- State Key Laboratory of Natural Medicines, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing, China
Chinese herbal polysaccharides (CHPs) are natural polymers composed of monosaccharides, which are widely found in Chinese herbs and work as one of the important active ingredients. Its biological activity is attributed to its complex chemical structure with diverse spatial conformations. However, the structural elucidation is the foundation but a bottleneck problem because the majority of CHPs are heteropolysaccharides with more complex structures. Similarly, the studies on the relationship between structure and function of CHPs are even more scarce. Therefore, this review summarizes the structure-activity relationship of CHPs. Meanwhile, we reviewed the structural elucidation strategies and some new progress especially in the advanced structural analysis methods. The characteristics and applicable scopes of various methods are compared to provide reference for selecting the most efficient method and developing new hyphenated techniques. Additionally, the principle structural modification methods of CHPs and their effects on activity are summarized. The shortcomings, potential breakthroughs, and developing directions of the study of CHPs are discussed. We hope to provide a reference for further research and promote the application of CHPs.
Introduction
The polysaccharides derived from Chinese herbs are mostly heteropolysaccharides which consist of different kinds of monosaccharides. Modern pharmacological studies reported that CHPs had functions such as anti-tumor (1), immunologic enhancement (2), intestinal microenvironment regulation (3), and anti-oxidation (4). As a drug, it is related to the occurrence and treatment of a variety of diseases and is favored on clinical settings. CHP preparations such as Astragalus polysaccharide injection, Ginseng polysaccharide injection, and Poria cocos polysaccharide oral liquid have been widely applied for clinical use.
Previous research focused on small molecules in Chinese herbs, while ignoring the research on macromolecular polysaccharides, resulting in a waste of resources. The complexity and instability of polysaccharides' structure make the process of revealing its mechanism and the development of products or drugs more complicated. As a result, it will impede the development of new polysaccharide drugs and reduce the application scope of polysaccharides. To play the better role of CHPs, the structure-activity relationship of polysaccharides must be clarified.
The primary structure and chain conformation of polysaccharides are closely related to the construction of various functions. However, due to the intricate structure and imperfection of technical support, current methods cannot fully clarify the exact structure of CHPs, especially the three-dimensional structure. There are fewer reports on the structure-activity relationship. In addition, some studies have shown that not all polysaccharides can express their ideal bio-activities (5). Nevertheless, the above problem is effectively solved by modifying its structure. Biological activity of polysaccharides after modification can be enhanced greatly and even new biological activity can be produced (6, 7). Structural modification can improve the physicochemical properties and activities of polysaccharides, which promotes the exploration of the structure-activity relationship of polysaccharides. Therefore, we summarized the structural elucidation, modification, and structure-activity relationship of CHPs, so as to provide theoretical reference and technical support for the development and utilization of CHPs.
Structure-Activity Relationship
Various polysaccharides show certain chemical structures. Wherein their biological activities depend on events that occur at the molecular structure level (8). Therefore, exploring the structure-activity relationship of polysaccharides in CHPs is of great significance for the development of new-carbohydrate drugs or pharmaceutical excipients. For example, different structural parts of Marine algae polysaccharides can directly or indirectly interact with the immune system and trigger several signal pathways, which lead to immune system activation (Figure 1) (9).
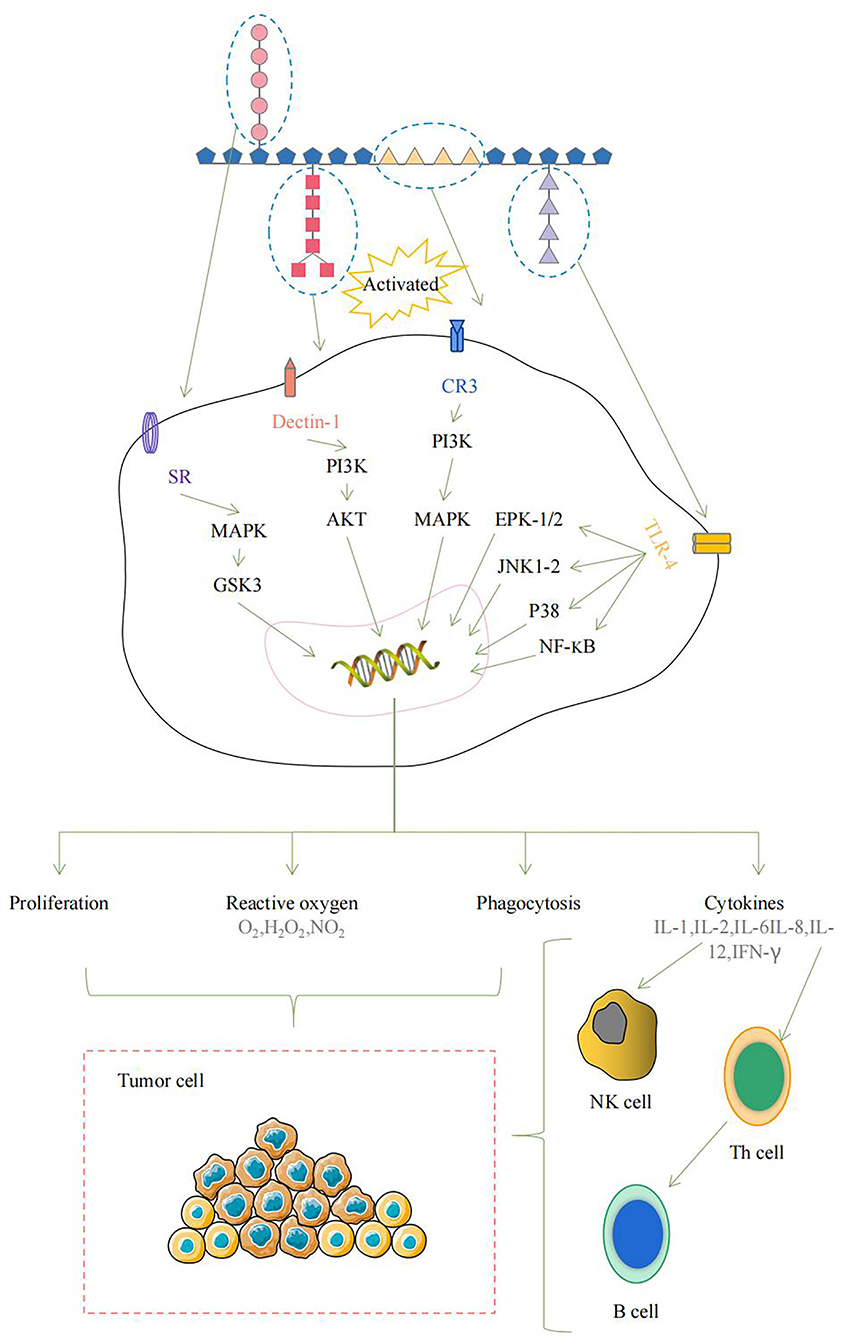
Figure 1. The schematic diagram of the immune system activated by Marine algae polysaccharides after interaction of several molecular events.
Relationship Between Molecular Weight and Activity
The molecular weight of polysaccharides only represents the average distribution within a certain range of relative molecular mass, which is mainly expressed by the mass average molar mass (Mw) and number-average molecular weight (Mn). Polydispersity (α) is generally used to describe the molecular weight distribution of polysaccharides, that is, α = Mw/Mn.
Firstly, molecular weight is an important feature affecting the therapeutic action of polysaccharides. The functional feature of molecular weight is related to amounts of active groups, and molecular weight indirectly affects the physicochemical properties such as solubility and viscosity, thereby affecting the absorption of polysaccharides in vivo. Secondly, the higher or lower molecular weight might reduce the activity of CHPs. It should be noted that a high molecular weight of polysaccharide normally has a large excluded volume that promotes intermolecular interaction of polysaccharide and impedes its uptake. On the contrary, if the relative molecular weight is too low, polysaccharides cannot form active-polymer structures such as triple-helical conformation (10). For example, the polysaccharide with moderate molecular weight from Dendrobium officinale showed the strongest inhibitory effect on the cells (11). Polysaccharides with less than 5 kDa or more than 400 kDa showed marginal immunomodulatory activity or lost the activity directly (12). Therefore, the dimension of molecular weight is closely related to biological activities in polysaccharides including antioxidation, lipid-lowering, antiviral, and so on.
Thirdly, the molecular weight shows a certain tendency of efficacy in an appropriate range. The low molecular weight of polysaccharides can play better exerting their antioxidant activity with containing more free hydroxyl groups to accept and eliminate more hydrogen radicals (13). It was found that a polysaccharide with the lower molecular weight obtained from corn whiskers showed the stronger antioxidant activity (14). It is worth noting that CHPs with intestinal barrier protection have higher molecular weight (15), which may be because high molecular weight polysaccharides can maintain the integrity of intestinal barrier structure by forming something similar to the sticky gel (16).
Taken together, polysaccharides in CHPs perform the best only on specific biological activities within the optimal range of relative molecular weight.
Relationship Between Monosaccharide Composition and Activity
The monosaccharide composition is divided into the type and proportion of monosaccharide, which is closely associated with biological activity. As an illustration, Angelica sinensis polysaccharides with radio-protective activity tended to be richer in galacturonic acid, galactose, and arabinose (17). It is generally considered that the monosaccharide composition with more complex shows the better biological activity (18). Two kinds of polysaccharides, RLP-1 and RLP-2, from Rosa Laevigata Fructus, had different monosaccharide compositions and activities. RLP-1 consisted of xylose, mannose, and galactose reducing hyperlipidemia of model rats, while RLP-2 only contained glucose having no such activity (19). Some studies demonstrated the monosaccharide composition in polysaccharide with intestinal barrier protection function is galactose, mannose, arabinose, xylose, and rhamnose (16, 20). In addition, the high content of uronic acid showed good antioxidant activity of polysaccharide (21). The mechanism may be the breakage of uronic acid chain caused by free radicals (22). Similarly, polysaccharides with amounts of uronic acid were beneficial to its hepatoprotective activity (18). In addition, the polysaccharides containing mannose and rhamnose were proved to have tumor-inhibitory and antioxidant activities, individually (23, 24). However, the specific rules and mechanisms have not been clearly set forth and need further exploration.
Relationship Between Glycosidic-Bond Type and Activity
The flexible connection between monosaccharides causes the complex structure of polysaccharides. The glycosidic bond is divided into α-type or β-type because the configuration of the glycosidic bond is determined by the configuration of the hemiacetal (ketone) hydroxyl group. It is generally considered that polysaccharides with β configuration have the higher activity, while most of polysaccharides with α configurations have no biological activity (25, 26). This is due to the existence of α-glucoamylase in the human body, which can hydrolyze α-glycosidic bonds under certain conditions. However, with the depth of research, it was found that α-glucan as vaccine adjuvants had good biocompatibility and biodegradability to maintain the homeostasis of the intestinal environment (27, 28). In addition, α-(1 → 4)-GalpA and α-(1 → 4)-Galp in the main chain of Ginseng polysaccharides were essential to biological activities such as anti-tumor (29).
The main glycosidic bond types of polysaccharides with different activities are also different. Most of the glucans with anti-tumor are mainly composed of the β- (1 → 3)-D-glucan as the main chain, with the β-(1 → 6)-D-glucan randomly as the branched chain (30), while the antitumor effect of glucans composed of the β-(1 → 6)-D-glucan as the main chain is much weaker. Some CHPs regulating intestinal flora activity are mostly connected by (1 → 3) glycosidic bond (31). Additionally, CHPs with hypoglycemic effect mostly have (1 → 3), (1 → 4), (1 → 6) glycosidic bonds (32–34).
Relationship Between Branching Degree and Activity
The degree of branching (DB) of CHPs influences the biological activity by affecting the molecular weight and conformation (35). Generally speaking, the higher the complexity of the branch, the stronger the activity of CHPs (36, 37). Three kinds of polysaccharides with different DBs were obtained from Ganoderma atrum. Their antioxidant activity and anti-tumor cell proliferation were positively correlated with DB (38). This may be related to the effect of DB on the binding ability of polysaccharides to specific receptors (39, 40). In recent years, the hyperbranched polysaccharides with highly branched structure have received extensive attention due to their diverse biological activities and applications, which may be because the hyperbranched structure has good water solubility, low viscosity, and high chain end density (41). A hyperbranched polysaccharide from Cordyceps sinensis with DB of 43% could stimulate macrophage function, which was thought to attribute to its hyperbranched structure (42). However, it was found that polysaccharide from ginseng with small branching degrees showed better immune-enhancing activity (43). Accordingly, the activity of CHPs is related to DB, and there may be an optimal DB value (44). This may be because too high DB value would lead to the decrease of water solubility, while the DB value is too low, resulting in fewer binding sites (45).
Relationship Between Chain Conformation and Activity
Compared with the primary structure, advanced structure of polysaccharides is more likely to affect their functions (46, 47). Three-dimensional network structures of polysaccharide molecules are easily formed via van der Waals forces, hydrogen bonds, and covalent bonds. Moreover, the inter-chain association of structures is common because of the large degree of freedom and flexibility. Polysaccharides have multiple conformational forms in solutions, including single random coil (48, 49), helix (50), double helix (51), triple helix (52), rod-like structures (53), worm-like (54), semiflexible chains (55), and sphere-like structures (56). At present, the triple-helical structure of polysaccharides is one of the most studied chain conformations with some specific biological activities, especially anti-tumor (57). If triple-helical structure is destroyed, the anti-tumor activity would be decreased. The lentinan had a good antitumor activity with triple-helical stereo-configuration, but when the stereo-configuration was destroyed, the anti-tumor activity disappeared as consequence (58). The antiviral activity could be affected by the change of triple-helical structure of pollen polysaccharides in Pinus massoniana (59), which might be related to the chain rigidity and exposure sites of the triple-helical structure. Furthermore, the polysaccharide chain with triple-helical conformation is more rigid and easier to be recognized by the receptor (60). When the polysaccharide with triple-helical structure is linearly distributed, more exposure sites were utilized to strengthen the activity. The research substantiated that the polysaccharide having extended linear conformation showed the better good hypolipidemic activity on four sulfated polysaccharides in sea cucumber (61).
At present, there are few studies about the relationship between the chain conformation and biological activity in polysaccharides, which is one of the key breakthroughs for the advancement of polysaccharides in CHPs.
Primary Structure Identification
Determination of Molecular Weight
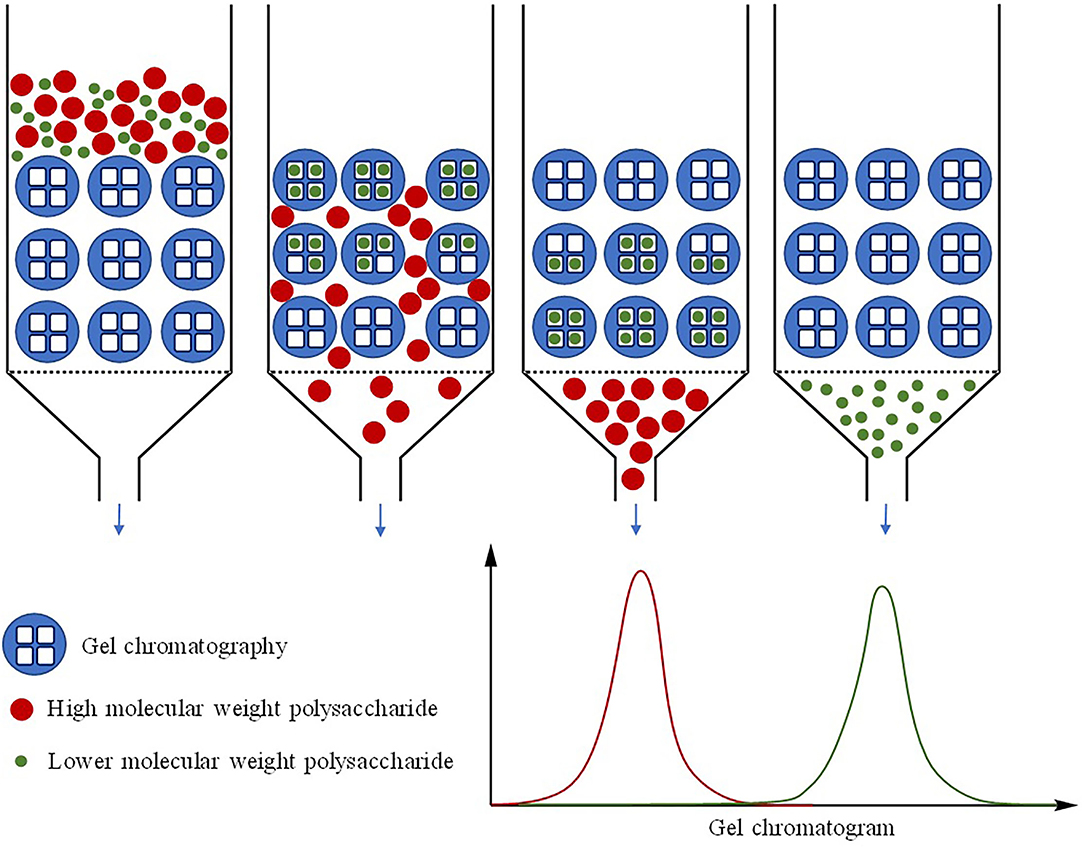
In recent years, gel permeation chromatography (GPC) has been widely used in laboratories to calculate the molecular weight of the polysaccharide as well as detect the homogeneity of the polysaccharide (Figure 2) (62, 63). Most of the current studies employ high-performance GPC (HPGPC) or high-performance exclusion chromatography (HPSEC) (64), which is based on the characteristic that polysaccharide with certain molecular weight has a corelated elution retention time (tR) on a gel column. Polysaccharides with known molecular weight are used to make a standard curve, and then the molecular weight of the sample can be calculated according to the tR and the standard curve. In this method, if a single and symmetrical peak appears, the component is usually considered to be a homogeneous polysaccharide (65). Differential refraction index detector (RID) in HPGPC was applied to determine the molecular weight of polysaccharides. As long as the refractive index of the detected compound is different from the liquid solvent system, it can be detected. The universal detector evaporative light scattering detector (ELSD) can also be used to determine the molecular weight of polysaccharides (66).
In addition, some hyphenated techniques have been developed to determine the molecular weight of polysaccharides, such as size exclusion chromatography (SEC)/GPC combined with a multi-angle laser light scattering (MALLS) detector (67, 68). A small-angle laser scattering device can be used to determine the molecular weight of macromolecular compounds quickly and accurately, without extrapolating the angle and concentration. The comparison of popular methods on determination molecular weight is shown in Table 1.
Determination of Monosaccharide Composition
The combination of gas chromatography (GC) and mass spectrometry (MS) or flame ionization detection (FID) is usually used as a conventional analysis method for monosaccharide composition (72, 73). The first step in determining the composition of monosaccharides is to degrade polysaccharides into monosaccharides. The acid hydrolysis method is the most widely used, and its process varies depending on the type of polysaccharide. Next, due to the lack of volatility of carbohydrates, it is necessary to use sugar alcohol acetate, sugar acetonitrile acetate derivatives, methyl ether, trimethylsilyl oxime, trimethylsilyl ether, and other reagents to derivatize the hydrolysate, making it suitable for GC.
High performance liquid chromatography (HPLC) with ultraviolet detector (UVD) is becoming more commonly used for quantifying monosaccharides (74). Separation rate of monosaccharides can be accelerated accordingly in reversed-phase liquid chromatography with stationary phase of C18 (75). Polysaccharides are not retained on the C18 chromatographic column and do not contain chromophoric groups. Monosaccharides need to be derivatized so that the separation efficiency of chromatography and sensitivity of UVD can be improved to observable level. 1-phenyl-3-methyl-5-pyrazolone (PMP) is usually used as a probe molecule for its characteristic of quantitative reaction with carbohydrates under mild conditions (76). Besides, the monosaccharide composition of polysaccharides can be determined without derivatization by using sugar analysis column or amino column and equipped with RID or ELSD or pulsed amperometric detector (PAD).
High performance anion exchange chromatography-pulsed amperometric detector (HPAEC-PAD) is a technology used to analyze polysaccharide currently (77–79), as it can analyze the hydrolytic products without sample derivation (80). The mechanism of HPAEC-PAD method is to use the dissociation of mono or oligosaccharides by elution in medium with pH above 12. Then the anions are fully exchanged on an agglomerated shell type anion exchange resin column. The separated components go into PAD for detection. The composition of monosaccharides can be determined referring to standard samples (81).
Capillary electrophoresis can also be used to determine the monosaccharide composition of CHPs. Samples are labeled by reagents with acidic groups, and then assayed by high performance capillary electrophoresis (HPCE) and detected by laser induced fluorescence detector (LIFD) (82). The advantages and disadvantages of various methods are listed in Table 2. The general process of determining the composition of monosaccharides is shown in Figure 3.
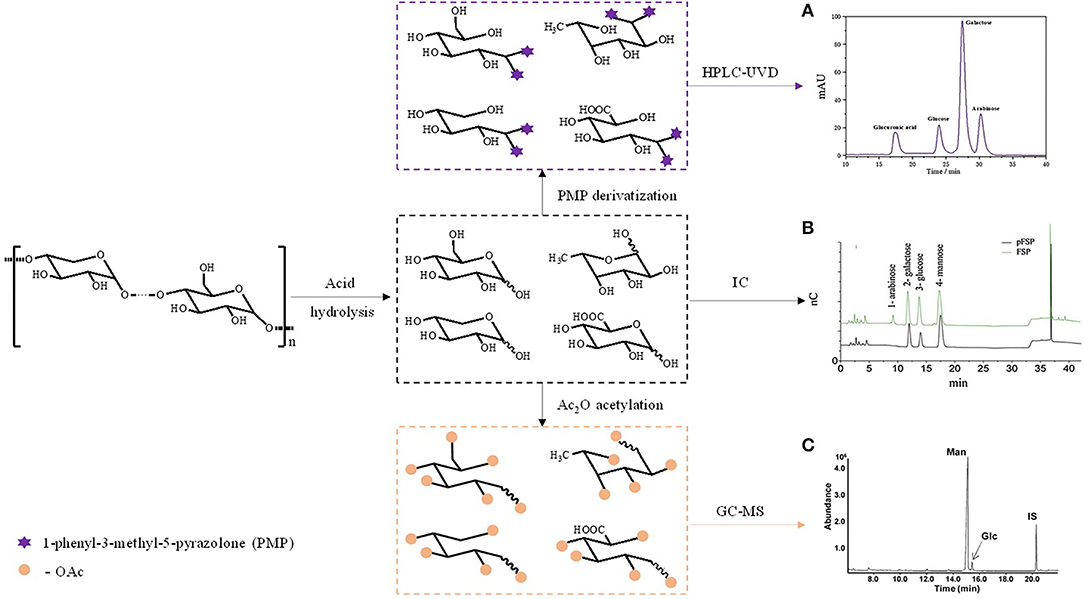
Figure 3. The general process of determining the composition of monosaccharides. (A) Monosaccharide composition of Angelica sinensis polysaccharide by HPLC-UVD; (B) Monosaccharide composition of polysaccharide from the fibrous roots and tuber of Bletilla striata by IC; (C) Monosaccharide composition of polysaccharide from Dendrobium devonianum by GC-MS.
Glycosidic Bonds Identification
Chemical reaction is an important step in glycosidic bond identification methods, including acid hydrolysis, periodic acid oxidation, Smith cleavage, and methylation reactions (Figure 4). Partial acid hydrolysis is currently the most commonly used method (86), which can be used to detect the sequence of glycosidic linkages breaks and preliminarily infer the possible glycosidic bond types. By measuring the usage of periodic acid and the release of formic acid, the position of glycosidic bonds, the degree of polymerization of linear polysaccharides and the number of branches of branched polysaccharides can be judged. The characteristic of Smith's degradation is that only glycosidic bonds are broken by periodic acid, while the sugar residues that are not oxidized are still attached to the sugar chain. The key point to the methylation reaction is whether the methylation is complete (87). For polysaccharides containing uronic acid and aminohexose, whether methylated acid hydrolysis will cause side reactions. It is necessary to reduce the polysaccharide before methylation, if the samples contain uronic acid.

Figure 4. Chemical reactions related to the determination of glycosidic bonds. (A) Different polysaccharides are oxidized by HIO4, reduced by NaBH4, and then hydrolyzed with dilute acid to obtain different products; (B) Products obtained by methylation and acid hydrolysis of linear glucans and branched polysaccharides.
The common determination methods of glycosidic bonds in polysaccharides are shown in Table 3. And there are some new methods to identify the glycosidic bonds of polysaccharides. Amicucci described the development and application of a chemical method for producing oligosaccharides from polysaccharides. The released oligosaccharides are characterized by the advanced LC-MS methods with high sensitivity, accuracy, and throughput. The technique is first used to identify polysaccharides by oligosaccharide fingerprinting. The Fenton's initiation toward defined oligosaccharide groups (FITDOG) process was developed to produce oligosaccharides from polysaccharides. The process was initiated by a reaction between a metal catalyst, Fe3+, and an oxidizing agent, hydrogen peroxide, to produce reactive radical species that cleave glycosidic bonds. The radicals induce oxidative cleavage of the polysaccharide backbone to produce oligosaccharides that are representative of the parent polysaccharide structure (96).
Spectroscopy Techniques
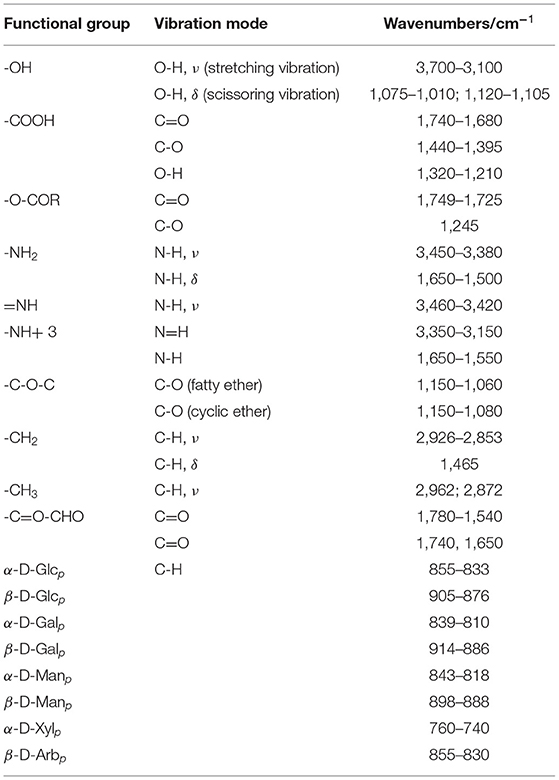
IR is mainly used to identify various functional groups, determine the α/β configuration of glycosidic bonds, distinguish five-carbon and six-carbon sugars, and identify different monosaccharides in polysaccharides (Table 4) (97, 98). The characteristic absorption peaks of polysaccharides are at 3,600–3,200, 3,000–2,800, and 1,200–1,000 cm−1 (99). Among them, polysaccharides contain lots of hydroxyl groups, which usually has a distinct broad stretching vibration absorption peak at the wavelength of 3,500–3,000 cm−1. The C-H stretching vibration can form a weak peak near 2,935 cm−1 (100). The absorption peak of sugar ring C-O-H, C-O-C is 1,000–1,100 cm−1. In addition, according to the absorption peak in the 1,170–700 cm−1 interval of the infrared spectrum, the size of sugar ring and the configuration of the glycosidic bond can be judged. Pyrannoside has 3 strong absorption peaks in the interval of 1,100–1,010 cm−1, while furanoside only has 2 absorption peaks in the corresponding region. The vibrations of the pyranose ring are at 917 and 770 cm−1, while the furanose ring is at 924 and 799 cm−1. Generally, 890 cm−1 is the characteristic peak of β-pyranoside bonds, and 840 cm−1 is the characteristic peak of α-pyranoside bonds.
Nuclear magnetic resonance spectroscopy (NMR) provides information about the structure of polysaccharides, which involves the identification of monosaccharides, α or β anomeric confirmation, glycosidic bonds type, and the sequence of repeating units in the polysaccharide chain. In the 1H-NMR spectrum, the chemical shifts of the polysaccharide signal are mainly distributed in δ 3.5–4.4 and δ 4.4–5.8 (anomeric proton region). Among them, the anomeric proton region plays a significant part of the structure analysis of polysaccharide, such as providing information on the configuration of polysaccharide residues. Generally, the chemical shift δ of the anomeric hydrogen of the pyranose residue with α configuration is larger than 5.0. For β configuration, the δ is less than 5.0. The configuration of the anomeric hydrogen can also be judged by combining the coupling constant J1,2 of the anomeric hydrogen and the vicinal hydrogen. For the β configuration, J1,2 = 7–9 Hz, and for the α configuration, J1,2 = 2–4 Hz. Unsaturated double bond in non-reducing end H4 falls in 5.9–6.0 ppm, H1 in 4.8–5.5 ppm and H2–H6 in 4.0–4.8 ppm. There are also some special proton signals that are helpful to determine the type of monosaccharide residues, such as δ 1.1–1.3, which may be the hydrogen at C6 position of fucose. When methyl (around 1.0 ppm), acetyl (around 2.0 ppm), sulfate and phosphate groups were substituted, the 1H chemical shift often shifted downward by about 0.2–0.5 ppm.
The 13C-NMR spectrum can confirm various carbon nuclei and distinguish the configuration and conformation of molecules in polysaccharide structure analysis. It can also be used to determine the substitution position and branch point of polysaccharide residues. In the 13C-NMR spectrum, the characteristic peaks of polysaccharides fall in 60–110 ppm. Among them, monosaccharide composition and sugar ring configuration can be determined by the isomeric carbon signal of 95–105 ppm. Allocarbons above 101 ppm can be distinguished as β-configuration, and between 95 to 103 as α-configuration. For C2–C5, 78–85 ppm is the carbon signal at the substituted position and 65–80 ppm is the one at unsubstituted position. Signals around 61 ppm belong to unsubstituted C6, and the substituted C6 signal moves to lower around 69 ppm. The signals in 96–110 ppm are the peaks of terminal carbons of sugar, and the other signals are the peaks of non-terminal carbons. The signals of unsubstituted C2, C3, C4, and C5 are at 78–70 ppm, while the unsubstituted C6 signals are at 64–60 ppm. The chemical shift of methyl carbon is about 35–40 ppm (100). Chemical shift of polysaccharide 13C substituted by methyl, acetyl, sulfate, or phosphate group shifts downward 6–7 ppm. Two-dimensional NMR (2D NMR) plays an indispensable role in the full attribution of 13C-NMR spectra of polysaccharides (101). Currently, NMR is a tool that can be used to analyze molecular structure completely independently, but it also has the problems of low sensitivity. Additionally, the severe overlap of the peaks makes the elucidation extremely complex, especially for Chinese herbal heteropolysaccharides.
The LC-MS methods have been developed and have brought remarkable advances in terms of sensitivity and specificity to the general analysis of polysaccharides, which can provide reliable molecular weights, and the information of fragments (102, 103). Xu et al. have developed a comprehensive method for quantitation of both neutral and acidic monosaccharides using ultra-high performance liquid chromatography triple quadrupole mass spectrometry (UHPLC/QqQ-MS) in dynamic multiple reaction monitoring (dMRM) mode. This method can achieve the separation, detection, and quantification of 14 PMP-derived monosaccharides (including fructose) and two sialic acids with label-free within 10 min, which has high sensitivity and wide linear range (104). Amicucci et al. presented a general LC-MS-based workflow for the de novo characterization of structurally diverse polysaccharides. This report presented the characterization of the maize polysaccharide by employing new analytical strategies, which is quantifying monosaccharide and glycosidic linkages by combining chemical depolymerization and UHPLC/QqQ-MS analysis. Partial acid hydrolysis paired with nano-HPLC/QTOF MS was used to analyze oligosaccharides sequencing. The elucidation of this complicated structure illustrates the high sensitivity, good reproducibility and fast speed of the analytical methods, which may serve as a general platform for polysaccharide analysis in the future (105).
Advanced Structure Analysis
The traditional methods of studying the high-level structure of polysaccharides have limitations in application. A new technique as X-ray diffraction (XRD) method can obtain various information such as bond angle, bond length, configuration angle at the same time (106). But this technology requires that the polysaccharide sample must have high purity and crystallization. In other words, amorphous polysaccharides are not applicable. Except for polymers with triple helical structure, there is no other report using XRD to study chain conformation (57). Additionally, the Congo red experiment does not require special equipment and is easy to popularize. However, this method can only be used to determine whether the sample has a triple helix structure. It cannot elucidate the precise structure and has low sensitivity.
Analytical Methods Based on the Theory of Polymer Dilute Solution
At present, the most commonly used analysis methods of advanced structure are HPLC combined with dynamic light scattering (DLS), static light scattering (SLS) or viscosity determination based on the theory of polymer dilute solution (107, 108). Normally, four main characteristics are considered when identifying polymer conformations, which concludes persistence length (q), molar mass per unit contour length (ML), diameter of chain (d), and contour length of chain (L). These four key parameters can be calculated by thermodynamic and hydrodynamic properties parameters including molecular weight (Mw), radius of gyration (Rg), intrinsic viscosity (η), diffusion coefficient (D), and hydrodynamic radius (Rh), which can be obtained through the Kratky-Porod model, helical worm-like chain model, or models derived from them (109).
The HPLC-SLS technology is a simple but effective analysis method for advanced structure of polysaccharides (110). It is based on the light scattering properties of polymer solutions and the related characteristics of molecular mass, size and concentration. Various structural information such as Mw, molecular mass distribution (MWD), and Rg can be obtained through formula (1) (111). Majority of studies still use Zimm mapping for data processing (112), while the Debye method can not only ensure accuracy but also shorten the experimental period (113). The exponent v of formula (2) is the slope of the linear regression of Rg to Mw, which is one of the parameters that characterize the conformation of the polymer. Similar to v, the fractal dimension df is also a parameter that characterizes the tightness of the internal structure of the polymer, and it is calculated with formula (3).
Because of the molecular thermal motion or Brownian motion in polymer solution, the phase of scattered particles in solution changes with time. The basic principle of DLS is that the frequency and the intensity of scattered light change and fluctuate with time. The values of Rh and D can be determined by DLS. Rh is an important parameter describing the size of a polymer in solution, and Rg is the root mean square value of the distance between the center of mass of the polymer and the axis of rotation. The form and rigidity of the polymer in a dilute solution can also be described by ρ (Rg/Rh) (114).
Viscometry is another simple method for measuring. The exponent α is a characteristic parameter of the high-level structure of polymers, which can be calculated according to formula (9). And then the intrinsic viscosity η can be obtained by extrapolation using a viscometer according to Huggins equation (10) and Kraemer equation (11). A large number of studies have combined these three methods for the analysis of the advanced structure of polysaccharides. Li S. et al. established an analytical method combining HPLC with multiple detectors to realize the analysis of β-Online separation and real-time detection of dextran aggregates and non-aggregates (115). The parameters, calculation formulas and the chain conformation information reflected by them are listed in Table 5.
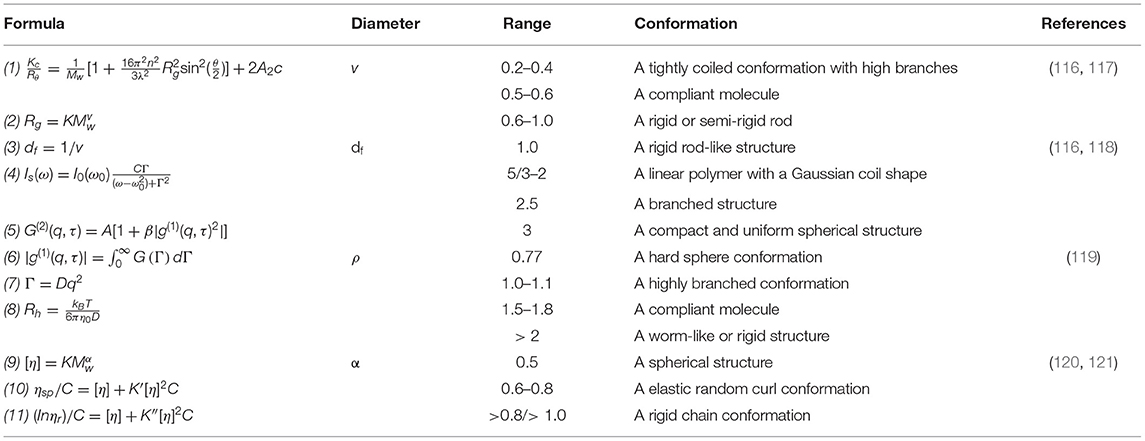
Table 5. The parameters, calculation formulas, and the chain conformation information reflected by them.
Microscope Observation Methods
High resolution and large depth of field are two main features of scanning electron microscope (SEM). The rough surface with certain fluctuations of polysaccharides can be directly observed by it (122). The surface morphology of polysaccharides significantly differs from each other due to various sources of polysaccharides. Spherical, flake and branched structures have been observed before, among which, spherical or flake morphology are most common (123, 124). And the extraction, purification, and preparation conditions could also influence on the surface morphology of polysaccharides (125). For example, three polysaccharides from Sagittaria sagittifolia L. separately obtained with hot water, ultrasound-assisted, and subcritical water extraction presented different SEM images (21). The different surface morphology of polysaccharides acquired by SEM can preliminarily infer the force between molecules. Polysaccharides usually have honeycomb-like structures, rough surfaces, and porous structures, indicating that the interaction among molecules is weak (126). A flake layer with unregular curls was observed indicating the strong attractions between functional groups on the surface to create the polysaccharide chains aggregation (127). The variation on morphology and shape mainly contributed to the changes of intramolecular hydrogen bonds (127). And the morphology and shape will affect the physical and chemical properties of polysaccharides. For instance, the smooth surface of polysaccharide probably had negative effect on the rehydration performance to reduce the solubility of polysaccharide itself (4).
Atomic force microscopy (AFM) is an analytical instrument that can study the three-dimensional (3D) surface morphology of polymers on the nanoscale in air and liquid (128). In AFM, when the probe scans the surface of the sample with a constant force between the probe and the sample, its motion trajectory can be recorded and converted into an integral 3D image. AFM does not require any special treatment on the sample, such as copper or carbon plating, which will cause irreversible damage to the sample. The disadvantage of AFM is that the imaging range is too small, the speed is slow, and it is too much affected by the probe. In early research, AFM was usually used to calculate the shape of the chain, including the diameter of the chain, the length of the chain, and the chain distribution of the polymer (129, 130). Zhao et al. used an AFM to observe the aggregation state, morphology and size of Schisandra polysaccharide particles in pure water. The heights of the particle were in the range of 1–5 nm and found that the polysaccharide particles had agglomeration phenomenon at 100 °C (131). The agglomerations suggest polysaccharide molecules have gathered and that their structures were branched and entangled (132).
Other Methods
According to the different characteristics of the sample's absorption of left-handed and right-handed circularly polarized light, circular dichroism (CD) can be used to observe the structure of polysaccharides (133, 134). Zhang et al. analyzed the CD spectra of the Cistanche deserticola polysaccharide in the range of 190–300 nm. They found that some fragments of the Cistanche deserticola polysaccharide mainly exist in an ordered structure, and locally form a spiral structure (135). The results of CD analysis of the Artemisia sphaeroides polysaccharide before and after the sulfonation showed that the shielding effect of the charged components promoted the transformation of the polysaccharide chain to a more rigid conformation (136).
The differential scanning calorimetry (DSC) can not only describe the change about temperature between the sample and the reference but also record the change law between the heat difference and the temperature in real time. Liu et al. used this method to study the conformational transition process of Ganoderma lucidum polysaccharide 20 (GLP20). As a result, in the DSC heating curve, the endothermic peak indicated that the intermolecular hydrogen bond was broken, and GLP20 changed from a triple helical structure to a single-stranded random coil (137).
Polysaccharide macromolecules are usually in a dynamic equilibrium state of multiple spatial conformations, and the structural information provided by NMR is the average information of these dynamic conformations. A series of spatial structures that meet the NMR data and truly reflect the dynamic equilibrium of the sugar chain are obtained through the method of molecular calculation. Usually, molecular dynamics simulation (MDS) can depict the chain conformation of macromolecules with more than 10 repeating units. Based on the lowest energy state, the optimal conformation of the polysaccharide could be confirmed after simulated annealing (57). For instance, the complex 3D conformation of lentinan that can retain its triple-helix through hydrogen network was revealed using MDS and rigid macromolecule docking, as well as spectral methods (40).
Structural Modification
The common methods of structural modification in CHPs are mainly divided into three categories, namely chemical modification, physical modification, and biological modification (Figure 5).
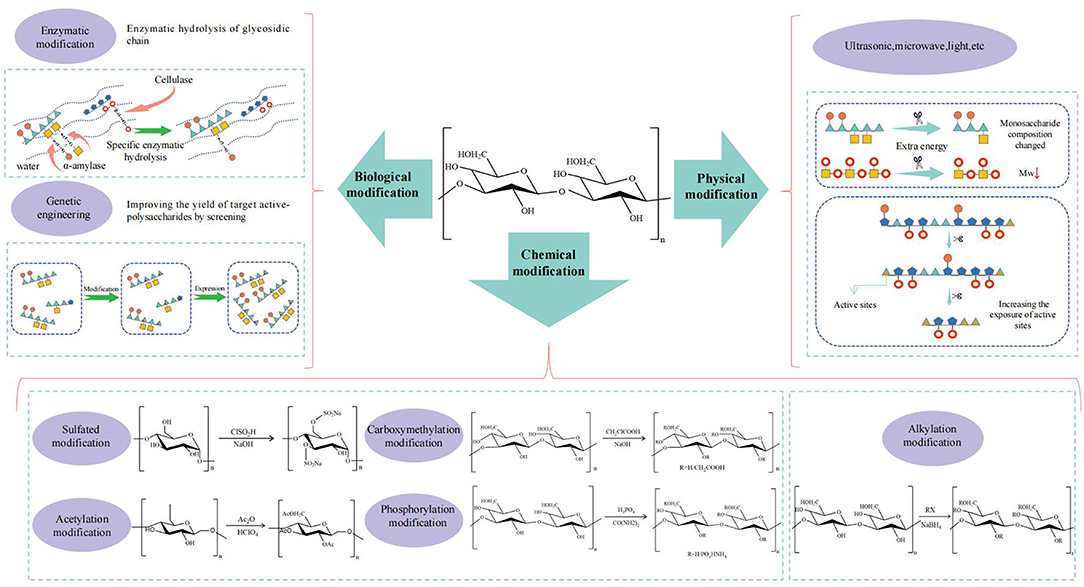
Figure 5. The schematic diagram of relationship between structural modification and biological activity of CHPs.
Chemical Modification
Chemical modification is the most common approach to tailor polysaccharide structure by inletting the required groups and thereby changing its original activity as well as producing new bioactivities (138). For instance, it was reported the anti-tumor activity of Poria cocos polysaccharide after modifications exhibit the stronger than the former (139). The modification was performed to achieve good water solubility, high chain rigidity and moderate molecular weight, which may be the reason for the improvement of its anti-tumor activity.
The branched chains of the polysaccharide can be modified by sulfation, phosphorylation, carboxymethylation, acetylation, etc., to enhance the bio-activity.
In sulfated modification, sulfuric acid groups are used to replace the hydroxyl, carboxyl, and amino ending on the sugar chain to exert activities. The degree of sulfation is positively related to anti-HIV and anticoagulant activity of CHPs. Firstly, sulfate groups are considered to be one of the determinants of anti-HIV activity of CHPs (8, 140). Then, sulfate groups can enhance the anticoagulant activity of CHPs by generating the high level of negative charge density. The sulfated polysaccharides obtained from Zingiber officinale with the higher degree of sulfation and suitable molecular weight showed better anticoagulant activities (141). In addition, CHPs exert their antioxidant activity by providing hydrogen atoms from polysaccharide chain and the presence of sulfated groups could increase the ability to provide hydrogen atoms (142, 143). In summary, sulfated polysaccharide are increasingly causing more attention, as they have been proved to improve structural properties and promote a variety of bioactivity.
The water solubility of polysaccharide can be improved by carboxymethylation with introduction of carboxymethyl into polysaccharide chain and thereby enhancing the biological activities of unmodified polysaccharides. After carboxymethylation, the molecular weight of polysaccharide from Cyclocarya paliurus decreased and correspondingly the molar ratio of monosaccharide composition changed (144). Among carboxymethylated polysaccharides, the antioxidant activity aims to terminate free radicals against oxidation reactions from occurring by increasing the ability of chelating transition metal ions and providing single electron or hydrogen atoms with the increased content of -COOH (145). It is worth mentioning that there is a positive correlation between antioxidant activity and the degree of carboxymethylation within a certain range (146). In addition, carboxymethylation can also enhance other biological activities of CHPs, such as anti-tumor and immune regulation activity (147). However, the mechanisms of action of carboxymethylated polysaccharides are still unclear and need to be explored forward.
On top of carboxymethylation, acetylation with the hydroxyl oxygen or amino nitrogen as the acetyl substitution sites can also enhance the water solubility of CHPs, mainly due to the exposure of hydroxyl groups from polysaccharide caused by the extension of polysaccharide chains (148). After the exposure of hydroxyl, the supply of hydrogen could be raised up, thereby enhancing the antioxidant activity of CHPs (149). It was found that the acetylated pumpkin polysaccharides with high degree of substitution had better antioxidant activity than those with low degree of substitution, and all of them perform better than those without modification (150). In addition, the potential application of acetylated CHPs is a feasible strategy to be an immunotherapeutic adjuvant (151).
A specific method used to modify the main chain of polysaccharides is the alkylation reaction by alkyl and long-chain aromatic alcohol for improving water solubility, reducing the viscosity of the solution, and thus improving the bioavailability. A good example was that Ganoderma lucidum polysaccharide was hydroxypropylated to improve the aqueous solubility and its antioxidant activity was significantly enhanced (152). An acceptable reason is that the hydroxyl groups in the molecules are more easily exposed to the reactive oxygen species (ROS), including free radicals and oxidants.
However, it should be noted that the appropriated degree of substitution is crucial to the activity. The crude polysaccharides of Cordyceps militaris were successfully modified by carboxymethylation and acetylation, but the expressed α-glucosidase inhibitory activity did not change significantly (153). To sum up, the change of the structure in polysaccharides has certain impacts on the biological activity with the degree of substitution and the substituent position as important factors affecting the result.
Physical Modification
In the physical modification, the degradation products with low molecular weight were obtained by serials of ultrasound, high energy radiation, microwave, and light to cut off some chemical bonds in the main chain due to high molecular weight of polysaccharides showing characteristics of high viscosity and poor solubility against absorption and utilization in vivo (154).
Ultrasonic treatment is the most commonly used method in physical modification. The ultrasonic treatment in the process of polysaccharide can break glycosidic bond to reduce the molecular weight, the viscosity, and enhance the solubility (155). It has been reported that the decreased molecular weight is positively correlated with ultrasonic intensity (154). In the process of ultrasonic, monosaccharide composition and uronic acid content would be also monitored as important indexes (156). The molecular weight of corn whisker polysaccharides after ultrasonic degradation decreased significantly, but the monosaccharide composition did not change. Interestingly, the molar ratio of mannose to galactose decreased significantly, suggesting that mannose and galactose residues may be the main active sites in the process of ultrasonic degradation. In the process, ultrasonic treatment also exposed more groups such as uronic acid, and thus provided more binding sites for increasing of α-glucosidase inhibitory activity (157).
Biological Modification
The biological modification for polysaccharides mainly involves enzymatic modification and genetic engineering. In enzymatic modification, specific biological enzymes are often used to degrade polysaccharides, and in which the non-reducing ends are cleaved resulting in double bonds forming. The molecular weight and solution viscosity of polysaccharides are decreased after side-chain detachment occurring by enzyme-modified treatment beyond the change of monosaccharide composition and biological activities enhancing (125, 158). It was found that the antioxidant activity of Morus alba L. polysaccharides hydrolyzed by cellulase was higher than original polysaccharides, which may be attributed to the exposure of more active groups of polysaccharides (159). After enzymatic hydrolysis, the primary structure of polysaccharide from Hericium erinaceus with the shorter branched chains did not change, which led to the decrease of molecular weight, the increase of glucose content and the enhancement of immunomodulatory activity of the polysaccharide (160). At present, it should be noted that most of the enzymes can be used in enzymatic modification as degradable enzymes, and the applicable types are limited, which blocks the usage of this method.
To promote the industrial production of polysaccharides, genetic engineering is employed to control the biosynthesis pathway of polysaccharides by manipulating the gene expression of microorganisms or introducing exogenous genes in microorganisms for obtaining target active-polysaccharides (161). The proportion of galactose and mannose in polysaccharides and the antioxidant activity of polysaccharides were greatly boosted by efficient expression of the Vitreoscilla hemoglobin gene in Ganoderma lucidum (162). However, genetic engineering is difficult to be operated and foreign genes are not easy to obtain, which still needs to be further developed.
Conclusions and Prospects
Up to now, researches on CHPs are still in the initial stage with many shortcomings. It can be concluded that there has been a conventional structure analysis procedure and common structure-modified approach, but no unified conclusion on the structure-activity relationship of CHPs.
The biological activities of CHPs are closely related to its structure. Firstly, according to the facts we consolidated, the activity of polysaccharide performs the best only in the relative optimum molecular weight range while the determination of the specific range still needs more work. Secondly, the type and proportion of monosaccharide composition has a certain influence on the activity of polysaccharides. Nevertheless, the rules reflected different polysaccharides on activities are multifarious. Thirdly, as for the effect of glycosidic bonds on the activity of CHPs, CHPs with anti-tumor effect composed of the β-(1 → 3)-D-glucan as the main chain and the β-(1 → 6)-D-glucan randomly as the branched chain were in-depth studied. However, the studies on the glycosidic bonds of CHPs with other activities are not systematic enough, and most of them are in a state of scattered research. Fourthly, the DB of CHPs affects biological activity by changing molecular weight and conformation, and an ideal DB value may exist. This might be due to the fact that a high DB value reduces water solubility, whereas a low DB value results in fewer binding sites. The attention should be paid to the study of the type and length of branches, so as to reveal the mechanism of their influence on biological activity. Finally, the polysaccharides of triple helix conformation show excellent activities. On the one hand, the study of mechanism between triple helix conformation and activities should be strengthened. On the other hand, the research of CHPs with other conformations should not be ignored.
With regard to molecular modification, chemical modifications are much more focused due to obviously enhancing the activity of CHPs by inletting the required groups, wherein degree of substitution and position of substituent play the important role. In addition, physical and biological modification could affect the activity of polysaccharides by changing their molecular weight, monosaccharide composition, and physicochemical properties. Physical modification can degrade the main chain of polysaccharides, which is relatively friendly to the acquisition for small molecule-weight of polysaccharides, but the method is too unstable. In addition, enzymes are widely used in biological modification with high efficiency and controllability, but the categories and amounts of available enzymes are finite. Finally, genetic engineering technology can achieve a large number of targeted polysaccharides, but this method is difficult to operate and exogenous genes are not easy to obtain.
In light of the aforementioned issues, some modern equipment also promoted the development of structural analysis techniques for polysaccharide. Besides, multidisciplinary approaches can help researchers think outside the box. MDS and other calculation techniques can better examine the three-dimensional structure of polysaccharides. Through the combined use of multiple technologies, interdisciplinary methods are applicable to the structural characterization, especially the analysis of chain conformation of CHPs. Meanwhile, combined with the structural modification, the desired biological activity could be obtained by degrading or introducing the target groups. Then, in wake of structural elucidation, some rules are deduced among structure-activity relationship of polysaccharide, which may speed up the application of CHPs.
Author Contributions
BW and LY: investigation and writing original draft. SG, LW, and MY: writing. LF and XJ: reviewing. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Key research and development program of China (2018YFC1706906), Double First-Class University project of China Pharmaceutical University (CPU2018GF07 and CPU2018PZQ19), National Natural Science Foundation Committee of P.R. China (Nos. 81703775 and 81973536), and the Special Fund for Transformation of Scientific and Technological Achievements in Jiangsu Province (BA2020077) and the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZ202025).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bian Y, Zeng H, Tao H, Huang L, Du Z, Wang J, et al. A pectin-like polysaccharide from polygala tenuifolia inhibits pancreatic cancer cell growth in vitro and in vivo by inducing apoptosis and suppressing autophagy. Int J Biol Macromol. (2020) 162:107–15. doi: 10.1016/j.ijbiomac.2020.06.054
2. Cui L, Chen L, Yang G, Li Y, Qiao Z, Liu Y, et al. Structural characterization and immunomodulatory activity of a heterogalactan from panax ginseng flowers. Food Res Int. (2021) 140:109859. doi: 10.1016/j.foodres.2020.109859
3. Guo C, Wang Y, Zhang S, Zhang X, Du Z, Li M, et al. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and scfas metabolism. Int J Biol Macromol. (2021) 181:357–68. doi: 10.1016/j.ijbiomac.2021.03.137
4. Chen Z, Zhao Y, Zhang M, Yang X, Yue P, Tang D, et al. Structural characterization and antioxidant activity of a new polysaccharide from bletilla striata fibrous roots. Carbohydr Polym. (2020) 227:115362. doi: 10.1016/j.carbpol.2019.115362
5. Xie L, Shen M, Hong Y, Ye H, Huang L, Xie J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr Polym. (2020) 229:115436. doi: 10.1016/j.carbpol.2019.115436
6. Chen F, Huang G. Preparation and immunological activity of polysaccharides and their derivatives. Int J Biol Macromol. (2018) 112:211–6. doi: 10.1016/j.ijbiomac.2018.01.169
7. Cao Y-Y, Ji Y-H, Liao A-M, Huang J-H, Thakur K, Li X-L, et al. Effects of sulfated, phosphorylated and carboxymethylated modifications on the antioxidant activities in-vitro of polysaccharides sequentially extracted from amana edulis. Int J Biol Macromol. (2020) 146:887–96. doi: 10.1016/j.ijbiomac.2019.09.211
8. Li S, Xiong Q, Lai X, Li X, Wan M, Zhang J, et al. Molecular modification of polysaccharides and resulting bioactivities. Compr Rev Food Sci Food Saf. (2016) 15:237–50. doi: 10.1111/1541-4337.12161
9. Xu S-Y, Huang X, Cheong K-L. Recent advances in marine algae polysaccharides: isolation, structure, and activities. Mar. Drugs. (2017) 15. doi: 10.3390/md15120388
10. Wang C, Li W, Chen Z, Gao X, Yuan G, Pan Y, et al. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from inonotus obliquus. Food Res Int. (2018) 103:280–8. doi: 10.1016/j.foodres.2017.10.058
11. Zhang X, Duan S, Tao S, Huang J, Liu C, Xing S, et al. Polysaccharides from dendrobium officinale inhibit proliferation of osteosarcoma cells and enhance cisplatin-induced apoptosis. J Funct Foods. (2020) 73:104143. doi: 10.1016/j.jff.2020.104143
12. Im SA, Oh ST, Song S, Kim MR, Kim DS, Woo SS, et al. Identification of optimal molecular size of modified aloe polysaccharides with maximum immunomodulatory activity. Int Immunopharmacol. (2005) 5:271–9. doi: 10.1016/j.intimp.2004.09.031
13. Jia Y, Xue Z, Wang Y, Lu Y, Li R, Li N, et al. Chemical structure and inhibition on α-glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohydr Polym. (2021) 252:117185. doi: 10.1016/j.carbpol.2020.117185
14. Jia Y, Gao X, Xue Z, Wang Y, Lu Y, Zhang M, et al. Characterization, antioxidant activities, and inhibition on α-glucosidase activity of corn silk polysaccharides obtained by different extraction methods. Int J Biol Macromol. (2020) 163:1640–8. doi: 10.1016/j.ijbiomac.2020.09.068
15. Błaszczyk K, Wilczak J, Harasym J, Gudej S, Suchecka D, Królikowski T, et al. Impact of low and high molecular weight oat beta-glucan on oxidative stress and antioxidant defense in spleen of rats with lps induced enteritis. Food Hydrocoll. (2015) 51:272–80. doi: 10.1016/j.foodhyd.2015.05.025
16. Huo J, Wu Z, Sun W, Wang Z, Wu J, Huang M, et al. Protective effects of natural polysaccharides on intestinal barrier injury: a review. J Agric Food Chem. (2022) 70:711–35. doi: 10.1021/acs.jafc.1c05966
17. Hou C, Yin M, Lan P, Wang H, Nie H, Ji X. Recent progress in the research of angelica sinensis (oliv) diels polysaccharides: extraction, purification, structure and bioactivities. Chem Biol Technol Agric. (2021) 8:13. doi: 10.1186/s40538-021-00214-x
18. Qu J, Huang P, Zhang L, Qiu Y, Qi H, Leng A, et al. Hepatoprotective effect of plant polysaccharides from natural resources: a review of the mechanisms and structure-activity relationship. Int J Biol Macromol. (2020) 161:24–34. doi: 10.1016/j.ijbiomac.2020.05.196
19. Yu CH Dai XY, Chen Q, Zang JN, Deng LL, Liu YH, et al. Hypolipidemic and antioxidant activities of polysaccharides from rosae laevigatae fructus in rats. Carbohydr Polym. (2013) 94:56–62. doi: 10.1016/j.carbpol.2013.01.006
20. Li F, Du P, Yang W, Huang D, Nie S, Xie M. Polysaccharide from the seeds of plantago asiatica l. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human caco-2 cell line. Int J Biol Macromol. (2020) 164:2134–40. doi: 10.1016/j.ijbiomac.2020.07.259
21. Gu J, Zhang H, Yao H, Zhou J, Duan Y, Ma H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria Sagittifolia L. Carbohydr Polym. (2020) 235:115939. doi: 10.1016/j.carbpol.2020.115939
22. Zheng L, Ma Y, Zhang Y, Meng Q, Yang J, Wang B, et al. Increased antioxidant activity and improved structural characterization of sulfuric acid-treated stepwise degraded polysaccharides from pholiota nameko Pn-01. Int J Biol Macromol. (2021) 166:1220–9. doi: 10.1016/j.ijbiomac.2020.11.004
23. Saito Y, Kinoshita M, Yamada A, Kawano S, Liu HS, Kamimura S, et al. Mannose and phosphomannose isomerase regulate energy metabolism under glucose starvation in leukemia. Cancer Sci. (2021) 112:4944–56. doi: 10.1111/cas.15138
24. Sorourian R, Khajehrahimi AE, Tadayoni M, Azizi MH, Hojjati M. Ultrasound-assisted extraction of polysaccharides from typha domingensis: structural characterization and functional properties. Int J Biol Macromol. (2020) 160:758–68. doi: 10.1016/j.ijbiomac.2020.05.226
25. Du B, Meenu M, Liu H, Xu B. A concise review on the molecular structure and function relationship of β-glucan. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20164032
26. Jin Y, Li P, Wang F. B-Glucans as potential immunoadjuvants: a review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine. (2018) 36:5235–44. doi: 10.1016/j.vaccine.2018.07.038
27. Mutaillifu P, Bobakulov K, Abuduwaili A, Huojiaaihemaiti H, Nuerxiati R, Aisa HA, et al. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza Glabra. Int J Biol Macromol. (2020) 144:751–9. doi: 10.1016/j.ijbiomac.2019.11.245
28. Moreno-Mendieta S, Guillén D, Hernández-Pando R, Sánchez S, Rodríguez-Sanoja R. Potential of glucans as vaccine adjuvants: a review of the α-glucans case. Carbohydr Polym. (2017) 165:103–14. doi: 10.1016/j.carbpol.2017.02.030
29. Ji X, Hou C, Shi M, Yan Y, Liu Y. An insight into the research concerning panax ginseng C. A. meyer polysaccharides: a Review. Food Rev Int. (2020) 1–17. doi: 10.1080/87559129.2020.1771363
30. Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. (2002) 60:258–74. doi: 10.1007/s00253-002-1076-7
31. Zhao D, Dai W, Tao H, Zhuang W, Qu M. Chang YN. Polysaccharide isolated from auricularia auricular-judae (bull) prevents dextran sulfate sodium-induced colitis in mice through modulating the composition of the gut microbiota. J Food Sci. (2020) 85:2943–51. doi: 10.1111/1750-3841.15319
32. Ai J, Bao B, Battino M, Giampieri F, Chen C, You L, et al. Recent advances on bioactive polysaccharides from mulberry. Food Funct. (2021) 12:5219–35. doi: 10.1039/D1FO00682G
33. Wang J, Jia J, Song L, Gong X, Xu J, Yang M, et al. Extraction, structure, and pharmacological activities of astragalus polysaccharides. Appl Sci. (2019) 9. doi: 10.3390/app9010122
34. Ji X, Peng B, Ding H, Cui B, Nie H, Yan Y. Purification, structure and biological activity of pumpkin polysaccharides: a review. Food Rev Int. (2021) 1–13. doi: 10.1080/87559129.2021.1904973
35. Ye J, Zhang C, Lyu X, Hua X, Zhao W, Zhang W, et al. Structure and physicochemical properties of arabinan-rich acidic polysaccharide from the by-product of peanut oil processing. Food Hydrocoll. (2021) 117:106743. doi: 10.1016/j.foodhyd.2021.106743
36. Gan T, Feng C, Lan H, Yang R, Zhang J, Li C, et al. Comparison of the structure and immunomodulatory activity of polysaccharides from fresh and dried longan. J Funct Foods. (2021) 76:104323. doi: 10.1016/j.jff.2020.104323
37. Li M, Li T, Hu X, Ren G, Zhang H, Wang Z, et al. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (murus alba l) based on different extraction techniques with superfine grinding pretreatment. Int J Biol Macromol. (2021) 183:1774–83. doi: 10.1016/j.ijbiomac.2021.05.108
38. Zhang H, Wang J-Q, Nie S-P, Wang Y-X, Cui SW, Xie M-Y. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma Atrum. Int J Biol Macromol. (2015) 79:248–55. doi: 10.1016/j.ijbiomac.2015.04.070
39. Zheng Z, Pan X, Luo L, Zhang Q, Huang X, Liu Y, et al. Advances in oral absorption of polysaccharides: mechanism, affecting factors, and improvement strategies. Carbohydr Polym. (2022) 282:119110. doi: 10.1016/j.carbpol.2022.119110
40. Wu X, Zheng Z, Guo T, Wang K, Zhang Y. Molecular dynamics simulation of lentinan and its interaction with the innate receptor dectin-1. Int J Biol Macromol. (2021) 171:527–38. doi: 10.1016/j.ijbiomac.2021.01.032
41. Chen L, Ge MD, Zhu YJ, Song Y, Cheung PCK, Zhang BB, et al. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr Polym. (2019) 223:115076. doi: 10.1016/j.carbpol.2019.115076
42. Wu D-T, Meng L-Z, Wang L-Y, Lv G-P, Cheong K-L, Hu D-J, et al. Chain conformation and immunomodulatory activity of a hyperbranched polysaccharide from cordyceps sinensis. Carbohydr Polym. (2014) 110:405–14. doi: 10.1016/j.carbpol.2014.04.044
43. Li B, Zhang N, Feng Q, Li H, Wang D, Ma L, et al. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity. Int J Biol Macromol. (2019) 123:713–22. doi: 10.1016/j.ijbiomac.2018.11.140
44. Bae IY, Kim HW, Yoo HJ, Kim ES, Lee S, Park DY, et al. Correlation of branching structure of mushroom β-glucan with its physiological activities. Food Res Int. (2013) 51:195–200. doi: 10.1016/j.foodres.2012.12.008
45. Li N, Wang C, Georgiev MI, Bajpai VK, Tundis R, Simal-Gandara J, et al. Advances in dietary polysaccharides as anticancer agents: structure-activity relationship. Trends Food Sci Technol. (2021) 111:360–77. doi: 10.1016/j.tifs.2021.03.008
46. Patel BK, Campanella OH, Janaswamy S. Impact of urea on the three-dimensional structure, viscoelastic and thermal behavior of iota-carrageenan. Carbohydr Polym. (2013) 92:1873–9. doi: 10.1016/j.carbpol.2012.11.026
47. Tao Y, Zhang R, Yang W, Liu H, Yang H, Zhao Q. Carboxymethylated hyperbranched polysaccharide: synthesis, solution properties, and fabrication of hydrogel. Carbohydr Polym. (2015) 128:179–87. doi: 10.1016/j.carbpol.2015.04.012
48. Chen X, Xu X, Zhang L, Kennedy JF. Flexible chain conformation of (1 → 3)-B-D-Glucan from poria cocos sclerotium in naoh/urea aqueous solution. Carbohydr Polym. (2009) 75:586–91. doi: 10.1016/j.carbpol.2008.08.027
49. Morris ER, Cutler AN, Ross-Murphy SB, Rees DA, Price J. Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydr Polym. (1981) 1:5–21. doi: 10.1016/0144-8617(81)90011-4
50. Kido S, Nakanishi T, Norisuye T, Kaneda I, Yanaki T. Ordered conformation of succinoglycan in aqueous sodium chloride. Biomacromolecules. (2001) 2:952–7. doi: 10.1021/bm010064h
51. Anderson NS, Campbell JW, Harding MM, Rees DA, Samuel JW. X-Ray diffraction studies of polysaccharide sulphates: double helix models for K- and L-carrageenans. J Mol Biol. (1969) 45:85–99. doi: 10.1016/0022-2836(69)90211-3
52. Meng Y, Shi X, Cai L, Zhang S, Ding K, Nie S, et al. Triple-Helix conformation of a polysaccharide determined with light scattering, afm, and molecular dynamics simulation. Macromolecules. (2018) 51:10150–9. doi: 10.1021/acs.macromol.8b02017
53. Jana NR, Gearheart L, Murphy CJ. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater. (2001) 13:1389–93. doi: 10.1002/1521-4095(200109)13:18<1389::AID-ADMA1389>3.0.CO;2-F.3.0.CO;2-F
54. Fang Y, Duan B, Lu A, Liu M, Liu H, Xu X, et al. Intermolecular interaction and the extended wormlike chain conformation of chitin in naoh/urea aqueous solution. Biomacromolecules. (2015) 16:1410–7. doi: 10.1021/acs.biomac.5b00195
55. Kök MS, Abdelhameed AS, Ang S, Morris GA, Harding SE. A novel global hydrodynamic analysis of the molecular flexibility of the dietary fibre polysaccharide konjac glucomannan. Food Hydrocoll. (2009) 23:1910–7. doi: 10.1016/j.foodhyd.2009.02.002
56. Gu J, Catchmark JM. Impact of hemicelluloses and pectin on sphere-like bacterial cellulose assembly. Carbohydr Polym. (2012) 88:547–57. doi: 10.1016/j.carbpol.2011.12.040
57. Meng Y, Lyu F, Xu X, Zhang L. Recent advances in chain conformation and bioactivities of triple-helix polysaccharides. Biomacromolecules. (2020) 21:1653–77. doi: 10.1021/acs.biomac.9b01644
58. Maeda YY, Watanabe ST, Chihara C, Rokutanda M. Denaturation and renaturation of a beta-1,6;1,3-glucan, lentinan, associated with expression of t-cell-mediated responses. Cancer Res. (1988) 48:671–5. doi: 10.1016/0192-0561(88)90374-8
59. Cui W, Huang J, Niu X, Shang H, Sha Z, Miao Y, et al. Screening active fractions from pinus massoniana pollen for inhibiting alv-j replication and their structure activity relationship investigation. Vet Microbiol. (2021) 252:108908. doi: 10.1016/j.vetmic.2020.108908
60. Yuan H, Lan P, He Y, Li C, Ma X. Effect of the modifications on the physicochemical and biological properties of B-Glucan-a critical review. Molecules. (2019) 25. doi: 10.3390/molecules25010057
61. Li S, Li J, Zhi Z, Wei C, Wang W, Ding T, et al. Macromolecular properties and hypolipidemic effects of four sulfated polysaccharides from sea cucumbers. Carbohydr Polym. (2017) 173:330–7. doi: 10.1016/j.carbpol.2017.05.063
62. Lv X, Wang C, Cheng Y, Huang L, Wang Z. Isolation and structural characterization of a polysaccharide Lrp4-a from Lycium Ruthenicum Murr. Carbohydr Res. (2013) 365:20–5. doi: 10.1016/j.carres.2012.10.013
63. Mirhosseini H, Amid BT, Cheong KW. Effect of Different drying methods on chemical and molecular structure of heteropolysaccharide–protein gum from durian seed. Food Hydrocoll. (2013) 31:210–9. doi: 10.1016/j.foodhyd.2012.11.005
64. Zhang X, Liu L, Lin C. Isolation, structural characterization and antioxidant activity of a neutral polysaccharide from sisal waste. Food Hydrocoll. (2014) 39:10–8. doi: 10.1016/j.foodhyd.2013.12.012
65. Zeng H, Miao S, Zhang Y, Lin S, Jian Y, Tian Y, et al. Isolation, preliminary structural characterization and hypolipidemic effect of polysaccharide fractions from fortunella margarita (Lour) swingle. Food Hydrocoll. (2016) 52:126–36. doi: 10.1016/j.foodhyd.2015.05.028
66. Chen F, Ran L, Mi J, Yan Y, Lu L, Jin B, et al. Isolation, characterization and antitumor effect on Du145 cells of a main polysaccharide in pollen of chinese wolfberry. Molecules. (2018) 23. doi: 10.3390/molecules23102430
67. Yang C, Gou Y, Chen J, An J, Chen W, Hu F. Structural characterization and antitumor activity of a pectic polysaccharide from Codonopsis Pilosula. Carbohydr Polym. (2013) 98:886–95. doi: 10.1016/j.carbpol.2013.06.079
68. Zhou S, Rahman A, Li J, Wei C, Chen J, Linhardt RJ, et al. Extraction methods affect the structure of goji (Lycium Barbarum) polysaccharides. Molecules. (2020) 25. doi: 10.3390/molecules25040936
69. Guo L, Du X, Lan J, Liang Q. Study on molecular structural characteristics of tea polysaccharide. Int J Biol Macromol. (2010) 47:244–9. doi: 10.1016/j.ijbiomac.2010.03.026
70. Wang W, Zhang F, Li Q, Chen H, Zhang W, Yu P, et al. Structure characterization of one polysaccharide from lepidium meyenii walp., and its antioxidant activity and protective effect against H[[sb]]2[[/s]]O[[sb]]2[[/s]]-Induced Injury Raw264.7 Cells. Int J Biol Macromol. (2018) 118:816–33. doi: 10.1016/j.ijbiomac.2018.06.117
71. Zhang S, An L, Li Z, Wang X, Wang H, Shi L, et al. Structural elucidation of an immunological arabinan from the rhizomes of ligusticum chuanxiong, a traditional Chinese medicine. Int J Biol Macromol. (2021) 170:42–52. doi: 10.1016/j.ijbiomac.2020.12.069
72. Medeiros PM, Simoneit BR. Analysis of sugars in environmental samples by gas chromatography-mass spectrometry. J Chromatogr A. (2007) 1141:271–8. doi: 10.1016/j.chroma.2006.12.017
73. Ma J, Adler L, Srzednicki G, Arcot J. Quantitative determination of non-starch polysaccharides in foods using gas chromatography with flame ionization detection. Food Chem. (2017) 220:100–7. doi: 10.1016/j.foodchem.2016.09.206
74. Li H, Long C, Zhou J, Liu J, Wu X, Long M. Rapid analysis of mono-saccharides and oligo-saccharides in hydrolysates of lignocellulosic biomass by Hplc. Biotechnol Lett. (2013) 35:1405–9. doi: 10.1007/s10529-013-1224-4
75. Saba JA, Shen X, Jamieson JC, Perreault H. Investigation of different combinations of derivatization, separation methods and electrospray ionization mass spectrometry for standard oligosaccharides and glycans from ovalbumin. J Mass Spectrom. (2001) 36:563–74. doi: 10.1002/jms.158
76. Honda S, Akao E, Suzuki S, Okuda M, Kakehi K, Nakamura J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-Phenyl-3-Methyl-5-Pyrazolone derivatives. Anal Biochem. (1989) 180:351–7. doi: 10.1016/0003-2697(89)90444-2
77. Zhang Z, Khan NM, Nunez KM, Chess EK, Szabo CM. Complete monosaccharide analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. Anal Chem. (2012) 84:4104–10. doi: 10.1021/ac300176z
78. Hentati F, Delattre C, Ursu AV, Desbrières J, Le Cerf D, Gardarin C, et al. Structural characterization and antioxidant activity of water-soluble polysaccharides from the tunisian brown seaweed Cystoseira Compressa. Carbohydr Polym. (2018) 198:589–600. doi: 10.1016/j.carbpol.2018.06.098
79. Du X, Zhang Y, Mu H, Lv Z, Yang Y, Zhang J. Structural elucidation and antioxidant activity of a novel polysaccharide (Tapb1) from Tremella Aurantialba. Food Hydrocoll. (2015) 43:459–64. doi: 10.1016/j.foodhyd.2014.07.004
80. Ren Y, Bai Y, Zhang Z, Cai W, Del Rio Flores A. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: a review of recent development. Molecules. (2019) 24. doi: 10.3390/molecules24173122
81. Miao M, Bai A, Jiang B, Song Y, Cui SW, Zhang T. Characterisation of a novel water-soluble polysaccharide from leuconostoc citreum Sk24002. Food Hydrocoll. (2014) 36:265–72. doi: 10.1016/j.foodhyd.2013.10.014
82. Xiao-Li M, Feng Feng S, Hui Z, Xie H, Song Ya L. Compositional monosaccharide analysis of morus nigra linn by hplc and HPCE quantitative determination and comparison of polysaccharide from Morus Nigra Linn by Hpce and Hplc. Curr Pharm Anal. (2017) 13:433–7. doi: 10.2174/1573412913666170330150807
83. Zhou Y, Duan Y, Huang S, Zhou X, Zhou L, Hu T, et al. Polysaccharides from Lycium Barbarum ameliorate amyloid pathology and cognitive functions in App/Ps1 transgenic mice. Int J Biol Macromol. (2020) 144:1004–12. doi: 10.1016/j.ijbiomac.2019.09.177
84. Liu Y, Yang L, Zhang Y, Liu X, Wu Z, Gilbert RG, et al. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liverglycogen structure. J Ethnopharmacol. (2020) 248:112308. doi: 10.1016/j.jep.2019.112308
85. Chen R, Liu Z, Zhao J, Chen R, Meng F, Zhang M, et al. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax Senticosu. Food Chem. (2011) 127:434–40. doi: 10.1016/j.foodchem.2010.12.143
86. Amicucci MJ, Galermo AG, Nandita E, Vo T-TT, Liu Y, Lee M, et al. A rapid-throughput adaptable method for determining the monosaccharide composition of polysaccharides. Int J Mass Spectrom. (2019) 438:22–8. doi: 10.1016/j.ijms.2018.12.009
87. Ciucanu I, Caprita R. Per-O-Methylation of neutral carbohydrates directly from aqueous samples for gas chromatography and mass spectrometry analysis. Anal Chim Acta. (2007) 585:81–5. doi: 10.1016/j.aca.2006.12.015
88. Guan J, Li SP. Discrimination of polysaccharides from traditional chinese medicines using saccharide mapping—enzymatic digestion followed by chromatographic analysis. J Pharm Biomed Anal. (2010) 51:590–8. doi: 10.1016/j.jpba.2009.09.026
89. Neeser JR, Schweizer TF. A quantitative determination by capillary gas-liquid chromatography of neutral and amino sugars (as O-Methyloxime Acetates), and a study on hydrolytic conditions for glycoproteins and polysaccharides in order to increase sugar recoveries. Anal Biochem. (1984) 142:58–67. doi: 10.1016/0003-2697(84)90516-5
90. Ip CC, Manam V, Hepler R, Hennessey JP. Carbohydrate composition analysis of bacterial polysaccharides: optimized acid hydrolysis conditions for hpaec-pad analysis. Anal Biochem. (1992) 201:343–9. doi: 10.1016/0003-2697(92)90349-C
91. Tang Y, Zhu ZY, Pan LC, Sun H, Song QY, Zhang Y. Structure analysis and anti-fatigue activity of a polysaccharide from lepidium meyenii walp. Nat Prod Res. (2019) 33:2480–9. doi: 10.1080/14786419.2018.1452017
92. Ciucanu I, Kerek F. A Simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. (1984) 131:209–17. doi: 10.1016/0008-6215(84)85242-8
93. Hakomori S-I. A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J Biol Chem. (1964) 55:205–8.
94. Shao P, Chen X, Sun P. Improvement of antioxidant and moisture-preserving activities of sargassum horneri polysaccharide enzymatic hydrolyzates. Int J Biol Macromol. (2015) 74:420–7. doi: 10.1016/j.ijbiomac.2014.12.021
95. Xia YG, Wang TL, Sun LM, Liang J, Yang BY, Kuang HX, et al. New Uplc-Ms/Ms method for the characterization and discrimination of polysaccharides from genus ephedra based on enzymatic digestions. Molecules. (2017) 22:12. doi: 10.3390/molecules22111992
96. Amicucci MJ, Nandita E, Galermo AG, Castillo JJ, Chen S, Park D, et al. A nonenzymatic method for cleaving polysaccharides to yield oligosaccharides for structural analysis. Nat Commun. (2020) 11:3963. doi: 10.1038/s41467-020-17778-1
97. Niu L-L, Wu Y-R, Liu H-P, Wang Q, Li M-Y, Jia Q. Optimization of extraction process, characterization and antioxidant activities of polysaccharide from Leucopaxillus Giganteus. J Food Meas Charact. (2021) 15:2842–53. doi: 10.1007/s11694-021-00865-2
98. Zhang X, Kong X, Hao Y, Zhang X, Zhu Z. Chemical Structure and Inhibition on Alpha-Glucosidase of Polysaccharide with Alkaline-Extracted from Glycyrrhiza Inflata Residue. Int J Biol Macromol. (2019) 147:1125–35. doi: 10.1016/j.ijbiomac.2019.10.081
99. Zhang G, Yin Q, Han T, Zhao Y, Su J, Li M, et al. Purification and antioxidant effect of novel fungal polysaccharides from the stroma of cordyceps kyushuensis. Ind Crops Prod. (2015) 69:485–91. doi: 10.1016/j.indcrop.2015.03.006
100. Huang H, Huang G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem Biol Drug Des. (2020) 96:1209–22. doi: 10.1111/cbdd.13794
101. Zhang H, Zou P, Zhao H, Qiu J, Regenstein JM, Yang X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of pinus koraiensis. Carbohydr Polym. (2021) 251:117078. doi: 10.1016/j.carbpol.2020.117078
102. Wu X, Jiang W, Lu J, Yu Y, Wu B. Analysis of the monosaccharide composition of water-soluble polysaccharides from sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem. (2014) 145:976–83. doi: 10.1016/j.foodchem.2013.09.019
103. Rühmann B, Schmid J, Sieber V. Fast carbohydrate analysis via liquid chromatography coupled with ultra violet and electrospray ionization ion trap detection in 96-well format. J Chromatogr A. (2014) 1350:44–50. doi: 10.1016/j.chroma.2014.05.014
104. Xu G, Amicucci MJ, Cheng Z, Galermo AG, Lebrilla CB. Revisiting monosaccharide analysis - quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring. Analyst. (2017) 143:200–7. doi: 10.1039/C7AN01530E
105. Amicucci MJ, Galermo AG, Guerrero A, Treves G, Nandita E, Kailemia MJ, et al. Strategy for structural elucidation of polysaccharides: elucidation of a maize mucilage that harbors diazotrophic bacteria. Anal Chem. (2019) 91:7254–65. doi: 10.1021/acs.analchem.9b00789
106. Chuang L, Panyoyai N, Shanks R, Kasapis S. Effect of sodium chloride on the glass transition of condensed starch systems. Food Chem. (2015) 184:65–71. doi: 10.1016/j.foodchem.2015.03.031
107. Guérin G, Raez J, Manners I, Winnik MA. Light scattering study of rigid, rodlike organometallic block copolymer micelles in dilute solution. Macromolecules. (2005) 38:7819–27. doi: 10.1021/ma0498870
108. Ioan CE, Aberle T, Burchard W. Light scattering and viscosity behavior of dextran in semidilute solution. Macromolecules. (2001) 34:326–36. doi: 10.1021/ma992060z
109. Kratky O, Porod G. Röntgenuntersuchung gelöster fadenmoleküle. Recl Trav Chim Pays-Bas. (1949) 68:1106–22. doi: 10.1002/recl.19490681203
110. Jayme M, Ames F, Bersani-Amado C, Machado M, Mangolin C, Gonçalves R, et al. Primary characterization and evaluation of anti ulcerogenic activity of an aqueous extract from callus culture of cereus peruvianus mill. (Cactaceae). Curr Pharm Biotechnol. (2015) 16. doi: 10.2174/1389201016666150303154342
111. Xu S, Xu X, Zhang L. Effect of heating on chain conformation of branched β-glucan in Water. J Phys Chem B. (2013) 117:8370–7. doi: 10.1021/jp403202u
112. Tao Y, Yan Y, Xu W. Shrinking factors of hyperbranched polysaccharide from fungus. Carbohydr Res. (2009) 344:1311–8. doi: 10.1016/j.carres.2009.05.004
113. Renard D, Lepvrier E, Garnier C, Roblin P, Nigen M, Sanchez C. Structure of glycoproteins from acacia gum: an assembly of ring-like glycoproteins modules. Carbohydr Polym. (2014) 99:736–47. doi: 10.1016/j.carbpol.2013.08.090
114. Shao L, Wu Z, Tian F, Zhang H, Liu Z, Chen W, et al. Molecular characteristics of an exopolysaccharide from lactobacillus rhamnosus Kf5 in Solution. Int. J. Biol. Macromol. (2015) 72:1429–34. doi: 10.1016/j.ijbiomac.2014.10.015
115. Li S, Huang Y, Wang S, Xu X, Zhang L. Determination of the triple helical chain conformation of β-glucan by facile and reliable triple-detector size exclusion chromatography. J Phys Chem B. (2014) 118:668–75. doi: 10.1021/jp4087199
116. Shakun M, Maier H, Heinze T, Kilz P, Radke W. Molar Mass Characterization of Sodium Carboxymethyl Cellulose by Sec-Malls. Carbohydr Polym. (2013) 95:550–9. doi: 10.1016/j.carbpol.2013.03.028
117. Deng Y, Li M, Chen L-X, Chen X-Q, Lu J-H, Zhao J, et al. Chemical Characterization and Immunomodulatory Activity of Acetylated Polysaccharides from Dendrobium Devonianum. Carbohydr Polym. (2018) 180:238–45. doi: 10.1016/j.carbpol.2017.10.026
118. Sillrén P, Swenson J, Mattsson J, Bowron D, Matic A. The temperature dependent structure of liquid 1-propanol as studied by neutron diffraction and epsr simulations. J Chem Phys. (2013) 138:214501. doi: 10.1063/1.4807863
119. Feng L, Yin J, Nie S, Wan Y, Xie M. Structure and conformation characterization of galactomannan from seeds of Cassia Obtusifolia. Food Hydrocoll. (2018) 76:67–77. doi: 10.1016/j.foodhyd.2017.06.008
120. Arinaitwe E, Pawlik M. Dilute solution properties of carboxymethyl celluloses of various molecular weights and degrees of substitution. Carbohydr Polym. (2014) 99:423–31. doi: 10.1016/j.carbpol.2013.08.030
121. Zhang Z, Guo L, Yan A, Feng L, Wan Y. Fractionation, structure and conformation characterization of polysaccharides from Anoectochilus Roxburghii. Carbohydr Polym. (2020) 231:115688. doi: 10.1016/j.carbpol.2019.115688
122. Wang Y, Li Y, Ma X, Ren H, Fan W, Leng F, et al. Extraction, purification, and bioactivities analyses of polysaccharides from Glycyrrhiza Uralensis. Ind Crops Prod. (2018) 122:596–608. doi: 10.1016/j.indcrop.2018.06.011
123. Zhao S, Han Z, Yang L, Hong B, Zhu H. Extraction, characterization and antioxidant activity evaluation of polysaccharides from Smilacina Japonica. Int J Biol Macromol. (2020) 151:576–83. doi: 10.1016/j.ijbiomac.2020.02.015
124. Hu J-L, Nie S-P, Li C, Wang S, Xie M-Y. Ultrasonic irradiation induces degradation and improves prebiotic properties of polysaccharide from seeds of plantago asiatica l. during in vitro fermentation by human fecal microbiota. Food Hydrocoll. (2018) 76:60–6. doi: 10.1016/j.foodhyd.2017.06.009
125. Ma F, Wang D, Zhang Y, Li M, Qing W, Tikkanen-Kaukanen C, et al. Characterisation of the mucilage polysaccharides from dioscorea opposita thunb. With Enzymatic Hydrolysis Food Chem. (2018) 245:13–21. doi: 10.1016/j.foodchem.2017.10.080
126. Rozi P, Abuduwaili A, Mutailifu P, Gao Y, Rakhmanberdieva R, Aisa HA, et al. Sequential extraction, characterization and antioxidant activity of polysaccharides from fritillaria pallidiflora schrenk. Int J Biol Macromol. (2019) 131:97–106. doi: 10.1016/j.ijbiomac.2019.03.029
127. Li Q, Wang W, Zhu Y, Chen Y, Zhang W, Yu P, et al. Structural elucidation and antioxidant activity a novel se-polysaccharide from se-enriched Grifola Frondosa. Carbohydr Polym. (2017) 161:42–52. doi: 10.1016/j.carbpol.2016.12.041
128. Kong L, Yu L, Feng T, Yin X, Liu T, Dong L. Physicochemical characterization of the polysaccharide from bletilla striata: effect of drying method. Carbohydr Polym. (2015) 125:1–8. doi: 10.1016/j.carbpol.2015.02.042
129. McIntire TM, Brant DA. Observations of the (1 → 3)-B-D-Glucan linear triple helix to macrocycle interconversion using noncontact atomic force microscopy. J Am Chem Soc. (1998) 120:6909–19. doi: 10.1021/ja981203e
130. Schefer L, Adamcik J, Mezzenga R. Unravelling secondary structure changes on individual anionic polysaccharide chains by atomic force microscopy. Angew Chem Int Ed. (2014) 53:5376–9. doi: 10.1002/anie.201402855
131. Zhao T, Mao G, Zhang M, Feng W, Mao R, Zhu Y, et al. Structure analysis of a bioactive heteropolysaccharide from schisandra chinensis (Turcz) Baill. Carbohydr Polym. (2014) 103:488–95. doi: 10.1016/j.carbpol.2013.12.058
132. Gao Y, Zhou Y, Zhang Q, Zhang K, Peng P, Chen L, et al. Hydrothermal extraction, structural characterization, and inhibition hela cells proliferation of functional polysaccharides from chinese tea zhongcha 108. J Funct Foods. (2017) 39:1–8. doi: 10.1016/j.jff.2017.09.057
133. Duan B, Zou S, Sun Y, Xu X. Nanoplatform constructed from a β-glucan and polydeoxyadenylic acid for cancer chemotherapy and imaging. Biomacromolecules. (2019) 20:1567–77. doi: 10.1021/acs.biomac.8b01780
134. Liu Q, Xu X, Zhang L. Variable chain conformations of renatured β-glucan in dimethylsulfoxide/water mixture. Biopolymers. (2012) 97:988–97. doi: 10.1002/bip.22115
135. Zhang W, Huang J, Wang W, Li Q, Chen Y, Feng W, et al. Extraction, Purification, Characterization and Antioxidant Activities of Polysaccharides from Cistanche Tubulosa. Int J Biol Macromol. (2016) 93:448–58. doi: 10.1016/j.ijbiomac.2016.08.079
136. Wang J, Niu S, Zhao B, Luo T, Liu D, Zhang J. Catalytic synthesis of sulfated polysaccharides. ii: comparative studies of solution conformation and antioxidant activities. Carbohydr Polym. (2014) 107:221–31. doi: 10.1016/j.carbpol.2014.02.074
137. Liu Y, Zhang J, Tang Q, Yang Y, Guo Q, Wang Q, et al. Physicochemical characterization of a high molecular weight bioactive β-d-glucan from the fruiting bodies of Ganoderma Lucidum. Carbohydr Polym. (2014) 101:968–74. doi: 10.1016/j.carbpol.2013.10.024
138. Wang B, Wang X, Xiong Z, Lu G, Ma W, Lv Q, et al. A review on the applications of traditional chinese medicine polysaccharides in drug delivery systems. Chinese Med. (2022) 17:12. doi: 10.1186/s13020-021-00567-3
139. Wang Y, Zhang L, Li Y, Hou X, Zeng F. Correlation of structure to antitumor activities of five derivatives of a beta-glucan from poria cocos sclerotium. Carbohydr Res. (2004) 339:2567–74. doi: 10.1016/j.carres.2004.08.003
140. Zhao Y, Tian N, Wang H, Yan H. Chemically sulfated polysaccharides from agaricus blazei murill: synthesis, characterization and anti-hiv activity. Chem Biodiversity. (2021) 18:e2100338. doi: 10.1002/cbdv.202100338
141. Wang C, He Y, Tang X, Li N. Sulfation, structural analysis, and anticoagulant bioactivity of ginger polysaccharides. J Food Sci. (2020) 85:2427–34. doi: 10.1111/1750-3841.15338
142. Wang X, Zhang Z, Yao Q, Zhao M, Qi H. Phosphorylation of low-molecular-weight polysaccharide from enteromorpha linza with antioxidant activity. Carbohydr Polym. (2013) 96:371–5. doi: 10.1016/j.carbpol.2013.04.029
143. Abuduwaili A, Nuerxiati R, Mutailifu P, Gao Y, Lu C, Yili A. Isolation, structural modification, characterization, and bioactivity of polysaccharides from folium isatidis. Ind Crops Prod. (2022) 176:114319. doi: 10.1016/j.indcrop.2021.114319
144. Wang Z-J, Xie J-H, Shen M-Y, Tang W, Wang H, Nie S-P, et al. Carboxymethylation of polysaccharide from cyclocarya paliurus and their characterization and antioxidant properties evaluation. Carbohydr Polym. (2016) 136:988–94. doi: 10.1016/j.carbpol.2015.10.017
145. Li CY, Liu L, Zhao YW, Chen JY, Sun XY, Ouyang JM. Inhibition of calcium oxalate formation and antioxidant activity of carboxymethylated Poria Cocos Polysaccharides. Oxid Med Cell Longevity. (2021) 2021:6653593. doi: 10.1155/2021/6653593
146. Wang Y, Mo Q, Li Z, Lai H, Lou J, Liu S, et al. Effects of degree of carboxymethylation on physicochemical and biological properties of pachyman. Int J Biol Macromol. (2012) 51:1052–6. doi: 10.1016/j.ijbiomac.2012.08.022
147. Liu X, Wang X, Xu X, Zhang X. Purification, antitumor and anti-inflammation activities of an alkali-soluble and carboxymethyl polysaccharide cmp33 from Poria Cocos. Int J Biol Macromol. (2019) 127:39–47. doi: 10.1016/j.ijbiomac.2019.01.029
148. Chen F, Huang G. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int J Biol Macromol. (2019) 141:14–20. doi: 10.1016/j.ijbiomac.2019.08.239
149. Chen Y, Zhang H, Wang Y, Nie S, Li C, Xie M. Acetylation and carboxymethylation of the polysaccharide from ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. (2014) 156:279–88. doi: 10.1016/j.foodchem.2014.01.111
150. Song Y, Yang Y, Zhang Y, Duan L, Zhou C, Ni Y, et al. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita Pepo, Lady Godiva). Carbohydr Polym. (2013) 98:686–91. doi: 10.1016/j.carbpol.2013.06.049
151. Liu X, Xie J, Jia S, Huang L, Wang Z, Li C, et al. Immunomodulatory Effects of an Acetylated Cyclocarya Paliurus Polysaccharide on Murine Macrophages Raw2647. Int J Biol Macromol. (2017) 98:576–81. doi: 10.1016/j.ijbiomac.2017.02.028
152. Liu W, Xu J, Jing P, Yao W, Gao X, Yu L. Preparation of a Hydroxypropyl Ganoderma Lucidum Polysaccharide and Its Physicochemical Properties. Food Chem. (2010) 122:965–71. doi: 10.1016/j.foodchem.2009.11.087
153. Ren YY, Sun PP Ji YP, Wang XT Dai SH, Zhu ZY. Carboxymethylation and acetylation of the polysaccharide from cordyceps militaris and their α-glucosidase inhibitory activities. Nat Prod Res. (2020) 34:369–77. doi: 10.1080/14786419.2018.1533830
154. Yuan D, Li C, Huang Q, Fu X. Ultrasonic degradation effects on the physicochemical, rheological and antioxidant properties of polysaccharide from sargassum pallidum. Carbohydr Polym. (2020) 239:116230. doi: 10.1016/j.carbpol.2020.116230
155. Yan J-K, Wang Y-Y, Ma H-L, Wang Z-B. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from phellinus linteus mycelia. Ultrason Sonochem. (2016) 29:251–7. doi: 10.1016/j.ultsonch.2015.10.005
156. Xiao J, Chen X, Zhan Q, Zhong L, Hu Q, Zhao L. Effects of ultrasound on the degradation kinetics, physicochemical properties and prebiotic activity of flammulina velutipes polysaccharide. Ultrason Sonochem. (2022) 82:105901. doi: 10.1016/j.ultsonch.2021.105901
157. Jia Y, Lu Y, Wang Y, Zhang M, He C, Chen H. Spheroidization of ultrasonic degraded corn silk polysaccharide to enhance bioactivity by the anti-solvent precipitation method. J Sci Food Agric. (2022) 102:53–61. doi: 10.1002/jsfa.11329
158. Wu Q, Qin D, Cao H, Bai Y. Enzymatic hydrolysis of polysaccharide from auricularia auricula and characterization of the degradation product. Int J Bio. Macromol. (2020) 162:127–35. doi: 10.1016/j.ijbiomac.2020.06.098
159. Hu TG, Zou YX Li EN, Liao ST, Wu H, Wen P. Effects of enzymatic hydrolysis on the structural, rheological, and functional properties of mulberry leaf polysaccharide. Food Chem. (2021) 355:129608. doi: 10.1016/j.foodchem.2021.129608
160. Liu X, Ren Z, Yu R, Chen S, Zhang J, Xu Y, et al. Structural characterization of enzymatic modification of hericium erinaceus polysaccharide and its immune-enhancement activity. Int J Biol Macromol. (2021) 166:1396–408. doi: 10.1016/j.ijbiomac.2020.11.019
161. Huang S, Chen F, Cheng H, Huang G. Modification and application of polysaccharide from traditional chinese medicine such as dendrobium officinale. Int J Biol Macromol. (2020) 157:385–93. doi: 10.1016/j.ijbiomac.2020.04.141
Keywords: Chinese herbal polysaccharides, structure-activity relationship, structural elucidation, modification, bioactivity
Citation: Wang B, Yan L, Guo S, Wen L, Yu M, Feng L and Jia X (2022) Structural Elucidation, Modification, and Structure-Activity Relationship of Polysaccharides in Chinese Herbs: A Review. Front. Nutr. 9:908175. doi: 10.3389/fnut.2022.908175
Received: 30 March 2022; Accepted: 22 April 2022;
Published: 20 May 2022.
Edited by:
Xiaolong Ji, Zhengzhou University of Light Industry, ChinaCopyright © 2022 Wang, Yan, Guo, Wen, Yu, Feng and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Feng, d2VubW94aXVzaGlAMTYzLmNvbQ==; Xiaobin Jia, amlheGlhb2JpbjIwMTVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Bei Wang†
Bei Wang† Lingling Yan
Lingling Yan Liang Feng
Liang Feng