
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Nutr. , 02 September 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.902986
This article is part of the Research Topic Intensive Care Unit Acquired Weakness: Potential Role of Medical Nutrition Treatment Quantity, Timing, and Composition View all 9 articles
 Cheng Lv1†
Cheng Lv1† Xingwei Jiang2†
Xingwei Jiang2† Yi Long3
Yi Long3 Zirui Liu1
Zirui Liu1 Jiajia Lin1
Jiajia Lin1 Cuili Wu4
Cuili Wu4 Xianghong Ye4
Xianghong Ye4 Ruiling Ye3
Ruiling Ye3 Yuxiu Liu1
Yuxiu Liu1 Man Liu1
Man Liu1 Yang Liu1
Yang Liu1 Wensong Chen5
Wensong Chen5 Lin Gao1
Lin Gao1 Zhihui Tong1
Zhihui Tong1 Lu Ke1,6*
Lu Ke1,6* Zhengying Jiang3*
Zhengying Jiang3* Weiqin Li1,6*
Weiqin Li1,6*  for the Chinese Critical Care Nutrition Trials Group (CCCNTG)
for the Chinese Critical Care Nutrition Trials Group (CCCNTG)Background: There is controversy over the optimal energy delivery in intensive care units (ICUs). In this study, we aimed to evaluate the association between different caloric adequacy assessed by a weight-based equation and short-term clinical outcomes in a cohort of critically ill patients.
Methods: This is a secondary analysis of a cluster-randomized controlled trial (N = 2,772). The energy requirement was estimated as 25 kcal/kg of body weight. The study subjects were divided into three groups according to their caloric adequacy as calculated by the mean energy delivered from days 3 to 7 of enrollment divided by the estimated energy requirements: (1) received < 70% of energy requirement (hypocaloric), (2) received 70–100% of energy requirement (normocaloric), and (3) received > 100% of energy requirement (hypercaloric). Cox proportional hazards models were used to analyze the association between caloric adequacy and 28-day mortality and time to discharge alive from the ICU.
Results: A total of 1,694 patients were included. Compared with normocaloric feeding, hypocaloric feeding significantly increased the risk of 28-day mortality (hazard ratio [HR] = 1.590, 95% confidence interval [CI]: 1.162–2.176, p = 0.004), while hypercaloric feeding did not. After controlling for potential confounders, the association remained valid (adjusted HR = 1.596, 95% CI: 1.150–2.215, p = 0.005). The caloric adequacy was not associated with time to discharge alive from the ICU in the unadjusted and the adjusted models.
Conclusion: Energy delivery below 70% of the estimated energy requirement during days 3–7 of critical illness is associated with 28-day mortality.
Clinical trial registration: [https://www.isrctn.com/ISRCTN12233792], identifier [ISRCTN12233792].
Nutrition therapy is essential in the management of critically ill patients, as they are vulnerable to energy/protein deficits due to severe catabolism and inadequate intake, which may result in increased infectious complications and prolonged intensive care unit (ICU) stay (1, 2). However, the optimal energy delivery for critical illness remains controversial, particularly during the early phase of ICU admission (3, 4). Notably, both insufficient and excessive calorie intakes are reported to be associated with poor outcomes (5–8).
The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines (9) recommend progressively providing energy to avoid overfeeding and cover at least 70% of the needs within 3–7 days. The recommendation rests on a large retrospective study including 1,171 critically ill patients, which revealed that the optimal energy intake appeared to be between 70 and 100% of the energy requirement (8). Since they used indirect calorimetry (IC) for energy requirement measurement, the generalizability of this finding in patients without IC was unknown (10). Given that IC is not readily available in all settings, especially in medium and low-income countries, weight-based equations are commonly applied in the absence of IC measurement (9, 11–14).
In the present study, we classified caloric adequacy using the three-category system (<70% as hypocaloric, 70–100% as normocaloric, and >100% as hypercaloric) based on a weight-based equation (25 kcal/kg/day). We aimed to investigate the association between caloric adequacy and short-term clinical outcomes in a cohort of critically ill patients from a large trial.
This study is a post hoc analysis of a multicenter, cluster-randomized, controlled trial assessing the impact of an evidence-based nutrition guideline in critically ill patients (15). The trial was approved by the local hospital ethics committees of all the participating ICUs and registered at the ISRCTN registry (ISRCTN12233792). Briefly, a total of 2,772 patients with an expected ICU stay longer than 7 days were enrolled within 24 h of ICU admission from 90 ICUs between March 26, 2018 and July 4, 2019. Our analysis is conducted on a subset of the participants. Inclusion criteria were (1) an ICU stay longer than 3 days and (2) having at least one evaluable nutrition day. We excluded patients who received PN during the first 48 h in light of the mainstream guidelines (9, 16). Additionally, patients who received an oral diet were also excluded because the calories from the oral diet cannot be calculated. Evaluable nutrition days were defined as the days when patients only received artificial nutrition (EN or PN) from days 3 to 7 after enrollment.
Energy intake was calculated only on the evaluable days because the calories from the oral diet were impossible to calculate precisely. The caloric adequacy was determined by comparing the mean actual mean daily energy delivery from days 3 to 7 of enrollment with the target energy requirement as a percentage. The target energy requirements were estimated based on a simple weight-based equation (25 kcal/kg of actual body weight on admission day).
All eligible patients were divided into three groups according to their caloric adequacy: (1) received < 70% of energy requirement (hypocaloric group), (2) received 70–100% of energy requirement (normocaloric group), and (3) received > 100% of energy requirement (hypercaloric group).
The coprimary outcomes are 28-day mortality and time to discharge alive from the ICU. The secondary outcomes include the length of ICU stay and new receipt of organ support therapy within the first 7 days after enrollment. Time to discharge alive from the ICU was only considered in survivors and censored at 28 days after enrollment. New receipt of organ support therapy was defined as a requirement of organ support therapy (mechanical ventilation, renal replacement therapy, and vasoactive agents) not applied at enrollment.
All data were extracted from the electronic database, such as de-identified data on patient characteristics, daily nutritional therapy, and utilization of organ support. The baseline characteristics included age, gender, weight, body mass index (BMI), modified Nutrition Risk in the Critically Ill (mNUTRIC) score (17), site before ICU admission, comorbidities, Acute Gastrointestinal Injury (AGI) score (18), and severity scores, such as Acute Physiology and Chronic Health Evaluation II (APACHE II) score (19) and sequential organ failure assessment (SOFA) score (20) at enrollment. Nutrition therapy variables included daily nutritional route (oral, enteral, and parenteral), enteral or parenteral formulas, protein supplements, hours with feed, and the feed-volume delivered in milliliters. In addition, energy provided by dextrose-containing intravenous fluids was included in the calculation of total energy intake. Nutritional intake was recorded within the first 7 days after enrollment or until discharge from the ICU or death, whichever occurred first.
The analysis was performed using SPSS version 25 (SPSS Inc., Chicago, IL, United States) and R software version 4.1.0. The normality of continuous variables was examined by the Kolmogorov–Smirnov test. Continuous data were presented as mean ± standard deviation (SD) or median (interquartile range, IQR). Categorical data were presented as numbers and percentages. Differences in baseline and nutritional characteristics among the three energy delivery categories were compared using the one-way analysis of variance (ANOVA) or the Kruskal–Wallis tests for continuous data and the chi-square test for categorical data. A Bonferroni correction was applied for the post hoc pairwise comparisons. The Kaplan–Meier methods were used to display survival curves for time to discharge alive from the ICU within 28 days after enrollment and 28-day mortality. Noncross- and cross-survival curves were compared by the log-rank test and two-stage test (21), respectively.
Cox proportional hazards models were used to analyze the effect of caloric adequacy on 28-day mortality and time to discharge alive from the ICU. The covariates, such as age, gender, BMI, APACHE II score, SOFA score, number of comorbidities, mNUTIRC score, the number of evaluable nutrition days, initiation of enteral nutrition within 48 h, mean parental nutrition intake from days 3 to 7, and AGI score were entered separately in the multivariable model. The relationships between caloric adequacy and the two coprimary outcomes were tested in the unadjusted and the adjusted models. Based on unadjusted analyses, variables of clinical significance or with p < 0.1 were considered covariates and included in the adjusted model 1.
The multicollinearity between potential confounding factors was evaluated in the multivariable model by assessing the variance inflation factor (VIF) (22, 23). The fit of the Cox model was assessed using Cox-Snell residuals. The proportional hazards assumption was tested by plotting Schoenfeld residuals. A two-tailed p-value of <0.05 was considered significant.
All the 2,772 trial participants were screened for potential inclusion, and 1,694 patients were included in the current analysis (Figure 1). A total of 823 patients were assigned to the hypocaloric group, 571 to the normocaloric group, and 300 to the hypercaloric group. Table 1 describes the baseline characteristics and clinical outcomes of the study population among three different categories of energy adequacy. The majority of patients had a mild gastrointestinal dysfunction with AGI grade I (n = 1,251, 73.8%) and were admitted to the emergency department (n = 706, 41.7%). The overall 28-day mortality was 13.1% (222/1694), and the median length of ICU stay was 16 days (interquartile range: 9–28 days). The variables of age, gender, BMI, and SOFA score were different among the three groups, whereas others were similar among the groups.
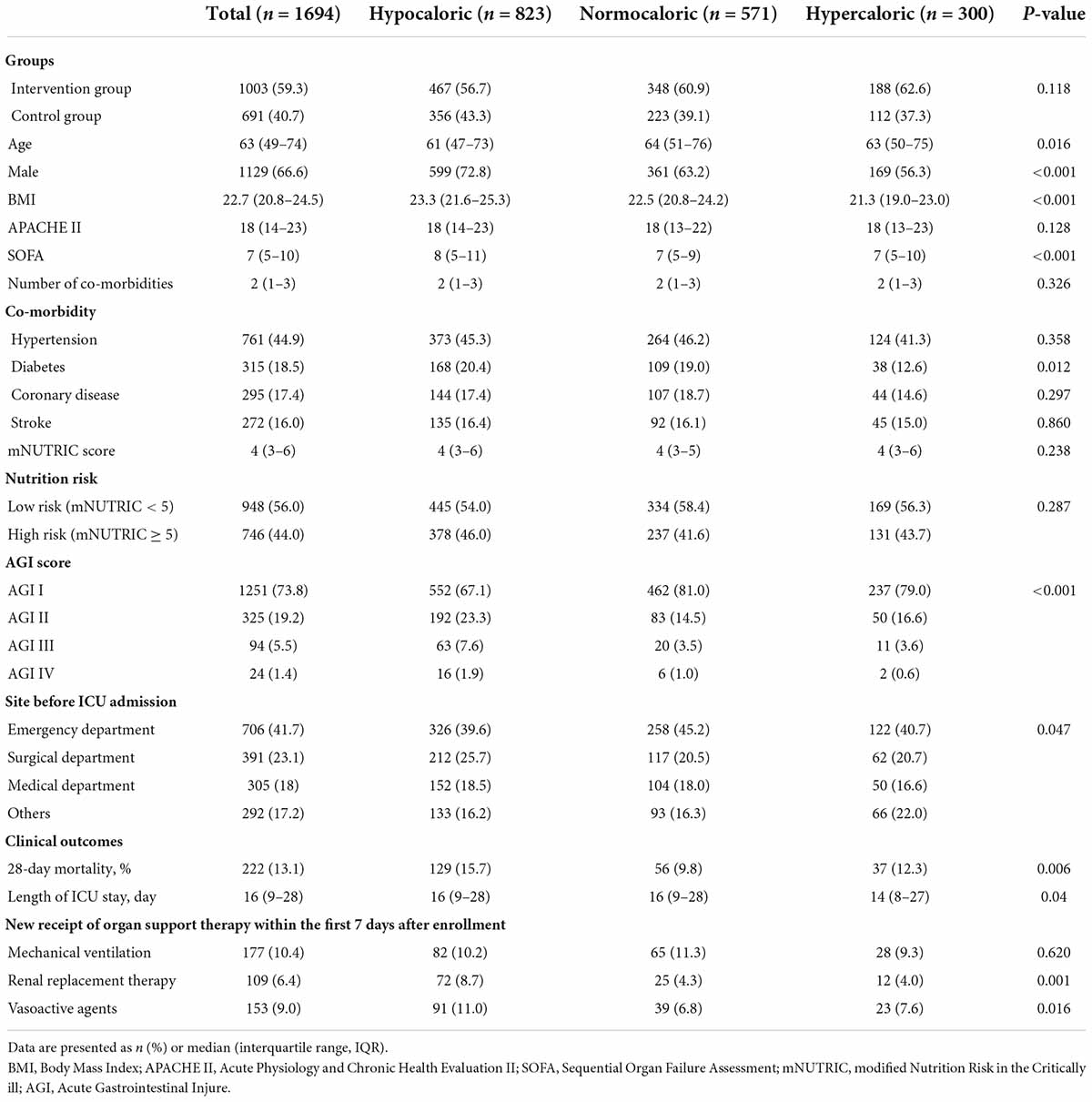
Table 1. Baseline characteristics and clinical outcomes in the study population and among three different categories of energy adequacy.
Data regarding nutrition therapy are shown in Table 2. The mean number of evaluable nutrition days was 4.49 days during the study period. The mean time of EN initiation was 2.17 days from study enrollment. There was no significance in prokinetics use among the three groups. The daily calories from days 3 to 7 after enrollment are shown in Figure 2. Overall, study patients received mean energy of 1,155.4 ± 456.5 kcal/day accounting for 73.8% of their estimated energy requirement. The mean protein intake was 45.3 ± 18.7 g/day.
After the multicollinearity analysis for coprimary outcomes, the mNUTRIC score and protein intake were removed from the adjusted models given to its collinearity with SOFA score and calorie intake, respectively. After performing a univariate Cox analysis for coprimary outcomes, factors of clinical importance or those with a p-value of < 0.1 were then taken into the multivariate model as potential confounders. In terms of the 28-day mortality, the variables, such as age, gender, BMI, initiation of enteral nutrition within 48 h, mean parental nutrition intake from days 3 to 7, the number of evaluable nutrition days, SOFA score, and the number of comorbidities were entered into a multivariable model. For the outcome of time to discharge alive from the ICU, the covariates include age, gender, BMI, initiation of enteral nutrition within 48 h, the mean parental nutrition intake from days 3 to 7, and SOFA score. The relationships of caloric adequacy with 28-day mortality and time to discharge alive from the ICU are presented in Table 3. Compared with normocaloric feeding, hypocaloric feeding significantly increased the risk of 28-day mortality (hazard ratio [HR] = 1.590, 95% CI: 1.162–2.176, p = 0.004), while hypercaloric feeding did not (HR = 1.394, 95% CI: 0.920–2.112, p = 0.117). The association remains valid after controlling for potential confounders (age, gender, BMI, the number of evaluable nutrition days, initiation of enteral nutrition within 48 h, the mean parental nutrition intake from days 3 to 7, SOFA score, and the number of comorbidities), with an adjusted HR of 1.596 (95% CI: 1.150–2.215, p = 0.005). The caloric adequacy was not associated with time to discharge alive from the ICU in the unadjusted and the adjusted models.
The Kaplan–Meier survival curves for the association of caloric adequacy with coprimary outcomes are shown in Figure 3. In terms of the 28-day mortality, the hypocaloric group was associated with a poor outcome compared with the normocaloric group (Figure 3A, ptwo–stage = 0.01), while other comparisons did not differ. Meanwhile, there was no significant difference in the time to discharge alive from the ICU among the three groups (Figure 3B, plog–rank = 0.0633).
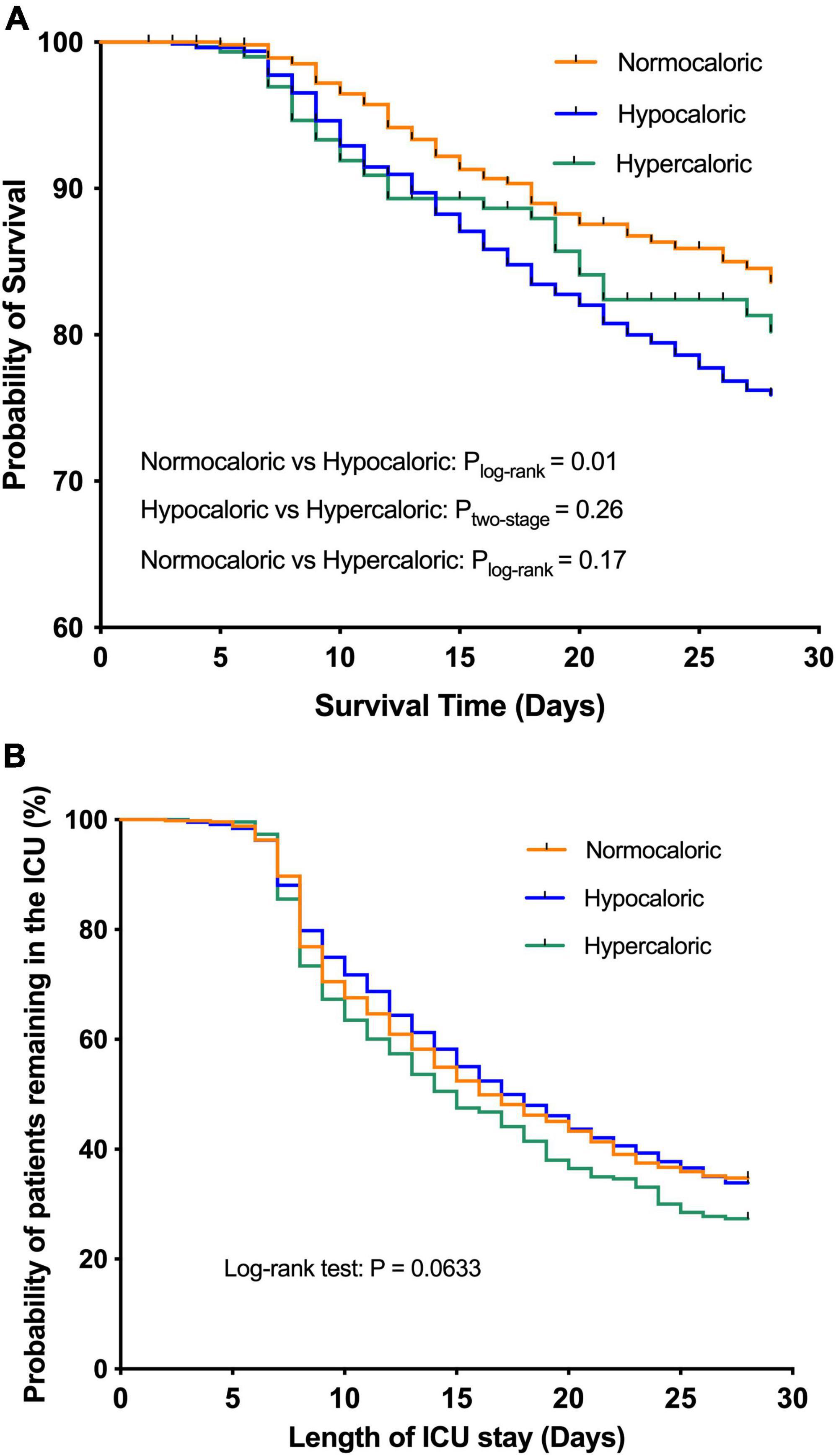
Figure 3. The Kaplan–Meier survival curves for the association of caloric adequacy with coprimary outcomes. (A) 28-day mortality. (B) Time to discharge alive from intensive care unit (ICU).
Our study found that hypocaloric feeding (below 70% of energy requirement estimated by a weight-based equation) was associated with increased 28-day mortality, but caloric adequacy was not associated with time to discharge alive from the ICU in the unadjusted and adjusted models.
The optimal calorie intake remains controversial since many studies have shown conflicting results regarding the impact of different caloric adequacy on outcomes in critically ill patients. Several observational studies demonstrated that early target reaching is beneficial (24–26), whereas others reported that near-target caloric intake is associated with adverse outcomes (27, 28) and suggested hypocaloric nutrition (29, 30). Two randomized clinical trials (RCTs) found that low-dose caloric feeding during the first few days of ICU admission improved patient outcomes (5, 28), while others failed to detect a difference between underfeeding and full-energy feeding (31, 32). The heterogeneity in the study populations, different methods for resting energy expenditure (REE) measurements, and other clinical confounders, such as protein intake may account for these disputable conclusions.
The time range we selected in this study is based on the 2019 ESPEN guidelines (9), which divide the acute phase of critical illness into an early period (days 1–2) and a late period (days 3–7) and recommend progressively providing energy to cover at least 70% of the needs within days 3–7 (8). This definition is based on the pathophysiology of critical illness and previous studies. To make our results comparable with previous major studies and guidelines, we chose the same time range for this study. Our results showed that energy delivery below 70% of the energy requirement was associated with increased mortality, which is in line with Zusman et al.’s study (8). However, there was no difference between the hypercaloric feeding and the normocaloric feeding groups. A possible explanation for that is the different methods adopted for energy requirement estimation (IC vs. equation). According to the guidelines, IC is recommended as the optimum method for determining energy requirements among critically ill patients (9, 11). It can facilitate personalized, targeted nutrition therapy for critically ill patients (33). However, IC equipment is not readily accessible everywhere due to its high cost and technical clumsiness (10, 34, 35). Alternatively, the weight-based predictive equation, also recommended by the ASPEN guidelines (11), was widely used to estimate energy requirements because of its simplicity (10), although predictive equations might result in inaccurate energy requirement estimation (36). Previous studies showed that the weight-based predictive equation (25 kcal/kg/day) could provide a relatively accurate estimation of energy requirement (37, 38).
For nutrition therapy in critically ill patients, several observational studies have shown that adequate protein intake might outweigh calorie intake (39, 40). Two large-scale RCTs also confirmed the same (32, 41). The PERMIT trial compared permissive underfeeding (40–60% of caloric target) with standard enteral (70–100% of caloric target) while ensuring similar protein intake by supplements and found no differences in outcomes (32). Similarly, the TARGET trial failed to find a difference in 90-day survival between hypocaloric (69 ± 18% of calculated caloric needs) and eucaloric feeding (103 ± 27% of calculated caloric needs) when the protein intake was kept equal (41). However, in this study, protein intake could not be independently added for adjustment due to its collinearity with calorie intake. That is because “calorie intake (kcal/day)” and “protein intake (g/day)” usually have an inherent relationship in the commercially available enteral or parenteral formulas because both of them are regulated based on the concept of “Non-Protein Calorie: Nitrogen Ratio.”
This study has several strengths and limitations. First, the nutritional data in the analysis were prospectively collected during a large-scale RCT conducted in 90 ICUs, implicating good generalizability of the findings. Second, to minimize the bias, we did not calculate the energy and protein delivery when patients received oral intake. However, due to the nature of this study (a post hoc analysis), a causal relationship cannot be inferred. Third, since most of the study subjects did not undergo indirect calorimetry (IC) to evaluate energy expenditure, the generalizability of our findings was unknown in patients using IC to determine their energy requirements.
This study showed that energy delivery below 70% of energy requirement during days 3–7 of critical illness is associated with increased 28-day mortality when using the weight-based equation to estimate the energy requirement.
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
The studies involving human participants were reviewed and approved by the Ethics Committee of Jinling Hospital. The patients/participants provided their written informed consent to participate in this study.
LK, ZT, ZJ, and WL: conceptualization. XJ, ZL, and YiL: data curation. CL: formal analysis. RY, YXL, ML, YaL, and WC: methodology. CW, XY, and LG: resources. WL: supervision. JL and LK: writing – original draft. CL, XJ, and YXL: writing – review, and editing. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (82070665) and the Major Program of Military Logistics Research Plan (ALB18J001).
The authors would like to acknowledge all the patients and health staff who participated in the original trial.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.902986/full#supplementary-material
1. Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux RNM, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. (2005) 24:502–9. doi: 10.1016/j.clnu.2005.03.006
2. Dvir D, Cohen J, Singer P. Computerized energy balance and complications in critically ill patients: an observational study. Clin Nutr. (2006) 25:37–44. doi: 10.1016/j.clnu.2005.10.010
3. Berger MM, Chiolero RL. Hypocaloric feeding: pros and cons. Curr Opin Crit Care. (2007) 13:180–6. doi: 10.1097/MCC.0b013e3280895d47
4. McClave SA, Codner P, Patel J, Hurt RT, Allen K, Martindale RG. Should we aim for full enteral feeding in the first week of critical illness? Nutr Clin Pract. (2016) 31:425–31. doi: 10.1177/0884533616653809
5. Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. (2011) 365:506–17. doi: 10.1056/NEJMoa1102662
6. Weijs PJ, Looijaard WG, Beishuizen A, Girbes AR, Oudemans-van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care. (2014) 18:701. doi: 10.1186/s13054-014-0701-z
7. Mogensen KM, Robinson MK, Casey JD, Gunasekera NS, Moromizato T, Rawn JD, et al. Nutritional status and mortality in the critically Ill. Crit Care Med. (2015) 43:2605–15. doi: 10.1097/CCM.0000000000001306
8. Zusman O, Theilla M, Cohen J, Kagan I, Bendavid I, Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. (2016) 20:367. doi: 10.1186/s13054-016-1538-4
9. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 38:48–79. doi: 10.1016/j.clnu.2018.08.037
10. McClave SA, Omer E. Point-counterpoint: indirect calorimetry is not necessary for optimal nutrition therapy in critical illness. Nutr Clin Pract. (2021) 36:268–74. doi: 10.1002/ncp.10657
11. Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). Crit Care Med. (2016) 44:390–438. doi: 10.1097/CCM.0000000000001525
12. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2009) 33:277–316. doi: 10.1177/0148607109335234
13. Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. (2006) 25:210–23. doi: 10.1016/j.clnu.2006.01.021
14. Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake! Crit Care Med. (2011) 39:2619–26. doi: 10.1097/CCM.0b013e318226641d
15. Ke L, Lin J, Doig GS, van Zanten ARH, Wang Y, Xing J, et al. Actively implementing an evidence-based feeding guideline for critically ill patients (NEED): a multicenter, cluster-randomized, controlled trial. Crit Care. (2022) 26:46.
16. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2016) 40:159–211. doi: 10.1177/0148607115621863
17. Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. (2016) 35:158–62. doi: 10.1016/j.clnu.2015.01.015
18. Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM working group on abdominal problems. Int Care Med. (2012) 38:384–94. doi: 10.1007/s00134-011-2459-y
19. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29. doi: 10.1097/00003246-198510000-00009
20. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Int Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
21. Qiu P, Sheng J. A two-stage procedure for comparing hazard rate functions. J R Statist Soc. (2010) 70:191–208.
22. Schroeder MA. Diagnosing and dealing with multicollinearity. West J Nurs Res. (1990) 12:175–84; discussion184–7. doi: 10.1177/019394599001200204
23. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. (2019) 72:558–69. doi: 10.4097/kja.19087
24. Heyland DK, Stephens KE, Day AG, McClave SA. The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr. (2011) 30:148–55. doi: 10.1016/j.clnu.2010.09.011
25. Elke G, Wang M, Weiler N, Day AG, Heyland DK. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: secondary analysis of a large international nutrition database. Crit Care. (2014) 18:R29. doi: 10.1186/cc13720
26. Wei X, Day AG, Ouellette-Kuntz H, Heyland DK. The association between nutritional adequacy and long-term outcomes in critically ill patients requiring prolonged mechanical ventilation: a multicenter cohort study. Crit Care Med. (2015) 43:1569–79. doi: 10.1097/CCM.0000000000001000
27. Arabi YM, Haddad SH, Tamim HM, Rishu AH, Sakkijha MH, Kahoul SH, et al. Near-target caloric intake in critically ill medical-surgical patients is associated with adverse outcomes. JPEN J Parenter Enteral Nutr. (2010) 34:280–8. doi: 10.1177/0148607109353439
28. Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr. (2011) 93:569–77. doi: 10.3945/ajcn.110.005074
29. Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest. (2003) 124:297–305. doi: 10.1378/chest.124.1.297
30. Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. (2004) 32:350–7. doi: 10.1097/01.CCM.0000089641.06306.68
31. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (Ards) Clinical Trials Network, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the eden randomized trial. JAMA. (2012) 307:795–803. doi: 10.1001/jama.2012.137
32. Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive underfeeding or standard enteral feeding in critically Ill adults. N Engl J Med. (2015) 372:2398–408. doi: 10.1056/NEJMoa1502826
33. Oshima T, Berger MM, De Waele E, Guttormsen AB, Heidegger CP, Hiesmayr M, et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. (2017) 36:651–62. doi: 10.1016/j.clnu.2016.06.010
34. Lev S, Cohen J, Singer P. Indirect calorimetry measurements in the ventilated critically ill patient: facts and controversies–the heat is on. Crit Care Clin. (2010) 26:e1–9. doi: 10.1016/j.ccc.2010.08.001
35. De Waele E, Spapen H, Honoré PM, Mattens S, Van Gorp V, Diltoer M, et al. Introducing a new generation indirect calorimeter for estimating energy requirements in adult intensive care unit patients: feasibility, practical considerations, and comparison with a mathematical equation. J Crit Care. (2013) 28:884. doi: 10.1016/j.jcrc.2013.02.011
36. Tah PC, Lee ZY, Poh BK, Abdul Majid H, Hakumat-Rai VR, Mat Nor MB, et al. A single-center prospective observational study comparing resting energy expenditure in different phases of critical illness: indirect calorimetry versus predictive equations. Crit Care Med. (2020) 48:e380–90. doi: 10.1097/CCM.0000000000004282
37. Singer P, Anbar R, Cohen J, Shapiro H, Shalita-Chesner M, Lev S, et al. The tight calorie control study (TICACOS): a prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Int Care Med. (2011) 37:601–9. doi: 10.1007/s00134-011-2146-z
38. Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Int Care Med. (2017) 43:1637–47. doi: 10.1007/s00134-017-4880-3
39. Weijs PJ, Stapel SN, de Groot SD, Driessen RH, de Jong E, Girbes AR, et al. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational cohort study. JPEN J Parenter Enteral Nutr. (2012) 36:60–8. doi: 10.1177/0148607111415109
40. Nicolo M, Heyland DK, Chittams J, Sammarco T, Compher C. Clinical outcomes related to protein delivery in a critically Ill population: a multicenter, multinational observation study. JPEN J Parenter Enteral Nutr. (2016) 40:45–51. doi: 10.1177/0148607115583675
Keywords: energy intake, mortality, hypocaloric feeding, resting energy expenditure, critical illness
Citation: Lv C, Jiang X, Long Y, Liu Z, Lin J, Wu C, Ye X, Ye R, Liu Y, Liu M, Liu Y, Chen W, Gao L, Tong Z, Ke L, Jiang Z and Li W (2022) Association between caloric adequacy and short-term clinical outcomes in critically ill patients using a weight-based equation: Secondary analysis of a cluster-randomized controlled trial. Front. Nutr. 9:902986. doi: 10.3389/fnut.2022.902986
Received: 23 March 2022; Accepted: 01 August 2022;
Published: 02 September 2022.
Edited by:
Kursat Gundogan, Emory University, United StatesReviewed by:
Annika Reintam Blaser, University of Tartu, EstoniaCopyright © 2022 Lv, Jiang, Long, Liu, Lin, Wu, Ye, Ye, Liu, Liu, Liu, Chen, Gao, Tong, Ke, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Ke, Y3Rna2VsdUBuanUuZWR1LmNu; Zhengying Jiang, emhlbmd5aW5namlhbmdAY3F1LmVkdS5jbg==; Weiqin Li, Y3RnY2hpbmFAbWVkYml0LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.