- 1Department of Hematology, The Affiliated Huaian No.1 People's Hospital of Nanjing Medical University, Huaian, China
- 2Key Laboratory of Hematology of Nanjing Medical University, Nanjing, China
- 3Department of Hematology, The Huai'an Clinical College of Xuzhou Medical University, Huaian, China
Background: Myelodysplastic syndromes (MDS) are a heterogeneous spectrum of clonal hematopoietic disorders with varying degrees of cytopenia and morphologic dysplasia. The controlling nutritional status (CONUT) score, an easy-to-use tool for assessing the nutritional status, was reported as an independent prognostic factor in cancer patients. However, its role in patients with MDS is unclear.
Objective: We aimed to explore the impact of CONUT score on the prognosis of patients with MDS, which is of great significance for clinical treatment.
Methods: A total of 121 patients with MDS were analyzed. The CONUT score was calculated prior to therapy. The bio-informatics tool X-tile was used to define the CONUT score and the threshold of 4 points was determined to predict the prognosis. Patients were divided into CONUTlow and CONUThigh groups, and the characteristics were compared between two groups.
Results: Results show that CONUTlow was associated with better overall survival (OS) than CONUThigh patients (Median OS, 30.20 vs. 19.63 months, p = 0.0003). However, there were no statistical differences in progression-free survival (PFS) between the two groups (p = 0.2683). Results of univariate and multivariate COX proportional hazard analysis adjusted for bone marrow blasts level, platelet count, International Prognostic Scoring System (IPSS) scores, gender, and hemoglobin (Hb) level showed that the CONUT score was useful in the evaluation standard of OS of MDS (hazard ratio (HR) 2.297, 95% CI 1.441–3.663, p < 0.001).
Conclusions: The CONUT, as a novel immuno-nutritional biomarker, may be useful in predicting the OS of MDS.
Introduction
Myelodysplastic syndromes (MDS) are a very heterogeneous group of clonal myeloid neoplasms characterized by the risk of developing acute myeloid leukemia (AML) (1, 2). Treatment of MDS is risk adapted, involving the definition of different goals of therapy according to the risk status of the patient (3). Diagnostics and specific risk stratification are the first steps toward an individual prognostication and treatment. Several prognostic indices such as the International Prognostic Scoring System (IPSS) (4) and Revised IPSS (IPSS-R) (5) have been established and are the most commonly used prognostic models. Nevertheless, the current prognostic staging systems do not consider the role of the patient's nutritional status on the prognosis of MDS.
Nutritional status has been found to be related to the clinical outcomes of patients with cancer (6, 7). In the field of hematologic malignancies, recent studies have suggested that nutritional status is a potential parameter influencing the prognosis of acute leukemia, diffuse large B-cell lymphoma (DLBCL), and multiple myeloma (MM) (8–11). However, the role of nutritional status on the prognosis of MDS is unclear.
The controlling nutritional status (CONUT), calculated based on serum albumin (ALB), total cholesterol concentration (CHO), and total lymphocyte count (ALC), is an easy-to-use and validated tool for assessing the nutritional status of patients (12). Several studies have revealed that the CONUT score is an independent prognostic factor in cancer patients, such as bladder cancer, pancreatic cancer, and hepatocellular carcinoma (13–15). In the field of hematologic malignancies, recent studies have suggested that the CONUT score is a potential parameter influencing the prognosis of acute myeloid leukemia (AML), DLBCL, and MM (9, 16, 17). In the field of DLBCL, Nagata et al. (16) first reported that high CONUT score was associated with poor overall survival (OS) of DLBCL patients. They retrospectively analyzed 476 DLBCL patients and divided them into two groups according to their CONUT scores. The results showed that patients with high CONUT scores (≥4) had poorer 5-year OS (49.0 vs. 83.2%, p < 0.001) and 5-year progression-free survival (PFS) (46.1 vs. 73.1%, p < 0.001) compared to those with low CONUT scores (<4). The similar conclusions were also found in acute leukemia and MM that high CONUT score indicated poor outcome. However, the role of CONUT on the prognosis of MDS is unclear.
In this study, we retrospectively evaluated the relationship between baseline CONUT Score and clinical outcomes of MDS patients.
Materials and methods
Study design and patient selection
Myelodysplastic syndromes are a group of diverse bone marrow disorders in which the bone marrow does not produce enough healthy blood cells. One hundred and sixty-four patients with newly diagnosed MDS between March 2010 and December 2020 were reviewed in the Huai'an No.1 people's hospital. The study population was selected according to the following criteria and followed up to April 2021. This study was carried in accordance with the Helsinki Declaration. All the patients were anonymous. Informed consent was waived because of the retrospective design of the data collection. PFS, primary end point, was defined as the duration from the first treatment to progression of MDS, death of any cause, or the end of clinical follow-up. OS, secondary end point, was defined as the duration from the first treatment to all-cause death or the end of follow-up.
The inclusion criteria: (a) Diagnosed with MDS according to 2008 and 2016 World Health Organization (WHO) definitions; (b) Complete blood samples were approved for experimental analysis; (c) Detailed clinical data were available.
The exclusion criteria: (a) Age <18 years; (b) Incomplete patient information.
The CONUT scores
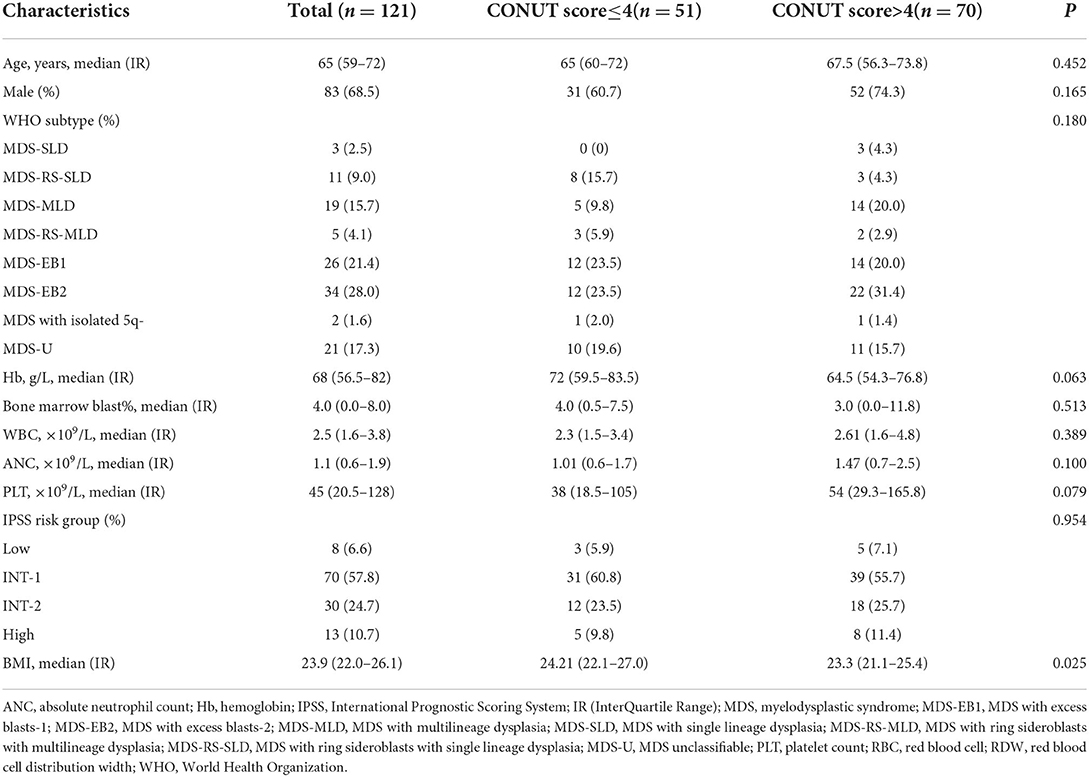
The CONUT scores were calculated based on the ALB, CHO, and ALC. Whole-blood samples collected into tubes were used for measurement (Supplementary Table S1). The baseline ALB, CHO, and ALC level at diagnosis was defined as the value that was obtained on the nearest day before the diagnosis. The ALC was measured using XN-9000 (Sysmex, Kobe, Japan). The ALB and CHO were measured using Cobas c 702(Roche, Switzerland). The bio-informatics tool X-tile (12) was used to analyze the CONUT score threshold, and four points was determined as the cut-off point to predict the prognosis. Subjects were classified as CONUT low ( ≤ 4; N = 51) or CONUT high (>4; N = 70) cohorts.
Statistical analysis
All analyses were performed with the Statistical Package (SPSS 26.0 Inc., Chicago, Illinois) and Graphpad Prism six (Graphpad Software, CA, USA). Differences of categorical variables were compared by Mann–Whitney U-test or chi-Squared test. OS/PFS were estimated adopting Kaplan–Meier method and survival curves were compared by the log-rank test. The X-tile software (Version 3.6.1, Yale University, New Haven, CT, USA) was used to evaluate the optimal cut-off CONUT scores. Univariate and multivariate Cox proportional hazards models were performed to identify the significant prognostic predictors. The hazard ratio (HR) and 95% confidence interval (CI) were calculated. The significant variables with p < 0.05 defined in univariate survival analyses were included for the multivariate analyses to validate the prognostic value of the CONUT. The p-value < 0.05 (two-tailed) indicated the statistical significance.
Results
Patient characteristics
A total of 121 patients with newly diagnosed MDS were included in the analyses. The characteristics of them were summarized in Table 1. The median age was 65 (59–72) years and 83 (68.5%) were male. Subjects were classified as CONUTlow (≤ 4; N = 51) and CONUThigh (>4; N = 70) cohorts. As a closely related to the nutritional characteristics parameters, body mass index (BMI) is also different between two group (p = 0.025). The distribution of characteristics such as age, gender, WHO subtype, hemoglobin (Hb) level, Bone marrow blast count, white blood cell (WBC) count, absolute neutrophil count (ANC), platelet (PLT) count, and IPSS subtypes were not significantly different between two groups.
Association between CONUT scores and clinical outcomes
The data showed that the CONUTlow was associated with better OS compared to CONUThigh patients (median OS, 30.20 vs. 19.63 months, p = 0.0003, Figure 1A). Although there was a similar tendency in PFS between the two groups, but with no statistically significant difference (p = 0.2683, Figure 1B). To determine whether the different risk group (defined by IPSS risk group) will affect the impact of CONUT score on the prognosis of MDS, subgroup analysis was done in lower risk groups (such as low risk and INT-1) and higher risk groups (such as INT-2 and high risk). Patients with lower risk were considered have better prognosis. The results showed that there is a statistical difference (p = 0.0048, Figure 2A) in lower risk patients. The same results were found in higher risk subgroups analysis. Patients in higher risk groups who with low CONUT score have better OS compared high CONUT score, respectively (p = 0.0105, Figure 3A). We did not find an association of CONUT score with PFS when patients were stratified by the IPSS risk categories (p = 0.2136 and p = 0.8565, Figures 2B, 3B).
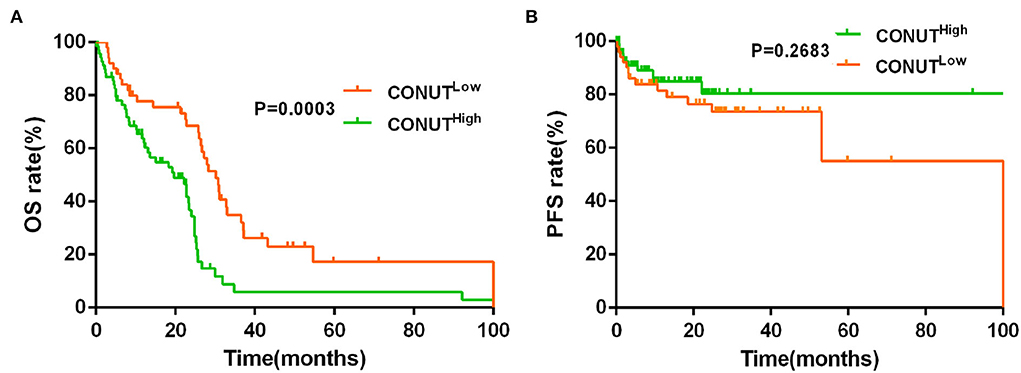
Figure 1. Lower controlling nutritional status (CONUT) was associated with better overall survival (OS) (A), but no with better progression-free survival (PFS) (B).
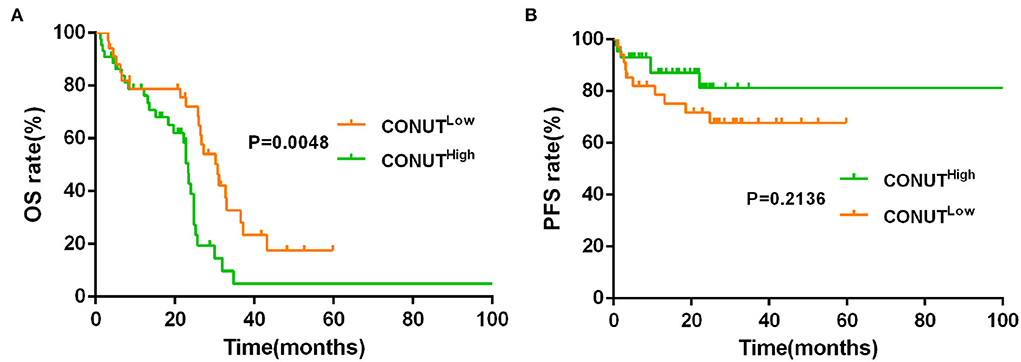
Figure 2. Lower CONUT was associated with better OS (A), but no with better PFS (B) in lower risk myelodysplastic syndromes (MDS) groups (lower risk MDS group was defined as International Prognostic Scoring System (IPSS) = low risk + INT-1).
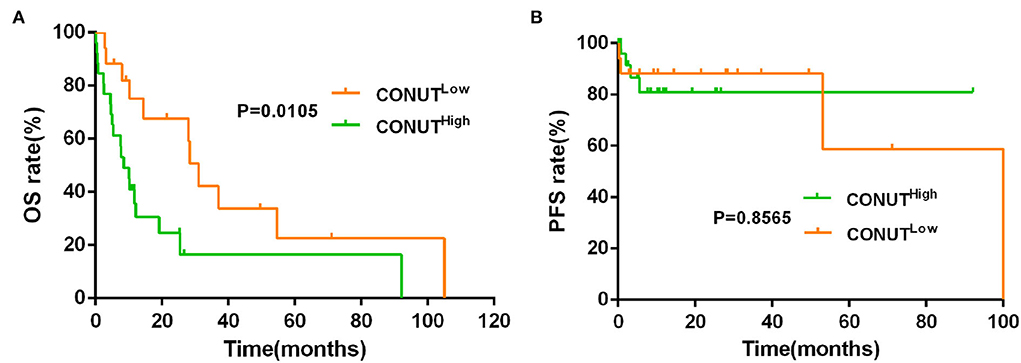
Figure 3. Lower CONUT was associated with better OS (A), but no with better PFS (B) in higher risk MDS groups (higher risk MDS group was defined as IPSS = INT-2 + high risk).
Univariate and multivariable analyses for OS
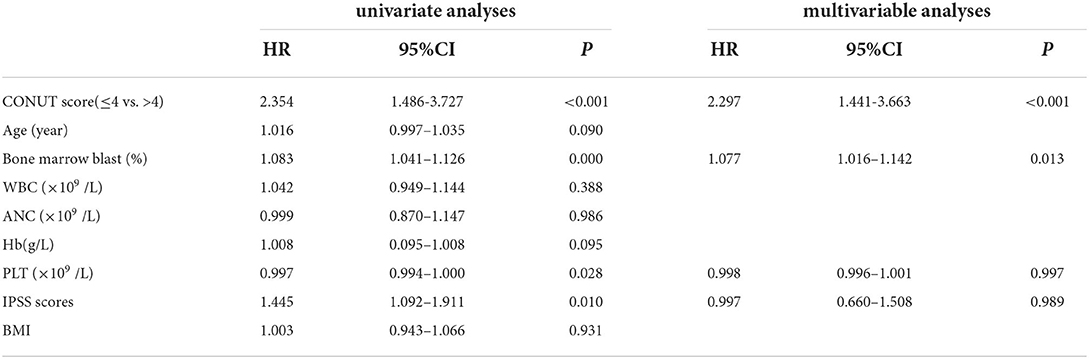
Univariate and multivariable analyses were performed to investigate the prognostic factors affecting death (Table 2). In a univariate analysis, CONUT score (HR 2.354, 95% CI 1.486–3.727, p < 0.001), bone marrow blast (HR 1.083, 95% CI 1.041–1.126, p < 0.001), PLT count (HR 0.997, 95% CI 0.994–1.000, p = 0.028), and IPSS scores (HR 1.445, 95% CI 1.092–1.911, p = 0.010) were associated with OS. In a multivariate analysis, parameter with independent adverse significance for the OS was CONUT score (HR 2.297, 95% CI 1.441–3.663, p < 0.001).
Discussion
In our study, we performed a retrospective analysis of 121 patients with MDS. The CONUT score was determined prior to therapy. We compared the survival between CONUTlow group and CONUThigh group and found that high CONUT scores were found to be strongly associated with poor outcome in MDS patients. Further external validations are needed to clarify the accurate prognostic role of the CONUT score for MDS patients.
The previous studies showed that the CONUT score at diagnosis was associated with the prognosis of patients with hematologic malignancies. In the field of MM, Okamoto et al. (17) found that transplant-eligible MM patients with high CONUT scores showed worse OS than those with low scores, and high CONUT score (>4) was an independent prognostic factor in MM patients. In the field of leukemia, Ureshino et al. (18) found that low CONUT score (≤ 3) was correlated with better OS in younger patients with adult T-cell leukemia (ATL). Among 14 younger patients who received allo-HSCT, low CONUT score group had better OS than high group. All those results indicated that the CONUT score could be a prognostic tool for patients with hematologic malignancies. In this study, we found that MDS patients whose CONUT score more than four at diagnosis experienced shorter OS compared to those lower than four. There is seemingly similar tendency in Kaplan–Meier curves of PFS, however, the difference was not significant, maybe due to the small sample sizes and bias in the study.
Univariate and multivariate analyses were also performed to investigate the prognostic factors affecting disease progression and death. Results indicated that CONUT score is a prognostic item in patients with MDS. Although the underline mechanism of action and how high CONUT score indicates true undernutrition or cachexia are still unclear, but it becomes a consensus that CONUT score is a useful tool (19–21), which can be quickly and accurately detected in peripheral blood test at diagnosis.
The CONUT score, as an immuno-nutritional index, is calculated based on ALB, ALC, and CHO. ALB affects not only by nutritional status, but also by liver synthesis capacity (22). It has been shown that ALB synthesis is reduced in many malignancies via proinflammatory cytokines such as TNF-a and IL-6 (23). ALC, another component of the CONUT score, is responsible for the cellular immune response of the host (24, 25). Lymphocytes control the host's antitumor activity by inducing apoptosis with their cytotoxic effects in the tumoral microenvironment, which inhibit cancer cell proliferation, invasion, and migration (26, 27). CHO is the main component of the cell membrane. Hypocholesterolemia is associated with the reduction of all lymphocytes, such as CD8 T-cells and suppresses the immune system (28). Each of the three parameters in the CONUT score was reported to reflect cancer prognosis in various types of cancer and there are several reasons for significant prognostic role of the CONUT score in cancer patients (29–31).
Firstly, low ALB was proved to be correlated with poor survival in gastric cancer patients (32). The same conclusions were identified in hematological malignancies, such as MM, AML, and MDS (11, 33). ALB is the most abundant protein in serum and contributes to the maintenance of oncotic pressure as well as to transport of hydrophobic molecules (34). ALB has mainly been considered a biomarker of immunocompetence status and fundamental to nutritional assessment (35). Persistent inflammation contributes to decreased ALB levels (36), plays a central role in the malnutrition, inflammation, which predicts poor clinical outcomes (37). Moreover, as a component of systemic inflammation, ALB is correlated with cancer-related systemic inflammation and tumor progression, which may be caused by the reduced production of ALB by hepatocytes due to the inflammatory cytokines released by the tumor cells (38). Secondly, ALC and absolute monocyte (AMC) counts at the time of treatment initiation were regarded as biomarkers of the tumor microenvironment and immune surveillance in several hematologic malignancies of both myeloid and lymphoid origin (39). Decreasing of ALC counts was shown to be associated with the increasing drug resistance in DLBCL (40). Third, CHO has been found to be related to cancer progression and metastasis, such as colorectal cancer, gallbladder cancer, and MM (41–43). The previous studies have found that lower serum total CHO levels were linked to worse prognosis in renal cell carcinoma and non-small-cell lung cancer (44, 45). It is not clear whether tumor progression causes hypocholesterolemia or hypocholesterolemia triggers tumor progression. Increased levels of cytokines such as IL-6 and activation of NF-kB associated with increased inflammation because of proliferation of tumor cells can cause hypocholesterolemia (46). There was no research exploring the role of CHO on the prognosis in patients with MDS.
Accordingly, the CONUT score as a combination of these three factors that might be a better prognostic factor for MDS. The status of nutrition is associated with the treatment response and the therapy-related side effects and toxicity, which also are factors associated with the prognosis of diseases. Therefore, early nutritional status screening and assessment may be important to provide comprehensive algorithms for individualized clinical treatment in cancer patients. As an easy get index in hospital, CONUT score can assess the nutritional status of patients more comprehensively.
There are some limitations in this study. First, as in most retrospective analyses, selection bias in collection cannot be completely avoided. Second, we could not find the significant correlation between the type of therapy and the survival, which may because of substandard treatment and small sample size.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Affiliated Huaian No.1 People's Hospital of Nanjing Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
LY designed the study. QC and KC wrote the manuscript. SW collected data. LZ was responsible for the tables. ST and ZH were responsible for the figures. CW and LY modified the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Science and Technology Fund of Huaian City [grant # HAB202020] and Commission of Health of Jiangsu Province [grant # 2019082].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.902704/full#supplementary-material
References
1. Karantanos T, DeZern AE. Biology and clinical management of hypoplastic MDS: MDS as a bone marrow failure syndrome. Best Pract Res Clin Haematol. (2021) 34:101280. doi: 10.1016/j.beha.2021.101280
2. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al.The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127 (2016) 2391–405. doi: 10.1182/blood-2016-03-643544
4. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. (1997) 89:2079–88. doi: 10.1182/blood.V89.6.2079
5. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. (2012) 120:2454–65. doi: 10.1182/blood-2012-03-420489
6. Sun GLi. J Zhang, J Zhu, and Z Zhang. The role of nutritional assessment for predicting Radiotherapy-induced adverse events in patients with gastric cancer. Br J Radiol. (2021) 95:20201004. doi: 10.1259/bjr.20201004
7. Xue W, Zhang Y, Wang H, Zhang Y, Hu X. Multicenter study of Controlling Nutritional Status (CONUT) score as a prognostic factor in patients with HIV-related renal cell carcinoma. Front Immunol. (2021) 12:778746. doi: 10.3389/fimmu.2021.778746
8. Sonowal R, Gupta V. Nutritional status in children with acute lymphoblastic leukemia, and its correlation with severe infection. Indian J Cancer. (2021) 58:190–4. doi: 10.4103/ijc.IJC_110_19
9. Senjo H, Onozawa M, Hidaka D, Yokoyama S, Yamamoto S, Tsutsumi Y, et al. A novel nutritional index “simplified CONUT” and the disease risk index independently stratify prognosis of elderly patients with acute myeloid leukemia. Sci Rep. (2020) 10:19400. doi: 10.1038/s41598-020-76250-8
10. Shen Z, Wang F, He C, Li D, Nie S, Bian Z, et al. The Value of Prognostic Nutritional Index (PNI) on newly diagnosed diffuse large B-Cell lymphoma patients: a multicenter retrospective study of HHLWG based on propensity score matched analysis. J Inflamm Res. (2021) 14:5513–22. doi: 10.2147/JIR.S340822
11. Zhou X, Lu Y, Xia J, Mao J, Wang J, Guo H. Association between baseline Controlling Nutritional Status score and clinical outcomes of patients with multiple myeloma. Cancer Biomark. (2021) 32:65–71. doi: 10.3233/CBM-210073
12. Ignacio de. Ulíbarri J, González-Madroño A, de Villar, P González NG, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status first validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
13. Zhao L, Sun J, Wang K, Tai S, Hua R, Yu Y, et al. Development of a new recurrence-free survival prediction nomogram for patients with primary non-muscle-invasive bladder cancer based on preoperative Controlling Nutritional status score. Cancer Manag Res. (2021) 13:6473–87. doi: 10.2147/CMAR.S323844
14. Dang C, Wang M, Zhu F, Qin T, Qin R. Controlling Nutritional status (CONUT) score-based nomogram to predict overall survival of patients with pancreatic cancer undergoing radical surgery. Asian J Surg. (2022) 45:1237–45. doi: 10.1016/j.asjsur.2021.08.011
15. Peng W, Yao M, Zou K, Li C, Wen T, Sun X. Postoperative controlling nutritional status score is an independent risk factor of survival for patients with small hepatocellular carcinoma: a retrospective study. BMC Surg. (2021) 21:338. doi: 10.1186/s12893-021-01334-9
16. Nagata A, Kanemasa Y, Sasaki Y, Nakamura S, Okuya T, Funasaka C, et al. Clinical impact of controlling nutritional status score on the prognosis of patients with diffuse large B-cell lymphoma. Hematol Oncol. (2020) 38:309–17. doi: 10.1002/hon.2732
17. Okamoto S, Ureshino H, Kidoguchi K, Kusaba K, Kizuka-Sano H, Sano H, et al. Clinical impact of the CONUT score in patients with multiple myeloma. Ann Hematol. (2020) 99:113–9. doi: 10.1007/s00277-019-03844-2
18. Ureshino H, Kusaba K, Kidoguchi K, Sano H, Nishioka A, Itamura H, et al. Clinical impact of the CONUT score and mogamulizumab in adult T-cell leukemia/lymphoma. Ann Hematol. (2019) 98:465–71. doi: 10.1007/s00277-018-3502-7
19. Dalmiglio C, Brilli L, Campanile M, Ciuoli C, Cartocci A, Castagna MG, et al. score: a new tool for predicting prognosis in patients with advanced thyroid cancer treated with TKI. Cancers. (2022) 14:724. doi: 10.3390/cancers14030724
20. Dong X, Tang S, Liu W, Qi W, Ye L, Yang X, et al. Prognostic significance of the Controlling Nutritional Status (CONUT) score in predicting postoperative complications in patients with Crohn's disease. Sci Rep. (2020) 10:19040. doi: 10.1038/s41598-020-76115-0
21. Suzuki H, Ito M, Takemura K, Nakanishi Y, Kataoka M, Sakamoto K, et al. Prognostic significance of the controlling nutritional status (CONUT) score in advanced urothelial carcinoma patients. Urol Oncol. (2020) 38:76.e11–7. doi: 10.1016/j.urolonc.2019.10.014
22. Tanriverdi O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: a medical oncologist's perspective. Med Oncol. (2014) 31:282. doi: 10.1007/s12032-014-0282-3
23. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. (2005) 39:S143–6. doi: 10.1097/01.mcg.0000155514.17715.39
24. Lamano JB, Ampie L, Choy W, Kesavabhotla K, Di Domenico JD, Oyon DE, et al. Immunomonitoring in glioma immunotherapy: current status and future perspectives. J Neurooncol. (2016) 127:1–13. doi: 10.1007/s11060-015-2018-4
25. Müller I, Munder MP. Kropf, and GM Hänsch Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. (2009) 30:522–30. doi: 10.1016/j.it.2009.07.007
26. Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. (2006) 90:1–50. doi: 10.1016/S0065-2776(06)90001-7
27. Lim JA, Oh CS, Yoon TG, Lee JY, Lee SH, Yoo YB, et al. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T-lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer. (2018) 18:159. doi: 10.1186/s12885-018-4064-8
28. Muldoon MF, Marsland A, Flory JD, Rabin BS, Whiteside TL, Manuck SB. Immune system differences in men with hypo- or hypercholesterolemia. Clin Immunol Immunopathol. (1997) 84:145–9. doi: 10.1006/clin.1997.4382
29. Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. (2018) 21:204–12. doi: 10.1007/s10120-017-0744-3
30. Takagi K, Buettner S, Ijzermans JN, Wijnhoven BP. Systematic review on the Controlling Nutritional Status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res. (2020) 40:5343–9. doi: 10.21873/anticanres.14541
31. Li Y, Zhang C, Ji R, Lu H, Zhang W, Li LL, et al. Prognostic significance of the controlling nutritional status (CONUT) score in epithelial ovarian cancer. Int J Gynecol Cancer. (2020) 30:74–82. doi: 10.1136/ijgc-2019-000865
32. Ouyang X, Dang Y, Zhang F, Huang Q. Low serum albumin correlates with poor survival in gastric cancer patients. Clin Lab. (2018) 64:239–45. doi: 10.7754/Clin.Lab.2017.170804
33. Sakurai. and T. Nakazato The prognostic value of the controlling nutritional status score in patients with myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia related changes treated with azacitidine. Leuk Lymphoma. (2020) 61:2995–7. doi: 10.1080/10428194.2020.1791847
34. Gekle M. Renal tubule albumin transport. Annu Rev Physiol. (2005) 67:573–94. doi: 10.1146/annurev.physiol.67.031103.154845
35. Seltzer MH, Bastidas JA, Cooper DM, Engler P, Slocum B, Fletcher HS. Instant nutritional assessment. JPEN J Parenter Enteral Nutr. (1979) 3:157–9. doi: 10.1177/014860717900300309
36. Kaysen GA, Chertow GM, Adhikarla R, Young B, Ronco C. Levin NW. Inflammation and dietary protein intake exert competing effects on serum albumin and creatinine in hemodialysis patients. Kidney Int. (2001) 60:333–40. doi: 10.1046/j.1523-1755.2001.00804.x
37. Mukai H, Villafuerte H, Qureshi AR, Lindholm B, Stenvinkel P. Serum albumin, inflammation, and nutrition in end-stage renal disease: C-reactive protein is needed for optimal assessment. Semin Dial. (2018) 31:435–9. doi: 10.1111/sdi.12731
38. Oh JS, Park DJ, Byeon KH, Ha YS, Kim TH, Yoo ES, et al. Decrease of preoperative serum albumin-to-globulin ratio as a prognostic indicator after radical cystectomy in patients with urothelial bladder. Cancer Urol J. (2021) 18:66–73. doi: 10.22037/uj.v16i7.6350
39. Binder M, Rajkumar SV, Lacy MQ, Gertz MA, Buadi FK, Dispenzieri A, et al. Peripheral blood biomarkers of early immune reconstitution in newly diagnosed multiple myeloma. Am J Hematol. (2019) 94:306–11. doi: 10.1002/ajh.25365
40. Laddaga FE, Ingravallo G, Mestice A, Tamma R, Perrone T, Maiorano E, et al. Correlation between circulating blood and microenvironment T-lymphocytes in diffuse large B-cell lymphomas. J Clin Pathol. (2022) 75:493–7. doi: 10.1136/jclinpath-2020-207048
41. Jun SY, Brown AJ, Chua NK, Yoon JY, Lee JJ, Yang JO, et al. Reduction of squalene epoxidase by cholesterol accumulation accelerates colorectal cancer progression and metastasis. Gastroenterology. (2021) 160 1194–207.e28. doi: 10.1053/j.gastro.2020.09.009
42. Yuan B, Fu J, Yu WL, Fu XH, Qiu YH, Yin L, et al. Prognostic value of serum high-density lipoprotein cholesterol in patients with gallbladder cancer. Rev Esp Enferm Dig. (2019) 111:839–45. doi: 10.17235/reed.2019.6201/2019
43. Liu X, Xu P, Wang L, Zhang C, Wang M, Ouyang J. Cholesterol levels provide prognostic information in patients with multiple myeloma. Clin Lab. (2020) 66:621–30. doi: 10.7754/Clin.Lab.2019.190824
44. Kang HW, Seo SP, Kim WT, Yun SJ, Lee SC, Kim WJ, et al. Low preoperative serum cholesterol level is associated with aggressive pathologic features and poor cancer-specific survival in patients with surgically treated renal cell carcinoma. Int J Clin Oncol. (2018) 23:142–50. doi: 10.1007/s10147-017-1172-4
45. Li JR, Zhang Y, Zheng JL. Decreased pretreatment serum cholesterol level is related with poor prognosis in resectable non-small cell lung cancer. Int J Clin Exp Pathol. (2015) 8:11877−83.
Keywords: myelodysplastic syndromes (MDS), controlling nutritional status (CONUT), prognosis, nutritional status, retrospective analysis
Citation: Chen Q, Chen K, Wang S, Zhang L, Shi Y, Tao S, He Z, Wang C and Yu L (2022) Prognostic value of the controlling nutritional status score in patients with myelodysplastic syndromes. Front. Nutr. 9:902704. doi: 10.3389/fnut.2022.902704
Received: 23 March 2022; Accepted: 30 June 2022;
Published: 27 July 2022.
Edited by:
Silvia Tejada, University of the Balearic Islands, SpainReviewed by:
Diego Fernández Lázaro, University of Valladolid, SpainGiuseppe Annunziata, University of Naples Federico II, Italy
Copyright © 2022 Chen, Chen, Wang, Zhang, Shi, Tao, He, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunling Wang, d2NsNjUwNkAxNjMuY29t; Liang Yu, eXVsaWFuZ2hhQDE2My5jb20=
†These authors have contributed equally to this work
 Qiuni Chen
Qiuni Chen Kankan Chen1,2†
Kankan Chen1,2† Lijuan Zhang
Lijuan Zhang Liang Yu
Liang Yu