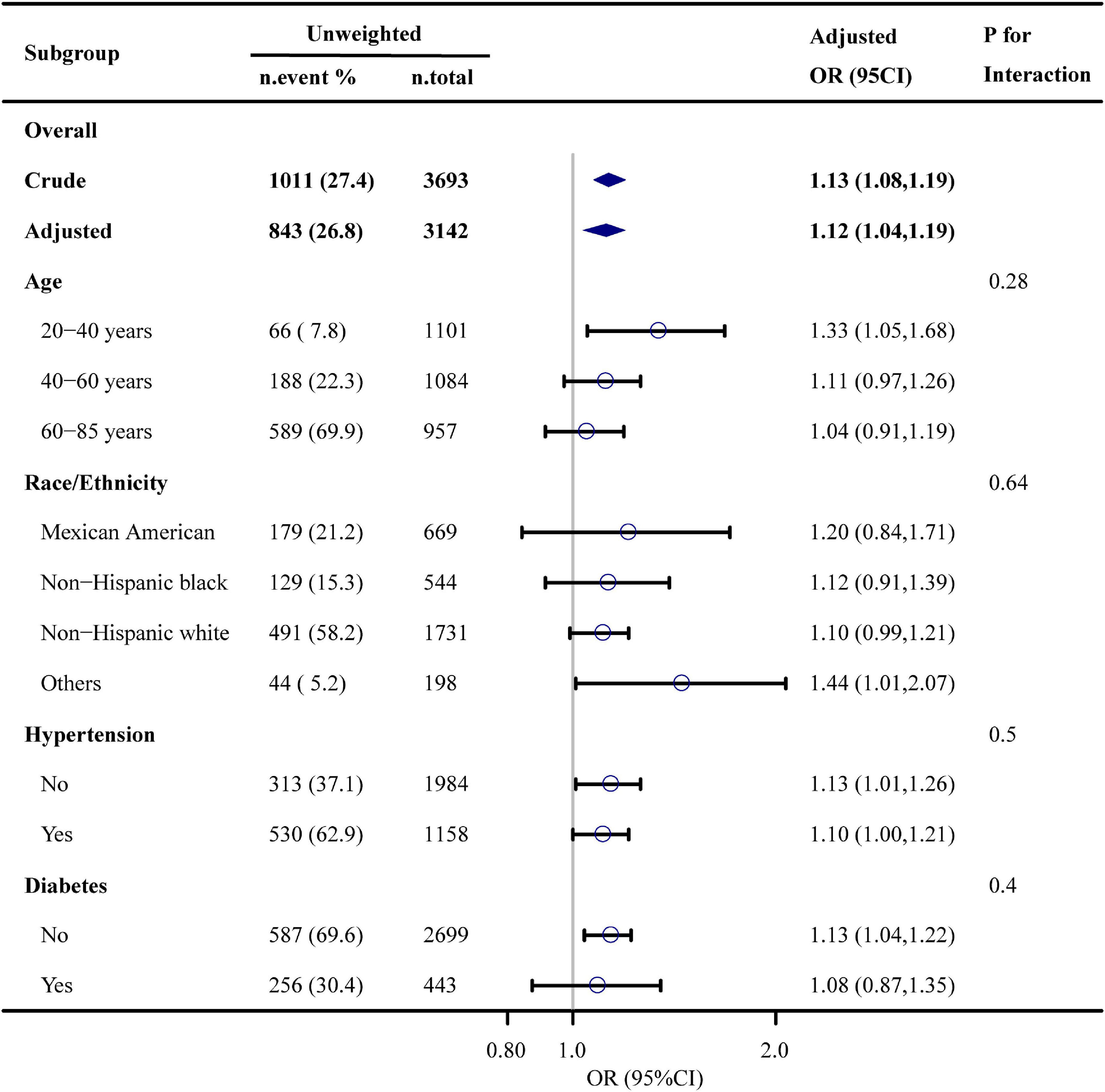
Figure 2. Association between dietary inflammation index and erectile dysfunction. Each stratification was adjusted for age, race and ethnicity, educational level, physical activity, smoking status, drinking status, BMI, hypertension, diabetes, cardiovascular disease, hypercholesterolemia, and eGFR, except the stratification factor itself.
This nationally representative study found robust association between DII and ED in US adult males. In the present study, after adjusting for the baseline imbalance, participants in the highest DII tertiles still had an approximately 1.5-time higher odds of having ED compared with those without ED. Besides, this relationship remained stable in the full-adjusted model that additionally adjusted for a large set of covariates. Notably, the slight variations of the odds ratio between the full-adjusted model and model 2 may indicate that some risk factors of ED (like diabetes, cardiovascular disease, and hypercholesterolemia) may not play a key role here. However, it should be interpreted cautiously, due to the potential residual confounding. Interestingly, in our subgroup analyses, males who are younger and without diabetes seemed to have a higher risk of ED, although no significant interaction was detected. Considering the cross-sectional nature, this may be explained by the reverse causation. ED is a common situation in the elderly persons who are more prone to chronic diseases, like diabetes. The higher risk of developing many chronic diseases may encourage the old to choose a healthier dietary pattern which may content more anti-inflammatory component. In our sensitivity analysis that ruled out the impact of some medication on ED, the odds ratio remained. In the other sensitivity analysis that redefined ED by using a stricter criterion, we noticed a higher odds ratio. This could be explained by a reduction of the misclassification of cases into non-cases which can bias the odds ratio to null. To conclude, the present study provides evidence of a robust association between DII and ED.
Most existing studies focus on the protective effect of specific anti-inflammatory nutrients or diet patterns on ED. As listed in Supplementary Table 1, both caffeine and flavonoids are anti-inflammatory food parameters. A study by Lopez et al. noticed a protective effect of caffeine against the risk of developing ED (22), and Cassidy et al. reported a protective effect of flavonoids against the risk of developing ED (46). Current evidence also suggested an anti-inflammatory effect of vitamin D (47, 48), and Farag et al. found a positive association between vitamin D deficiency and ED (21). These studies support our findings very well. For pro-inflammatory dietary components, it is hard to judge its impact on ED since only limited relevant studies. Saturated fatty acids (SFA) and fat are widely considered as pro-inflammatory dietary components. Medeiros Júnior et al. has found that a high-SFA diet could led to an increase in collagen fibers and decline in corpus cavernosum cell proliferation in rat penile tissue (49). Nguyen et al. also found that a high-fat diet in combination with marijuana can lead to an accelerated corporal fibrosis in mouse (50). However, those are all indirect evidence and further studies are needed in this field. Both Mediterranean diet and plant-based diet are previously found to be inversely associated with DII score and they are also suggested to play a role in maintaining erectile health (51, 52). In 2010, Giugliano et al. found that a greater adherence to Mediterranean diet was associated with a lower risk of ED in Italian male that with T2DM (53). To further assess the effect of Mediterranean diet on ED, they conducted a long-term dietary trial in participants with newly diagnosed T2DM (11). It shows that over the entire follow-up, men in the Mediterranean diet group had a better ED as well as a lower C-reactive protein levels compared with those in the low-fat group. However, since those studies were conducted in participants with T2DM, application of the results to the general population should be carefully considered. Recently, a cross-sectional study by Carto et al. has reported that a healthful plant-based diet was negatively associated with ED among the US population (54). A cohort study by Yang et al. also found that healthy plant-based diet indices was inversely associated with incident ED and unhealthy plant-based diet indices was positively associated with incident ED among US elderly males (55). A possible explanation for those studies could be both of those two diet patterns share some similar anti-inflammatory food groups, which may have protective effect on ED. Results of our study complement the previous research and may provide valuable information for understanding the pathology of diet on ED.
Although previous studies have noticed an elevation of inflammatory biomarkers in both animal models and humans with ED (56, 57), the exact mechanism is still unclear. A possible explanation is that the pro-inflammatory diet mediated ED by increasing the vascular endothelial injury. As is known, endothelial nitric oxide is a molecule that regulates vascular tone and can protect endothelial cells from oxidative damage. Previous studies showed that inflammatory biomarkers, such as TNF-α, can inhibit endothelial nitric-oxide synthase (eNOS) gene expression in endothelial cells (58–60), leading to vascular endothelial injury and a higher risk of ED. In addition, a pro-inflammatory diet can contribute to the pathogenesis of diabetes and cardiovascular disease. Both are potential risk factors of ED.
The present study has serval strengths. First, it used large high-quality data from the NHANES and considered many potential covariates, which strengthens the reliability of the results. Furthermore, since all analyses were accounted for the NHANES complex sampling design, these findings are generalizable to general US males. Finally, to our knowledge, this is the first study to explore the association between DII and ED. It provides data for future studies. However, some limitations should be mentioned in this study. First, due to the cross-sectional nature, causal inference about the association between DII and ED could not be established. Thus, further well-designed cohort studies are needed for future studies. Second, although the single question to access ED was validated in the previous study, there could still be a recall bias. Thus, we also conducted a sensitivity analysis that re-defined ED by using a stringent criterion to further verify the reliability of our results. Third, although we adjusted for potential confounders as far as possible, there could be some residual or unmeasured confounders, such as glucocorticoid use (45). Thus, we conducted a sensitivity analysis that excluded participants taking medicines that potentially affect erectile function. Finally, the DII was calculated based on a 24-h dietary recall interview in the present study. Although a prospective investigation found that DII is relatively constant during several years of observation in females (61), it is not necessarily generalizable to males. Nevertheless, since the present study is the first one to investigate the DII–ED relationship, it still provides preliminary evidence in this direction.
In summary, this cross-sectional analysis suggests that dietary inflammatory potential, as estimated by the DII score, is positively associated with ED in non-institutionalized US males. Since a pro-inflammatory diet may be a modifiable risk factor of ED, we expect more studies on this field.
Publicly available datasets were analyzed in this study. This data can be found here: CDC National Center for Health Statistics NHANES database: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
ZR contributed to study planning, data analyses, and drafting of the manuscript. XX, HY, RL, WJ, and TL contributed to study planning and manuscript development. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.930272/full#supplementary-material
1. Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. (2010) 7:1598–607. doi: 10.1111/j.1743-6109.2010.01778.x
2. Kessler A, Sollie S, Challacombe B, Briggs K, Van Hemelrijck M. The global prevalence of erectile dysfunction: a review. BJU Int. (2019) 124:587–99. doi: 10.1111/bju.14813
3. Goldstein I, Goren A, Li VW, Maculaitis MC, Tang WY, Hassan TA. The association of erectile dysfunction with productivity and absenteeism in eight countries globally. Int J Clin Pract. (2019) 73:e13384. doi: 10.1111/ijcp.13384
4. Wessells H, Joyce GF, Wise M, Wilt TJ. Erectile dysfunction. J Urol. (2007) 177:1675–81. doi: 10.1016/j.juro.2007.01.057
5. Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex Med Rev. (2020) 8:48–58. doi: 10.1016/j.sxmr.2019.06.008
6. Maiorino MI, Bellastella G, Esposito K. Lifestyle modifications and erectile dysfunction: what can be expected? Asian J Androl. (2015) 17:5–10. doi: 10.4103/1008-682X.137687
7. Calogero AE, Burgio G, Condorelli RA, Cannarella R, La Vignera S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. (2019) 22:12–9. doi: 10.1080/13685538.2018.1434772
8. Kaya-Sezginer E, Gur S. The inflammation network in the pathogenesis of erectile dysfunction: attractive potential therapeutic targets. Curr Pharm Des. (2020) 26:3955–72. doi: 10.2174/1381612826666200424161018
9. Defeudis G, Mazzilli R, Di Tommaso AM, Zamponi V, Carlomagno F, Tuccinardi D, et al. Effects of diet and antihyperglycemic drugs on erectile dysfunction: a systematic review. Andrology. (2022). doi: 10.1111/andr.13192
10. Bauer SR, Breyer BN, Stampfer MJ, Rimm EB, Giovannucci EL, Kenfield SA. Association of diet with erectile dysfunction among men in the health professionals follow-up study. JAMA Netw Open. (2020) 3:e2021701. doi: 10.1001/jamanetworkopen.2020.21701
11. Maiorino MI, Bellastella G, Caputo M, Castaldo F, Improta MR, Giugliano D, et al. Effects of Mediterranean diet on sexual function in people with newly diagnosed type 2 diabetes: the MÈDITA trial. J Diabetes Complications. (2016) 30:1519–24. doi: 10.1016/j.jdiacomp.2016.08.007
12. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
13. Millar SR, Navarro P, Harrington JM, Shivappa N, Hébert JR, Perry IJ, et al. Dietary score associations with markers of chronic low-grade inflammation: a cross-sectional comparative analysis of a middle- to older-aged population. Eur J Nutr. (2022) 61:3377–90. doi: 10.1007/s00394-022-02892-1
14. Shivappa N, Hebert JR, Marcos A, Diaz L-E, Gomez S, Nova E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Amp Food Res. (2017) 61:1600707. doi: 10.1002/mnfr.201600707
15. Shin D, Lee KW, Brann L, Shivappa N, Hébert JR. Dietary inflammatory index is positively associated with serum high-sensitivity C-reactive protein in a Korean adult population. Nutrition. (2019) 6:155–61. doi: 10.1016/j.nut.2018.11.016
16. Wirth MD, Sevoyan M, Hofseth L, Shivappa N, Hurley TG, Hébert JR. The dietary inflammatory index is associated with elevated white blood cell counts in the national health and nutrition examination survey. Brain Behav Immun. (2018) 69:296–303. doi: 10.1016/j.bbi.2017.12.003
17. Suzuki K, Shivappa N, Kawado M, Yamada H, Hashimoto S, Wakai K, et al. Association between dietary inflammatory index and serum C-reactive protein concentrations in the Japan Collaborative Cohort Study. Nagoya J Med Sci. (2020) 82:237–49. doi: 10.18999/nagjms.82.2.237
18. Shivappa N, Hébert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. (2015) 113:665–71. doi: 10.1017/S000711451400395X
19. Na W, Kim M, Sohn C. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in Korean: data from the health examinee cohort. J Clin Biochem Nutr. (2018) 62:83–8. doi: 10.3164/jcbn.17-22
20. NHANES. About the National Health and Nutrition Examination Survey. (2020). Available online at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed April 7, 2021).
21. Farag YMK, Guallar E, Zhao D, Kalyani RR, Blaha MJ, Feldman DI, et al. Vitamin D deficiency is independently associated with greater prevalence of erectile dysfunction: the National Health and Nutrition Examination Survey (NHANES) 2001-2004. Atherosclerosis. (2016) 252:61–7. doi: 10.1016/j.atherosclerosis.2016.07.921
22. Lopez DS, Wang R, Tsilidis KK, Zhu H, Daniel CR, Sinha A, et al. Role of caffeine intake on erectile dysfunction in US men: results from NHANES 2001-2004. PLoS One. (2014) 10:e0123547. doi: 10.1371/journal.pone.0123547
23. O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male aging study. J Gen Intern Med. (2005) 20:515–9. doi: 10.1111/j.1525-1497.2005.0076.x
24. Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. (2007) 120:151–7. doi: 10.1016/j.amjmed.2006.06.010
25. Loprinzi PD, Nooe A. Erectile dysfunction and mortality in a national prospective cohort study. J Sex Med. (2015) 12:2130–3. doi: 10.1111/jsm.13032
26. Botelho J, Leira Y, Viana J, Machado V, Lyra P, Aldrey JM, et al. The role of inflammatory diet and vitamin D on the link between periodontitis and cognitive function: a mediation analysis in older adults. Nutrients. (2021) 13:924. doi: 10.3390/nu13030924
27. Araujo AB, Mohr BA, McKinlay JB. Changes in sexual function in middle-aged and older men: longitudinal data from the Massachusetts male aging study. J Am Geriatr Soc. (2004) 52:1502–9. doi: 10.1111/j.0002-8614.2004.52413.x
28. Saigal CS. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. (2006) 166:207. doi: 10.1001/archinte.166.2.207
29. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States. JAMA. (1999) 281:537. doi: 10.1001/jama.281.6.537
30. Li H, Xu W, Wang T, Wang S, Liu J, Jiang H. Effect of weight loss on erectile function in men with overweight or obesity: a meta-analysis of randomised controlled trials. Andrologia. (2021) 54:e14250. doi: 10.1111/and.14250
31. Gazzaruso C, Solerte SB, Pujia A, Coppola A, Vezzoli M, Salvucci F, et al. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: a potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol. (2008) 51:2040–4. doi: 10.1016/j.jacc.2007.10.069
32. Mostafaei H, Mori K, Hajebrahimi S, Abufaraj M, Karakiewicz PI, Shariat SF. Association of erectile dysfunction and cardiovascular disease: an umbrella review of systematic reviews and meta-analyses. BJU Int. (2021) 128:3–11. doi: 10.1111/bju.15313
33. Fryar CD, Hirsch R, Eberhardt MS, Yoon SS, Wright JD. Hypertension, high serum total cholesterol, and diabetes: racial and ethnic prevalence differences in U.S. adults, 1999-2006. NCHS Data Brief. (2010) 36:1–8.
34. Rosas SE, Joffe M, Franklin E, Strom BL, Kotzker W, Brensinger C, et al. Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int. (2001) 59:2259–66. doi: 10.1046/j.1523-1755.2001.00742.x
35. van Ek GF, Krouwel EM, Nicolai MP, Bouwsma H, Ringers J, Putter H, et al. Discussing sexual dysfunction with chronic kidney disease patients: practice patterns in the office of the nephrologist. J Sex Med. (2015) 12:2350–63. doi: 10.1111/jsm.13062
36. Sivaratnam L, Selimin DS, Abd Ghani SR, Nawi HM, Nawi AM. Behavior-related erectile dysfunction: a systematic review and meta-analysis. J Sex Med. (2021) 18:121–43. doi: 10.1016/j.jsxm.2020.09.009
37. Chew K-K. Alcohol consumption and male erectile dysfunction: an unfounded reputation for risk? J Sex Med. (2009) 6:2340. doi: 10.1111/j.1743-6109.2009.01333.x
38. Lamina S, Okoye CG, Dagogo TT. Therapeutic effect of an interval exercise training program in the management of erectile dysfunction in hypertensive patients. J Clin Hypertens. (2009) 11:125–9. doi: 10.1111/j.1751-7176.2009.00086.x
39. NHIS. Adult Tobacco Use - Glossary. (2019). Available online at: https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm (accessed August 15, 2022).
40. CDC,. (2021) Biomonitoring Summary Available online at: https://www.cdc.gov/biomonitoring/Cotinine_BiomonitoringSummary.html (accessed August 30, 2022).
41. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. (2013) 2:1–24.
42. Montejo AL, Prieto N, de Alarcón R, Casado-Espada N, de la Iglesia J, Montejo L. Management strategies for antidepressant-related sexual dysfunction: a clinical approach. J Clin Med. (2019) 8:E1640. doi: 10.3390/jcm8101640
43. Dumontaud M, Korchia T, Khouani J, Lancon C, Auquier P, Boyer L, et al. Sexual dysfunctions in schizophrenia: beyond antipsychotics. A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 98:109804. doi: 10.1016/j.pnpbp.2019.109804
44. Defeudis G, Di Tommaso AM, Di Rosa C, Cimadomo D, Khazrai YM, Faggiano A, et al. The role of antihyperglycemic drugs and diet on erectile function: results from a perspective study on a population with prediabetes and diabetes. J Clin Med. (2022) 11:3382. doi: 10.3390/jcm11123382
45. Nieschlag E, Vorona E. Mechanisms in endocrinology: medical consequences of doping with anabolic androgenic steroids: effects on reproductive functions. Eur J Endocrinol. (2015) 173:R47–58. doi: 10.1530/EJE-15-0080
46. Cassidy A, Franz M, Rimm EB. Dietary flavonoid intake and incidence of erectile dysfunction. Am J Clin Nutr. (2016) 103:534–41. doi: 10.3945/ajcn.115.122010
47. Chen N, Wan Z, Han S-F, Li B-Y, Zhang Z-L, Qin L-Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients. (2014) 6:2206–16. doi: 10.3390/nu6062206
48. Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. (2015) 10:e0141770. doi: 10.1371/journal.pone.0141770
49. Medeiros Júnior JL, Oliveira FA, de Silva PC, Furriel A, Sampaio FJB, Gregório BM. Lard and/or canola oil-rich diets induce penile morphological alterations in a rat model. Acta Cir Bras. (2014) 29(Suppl. 1):39–44. doi: 10.1590/s0102-86502014001300008
50. Nguyen S, Mangubat M, Eleswarapu S, Wilson JB, Molina J, Abraham A, et al. The combination of high-fat diet and oral marijuana promotes the development of fibrosis in the mouse corpora cavernosa. Sex Med. (2021) 9:100312. doi: 10.1016/j.esxm.2020.100312
51. Hodge AM, Bassett JK, Shivappa N, Hébert JR, English DR, Giles GG, et al. Dietary inflammatory index. Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Amp Control. (2016) 27:907–17. doi: 10.1007/s10552-016-0770-1
52. Turner-McGrievy GM, Wirth MD, Shivappa N, Wingard EE, Fayad R, Wilcox S, et al. Randomization to plant-based dietary approaches leads to larger short-term improvements in Dietary Inflammatory Index scores and macronutrient intake compared with diets that contain meat. Nutr Res. (2015) 35:97–106. doi: 10.1016/j.nutres.2014.11.007
53. Giugliano F, Maiorino MI, Bellastella G, Autorino R, De Sio M, Giugliano D, et al. Adherence to Mediterranean diet and erectile dysfunction in men with type 2 diabetes. J Sex Med. (2010) 7:1911–7. doi: 10.1111/j.1743-6109.2010.01713.x
54. Carto C, Pagalavan M, Nackeeran S, Blachman-Braun R, Kresch E, Kuchakulla M, et al. Consumption of a healthy plant-based diet is associated with a decreased risk of erectile dysfunction: a cross-sectional study of the National Health and Nutrition Examination Survey. Urology. (2022) 161:76–82. doi: 10.1016/j.urology.2021.12.021
55. Yang H, Breyer BN, Rimm EB, Giovannucci E, Loeb S, Kenfield SA, et al. Plant-based diet index and erectile dysfunction in the Health Professionals Follow-Up Study. BJU Int. (2022) 130:514–21. doi: 10.1111/bju.15765
56. Yamashita S, Kato R, Kobayashi K, Hisasue S-I, Arai Y, Tsukamoto T. Inhibition of interleukin-6 attenuates erectile dysfunction in a rat model of nerve-sparing radical prostatectomy. J Sex Med. (2011) 8:1957–64. doi: 10.1111/j.1743-6109.2011.02283.x
57. Billups KL, Kaiser DR, Kelly AS, Wetterling RA, Tsai MY, Hanson N, et al. Relation of C-reactive protein and other cardiovascular risk factors to penile vascular disease in men with erectile dysfunction. Int J Impot Res. (2003) 15:231–6. doi: 10.1038/sj.ijir.3901012
58. Anderson HDI, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. (2004) 279:963–9. doi: 10.1074/jbc.M309552200
59. Yoshizumi M, Perrella MA, Burnett JC, Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. (1993) 73:205–9. doi: 10.1161/01.res.73.1.205
60. Neumann P, Gertzberg N, Johnson A. TNF-α induces a decrease in eNOS promoter activity. Am J Physiol Lung Cell Mol Physiol. (2004) 286:L452–9. doi: 10.1152/ajplung.00378.2002
Keywords: erectile dysfunction, inflammation, dietary score, dietary recall, NHANES, cross-sectional study
Citation: Ruan Z, Xie X, Yu H, Liu R, Jing W and Lu T (2022) Association between dietary inflammation and erectile dysfunction among US adults: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2001–2004. Front. Nutr. 9:930272. doi: 10.3389/fnut.2022.930272
Received: 27 April 2022; Accepted: 27 October 2022;
Published: 11 November 2022.
Edited by:
Rossella Mazzilli, Sapienza University of Rome, ItalyReviewed by:
Justin La Favor, Florida State University, United StatesCopyright © 2022 Ruan, Xie, Yu, Liu, Jing and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Lu, bHV0YW9paEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.