- 1National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng, China
- 2Shenzhen Research Institute of Henan University, Shenzhen, China
- 3Joint International Research Laboratory of Food & Medicine Resource Function, Henan Province, Kaifeng, China
- 4Medicinal and Aromatic Plants Researches Department, Horticulture Research Institute, Agricultural Research Center, Giza, Egypt
- 5Hebei Food Safety Key Laboratory, Hebei Food Inspection and Research Institute, Shijiazhuang, China
Nigella sativa is a valuable herb for its functional compositions in both food and medication. N. sativa seeds can enhance immunity, anti-inflammation and analgesia and hypoglycemia, but most of the related researches are related to volatile oil and extracts, and the activity and mechanism of compounds is not clear. In this study, Ethyl-α-D-galactopyranoside (EG), Methyl-α-D-glucoside (MG), 3-O-[β-D-xylopyranose-(1 → 3)-α-L-rhamnose-(1 → 2)-α-L-arabinose]-28-O-[α-L-rhamnose-(1 → 4)-β-D-glucopyranose-L-(1 → 6)-β-D-glucopyranose]-hederagenin (HXRARG) and 3-O-[β-D-xylopyranose-(1 → 3)-α-L-rhamnose-(1 → 2)-α-L-arabinose]-hederagenin (HXRA) were isolated and identified from N. sativa seeds. In addition, four compounds could activate NF-κB pathway by promoting the expression of phosphorylation of P65 and IκBα, promoting the phosphorylation of JNK, Erk and P38 to activate MAPK signaling pathway, enhancing the proliferation and phagocytic activity of RAW264.7 cells, and promoting the release of NO, TNF-α and IL-6 on RAW264.7 cell in vitro. The results showed that N. sativa can be used as dietary supplement to enhance immune.
Introduction
Hippocrates put forward the creed of “make food your medicine, and make medicine be your food” nearly 2, 500 years ago (1, 2). This creed revived interest and spawned terms such as pharmaceuticals, nutritional medicines and functional foods. These terms are not only becoming more and more popular among researchers, but also arouse consumer interest in health regimens based on a natural diet. In recent decades, our understanding of the basic principles and correlation between nutrition and human health has been greatly enhanced (3, 4). It is increasingly clear that diet-based natural compounds represent the basic categories of regulatory factors that affect human health (5). Historical evidence in the Chinese traditional medical system emphasizes the role of natural dietary factors in health and disease management, and natural products can be used as immune enhancers to improve the quality of life (6, 7). In addition, the safety, effectiveness and affordability of nutritional drugs stimulate consumer confidence. As a consequence, the global focus is on the study of bioactive natural regulators for human health. This seems to be more important in modern times, because a diet-dependent preventive lifestyle may reduce our growing dependence on antibiotics or may combine to increase the efficacy of antibiotics (8).
Immune system is an important part of the body. It is a defense system for the body to resist pathogen infection, avoid the invasion of various external dangerous invaders, maintain the stability of internal environment, and monitor the mutation of its own cells (9). Besides, immunity can be subdivided into native immunity and adaptive immunity according to the way of acquisition. Native immunity is an innate ability of the human body to resist foreign infections in a non-specific manner. (10). Moreover, adaptive immunity is the body's third line of defense to protect itself, characterized by its protection function only against a specific pathogen. Macrophages are key innate immune cells found in practically all of the body's major organs (11). They are the initial line of defense against infections and cancerous cells infiltrating the body (12, 13). Macrophages drive other immune cells to the infected location, where they produce cytokines, chemokines, and inflammatory chemicals to stimulate inflammation (14, 15). In addition, macrophages initiate adaptive immune responses through antigen presentation and cytokine secretion. As a consequence, macrophages are considered to be some key target cells for immune regulation (16).
Pattern recognition receptors (PRR) is a protein that interacts with the innate immune system and is responsible for identifying pathogen and antigen molecular patterns (17). When PRR are activated, intracellular signaling cascades like as mitogen-activated protein kinases (MAPKs) and nuclear factor (NF)-κB are subsequent activated, resulting in gene transcription that is involved in the inflammatory response (18, 19). Macrophages are activated in this process, which results in greater phagocytosis, enhanced synthesis of immunomodulatory signal molecules like nitric oxide (NO), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and reactive oxygen species (ROS) (20). RAW264.7 cells are utilized to investigate how different medications or immunomodulators affect immune response, mostly to detect the proliferation ability, phagocytosis ability, secretion level of various cytokines and killing effect on tumor cells of RAW264.7 cells, and to evaluate the regulatory response of some immunomodulators to initial immunity (21, 22).
Nigella sativa belongs to Ranunculaceae family that can be found in southern Europe, southwest Asia, and North Africa (23). Anti-cancer, bacteriostasis, anti-hypertension, liver protection, anti-inflammation, antipyretic, analgesia, and elimination of ROS are just a few of the biological properties of N. sativa seeds. So, they are widely used in food and medicine (24, 25). Alshatwi A.A found that N. sativa seeds have immunostimulatory effect on the growth of human peripheral blood monon-uclear cells triggered by non-phytohemagglutinin (26). Ebaid H found N. sativa oil could slightly improve the damaged spleen tissue, repair thymus tissue in a time-dependent manner, and restore cellular and humoral immunity (27).
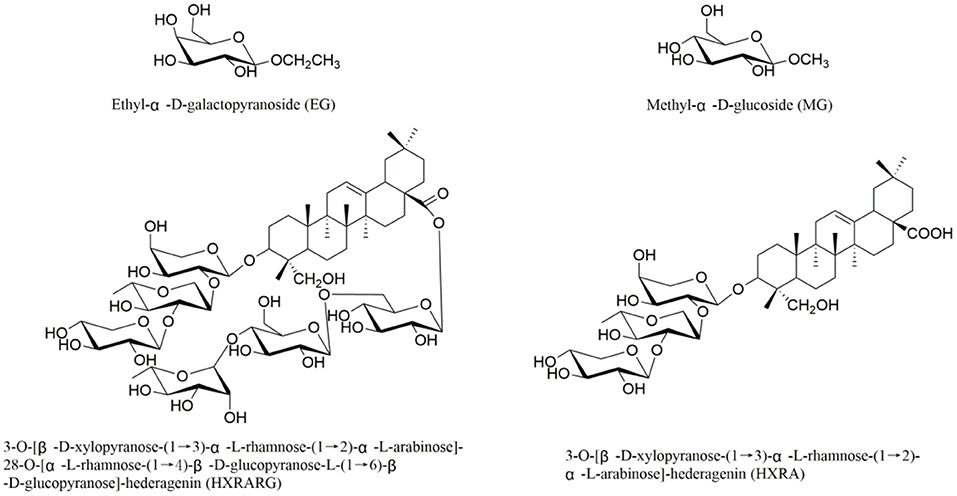
Our ongoing research (28–31), four compounds, (Figure 1) Ethyl-α-D-galactopyranoside (EG), Methyl-α-D-glucoside (MG), 3-O-[β-D-xylopyranose-(1 → 3)-α-L-rhamnose-(1 → 2)-α-L-arabinose]-28-O-[α-L-rhamnose-(1 → 4)-β-D-glucopyranose-L-(1 → 6)-β-D-glucopyranose]-hederagenin (HXRARG) and 3-O-[β-D-xylopyranose-(1 → 3)-α-L-rhamnose-(1 → 2)-α-L-arabinose]-hederagenin (HXRA), were isolated and identified and they were found to stimulate NO production in RAW264.7 cells. It is speculated that EG, MG, HXRA and HXRARG have immunomodulatory effects. According to the literatures, although pharmacological studies have shown that N. sativa seeds have immune enhancement, but there is no study on the active compounds. Thus, in this paper, the immune activity and mechanism of EG, MG, HXRARG and HXRA were carried out.
Materials and Methods
Chemicals and Reagents
The following reagents were used in this research: Mouse macrophage cell line RAW264.7 was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China); Dulbecco's Modified Eagle's Medium (DMEM) and neutral red staining solution were bought from Solarbio (Beijing, China); Lipopolysaccharide (LPS) were obtained from Sigma-Aldrich (St. Louis, MO, USA); Griess kit and ROS kit were purchased from Beyotime Biotechnology (Shanghai, China); Mouse TNF-α and IL-6 ELISA kit were purchased from Beijing 4A Biotech Co, Ltd (Beijing, China); Primer iNOS, TNF-α, IL-6 and Cox-2 were designed and synthesized by Thermo Fisher Scientific (Shanghai, China Thermo Fisher scientific (Shanghai, China); Evo M-MLV RT Kit with gDNA Clean and TB Green TM Ex TaqTM II (Tli RNadeH Plus), Bulk kit were obtained from TaKaRa; anti-NF-κB p65 antibody, anti-phospho-NF-κB p65 antibody, anti-iNOS antibody, anti-COX-2 antibody, anti-IκBα antibody, anti-phospho-IκBα antibody, anti-P38 MAPK antibody, anti-phospho-P38 MAPK antibody, anti-Erk antibody, anti-phospho-Erk antibody, anti-JNK antibody and anti-phospho-JNK antibody were bought from Cell Signaling Technology (Danvers, MA, USA).
Cell Culture
RAW264.7 cells were cultured by the assay of Senye Wang (32). At 37°C in a humidified incubator with a 5% CO2 atmosphere, cells were grown in DEME high glucose medium with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/mL penicillin and 100 μg/mL streptomycin. LPS was adopted as a positive control.
Cell Viability Assay
Cell viability assay was consistent with the method previously reported (33). Briefly, In 96-well-plates, 1.0 × 104 cells were plated. EG, MG, HXRARG and HXRA were added at increasing concentrations over the next 24 h. Each well-received 20 μL of MTT reagent 4 h before the end of the experiments. To dissolve the formazan, 150 μL of dimethyl sulfoxide (DMSO) was added at the end. At a wavelength of 570 nm, absorbance was recorded.
Phagocytic Activity
The phagocytic capacity of macrophages was measured using the previously described approach (34). RAW264.7 cells were seeded at a density of 1.0 × 104 cells per well in 96-well-plates and incubated overnight. RAW264.7 cells were incubated with increasing concentrations of EG (80, 60 and 40 μM), MG (80, 60 and 40 μM), HXRARG (80, 60 and 40 μM), HXRA (8, 6 and 4 μM) or LPS (1 μg/mL) for 24 h. We discarded the old culture medium, washed gently with PBS solution for 2–3 times, 100 μL of 0.1 percent neutral red solution was added to each well, and cultivation was continued for 30 min. After being washed with PBS solution for three times, the cells were photographed under fluorescence microscope. Each well-added cell lytic solution comprising acetic acid and anhydrous ethanol (1:1, V/V) and was kept at 4°C overnight. A micro-plate reader was used to measure the absorbance of each hole at 550 nm.
Measurement of NO
RAW264.7 cells were seeded at a density of 1.0 × 105 cells per well in 24-well-plates overnight. Then RAW264.7 cells were treated with the above methods for 24 h. After culture, the medium (50 μL) was transferred to a new 96-well-plate, 50 μL Griess reagent I and II were added, and the absorbance of each well was measured at 540 nm.
Determination of IL-6 and TNF-α
For 24 h, RAW264.7 cells were pre-incubated at a density of 1.5 × 105 cells per well in 24-well-plates, and then cultured with different concentrations of EG (80, 60, and 40 μM), MG (80, 60, and 40 μM), HXRARG (80, 60, and 40 μM), HXRA (8, 6, and 4 μM) or LPS (1 μg/mL) for 24 h. The cell culture medium was centrifuged using ELISA kit to determine the concentration of TNF-α and IL-6.
Determination of Intracellular ROS
RAW264.7 cells were seeded at a density of 5.0 × 104 cells per well on a 6-well plate overnight, and then each well was exchanged with DMEM medium containing different concentrations of EG (80, 60, and 40 μM), MG (80, 60, and 40 μM), HXRARG (80, 60 and 40 μM), HXRA (8, 6, and 4 μM) or LPS (1 μg/mL). After gently washing the cells with serum-free DMEM, the 20 min was incubated with 10 μM DCFH-DA and flipped every 3–5 min, so that the DCFH-DA could be in full contact with the cells. Subsequently, after passing through a 300 mesh nylon screen, flow cytometry was used to identify the change in ROS.
Western Blot
The cells after treatment for 24 h using the above experimental procedure, and Western Blot experiment was conducted according to the reference (31). Total protein was extracted using the proper volume of a weak radioimmune precipitation assay (RIPA) lysis buffer based on the density of the cultivated cells (about 100 μL per well). The BCA method was utilized to determine protein concentrations 0.20 μg of proteins from various groups were separated by 12% SDS-polyacrylamidegel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride (PVDF) membrane after boiling at 100 °C for 5 min. The PVDF membranes were exposed with the relevant primary antibodies at 4°C overnight after being blocked with 5 percent dry skimmed milk, and then treated with the corresponding HRP-linked secondary antibodies at room temperature for 2 h. Enhanced chemiluminescence (ECL) detection reagent was performed to visualize the protein bands, which were scanned using ImageJ software.
Quantitative Real-time PCR (qRT-PCR)
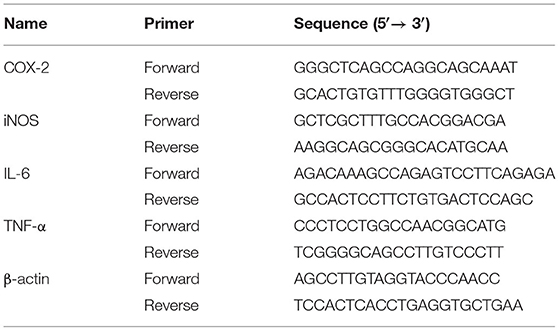
In the same way as the previous cell processing approaches, then qRT-PCR were used as previously reported (35). In brief, RAW264.7 cells were inoculated for 12 h on 6-well-plates and then treated for 24 h with LPS or different doses of EG, MG, HXRARG, and HXRA. Total RNA was extracted using Trizol reagent and reversed to cDNA using the Evo M-ML V RT Kit with gDNA Clean, following the manufacturer's instructions. A spectrophotometer was used to calculate the concentration of extracted RNA. The SYBR Green Pro Taq HS Premix qPCR kit was used to detect the expression of mRNAs. β-actin was assessed as on normalization control and the sequences were listed in Table 1.
Statistical Analysis
The numerical statistics were examined using one-way ANOVA in SPSS 19.0 software, and the experimental results were reported as mean standard deviation. The software GraPhPad Prism 5.0 was used to analyze all of the column images.
Results
Effects of EG, MG, HXRARG, and HXRA on Cell Viability of RAW264.7 Cells
Compared with the control group, EG, MG or HXRARG with 200 μM could significantly inhibit the proliferation of macrophages, while the concentration of 40–80 μM could promote the proliferation of RAW264.7 cells (Figures 2A–C). After treated with HXRA (20 μM) for 24 h, the survival rate of RAW264.7 macrophages was only about 50%, but when the concentration was 2–10 μM, it could promote the proliferation of RAW264.7 cells (Figure 2D). Macrophages that have been activated serve a vital part in the immune response, and their proliferative activity can be adopted as an index of immunoregulation and cytotoxicity. So, 80, 60, and 40 μM were selected as the drug concentrations of EG, MG, HXRARG and 8, 6, and 4 μM were selected as the concentrations of HXRA in the next experiments.
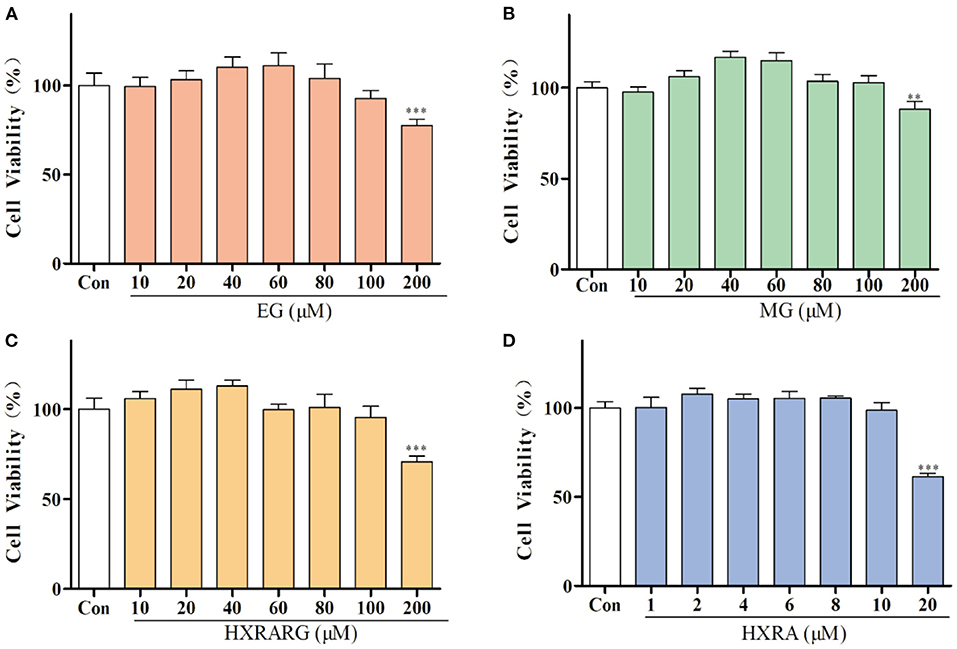
Figure 2. Effects of EG (A), MG (B), HXRARG (C), and HXRA (D) on the viability of RAW264.7 cells. Data are expressed as the means ± SD. Compared with the control, **P < 0.01, ***P < 0.001.
Effects of EG, MG, HXRARG, and HXRA on Phagocytic Function of RAW264.7 Cells
Compared with the control group, after being stimulated by EG, MG, HXRARG, or HXRA, more neutral red enters the cells (Figures 3A–E), indicating that EG, MG, HXRARG, HXRA, or LPS could enhance the phagocytic ability of macrophages. In addition, under the microscope, the volume of RAW264.7 cells increased after LPS treatment, showing irregular polygons and some cells protruding dendritic pseudopodia. The morphological changes of EG, MG, HXRARG or HXRA group were similar to those of LPS group, but the proportion of amoeba cells was significantly lower than that of LPS group. The morphological changes of macrophages may mean the enhancement of cell adhesion to matrix and phagocytic activity to pathogenic microorganisms. The phagocytic index of the LPS group was nearly double that of the control group after cell lysis, as expected. Compared with the control group, except HXRA (4 μM), the ability of RAW264.7 cells to phagocytize when exposed to various doses EG, MG, HXRARG and HXRA was significantly enhanced (Figures 3F–I), which was consistent with the results of microscope photography.
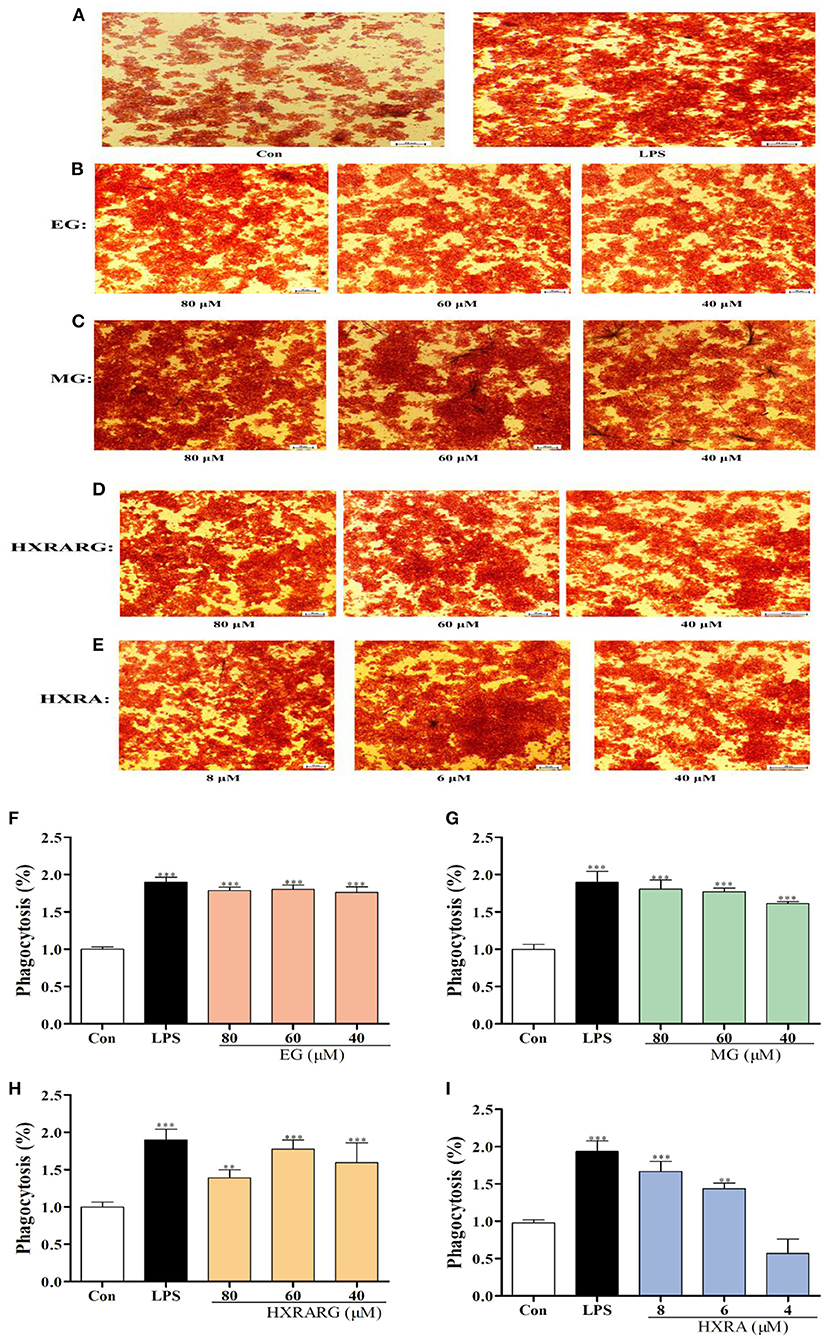
Figure 3. Effects of EG, MG, HXRARG, and HXRA on phagocytosis of neutral red in RAW264.7 cells. (A–E) microscope picture, (F–I) quantitative analysis picture. Data are expressed as the means ± SD. Compared with the control, **P < 0.01, ***P < 0.001.
Effects of EG, MG, HXRARG, and HXRA on No Secretion of RAW264.7 Cells
The content of NO in the LPS group increased approximately 1.5 times when compared to the control group. At the same time, MG (80, 60, and 40 μM), HXRARG (80, 60, and 40 μM) and HXRA (8, 6, and 4 μM) also remarkably boosted the potential of RAW264.7 cells to produce NO in a dose-dependent manner. The generation of NO was comparable to that of the LPS group when the concentration of EG was 60 μM (Figure 4).
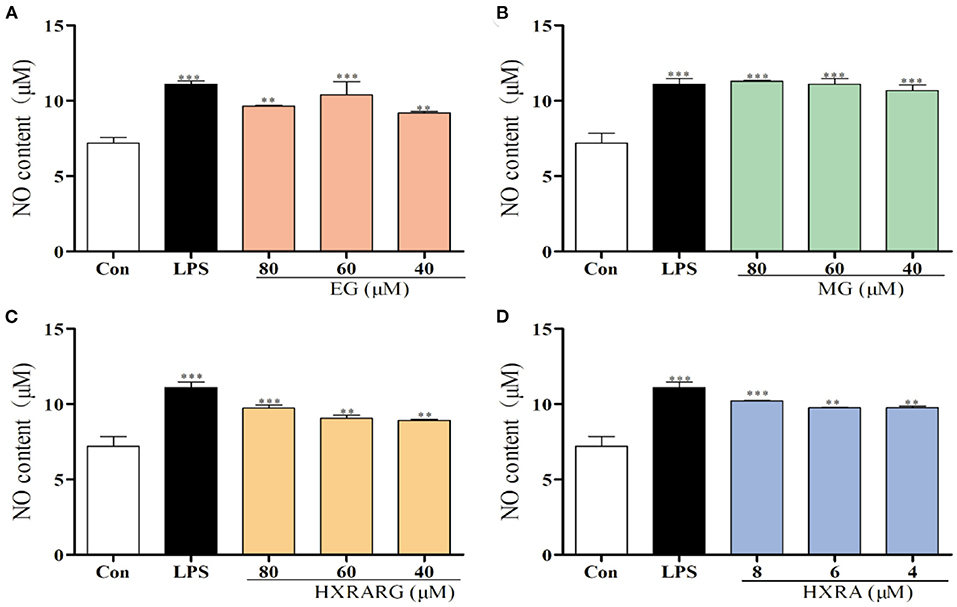
Figure 4. Effects of EG (A), MG (B), HXRARG (C), and HXRA (D) on nitric oxide (NO) production in RAW264.7 cells. Data are expressed as the means ± SD. Compared with the control, **P < 0.01, ***P < 0.001.
Effects of EG, MG, HXRARG, and HXRA on iNOS, COX-2 Protein and mRNA Expression in RAW264.7 Cells
In RAW264.7 cells stimulated by LPS, the protein expression of iNOS and COX-2 was dramatically up-regulated (Figures 5A–D). When RAW264.7 cells were treated with compound for 24 h, the activities of total iNOS and Cox-2 were significantly increased when the expression of intracellular β-actin was almost unchanged. LPS greatly enhance the mRNA transcription of iNOS and COX-2, as seen in Figures 5E,F, and different concentrations of EG, MG, HXRARG or HXRA dramatically increase the transcription of COX-2 and iNOS mRNA in macrophages. In general, at the protein and gene levels, EG, MG, HXRARG, and HXRA can significantly enhance iNOS and COX-2 synthesis.
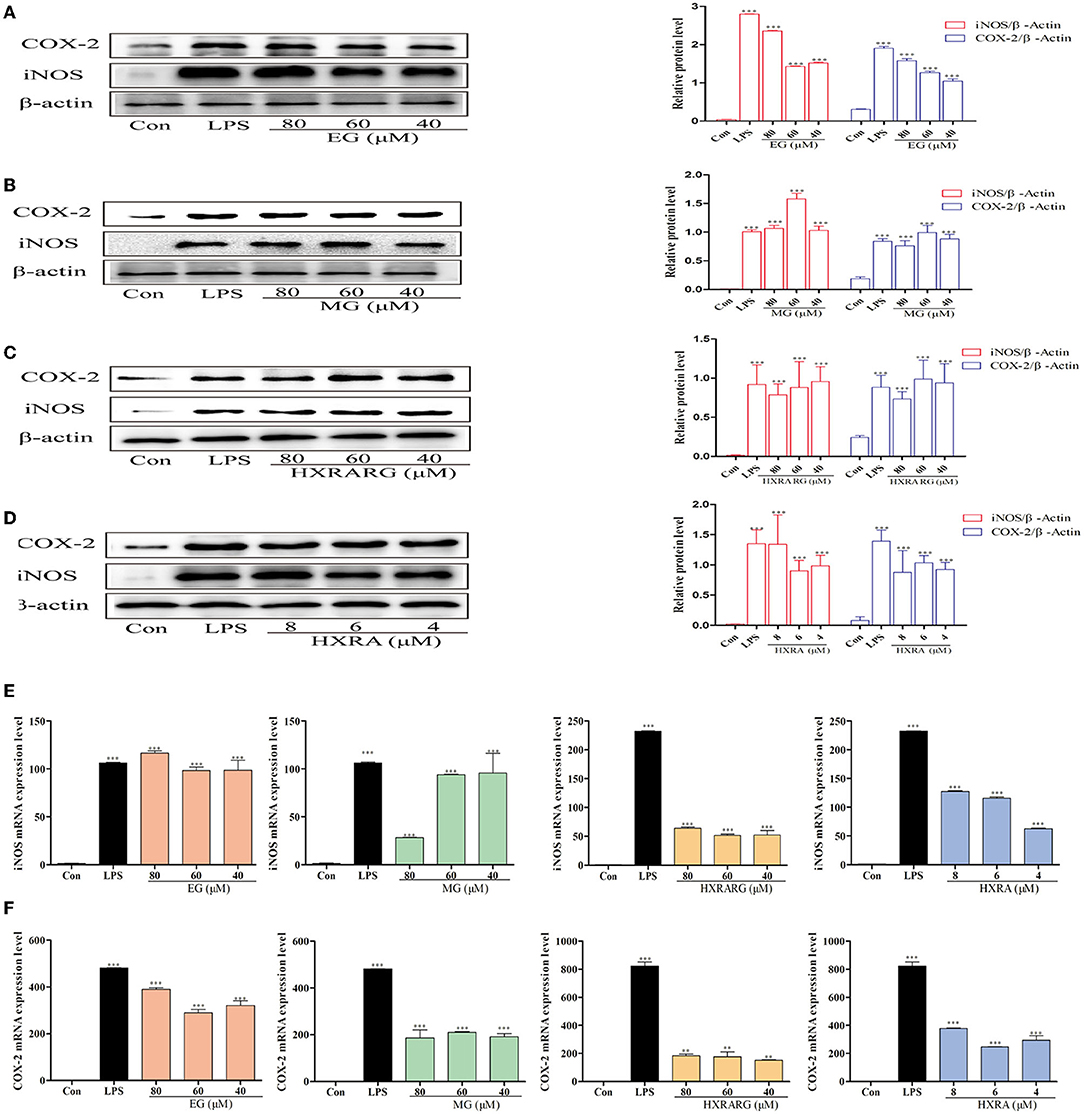
Figure 5. Effects of different concentrations of EG, MG, HXRARG, and HXRA on iNOS and Cox-2 protein (A–D) and mRNA (E,F) expression in RAW264.7 cells. (A–D): the left side was the protein band diagram, and the right side was the protein quantification diagram. (E) mRNA expression of iNOS. (F) mRNA expression of COX-2. Data are expressed as the means ± SD. Compared with the control, **P < 0.01, ***P < 0.001.
Effects of EG, MG, HXRARG, and HXRA on the Expression of TNF-α, IL-6, and mRNA in RAW264.7 Cells
TNF-α and IL-6 secretion increased dramatically in RAW264.7 cells induced by LPS, as shown in Figure 6, and treatment of RAW264.7 cells with various concentrations of EG, MG, HXRARG, or HXRA for 24 h likewise enhance TNF-α secretion. In the Figures 6A,B, compared with the high and low dose groups, EG, MG, HXRARG, and HXRA could more effectively promote the secretion of IL-6 in the middle dose group. In Figures 6C,D, the mRNA expression of TNF-α and IL-6 increased significantly after LPS stimulation. TNF- and IL-6 mRNA expression was also considerably elevated after stimulation with various concentrations of EG, MG, HXRARG, or HXRA compared to the control group. These results suggested that EG, MG, HXRARG, or HXRA stimulate immune cells to secrete more TNF- α and IL-6 by promoting the mRNA translocation of TNF-α and IL-6.
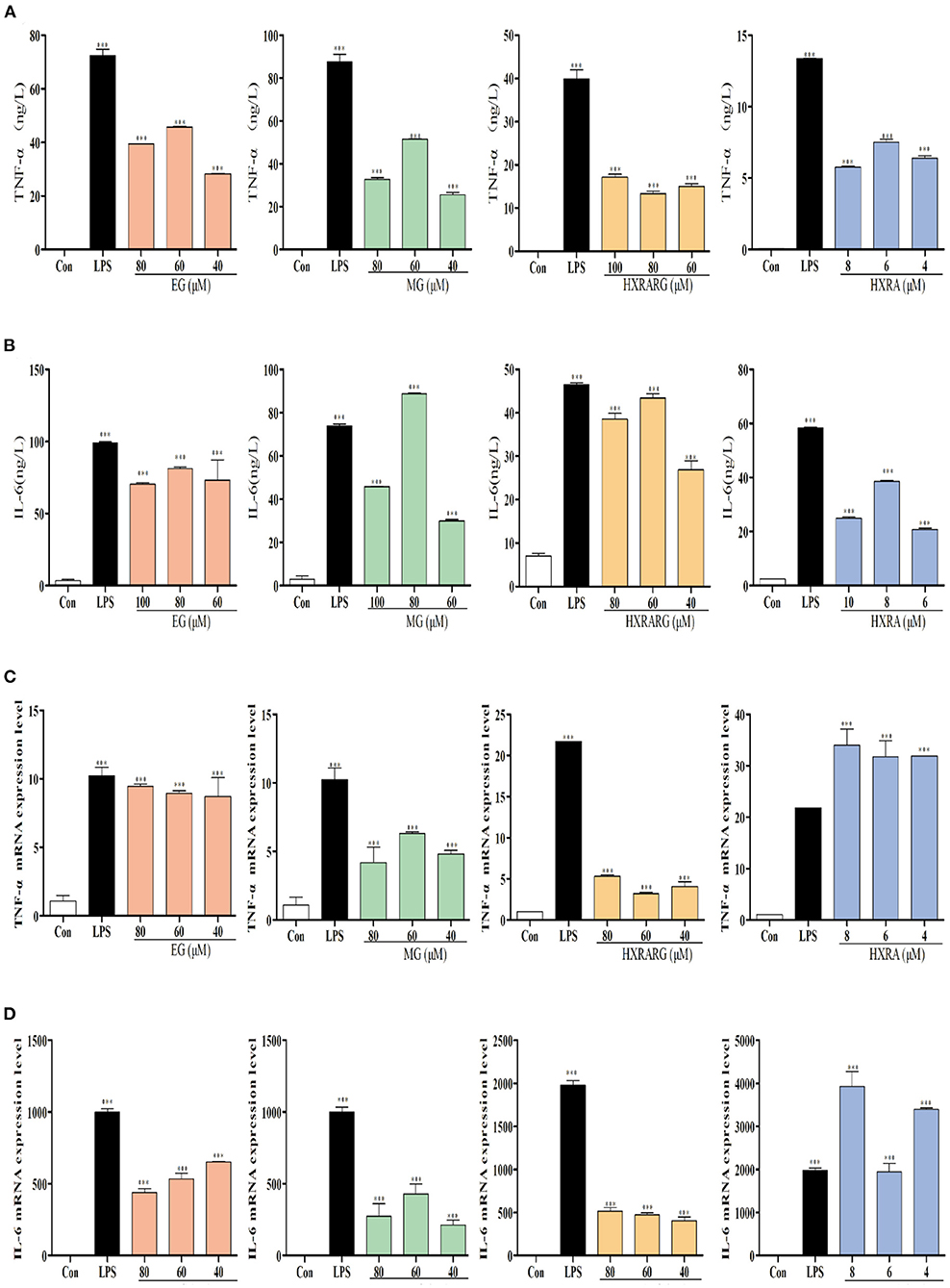
Figure 6. Effects of EG, MG, HXRARG and HXRA on the expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and mRNA in RAW264.7 cells. (A) secretion of IL-6 (B) secretion of TNF-α (C) TNF-α mRNA transcription (D) IL-6 mRNA transcription. Data are expressed as the means ± SD. Data are expressed as the means ±SD. Compared with the control, ***P < 0.001.
Effects of EG, MG, HXRARG, and HXRA on the Production of ROS in RAW264.7 Cells
The peak shape of the LPS group shifted to the right in comparison to the control group in Figure 7, suggesting that the ROS level of RAW264.7 cells increased following LPS stimulation. Compared with the control group, the peak shape also shifted to the right after stimulation with different concentrations of EG, MG, HXRARG, or HXRA, indicating that the production of ROS was significantly increased. Thus, we speculate that EG, MG, HXRARG, or HXRA enhance the immune function of macrophages in many ways.
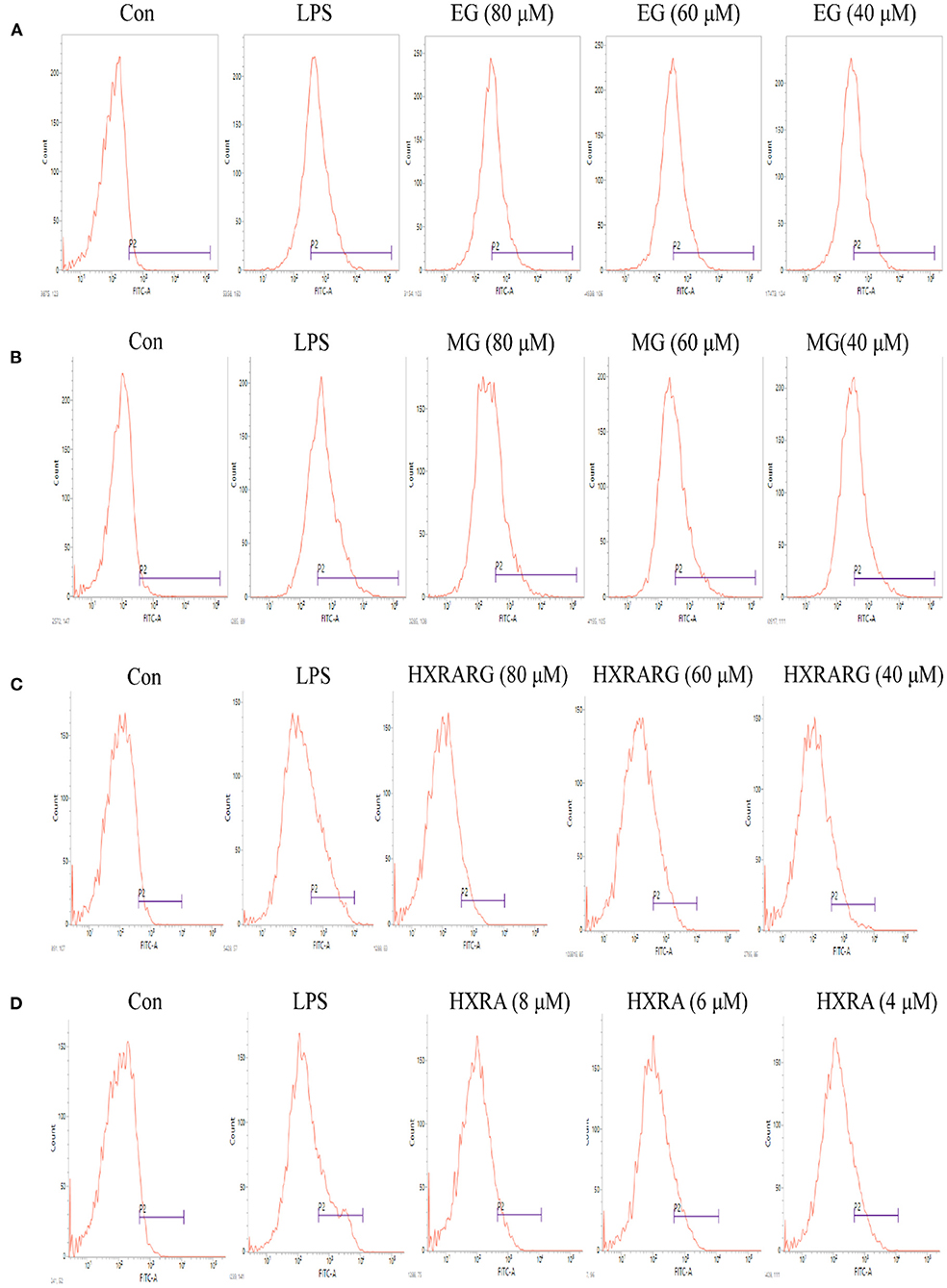
Figure 7. Effect of different concentrations of EG (A), MG (B), HXRARG (C), and HXRA (D) on ROS production ability of RAW 264.7 cells.
Effects of EG, MG, HXRARG, and HXRA on the Activation of NF-κB Pathways in RAW264.7 Cells
The detection of NF-κB P65 in the nucleus and IκB in the cytoplasm is an important sign to judge whether the NF-κB pathway is activated or not. The expression levels of phosphorylated P65 and phosphorylated IκB in the LPS group were substantially higher than in the control group. After RAW264.7 cells were treated with EG, MG, HXRARG or HXRA, there was no significant effect on the expression of NF-κB P65 and IκB-α, but the phosphorylated expression of IκB-α and P65 was significantly increased, Figure 8 indicating that EG, MG, HXRARG, or HXRA activate NF-κB signal pathway so as to induce the activation of RAW264.7 cells.
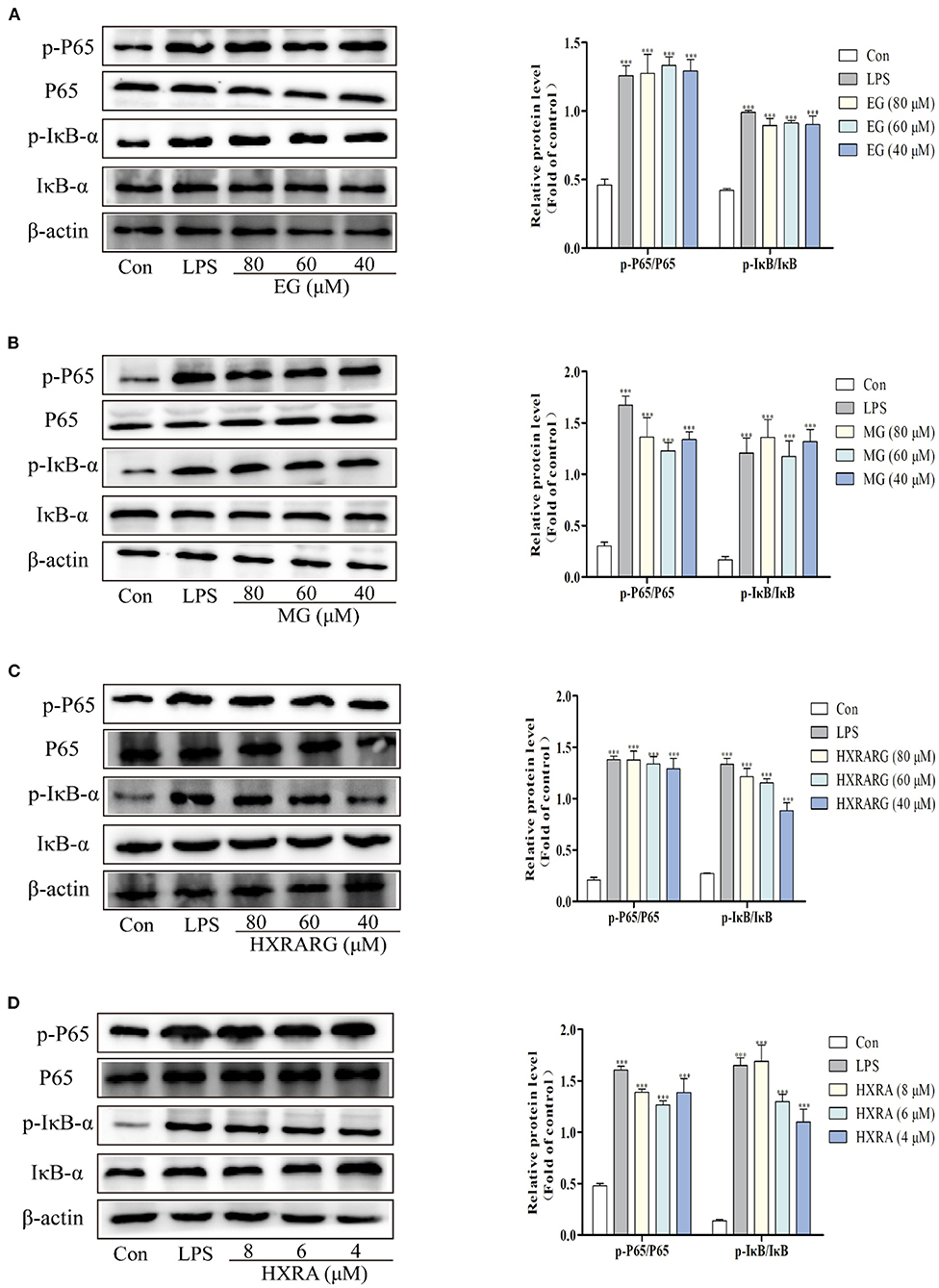
Figure 8. Effects of different concentrations of EG (A), MG (B), HXRARG (C), and HXRA (D) on the expression of related proteins in NF-κB signaling pathway in RAW264.7 cells. The left side was the protein band diagram, and the right side was the protein quantification diagram. Data are expressed as the means ± SD. Compared with the control, ***P < 0.001.
Effects of EG, MG, HXRARG, and HXRA on the Activation of MAPK Pathways in RAW264.7 Cells
MAPK signal pathway is the convergence of many signal transduction pathways in cells. In addition, many bioactive components can regulate the production and secretion of NO and cytokines in macrophages by activating MAPK (36). In Figure 9, the LPS group had considerably higher phosphorylation levels of P38, ERK, and JNK than the control group. The ratios of p-P38/P38, p-Erk/Erk, and p-JNK/JNK were notably greater after stimulation with EG, MG, HXRARG, or HXRA than in the control group. These results suggest that EG, MG, HXRARG, or HXRA can activate MAPK signal pathway and induce immune response.
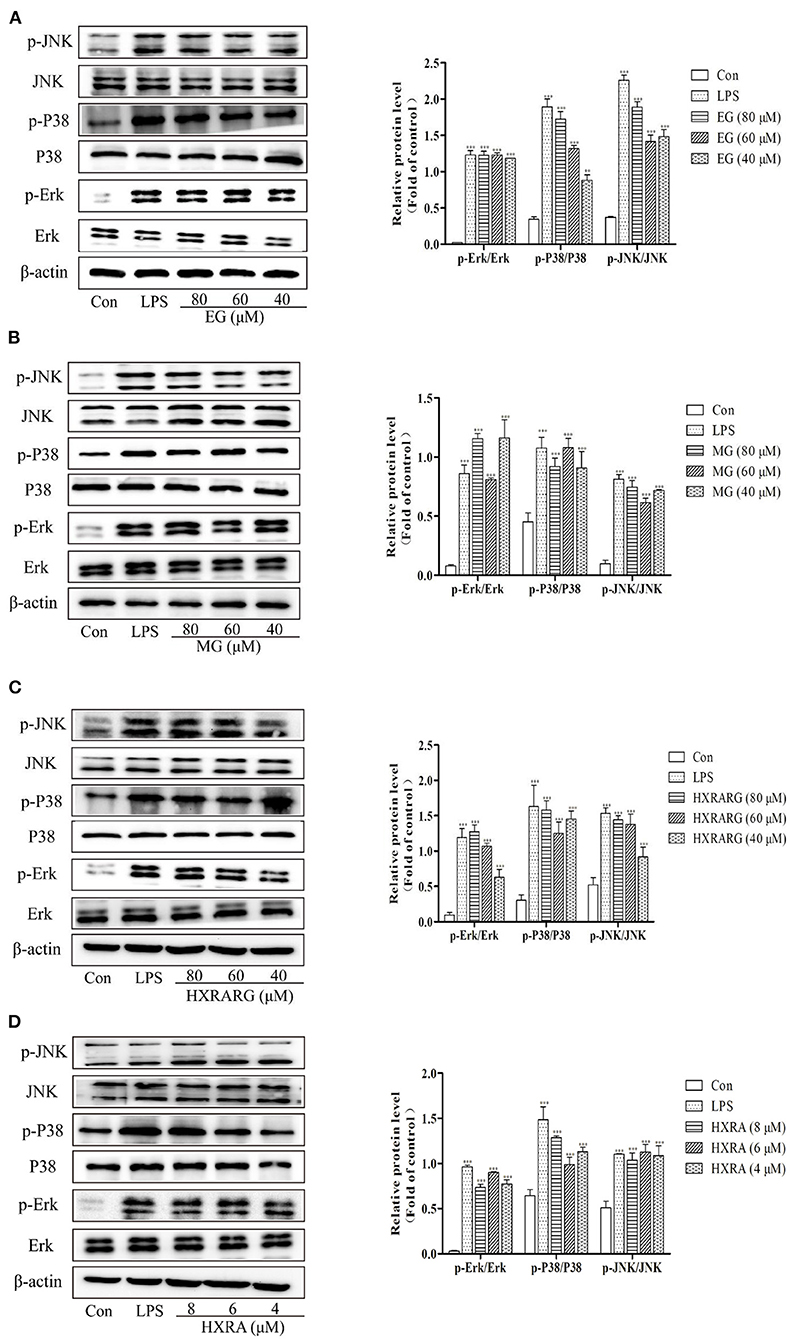
Figure 9. Effects of different concentrations of EG (A), MG (B), HXRARG (C), and HXRA (D) on the expression of related proteins in MAPK signaling pathway in RAW264.7 cells. The left side was the protein band diagram, and the right side was the protein quantification diagram. Data are expressed as the means ± SD. Compared with the control, ***P < 0.001.
Discussion
Plants have long been thought to be the foundation of nutrient-dense and useful diets, as well as the source of modern medicines throughout human history, which is not only because of their easy availability and low cost, but also because they are believed to have less harmful effects than synthetic drugs (37, 38). In addition, when used in combination with different conventional chemotherapy drugs, they produce synergistic effects, thereby reducing the dose of drugs used at the same time, optimizing efficacy and toxicity, and may overcome the problem of drug resistance (39). Therefore, it has a greater margin of safety and praiseworthy efficacy for a variety of diseases, and can be evaluated in clinical trials in a variety of cases (40, 41). However, it is estimated that only 15 percent of the 3,00,000 herbaceous species that exist in the world have been developed into pharmacological potential. Among several medicinal plants, N. sativa is regarded as one of the most valuable nutrient-dense herbs in human history, and numerous scientific investigations are underway to confirm its traditional use (42). At the same time, researchers found that N. sativa seeds can be utilized as nutritional supplements for COVID-19 patients as an adjuvant treatment, which set off an upsurge of research on N. sativa seeds (43–45).
Immunomodulatory effect is one of the most valuable characteristics of N. sativa seeds. In the last 20 years, significant progress has been made in the study of the health benefits of N. sativa seeds and its principal active ingredient, thymoquinone (TQ), on the immune system. TQ enhances immunomodulatory properties through T cells and NK cells (46). In most of the subjects who participated in the trial, after treatment with TQ, the ratio of CD4 and CD8 T cells increased by 55%, and the function of NK cells was improved, showing a significant effect (47). On BALB/c mice, Ghonime M investigated the therapeutic potential of a variety of medicinal herbs, including N. sativa seeds (48). Treated with various doses of N. sativa seed methanol extract (intraperitoneal injection), it was found that it could increase the total leukocyte count (up to 1.2 × 104 cells/mm3). After administration of N. sativa seed extract, the activity of bone marrow cells also increased significantly. The spleen weight of N. sativa seed extract group increased significantly. Mice were immunosuppressed by cyclophosphamide, and the resistance of mice treated with N. sativa seed extract to lethal infection of Candida albicans was significantly recovered. The most of the studies on the immune activity of N. sativa seeds are based on animal models and are usually related to TQ, while there are few reports on the contribution of other active components to immune enhancement and action mechanism. In this study, RAW264.7 macrophages were used and cytotoxicity, phagocytosis, release of cytokines and effects of immune-related signaling pathways were used to evaluate the immune activity and immune mechanism of EG, MG, HXRA and HXRARG, which were isolated in N. sativa seeds.
The attention of people looking for new immune targets has shifted from extracellular to intracellular molecules with the in-depth study of cytokine transduction pathway (49). NF-κB is a ubiquitous and important nuclear transcription factor in the process of immunity. NF-κB interacts to IκBα in the cytoplasm in an inactive state under normal conditions (50–52). When the cell is activated, IκBα is ubiquitinated and then degraded, and then some specific sites of NF-κB subunits are phosphorylated to make NF-κB into an active form. Then, transfer to the nucleus and DNA binds to activate transcription, which participates in many biological processes such as apoptosis, cellular immune regulation, inflammation and tumorigenesis by regulating the expression of related chemokines, growth factors, COX-2 and nitric oxide synthase (53). P65 is the first NF-κB subunit found to be phosphorylated after the degradation of IκBα (54). The degradation of IκBα and the phosphorylation of p65 are important events in the activation of NF-κB signal pathway. The MAPK family contains three main kinases, namely Erk1/2, JNK and P38 MAPK. The co-activation of these kinases promotes cell proliferation, migration, invasion, angiogenesis, metastasis and apoptosis, which is very important for the development and activation of macrophages and T cells (55). The three kinases of MAPK are closely related but relatively independent, and are activated in the form of step-by-step phosphorylation.
The results showed that the safe doses of EG, MG, HXRARG and HXRA could activate the NF-κB pathway by promoting the phosphorylation of NF-κB p65 and IκBα in RAW264.7 cells, and activate the MAPK pathway by promoting the phosphorylation of JNK, Erk and P38, which leads to the changes of proliferation, phagocytosis and secretion of cytokines (Figure 10). He obtained two kinds of homogeneous polysaccharides PCSPA and PCSPB from Crocus sativus, and found that MAPK and NF-κB pathway can enhance cell phagocytosis, up-regulate the expression of surface molecules, promote the production of cytokines and chemokine and mRNA expression, and significantly activate RAW264.7 cells (56). Yan found that a new type of heterogalactan (WPEP-N-b) isolated from the Pleurotus eryngi fruiting body activates macrophages by increasing the production and phagocytosis of NO, TNF-α, IL-6, and IL-1β. At the molecular level, WPEP-N-b activates macrophages through TLR2-mediated MAPK and NF-κB signaling pathways (57). El-Obeid reported that melanin extracted from N. sativa seeds can enhance the mRNA expression of TNF-α, IL-6 and vascular endothelial growth factor (VEGF) in monocytes, peripheral blood mono-nuclear cells (PBMC) and THP-1 cells (58), which is consistent with our results.
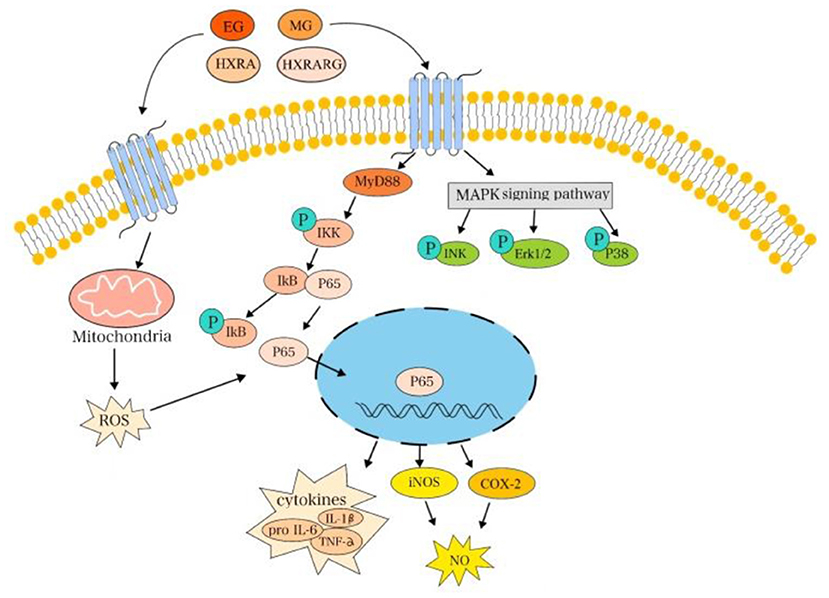
Figure 10. EG, MG, HXRARG, and HXRA activate NF-κB and MAPK signaling pathways to promote the activation of RAW264.7 cells.
The medicinal and health promotion characteristics of plant products are related to the existence of phytochemical components such as some secondary metabolites, phenolic compounds and terpenes in natural essential oils, which show many positive biological activities (59). N. sativa is a good source of essential nutrients, and supplementation of N. sativa can improve the overall antioxidant status of the body. Immune enhancement and immunomodulatory effects may be due to its interference with the ability of immunomodulators (60).
EG and MG, as monosaccharide derivatives isolated from N. sativa seeds, showed good potential to regulate and enhance the immune system. Huang found that monosaccharide derivative GPP could activate RAW264.7 cells by increasing phagocytosis, NO production and mRNA expression of iNOS and TNF-α (61, 62). Although the mechanism of immune enhancement of GPP was not studied in vitro, GPP could significantly improve the activity of natural killer (NK) cells and macrophage phagocytosis in mice after oral administration of 0, 3.6, 120, and 360 mg/kg body weight (mg/kg BW) for 30 days. Tsvetkov YE's immunobiological evaluation of chitin and chitosan-derived oligosaccharides on RAW264.7 cells in vitro showed that the beneficial immunomodulatory effects were related to cell proliferation, phagocytosis, cytokine release and cellular respiratory burst, which was consistent with the results of this experiment (63). Levine R and other studies have found that arabinoxylan with immunomodulatory effect can be regarded as an effective bioactive food supplement related to many health improvement functions (64).
Triterpenoid saponins have a variety of pharmacological effects, including hypoglycemia, anticancer, neuroprotection, liver protection, inhibition of lipid production, antibacterial, antioxidation, anti-angiogenesis and so on (65, 66). However, most of the studies on triterpenoid saponins are focused on anti-inflammatory effect, and there are few studies on immune effect. The anti-inflammatory activity of ginsenoside PG-RT8 was evaluated by RAW264.7 macrophages and THP-1 cells stimulated by LPS (67). It was found that PG-RT8 could attenuate the release of LPS-mediated pro-inflammatory factors (IL-1b, IL-6, iNOS, COX2 and MMP-9) on a dose-dependent manner. In addition, PG-RT8 strongly inhibited the production of ROS and NO. Salidroside derivative (PTs29) significantly inhibited LPS-induced secretion of pro-inflammatory factors TNF-α and IL-6 in THP-1 cells by stimulating the phosphorylation of AMPK and acetyl-CoA carboxylase (ACC). It indicated that triterpene saponins have good immune enhancement effect (68).
It is very important to improve personal immunity by eating nutritionally balanced food. So, balanced food with therapeutic functions is very beneficial. N. sativa seeds are very safe and effective as immune supplements. Ali and Blunden proved that N. sativa seed extracts or oils did not have significant toxic or adverse effects on liver or kidney function (69). In addition, monosaccharide derivatives or saponins derivatives are usually low in our diets, which is an important contribution to some dietary habits. Our investigation also proved that N. sativa seed contains the active components to improve immunity and it will be the beneficial supplements for immunodeficient human.
Conclusion
Four compounds, Ethyl-α-D-galactopyranoside (EG), Methyl-α-D-glucoside (MG), 3-O-[β-D-xylopyranose-(1 → 3)-α-L-rhamnose-(1 → 2)-α-L-arabinose]-28-O-[α-L-rhamnose-(1 → 4)-β-D-glucopyranose-L-(1 → 6)-β-D-glucopyranose]-hederagenin (HXRARG) and 3-O-[β-D-xylopyranose-(1 → 3)-α-L-rhamnose-(1 → 2)-α-L-arabinose]-hederagenin (HXRA), were isolate and identified in N. sativa seeds. They could significantly increase the production of NO, COX-2, TNF-α, and IL-6 by activating the JNK, ERK1/2, P38, and NF-κB pathways. Therefore, N. sativa seeds exhibited immune-enhancing potential, and might be developed as an immunopotentiator in the near future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JW designed the experiments and wrote the manuscript. BW performed experiments. QW analyzed and summarized data. YC was responsible for formal analysis and visualization. AA contributed to the data acquisition. YZ supervised project administration and critically reviewed the manuscript. WK provided resources, funding, and reviewed manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Research on Precision Nutrition and Health Food, Department of Science and Technology of Henan Province (CXJD2021006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.899797/full#supplementary-material
References
1. Butt MS, Sultan MT. Nigella sativa: reduces the risk of various maladies. Crit Rev Food Sci Nutr. (2010) 50:654–65. doi: 10.1080/10408390902768797
2. Sultan MT, Butt MS, Qayyum MMN, Suleria HAR. Immunity: plants as effective mediators. Crit Rev Food Sci Nutr. (2014) 54:1298–308. doi: 10.1080/10408398.2011.633249
3. Sultan MT, Butt MS, Anjum FM, Jamil A, Nasir M. Nutritional profile of indigenous cultivar of black cumin seeds and antioxidant potential of its fixed and essential oil. Pak J Bot. (2009) 41:1321–30. doi: 10.3417/2007082
4. Bourgeon S, Raclot T, Maho YL, Ricquier D, Criscuolo F. Innate immunity, assessed by plasma no measurements, is not suppressed during the incubation fast in eiders. Dev Comp Immunol. (2007) 31:720–8. doi: 10.1016/j.dci.2006.11.009
5. Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sánchez E, Nabavi SF, et al. Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol Res. (2017) 196:44–68. doi: 10.1016/j.micres.2016.12.003
6. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. (2015) 33:1582–614. doi: 10.1016/j.biotechadv.2015.08.001
7. Li FS, Weng JK. Demystifying traditional herbal medicine with modern approach. Nature plants. (2017) 3:17109. doi: 10.1038/nplants.2017.109
8. Nourbakhsh F, Lotfalizadeh M, Badpeyma M, Shakeri A, Soheili V. From plants to antimicrobials: natural products against bacterial membranes. Phytotherapy Res: PTR. (2022) 36:33–52. doi: 10.1002/ptr.7275
9. Wolf AJ, Underhill DM. Peptidoglycan recognition by the innate immune system. Nat Rev Immunol. (2018) 18:243–54. doi: 10.1038/nri.2017.136
10. Kumar J, Ramlal A, Kumar K, Rani A, Mishra V. Signaling Pathways and Downstream Effectors of Host Innate Immunity in Plants. Int J Mol Sci. (2021) 22:9022. doi: 10.3390/ijms22169022
11. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. (2019) 19:369–82. doi: 10.1038/s41577-019-0127-6
12. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
13. Oishi Y, Manabe I. Macrophages in inflammation, repair, and regeneration. Int Immunol. (2018) 30:511–28. doi: 10.1093/intimm/dxy054
14. Ahmad W, Jantan I, Kumolosasi E, Haque MA, Bukhari SNA. Immunomodulatory effects of tinospora crispa extract and its major compounds on the immune functions of RAW2647 macrophages international. Immunopharmacol. (2018) 60:141–51. doi: 10.1016/j.intimp.2018.04.046
15. Palmieri EM, McGinity C, Wink DA, McVicar DW. Nitric oxide in macrophage immunometabolism: hiding in plain sight. Metabolites. (2020) 10:429. doi: 10.3390/metabo10110429
16. Sun H, Zhang J, Chen F, Chen X, Zhou Z, Wang H. Activation of raw2647 macrophages by the polysaccharide from the roots of actinidia eriantha and its molecular mechanisms. Carbohyd Pol. (2015) 121:388–402. doi: 10.1016/j.carbpol.2014.12.023
17. Li D, Wu M. Pattern recognition receptors in health and diseases. Sig Trans Target Therapy. (2021) 6:291. doi: 10.1038/s41392-021-00687-0
18. Ren Z, Qin T, Qiu F, Song Y, Lin D, Ma Y, et al. Immunomodulatory effects of hydroxyethylated hericium erinaceus polysaccharide on macrophages RAW2647.7. Int J Biol Macromol. (2017) 105:879–85. doi: 10.1016/j.ijbiomac.2017.07.104
19. Rod-In W, Monmai C, Lee SM, Jung SK, You S, Park WJ. Anti-inflammatory effects of lipids extracted from Arctoscopus Japonicus eggs on lps-stimulated RAW2647.7. Cells. Marine Drugs. (2019) 17:580. doi: 10.3390/md17100580
20. Ghonime M, Emara M, Shawky R, Soliman H, El-Domany R, Abdelaziz A. Immunomodulation of RAW2647 murine macrophage functions and antioxidant activities of 11 plant extracts. Immunol Invest. (2015) 44:237–52. doi: 10.3109/08820139.2014.988720
21. Li H, Xie W, Qiao X, Cui H, Yang X, Xue C. Structural characterization of arabinogalactan extracted from Ixeris Chinensis (Thunb) Nakai and its immunomodulatory effect on RAW2647 macrophages. Int J Biol Macromol. (2020) 143:977–83. doi: 10.1016/j.ijbiomac.2019.09.158
22. Zeng F, Chen W, He P, Zhan Q, Wang Q, Wu H, et al. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from chinese water chestnut peels. Carbohydr Polym. (2020) 246:116551. doi: 10.1016/j.carbpol.2020.116551
23. Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin J Nat Med. (2016) 14:732–45. doi: 10.1016/s1875-5364(16)30088-7
24. Amin B, Hosseinzadeh H. Black Cumin (Nigella stiva) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med. (2016) 82:8–16. doi: 10.1055/s-0035-1557838
25. Gholamnezhad Z, Havakhah S, Boskabady MH. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: a review. J Ethnopharmacol. (2016) 190:372–86. doi: 10.1016/j.jep.2016.06.061
26. Alshatwi AA. Bioactivity-guided identification to delineate the immunomodulatory effects of methanolic extract of nigella sativa seed on human peripheral blood monon-uclear cells. Chin J Integr Med. (2014). doi: 10.1007/s11655-013-1534-3
27. Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol. (2015) 28:295–304. doi: 10.1016/j.intimp.2015.06.023
28. Liang Q, Dong J, Wang S, Shao W, Ahmed AF, Zhang Y, et al. Immunomodulatory effects of Nigella sativa seed polysaccharides by gut microbial and proteomic technologies. Int J Biol Macromol. (2021) 184:483–96. doi: 10.1016/j.ijbiomac.2021.06.118
29. Dong J, Liang Q, Niu Y, Jiang S, Zhou L, Wang J, et al. Effects of Nigella Sativa seed polysaccharides on type 2 diabetic mice and gut microbiota. Int J Biol Macromol. (2020) 159:725–38. doi: 10.1016/j.ijbiomac.2020.05.042
30. Niu Y, Zhou L, Meng L, Chen S, Ma C, Liu Z, et al. Recent progress on chemical constituents and pharmacological effects of the genus Nigella. Evidence-based complementary and alternative medicine: eCAM. (2020) 2020:6756835. doi: 10.1155/2020/6756835
31. Niu Y, Wang B, Zhou L, Ma C, Waterhouse GIN, Liu Z, et al. Nigella Sativa: a dietary supplement as an immune-modulator on the basis of bioactive components. Front Nutr. (2021) 8:722813. doi: 10.3389/fnut.2021.722813
32. Wang S, Zhou L, Attia FA-ZKK, Tang Q, Wang M, Liu Z, et al. Origanum Majorana L: a nutritional supplement with immunomodulatory effects. Front Nutr. (2021) 8:748031. doi: 10.3389/fnut.2021.748031
33. Wang J, Wang H, Zhang H, Liu Z, Ma C, Kang W. Immunomodulation of Adps-1a and Adps-3a on Raw2647 Cells through Nf-?b/Mapk signaling pathway. Int J Biol Macromol. (2019) 132:1024–30. doi: 10.1016/j.ijbiomac.2019.04.031
34. Repetto G. Peso Ad, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Prot. (2008) 3:1125–31. doi: 10.1038/nprot.2008.75
35. Xu X, Wang P, Wang B, Wang M, Wang S, Liu Z, et al. Glucose absorption regulation and mechanism of the compounds in Lilium Lancifolium thunb on Caco-2 cells. Food and Chem Toxicol: Int J Pub Br Ind Biol Res Assoc. (2021) 149:112010. doi: 10.1016/j.fct.2021.112010
36. Xu Z, Lin R, Hou X, Wu J, Zhao W, Ma H, et al. Immunomodulatory mechanism of a purified polysaccharide isolated from Isaria cicadae miquel on RAW2647 cells via activating Tlr4-Mapk-Nf-?b signaling pathway. Int J Biol Macromol. (2020) 164:4329–38. doi: 10.1016/j.ijbiomac.2020.09.035
37. Gul K, Singh AK, Jabeen R. Nutraceuticals and functional foods: the foods for the future world. Crit Rev Food Sci Nutr. (2016) 56:2617–27. doi: 10.1080/10408398.2014.903384
38. Zhang JY, Deng H, Bai J, Zhou XH, Zhao YS, Zhu Y, et al. Health-promoting properties of barley: a review of nutrient and nutraceutical composition, functionality, bioprocessing, and health benefits. Crit Rev Food Sci Nutr. (2021). doi: 10.1080/10408398.2021.1972926
39. Meng X, Li Q, Shi R, Chang J, Chang H, Li M. Food supplements could be an effective improvement of diabetes mellitus: a review. J Future Foods. (2021) 1:67–81. doi: 10.1016/j.jfutfo.2021.09.003
40. Guo BJ, Bian ZX, Qiu HC, Wang YT, Wang Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann N Y Acad Sci. (2017) 1401:37–48. doi: 10.1111/nyas.13414
41. Ramaa CS, Shirode AR, Mundada AS, Kadam VJ. Nutraceuticals–an emerging era in the treatment and prevention of cardiovascular diseases. Curr Pharm Biotechnol. (2006) 7:15–23. doi: 10.2174/138920106775789647
42. Gull T, Anwar F, Sultana B, Alcayde M, Nouman W. Capparis species: a potential source of bioactives and high-value components: a review. Industrial Crops & Products. (2015) 67:81–96. doi: 10.1016/j.indcrop.2014.12.059
43. Maiti S, Banerjee A, Kanwar M. In Silico Nigellidine (N. Sativa) bind to viral spike/active-sites of ace1/2, At1/2 to prevent Covid-19 induced vaso-tumult/vascular-damage/comorbidity. Vascular Pharmacol. (2021) 138:106856. doi: 10.1016/j.vph.2021.106856
44. Kulyar MFEA, Li R, Mehmood K, Waqas M, Li K, Li J. Potential influence of Nagella sativa (Black Cumin) in reinforcing immune system: a hope to decelerate the Covid-19 pandemic. Phytomedicine: Int J Phytotherapy Phytopharmacol. (2021) 85:153277. doi: 10.1016/j.phymed.2020.153277
45. Islam MN, Hossain KS, Sarker PP, Ferdous J, Hannan MA, Rahman MM, et al. Revisiting pharmacological potentials of Nigella sativa seed: a promising option for Covid-19 prevention and cure. Phytotherapy research: PTR. (2021) 35:1329–44. doi: 10.1002/ptr.6895
46. Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. (2000) 22:729–40. doi: 10.1016/s0192-0561(00)00036-9
47. Demir H, Kanter M, Coskun O, Uz YH, Koc A, Yildiz A. Effect of black cumin (Nigella sativa) on heart rate, some hematological values, and pancreatic beta-cell damage in cadmium-treated rats. Biol Trace Elem Res. (2006) 110:151–62. doi: 10.1385/bter:110:2:151
48. Ghonime M, Eldomany R, Abdelaziz A, Soliman H. Evaluation of Immunomodulatory effect of three herbal plants growing in Egypt. Immunopharmacol Immunotoxicol. (2011) 33:141–5. doi: 10.3109/08923973.2010.487490
49. Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. (2002) 111:927–30. doi: 10.1016/s0092-8674(02)01201-1
50. Kawai T, Akira S. Signaling to Nf-Kappab by toll-like receptors. Trends Mol Med. (2007) 13:460–9. doi: 10.1016/j.molmed.2007.09.002
51. Dorrington MG, Fraser IDC. Nf-?b signaling in macrophages: dynamics, crosstalk, and signal integration. Front Immunol. (2019) 10:705. doi: 10.3389/fimmu.2019.00705
53. Yao C, Narumiya S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br J Pharmacol. (2019) 176:337–54. doi: 10.1111/bph.14530
54. Pflug KM, Sitcheran R. Targeting Nf-?b-inducing kinase (Nik) in immunity, inflammation, and cancer. Int J Mol Sci. (2020) 21:8470. doi: 10.3390/ijms21228470
55. Fan R, Zhu C, Qiu D, Mao G, Zeng J. Activation of raw2647 macrophages by an acidic polysaccharide derived from citrus grandis ‘Tomentosa'. Int J Biol Macromol. (2020) 156:1323–9. doi: 10.1016/j.ijbiomac.2019.11.172
56. He Y, Peng H, Zhang H, Liu Y, Sun H. Structural characteristics and immunopotentiation activity of two polysaccharides from the petal of crocus sativus. Int J Bio Macromol. (2021) 180:129–42. doi: 10.1016/j.ijbiomac.2021.03.006
57. Yan J, Meng Y, Zhang M, Zhou X, Cheng H, Sun L, et al. A 3-O-Methylated heterogalactan from pleurotus eryngii activates macrophages. Carbohydrate Pol. (2019) 206:706–15. doi: 10.1016/j.carbpol.2018.11.063
58. El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A. Herbal melanin modulates tumor necrosis factor alpha (Tnf-Alpha), interleukin 6 (Il-6), and vascular endothelial growth factor (Vegf) production. Phytomed: Int J Phytotherapy Phytopharmacol. (2006) 13:324–33. doi: 10.1016/j.phymed.2005.03.007
59. Wu X, Schauss AG. Mitigation of inflammation with foods. J Agric Food Chem. (2012) 60:6703–17. doi: 10.1021/jf3007008
60. Elmowalid G, Amar AM, Ahmad AAM. Nigella Sativa seed extract: 1. Enhancement of sheep macrophage immune functions in vitro. Res Vet Sci. (2013) 95:437–43. doi: 10.1016/j.rvsc.2013.02.015
61. Huang Q, Li L, Chen H, Liu Q, Wang Z. Gpp (Composition of Ganoderma Lucidum Poly-Saccharides and Polyporus Umbellatus Poly-Saccharides) Enhances innate immune function in mice. Nutrients. (2019) 11:1480. doi: 10.3390/nu11071480
62. Yin Z, Liang Z, Li C, Wang J, Ma C, Kang W. Immunomodulatory effects of polysaccharides from edible fungus: a review. Food Sci Human Wellness. (2021) 10:393–400. doi: 10.1016/j.fshw.2021.04.001
63. Tsvetkov YE, Paulovičová E, Paulovičová L, Farkaš P, Nifantiev NE. Synthesis of biotin-tagged chitosan oligosaccharides and assessment of their immunomodulatory activity. Front Chem. (2020) 8:554732. doi: 10.3389/fchem.2020.554732
64. Levine R. Monosaccharides in health and disease. Annu Rev Nutr. (1986) 6:211–24. doi: 10.1146/annurev.nu.06.070186.001235
65. Li C, Cui Y, Lu J, Meng L, Ma C, Liu Z, et al. Spectrum-effect relationship of immunologic activity of Ganoderma Lucidum by UPLC-Ms/Ms and component knock-out method. Food Science and Human Wellness. (2021) 10:278–88. doi: 10.1016/j.fshw.2021.02.019
66. Liu Y, Chen R, Li L, Dong R, Yin H, Wang Y, et al. The triterpenoids-enriched extracts from Antrodia Cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57bl/6 mice. Food Sci Human Wellness. (2021) 10:497–507. doi: 10.1016/j.fshw.2021.04.012
67. Rho T, Jeong HW, Hong YD, Yoon K, Cho JY, Yoon KD. Identification of a novel triterpene saponin from Panax Ginseng Seeds, Pseudoginsenoside Rt8, and its antiinflammatory activity. J Ginseng Res. (2020) 44:145–53. doi: 10.1016/j.jgr.2018.11.001
68. Liu L, Li H, Hu K, Xu Q, Wen X, Cheng K, et al. Synthesis and anti-inflammatory activity of saponin derivatives of δ-oleanolic acid. Eur J Med Chem. (2021) 209:112932. doi: 10.1016/j.ejmech.2020.112932
Keywords: Nigella sativa, immunoregulation, NF-κB, MAPK, RAW264.7 cells
Citation: Wei J, Wang B, Chen Y, Wang Q, Ahmed AF, Zhang Y and Kang W (2022) The Immunomodulatory Effects of Active Ingredients From Nigella sativa in RAW264.7 Cells Through NF-κB/MAPK Signaling Pathways. Front. Nutr. 9:899797. doi: 10.3389/fnut.2022.899797
Received: 19 March 2022; Accepted: 22 April 2022;
Published: 31 May 2022.
Edited by:
Quancai Sun, Jiangsu University, ChinaReviewed by:
Guitang Chen Chen, China Pharmaceutical University, ChinaMinhui Li, Baotou Medical College, China
Copyright © 2022 Wei, Wang, Chen, Wang, Ahmed, Zhang and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adel F. Ahmed, YWRlbGZhaG1pXzIwMDhAeWFob28uY29t; Yan Zhang, c25vd3dpbmdsdkAxMjYuY29t; Wenyi Kang, a2FuZ3dlbnlAaG90bWFpbC5jb20=
 Jinfeng Wei1,2
Jinfeng Wei1,2 Baoguang Wang
Baoguang Wang Yixiao Chen
Yixiao Chen Qiuyi Wang
Qiuyi Wang Adel F. Ahmed
Adel F. Ahmed Yan Zhang
Yan Zhang Wenyi Kang
Wenyi Kang