- Research Center for Biochemistry and Nutrition in Metabolic Diseases, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran
Background: COVID-19 is a highly transmissible viral infection with high morbidity. Few studies have been done about dietary intakes in patients with COVID-19. This study aimed to evaluate the association between major dietary patterns before COVID-19 diagnosis in recovered patients and the risk of disease severity and symptoms after the disease begins.
Methods: Overall, 250 recovered cases with both genders completed study questionnaires providing data on demographic characteristics, self-reported web-based 168-item food frequency questionnaire (FFQ), and COVID-19 outcomes in Shahid Beheshti Hospital, Kashan. PCR was used to determine a positive diagnosis of COVID-19. We used multivariable logistic regression models to assess the association between major dietary patterns and study outcomes. All statistical analyses were done by SPSS version 16.
Results: We identified three major dietary patterns—unhealthy, traditional, and healthy dietary patterns. Serum levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were significantly higher in patients with unhealthy and traditional dietary patterns and lower in those with healthy dietary patterns. There was a significant direct relationship between unhealthy and traditional patterns with risk of severe COVID-19 and hospitalization duration and a significant direct association between an unhealthy pattern and the odds ratio (OR) of convalescence duration. A significant inverse relationship was found between healthy pattern and risk of severe COVID-19 and OR of convalescence duration. We found a significant direct association between unhealthy pattern and OR of cough, fever, chilling, weakness, myalgia, nausea and vomiting, and sore throat and between traditional pattern and OR of cough, fever, and chilling. In contrast, a significant inverse association was seen between healthy pattern and OR of dyspnea, weakness, and sore throat.
Conclusion: This study showed that high adherence to an healthy pattern was associated with lower CRP and ESR levels and lower risk of severe COVID-19, hospitalization, and convalescence duration in patients who recovered from COVID-19. More adherence to unhealthy or traditional dietary patterns was associated with higher CRP and ESR levels and a higher risk of severe COVID-19 and hospitalization duration. A direct association was found between unhealthy and traditional patterns and the risk of some COVID-19 symptoms, while an inverse association was found for a healthy dietary pattern.
Introduction
Coronaviruses are a large family of viruses that can cause respiratory infections in animals and humans (1). COVID-19 is an infectious disease caused by a new coronavirus and was first observed in Wuhan, China (2). The disease was unknown before it began to spread in Wuhan in December 2019 (2). So far, it has affected more than 190 countries around the world (3, 4). The clinical and laboratory features of COVID-19 are similar to the severe acute respiratory syndrome (SARS), which was first observed in China, and Middle East respiratory syndrome (MERS), which was first observed in Saudi Arabia (5).
Acute respiratory syndrome in COVID-19 is the main cause of hospitalization in the intensive care unit (ICU) and death (6). Cytokine storm is the main cause of organ dysfunction among these patients (7). Among environmental factors, dietary intake is an important factor affecting inflammation in the body (8–10). Therefore, it seems that the dietary intake of patients with COVID-19 before the beginning of the disease might influence the disease outcomes (11). So far, numerous studies have indicated that deficiency in vitamins and minerals might influence susceptibility to infectious diseases (12). In addition, some studies have shown the special role of some vitamins such as vitamin D in the immune function through infectious diseases (13, 14). However, it must be kept in mind that interactions between nutrients might confound the association of a specific nutrient with COVID-19. Therefore, the dietary pattern can be used as a new direction in nutritional epidemiology to find diet–disease relationships.
A recent population-based case–control study among six countries indicated that consumption of a plant-based diet was associated with a lower odds ratio (OR) of moderate to severe COVID-19 (15). Another study about COVID-19 symptoms and habitual food intake in adult outpatients indicated that an increase in habitual intake of legumes and grains, bread, and cereals was associated with reduced overall symptom severity in patients with COVID-19 (11). A recent study about diet and duration of recovery from COVID-19 showed that adherence to a healthy diet was associated with a shorter duration of recovery from COVID-19 (16).
Although the association of several nutrients with COVID-19 outcomes has received great attention, we are unaware of any study linking major dietary patterns to COVID-19 outcomes. Therefore, this study is conducted to determine the relationship between major dietary patterns and COVID-19 outcomes in Kashan, Iran.
Methods
This retro-prospective study was performed among 250 recovered cases of COVID-19 aged 18–65 years of both genders, who were selected using a simple random sampling method from Shahid Beheshti Hospital, Kashan, Iran. This study was performed from June to September 2021. The study protocol was approved by the Ethics Committee of Kashan University of Medical Sciences (Registration No. IR.KAUMS.MEDNT.REC.1400.048). All participants were requested to complete informed consent.
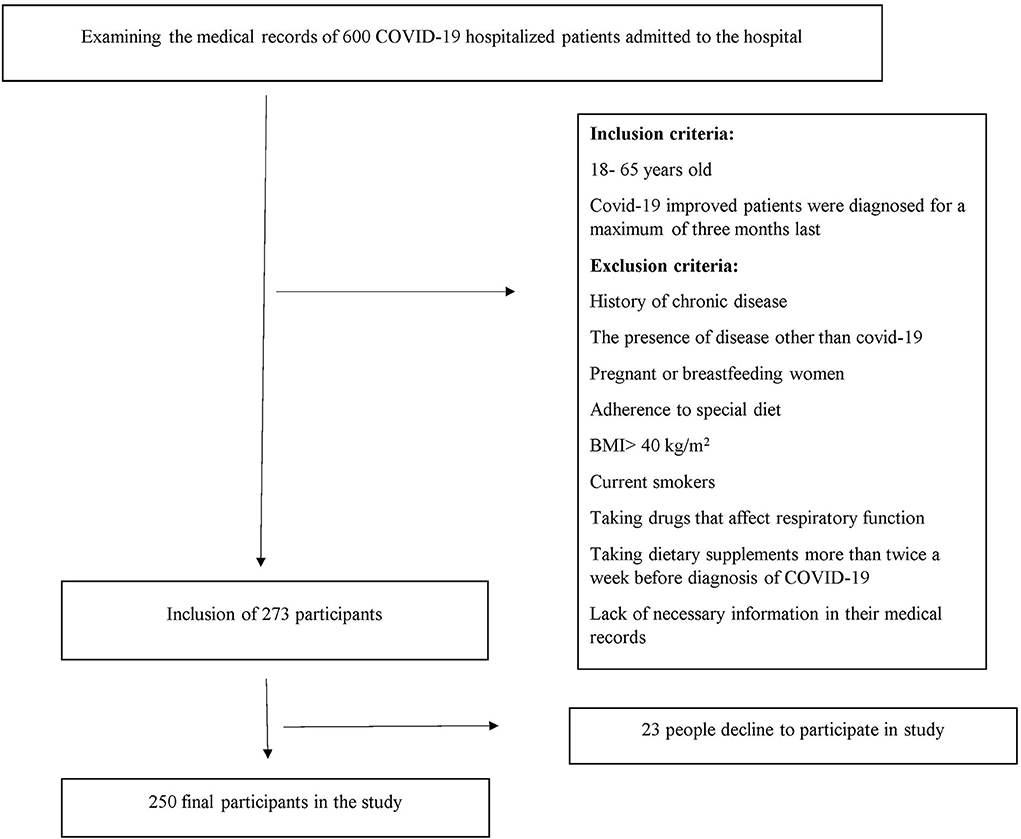
All patients with COVID-19 who had medical records in the Shahid Beheshti Hospital with a maximum of 3 months from the beginning of their COVID-19 diagnosis were included. Patients were excluded if any of the following conditions existed: 1- Patients with other diseases than COVID-19. 2- those who had a history of chronic diseases such as diabetes and heart disease as well as diseases that affect the severity of COVID-19. 3- patients with a body mass index of more than 40. 4- pregnant or breastfeeding women; 5- current smokers. 6- patients who were consuming dietary supplements more than two times in a week before the first diagnosis of COVID-19. 7- patients who were on specific diets. 8- patients who were consuming medicines that influence respiratory function including fluticasone and flunisolide; and 9- subjects with insufficient data in their medical records (Figure 1).
Assessment of dietary intake
A 168-item food frequency questionnaire (FFQ) was obtained from patients through a web-based online questionnaire to collect information on their dietary intakes during the past year before the diagnosis of COVID-19. Participants were asked to report their dietary intakes as daily, monthly, or annually. Finally, we converted their intakes of food items into grams per day using “household measures.” Dietary intakes of micro- and macro-nutrients were calculated by the use of the Nutritionist 4 (N4) software.
Measurement of COVID-19 severity
COVID-19 severity was assessed by the COVID-19 Treatment Guidelines (CTG) (17), updated on 19 October 2021. According to the CTG, the severity of COVID-19 was categorized into five levels. Asymptomatic or presymptomatic infection: individuals with a positive test for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test [NAAT] or an antigen test) but without the symptoms of COVID-19. Mild illness: individuals with any of the various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, and loss of taste and smell) but without breath shortness, dyspnea, or abnormal chest imaging. Moderate illness: individuals with evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO2) ≥94% on room air at sea level. Severe illness: individuals with SpO2 <94% on room air at sea level, a ratio of arterial partial oxygen pressure to the fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, and a respiratory rate >30 breaths/min or lung infiltrates >50%. Critical illness: individuals who had respiratory failure, septic shock, and/or multiple organ dysfunctions. We considered mild and moderate illnesses as a non-severe illness.
Measurement of COVID-19 symptoms
We asked patients to fulfill a general questionnaire including a question about the presence of each common symptom of COVID-19. These symptoms were dyspnea, cough, fever, chilling, weakness, myalgia, sore throat, nausea, and vomiting.
Assessment of inflammatory markers
Data on erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were obtained from medical records. First measurements of CRP and ESR at the beginning of the disease were obtained.
Assessment of other variables
Required information on demographic characteristics, physical activity, convalescence duration, supplements intake, corticosteroids use, antiviral drug use, and participants' height and weight were obtained for each subject by a general questionnaire.
Statistical analysis
Normal distribution of data was explored by the Kolmogorov-Smirnov test. We classified 168 food items in FFQ into 21 predefined food groups to identify dietary patterns. The similarity of nutrients in food items was the basis for this classification: eggs, processed meat, sweets and desserts, sweetened drinks, meats, solid oils, junk foods, liquid oils, salt, refined grains, whole grains, flavors and pickles, chicken and fish, caffeine-containing drinks, red meat, vegetables, fruits and juices, low-fat dairy products, nuts, high-fat dairy products, and legumes (18–20). We conducted varimax rotation to generate a simple and differential varimax. The scree plot test and eigenvalues>1 were used to determine major dietary patterns. Adherence score for each dietary pattern was obtained and participants were categorized as tertiles based on these scores. We used an independent t-test to compare quantitative variables between categories of dietary patterns. A chi-square test was used to compare qualitative variables between categories. The correlation between adherence to each dietary pattern with outcomes of interest by considering confounding variables was assessed by the multivariable regression test. All statistical analyses were performed by the Statistical Package for Social Sciences software (SPSS Inc., version 16). A p-value of <0.05 was considered statistically significant.
Results
Major dietary patterns
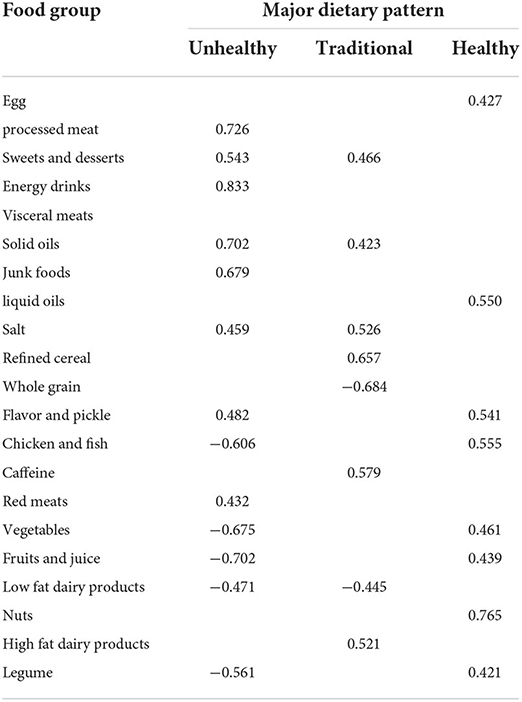
We identified three major dietary patterns – unhealthy, traditional, and healthy dietary patterns (21). The unhealthy pattern was mainly characterized by a high intake of processed meats, sweets and desserts, energy drinks, red meats, solid oils, and junk foods. Participants in the traditional pattern had a high load of sweets and desserts, solid oils, salt, refined cereals, caffeine, and high-fat dairy product consumption. The healthy pattern was highly characterized by the intake of eggs, liquid oils, flavors and pickles, chicken and fish, vegetables, fruits and fruit juices, and nuts and legumes. Factor loadings of food groups in these major patterns are shown in Table 1.
Characteristics of participants according to tertiles of major dietary patterns
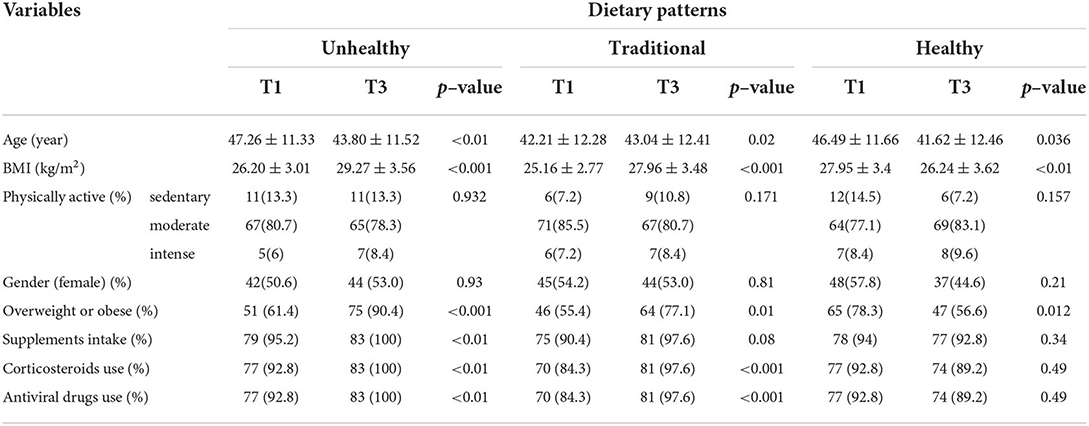
The characteristics of participants according to tertiles of major dietary patterns are shown in Table 2. We found significant differences in age (43.80 ± 11.52 vs. 47.26 ± 11.33, p = <0.01), body mass index (BMI) (29.27 ± 3.56 vs. 26.20 ± 3.01, p = <0.001), likelihood of overweight or obesity (75 vs. 51, p = <0.001), supplements intake (83 vs. 79, p = <0.01), and corticosteroids use and antiviral drug use (83 vs. 77, p = <0.01) in the highest vs. lowest tertiles of unhealthy dietary pattern. However, there were no significant differences in physical activity level and gender between tertiles of the unhealthy pattern. With regard to traditional pattern, significant differences were observed in age (43.04 ± 12.41 vs. 42.21 ± 12.28, p = 0.02), BMI (27.96 ± 3.48 vs. 25.16 ± 2.77, p = <0.001), likelihood of overweight or obesity (64 vs. 46, p = 0.01), and corticosteroids use and antiviral drug use (81 vs. 70, p = <0.001) between the highest and lowest tertiles of the traditional pattern. There were no significant differences in physical activity level, gender, and percentage of participants with supplement use across tertiles of the dietary pattern. Finally, significant differences were observed in age (41.62 ± 12.46 vs. 46.49 ± 11.66, p = 0.03), BMI (26.24 ± 3.62 vs. 27.95 ± 3.4, p = <0.01), and the likelihood of overweight or obesity (47 vs. 65, p = 0.01) between the highest and lowest tertiles of healthy pattern, while no significant differences were found in physical activity level, gender, supplements intake, and corticosteroids or antiviral drug use.
Dietary intake of nutrients across tertiles of major dietary patterns
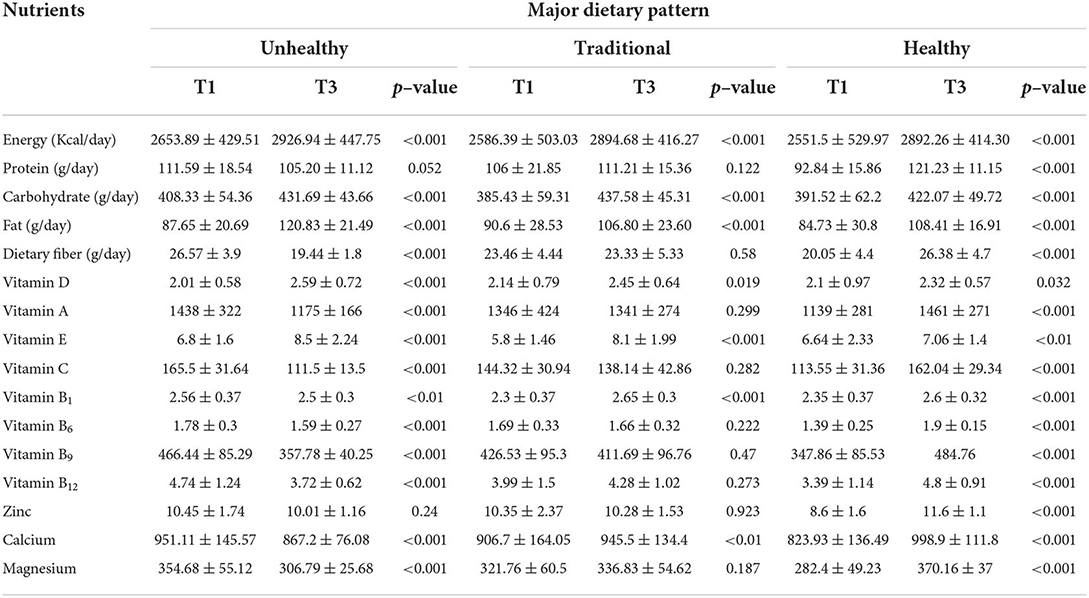
Dietary intake of nutrients across tertiles of major dietary patterns is shown in Table 3. Comparing the highest to the lowest tertile of unhealthy pattern, significant differences were observed in total energy (2926.94 ± 447.75 vs. 2653.89 ± 429.51, p = <0.001), carbohydrate (431.69 ± 43.66 vs. 408.33 ± 54.36, p = <0.001), fat (120.83 ± 21.49 vs. 87.65 ± 20.69, p = <0.001), dietary fiber (19.44 ± 1.8 vs. 26.57 ± 3.9, p = <0.001), vitamins B1 (2.5 ± 0.3 vs. 2.56 ± 0.37, p = <0.01), B6 (1.59 ± 0.27 vs. 1.78 ± 0.3, p = <0.001), B9 (357.78 ± 40.25 vs. 466.44 ± 85.29, p = <0.001), B12 (3.72 ± 0.62 vs. 4.74 ± 1.24, p = <0.001), C (111.5 ± 13.5 vs. 165.5 ± 31.64, p = <0.001), E (8.5 ± 2.24 vs. 6.8 ± 1.6, p = <0.001), D (2.59 ± 0.72 vs. 2.01 ± 0.58, p = <0.001), and A (1,175 ± 166 vs. 1,438 ± 322, p = <0.001), and calcium (867.2 ± 76.08 vs. 951.11 ± 145.57, p = <0.001) and magnesium (306.79 ± 25.68 vs. 354.68 ± 55.12, p = <0.001) intakes. In addition, dietary intakes of energy (2,894.68 ± 416.27 vs. 2,586.39 ± 503.03, p = <0.001), carbohydrate (437.58 ± 45.31 vs. 385.43 ± 59.31, p = <0.001), fat (106.80 ± 23.60 vs. 90.6 ± 28.53, p = <0.001), vitamins D (2.45 ± 0.64 vs. 2.14 ± 0.79, p = 0.019), E (8.1 ± 1.99 vs. 5.8 ± 1.46, p = <0.001), and B1 (2.65 ± 0.3 vs. 2.3 ± 0.37, p = <0.001), and calcium (945.5 ± 134.4 vs. 906.7 ± 164.05, p = <0.01) were significantly different between the highest in contrast to the lowest tertile of traditional pattern. Comparing the highest to the lowest tertiles of healthy pattern, we found significant differences in total energy (2,892.26 ± 414.30 vs. 2,551.5 ± 529.97, p = <0.001), protein (121.23 ± 11.15 vs. 92.84 ± 15.86, p = <0.001), carbohydrate (422.07 ± 49.72 vs. 391.52 ± 62.2, p = <0.001), fat (108.41 ± 16.91 vs. 84.73 ± 30.8, p = <0.001), dietary fiber (26.38 ± 4.7 vs. 20.05 ± 4.4, p = <0.001), vitamins B1 (2.6 ± 0.32 vs. 2.35 ± 0.37, p = <0.001), B6 (1.9 ± 0.15 vs. 1.39 ± 0.25, p = <0.001), B9 (484.76 vs. 347.86 ± 85.53, p = <0.001), B12 (4.8 ± 0.91 vs. 3.39 ± 1.14, p = <0.001), C (162.04 ± 29.34 vs. 113.55 ± 31.36, p = <0.001), E (7.06 ± 1.4 vs. 6.64 ± 2.33, p = <0.01), D (2.32 ± 0.57 vs. 2.1 ± 0.97, p = 0.032), and A (1,461 ± 271 vs. 1,139 ± 281, p = <0.001), and zinc (11.6 ± 1.1 vs. 8.6 ± 1.6, p = <0.001), calcium (998.9 ± 111.8 vs. 823.93 ± 136.49, p = <0.001), and magnesium (370.16 ± 37 vs. 282.4 ± 49.23, p = <0.001) intakes.
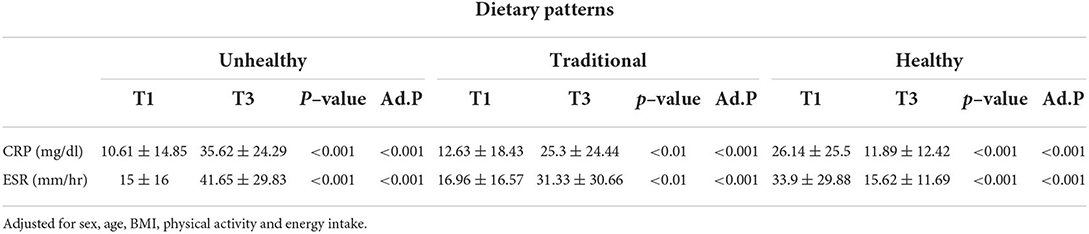
The association between major dietary patterns, CRP, and ESR
The association between major dietary patterns and inflammatory markers after adjustment for sex, age, BMI, physical activity, and energy intake in patients with COVID-19 is shown in Table 4. Serum levels of CRP and ESR were significantly higher in patients at top tertiles of unhealthy (35.62 ± 24.29 vs. 10.61 ± 14.85, p = <0.001 and 41.65 ± 29.83 vs. 15 ± 16, p = <0.001, respectively) and traditional (25.3 ± 24.44 vs. 12.63 ± 18.43, p = <0.001 and 31.33 ± 30.66 vs. 16.96 ± 16.57, p = <0.001, respectively) dietary patterns than those at the bottom. In contrast, our analysis indicated lower levels of CRP and ESR in those at the third tertile vs. those at the first tertile of the healthy dietary pattern (11.89 ± 12.42 vs. 26.14 ± 25.5, p = <0.001 and 15.62 ± 11.69 vs. 33.9 ± 29.88, p = <0.001, respectively).
The relationship between major dietary patterns and the risk of severe COVID-19
Multivariable binary logistic regression for the relationship between major dietary patterns and risk of severe COVID-19 is indicated in Table 5. In the crude model, we found a significant direct association between adherence to unhealthy and traditional dietary patterns and risk of severe COVID-19 (OR: 4.34; 95% confidence interval (CI): 2.26, 8.34, p = <0.001 and 3.37; 95% CI: 1.77, 6.42, p = <0.001, respectively). Such relationship was also observed after controlling for age, sex, and energy intake (OR: 5.06; 95% CI: 2.51, 10.21, p = <0.001 and 3.61; 95% CI: 1.81, 7.19, p = <0.001, respectively). Additional adjustments for other potential confounders including physical activity, supplement use, corticosteroids use, and antiviral drug use had no effect on the association (OR: 4.57; 95% CI: 2.34, 9.64, p = <0.01 and 3.13; 95% CI: 1.55, 6.3, p = <0.01, respectively). In the fully adjusted model, this association also remained significant (OR: 3.23; 95% CI: 1.53, 6.81, p = <0.01 and 2.17; 95% CI: 1.03, 4.54, p = 0.04, respectively). We found a significant inverse relationship between adherence to healthy pattern and risk of severe COVID-19 (OR: 0.25; 95% CI: 0.13, 0.49, p = <0.001). After adjustments for the potential confounders in three models, the association remained as statistically significant (model 1: 0.22, 95% CI: 0.1, 0.45, p = <0.001; model 2: 0.21, 95% CI: 0.1, 0.45, p = <0.01; model 3: 0.31, 95% CI: 0.14, 0.68, p = <0.01).
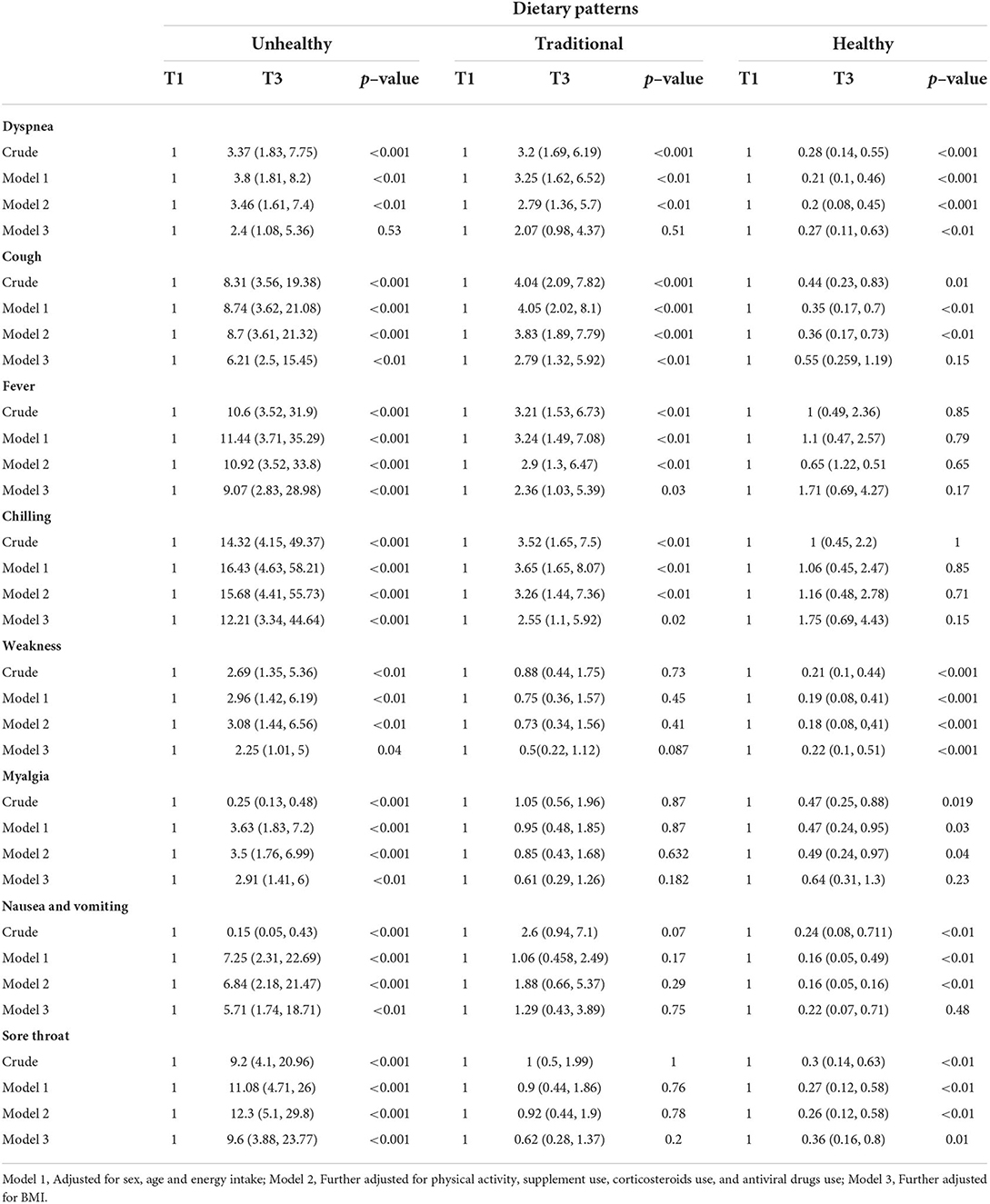
The association between major dietary patterns and the risk of each COVID-19 symptom
Multivariable binary logistic regression for the association between major dietary patterns and the risk of each COVID-19 symptom is shown in Table 6. After controlling for potential confounders, we found a significant direct association between unhealthy pattern and OR of cough (6.21, 95% CI: 2.5, 15.45, p = <0.01), fever (9.07, 95% CI: 2.83, 28.98, p = <0.001), chilling (12.21, 95% CI: 3.34, 44.64, p = <0.001), weakness (2.25, 95% CI: 1.01, 5, p = 0.04), myalgia (2.91, 95% CI: 1.41, 6, p = <0.01), nausea and vomiting (5.71, 95% CI: 1.74, 18.71, p = <0.01), and sore throat (9.6, 95% CI: 3.88, 23.77, p = <0.001). A significant direct association was also found between traditional pattern and OR of cough (2.79, 95% CI: 1.32, 5.92, p = <0.01), fever (2.36, 95% CI: 1.03, 5.39, p = 0.03), and chilling (2.55, 95% CI: 1.1, 5.92, p = 0.02) at the fully adjusted model. In contrast, a significant inverse association was seen between healthy pattern and OR of dyspnea (0.27, 95% CI: 0.11, 0.63, p = <0.01), weakness (0.22, 95% CI: 0.1, 0.51, p = <0.001), and sore throat (0.36, 95% CI: 0.16, 0.8, p = 0.01) at the third model of adjustment.
The association of dietary patterns and OR of lasted hospitalization and convalescence duration
Multivariable binary logistic regression for the association of each dietary pattern and OR of lasted hospitalization and convalescence duration is presented in Table 7. There were significant associations between unhealthy and traditional patterns with OR of long-term hospitalization after adjusting for confounder variables (2.87, 95% CI: 1.28, 6.45, p = 0.01 and 2.97, 95% CI: 1.23, 7.15, p = 0.01, respectively). After controlling for the potential confounders, there was no significant association between healthy pattern and OR of lasted hospitalization (0.44, 95% CI: 0.18, 1.06, p = 0.07). Furthermore, we did not find a significant association between the traditional pattern and OR of convalescence duration after adjustment for the potential confounders (1.04, 95% CI: 0.51, 2.11, p = 0.9). However, a significant direct association was found between the unhealthy pattern and OR of convalescence duration in the final model (1.06, 95% CI: 0.97, 1.16, p = <0.01). Moreover, a significant inverse association was seen between healthy pattern and OR of convalescence duration (0.32, 95% CI: 0.15, 0.68, p = <0.01).
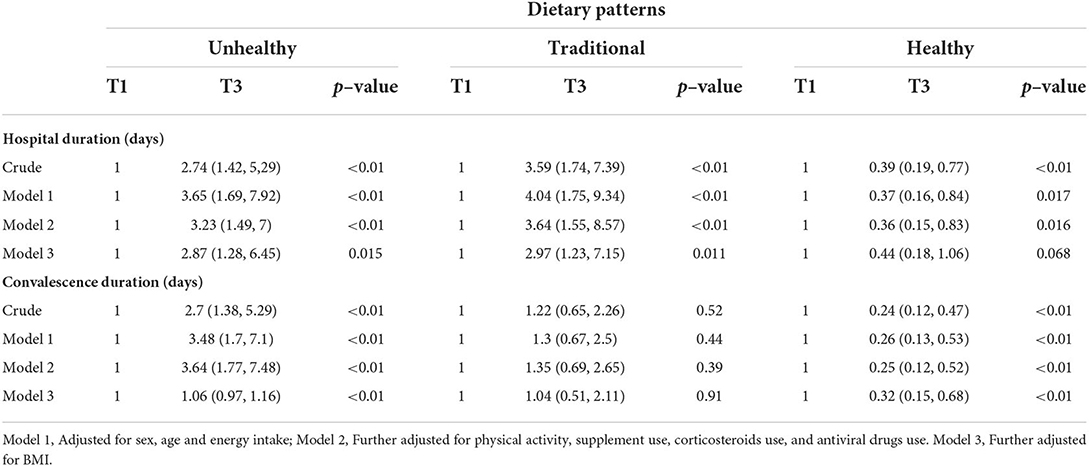
Table 7. Odds ratio (95% CI) for hospital stay and convalescence duration according to major dietary patterns.
Discussion
This study aimed to evaluate the association between major dietary patterns before COVID-19 diagnosis in recovered patients and the risk of disease severity and symptoms after the disease begins.
More adherence to the healthy dietary pattern was associated with lower concentrations of CRP and ESR. In contrast, more adherence to unhealthy and traditional patterns was associated with higher concentrations of those pro-inflammatory markers. To the best of our knowledge, there is no study investigating the relationship between dietary patterns and inflammatory markers in patients with COVID-19. Findings from a case–control study showed a positive association between the dietary inflammatory index and serum levels of CRP and ESR in patients with COVID-19 (22). Other studies evaluated the relationship between an individual nutrient and the levels of CRP and ESR. For example, a meta-analysis suggested that consumption of healthy foods rich in antioxidant vitamins and phytochemicals was associated with lower CRP levels in men (23).
Our study indicated a positive relationship between unhealthy and traditional dietary patterns and the risk of severe COVID-19, while an inverse association was found for the healthy dietary pattern. A cross-sectional study on 236 patients with COVID-19 showed that an increment in habitual intake of legumes, grains, and bread and cereals decreased overall symptom severity in patients with COVID-19 (11). Moreover, a population-based case–control study in six countries indicated that individuals who consumed plant-based diets with a higher intake of vegetables, legumes, and nuts, and a lower intake of poultry and red or processed meats had lower OR of severe COVID-19-like illness (15). Furthermore, the results of a prospective cohort study supported the finding of our study, in which adherence to plant-based foods was associated with lower risk and severity of COVID-19 (24). Due to common food items in such dietary patterns, it seems that more detailed studies are needed to illustrate which food items are more important and which are the main anti-COVID-19 micronutrients or bioactive components in those foods.
With regard to the symptoms of COVID-19, we found a significant increment in the risk of dyspnea, cough, fever, chilling, weakness, myalgia, nausea and vomiting, and sore throat with more adherence to the unhealthy pattern. Adjustment for BMI disappeared the association for dyspnea. It seems that BMI is an important confounding factor in the relationship between dietary intake and the risk of COVID-19 symptoms. A recent systematic review and meta-analysis including 46 studies involving 625,153 patients indicated a greater risk of infection, hospitalization, clinically severe disease, mechanical ventilation, ICU admission, and mortality due to COVID-19 in patients with obesity (25). A significant positive relation was found between the traditional dietary pattern and dyspnea, cough, fever, and chilling. The association for dyspnea disappeared after adjustment for BMI. A lack of significant association for some symptoms might be due to the effect of other confounding factors that need to be taken into account in future investigations. For instance, patients' physical activity before COVID-19 diagnosis, their medical history, and family history of different diseases are among the most important confounders that should be attended to with more detail. In contrast to the two aforementioned dietary patterns, higher adherence to the healthy dietary pattern was associated with a lowered risk of dyspnea, weakness, and sore throat. To the best of our knowledge, this study is the first investigation into the association between dietary patterns and symptoms of COVID-19. A recent case–control study indicated that more intake of legumes, grains, and bread and cereals was associated with a reduction in overall symptom severity in patients with COVID-19 (11).
More adherence to unhealthy or traditional patterns was associated with increased duration of hospitalization in patients with COVID-19. Although an inverse association was seen between adherence to the healthy dietary pattern and hospitalization time in our study, the association was removed after additional adjustment for BMI in the third model. Similar to what we said for COVID-19 symptoms, it can be suggested that BMI is an important confounder in the relationship between dietary intake and hospital duration. Findings from a retrospective cohort study indicated that subjects with obesity who were affected by COVID-19 required longer hospitalization and more intensive and longer oxygen treatments (26).
Finally, we found a direct association between more adherence to the unhealthy dietary pattern and convalescence duration, while an inverse association was found for more adherence to the healthy dietary pattern. However, no significant association was found between the traditional dietary pattern and convalescence duration in patients with COVID-19. In line with our findings, a cross-sectional study on COVID-19 survivors in Saudi Arabia indicated that more adherence to a healthy diet was associated with a shorter duration of recovery from COVID-19 (16). Further studies about different common known dietary patterns are needed to expand the current finding.
The exact mechanisms through which dietary patterns might affect COVID-19 severity and symptoms are unknown. It is suggested that micro-nutrients in a diet might affect COVID-19 prognosis (27). Vitamin A has various functions in the body's immune system (28). Growth, development, and function of neutrophils, monocytes and macrophages, apoptosis, and gene expression of B and T lymphocytes are examples of vitamin A functions in the immune system (29). Vitamin B6 is another important factor in a diet that strengthens the immune system and increases the production of white blood cells including IL-2 and T cells (30). In addition, numerous studies have indicated the effective roles of vitamin C in the prevention of infections, such as SARS coronavirus (31, 32). A recent meta-analysis indicated that low serum vitamin D concentration was associated with more risk of in-hospital mortality among patients with COVID-19 (13). Roles of vitamin D in immune responses and protecting the body against various viruses have been reported previously (33).
For example, a recent meta-analysis indicated that vitamin D supplementation was associated with a reduction in the ICU admission rate, a reduction in the need for mechanical ventilation, and a reduction in mortality from COVID-19 (34).
Furthermore, vitamin E deficiency has been associated with lipid peroxidation (35), and omega-3 fatty acid has protective roles against infectious diseases by removing body inflammation (36). Cytokine storm in response to viral infections can lead to multi-organ failure in patients with COVID-19 (37). Furthermore, fibers are fermented by the gut flora to produce short-chain fatty acids, which have anti-inflammatory functions (38).
This study is the first investigation into the association of major dietary patterns with the risk of COVID-19 symptoms and severity. However, some limitations should also be taken into account when interpreting the findings of this study. This is a single-center study. Although the study population included adults, it would be prudent to consider their sample size and the fact that they were all drawn from the same center when determining their generalizability to the general population. We did not examine the socioeconomic status of participants, which may influence their dietary intake. Our study had a limited sample size, which highlights the need for larger studies. In addition, differences in virus variants can affect the severity and symptoms of COVID-19 (39, 40). Insufficient information in the medical records of some patients was another limitation of our study. Furthermore, we excluded patients with acute and very high severe diseases from our study. This was because of a lack of information about their dietary intake before the disease diagnosis and also due to their inability to fill out the questionnaires. We assessed the dietary intakes of participants with a self-reported web-based 168-FFQ. Therefore, recall bias and misclassification of participants by the dietary intakes should not be neglected. Finally, due to the cross-sectional design of this study, it is impossible to confer causality.
In conclusion, this study showed that high adherence to a healthy pattern was associated with less CRP and ESR and lower risk of severe COVID-19, and hospitalization and convalescence durations in patients who recovered from COVID-19. However, more adherence to unhealthy or traditional dietary patterns was associated with higher CRP and ESR, risk of severe COVID-19, and hospitalization duration. A direct association was found between adherence to the unhealthy pattern and risk of cough, fever, chilling, weakness, myalgia, nausea and vomiting, and sore throat, and between the traditional pattern with risk of dyspnea, cough, fever, and chilling. A healthy dietary pattern was inversely associated with the risk of dyspnea, cough, weakness, myalgia, nausea and vomiting, and sore throat.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Kashan University of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AE: conceptualization, formal analysis, writing–original draft, writing–review and editing, and data collection. AM: supervision, conceptualization, methodology, investigation, funding acquisition, formal analysis, writing–original draft, and writing–review and editing. MT: supervision, conceptualization, formal analysis, writing–original draft, and writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Kashan University of Medical Sciences in Kashan. This paper is extracted from the results of a research project (Registration No. IR.KAUMS.MEDNT.REC.1400.048), which was conducted at Shahid Beheshti Hospital, Kashan University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.929384/full#supplementary-material
References
1. Kunz R, Minder M. COVID-19 pandemic: palliative care for elderly and frail patients at home and in residential and nursing homes. Swiss Med Wkly. (2020) 150:w20235. doi: 10.4414/smw.2020.20235
2. Swiss Academy Of Medical S. COVID-19 pandemic: triage for intensive-care treatment under resource scarcity. Swiss Med Wkly. (2020) 150:w20229. doi: 10.4414/smw.2020.20229
3. Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. (2020) 101:138–48. doi: 10.1016/j.ijid.2020.09.1464
4. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the department of radiology should know. J Am Coll Radiol. (2020) 17:447–51. doi: 10.1016/j.jacr.2020.02.008
5. Guan W, Liu J, Yu C. CT Findings of Coronavirus Disease (COVID-19) severe pneumonia. AJR Am J Roentgenol. (2020) 214:W1–2. doi: 10.2214/AJR.20.23035
6. Nabavi S, Javidarabshahi Z, Allahyari A, Ramezani M, Seddigh-Shamsi M, Ravanshad S, et al. Clinical features and disease severity in an Iranian population of inpatients with COVID-19. Sci Rep. (2021) 11:8731. doi: 10.1038/s41598-021-87917-1
7. Mustafa MI, Abdelmoneim AH, Mahmoud EM, Makhawi AM. Cytokine storm in COVID-19 patients, its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediators Inflamm. (2020) 2020:8198963. doi: 10.1155/2020/8198963
8. Saghafi-Asl M, Mirmajidi S, Asghari Jafarabadi M, Vahid F, Shivappa N, Hébert JR, et al. The association of dietary patterns with dietary inflammatory index, systemic inflammation, and insulin resistance, in apparently healthy individuals with obesity. Sci Rep. (2021) 11:7515. doi: 10.1038/s41598-021-86993-7
9. Ebrahimzadeh A, Ebrahimzadeh A, Mirghazanfari SM, Hazrati E, Hadi S, Milajerdi A. The effect of ginger supplementation on metabolic profiles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2022) 65:102802. doi: 10.1016/j.ctim.2022.102802
10. Milajerdi A, Mousavi SM, Sadeghi A, Salari-Moghaddam A, Parohan M, Larijani B, et al. The effect of probiotics on inflammatory biomarkers: a meta-analysis of randomized clinical trials. Eur J Nutr. (2020) 59:633–49. doi: 10.1007/s00394-019-01931-8
11. Salazar-Robles E, Kalantar-Zadeh K, Badillo H, Calderón-Juárez M, García-Bárcenas CA, Ledesma-Pérez PD, et al. Association between severity of COVID-19 symptoms and habitual food intake in adult outpatients. BMJ Nutr Prev Health. (2021) 4:e000348. doi: 10.1136/bmjnph-2021-000348
12. Wang MX, Koh J, Pang J. Association between micronutrient deficiency and acute respiratory infections in healthy adults: a systematic review of observational studies. Nutr J. (2019) 18:80. doi: 10.1186/s12937-019-0507-6
13. Ebrahimzadeh A, Mohseni S, Narimani B, Ebrahimzadeh A, Kazemi S, Keshavarz F, et al. Association between vitamin D status and risk of covid-19 in-hospital mortality: a systematic review and meta-analysis of observational studies. J Am Coll Nutr. (2021) 31:1–11. doi: 10.1080/10408398.2021.2012419
14. Gunville CF, Mourani PM, Ginde AA. The role of vitamin D in prevention and treatment of infection. Inflamm Allergy Drug Targets. (2013) 12:239–45. doi: 10.2174/18715281113129990046
15. Kim H, Rebholz CM, Hegde S, LaFiura C, Raghavan M, Lloyd JF, et al. Plant-based diets, pescatarian diets and COVID-19 severity: a population-based case–control study in six countries. BMJ Nutr Prev Health. (2021) 4:257–66. doi: 10.1136/bmjnph-2021-000272
16. Alamri FF, Khan A, Alshehri AO, Assiri A, Khan SI, Aldwihi LA, et al. Association of healthy diet with recovery time from COVID-19: results from a nationwide cross-sectional study. Int J Environ Res Public Health. (2021) 18:248. doi: 10.3390/ijerph18168248
17. Clinical Spectrum of SARS-CoV-2 Infection 2021. Available online at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed June 10, 2021).
18. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. (1999) 69:243–9. doi: 10.1093/ajcn/69.2.243
19. Esmaillzadeh A, Azadbakht L. Major dietary patterns in relation to general obesity and central adiposity among Iranian women. J Nutr. (2008) 138:358–63. doi: 10.1093/jn/138.2.358
20. Rezazadeh A, Rashidkhani B, Omidvar N. Association of major dietary patterns with socioeconomic and lifestyle factors of adult women living in Tehran, Iran. Nutrition (Burbank, Los Angeles County, Calif). (2010) 26:337–41. doi: 10.1016/j.nut.2009.06.019
21. Shahavandi M, Amini MR, Shahinfar H, Shab-Bidar S. Major dietary patterns and predicted cardiovascular disease risk in an Iranian adult population. Nutr Health. (2020) 27:27–37. doi: 10.1177/0260106020952591
22. Moludi J, Qaisar SA, Alizadeh M, Jafari Vayghan H, Naemi M, Rahimi A, et al. The relationship between dietary inflammatory index and disease severity and inflammatory status: a case-control study of COVID-19 patients. Br J Nutr. (2022) 127:773–81. doi: 10.1017/S0007114521003214
23. Bonaccio M, Cerletti C, Iacoviello L, de Gaetano G. Mediterranean diet and low-grade subclinical inflammation: the Moli-sani study. Endocr Metab Immune Disord Drug Targets. (2015) 15:18–24. doi: 10.2174/1871530314666141020112146
24. Merino J, Joshi AD, Nguyen LH, Leeming ER, Mazidi M, Drew DA, et al. Diet quality and risk and severity of COVID-19: a prospective cohort study. Gut. (2021) 70:2096–104. doi: 10.1136/gutjnl-2021-325353
25. Cai Z, Yang Y, Zhang J. Obesity is associated with severe disease and mortality in patients with coronavirus disease 2019 (COVID-19): a meta-analysis. BMC Public Health. (2021) 21:1505. doi: 10.1186/s12889-021-11546-6
26. Moriconi D, Masi S, Rebelos E, Virdis A, Manca ML, De Marco S, et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-CoV-2 shedding. Obes Res Clin Pract. (2020) 14:205–9. doi: 10.1016/j.orcp.2020.05.009
27. Gasmi A, Tippairote T, Mujawdiya PK, Peana M, Menzel A, Dadar M, et al. Micronutrients as immunomodulatory tools for COVID-19 management. Clinical Immunology. (2020) 220:108545. doi: 10.1016/j.clim.2020.108545
28. Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of vitamin A in the immune system. J Clin Med. (2018) 7:258. doi: 10.3390/jcm7090258
29. Semba RD. The role of vitamin A and related retinoids in immune function. Nutr Rev. (1998) 56:S38–48. doi: 10.1111/j.1753-4887.1998.tb01643.x
30. Rall LC, Meydani SN. Vitamin B6 and immune competence. Nutr Rev. (1993) 51:217–25. doi: 10.1111/j.1753-4887.1993.tb03109.x
32. Hemilä H. Vitamin C and SARS coronavirus. J Antimicrob Chemother. (2003) 52:1049–50. doi: 10.1093/jac/dkh002
33. Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. (2018) 10:614. doi: 10.3390/nu10111614
34. Hariyanto TI, Intan D, Hananto JE, Harapan H, Kurniawan A. Vitamin D supplementation and Covid-19 outcomes: a systematic review, meta-analysis and meta-regression. Rev Med Virol. (2022) 32:e2269. doi: 10.1002/rmv.2269
35. Galmés S, Serra F, Palou A. Vitamin E metabolic effects and genetic variants: a challenge for precision nutrition in obesity and associated disturbances. Nutrients. (2018) 10:1919. doi: 10.3390/nu10121919
36. Husson MO, Ley D, Portal C, Gottrand M, Hueso T, Desseyn JL, et al. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect. (2016) 73:523–35. doi: 10.1016/j.jinf.2016.10.001
37. Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID-19. Immunity. (2020) 53:19–25. doi: 10.1016/j.immuni.2020.06.017
38. Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. (2020) 12:1562. doi: 10.3390/nu12061562
39. Grint DJ, Wing K, Williamson E, McDonald HI, Bhaskaran K, Evans D, et al. Case fatality risk of the SARS-CoV-2 variant of concern B. 117 in England, 16 November to 5 February. Euro Surveill. (2021) 26:2100256. doi: 10.2807/1560-7917.ES.2021.26.11.2100256
Keywords: dietary patterns, COVID-19, inflammation, symptoms, disease severity
Citation: Ebrahimzadeh A, Taghizadeh M and Milajerdi A (2022) Major dietary patterns in relation to disease severity, symptoms, and inflammatory markers in patients recovered from COVID-19. Front. Nutr. 9:929384. doi: 10.3389/fnut.2022.929384
Received: 26 April 2022; Accepted: 01 August 2022;
Published: 22 August 2022.
Edited by:
Takashi Shigematsu, Rinku General Medical Center, JapanReviewed by:
Timotius Ivan Hariyanto, University of Pelita Harapan, IndonesiaOmid Sadeghi, Isfahan University of Medical Sciences, Iran
Copyright © 2022 Ebrahimzadeh, Taghizadeh and Milajerdi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohsen Taghizadeh, bW9oc2VudGE0NEB5YWhvby5jb20=; Alireza Milajerdi, YW1raHZAeWFob28uY29t
 Armin Ebrahimzadeh
Armin Ebrahimzadeh Mohsen Taghizadeh*
Mohsen Taghizadeh*