- 1Department of Transfusion Medicine, Wuhan Hospital of Traditional Chinese and Western Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pharmacy, Wuhan Fourth Hospital, Wuhan, China
- 3Tongji School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Wuhan Third Hospital, Tongren Hospital of Wuhan University, Wuhan, China
- 5Tongji-Rongcheng Center for Biomedicine, Huazhong University of Science and Technology, Wuhan, China
The Coronavirus Disease 2019 (COVID-19) showed worse prognosis and higher mortality in individuals with obesity. Dyslipidemia is a major link between obesity and COVID-19 severity. Statins as the most common lipid regulating drugs have shown favorable effects in various pathophysiological states. Importantly, accumulating observational studies have suggested that statin use is associated with reduced risk of progressing to severe illness and in-hospital death in COVID-19 patients. Possible explanations underlie these protective impacts include their abilities of reducing cholesterol, suppressing viral entry and replication, anti-inflammation and immunomodulatory effects, as well as anti-thrombosis and anti-oxidative properties. Despite these benefits, statin therapies have side effects that should be considered, such as elevated creatinine kinase, liver enzyme and serum glucose levels, which are already elevated in severe COVID-19. Concerns are also raised whether statins interfere with the efficacy of COVID-19 vaccines. Randomized controlled trials are being conducted worldwide to confirm the values of statin use for COVID-19 treatment. Generally, the results suggest no necessity to discontinue statin use, and no evidence suggesting interference between statins and COVID-19 vaccines. However, concomitant administration of statins and COVID-19 antiviral drug Paxlovid may increase statin exposure and the risk of adverse effects, because most statins are metabolized mainly through CYP3A4 which is potently inhibited by ritonavir, a major component of Paxlovid. Therefore, more clinical/preclinical studies are still warranted to understand the benefits, harms and mechanisms of statin use in the context of COVID-19.
Introduction
Obese individuals are more vulnerable to the SARS-CoV-2 caused Coronavirus Disease 2019 (COVID-19) (1–3). Obese people have ~46% higher risk for SARS-CoV-2 positive, ~74% increased odds for intense care unit (ICU) admission and ~48% increased risk in deaths (4). Severe obesity [body mass index (BMI) ≥35] was significantly associated with the need for invasive mechanical ventilation (IMV) (5), and was an independent predictor for intubation outcome (6). Moreover, with hyperlipidemia as a major link, obese individuals are prone to cardiovascular disease, hypertension, diabetes, myocardial infarction and stroke, which are among recognized risk factors for adverse COVID-19 outcomes (7–12) (Figure 1).
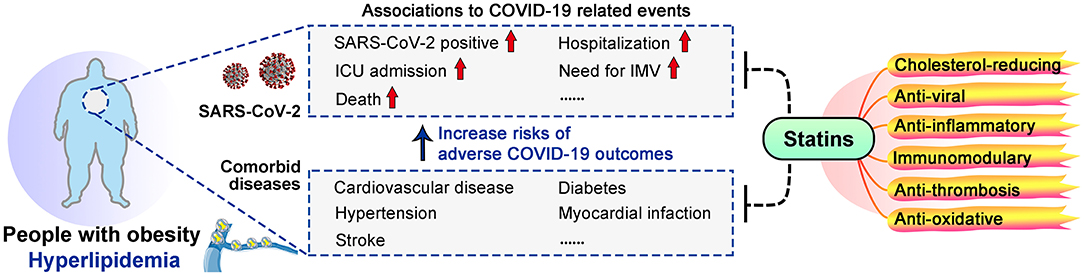
Figure 1. Links between obesity, COVID-19 and statins. Obesity increased the risks of adverse COVID-19 outcomes, and dyslipidemia is a major link between obesity and COVID-19 severity. Statins may benefit COVID-19 patients, especially those with obesity and dyslipidemia, due to their multiple effects.
Prevalence of dyslipidemia was 18–39.7% as a comorbid condition in hospitalized COVID-19 patients (13–15). A population-based analysis on 61.4 million adult patients suggested that patients with hyperlipidemic state had 70% increased odds for catching COVID-19 (16). Moreover, COVID-19 patients may develop dyslipidemia that leads to life-threatening metabolic diseases and thrombotic complications (17–19), lipid-regulating agents are thus considered for possible therapeutic effects against COVID-19.
Statins are the most commonly used lipid-regulating drugs, 145.8 million people used statins in 2018 (20). Statins are HMG-CoA reductase inhibitors that can reduce serum total cholesterol, low-density lipoprotein cholesterol (LDL-C) and triglyceride levels, and have other pleiotropic effects such as modulating immune response and alleviating inflammation (21–23). Structurally, statins are classified as lipophilic (atorvastatin, lovastatin, simvastatin, and fluvastatin) and hydrophilic (pravastatin and pitavastatin), while rosuvastatin has an intermediate behavior (24). Statin prescription is majorly under consideration for primary preventions of cardiovascular disease, and other pathologies such as thrombosis (25, 26). Rational use of different types of statins are recommended according to LDL-C lowering need, pre-existing cardiovascular events or related risk factors including dyslipidemia, diabetes, hypertension, and age (25, 26).
Observational studies have suggested protective effects of statins in COVID-19 patients. Statin use was associated with a 55% decreased risk for IMV (27), 22–30% reduced risk of ICU admission (28), and 30–47% lower risk for death (28–33) (Table 1). Moreover, statin use prior to admission was associated with 71% reduction in the odds of developing severe COVID-19 (34) and 73% for death (31). High-intensity statin use reduced the risk of death by 49% in COVID-19 patients with coronary artery disease patients (32) (Table 1). Possible explanations for these benefits of statins include their recognized cholesterol-reducing, anti-inflammatory and immunomodulatory capacities (21, 23, 49), and also their anti-viral, anti-thrombosis and anti-oxidative abilities (50–53) (Figure 1). However, possible side effects of statin should be considered, such as elevated creatine kinase (CK) and serum glucose levels, which are already elevated in severe COVID-19 (19, 54–56). Currently, clinical trials are being conducted worldwide to confirm the safety and benefits of statin use for COVID-19 patients, with criteria including mortality, thrombosis formation, need for ECMO or IMV, viral load etc. (Table 1).
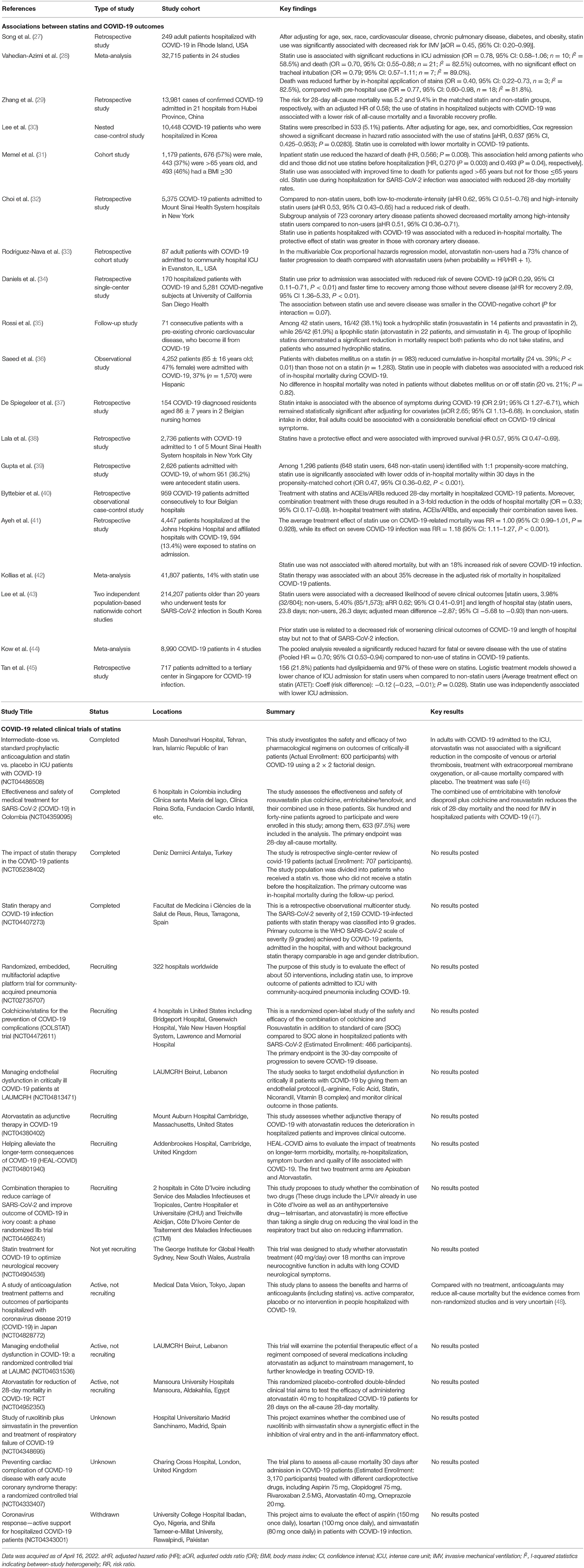
Table 1. Associations between statins and COVID-19 outcomes, and clinical trials regarding statin use in COVID-19.
Different SARS-CoV-2 variants cause resurges of infections (57–60). Vaccines and antiviral therapies are powerful tools against COVID-19 (61, 62), yet data regarding the responses of obese individuals or statin users to these agents remain limited. It has been hypothesized that vaccines would offer reduced protection in obese individuals, based on evidence of immune cell dysregulation and alterations in inflammatory signaling pathways (4, 63). Given the immunomodulatory effects of statins, concerns have also been raised regarding possible interferences with COVID-19 vaccines. Moreover, cautions should be take that drug interactions between statins and some agents used in COVID-19 treatment, may lead to adverse effects (64–66).
Here, we provide a comprehensive update of the values, possible mechanisms, and noteworthy cautions regarding statin use in COVID-19. This review was conducted by consulting resources from peer-reviewed articles and/or official websites like WHO. Common search terms included “COVID-19 OR SARS-CoV-2” AND “statin OR lipid lowering”, etc.
Values and Mechanisms of Statins in COVID-19
As effective drugs for reducing cholesterol, statins can prevent cardiovascular events which are key risk factors for COVID-19 infection and poor prognosis (9, 67–69). Cholesterol reduction allows statins to affect cell membrane structure and function, particularly lipid rafts that play important roles in viral entry and cellular processes like signal transduction (70–72). Moreover, by reducing intermediate products of cholesterol biosynthesis, statins downregulate protein isoprenylation to regulate numerous signaling pathways including immune responses (73). Hyperactivation of immune responses, elevated systematic inflammation, increased oxidative stress, and thrombosis events have been observed in severe COVID-19, especially among those with obesity or cardiovascular diseases, while statins have shown suppressive effects against these processes (Figure 1).
Antiviral Effects
SARS-CoV-2 infection initiates from cell entry by attaching to its receptor ACE2 (70, 71). The cholesterol-rich membrane lipid rafts is crucial for this process. By reducing cholesterol, disrupting lipid raft composition, altering membrane receptor assembly and localization, statins interfere with virus fusion and entry in HIV models (51, 74). Potential mechanism is that statins-mediated blockade of HMG-CoA reductase leads to inhibition of Rho guanosine triphosphatase, a key contributor to clustering of lipid raft-associated receptors (74, 75). Statins may increase ACE2 levels under disease situations with unknown clinical relevance (76), and simvastatin significantly affected SARS-CoV-2 cell entry through displacing ACE2 on lipid rafts (77) (Figure 2). Simvastatin can also reduce SARS-CoV-2 replication (77). Viral infection increases HMG-CoA reductase activity and cholesterol synthesis to assist viral replication, which explains the negative impact of statins on viral replication (78, 79) (Figure 2). Viral assembly and release following replication can be suppressed by statins through inhibiting mevalonate synthesis and intracellular cholesterol levels (80, 81). Whether statins similarly affect the assembly and release of SARS-CoV-2 remain unknown.
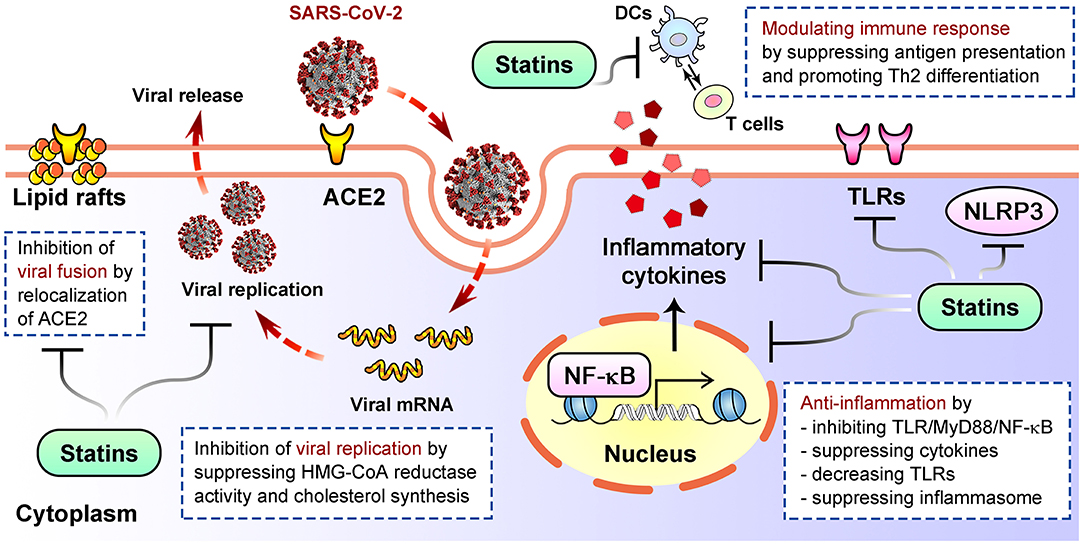
Figure 2. Well established mechanisms of how statins inhibit viral life cycle, alleviate inflammation and modulate immune response after infection. DCs, dendritic cells; TLRs, Toll-like receptors.
Although multiple statins have showed antiviral effects against different viruses, a study suggested higher efficacy for lipophilic statins against Zika viral replication, possibly because they can enter cells via passive transport to reach higher intracellular concentrations (82). Moreover, comparison between the survival curves of patients with a pre-existing chronic cardiovascular disease indicated a significant reduction in mortality in lipophilic statin users vs. hydrophilic group and non-users group (35). Therefore, it will be critical to understand whether and how the type, dose and duration of statin therapy affect antiviral effects and subsequently the outcome of SARS-CoV-2 infection.
Anti-inflammatory and Immunomodulatory Effects
Exacerbated inflammation is a pathological hallmark of COVID-19 (83). During severe COVID-19, a generalized inflammatory state is caused by cytokine storm due to hyperactivation of host immune system, leading to lesions in multiple organs and even death (84). Upon SARS-CoV-2 invasion, antigen-presenting cells recognize the pathogen via Toll-like receptors (TLRs) and activates two main downstream pathways, MyD88- and TRIF-dependent pathways, both leading to NF-κB activation (85–88). NF-κB initiates the first stage of inflammasome activation and induces the production of pro-inflammatory factors, including interleukin-6 (IL-6), a key cytokine associated with COVID-19 severity and mortality (89–91). Activation of NLRP3 inflammasome involves in response to infection of RNA viruses including SARS-CoV-2 (92–95). Patients with a reduced immune fitness and pre-existing systemic inflammatory state, such as obesity or cardiovascular diseases, are prone to demonstrate dysregulated NLRP3 inflammasome activity and pro-inflammatory cytokines expression, resulting in severe COVID-19 (92, 96, 97).
Statins are known for anti-inflammatory and immunomodulatory effects (Figure 2). Statins can decrease TLRs expression, suppress MYD88-NF-κB pathway and the levels of pro-inflammatory cytokines like IL-6, IL-8, TNF-α and MCP-1, thereby altering inflammatory pathway to reduce cell damage (21, 98–100). Statins directly regulate NLRP3 inflammasome (101), or suppress TLR4-MyD88-NF-κB pathway to inhibit its activation (102), thus downregulate cytokines including IL-18 and IL-1β (103, 104). Immunomodulatory actions also underlie statins' beneficial effects in pathologic status. For examples, statins block mevalonate generation required for T cell proliferation (105), repress MHC-II molecules that are critical for presenting antigen to T cells (23, 106), and suppress maturation of dendritic cells (107), therefore may alleviate hyperactivation of immune response. Rosuvastatin promotes the differentiation of peripheral blood monocytes into anti-inflammatory M2 macrophages (49, 108); atorvastatin suppresses proliferation of naïve Th0 cells and secretion of Th1 pro-inflammatory cytokines, while enhances secretion of Th2 anti-inflammatory cytokines (109).
Clinical studies indicate that statins decrease serum CRP levels (110), an inflammatory biomarker and risk factor for adverse COVID-19 outcomes (111). Importantly, in-hospital statin use is significantly associated with ameliorated inflammatory responses, as reflected by lower levels of circulating CRP, IL-6 and neutrophil counts in statin users (29). Correspondingly, simvastatin downregulated SARS-CoV-2-infection-triggered inflammation in human neutrophils, peripheral blood monocytes, and lung epithelial Calu-3 cells, showing its anti-inflammatory effect both at the site of viral infection and systemically (77). Statin-mediated CRP reduction can be achieved by lowering LDL-C, suppressing Rac-1 activation and increasing apolipoprotein A-I, all of which alleviate inflammation and subsequent CRP generation (112). Notably, for PCSK9 inhibitors, another class of lipid-lowering drugs that significantly decreases LDL-C but not inflammatory markers like CRP (113, 114), evidence is lacked so far regarding their possible benefits on COVID-19 outcomes, while deeper investigation is needed. Since COVID-19 patients with obesity are prone to immune cell dysregulation and elevated inflammations, further studies are warranted to explore whether and how statins may protect them from COVID-19.
Anti-thrombosis Effects
Thrombosis are among the most frequent complications in COVID-19 patients, especially in critically ill cases (115–117), and elevated D-dimer levels show prognostic significance for poor outcomes (7, 116, 118). Therefore, prevention/alleviation of thrombosis is a key to COVID-19 treatment, especially for obese patients who are prone to ICU admission and thromboembolic events.
Statins can reduce the occurrence of deep vein thrombosis and pulmonary embolism (52, 119–122), common thrombotic events in COVID-19 cases (123, 124). The anti-thrombosis impact of statins not only relates to its cholesterol-lowering effects and to plaque stabilization (125), but also involves lipid-lowering independent inhibitory effect on platelets activation and coagulation cascade, major pathways for thrombosis formation (18, 52, 126). Statins exert antiplatelet effect via downregulating prothrombotic factors including platelet thromboxane A2, NOX2 (the catalytic subunit of NADPH oxidase), oxidized low-density lipoprotein (oxLDL) and its receptor CD36 (127–129), and via promoting endothelial nitric oxide synthase (eNOS) which improves production of platelet nitric oxide (NO), a potent inhibitor of platelet activation and aggregation (130, 131).
Statins also interfere with clotting system and coagulation cascade. Statins downregulate the expression and activity of tissue factor which initiates the extrinsic pathway of coagulation (132–136), and reduce the serum level of plasminogen activator inhibitor (137); meanwhile, statins upregulate KLF2 that has anticoagulant and atheroprotective effects (138, 139), promote thrombomodulin expression and fibrinolytic activity (140–143). Since a mutual relationship exists between immune activation and thrombus formation (144), the anti-inflammatory actions of statins may also contribute to thrombosis suppression (18).
The anti-thrombosis effect of statins has been widely investigated in patients at risk for cardiovascular disease or those with established atherosclerosis, and varies from different statins (145, 146), but their impacts on COVID-19-related thrombosis remain largely unknown. A clinical trial in ICU admitted COVID-19 patients observed lower rate of thrombosis event in atorvastatin group, although without significant association, suggesting possible anti-thrombosis role of atorvastatin in COVID-19 cases; assessment of outcomes after long-time follow-up is ongoing (46).
Anti-oxidative Effects
Excessive reactive oxygen species (ROS) is associated with high neutrophil to lymphocyte ratio in critically ill COVID-19 (147). In monocytes and macrophages, SARS-CoV-2 infection triggers mitochondrial ROS production, induces HIF1α stabilization and consequently promotes glycolysis which facilitates viral replication (148). Overwhelming oxidative stress also causes local or systemic damages, induces thrombosis, contributing to COVID-19 severity (149).
Statins exerts anti-oxidative effect by attenuating NF-κB activation, reducing circulating oxLDL and their uptake by macrophages, inhibiting oxidant enzymes such as NADPH oxidase and myeloperoxidase, and upregulating the activity of antioxidant enzymes like catalase and paraoxonase (53, 150). Additionally, statins downregulate NOX2-derived oxidative stress, ultimately exerting antiplatelet effects (128, 151–153). Despite these anti-oxidative effects which possibly benefits COVID-19 treatment, statins may induce ROS production, mediate redox imbalance and consequent cellular oxidative damage, especially under excessive or long-term statin use (154).
Clinical Trials Regarding Statin Use and COVID-19
Currently, among clinical trials regarding statin use and COVID-19, two have published results, while the others remain uncompleted or have not posted results (Table 1). In INSPIRATION/INSPIRATION-S study (NCT04486508) conducted in Iran ICU admitted COVID-19 patients, atorvastatin (20 mg/day) was not associated with a significant reduction in the composite of thrombosis, ECMO treatment, or all-cause mortality; however, atorvastatin treatment was safe, and may have clinical importance with lower overall event rates (46). Another study (NCT04359095) was conducted in Colombia (47), emtricitabine with tenofovir disoproxil (200/300 mg/day for 10 days) plus colchicine and rosuvastatin (0.5 mg and 40 mg/day for 14 days) combination reduced the risk of 28-day all-cause mortality by 22%, and lowered the need for IMV (47). These findings indicated safety and potential benefits of statins for COVID-19 treatment, yet the therapeutic effect varies from cohort and medications. More randomized controlled trials are therefore warranted to assess the effect of statin administration alone or in combination with regards of medication dose and duration, and to evaluate the influence of chronic statin use on COVID-19-related in-hospital events or long-term complications.
Cautions About Statins Use in COVID-19
Although statins are generally well-tolerated, for COVID-19 patients especially those with obese or chronic statin use, cautions are required for potential risks of statins-associated muscle symptoms, liver injury, new-onset diabetes, renal injury, and neurological and neurocognitive disorders, which may also result from severe COVID-19 (68, 84, 126, 155, 156) (Figure 3).
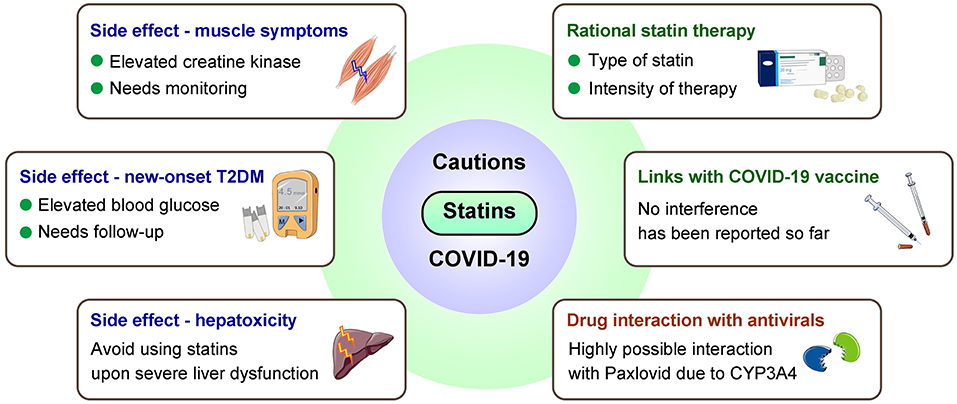
Figure 3. Major cautions for statin use in COVID-19 patients. Considerations are needed from aspects including side effects, interferences with COVID-19 vaccines/antivirals, and proper statin therapy. T2DM, type 2 diabetes mellitus.
Statin-associated muscular symptoms (SAMS) are principal cause of poor patient compliance that contribute to adverse outcomes (157, 158). SAMS include fatigue, weakness and pain, possibly accompanied by elevated serum CK levels and activity (54), while similar symptoms also present at early onset of COVID-19 (159). Therefore, for COVID-19 patients who use statins, careful monitoring muscle symptoms and CK levels are necessary; when muscle symptoms occur, assessment and approaches for statin intolerance may be considered (68, 160).
Statins-associated hepatotoxicity may add to COVID-19 related liver injury that potentially caused by psychological stress, systemic inflammation, etc., especially among obese individuals at higher risk of liver dysfunction (161–163). It has been suggested to avoid statin use in the case of severe liver damages, liver failure, and decompensated cirrhosis (24).
COVID-19 may induce or accelerate type 2 diabetes mellitus (T2DM) development as one of its acute and suspected long-term complications (19, 56), while statin may increase incidence of new-onset T2DM, which appears to be more common in obese patients (55, 164, 165). Despite the risk of T2DM, the cardiovascular benefits of statins should not be masked (166). Therefore, statin therapy can be continued in such patients with glucose monitoring, to achieve better glycemic control and avoid developing metabolic disorders after SARS-CoV-2 infection.
Clinicians should also be cautious when treating statin users with COVID-19 who show renal or neurological symptoms and relevant laboratory abnormalities. Renal dysfunction can be caused by SARS-CoV-2 infection and is associated with COVID-19 poor prognosis (167–169); whether statin-associated renal toxicity (170, 171) exacerbates COVID-19-related renal dysfunction remains unclear. There are also concerns regarding whether use of statin (especially lipophilic ones that can cross the blood-brain barrier) may worsen clinical manifestations of nervous system in COVID-19 patients, given their side effects of causing neurological disorders (171, 172).
Caution is also necessary for drug interaction during COVID-19 management. Most statins are predominantly metabolized by CYP450 enzymes, mainly through CYP3A4 (64). Therefore, concomitant administration of CYP3A4 inhibitors, such as some macrolides (clarithromycin/telithromycin/erythromycin) and antiretroviral drugs (lopinavir/ritonavir), with statins can increase the risk of adverse events (68, 173). For severe COVID-19 patients treated with IL-6 receptor blocker tocilizumab, rosuvastatin is recommended (68). Additionally, decreased LDL-C was observed in some severe COVID-19 cases (174, 175). Such finding may due to a more intensive lipid-lowering treatment in patients with high cardiovascular risk who are more vulnerable to COVID-19 (176), and deeper analysis is warranted to better prescribe appropriate intensity statin therapy.
Statins and COVID-19 Vaccines
The impact of statins on vaccine efficacy remain controversy. Take influenza vaccine for example, a clinical trial suggested immunosuppressive effect of chronic statin medication may weaken the immune response to vaccine (177), whereas another study indicated that statin did not modify influenza vaccine effectiveness (178). Presently, multiple COVID-19 vaccines have been approved, notably, vaccination participants with BMI ≥ 30 had a smaller infection risk reduction than those with BMI < 30 (179), and central obesity (higher waist circumference) is associated with lower neutralizing antibody titers following vaccination (180). Presently, no evidence suggests statin may affect COVID-19 vaccine effectiveness.
Statins and COVID-19 Antivirals
SARS-CoV-2 variants may have substantial immune evasiveness that weakens vaccine protection due to spike protein mutations (57–60). Small molecular antivirals have reduced hospitalization rate and mortality in patients with promising safety (181, 182), among which Paxlovid stands out by reducing the risk of COVID-19-related hospital admission or death by 89% (183, 184). Paxlovid consists of a SARS-CoV-2 main protease inhibitor PF-07321332, and an anti-HIV drug ritonavir that boosts the effectiveness of protease inhibitors (184). However, co-administration of statins and Paxlovid may increase statin exposure and the risk of adverse effects including muscle symptoms and liver toxicity, because ritonavir potently inhibits CYP3A4 through which lipophilic statins are predominantly metabolized (64–66). Therefore, when such concomitant use is needed, it is possible to continue rosuvastatin therapy starting a low dose and titrating up (68, 156). More studies are needed to clarify the possible risks regarding co-administration of statins and antivirals, to find a proper regimen, and to explore whether obesity and dyslipidemia may interfere the efficacy of antivirals.
Conclusion and Future Perspectives
Current data suggest that statins are safe for COVID-19 patients and may exert therapeutical benefits. Generally, there is no necessity to discontinue statin use, and no evidence suggesting interference between statins and COVID-19 vaccines. However, cautions should be taken to achieve proper medication for statin users with COVID-19, considering possible side effects and drug interaction (Figure 3). Two major cautions are: Proper type of statin. Compared to hydrophilic statins, lipophilic statins enter cells via passive transport to reach higher intracellular concentrations, and have a larger distribution volume, thus may be more protective in respect of anti-viral ability. Intensity of statin therapy. Hypolipidemia is harmful. Decreased LDL-C is observed in some COVID-19 patients and associated with COVID-19 severity (174, 175), yet such findings may due to a more intensive lipid-lowering treatment in patients with high cardiovascular risk who are more vulnerable to COVID-19 (176). Low-, moderate-, high-intensity statin therapy should be applied according to specific LDL-C lowering needs. Importantly, careful monitoring of LDL-C, CK, blood glucose and liver function is recommended in context of COVID-19.
Currently known impacts of statins in COVID-19 are mostly based on observational studies and may vary due to heterogenicity in different trials/cohorts. To address the effect of statins in COVID-19 patients, especially in those with obesity or dyslipidemia-related diseases, randomized controlled trials with proper patient stratification are warranted. Moreover, experimental evidence of how different statins act in COVID-19 models are rare, highlighting the importance of related studies.
Author Contributions
CL, WY, YC, and KH: conceptualization. CL, WY, YC, and JS: writing—original draft preparation. CL, YC, SW, AP, and KH: writing—review and editing. All authors have read and agreed to the final version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (31971066), the China Postdoctoral Science Foundation (2021M700050), the Natural Science Foundation of Hubei Province (2021CFA004 and 2021CFB250), and the Postdoctoral Innovation Research Program of Hubei Province.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely appreciate the investigators and authors who have contributed to this field and apologize that we could not discuss and cite all of them in this review due to space limitations.
References
1. Cai QX, Chen FJ, Wang T, Luo F, Liu XH, Wu QK, et al. Obesity and Covid-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. (2020) 43:1392–8. doi: 10.2337/dc20-0576
2. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20133 Uk patients in hospital with Covid-19 using the isaric who clinical characterisation protocol: prospective observational cohort study. BMJ. (2020) 369:m1985. doi: 10.1136/bmj.m1985
3. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. (2020) 71:896–7. doi: 10.1093/cid/ciaa415
4. Popkin BM, Du SF, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and Covid-19: a global perspective on the epidemiology and biological relationships. Obes Rev. (2020) 21:e13128. doi: 10.1111/obr.13128
5. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. (2020) 28:1195–9. doi: 10.1002/oby.22831
6. Palaiodimos L, Kokkinidis DG, Li WJ, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in -hospital outcomes, and higher in -hospital mortality, in a Cohort of patients with Covid-19 in the Bronx, New York. Metabolism. (2020) 108:154262. doi: 10.1016/j.metabol.2020.154262
7. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with Covid-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
8. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019. Pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934–43. doi: 10.1001/jamainternmed.2020.0994
9. Vuorio A, Kaste M, Kovanen PT. Familial hypercholesterolemia and statins in the Covid-19 era: mitigating the risk of ischemic stroke. eNeurologicalSci. (2021) 23:100344. doi: 10.1016/j.ensci.2021.100344
10. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. (2020) 382:e60. doi: 10.1056/NEJMc2009787
11. Zhang W, Yang D, Yuan Y, Liu C, Chen H, Zhang Y, et al. Muscular G9a regulates muscle-liver-fat axis by musclin under overnutrition in female mice. Diabetes. (2020) 69:2642–54. doi: 10.2337/db20-0437
12. Chen H, Liu C, Cheng C, Zheng L, Huang K. Effects of apelin peptides on diabetic complications. Curr Protein Pept Sci. (2018) 19:179–89. doi: 10.2174/1389203718666170918154728
13. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591. Patients infected with SARS-CoV-2 admitted to icus of the Lombardy Region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
14. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. doi: 10.1136/bmj.m1966
15. Casas-Rojo JM, Anton-Santos JM, Millan-Nunez-Cortes J, Lumbreras-Bermejo C, Ramos-Rincon JM, Roy-Vallejo E, et al. Clinical characteristics of patients hospitalized with covid-19 in Spain: results from the semi-Covid-19 registry. Rev Clin Esp. (2020) 220:480–94. doi: 10.1016/j.rceng.2020.07.003
16. Ghoneim S, Butt MU, Hamid O, Shah A, Asaad I. The incidence of Covid-19 in patients with metabolic syndrome and non-alcoholic steatohepatitis: a population-based study. Metabol Open. (2020) 8:100057. doi: 10.1016/j.metop.2020.100057
17. Gomez-Mesa JE, Galindo-Coral S, Montes MC, Munoz Martin AJ. Thrombosis and coagulopathy in Covid-19. Curr Probl Cardiol. (2021) 46:100742. doi: 10.1016/j.cpcardiol.2020.100742
18. Gasecka A, Borovac JA, Guerreiro RA, Giustozzi M, Parker W, Caldeira D, et al. Thrombotic complications in patients with covid-19: pathophysiological mechanisms, diagnosis, and treatment. Cardiovasc Drugs Ther. (2021) 35:215–29. doi: 10.1007/s10557-020-07084-9
19. Hayden MR. An immediate and long-term complication of covid-19 may be type 2 diabetes mellitus: the central role of cell dysfunction, apoptosis and exploration of possible mechanisms. Cells. (2020) 9:2475. doi: 10.3390/cells9112475
20. Blais JE, Wei Y, Yap KKW, Alwafi H, Ma TT, Brauer R, et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis. (2021) 328:44–51. doi: 10.1016/j.atherosclerosis.2021.05.016
21. Lefer DJ. Statins as potent antiinflammatory drugs. Circulation. (2002) 106:2041–2. doi: 10.1161/01.CIR.0000033635.42612.88
22. Igel M, Sudhop T, von Bergmann K. Pharmacology of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors (statins), including rosuvastatin and pitavastatin. J Clin Pharmacol. (2002) 42:835–45. doi: 10.1177/009127002401102731
23. Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. (2000) 6:1399–402. doi: 10.1038/82219
24. Meurer L, Cohen SM. Drug-induced liver injury from statins. Clin Liver Dis. (2020) 24:107–19. doi: 10.1016/j.cld.2019.09.007
25. Kazi DS, Penko JM, Bibbins-Domingo K. Statins for primary prevention of cardiovascular disease: review of evidence and recommendations for clinical practice. Med Clin North Am. (2017) 101:689–99. doi: 10.1016/j.mcna.2017.03.001
26. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., García FAR, et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA. (2016) 316:1997–2007. doi: 10.1001/jama.2016.15450
27. Song SL, Hays SB, Panton CE, Mylona EK, Kalligeros M, Shehadeh F, et al. Statin use is associated with decreased risk of invasive mechanical ventilation in covid-19 patients: a preliminary study. Pathogens. (2020) 9:759. doi: 10.3390/pathogens9090759
28. Vahedian-Azimi A, Mohammadi SM, Heidari Beni F, Banach M, Guest PC, Jamialahmadi T, et al. Improved covid-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch Med Sci. (2021) 17:579–95. doi: 10.5114/aoms/132950
29. Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with covid-19. Cell Metab. (2020) 32:176–87.e4. doi: 10.1016/j.cmet.2020.06.015
30. Lee HY, Ahn J, Park J, Kyung Kang C, Won SH, Wook Kim D, et al. Beneficial effect of statins in covid-19-related outcomes-brief report: a national population-based cohort study. Arterioscler Thromb Vasc Biol. (2021) 41:e175–e82. doi: 10.1161/ATVBAHA.120.315551
31. Memel ZN, Lee JJ, Foulkes AS, Chung RT, Thaweethai T, Bloom PP. Association of statins and 28-day mortality rates in patients hospitalized with severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. (2022) 225:19–29. doi: 10.1093/infdis/jiab539
32. Choi D, Chen Q, Goonewardena SN, Pacheco H, Mejia P, Smith RL, et al. Efficacy of statin therapy in patients with hospital admission for covid-19. Cardiovasc Drugs Ther. (2021). doi: 10.1007/s10557-021-07263-2. [Epub ahead of print].
33. Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, Chung CW, Trelles-Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in covid-19 patients admitted to an icu: a retrospective cohort study. Crit Care. (2020) 24:429. doi: 10.1186/s13054-020-03154-4
34. Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, et al. Relation of statin use prior to admission to severity and recovery among covid-19 inpatients. Am J Cardiol. (2020) 136:149–55. doi: 10.1016/j.amjcard.2020.09.012
35. Rossi R, Talarico M, Coppi F, Boriani G. Protective role of statins in covid 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Intern Emerg Med. (2020) 15:1573–6. doi: 10.1007/s11739-020-02504-y
36. Saeed O, Castagna F, Agalliu I, Xue X, Patel SR, Rochlani Y, et al. Statin use and in-hospital mortality in patients with diabetes mellitus and covid-19. J Am Heart Assoc. (2020) 9:e018475. doi: 10.1161/JAHA.120.018475
37. De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, et al. The effects of arbs, aceis, and statins on clinical outcomes of covid-19 infection among nursing home residents. J Am Med Dir Assoc. (2020) 21:909–14.e2. doi: 10.1016/j.jamda.2020.06.018
38. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with covid-19 infection. J Am Coll Cardiol. (2020) 76:533–46. doi: 10.1101/2020.04.20.20072702
39. Gupta A, Madhavan MV, Poterucha TJ, DeFilippis EM, Hennessey JA, Redfors B, et al. Association between antecedent statin use and decreased mortality in hospitalized patients with covid-19. Nat Commun. (2021) 12:1325. doi: 10.1038/s41467-021-21553-1
40. Byttebier G, Belmans L, Alexander M, Saxberg BEH, De Spiegeleer B, De Spiegeleer A, et al. Hospital mortality in covid-19 patients in belgium treated with statins, ace inhibitors and/or arbs. Hum Vaccin Immunother. (2021) 17:2841–50. doi: 10.1080/21645515.2021.1920271
41. Ayeh SK, Abbey EJ, Khalifa BAA, Nudotor RD, Osei AD, Chidambaram V, et al. Statins use and covid-19 outcomes in hospitalized patients. PLoS ONE. (2021) 16:e0256899. doi: 10.1371/journal.pone.0256899
42. Kollias A, Kyriakoulis KG, Kyriakoulis IG, Nitsotolis T, Poulakou G, Stergiou GS, et al. Statin use and mortality in covid-19 patients: updated systematic review and meta-analysis. Atherosclerosis. (2021) 330:114–21. doi: 10.1016/j.atherosclerosis.2021.06.911
43. Lee SW, Kim SY, Moon SY, Yoo IK, Yoo EG, Eom GH, et al. Statin use and covid-19 infectivity and severity in South Korea: two population-based nationwide cohort studies. JMIR Public Health Surveill. (2021) 7:e29379. doi: 10.2196/29379
44. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with covid-19. Am J Cardiol. (2020) 134:153–5. doi: 10.1016/j.amjcard.2020.08.004
45. Tan WYT, Young BE, Lye DC, Chew DEK, Dalan R. Statin use is associated with lower disease severity in covid-19 infection. Sci Rep. (2020) 10:17458. doi: 10.1038/s41598-020-74492-0
46. INSPIRATION-S Investigators. Atorvastatin versus placebo in patients with covid-19 in intensive care: randomized controlled trial. BMJ. (2022) 376:e068407. doi: 10.1136/bmj-2021-068407
47. Gaitan-Duarte HG, Alvarez-Moreno C, Rincon-Rodriguez CJ, Yomayusa-Gonzalez N, Cortes JA, Villar JC, et al. Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with covid-19: a pragmatic, open-label randomized trial. EClinicalMedicine. (2022) 43:101242. doi: 10.1016/j.eclinm.2021.101242
48. Flumignan RL, Civile VT, Tinôco JDS, Pascoal PI, Areias LL, Matar CF, et al. Anticoagulants for people hospitalised with covid-19. Cochrane Database Syst Rev. (2022) 3:Cd013739. doi: 10.1002/14651858.CD013739.pub2
49. Kashour T, Halwani R, Arabi YM, Sohail MR, O'Horo JC, Badley AD, et al. Statins as an adjunctive therapy for covid-19: the biological and clinical plausibility. Immunopharmacol Immunotoxicol. (2021) 43:37–50. doi: 10.1080/08923973.2020.1863984
50. Pawlos A, Niedzielski M, Gorzelak-Pabis P, Broncel M, Wozniak E. Covid-19: direct and indirect mechanisms of statins. Int J Mol Sci. (2021) 22:177. doi: 10.3390/ijms22084177
51. Gorabi AM, Kiaie N, Bianconi V, Jamialahmadi T, Al-Rasadi K, Johnston TP, et al. Antiviral effects of statins. Prog Lipid Res. (2020) 79:101054. doi: 10.1016/j.plipres.2020.101054
52. Violi F, Calvieri C, Ferro D, Pignatelli P. Statins as antithrombotic drugs. Circulation. (2013) 127:251–7. doi: 10.1161/CIRCULATIONAHA.112.145334
53. Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis. (2004) 15:251–8. doi: 10.1097/01.mca.0000131573.31966.34
54. Bouitbir J, Sanvee GM, Panajatovic MV, Singh F, Krähenbühl S. Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol Res. (2020) 154:104201. doi: 10.1016/j.phrs.2019.03.010
55. Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, et al. Statin Intolerance - an attempt at a unified definition. Position paper from an international lipid expert panel. Arch Med Sci. (2015) 11:1–23. doi: 10.5114/aoms.2015.49807
56. Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and covid-19. Diabetes Metab Syndr. (2020) 14:2211–7. doi: 10.1016/j.dsx.2020.11.012
57. Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. (2021) 592:616–22. doi: 10.1038/s41586-021-03324-6
58. Collier DA, De Marco A, Ferreira I, Meng B, Datir RP, Walls AC, et al. Sensitivity of SARS-CoV-2 B117 to mRNA vaccine-elicited antibodies. Nature. (2021) 593:136–41. doi: 10.1038/s41586-021-03412-7
59. Sokal A, Broketa M, Barba-Spaeth G, Meola A, Fernández I, Fourati S, et al. Analysis of mRNA vaccination-elicited Rbd-specific memory B cells reveals strong but incomplete immune escape of the SARS-CoV-2 omicron variant. Immunity. (2022). doi: 10.1016/j.immuni.2022.04.002. [Epub ahead of print].
60. Wang Z, Muecksch F, Cho A, Gaebler C, Hoffmann H-H, Ramos V, et al. Analysis of memory B cells identifies conserved neutralizing epitopes on the N-terminal domain of variant SARS-CoV-2 spike proteins. Immunity. (2022). doi: 10.1016/j.immuni.2022.04.003. [Epub ahead of print].
61. Kozlov M. Why scientists are racing to develop more covid antivirals. Nature. (2022) 601:496. doi: 10.1038/d41586-022-00112-8
62. Wang Z, Yang L. In the age of omicron variant: paxlovid raises new hopes of covid-19 recovery. J Med Virol. (2022) 94:1766–7. doi: 10.1002/jmv.27540
63. Townsend MJ, Kyle TK, Stanford FC. Covid-19 vaccination and obesity: optimism and challenges. Obesity. (2021) 29:634–5. doi: 10.1002/oby.23131
64. Sirtori CR. The pharmacology of statins. Pharmacol Res. (2014) 88:3–11. doi: 10.1016/j.phrs.2014.03.002
65. Kiortsis DN, Filippatos TD, Mikhailidis DP, Elisaf MS, Liberopoulos EN. Statin-associated adverse effects beyond muscle and liver toxicity. Atherosclerosis. (2007) 195:7–16. doi: 10.1016/j.atherosclerosis.2006.10.001
66. Lee KCH, Sewa DW, Phua GC. Potential role of statins in covid-19. Int J Infect Dis. (2020) 96:615–7. doi: 10.1016/j.ijid.2020.05.115
67. Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci. (2010) 86:484–93. doi: 10.2183/pjab.86.484
68. Banach M, Penson PE, Fras Z, Vrablik M, Pella D, Reiner Z, et al. Brief recommendations on the management of adult patients with familial hypercholesterolemia during the covid-19 pandemic. Pharmacol Res. (2020) 158:104891. doi: 10.1016/j.phrs.2020.104891
69. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the covid-19 pandemic. J Am Coll Cardiol. (2020) 75:2352–71. doi: 10.1016/j.jacc.2020.03.031
70. Li Y, Xiao Y, Chen Y, Huang K. Nano-based approaches in the development of antiviral agents and vaccines. Life Sci. (2021) 265:118761. doi: 10.1016/j.lfs.2020.118761
71. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res. (2020) 126:1456–74. doi: 10.1161/CIRCRESAHA.120.317015
72. Wang S, Zheng J, Ma L, Petersen RB, Xu L, Huang K. Inhibiting protein aggregation with nanomaterials: the underlying mechanisms and impact factors. Biochim Biophys Acta Gen Subj. (2022) 1866:130061. doi: 10.1016/j.bbagen.2021.130061
73. Matzinger P. The danger model: a renewed sense of self. Science. (2002) 296:301–5. doi: 10.1126/science.1071059
74. del Real G, Jiménez-Baranda S, Mira E, Lacalle RA, Lucas P, Gómez-Moutón C, et al. Statins inhibit Hiv-1 infection by down-regulating rho activity. J Exp Med. (2004) 200:541–7. doi: 10.1084/jem.20040061
75. Gower TL, Graham BS. Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro. Antimicrob Agents Chemother. (2001) 45:1231–7. doi: 10.1128/AAC.45.4.1231-1237.2001
76. South AM, Diz DI, Chappell MC. Covid-19, Ace2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. (2020) 318:H1084–h90. doi: 10.1152/ajpheart.00217.2020
77. Teixeira L, Temerozo JR, Pereira-Dutra FS, Ferreira AC, Mattos M, Goncalves BS, et al. Simvastatin downregulates the SARS-CoV-2-induced inflammatory response and impairs viral infection through disruption of lipid rafts. Front Immunol. (2022) 13:820131. doi: 10.3389/fimmu.2022.820131
78. Mackenzie JM, Khromykh AA, Parton RG. Cholesterol manipulation by west nile virus perturbs the cellular immune response. Cell Host Microbe. (2007) 2:229–39. doi: 10.1016/j.chom.2007.09.003
79. Rothwell C, Lebreton A, Young Ng C, Lim JY, Liu W, Vasudevan S, et al. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. (2009) 389:8–19. doi: 10.1016/j.virol.2009.03.025
80. Liao Z, Graham DR, Hildreth JE. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res Hum Retroviruses. (2003) 19:675–87. doi: 10.1089/088922203322280900
81. Shrivastava-Ranjan P, Flint M, Bergeron É, McElroy AK, Chatterjee P, Albariño CG, et al. Statins suppress ebola virus infectivity by interfering with glycoprotein processing. mBio. (2018) 9:e00660-18. doi: 10.1128/mBio.00660-18
82. Españo E, Nam JH, Song EJ, Song D, Lee CK, Kim JK. Lipophilic statins inhibit zika virus production in vero cells. Sci Rep. (2019) 9:11461. doi: 10.1038/s41598-019-47956-1
83. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. Covid-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
84. Vitiello A, Ferrara F. Plausible positive effects of statins in covid-19 patient. Cardiovasc Toxicol. (2021) 21:781–9. doi: 10.1007/s12012-021-09674-x
85. Zhang Y, Guo X, Yan W, Chen Y, Ke M, Cheng C, et al. ANGPTL8 negatively regulates NF-κB activation by facilitating selective autophagic degradation of Ikkγ. Nat Commun. (2017) 8:2164. doi: 10.1038/s41467-017-02355-w
86. Zhang Y, Zheng L, Huang K. A new way to regulate inflammation: selective autophagic degradation of Ikkγ mediated by Angptl8. Cell Stress. (2018) 2:66–8. doi: 10.15698/cst2018.03.128
87. Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. (2020) 180:1044–66. doi: 10.1016/j.cell.2020.02.041
88. Khan S, Shafiei MS, Longoria C, Schoggins JW, Savani RC, Zaki H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife. (2021) 10:e68563. doi: 10.7554/eLife.68563
89. Coomes EA, Haghbayan H. Interleukin-6 in covid-19: a systematic review and meta-analysis. Rev Med Virol. (2020) 30:1–9. doi: 10.1002/rmv.2141
90. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe covid-19 patients. Science. (2020) 369:718–24. doi: 10.1126/science.abc6027
91. Zhou Q, Cheng C, Wei Y, Yang J, Zhou W, Song Q, et al. Usp15 potentiates NF-κB activation by differentially stabilizing Tab2 and Tab3. FEBS J. (2020) 287:3165–83. doi: 10.1111/febs.15202
92. van den Berg DF, Te Velde AA. Severe covid-19: NLRP3 inflammasome dysregulated. Front Immunol. (2020) 11:1580. doi: 10.3389/fimmu.2020.01580
93. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. (2019) 20:328. doi: 10.3390/ijms20133328
94. Zhao C, Zhao W. NLRP3 Inflammasome-a key player in antiviral responses. Front in Immunol. (2020) 11:211. doi: 10.3389/fimmu.2020.00211
95. Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. (2019) 10:128. doi: 10.1038/s41419-019-1413-8
96. López-Reyes A, Martinez-Armenta C, Espinosa-Velázquez R, Vázquez-Cárdenas P, Cruz-Ramos M, Palacios-Gonzalez B, et al. NLRP3 inflammasome: the stormy link between obesity and covid-19. Front Immunol. (2020) 11:570251. doi: 10.3389/fimmu.2020.570251
97. Yang J, Song QY, Niu SX, Chen HJ, Petersen RB, Zhang Y, et al. Emerging roles of angiopoietin-like proteins in inflammation: mechanisms and potential as pharmacological targets. J Cell Physiol. (2022) 237:98–117. doi: 10.1002/jcp.30534
98. Zelvyte I, Dominaitiene R, Crisby M, Janciauskiene S. Modulation of inflammatory mediators and ppargamma and NFkappaB expression by pravastatin in response to lipoproteins in human monocytes in vitro. Pharmacol Res. (2002) 45:147–54. doi: 10.1006/phrs.2001.0922
99. Moutzouri E, Tellis CC, Rousouli K, Liberopoulos EN, Milionis HJ, Elisaf MS, et al. Effect of simvastatin or its combination with ezetimibe on toll-like receptor expression and lipopolysaccharide - induced cytokine production in monocytes of hypercholesterolemic patients. Atherosclerosis. (2012) 225:381–7. doi: 10.1016/j.atherosclerosis.2012.08.037
100. Koushki K, Shahbaz SK, Mashayekhi K, Sadeghi M, Zayeri ZD, Taba MY, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol. (2021) 60:175–99. doi: 10.1007/s12016-020-08791-9
101. Parsamanesh N, Moossavi M, Bahrami A, Fereidouni M, Barreto G, Sahebkar A. NLRP3 inflammasome as a treatment target in atherosclerosis: a focus on statin therapy. Int Immunopharmacol. (2019) 73:146–55. doi: 10.1016/j.intimp.2019.05.006
102. Kong F, Ye B, Lin L, Cai X, Huang W, Huang Z. Atorvastatin suppresses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB signaling in Pma-stimulated Thp-1 monocytes. Biomed Pharmacother. (2016) 82:167–72. doi: 10.1016/j.biopha.2016.04.043
103. Satoh M, Tabuchi T, Itoh T, Nakamura M. NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin Sci. (2014) 126:233–41. doi: 10.1042/CS20130043
104. Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. Il-1β and Il-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. (2014) 171:5589–602. doi: 10.1111/bph.12876
105. Chakrabarti R, Engleman EG. Interrelationships between mevalonate metabolism and the mitogenic signaling pathway in T lymphocyte proliferation. J Bio Chem. (1991) 266:12216–22. doi: 10.1016/S0021-9258(18)98884-8
106. Axelrod ML, Cook RS, Johnson DB, Balko JM. Biological consequences of Mhc-Ii expression by tumor cells in cancer. Clin Cancer Res. (2019) 25:2392–402. doi: 10.1158/1078-0432.CCR-18-3200
107. Yilmaz A, Reiss C, Tantawi O, Weng A, Stumpf C, Raaz D, et al. Hmg-Coa reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis. (2004) 172:85–93. doi: 10.1016/j.atherosclerosis.2003.10.002
108. Zhang T, Shao B, Liu GA. Rosuvastatin promotes the differentiation of peripheral blood monocytes into M2 macrophages in patients with atherosclerosis by activating PPAR-γ. Eur Rev Med Pharmacol Sci. (2017) 21:4464–71.
109. Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The Hmg-coa reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. (2002) 420:78–84. doi: 10.1038/nature01158
110. Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (Prince): a randomized trial and cohort study. JAMA. (2001) 286:64–70. doi: 10.1001/jama.286.1.64
111. Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, et al. C-reactive protein and clinical outcomes in patients with covid-19. Eur Heart J. (2021) 42:2270–9. doi: 10.1093/eurheartj/ehaa1103
112. Arévalo-Lorido JC. Clinical relevance for lowering C-reactive protein with statins. Ann Med. (2016) 48:516–24. doi: 10.1080/07853890.2016.1197413
113. Ruscica M, Ferri N, Macchi C, Corsini A, Sirtori CR. Lipid lowering drugs and inflammatory changes: an impact on cardiovascular outcomes? Ann Med. (2018) 50:461–84. doi: 10.1080/07853890.2018.1498118
114. Ruscica M, Tokgözoglu L, Corsini A, Sirtori CR. Pcsk9 inhibition and inflammation: a narrative review. Atherosclerosis. (2019) 288:146–55. doi: 10.1016/j.atherosclerosis.2019.07.015
115. Ali MAM, Spinler SA. Covid-19 and thrombosis: from bench to bedside. Trends Cardiovasc Med. (2021) 31:143–60. doi: 10.1016/j.tcm.2020.12.004
116. Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, et al. Covid-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. (2020) 136:489–500. doi: 10.1182/blood.2020006520
117. Ferrari F, Martins VM, Teixeira M, Santos RD, Stein R. Covid-19 and thromboinflammation: is there a role for statins? Clinics. (2021) 76:e2518. doi: 10.6061/clinics/2021/e2518
118. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with covid-19. Lancet Haematol. (2020) 7:e438–e40. doi: 10.1016/S2352-3026(20)30145-9
119. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. (2009) 360:1851–61. doi: 10.1056/NEJMoa0900241
120. Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM, HMG. CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost. (2004) 2:700–1. doi: 10.1111/j.1538-7836.2004.00696.x
121. Khemasuwan D, Divietro ML, Tangdhanakanond K, Pomerantz SC, Eiger G. Statins decrease the occurrence of venous thromboembolism in patients with cancer. Am J Med. (2010) 123:60–5. doi: 10.1016/j.amjmed.2009.05.025
122. Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD Jr., Wakefield TW, Diaz JA. Statins, inflammation and deep vein thrombosis: a systematic review. J Thromb Thrombolysis. (2012) 33:371–82. doi: 10.1007/s11239-012-0687-9
123. Mackman N. Triggers, targets and treatments for thrombosis. Nature. (2008) 451:914–8. doi: 10.1038/nature06797
124. Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in covid-19. Am J Hematol. (2020) 95:1578–89. doi: 10.1002/ajh.25982
125. Pucci A, Sheiban I, Formato L, Celeste A, Brscic E, Moretti C, et al. In vivo coronary plaque histology in patients with stable and acute coronary syndromes: relationships with hyperlipidemic status and statin treatment. Atherosclerosis. (2007) 194:189–95. doi: 10.1016/j.atherosclerosis.2006.07.026
126. Subir R, Jagat JM, Kalyan KG. Pros and cons for use of statins in people with coronavirus disease-19 (Covid-19). Diabetes Metab Syndr. (2020) 14:1225–9. doi: 10.1016/j.dsx.2020.07.011
127. Notarbartolo A, Davi G, Averna M, Barbagallo CM, Ganci A, Giammarresi C, et al. Inhibition of thromboxane biosynthesis and platelet function by simvastatin in type Iia hypercholesterolemia. Arterioscler Thromb Vasc Biol. (1995) 15:247–51. doi: 10.1161/01.ATV.15.2.247
128. Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, et al. Effects of atorvastatin and rosuvastatin on thromboxane-dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis. (2011) 214:122–8. doi: 10.1016/j.atherosclerosis.2010.10.006
129. Owens AP II, Mackman N. The antithrombotic effects of statins. Annu Rev Med. (2014) 65:433–45. doi: 10.1146/annurev-med-051812-145304
130. Laufs U, Gertz K, Huang P, Nickenig G, Bohm M, Dirnagl U, et al. Atorvastatin upregulates type iii nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. (2000) 31:2442–9. doi: 10.1161/01.STR.31.10.2442
131. Haramaki N, Ikeda H, Takenaka K, Katoh A, Sugano R, Yamagishi S, et al. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler Thromb Vasc Biol. (2007) 27:1471–7. doi: 10.1161/ATVBAHA.106.128793
132. Ferro D, Basili S, Alessandri C, Mantovani B, Cordova C, Violi F. Simvastatin reduces monocyte-tissue-factor expression type Iia hypercholesterolaemia. Lancet. (1997) 350:1222. doi: 10.1016/S0140-6736(05)63452-6
133. Colli S, Eligini S, Lalli M, Camera M, Paoletti R, Tremoli E. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol. (1997) 17:265–72. doi: 10.1161/01.ATV.17.2.265
134. Meisel SR, Xu XP, Edgington TS, Cercek B, Ong J, Kaul S, et al. Dose-dependent modulation of tissue factor protein and procoagulant activity in human monocyte-derived macrophages by oxidized low density lipoprotein. J Atheroscler Thromb. (2011) 18:596–603. doi: 10.5551/jat.7179
135. Markle RA, Han J, Summers BD, Yokoyama T, Hajjar KA, Hajjar DP, et al. Pitavastatin alters the expression of thrombotic and fibrinolytic proteins in human vascular cells. J Cell Biochem. (2003) 90:23–32. doi: 10.1002/jcb.10602
136. Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. (2007) 27:1687–93. doi: 10.1161/ATVBAHA.107.141911
137. Sahebkar A, Catena C, Ray KK, Vallejo-Vaz AJ, Reiner Ž, Sechi LA, et al. Impact of statin therapy on plasma levels of plasminogen activator inhibitor-1. A systematic review and meta-analysis of randomised controlled trials. Thromb Haemost. (2016) 116:162–71. doi: 10.1160/TH15-10-0770
138. Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, et al. Kruppel-like factor 2 (Klf2) regulates endothelial thrombotic function. Circ Res. (2005) 96:e48–57. doi: 10.1161/01.RES.0000159707.05637.a1
139. Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. (2005) 112:720–6. doi: 10.1161/CIRCULATIONAHA.104.525774
140. Lin SJ, Chen YH, Lin FY, Hsieh LY, Wang SH, Lin CY, et al. Pravastatin induces thrombomodulin expression in tnfalpha-treated human aortic endothelial cells by inhibiting Rac1 and Cdc42 translocation and activity. J Cell Biochem. (2007) 101:642–53. doi: 10.1002/jcb.21206
141. Undas A, Brummel-Ziedins KE, Mann KG. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. (2005) 25:287–94. doi: 10.1161/01.ATV.0000151647.14923.ec
142. Eto M, Kozai T, Cosentino F, Joch H, Lüscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. (2002) 105:1756–9. doi: 10.1161/01.CIR.0000015465.73933.3B
143. Bianconi V, Sahebkar A, Banach M, Pirro M. Statins, haemostatic factors and thrombotic risk. Curr Opin Cardiol. (2017) 32:460–6. doi: 10.1097/HCO.0000000000000397
144. Rawish E, Sauter M, Sauter R, Nording H, Langer HF. Complement, inflammation and thrombosis. Br J Pharmacol. (2021) 178:2892–904. doi: 10.1111/bph.15476
145. Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost. (2009) 7:514–20. doi: 10.1111/j.1538-7836.2008.03235.x
146. Hilgendorff A, Muth H, Parviz B, Staubitz A, Haberbosch W, Tillmanns H, et al. Statins differ in their ability to block Nf-KappaB activation in human blood monocytes. Int J Clin Pharmacol Ther. (2003) 41:397–401. doi: 10.5414/CPP41397
147. Laforge M, Elbim C, Frere C, Hemadi M, Massaad C, Nuss P, et al. Tissue damage from neutrophil-induced oxidative stress in covid-19. Nat Rev Immunol. (2020) 20:515–6. doi: 10.1038/s41577-020-0407-1
148. Codo AC, Davanzo GG, Monteiro LD, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1 alpha/glycolysis-dependent axis. Cell Metab. (2020) 32:437–6.e5. doi: 10.2139/ssrn.3606770
149. Schönrich G, Raftery MJ, Samstag Y. Devilishly radical network in covid-19: oxidative stress, neutrophil extracellular traps (Nets), and T cell suppression. Adv Biol Regul. (2020) 77:100741. doi: 10.1016/j.jbior.2020.100741
150. Yang S, Shih HJ, Chow YC, Wang TY, Tsai PS, Huang CJ. Simvastatin attenuates testicular injury induced by torsion-detorsion. J Urol. (2010) 184:750–6. doi: 10.1016/j.juro.2010.03.103
151. Pignatelli P, Sanguigni V, Lenti L, Loffredo L, Carnevale R, Sorge R, et al. Oxidative stress-mediated platelet Cd40 ligand upregulation in patients with hypercholesterolemia: effect of atorvastatin. J Thromb Haemost. (2007) 5:1170–8. doi: 10.1111/j.1538-7836.2007.02533.x
152. Pignatelli P, Carnevale R, Pastori D, Cangemi R, Napoleone L, Bartimoccia S, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation. (2012) 126:92–103. doi: 10.1161/CIRCULATIONAHA.112.095554
153. Violi F, Carnevale R, Pastori D, Pignatelli P. Antioxidant and antiplatelet effects of atorvastatin by Nox2 inhibition. Trends Cardiovasc Med. (2014) 24:142–8. doi: 10.1016/j.tcm.2013.09.006
154. Liu A, Wu Q, Guo J, Ares I, Rodriguez JL, Martinez-Larranaga MR, et al. Statins: adverse reactions, oxidative stress and metabolic interactions. Pharmacol Ther. (2019) 195:54–84. doi: 10.1016/j.pharmthera.2018.10.004
155. Rodrigues-Diez RR, Tejera-Muñoz A, Marquez-Exposito L, Rayego-Mateos S, Santos Sanchez L, Marchant V, et al. Statins: could an old friend help in the fight against covid-19? Br J Pharmacol. (2020) 177:4873–86. doi: 10.1111/bph.15166
156. Katsiki N, Banach M, Mikhailidis DP. Lipid-lowering therapy and renin-angiotensin-aldosterone system inhibitors in the era of the covid-19 pandemic. Arch Med Sci. (2020) 16:485–9. doi: 10.5114/aoms.2020.94503
157. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-Associated muscle symptoms: impact on statin therapy-european atherosclerosis society consensus panel statement on assessment, aetiology and management. Eur Heart J. (2015) 36:1012–22. doi: 10.1093/eurheartj/ehv043
158. Nguyen KA, Li L, Lu D, Yazdanparast A, Wang L, Kreutz RP, et al. A comprehensive review and meta-analysis of risk factors for statin-induced myopathy. Eur J Clin Pharmacol. (2018) 74:1099–109. doi: 10.1007/s00228-018-2482-9
159. Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, et al. Musculoskeletal consequences of covid-19. J Bone Joint Surg Am. (2020) 102:1197–204. doi: 10.2106/JBJS.20.00847
160. Rosenson RS, Baker S, Banach M, Borow KM, Braun LT, Bruckert E, et al. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol. (2017) 70:1290–301. doi: 10.1016/j.jacc.2017.07.752
161. Li J, Fan JG. Characteristics and mechanism of liver injury in 2019. Coronavirus Disease. J Clin Transl Hepatol. (2020) 8:13–7. doi: 10.14218/JCTH.2020.00019
162. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected - obesity, impaired metabolic health and covid-19. Nat Rev Endocrinol. (2021) 17:135–49. doi: 10.1038/s41574-020-00462-1
163. Zhang Y, Xue W, Zhang W, Yuan Y, Zhu X, Wang Q, et al. Histone methyltransferase G9a protects against acute liver injury through Gstp1. Cell Death Differ. (2020) 27:1243–58. doi: 10.1038/s41418-019-0412-8
164. Liu C, Wang J, Wei Y, Zhang W, Geng M, Yuan Y, et al. Fat-specific knockout of Mecp2 upregulates slpi to reduce obesity by enhancing browning. Diabetes. (2020) 69:35–47. doi: 10.2337/db19-0502
165. Chen H, Huang Y, Zhu X, Liu C, Yuan Y, Su H, et al. Histone demethylase Utx Is a therapeutic target for diabetic kidney disease. J Physiol. (2019) 597:1643–60. doi: 10.1113/JP277367
166. Galicia-Garcia U, Jebari S, Larrea-Sebal A, Uribe KB, Siddiqi H, Ostolaza H, et al. Statin treatment-induced development of type 2 diabetes: from clinical evidence to mechanistic insights. Int J Mol Sci. (2020) 21:725. doi: 10.3390/ijms21134725
167. Yang C, Zhang Y, Zeng X, Chen H, Chen Y, Yang D, et al. Kidney injury molecule-1 is a potential receptor for SARS-CoV-2. J Mol Cell Biol. (2021) 13:185–96. doi: 10.1093/jmcb/mjab003
168. Yang D, Xiao Y, Chen J, Chen Y, Luo P, Liu Q, et al. Covid-19 and chronic renal disease: clinical characteristics and prognosis. QJM. (2020) 113:799–805. doi: 10.1093/qjmed/hcaa258
169. Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical Characteristics and outcomes of patients with diabetes and covid-19 in association with glucose-lowering medication. Diabetes Care. (2020) 43:1399–407. doi: 10.2337/dc20-0660
170. Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, et al. Adverse effects of statin therapy: perception Vs. The evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. (2018) 39:2526–39. doi: 10.1093/eurheartj/ehy182
171. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. (2019) 124:328–50. doi: 10.1161/CIRCRESAHA.118.312782
172. Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurological involvement in covid-19 and potential mechanisms: a review. Neurocrit Care. (2021) 34:1062–71. doi: 10.1007/s12028-020-01049-4
173. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. (2019). Esc/Eas guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular. Risk Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
174. Zhao M, Luo Z, He H, Shen B, Liang J, Zhang J, et al. Decreased low-density lipoprotein cholesterol level indicates poor prognosis of severe and critical covid-19 patients: a retrospective, single-center study. Front Med. (2021) 8:585851. doi: 10.3389/fmed.2021.585851
175. Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. hypolipidemia is associated with the severity of covid-19. J Clin Lipidol. (2020) 14:297–304. doi: 10.1016/j.jacl.2020.04.008
176. Fogacci F, Borghi C, Cicero AFG. Misinterpreting data in lipidology in the era of covid-19. J Clin Lipidol. (2020) 14:543–4. doi: 10.1016/j.jacl.2020.07.004
177. Black S, Nicolay U, Del Giudice G, Rappuoli R. Influence of statins on influenza vaccine response in elderly individuals. J Infect Dis. (2016) 213:1224–8. doi: 10.1093/infdis/jiv456
178. Havers FP, Chung JR, Belongia EA, McLean HQ, Gaglani M, Murthy K, et al. Influenza vaccine effectiveness and statin use among adults in the United States, 2011-2017. Clin Infect Dis. (2019) 68:1616–22. doi: 10.1093/cid/ciy780
179. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the covid symptom study app in the Uk: a prospective observational study. Lancet Infect Dis. (2021) 21:939–49. doi: 10.1016/S1473-3099(21)00224-3
180. Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to Covid-19 mRNA vaccine. Diabetes Metab Res Rev. (2022) 38:e3465. doi: 10.1002/dmrr.3465
181. Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and paxlovid) for Covid-19: a meta-analysis. Ann Med. (2022) 54:516–23. doi: 10.1080/07853890.2022.2034936
182. Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral covid antiviral drugs. Clin Infect Dis. (2022). doi: 10.1093/cid/ciac180. [Epub ahead of print].
183. Mahase E. Covid-19: pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. (2021) 375:n2713. doi: 10.1136/bmj.n2713
Keywords: SARS-CoV-2, COVID-19, statins, obesity, dyslipidemia, inflammation, immune response, thrombosis
Citation: Liu C, Yan W, Shi J, Wang S, Peng A, Chen Y and Huang K (2022) Biological Actions, Implications, and Cautions of Statins Therapy in COVID-19. Front. Nutr. 9:927092. doi: 10.3389/fnut.2022.927092
Received: 23 April 2022; Accepted: 30 May 2022;
Published: 22 June 2022.
Edited by:
Timotius Ivan Hariyanto, University of Pelita Harapan, IndonesiaReviewed by:
Lorenzo Da Dalt, University of Milan, ItalyFederica Fogacci, University of Bologna, Italy
Copyright © 2022 Liu, Yan, Shi, Wang, Peng, Chen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuchen Chen, Y2hlbnljOTNAaHVzdC5lZHUuY24=
 Chengyu Liu
Chengyu Liu Wanyao Yan
Wanyao Yan Jiajian Shi
Jiajian Shi Shun Wang1
Shun Wang1 Yuchen Chen
Yuchen Chen Kun Huang
Kun Huang