
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 27 July 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.918754
This article is part of the Research Topic Dietary Intake, Eating Behavior and Health Outcomes View all 36 articles
Objective: Inflammatory bowel disease (IBD) and alcohol use has become a significant and growing public health concern. Alcohol use has been reported to be the most-avoided diet item among IBD patients. However, knowledge regarding the impact of different classes of alcoholic beverages on the management of IBD is limited. Our study aims to evaluate the association of different frequencies, amounts, and subtypes of alcoholic beverages with IBD risk.
Methods: The UK Biobank comprised 7,095 subjects with IBD and 4,95,410 subjects without IBD. Multivariate Logistic regression, stratifying analysis, and interaction terms were used to estimate the odds ratios (ORs) and 95% confidence intervals (95% CIs) of IBD. A generalized additive model was used to evaluate the linearity associations of the total amount of all alcoholic beverages or that of each of five alcoholic beverages with IBD risk.
Results: Compared with non-drinkers, the IBD risk was 12 to 16% lower in red wine consumers (1–2 glasses/week, OR [95%CI], 0.88 [0.80, 0.97]; 3–4 glasses/week, 0.84 [0.76, 0.93]; ≥5 glasses/week, 0.86 [0.78, 0.95]), whereas 12% higher in white wine and champagne consumers (1–2 glasses/week, 1.12 [1.03, 1.22]). Stratifying analysis showed low-frequency red wine consumers were associated with a lower IBD risk (0.85 [0.74, 0.97]), whereas spirits consumers were associated with a higher risk (1.28 [1.03, 1.59]). High doge of red wine consumers were associated with a lower IBD risk (above guidelines, 0.80 [0.67, 0.97]; double above, 0.83 [0.71, 0.97]), whereas high doge white wine and champagne (1.32 [1.09, 1.61]) and beer and cider (1.26 [1.02, 1.54]) consumers were associated with a higher IBD risk. White wine and champagne showed a significant interaction effect with high doge alcohol consumption (1.27 [1.03–1.58], p = 0.029). The dose-response association showed an increased IBD risk with more number of alcohol consumption of white wine and champagne, beer and cider, or the total amount of all alcoholic beverages. However, red wine is at low risk across the whole dose cycle.
Conclusions: The IBD risk appears to vary across different frequencies, amounts, and subtypes of alcoholic beverages. Overall, alcohol intake is not recommended.
Crohn's disease (CD) and ulcerative colitis (UC) are collectively known as inflammatory bowel disease (IBD). IBD has become a significant and growing public health concern and has conveyed a high rate of morbidity and mortality (1, 2). IBD is progressive and immune-mediated inflammatory disease of the gastrointestinal tract. The established importance of environmental factors (e.g., diet and gut microenvironment) in the development of IBD has been widely reported (3, 4). Dietary triggers have aroused significant interest, as identifying modifiable dietary factors could help reduce relapse frequency, thereby limiting steroid use and decreasing hospitalizations (5). However, the exact etiological mechanism of IBD still remains unknown.
Alcohol use is a leading risk factor for disease burden worldwide, accounting for nearly 10% of global deaths among populations aged 15 to 49 years, and poses dire ramifications for future population health in the absence of policy action today (6). Alcohol use in patients with IBD is common (7), and even though its prevalence in patients with IBD appears to be similar to the general population, it has been reported to be the most-avoided diet item among IBD patients (8). Niccum et al. found that alcohol consumption is associated with an increased risk of microscopic colitis (9). Jowett et al. found that alcohol consumption is associated with a higher risk of relapse in patients with UC (10). Mantzouranis et al. reviewed several literature and found that alcohol consumption is associated with worse IBD symptoms among patients who consumed alcoholic beverages compared with those who did not consume alcoholic beverages (11). Patients with alcohol abuse disorder have a similar microbial signature to that of patients with IBD, and ethanol ingested from alcoholic beverages has been widely known to impair gut barrier permeability and function (1, 12–15), which may suggest that the alcohol consumption is involved in modulating the microbiome and facilitating intestinal inflammation, and therefore could facilitate IBD pathogenesis. However, beneficial effect on our health of some classes of alcoholic beverages have also been reported in recent years (16–18). However, knowledge regarding the impact of different classes of alcoholic beverages on the management of IBD are limited.
The observed relationships between consumption of alcoholic beverages and diseases are often non-linear, for example, with low-to-moderate alcohol consumption being protective and heavy alcohol consumption being harmful, or J-shaped risk of diseases with the amount of consumption of alcoholic beverages (18–22). There is limited high-quality epidemiologic evidence for the effect of different classes of alcoholic beverages on IBD. In the present large longitudinal observational study, we systematically investigated the associations of different classes of alcoholic beverages with IBD risk, as well as their associations among different frequencies and amounts of alcohol consumption. We further examined the “dose”-response association between alcohol consumption and IBD risk by drawing the risk trajectory of IBD with different amounts of alcohol consumption.
The UK Biobank is a prospective cohort with a total of 5,02,505 subjects recruited from March 2006 to December 2010. We discovered 7,095 IBD cases, which consists of 2,027 cases who only have a diagnosis of Crohn's disease, 4,334 cases who only have a diagnosis of ulcerative colitis, and 734 cases who are both diagnosed with Crohn's disease and ulcerative colitis. Ethical approval of the UK Biobank was obtained from the National Health System Northwest Multicenter Research Ethics Committee (REC reference: 16/NW/0274).
IBD was ascertained by the International Classification of Diseases, Version 10 (ICD-10), terms from the UK Biobank data field 4,1270, which included Crohn's disease (code K50) and ulcerative colitis (code K51).
To assess the frequency of alcohol consumption (Field ID 1558), subjects were asked to answer the question: About how often do you drink alcohol? Seven responses were given: prefer not to answer, never, special occasions only, one to three times a month, once or twice a week, three or four times a week, and daily or almost daily. The frequency of alcohol consumption was classified into three categories: (1) high frequency (≥3 times/week); (2) low frequency (<3 times/week); and (3) never/special occasions only (18).
The average weekly intake of red wine (ID 1568), champagne plus white wine (ID 1578), beer and cider (ID 1588), spirits (ID 1598), and fortified wine (ID 1608) were calculated, respectively. For example, to assess the weekly intake of red wine, subjects were asked to answer the question: In an average week, how many glasses of red wine would you drink? A response with an exact value (e.g., 2 glasses/week) should be given.
The total amount of alcohol consumption was quantified by summing the average weekly intake of red wine, champagne plus white wine, beer and cider, spirits, and fortified wine. As shown in our previous study (18), the weekly intake level of alcohol was converted into units for wines (1 standard glass = 2 units), beer and cider (1 pint =2 units), and spirits (1 shot = 1 unit), and was classified into four categories: (1) non-drinker, previous drinker, or special occasions only; (2) within recommended guidelines: <14 UK units/week; (3) above recommended guidelines: ≥14 units/week and <28 units/week; and (4) 2-fold or more above the recommended guidelines: ≥28 units/week.
A wide range of sociodemographic factors, lifestyle factors, sleep phenotypes, and comorbidities was considered as covariates to adjust for any potential confounding. The covariates of sociodemographic factors included age, sex, ethnicity (white and non-white), education level (college or above, others), body mass index [BMI≥30 and <30 kg/m (2)], current employment status, Townsend deprivation index, and overall health rating. Current employment status was classified into employed (including those in paid employment or self-employed) and unemployed (including those in retired, looking after home and/or family, unable to work because of sickness or disability, doing unpaid or voluntary work, or being full- or part-time students, and unemployed).
The covariates of lifestyle factors included smoking status (never, previous, or current) and usually walking pace (normal, slow, and fast walking pace). The covariates of comorbidities included cerebrovascular diseases, cardiovascular diseases, diabetes, respiratory disease, and cancer. The covariates of sleep phenotype included sleep duration (normal, short, and long sleep duration), early awakening, napping during the day, daytime dozing/sleeping (narcolepsy), sleeplessness or insomnia, and snoring.
Continuous variables are presented as mean ± SD, and categorical variables are presented as a number (percentage). We used the unpaired t-test and χ2 test to compare differences between groups where appropriate.
We used Logistic regression analysis to estimate the odds ratios (ORs) and 95% confidence intervals (95% CIs). Multivariate Logistic regression analysis was used to evaluate the associations between consumption of alcoholic beverages and IBD risk after adjusting for sociodemographic factors, lifestyle factors, sleep phenotypes, comorbidities, frequency of alcohol consumption, amount of weekly intake level of alcohol consumption, and alcoholic beverages. Stratifying analysis was further used to examine the association of different subtypes of alcoholic beverages with the risk of IBD, separated by frequency of alcohol consumption and the total amount of alcohol consumption, respectively.
Interaction terms were employed for the overall sample to explore potential interactions between different subtypes of alcoholic beverages on the risk of IBD in the final model. A generalized additive model was used to evaluate the linearity associations of the total amount of alcohol consumption or that of alcoholic beverages with the risk of IBD.
All analyses were conducted with SPSS version 24.0 and R Statistical Software version 4.0. A two-tailed p-value <0.05 was considered significant.
The demographic characteristics of the study population are presented in Table 1. Compared with subjects without IBD, those with IBD had a poor socioeconomic status [a lower education level (p < 0.001), a poor employment status (p < 0.001), and a higher Townsend deprivation score (p < 0.001)], a poor overall health rating (p < 0.001), more sleep disorders (long and short sleep duration; p < 0.001, usually napping during the day, p < 0.001; narcolepsy, p < 0.001; sleeplessness, p < 0.001; snoring, p < 0.001), and more comorbidities (cerebrovascular diseases, p < 0.001; cardiovascular diseases, p < 0.001; diabetes, p < 0.001; respiratory disease, p < 0.001; and cancer, p < 0.001). Furthermore, they were more likely to be males (p < 0.001), white ethnicity (p = 0.004), smokers (p < 0.001), and slow walking pacers (p < 0.001), but they were less likely to be frequent alcohol drinkers (p < 0.001), heavy drinkers (p < 0.001), red wine drinkers (p < 0.001), white wine and champagne drinkers (p < 0.001), and beer and cider drinkers (p < 0.001), whereas they were more likely to be spirits drinkers (p < 0.001) and fortified wine drinkers (p < 0.001).
The associations of different alcoholic beverages with the risk of IBD are shown in Table 2. In the final multivariate model, compared with non-drinkers, the risk of IBD was 12 to 16% lower in red wine consumers (1–2 glasses/week, OR [95%CI], 0.88 [0.80, 0.97]; 3–4 glasses/week, 0.84 [0.76, 0.93]; ≥5 glasses/week, 0.86 [0.78, 0.95]) regardless of the amount of red wine, whereas 12% higher in white wine and champagne consumers of 1–2 glasses/week (1.12 [1.03, 1.22]). Furthermore, the higher risk was not significant when the amount of white wine and champagne was higher (3–4 glasses/week, 1.04 [0.94, 1.16]; ≥5 glasses/week, 1.04 [0.93, 1.15]). Compared with non-drinkers, beer and cider consumers, spirits wine consumers, and fortified wine consumers were not significantly associated with IBD risk regardless of the amount of alcohol.
We further analyzed the association of alcoholic beverages with IBD risk among those subjects who only consumed one alcoholic beverage (Table 3). Compared with subjects without IBD, those subjects with IBD were less likely to be red wine drinkers (p < 0.001) and white wine and champagne drinkers (p < 0.001). However, no significant differences were found in beer and cider drinkers (p = 0.1), spirits drinkers (p = 0.49), and fortified wine drinkers (p = 0.89) between subjects with and without IBD. In the final multivariate model, compared with non-drinkers, the risk of IBD was 24% lower in red wine consumers of 3–4 glasses/week (0.76 [0.59, 1.00]) and 23% lower in ≥5 glasses/week (0.77 [0.66, 0.91]). However, white wine and champagne drinkers were not significantly associated with the risk of IBD.
We further examined the association of different alcoholic beverages with IBD risk, separated by frequency of alcohol intake (Table 4), amount of alcohol consumption (Table 5), and overall health rating (Table 6), respectively. In the final multivariate model, sociodemographic factors, lifestyle factors, sleep phenotypes, comorbidities, frequency of alcohol consumption, amount of weekly intake level of alcohol consumption, and alcoholic beverages were adjusted.
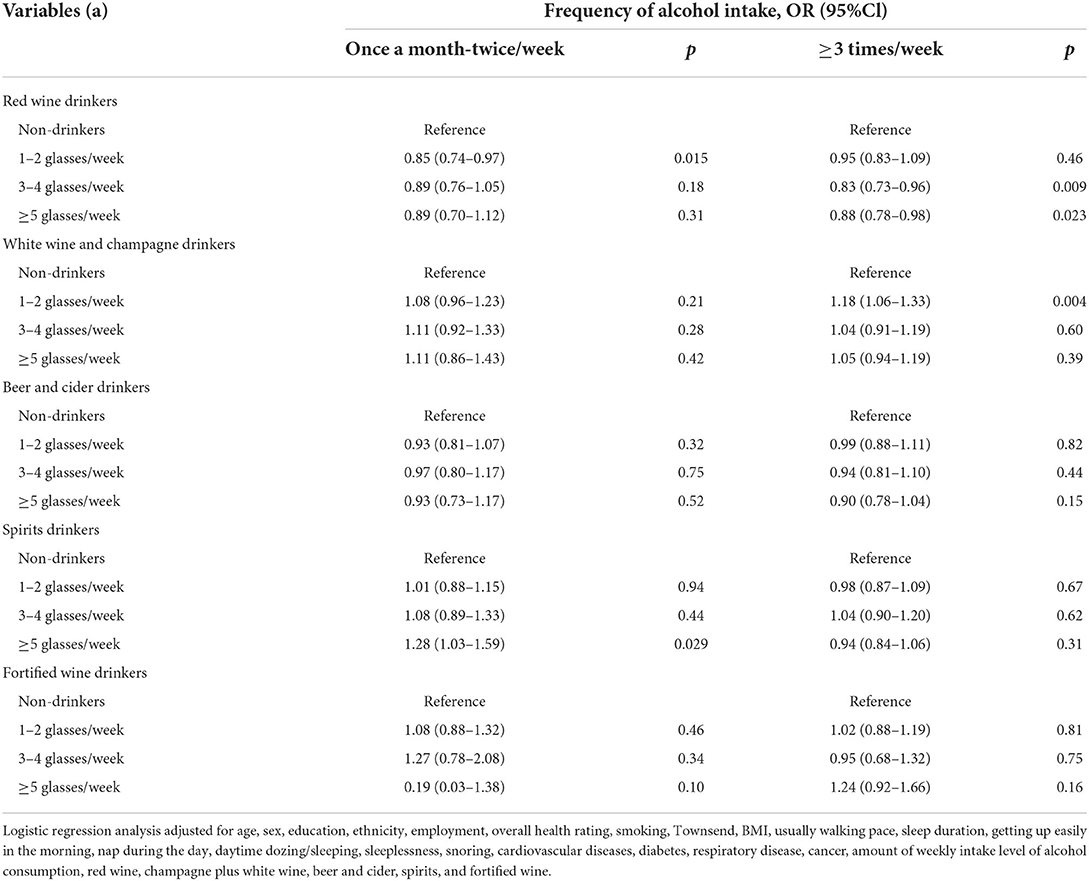
Table 4. Odds ratios and 95% CIs for the association between different alcoholic beverages and IBD risk, separated by frequency of alcohol intake.

Table 5. Odds ratios and 95% CIs for the association between different alcoholic beverages and IBD risk, separated by the amount of alcohol consumption.
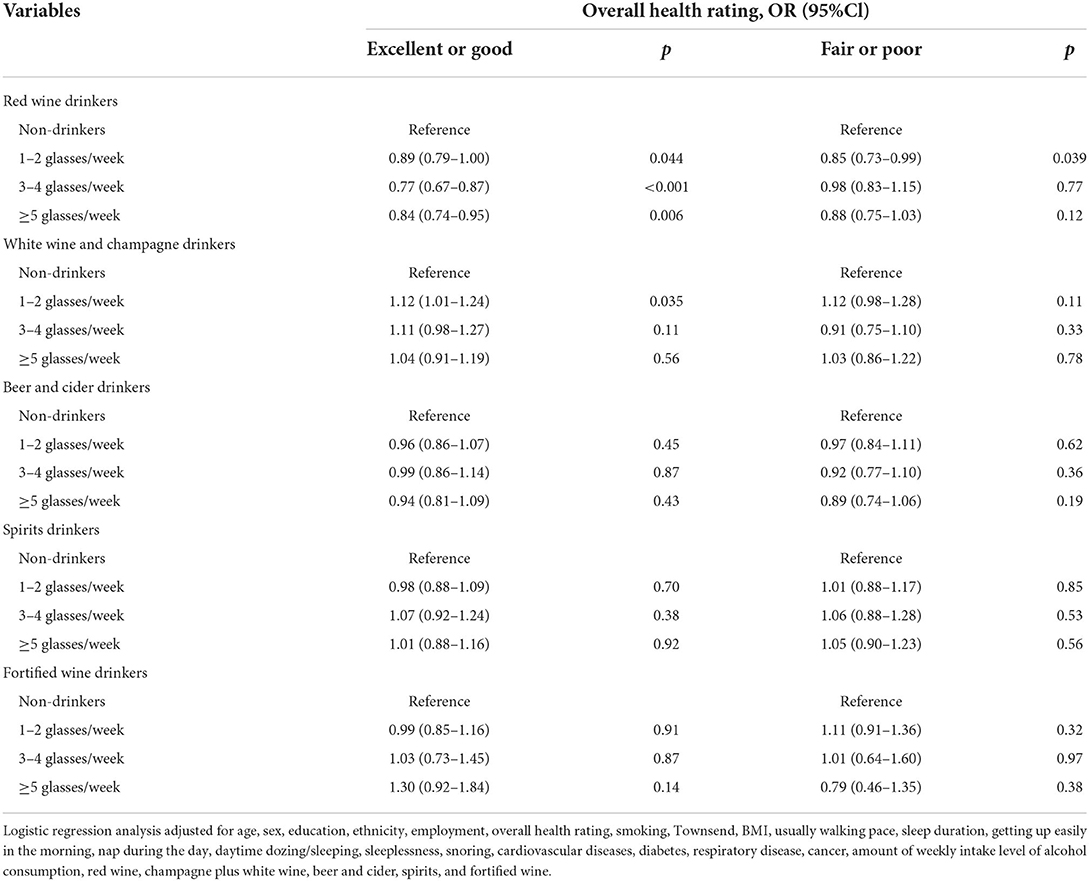
Table 6. Odds ratios and 95% CIs for the association between different alcoholic beverages and IBD risk, separated by overall health rating.
When stratifying our analysis by frequency of alcohol intake (Table 4), we found that, among the subjects who usually reported consumption of alcohol at a low frequency, consumption of red wine was associated with a lower risk of IBD (1–2 glasses/week, 0.85 [0.74, 0.97]), whereas consumption of spirits was associated with a higher risk of IBD (≥5 glasses/week, 1.28 [1.03, 1.59]). Among the subjects who usually reported consumption of alcohol at a high frequency, consumption of red wine was still associated with a lower risk of IBD (3–4 glasses/week, 0.83 [0.73, 0.96]; ≥5 glasses/week, 0.88 [0.78, 0.98]), whereas consumption of white wine and champagne was associated with a higher risk of IBD (1–2 glasses/week, 1.18 [1.06, 1.33]).
When stratifying our analysis by the amount of alcohol consumption (Table 5), we found that, among those subjects who usually reported consumption of alcohol within guidelines, all alcoholic beverages were not associated with IBD risk. Among those subjects who usually reported consumption of alcohol above guidelines, consumption of red wine was associated with a lower risk of IBD (3–4 glasses/week, 0.80 [0.67, 0.97]). Among those subjects who usually reported consumption of alcohol double above the guidelines, consumption of red wine was still associated with a lower risk of IBD (≥5 glasses/week, 0.83 [0.71, 0.97]), whereas consumption of white wine and champagne (1–2 glasses/week, 1.32 [1.09, 1.61]) and consumption of beer and cider (1–2 glasses/week,1.26 [1.02, 1.54]) were associated with a higher risk of IBD, respectively.
We also examined the association of different alcoholic beverages with IBD risk when stratifying our analysis by overall health rating (Table 6). Among those subjects with excellent or good overall health rating, consumption of red wine was associated with 11% to 23% lower risks of IBD (1–2 glasses/week, 0.89 [0.79, 1.00]; 3–4 glasses/week, 0.77 [0.67, 0.87]; ≥5 glasses/week, 0.84 [0.74, 0.95]) regardless of the amount of red wine, whereas consumption of white wine and champagne (1–2 glasses/week, 1.12 [1.01, 1.24]) was associated with a 12% higher risk of IBD. For those subjects with poor overall health rating, only consumption of 1–2 glasses/week of red wine was associated with a 15% lower risk of IBD (1–2 glasses/week, 0.85 [0.73, 0.99]), whereas the higher risk was not significant among white wine and champagne consumers. Other alcoholic beverages were not associated with IBD risk among those subjects with both good and poor overall health ratings. Therefore, our study suggests that the association between alcoholic beverages and the risk of IBD are not affected by overall health rating.
Tables 7–11 showed that there were significant interaction effects between different alcoholic beverages, overall health rating, and amount of alcohol consumption (alcoholic beverage × alcoholic beverage, alcoholic beverage × overall health rating, alcoholic beverage × amount of alcohol consumption) regarding the risk of IBD after adjusting for sociodemographic factors, lifestyle factors, sleep phenotypes, comorbidities, frequency of alcohol consumption, amount of alcohol consumption, and alcoholic beverages.
There is an approximate interaction effect between 1–2 glasses/week of red wine and 1–2 glasses/week of fortified wine (1.38 [1.00–1.92], p = 0.05). However, other interaction effects of red wine with overall health rating, amount of alcohol consumption, white wine and champagne, beer and cider, or spirits on IBD risk were not found (Table 7). Consumption of 1–2 glasses/week of white wine and champagne showed significant interaction effects with double above the guidelines of alcohol consumption (1.27 [1.03–1.58], p = 0.029) and more than five glasses/week of beer and cider (1.46 [1.20–1.79], p < 0.001) on a higher risk of IBD, respectively (Table 8). A similar interaction pattern was observed between 3–4 glasses/week of white wine and champagne and 3–4 glasses/week of beer and cider (1.45 [1.05–2.01], p = 0.024). Several interaction effects between beer and cider and poor health rating (3–4 glasses/week × poor health, p = 0.04; ≥5 glasses/week × poor health, p = 0.001) or spirits (1–2 glasses/week beer and cider ×3–4 glasses/week spirits, p = 0.026; 1–2 glasses/week × ≥5 glasses/week, p = 0.003; 3-4 glasses/week ×1–2 glasses/week, p = 0.023) on higher risks of IBD were observed (Table 9). There is an interaction effect between ≥5 glasses/week of spirits and 3–4 glasses/week of fortified wine (p = 0.032) (Table 10). However, this interaction pattern was not observed between fortified wine and overall health rating and between fortified wine and amount of alcohol consumption, respectively (Table 11).
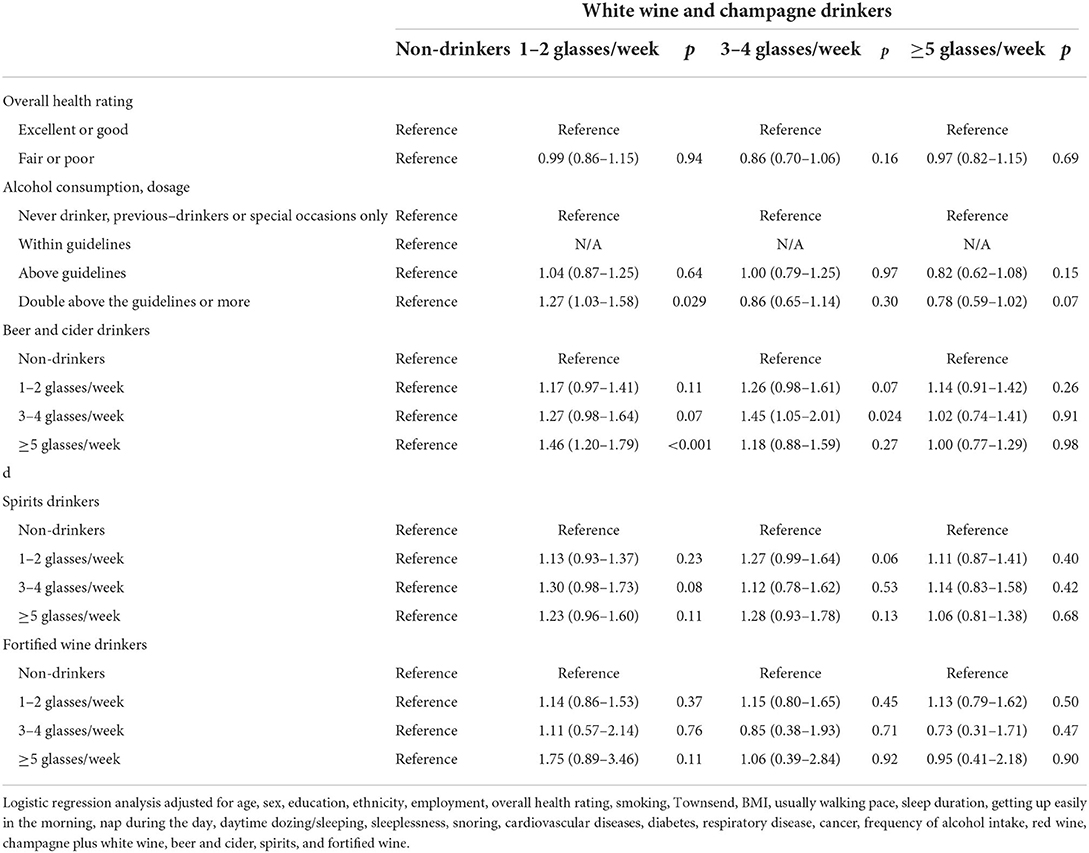
Table 8. Odds ratios and 95% CIs for the interaction effect between white wine and champagne and the risk of IBD.
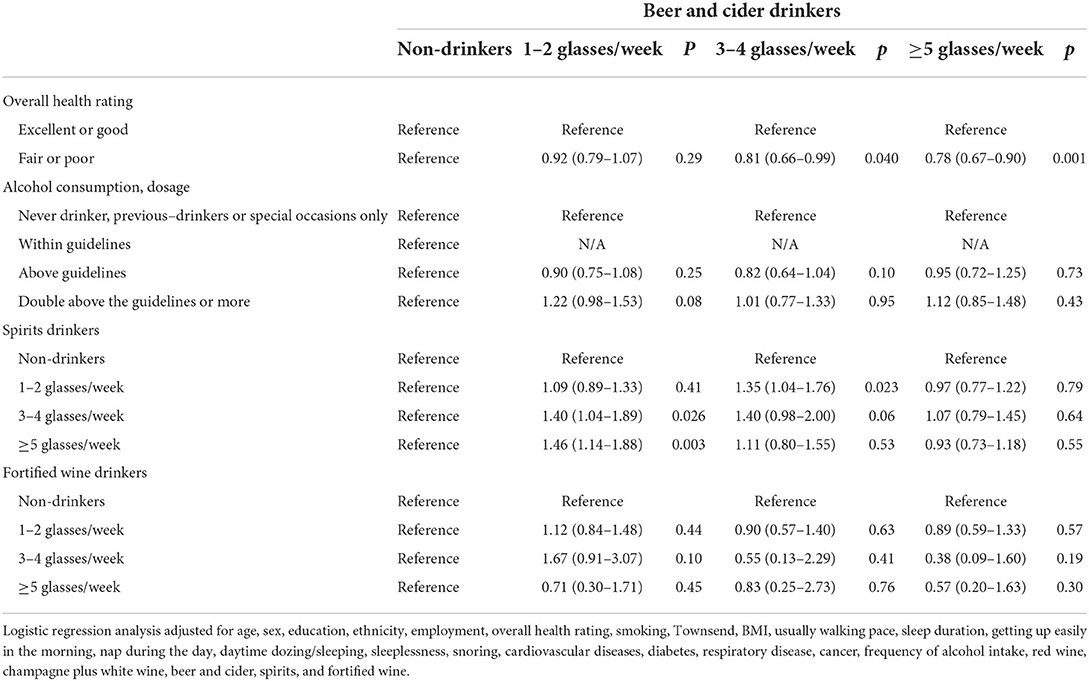
Table 9. Odds ratios and 95% CIs for the interaction effect between beer and cider and the risk of IBD.
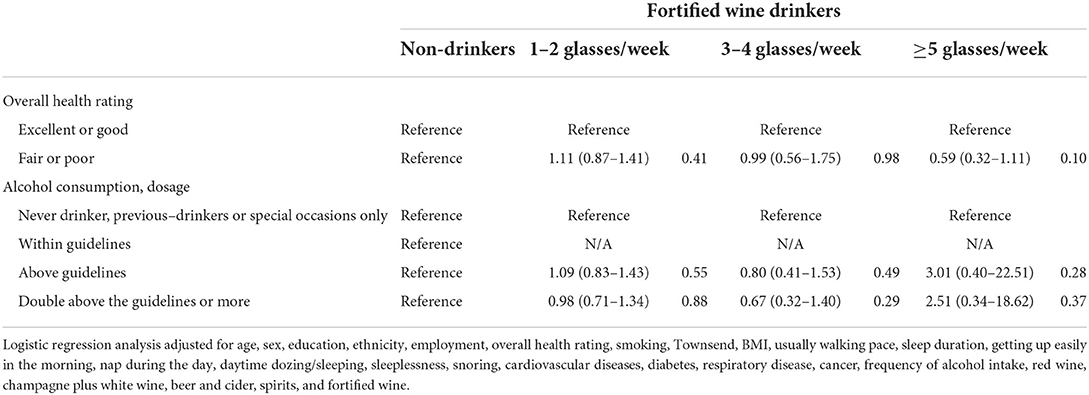
Table 11. Odds ratios and 95% CIs for the interaction effect between fortified wine and risk of IBD.
The dose-response associations between the amount of alcohol consumption and alcoholic beverages on IBD risk are shown in Figures 1A–F. The total amount of alcohol consumption (Figure 1A) and white wine and champagne (Figure 1C) showed a curvilinear J-shaped correlation with the risk of IBD, respectively. Red wine, beer and cider, spirits, and fortified wine showed a linear correlation with IBD risk, respectively (Figures 1B,D–F), where alcohol consumers had an increased risk with a greater number of consumptions of beer and cider, and the IBD risk was lower among red consumers across the whole dose cycle.
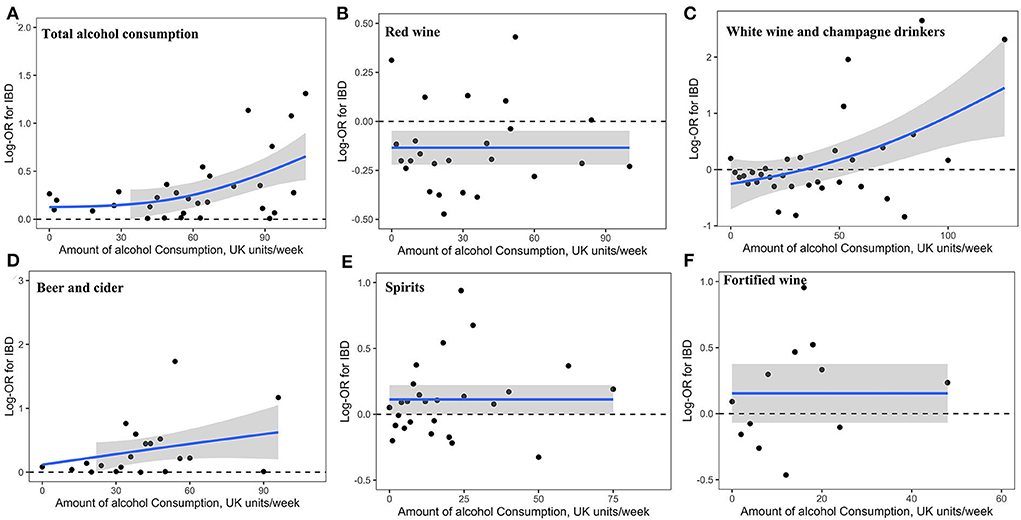
Figure 1. Association of alcohol consumption with risk of IBD. Linear and non-linear associations of the amount of total alcohol consumption (A) and that of alcoholic beverages (B, red wine; C white wine and champagne; D, beer and cider; E, spirits; F, fortified wine) with risk of IBD.
In this prospective study of a large-sized UK Biobank cohort, we documented four novel findings. First, red wine was identified as an independent protective factor for IBD. It played the protective effect on those subjects who consumed alcohol above or double above the guidelines and consumed alcohol both at high and low frequencies. Second, 1–2 glasses/week of white wine and champagne was identified as an independent risk factor for IBD, especially among those subjects who consumed alcohol double above the guidelines and who consumed alcohol at a high frequency. Third, consumption of ≥5 glasses/week of spirits at a low frequency and consumption of beer and cider double above the guidelines are associated with increased risk of IBD. Fourth, the dose-response associations showed an increased risk of IBD with more number of alcohol consumption. This association can be found in white wine and champagne, and beer and cider. However, red wine is still at a low risk across the whole dose cycle. Therefore, the IBD risk appears to vary across consumption of different subtypes, frequencies, and number of alcoholic beverages. Especially, our study suggests that the association between alcoholic beverages and the risk of IBD is not affected by overall health rating.
Our study found that alcohol intake was associated with a higher risk of IBD. The protective association of low-to-moderate alcohol consumption with lower risk of cataract (23), lower risk of myocardial infarction, (20) and better cognitive function (24) have been widely reported. Recent studies found that chronic alcoholism was not a risk or protective factor of IBD (25, 26). However, studies have shown that long-term alcohol abuse and acute binge drinking are associated with immunosuppression and increased susceptibility to both bacterial and viral infections (27, 28). The widely held view of the health benefits of alcohol needs revising, and Collaborators proposed that the safest level of drinking is none (6). Our results support this view. In our study, the total number of alcohol consumption showed a curvilinear J-shaped correlation with IBD risk, and the recommended intake of alcohol is also none. This level conflicts with most health guidelines, which espouse health benefits associated with low-to-moderate alcohol consumption. However, subgroup analyses for alcoholic beverages reported a new finding, which shows that red wine is safe to drink throughout the whole dose cycle as it was associated with a lower risk of IBD. Therefore, it's worth noting that, although our findings suggest that alcohol intake is not recommended because it was associated with a higher risk of IBD, there is no direct evidence that red wine is not recommended for IBD high-risk population.
The most striking and interesting finding was that consumption of red wine was associated with a lower chance of developing IBD. More and more research are trying to explore the potentially beneficial properties of wine or its components on IBD, and studies have shown that the beneficial properties of wine are attributed to their polyphenolic content, but are not independent of the presence of alcohol (29, 30). Polyphenols are present in varying degrees in different subtypes of alcoholic beverages, particularly in red wine which has the highest concentrations of phenolic compounds (31) because all grape parts are used during the winemaking process of red wine (32). Red wine provides additional benefits compared to other subtypes of alcoholic beverages probably due to its higher polyphenolic content, by decreasing blood pressure, improving endothelial function, reducing inflammation and cell adhesion, inhibiting the oxidation of low-density lipoprotein particles, and other favorable effects on the cellular redox state, inhibiting platelet aggregation, and activating proteins that prevent cell death (31). Polyphenols comprised several chemical compounds that are generally classified into flavonoids and non-flavonoids (33), which are considered the main bioactive components in wine that positively affect health (31, 34). The beneficial effect included antioxidant, anti-inflammatory, anti-cancer, and anti-microbial (35). As known, polyphenols could inhibit the effects of several types of viruses, including the Epstein–Barr virus (36, 37), herpes simplex virus (38), enterovirus (39, 40), COVID-19 (18), influenza virus (41), and other viruses causing respiratory tract-related infections (42, 43). Many of the phenolic compounds in wine have low bioavailability, and hence, reach low concentrations in the bloodstream, while their high content present in the gut can produce a more significant effect on enterocytes and the bacterial flora (44, 45). Therefore, these findings support the notion of the strong beneficial properties of red wine against developing IBD risk.
Our study suggests that consumption of some alcoholic beverages were associated with higher risks of developing IBD. We discovered risk factors of low frequency of ≥5 glasses/week spirit, high frequency and double above dose of the guidelines of 1–2 glasses/week of white wine and champagne, and double above dose of the guidelines of beer and cider for developing IBD. Sanja Radonjić et al. have reported the differences between wine and beer in the presence and the concentrations of phenolic substances (33). Furthermore, spirits had the highest alcohol concentration and the lowest polyphenolic concentration. These findings have shown the differences between these alcoholic beverages and red wine. Evidence have shown that red wine extract and wine digested fluids played a protective effect on the cellular barrier (46, 47) and led to increased intestinal permeability (48). However, this is in stark contrast to the evidence by Asai et al. that a low and acute dose of ethanol leads to apoptotic cell death in confluent Caco-2 cells and, therefore, impairs intestinal barrier function (49). Therefore, it is probable that the polyphenolic content per se has a positive effect on intestinal permeability, while the alcoholic content potentially negates this effect (17). These findings may suggest that the specific class of polyphenolic constituents may be responsible for the beneficial effect of alcoholic beverages on IBD, but not the alcohol concentration.
The major strengths of this study are the prospective and comprehensive study design, large UK population-based cohort, dose-response associations of alcohol consumption with IBD risk, and a focus on the association of different subtypes of alcoholic beverages with IBD risk. However, there are several limitations that should be addressed. First, subjects in the UK Biobank have a restricted age range, and therefore our data could not represent the whole population. Second, the data on alcohol drinking habits were derived from baseline, and we did not know about potential changes from baseline to outcome end-point. Third, recruiting heavy drinkers to test different alcoholic beverages for dose-response analyses is difficult. Fourth, although we adjusted for a wide range of potential confounders and did a series of rigorous statistical analyses, residual confounding factors may still exist, especially the interaction between different alcoholic beverages. Fifth, a relatively small number of IBD cases that only consumed one alcoholic beverage were included, which may reduce its reliability. Sixth, past trauma or stressful events may be a cause of alcohol intake, future studies should adjust for the effect of past trauma or stressful events.
In conclusion, our study suggests that the IBD risk appears to vary across different frequencies, amounts, and subtypes of alcoholic beverages. Overall, alcohol intake is not recommended because it was associated with a higher risk of IBD, and the safe level of drinking appears to be none. A focus on the association of different frequencies, amounts, and subtypes of alcoholic beverages with the risk of IBD provided an important addition to the existing research on alcohol intake and IBD risk. We found that consumption of red wine may reduce the risk of IBD, while high frequency and high dose of white wine and champagne, low frequency and acute dose of spirit, and high dose of beer and cider appear to increase the risk of IBD. Public health guidance should focus on reducing the risk of IBD by advocating healthy lifestyle habits and preferential policies among consumers.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethical approval of the UK Biobank was obtained from the National Health System Northwest Multicenter Research Ethics Committee (REC reference: 16/NW/0274). The patients/participants provided their written informed consent to participate in this study.
B-XL, CZ, X-JD, and YC had the idea for and designed this study. X-JD and B-XL had full access to all the data in this study, take responsibility for the integrity of the data and the accuracy of the data analysis, and drafted the paper. CZ, X-JD, and YC critically revised the manuscript for important intellectual content and gave final approval for the version to be published. JY, CZ, and YC take responsibility for double check of the data analysis. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82060448) and Sanming Project of Medicine in Shenzhen (Grant No. SZSM201812052). This research has been conducted using the UK Biobank Resource under Application Number 75732 and Basket 2013245.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.918754/full#supplementary-material
1. Cannon AR, Kuprys PV, Cobb AN, Ding X, Kothari AN, Kuo PC, et al. Alcohol enhances symptoms and propensity for infection in inflammatory bowel disease patients and a murine model of DSS-induced colitis. J Leukoc Biol. (2018) 104:543–55. doi: 10.1002/JLB.4MA1217-506R
2. Abubakar II, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2015) 385:117–71. doi: 10.1016/S0140-6736(14)61682-2
3. Vindigni SM, Zisman TL, Suskind DL. Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therap Adv Gastroenterol. (2016) 9:606–25. doi: 10.1177/1756283X16644242
4. Abegunde AT, Muhammad BH, Bhatti O. Ali T. Environmental risk factors for inflammatory bowel diseases: evidence based literature review World J Gastroenterol. (2016) 22:6296–317. doi: 10.3748/wjg.v22.i27.6296
5. White BA, Ramos GP, Kane S. The Impact of Alcohol in Inflammatory Bowel Diseases. Inflamm Bowel Dis. (2022) 28:466-473. doi: 10.1093/ibd/izab089
6. Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2018) 392:1015–35.
7. Swanson GR, Sedghi S, Farhadi A. Keshavarzian A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol. (2010) 44:223–8. doi: 10.1016/j.alcohol.2009.10.019
8. Miller N, Bernstein CN, Vagianos K, Clara I, Carr R, Graff LA, et al. What Are Adults With Inflammatory Bowel Disease (IBD) Eating? A closer look at the dietary habits of a population-based Canadian IBD cohort. JPEN J Parenter Enteral. (2014) 40:405. doi: 10.1177/0148607114549254
9. Niccum B, Casey K, Burke K, Lopes EW, Lochhead P, Ananthakrishnan A, et al. Alcohol consumption is associated with an increased risk of microscopic colitis: results from 2 prospective US cohort studies. Inflamm Bowel Dis. (2021) 2:1–9. doi: 10.1093/ibd/izab220
10. Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. (2004) 53:1479–84. doi: 10.1136/gut.2003.024828
11. Mantzouranis G, Fafliora E, Saridi M, Tatsioni A, Glanztounis G, Albani E. et al. Alcohol and narcotics use in inflammatory bowel disease. Ann Gastroenterol. (2018) 31:649–58. doi: 10.20524/aog.2018.0302
12. Keshavarzian A, Fields JZ, Vaeth J. Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol. (1994) 89:2205–11.
13. Piovezani Ramos G. Kane S. Alcohol use in patients with inflammatory bowel disease. J Gastroenterol Hepatol. (2021) 17:211–25.
14. Frank DN, Amand A, Feldman RA, Boedeker EC, Harpaz N. Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. In: Proceedings of the National Academy of Sciences. (2007) 104:13780–5. doi: 10.1073/pnas.0706625104
15. Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest. (2012) 302:966–78. doi: 10.1152/ajpgi.00380.2011
16. Jiang X, Zhu Z, Manouchehrinia A, Olsson T, Alfredsson L. Kockum I. Alcohol consumption and risk of common autoimmune inflammatory diseases-evidence from a large-scale genetic analysis totaling 1 million individuals. Front Genet. (2021) 12:687745. doi: 10.3389/fgene.2021.687745
17. Vrdoljak J, Kumric M, Ticinovic Kurir T, Males I, Martinovic D, Vilovic M, et al. Effects of wine components in inflammatory bowel diseases. Molecules. (2021) 28:26. doi: 10.3390/molecules26195891
18. Dai XJ, Tan L, Ren L, Shao Y, Tao W. Wang Y. COVID-19 risk appears to vary across different alcoholic beverages. Front Nutr. (2022) 8:772700. doi: 10.3389/fnut.2021.772700
19. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ. Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. (2011) 342:d671. doi: 10.1136/bmj.d671
20. Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA Jr, Stampfer MJ, Willett WC, et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. (2003) 348:109–18. doi: 10.1056/NEJMoa022095
21. Dai XJ, Shao Y, Ren L, Tao W. Wang Y. Risk factors of COVID-19 in subjects with and without mental disorders. J Affect Disord. (2022) 297:102–11. doi: 10.1016/j.jad.2021.10.024
22. Chikritzhs TN, Naimi TS, Stockwell TR. Liang W. Mendelian randomisation meta-analysis sheds doubt on protective associations between ‘moderate' alcohol consumption and coronary heart disease. Evid Based Med. (2015) 20:38. doi: 10.1136/ebmed-2014-110086
23. Chua SYL, Luben RN, Hayat S, Broadway DC, Khaw K-T, Warwick A, et al. Alcohol consumption and incident cataract surgery in two large UK cohorts. Ophthalmology. (2021) 128:837–47. doi: 10.1016/j.ophtha.2021.02.007
24. Zhang R, Shen L, Miles T, Shen Y, Cordero J, Qi Y, et al. Association of Low to Moderate Alcohol Drinking With Cognitive Functions From Middle to Older Age Among US Adults. JAMA Netw Open. (2020) 3:e207922–e207922. doi: 10.1001/jamanetworkopen.2020.7922
25. Amarapurkar AD, Amarapurkar DN, Rathi P, Sawant P, Patel N, Kamani P, et al. Risk factors for inflammatory bowel disease: a prospective multi-center study Indian. J Gastroenterol. (2018) 37:189–95. doi: 10.1007/s12664-018-0850-0
26. Casey K, Lopes EW, Niccum B, Burke K, Ananthakrishnan AN, Lochhead P, et al. Alcohol consumption and risk of inflammatory bowel disease among three prospective US cohorts. Aliment Pharmacol Ther. (2022) 55:225–33. doi: 10.1111/apt.16731
27. Barr T, Helms C, Grant K. Messaoudi I. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 65:242–51. doi: 10.1016/j.pnpbp.2015.09.001
28. Sarkar D, Jung MK. Wang HJ. Alcohol and the immune system. Alcohol Res Curr Rev. (2015) 37:153–5.
29. Ruf JC. Overview of epidemiological studies on wine, health and mortality. Drugs Exp Clin Res. (2003) 29:173–9.
30. Estruch R. Lamuela-Raventos RM. Alcohol, wine and cardiovascular disease, two sides of the same coin. Intern Emerg Med. (2010) 5:277–9. doi: 10.1007/s11739-010-0391-8
31. Arranz S, Chiva-Blanch G, Valderas-Martinez P, Medina-Remon A, Lamuela-Raventos RM, Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. (2012) 4:759–81. doi: 10.3390/nu4070759
32. Davis C, Bryan J, Hodgson J. Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients. (2015) 7:9139–53. doi: 10.3390/nu7115459
33. Radonjic S, Maras V, Raicevic J, Kosmerl T. Wine or Beer? Comparison, changes and improvement of polyphenolic compounds during technological phases. Molecules. (2020) 27:25. doi: 10.3390/molecules25214960
34. Gutierrez-Escobar R, Aliano-Gonzalez MJ. Cantos-Villar E. Wine polyphenol content and its influence on wine quality and properties: a review. Molecules. (2021) 30:26. doi: 10.3390/molecules26030718
35. Latruffe N. Rifler JP. Special issue: wine and vine components and health. Diseases. (2019) 19:7. doi: 10.3390/diseases7010030
36. Yiu C-Y, Chen S-Y, Chang L-K, Chiu Y-F. Lin T-P. Inhibitory effects of resveratrol on the Epstein-Barr virus lytic cycle. Molecules. (2010) 15:7115–24. doi: 10.3390/molecules15107115
37. De Leo A, Arena G, Lacanna E, Oliviero G, Colavita F. Mattia E. Resveratrol inhibits Epstein Barr Virus lytic cycle in Burkitt's lymphoma cells by affecting multiple molecular targets. Antiviral Res. (2012) 96:196–202. doi: 10.1016/j.antiviral.2012.09.003
38. Faith SA, Sweet TJ, Bailey E, Booth T. Docherty JJ. Resveratrol suppresses nuclear factor-κB in herpes simplex virus infected cells. Antiviral Res. (2006) 72:242–51. doi: 10.1016/j.antiviral.2006.06.011
39. Zhang L, Li Y, Gu Z, Wang Y, Shi M, Ji Y, et al. Resveratrol inhibits enterovirus 71 replication and pro-inflammatory cytokine secretion in rhabdosarcoma cells through blocking IKKs/NF-κB signaling pathway. PloS ONE. (2015) 10:e0116879. doi: 10.1371/journal.pone.0116879
40. Annunziata G, Maisto M, Schisano C, Ciampaglia R, Narciso V, Tenore GC, et al. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: an overview. Viruses. (2018) 10:473. doi: 10.3390/v10090473
41. Lin C-j, Lin H-J, Chen T-H, Hsu Y-A, Liu C-S, Hwang G-Y, et al. Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PLoS One. 10:e0117602. doi: 10.1371/journal.pone.0117602
42. Zang N, Xie X, Deng Y, Wu S, Wang L, Peng C, et al. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J virol. (2011) 85:13061–8. doi: 10.1128/JVI.05869-11
43. Liu T, Zang N, Zhou N, Li W, Xie X, Deng Y, et al. Resveratrol inhibits the TRIF-dependent pathway by upregulating sterile alpha and armadillo motif protein, contributing to anti-inflammatory effects after respiratory syncytial virus infection. J Virol. (2014) 88:4229–36. doi: 10.1128/JVI.03637-13
44. Biasi F, Deiana M, Guina T, Gamba P, Leonarduzzi G. Poli G. Wine consumption and intestinal redox homeostasis. Redox Biol. (2014) 2:795–802. doi: 10.1016/j.redox.2014.06.008
45. Giovinazzo G. Grieco F. Functional properties of grape and wine polyphenols. Plant Foods Hum Nutr. (2015) 70:454–62. doi: 10.1007/s11130-015-0518-1
46. Nunes C, Freitas V, Almeida L. Laranjinha J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: implications for intestinal inflammation. Food Funct. (2019) 10:1364–74. doi: 10.1039/C8FO02469C
47. Zorraquín-Peña I, Taladrid D, Tamargo A, Silva M. Moreno-Arribas MV. Effects of wine and its microbial-derived metabolites on intestinal permeability using simulated gastrointestinal digestion/colonic fermentation and Caco-2 intestinal cell models. Microorganisms. (2021) 9:1378. doi: 10.3390/microorganisms9071378
48. Swanson GR, Tieu V, Shaikh M, Forsyth C, Keshavarzian A. Is moderate red wine consumption safe in inactive inflammatory bowel disease? Digestion. (2011) 84:238–44. doi: 10.1159/000329403
Keywords: inflammatory bowel disease, Crohn's disease, ulcerative colitis, alcohol consumption, prospective cohort, risk factor, UK Biobank, alcoholic beverage
Citation: Liu B, Yang J, Zeng C, Dai X and Chen Y (2022) Risk of inflammatory bowel disease appears to vary across different frequency, amount, and subtype of alcoholic beverages. Front. Nutr. 9:918754. doi: 10.3389/fnut.2022.918754
Received: 12 April 2022; Accepted: 24 June 2022;
Published: 27 July 2022.
Edited by:
Rafaela Rosário, University of Minho, PortugalReviewed by:
Imma Pagano, University of Salerno, ItalyCopyright © 2022 Liu, Yang, Zeng, Dai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan Zeng, emN5ODk2QDE2My5jb20=; Xi-Jian Dai, ZGFpeGpkb2N0b3JAMTI2LmNvbQ==; Youxiang Chen, Y2hlbnl4MTAyQG5jdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.