- 1Department of Clinical Laboratory, Guangdong Women and Children Hospital, Guangzhou, China
- 2Department of Preventive Medicine, School of Public Health, Guangzhou Medical University, Guangzhou, China
- 3Medical Genetics Center, Guangdong Women and Children Hospital, Guangzhou, China
Evidence suggests a potential relationship between gestational weight gain (GWG) and adverse birth outcomes. However, the role of maternal genetic polymorphisms remains unclear. This study was conducted to investigate whether the relationship of GWG with risk of adverse birth outcomes was modified by methylenetetrahydrofolate reductase (MTHFR) polymorphisms. A total of 2,967 Chinese pregnant women were included and divided into insufficient, sufficient, and excessive groups based on the Institute of Medicine (IOM) criteria. Polymorphisms of C677T and A1298C in gene MTHFR were genotyped. Multivariable logistic regression models were introduced after controlling major confounders. Excessive GWG was found to increase the odds ratio (OR) for macrosomia [OR = 3.47, 95% confidence interval (CI): 1.86–6.48] and large-for-gestational age (LGA, OR = 3.25, 95% CI: 2.23–4.74), and decreased the OR for small-for-gestational age (SGA, OR = 0.60, 95% CI: 0.45–0.79). Pregnant women with insufficient GWG had a higher frequency of SGA (OR = 1.68, 95% CI: 1.32–2.13) and a lower rate of LGA (OR = 0.51, 95% CI: 0.27–0.96). Interestingly, significant associations of GWG categories in relation to low birth weight (LBW), macrosomia, and SGA were only suggested among pregnant women with MTHFR A1298C AA genotype. Among pregnant women with insufficient GWG group, an increased risk of 3.96 (95% CI: 1.57–10.01) for LBW was observed among subjects with the A1298C AA genotype, compared to the AC+CC genotype group. GWG categories are closely related to LBW, macrosomia, SGA and LGA, and the associations were modified by the polymorphism of MTHFR A1298C.
Introduction
Excessive weight and obesity have become major public health issues worldwide, especially for pregnant women who are in a special physiological condition. Nearly 31.8% of US women of reproductive ages are obese (1), and the prevalence of overweight and obesity for Chinese women aged 18–44 years are 26.4 and 11.0% (2). Gestational weight gain (GWG) is a valuable indicator of maternal nutritional status, reflecting maternal fat storage and maintaining the growth of the fetus, placenta, and uterus. In 2009, the Institute of Medicine (IOM) provided specific recommendations regarding the optimal GWG accounting for pre-pregnancy body mass index (BMI) categories (3). According to the data from the US Center for Disease Control, 48% of pregnant women gained excessive GWG, and 21% gained insufficient GWG (4). In China, excessive weight gain occurred in 57.9% of pregnant women, and insufficient weight gain was 12.5% (5). GWG is closely related to fetal growth restriction, pregnancy complications, fertility, pregnancy loss, infant mortality, and even childhood obesity.
Accumulated evidence suggests that genetic susceptibility is an important factor in fat accumulation and lipid metabolisms, possibly resulting in abnormal GWG for pregnant women. C677T and A1298C variants in the methylenetetrahydrofolate reductase (MTHFR) gene known to reduce enzyme function and ultimately lead to enhanced homocysteine levels were considered as potential candidates (6). It has been reported that irregular MTHFR activity can affect body fat storage via epigenetic mechanisms, as homocysteine plays key roles in the transfer of methyl groups in the activated methyl cycle (7). Several studies have investigated the potential associations of MTHFR C677T and A1298C polymorphisms with obesity/overweight or other metabolic syndromes (8–10). For instance, a Chinese study has reported significant weight gain related to C677T variants (11). A dietary study observed that MTHFR variants are associated with BMI at baseline, and obese individuals with C677T CC genotype lost more weight than the T allele carriers after nutritional intervention (12). Some researchers speculated that elevated homocysteine levels induced by MTHFR polymorphisms might affect the development of overweight/obesity through epigenetic control of gene expression in fat storage in the body, since methyl and homocysteine metabolism are closely related to DNA methylation (13, 14).
There is well-documented evidence on the associations between GWG and obstetric outcomes in human studies (9). However, the role of maternal genetic polymorphisms in these relationships requires further investigation. The present study aimed to investigate whether the relationship between GWG and adverse birth outcomes was modified by MTHFR C677T and A1298C polymorphisms, two loci in gene MTHFR that have been most widely investigated.
Materials and methods
Study population
This study was conducted at Guangdong Women and Children Hospital, a large teaching tertiary public hospital in Guangzhou city, Guangdong province, China. Pregnant women who delivered at the study hospital between January 2017 and December 2019 were considered for inclusion, and a total of 4,640 pregnant women were selected according to the following inclusion criteria: (i) > 18 years old, (ii) had regular prenatal examinations, (iii) gave a birth to singleton live baby, and (iv) had complete data on basic information, gestational weight during pregnancy, the genotype of MTHFR genes, and birth outcomes. In addition, we excluded women who underwent in vitro fertilization (n = 636), had multiple pregnancies (n = 340), stillbirths (n = 446), or abortions (n = 251). Finally, a total of 2,967 mother-infant pairs were included in the present study. This study protocol was approved by the Medical Ethical Committees of Guangdong Women and Children Hospital. All health care procedures were carried out in accordance with approved guidelines and regulations.
Weight measurements
Maternal pre-pregnancy BMI was calculated as self-reported pre-pregnancy weight in kilograms divided by the square of height in meters (kg/m2). According to Chinese BMI classification, pre-pregnancy BMI were assigned as underweight (<18.5 kg/m2), normal weight (18.5–23.9 kg/m2), overweight (24.0–27.9 kg/m2), and obesity (≥28.0 kg/m2), respectively (15). GWG was defined as the difference between the pre-pregnancy weight and weight at delivery for pregnant women. The IOM recommendations were used to classify the GWG group, which were 12.5–18 kg for pregnant women that were underweight, 11.5–16 kg for normal weight women, 7–11.5 kg for overweight women, and 5–9 kg for obese women, respectively (3). Based on IOM criteria, women were divided into an insufficient group (GWG < the recommended range), a sufficient group (GWG within the recommended range), and an excessive group (GWG > the recommended range).
Birth outcome and covariates
Anthropometric information of the newborns was extracted from the medical records. Once a newborn was born, obstetric nurses immediately determined and recorded its birth weight and length. Obstetric nurses abstracted gestational age, mode of delivery, infant sex, birth weight, length, and head circumference. In the present study, low birth weight (LBW) was defined as birth weight <2,500 g, and macrosomia was considered as birth weight >4,000 g. Based on the population-based birth weight reference percentiles for Chinese, birth weight was divided into small-for-gestational age (SGA; birth weight ≤ the gender-specific 10th percentile for gestational age) and large-for-gestational age (LGA; birth weight ≥ the gender-specific 90th percentile for gestational age) (16). Additionally, data on maternal age, gravidity, parity, education, smoking and drinking status, pregnancy complications [gestational diabetes mellitus (GDM) and hypertensive disorders in pregnancy (HDP)], and homocysteine were extracted for potential covariates.
MTHFR genotype
Venous blood samples were collected using vacuum tubes with potassium salt of ethylenediaminetetraacetic acid and stored at 4°C. Genomic DNA was extracted from whole blood samples using a DNA extraction kit (Magen, Guangzhou, China) on an automated nucleic acid extraction workstation (Hamilton, Sweden), according to the manufacturer's instructions. MTHFR C677CT and A1298C variants were genotyped using fluorescence quantitative polymerase chain reaction (PCR), where each reaction system contained Premix Ex Taq (TakaRa, Japan), TaqMan-MGB probes, deionized water, and genomic DNA. For MTHFR C677CT, the forward primer sequence was 5′-CTCTTCTACCTGAAGAGCAAGTCC-3′, and the reverse primer sequence was 5′-CACTCCAGCATCACTCACTTTGT-3′. For MTHFR A1298C, the forward primer sequence was 5′-CCGAAGCAGGGAGCTTTG-3′, and the reverse primer sequence was 5′-CGGTGCATGCCTTCACAA-3′. Reaction conditions are 95°C for 3 min to activate fluorescent groups, following by 40 cycles of amplification (95°C for 20 s, 58°C for 20 s, 65°C for 45 s). The endpoint fluorescence was read and analyzed by a ViiA 7 Dx PCR system (Applied Biosystems, USA).
Statistical analysis
First, general characteristics of our participants were described. Continuous variables were described as mean ± standard deviation or median (interquartile range). Categorical data were described by frequencies (%). The difference between the 3 GWG groups were compared using parametric or non-parametric methods for continuous or categorical data.
Multivariable logistic models were applied to assess the relationship between GWG categories and adverse birth outcomes, among which, pregnant women in sufficient group of GWG were considered the reference group. The cases included pregnant women who gave birth to LBW (n = 144), macrosomia (n = 49), SGA (n = 432), or LGA (n = 149) babies, and the controls were those who delivered infants without the above 4 birth outcomes (n = 2,365). We adjusted for potential covariates in the regression models based on previous reports or statistical considerations. The change-in-effect estimate method was applied to select confounders, in which covariates that changed the main effect estimates by ≥10% were introduced into the models. The inclusion of potential confounders in the final linear regression models for LBW and macrosomia was as follows: maternal age (continuous), education level (less than high school, high school or equivalent, and college or above), delivery mode (natural labor or cesarean section), parity (nulliparous or multiparous), gestational age at delivery (continuous), HDP (no or yes), GDM (no or yes), and infant sex (boys or girls). With regard to regression models for SGA and LGA, gestational age at delivery was not included in the case of co-linearity. To assess whether different pre-pregnancy BMI categories affect the observed associations, World Health Organization (WHO) criteria of BMI classification were introduced (BMI for underweight, <18.5; normal weight, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; and obesity, ≥30.0 kg/m2) and the analyses were repeated (17).
Deviation from Hardy–Weinberg expectation (HWE) was calculated among the control group, and P-value > 0.05 indicated that the two variants were in accordance with HWE. Co-dominant, dominant, recessive, and additive models were used to explore the associations between genetic polymorphisms and adverse birth outcomes. False discovery rate (FDR) corrections were introduced to adjust the P-values for multiple corrections. To investigate the potential modification effects of genetic polymorphisms on the associations between GWG categories and adverse birth outcomes, study subjects were divided into non-mutated and mutated groups, considering the dominating effect of mutated allele as well as for better statistical power, similar to previous studies (18, 19). The likelihood ratio test was used to analysis the interaction effect between GWG categories and genetic polymorphisms on birth outcomes. Differences in the likelihood scores of the two models with and without the interaction term of genotypes and GWG categories were compared. In addition, we further evaluated the associations of MTHFR polymorphisms and adverse birth outcomes within the strata of GWG categories. Generalized additive models were fitted to explore the dose-response relationship between GWG values and adverse birth outcomes with spline smoothing function among high-risk pregnant women with MTHFR A1298C AA genotype.
All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R version 3.3.3 (R Foundation for Statistical Computing). P-value < 0.05 (two-tailed) was considered statistically significant.
Results
Study population
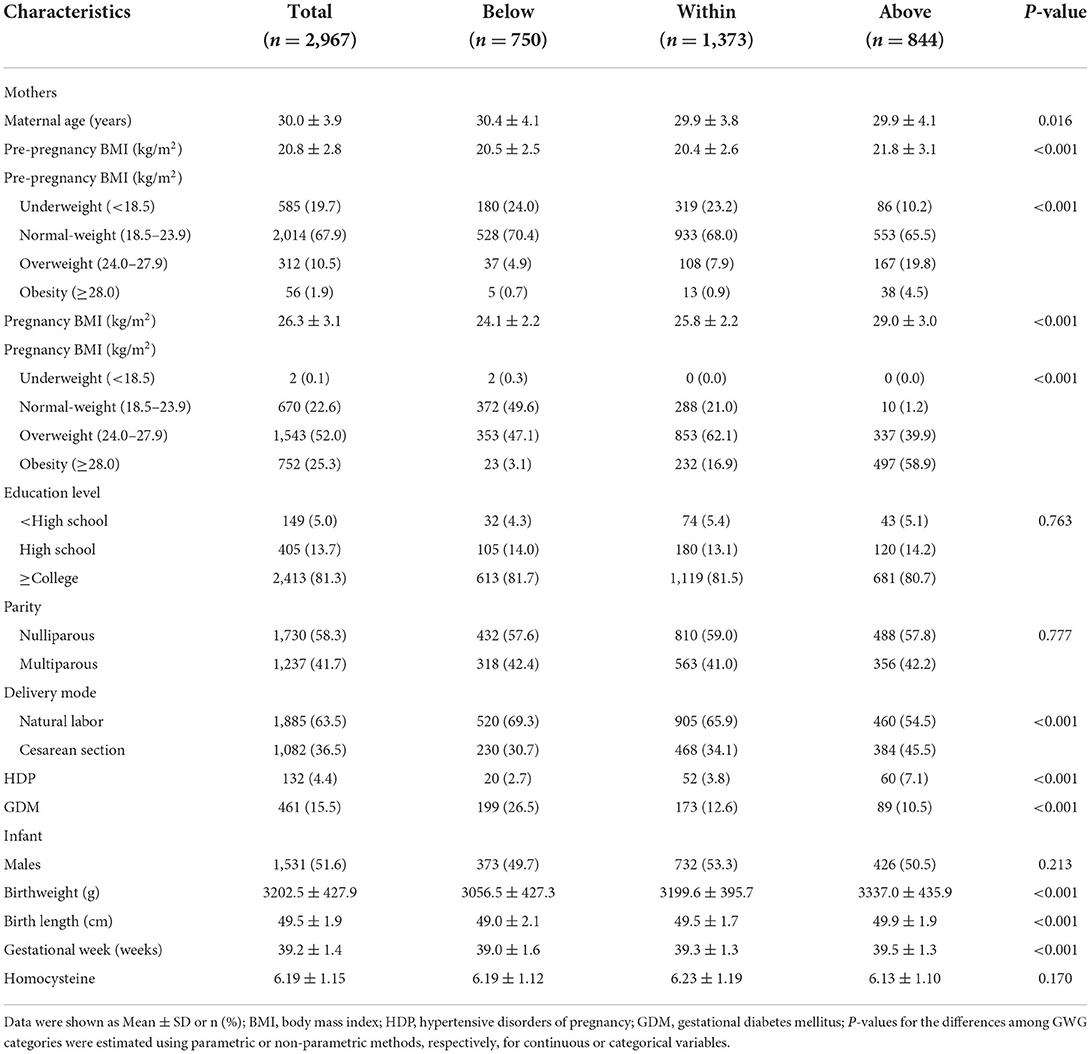
Descriptive variables are listed in Table 1. A total of 2,967 mother-infant pairs were selected for analysis. The average of maternal age was 30.0 ± 3.9 years. For pre-pregnancy BMI, there were 2014 (67.9%) pregnant women in the normal-weight group, 585 (19.7%) in the underweight group, 312 (10.5%) in the overweight group, and 56 (1.9%) in the obesity group. At the time of delivery, the normal-weight group, underweight group, overweight group, and obesity group had 670 (22.6%), 2 (0.1%), 1,543 (52.0%), and 752 (25.3%) pregnant women, respectively. When grouped according to the IOM guidelines, there were 1,373 (46.3%) pregnant women with GWG within the recommended range, 750 (25.3%) below the recommended range, and 844 (28.4) above the recommended range. Underweight and normal-weight women were more likely to have GWG within the IOM guidelines, while overweight and obese women were more likely to have GWG above the IOM guidelines (Figure 1). Most mothers were nulliparous (n = 1,730, 58.3%), and the deliver mode of 1,082 (36.5%) women was cesarean section. The present study reported that none of the mothers had a history of smoking or drinking during pregnancy. Additionally, 132 (4.4%) and 461 (15.5%) mothers were diagnosed with HDP and GDM, respectively, in this population. There were 51.6% (n = 1,531) boys and 48.4% (n = 1,436) girls among the infants. The mean weight, length, and gestational age at birth were 3202.5 ± 427.9 g, 49.5 ± 1.9 cm, and 39.2 ± 1.4 weeks, respectively.
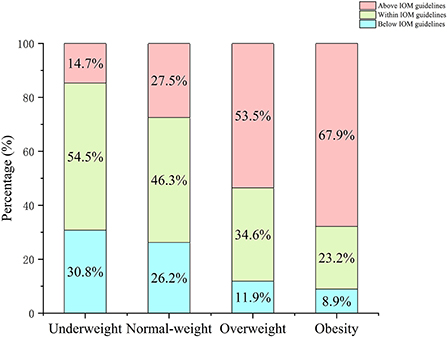
Figure 1. Percentage of pregnant women in each BMI category by IOM GWG guidelines. Pre-pregnancy BMI were categorized according to Chinese BMI classification (underweight, <18.5 kg/m2; normal weight, 18.5–23.9 kg/m2; overweight, 24.0–27.9 kg/m2; and obesity, ≥28.0 kg/m2).
GWG categories in relation to adverse birth outcomes
In our study population, the prevalence rates were 4.9% (n = 144), 1.7% (n = 49), 14.6% (n = 432), and 5.0% (n = 149) for LBW, macrosomia, SGA, and LGA, respectively (Table 2). According to the IOM criteria, 41.0% LBW cases (n = 59), 30.6% macrosomia cases (n = 15), 46.1% SGA cases (n = 199), and 30.2% LGA cases (n = 45) were included in the sufficient group for GWG, while the insufficient group consisted of 43.1% LBW cases (n = 62), no macrosomia cases, 36.3% SGA cases (n = 157), and 8.7% LGA cases (n = 13). After adjusting for potential confounders, women who had GWG below IOM guideline were more likely to have SGA [adjusted odds ratio (OR) = 1.68, 95% confidence interval (CI): 1.32–2.13] and less likely to have LGA (adjusted OR = 0.51, 95% CI: 0.27–0.96) than those who had GWG within IOM. There were 16.0% LBW cases (n = 23), 69.4% macrosomia cases (n = 34), 17.6% SGA cases (n = 76), and 61.1% LGA cases (n = 91) were included in the excessive group for GWG. Compared to those in the sufficient group, pregnant women with GWG above the IOM recommended range had decreased odds of SGA (adjusted OR = 0.60, 95% CI: 0.45–0.79). In addition, higher risks of macrosomia (adjusted OR = 3.47, 95% CI: 1.86–6.48) and LGA (adjusted OR = 3.25, 95% CI: 2.23–4.74) were also observed among pregnant women in the excessive group. Different pre-pregnancy BMI categories, according to WHO standards, were introduced to assess the robustness of our results.
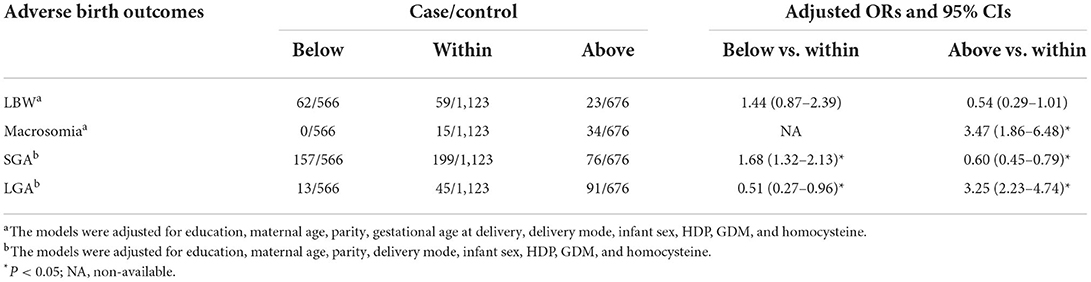
Table 2. Associations between adverse birth outcomes and GWG categories according to IOM guidelines.
MTHFR polymorphisms in relation to adverse birth outcomes
The minor allele frequency was 23.3% for MTHFR A1298C and 28.3% for MTHFR C677T in the control group, and all polymorphisms were consistent with HWE (P for HWE = 0.266 for A1298C and P for HWE = 0.065 for C677T). Dominant, recessive, and additive models were used to assess the association between MTHFR polymorphism and the 4 birth outcomes (Supplementary Tables S1, S2). According to the results from crude and adjusted regression models, no significant association was observed between MTHFR A1298C and C677T in relation to LBW, macrosomia, SGA and LGA (all P > 0.05).
Modification effects of MTHFR polymorphisms on the association of GWG with adverse birth outcomes
Modification effects of MTHFR polymorphisms on the association between GWG categories and birth outcomes were explored by dividing the study population according to various genotypes of MTHFR A1298C and C677T. As for the A1298C polymorphisms, although no interaction effect was suggested (P for interaction > 0.05), obvious differences were suggested between genotypes (see Table 3). Increased risks of LBW (adjusted OR = 2.10, 95% CI: 1.08–4.10) and SGA (adjusted OR = 1.95, 95% CI: 1.43–2.65) were only observed among women with the A1298C AA genotype and GWG below IOM guideline, while no significant association was found among women with the A1298C AC+CC genotype. Similarly, a higher risk of macrosomia was only observed in women with higher GWG and A1298C AA genotype (adjusted OR = 4.19, 95% CI: 1.89–9.29). With regard to the decreased odds of LGA in women with insufficient GWG, no significant association was observed when the population was stratified by the A1298C and C677T genotypes (see Table 4). When the population was stratified by IOM GWG categories, we found pregnant women with insufficient GWG and A1298C AA genotype had an increased risk of 3.96 (95% CI: 1.57–10.01) for LBW, compared with the AA+AC group, while no significant association of C677T polymorphism was observed (Table 5, Supplementary Table S3). Dose-response relationship between GWG values and 4 adverse birth outcomes with spline smoothing function among pregnant women with MTHFR A1298C AA genotype was shown in Supplementary Figure S2. A negative correlation of GWG was suggested in relation to LBW and SGA, while a positive correlation of GWG was indicated in relation to macrosomia and LGA.
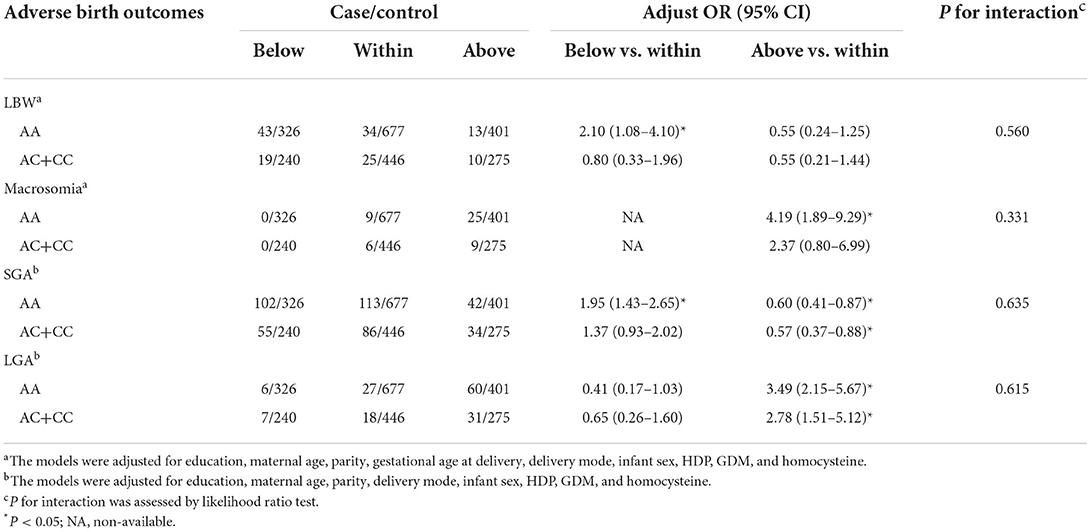
Table 3. Associations of GWG categories and adverse birth outcomes stratified by MTHFR A1298C polymorphisms.
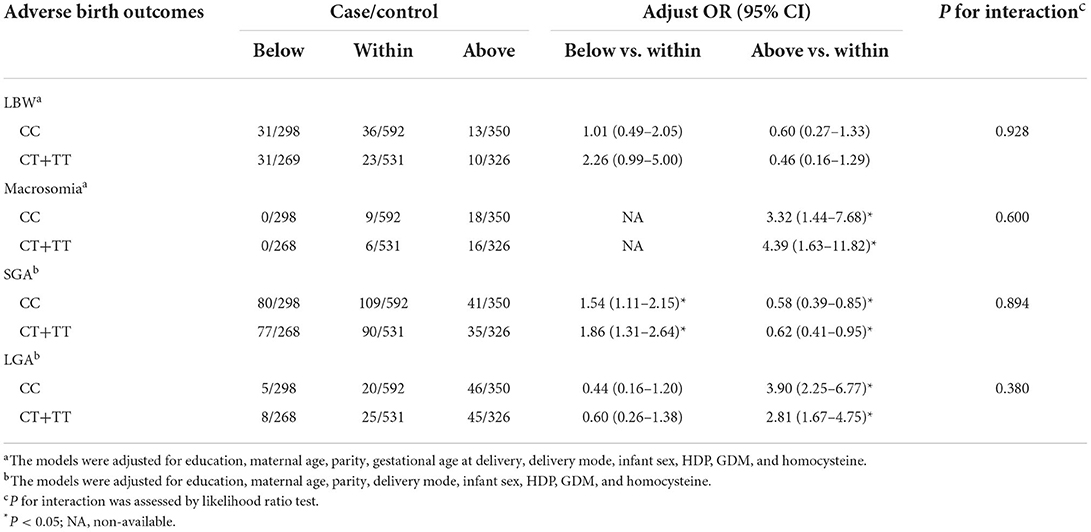
Table 4. Associations of GWG categories and adverse birth outcomes stratified by MTHFR C677T polymorphisms.
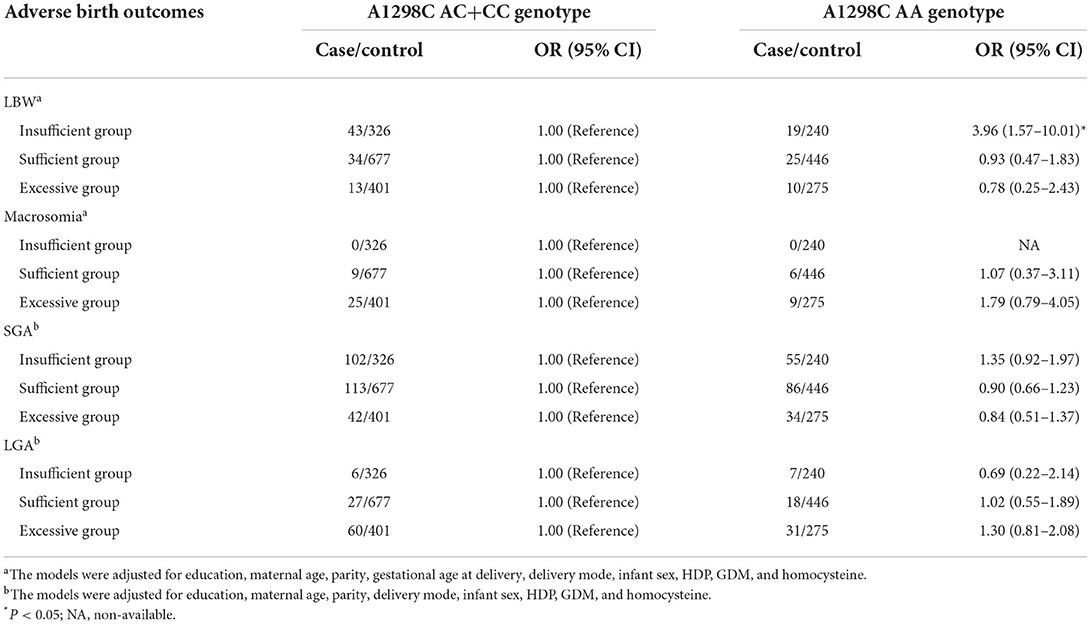
Table 5. Associations of MTHFR A1298C polymorphisms and adverse birth outcomes stratified by IOM GWG categories.
Sensitivity analyses
To assess the robustness of our results, sensitivity analyses were performed by repeating the analyses according to WHO criteria of BMI classifications. The obtained results were similar to the main analyses, and did not drastically change (Supplementary Tables S4–S7, Supplementary Figure S1).
Discussion
The prevalence of underweight, overweight and obesity for Chinese women before pregnancy in the present study were 19.7, 10.5, and 1.9%, respectively. Excessive GWG accounted for 28.4% of the participants based on the IOM standards, and the proportion was 25.3% for insufficient GWG. Compared to those with normal GWG, pregnant women with insufficient GWG were more likely to give birth to SGA and less likely to give birth to LGA babies, whereas pregnant women with excessive GWG had decreased odds of SGA. Pregnant women in the excessive group had a higher risk of macrosomia and LGA. Interestingly, significant associations of GWG categories in relation to LBW, macrosomia and SGA were only suggested among pregnant women with the MTHFR A1298C AA genotype. Among pregnant women with insufficient GWG group, an elevation in the risk of LBW was observed among subjects with the A1298C AA genotype compared to the AC+CC genotype group.
Accumulating evidence has suggested that Chinese people are likely to have higher percentages of body fat (20, 21) and higher rates of hypertension, type 2 diabetes, and dyslipidemia than Caucasian people at specific BMI values (22). The Working Group on obesity in China recommended BMI cutoffs of 24.0 kg/m2 to define overweight and 28.0 kg/m2 to define obesity (15), supported by evidence from the China Kadoorie Biobank (23, 24). In the present study, 12.4% women were overweight and obesity before pregnancy on the basis of Chinese standards, compared to 8.0% according to WHO criteria. The distributions were comparable with those of previous studies in Chinese women of reproductive age (25, 26), but lower than those in the USA (27) and several European countries (UK, Spain, Belgium, etc.) (28). Our findings did not support the evidence that Chinese BMI standards establish better sensitivity and specificity for identifying adverse birth outcomes than the WHO criteria because the direction and the strength of the obtained associations were similar according to these two standards. This is because of the analogous distributions in the GWG categories. In our study, 25.3 and 28.4% of the women showed insufficient and excessive GWG according to Chinese BMI standards, and the proportions were 26.6 and 26.7% with regard to WHO criteria. The GWG categories in the present study were different from other Chinese studies (15.2% < recommended range and 52.1% > recommended range) (26) and the US population (21.2% < recommended range and 51.0% > recommended range) (27).
GWG is mainly attributed to maternal fat accumulation, fluid expansion, and fetal, placental, and uterine development, which can partly reflect maternal nutrition and fetal development. It is reported that GWG is closely related to a majority of neonatal risks including fetal growth restriction, premature birth, GDM, HDP, and infant mortality, as well as with long-term offspring metabolic health outcomes (29). In the present study, excessive GWG was found to increase the OR for macrosomia and LGA, and decreased the OR for SGA, consistent with the previous studies. For instance, Gou et al. explored the associations of GWG categories with adverse birth weight in a Chinese population of pregnant women with GDM (n = 1,523) and demonstrated that excessive GWG could significantly increase a 2.20- and 2.06-fold risk, respectively, for macrosomia and LGA, and decrease the risk of SGA by 51.0% (30). Zhao et al. analyzed the data from 1,617 pregnant women and concluded that excessive GWG was associated with macrosomia and LGA, but no significant association was observed between excessive GWG and SGA risk (26). In addition, our study suggested that pregnant women with inadequate GWG had a higher frequency of SGA (adjusted OR = 1.68, 95% CI: 1.32–2.13) and a lower rate of LGA (adjusted OR = 0.51, 95% CI: 0.27–0.96). Similar conclusions were also reported in studies conducted in China (26), Japan (31), and Norway (32). Our conclusions were supported by a meta-analysis that included more than 1 million pregnancies. Excessive GWG was related to a lower risk of SGA (OR = 0.66), and higher risks of LGA (OR = 1.85) and macrosomia (OR = 1.95), while insufficient GWG was correlated with a higher risk of SGA (OR = 1.53) and a lower risk of LGA (OR = 0.59) (33). Similar to our findings, the relationship between the GWG categories and LBW risk was not statistically significant in this meta-analysis. However, Zhao et al. (26) and Hung et al. (34) reported that insufficient GWG increased the LBW risk. Considering the crucial role of the gestational week on fetal growth, SGA and LGA are thought to be more valuable outcomes compared to LBW and macrosomia, calculated by crude birthweight.
MTHFR is a crucial enzyme that catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, an important enzymatic process in folate metabolism and the remethylation of homocysteine to methionine. Two common single nucleotide polymorphisms, C677T and A1298C, are known to affect the enzyme function and homocysteine metabolism and have shown potential clinical significance. C677T causes an alanine to valine substitution, resulting in the thermolability of MTHFR. The specific enzyme activity in C677T homozygous mutated subjects decreased to 30% compared to that in heterozygous subjects (~65%) and non-mutated controls (100%) (35). Similarly, the A1298C polymorphism encodes glutamate to alanine substitution, leading to a decrease in enzyme activity to a lesser extent (36). Increased plasma homocysteine levels have been associated with the C677T polymorphism alone and in combination with the A1298C mutation (37, 38). In addition to maintaining a normal range of folate and homocysteine levels, the MTHFR enzyme is important in many biological reactions, including DNA synthesis, cell growth, implantation and invasion of the embryo, especially in fetal growth during pregnancy (39). Recent observations have indicated that MTHFR variants are independent risk factors for adverse birth outcomes. For instance, Tiwari et al. in an Indian population found that pregnant women with MTHFR C677T mutated subjects have an increased risk for preterm delivery, negative pregnancy outcomes, and LBW (40). Mo et al. conducted a study on two Chinese populations, indicating that the frequency of MTHFR A1298C CC genotype was significantly different between a group with adverse birth outcomes and healthy controls (41). However, other studies had reported contrary conclusions (42, 43), consistent with our findings. The present study suggested null associations between MTHFR polymorphisms and adverse birth outcomes, neither in crude models nor fully adjusted models. The inconsistent conclusions could be ascribed to the relatively low-frequency distribution of homozygous mutation of MTHFR C677T and A1298C and the small sample size in our study. In addition, this might be due to the small difference of homocysteine levels across genotypes. In our study, homocysteine concentrations among MTHFR C677T homozygous mutated subjects were comparable with heterozygous subjects (TT: 6.31 ± 1.23 μmol/L vs. CT: 6.32 ± 1.19 μmol/L), slightly higher than non-mutated participants (CC: 6.07 ± 1.10 μmol/L) (P < 0.001). With regard to A1298C mutation, consistent with a previous study (37), no significant elevation in homocysteine concentrations was observed, even for homozygous mutant subjects (CC: 6.10 ± 0.95 μmol/L vs. AC: 6.18 ± 1.20 μmol/L vs. AA: 6.20 ± 1.14 μmol/L, P = 0.559).
Homocysteine is crucial for the transfer of methyl groups in the activated methyl cycle. Genetic and animal studies had provided clues that homocysteine might regulate the expression of genes involved in body fat storage and lipid metabolisms via epigenetic mechanisms (13, 14). Recent evidence from human studies has indicated a potential relationship between MTHFR polymorphisms and obesity/overweight. According to the results of a meta-analysis of 38,317 participants, a strong relationship was suggested between homocysteine concentrations and obesity via the effect of MTHFR C677T polymorphism (TT vs. CC: OR = 1.13, 95% CI = 1.03–1.24) (8). Renzo et al. conducted a dietary study in an Italy population, and observed that participants with C677T CT or TT genotype had higher body weight, BMI, waist, abdomen, hip, waist/hip, fat and lean at baseline, and the ratio of total body lean to total body fat was significantly lower in mutated genotype group after dietary intervention (12). Furthermore, a genetic study by Terruzzi et al. suggested that DNA hypomethylation owing to the lower efficiency of polymorphic MTHFR enzymes could regulate the proliferation and differentiation of myoblasts, promoting muscle growth and increasing muscle mass (44). Considering the alteration effect of MTHFR polymorphisms on fat storage and lipid metabolisms, we hypothesized that MTHFR polymorphisms possess a potential modification effect on the association between GWG and adverse birth outcomes.
Surprisingly, according to our results, it was A1298C, not the C677T polymorphism, which showed modification effect on the association between GWG categories and adverse birth outcomes, as A1298C does not result in either a thermolabile protein or severely change homocysteine levels in the blood. Significant associations of insufficient GWG in relation to LBW and SGA and excessive GWG in relation to macrosomia were merely observed among pregnant women with A1298C AA genotype. These findings were further supported by the results of the subgroup analysis by the GWG category. Among pregnant women with insufficient GWG, an increased risk of LBW was suggested for A1298C non-mutated women compared to the mutated group, although null associations were indicated for SGA and macrosomia. It could be interpreted that A1298C non-mutated pregnant women were more susceptible to adverse birth outcomes than the mutant participants. Relevant functional studies on the A1298C polymorphism are scarce. The risk effect of the A1298C non-mutated genotype may be ascribed to the influence of the C677T polymorphisms. The MTHFR A1298C and C677T polymorphisms show a high degree of linkage disequilibrium (45). According to our data, there were, respectively, 43.2% C677T CT and 15.1% TT genotypes among A1298C AA subjects; and the percentages were 36.9 and 0.6% among A1298C AC subjects and 2.2 and 0.0% among A1298C CC subjects. A1298C non-mutated pregnant women were more likely to be homozygous or heterozygous mutant for the C677T polymorphism. Thus, A1298C non-mutated pregnant women might be at risk of the harmful effect of C677T mutated polymorphism, which has been reported to be related to a variety of adverse birth outcomes. However, in the present study, neither a direct effect nor a modification effect of the C677T polymorphism was indicated according to our analysis. Therefore, the above speculation should be further verified in human studies, along with the functional effect of the A1298C polymorphism. Our findings were similar to those of a previous cohort study (n = 2,034) by Said et al., who observed a significant reduction in the risk of severe fetal growth restriction among nulliparous women with the MTHFR A1298C homozygous polymorphism (46). In contrast, Chedraui et al. found that A1298C homozygous polymorphism was correlated with higher neck and mid-arm circumference and a higher risk of preeclampsia, which might affect birth outcomes (47). However, it should be noted that our findings may be due to chance regarding the relatively small sample size when stratified by genotypes. Further studies conducted in larger populations are required to confirm our conclusions.
Our work has several strengths. A total of 2,967 pregnant women in a Chinese population were included, and the potential association between GWG categories and adverse birth outcomes were explored by adjusting for major confounders. We assessed whether the Chinese BMI standards establish better sensitivity and specificity for identifying adverse birth outcomes than the WHO criteria in a Chinese population. In addition, we investigated, for the first time, whether MTHFR polymorphism modify the effect of maternal GWG categories on adverse birth outcomes. However, this study had several limitations. First, as our research had a retrospective design, we could not judge the causality of the observed associations. Therefore, future prospective studies are required. Second, self-reported maternal pre-pregnancy BMI was applied to calculate GWG, raising possible measurement misclassifications. Third, potential confounders such as diet, physical activity, and other genetic factors were not controlled in our analysis models, which might have affected the reliability of our results. In addition, effects of other SNPs in gene MTHFR, especially for those SNPs in 3′ or 5′ near gene, promoter, untranslated regions and exons, had not been studied, which might also influence the study associations. Future studies should focus the modification effects of more variants in gene MTHFR. Finally, all subjects were from Han population, and most of them had a high education level (81.3% of college or above). Therefore, the generalizability of our findings to other populations might be limited.
Conclusion
In summary, our research provides evidence of a potential association between GWG categories, MTHFR polymorphisms, and adverse birth outcomes. Women with insufficient GWG had a significantly increased risk of SGA and decreased risk of LGA. Pregnant women with excessive GWG were less likely to give birth to SGA infants and had higher risks of macrosomia and LGA. In addition, a modification effect of the A1298C polymorphism has been suggested. The A1298C non-mutated genotype is considered a risk factor for adverse birth outcome among pregnant women with insufficient GWG. Our findings contribute to a better understanding of the health effects of GWG and MTHFR polymorphisms on birth outcomes. However, further prospective studies with larger sample size are required to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study protocol was approved by the Medical Ethical Committees of Guangdong Women and Children Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XM contributed to the conception and design of the study. WW and DL conducted data analysis and wrote the manuscript. WL, XR, CG, and KL contributed to the analysis and interpretation of the data. All authors have critically reviewed the manuscript for important intellectual content and approved of the final version for publication.
Funding
This research was funded by the Natural Science Foundation of Guangdong Province of China (2020A1515010434), the Medical Scientific Research Foundation of Guangdong Province of China (A2020059), the Guangzhou Basic and Applied Basic Research Foundation (202102021190), and the National Natural Science Foundation of China (42107450).
Acknowledgments
The authors wanted to thank participators who volunteered to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.919651/full#supplementary-material
References
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. (2014) 311:806–14. doi: 10.1001/jama.2014.732
2. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:373–92. doi: 10.1016/S2213-8587(21)00045-0
3. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines, Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US) (2009).
4. Quickstats. Gestational Weight Gain* among women with full-term, singleton births, compared with recommendations - 48 states and the district of Columbia, 2015. MMWR Morb Mortal Wkly Rep. (2016) 65:1121. doi: 10.15585/mmwr.mm6540a10
5. Huang A, Ji Z, Zhao W, Hu H, Yang Q, Chen D. Rate of gestational weight gain and preterm birth in relation to prepregnancy body mass indices and trimester: a follow-up study in China. Reprod Health. (2016) 13:1–7. doi: 10.1186/s12978-016-0204-2
6. Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (Mthfr) C677t polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet. (2015) 58:1–10. doi: 10.1016/j.ejmg.2014.10.004
7. Williams KT, Schalinske KL. New insights into the regulation of methyl group and homocysteine metabolism. J Nutr. (2007) 137:311–4. doi: 10.1093/jn/137.2.311
8. Fu L, Li YN, Luo D, Deng S, Hu YQ. Plausible relationship between homocysteine and obesity risk via Mthfr gene: a meta-analysis of 38,317 individuals implementing Mendelian randomization. Diabetes Metab Syndr Obes. (2019) 12:1201–12. doi: 10.2147/DMSO.S205379
9. Zhi X, Yang B, Fan S, Wang Y, Wei J, Zheng Q, et al. Gender-specific interactions of Mthfr C677t and Mtrr A66g polymorphisms with overweight/obesity on serum lipid levels in a Chinese Han population. Lipids Health Dis. (2016) 15:185. doi: 10.1186/s12944-016-0354-9
10. Zhi X, Yang B, Fan S, Li Y, He M, Wang D, et al. Additive interaction of Mthfr C677t and Mtrr A66g polymorphisms with being overweight/obesity on the risk of type 2 diabetes. Int J Environ Res Public Health. (2016) 13:1243. doi: 10.3390/ijerph13121243
11. Liao J, Wang N, Ma M, Lu T, Yan H, Yue W. C677t polymorphism in the Mthfr gene is associated with risperidone-induced weight gain in schizophrenia. Front Psychiatry. (2020) 11:617. doi: 10.3389/fpsyt.2020.00617
12. Di Renzo L, Rizzo M, Iacopino L, Sarlo F, Domino E, Jacoangeli F, et al. Body composition phenotype: Italian mediterranean diet and C677t Mthfr gene polymorphism interaction. Eur Rev Med Pharmacol Sci. (2013) 17:2555–65. Available online at: https://www.europeanreview.org/wp/wp-content/uploads/2555-2565.pdf (accessed July 27, 2022).
13. Bordoni L, Petracci I, Mlodzik-Czyzewska M, Malinowska AM, Szwengiel A, Sadowski M, et al. Mitochondrial DNA and epigenetics: investigating interactions with the one-carbon metabolism in obesity. Oxid Med Cell Longev. (2022) 2022:9171684. doi: 10.1155/2022/9171684
14. Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. (2002) 132(8 Suppl):2393s−400s. doi: 10.1093/jn/132.8.2393S
15. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96. doi: 10.1046/j.1440-6047.11.s8.9.x
16. Dai L, Deng C, Li Y, Zhu J, Mu Y, Deng Y, et al. Birth weight reference percentiles for Chinese. PLoS ONE. (2014) 9:e104779. doi: 10.1371/journal.pone.0104779
17. WHO Consultation on Obesity. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253.
18. Wu W, Liu D, Jiang S, Zhang K, Zhou H, Lu Q. Polymorphisms in gene Mmp-2 modify the association of cadmium exposure with hypertension risk. Environ Int. (2019) 124:441–7. doi: 10.1016/j.envint.2019.01.041
19. Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, Parvez F, et al. Interaction between arsenic exposure from drinking water and genetic polymorphisms on cardiovascular disease in Bangladesh: a prospective case-cohort study. Environ Health Perspect. (2015) 123:451–7. doi: 10.1289/ehp.1307883
20. Wang D, Li Y, Lee SG, Wang L, Fan J, Zhang G, et al. Ethnic differences in body composition and obesity related risk factors: study in Chinese and white males living in China. PLoS ONE. (2011) 6:e19835. doi: 10.1371/journal.pone.0019835
21. Chen YM, Ho SC, Lam SS, Chan SS. Validity of body mass index and waist circumference in the classification of obesity as compared to percent body fat in Chinese middle-aged women. Int J Obes. (2006) 30:918–25. doi: 10.1038/sj.ijo.0803220
22. Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
23. Gao M, Wei YX, Lyu J, Yu CQ, Guo Y, Bian Z, et al. The cut-off points of body mass index and waist circumference for predicting metabolic risk factors in Chinese adults (in Chinese). Zhonghua Liu Xing Bing Xue Za Zhi. (2019) 40:1533–40. doi: 10.3760/cma.j.issn.0254-6450.2019.12.006
24. Li JC, Lyu J, Gao M, Yu CQ, Guo Y, Bian Z, et al. Association of body mass index and waist circumference with major chronic diseases in Chinese adults (in Chinese). Zhonghua Liu Xing Bing Xue Za Zhi. (2019) 40:1541–7. doi: 10.3760/cma.j.issn.0254-6450.2019.12.007
25. Wu Y, Wan S, Gu S, Mou Z, Dong L, Luo Z, et al. Gestational weight gain and adverse pregnancy outcomes: a prospective cohort study. BMJ Open. (2020) 10:e038187. doi: 10.1136/bmjopen-2020-038187
26. Zhao R, Xu L, Wu ML, Huang SH, Cao XJ. Maternal pre-pregnancy body mass index, gestational weight gain influence birth weight. Women Birth. (2018) 31:e20–5. doi: 10.1016/j.wombi.2017.06.003
27. Kominiarek MA, Saade G, Mele L, Bailit J, Reddy UM, Wapner RJ, et al. Association between gestational weight gain and perinatal outcomes. Obstet Gynecol. (2018) 132:875–81. doi: 10.1097/AOG.0000000000002854
28. Devlieger R, Benhalima K, Damm P, Van Assche A, Mathieu C, Mahmood T, et al. Maternal obesity in Europe: where do we stand and how to move forward?: A scientific paper commissioned by the European board and college of obstetrics and gynaecology (Ebcog). Eur J Obstet Gynecol Reprod Biol. (2016) 201:203–8. doi: 10.1016/j.ejogrb.2016.04.005
29. Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. (2016) 4:1025–36. doi: 10.1016/S2213-8587(16)30217-0
30. Gou BH, Guan HM, Bi YX, Ding BJ. Gestational diabetes: weight gain during pregnancy and its relationship to pregnancy outcomes. Chin Med J. (2019) 132:154–60. doi: 10.1097/CM9.0000000000000036
31. Enomoto K, Aoki S, Toma R, Fujiwara K, Sakamaki K, Hirahara F. Pregnancy outcomes based on pre-pregnancy body mass index in Japanese women. PLoS ONE. (2016) 11:e0157081. doi: 10.1371/journal.pone.0157081
32. Haugen M, Brantsæter AL, Winkvist A, Lissner L, Alexander J, Oftedal B, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth. (2014) 14:201. doi: 10.1186/1471-2393-14-201
33. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. (2017) 317:2207–25. doi: 10.1001/jama.2017.3635
34. Hung TH, Hsieh TT. Pregestational Body mass index, gestational weight gain, and risks for adverse pregnancy outcomes among Taiwanese women: a retrospective cohort study. Taiwan J Obstet Gynecol. (2016) 55:575–81. doi: 10.1016/j.tjog.2016.06.016
35. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. (1995) 10:111–3. doi: 10.1038/ng0595-111
36. Klai S, Fekih-Mrissa N, El Housaini S, Kaabechi N, Nsiri B, Rachdi R, et al. Association of Mthfr A1298c polymorphism (but not of Mthfr C677t) with elevated homocysteine levels and placental vasculopathies. Blood Coagul Fibrinolysis. (2011) 22:374–8. doi: 10.1097/MBC.0b013e328344f80f
37. Pereira AC, Schettert IT, Morandini Filho AA, Guerra-Shinohara EM, Krieger JE. Methylenetetrahydrofolate reductase (Mthfr) C677t gene variant modulates the homocysteine folate correlation in a mild folate-deficient population. Clin Chim Acta. (2004) 340:99–105. doi: 10.1016/j.cccn.2003.09.016
38. Pavarino-Bertelli EC, Sanches de Alvarenga MP, Goloni-Bertollo EM, Baptista MA, Haddad R, Hoerh NF, et al. Hyperhomocysteinemia and Mthfr C677t and A1298c polymorphisms are associated with chronic allograft nephropathy in renal transplant recipients. Transplant Proc. (2004) 36:2979–81. doi: 10.1016/j.transproceed.2004.12.002
39. Gaiday AN, Tussupkaliyev AB, Bermagambetova SK, Zhumagulova SS, Sarsembayeva LK, Dossimbetova MB, et al. Effect of homocysteine on pregnancy: a systematic review. Chem Biol Interact. (2018) 293:70–6. doi: 10.1016/j.cbi.2018.07.021
40. Tiwari D, Bose PD, Das S, Das CR, Datta R, Bose S. Mthfr (C677t) polymorphism and Pr (Progins) mutation as genetic factors for preterm delivery, fetal death and low birth weight: a northeast Indian population based study. Meta Gene. (2015) 3:31–42. doi: 10.1016/j.mgene.2014.12.002
41. Mo H, Rao M, Wang G, Long YX, Wang HW, Tang L. Polymorphism of Mthfr 1298a>C in relation to adverse pregnancy outcomes in Chinese populations. Mol Genet Genomic Med. (2019) 7:e642. doi: 10.1002/mgg3.642
42. Luo L, Chen Y, Wang L, Zhuo G, Qiu C, Tu Q, et al. Polymorphisms of genes involved in the folate metabolic pathway impact the occurrence of unexplained recurrent pregnancy loss. Reprod Sci. (2015) 22:845–51. doi: 10.1177/1933719114565033
43. Wang BJ, Liu MJ, Wang Y, Dai JR, Tao JY, Wang SN, et al. Association between Snps in genes involved in folate metabolism and preterm birth risk. Genet Mol Res. (2015) 14:850–9. doi: 10.4238/2015.February.2.9
44. Terruzzi I, Senesi P, Montesano A, La Torre A, Alberti G, Benedini S, et al. Genetic polymorphisms of the enzymes involved in DNA methylation and synthesis in elite athletes. Physiol Genom. (2011) 43:965–73. doi: 10.1152/physiolgenomics.00040.2010
45. Pilsner JR, Hu H, Wright RO, Kordas K, Ettinger AS, Sánchez BN, et al. Maternal Mthfr genotype and haplotype predict deficits in early cognitive development in a lead-exposed birth cohort in Mexico city. Am J Clin Nutr. (2010) 92:226–34. doi: 10.3945/ajcn.2009.28839
46. Said JM, Higgins JR, Moses EK, Walker SP, Borg AJ, Monagle PT, et al. Inherited thrombophilia polymorphisms and pregnancy outcomes in nulliparous women. Obstet Gynecol. (2010) 115:5–13. doi: 10.1097/AOG.0b013e3181c68907
Keywords: gestational weight gain, methylenetetrahydrofolate reductase polymorphisms, low birth weight, macrosomia, small-for-gestational age, large-for-gestational age
Citation: Wu W, Luo D, Ruan X, Gu C, Lu W, Lian K and Mu X (2022) Polymorphisms in gene MTHFR modify the association between gestational weight gain and adverse birth outcomes. Front. Nutr. 9:919651. doi: 10.3389/fnut.2022.919651
Received: 13 April 2022; Accepted: 22 July 2022;
Published: 08 August 2022.
Edited by:
Louise Brough, Massey University, New ZealandReviewed by:
Lixin Guo, Peking University, ChinaEwa Wender-Ozegowska, Poznan University of Medical Sciences, Poland
Dazhi Fan, Foshan Women and Children Hospital, China
Copyright © 2022 Wu, Luo, Ruan, Gu, Lu, Lian and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Mu, bXV4aWFvcGluZzcyMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Weixiang Wu1†
Weixiang Wu1† Chunming Gu
Chunming Gu Xiaoping Mu
Xiaoping Mu