- Department of Nephrology, The Second Xiangya Hospital of Central South University, Hunan Key Laboratory of Kidney Disease and Blood Purification, Changsha, China
Background: Diet management is a pivotal intervention for chronic kidney disease (CKD) patients. Dietary inflammation index (DII) is developed to evaluate the integral inflammatory potential of a diet pattern. However, research about the association between DII and mortality in CKD is limited.
Objective: We conducted a cohort study to investigate the relationship between energy-adjusted DII (E-DII) and the 5-year all-cause and cardiovascular mortality in CKD population.
Materials and Methods: CKD participants with complete E-DII data and death status from National Health and Nutrition Examination Survey (1999–2014) were involved in this study. E-DII was calculated based on dietary recall interviews. Smooth curve fitting, Kaplan–Meier survival analysis, and Cox proportional hazards models were used to evaluate the association between E-DII and the 5-year all cause and cardiovascular mortality. Subgroup analysis was also performed.
Results: A total of 7,207 participants were included (55.46% elderly and 46.54% male) in this study. The 5-year all-cause and cardiovascular mortality were 16.86 and 4.32%, respectively. Smooth curve fitting showed a “J” shape and near linear relationship between the E-DII score and the 5-year all-cause and cardiovascular mortality, respectively. In multivariate Cox proportional hazards models, the hazard ratios (95% confidence intervals [CI]) for the highest tertile of the E-DII were 1.33 (1.15, 1.54) for all-cause mortality, and 1.54 (1.15, 2.07) for cardiovascular mortality when compared with the lowest tertile of the E-DII. The subgroup analyses revealed relatively stronger associations between the E-DII and the mortality among CKD patients with other death risk factors.
Conclusions: Energy-adjusted dietary inflammatory index is independently related with the 5-year all-cause and cardiovascular mortality among CKD patients. Therefore, anti-inflammatory diet patterns should be recommended for CKD patients.
Introduction
Chronic kidney disease (CKD) has become one of the most common health and life-threatening disease. The global prevalence of all-stage CKD and the CKD-associated mortality increased 29.3 and 41.5%, respectively, between 1990 and 2017 (1). High burden of cardiovascular disease and cardiovascular mortality due to volume overload, hypertension, atherosclerosis, and vascular calcification is widely recognized in CKD patients (2).
Inflammation is one of the main systemic nature of CKD, characterized by high levels of proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin 1β (IL-1β), IL-6, and C-reactive protein (CRP) (3, 4). The systematic inflammation is correlated with many adverse events and outcomes in CKD including increase in all-cause and cardiovascular mortality. A prospective cohort study involving 3,875 CKD participants (2–4 stage) investigated the associations between the baseline plasma IL-6, CRP, fibroblast growth factor 23 (FGF23), and the all-cause mortality. Not surprisingly, they found that CKD patients with higher level of IL-6, CRP, and FGF23 suffered 35, 28, and 45% higher risk of death, respectively (5). Our group also demonstrated that platelet-to-lymphocyte ratio, an inflammatory biomarker, is independently associated with an increased 5-year all-cause and cardiovascular mortality in CKD patients (6).
Chronic kidney disease associated inflammation is influenced and regulated by multiple factors including accumulation of uremic toxins and infections like periodontal disease (3). Interestingly, diet is another important source of inflammation. It has been documented that inflammatory cytokines like CRP can be modulated by dietary components and specific nutrients (7). To evaluate the integral anti- and pro-inflammatory effect of diet patterns, dietary inflammatory index (DII) was developed (7). Furthermore, DII score is regarded to be positively associated with numerous inflammatory diseases such as obesity, diabetes, cardiovascular disease, nonalcoholic fatty liver disease, periodontitis, and chronic kidney disease (8–11). However, the correlation between DII and the risk of death among CKD patients has not been elucidated. Therefore, this study aimed to examine the relationship between the E-DII score and the 5-year mortality in CKD population based on the public database National Health and Nutrition Examination Surveys (NHANES). We hypothesized energy-adjusted DII (E-DII) is positively related with the risk of death in CKD population.
Materials and Methods
Study Population
The NHANES is a program evaluating the health and nutritional status of the people in the United States. NHANES collects demographics, dietary, medical examination, laboratory, and questionnaire data (12). This program was approved by The National Center for Health Statistics (NCHS) Research Ethics Review Board and the written informed consent was obtained from the participants (13).
In this study, we collected data from 8 continuous NHANES cycles, ranging from 1999 to 2014 (12). The inclusion criteria were (1) diagnosed with CKD (estimated glomerular filtration rate [eGFR] < 60 ml/min per 1.73 m2 or urine albumin-to creatinine ratio (ACR) ≥ 30 mg/g) (14), (2) had complete data for E-DII and death status. While the exclusion criteria were (1) had missing data for E-DII and death status, (2) age < 20 years old, (3) extreme energy intake (< 500 kcal/day or more than 5,000 kcal/day for women, and 8,000 kcal/day for men) (15). As shown in Figure 1, 7,207 participants were involved for analysis.
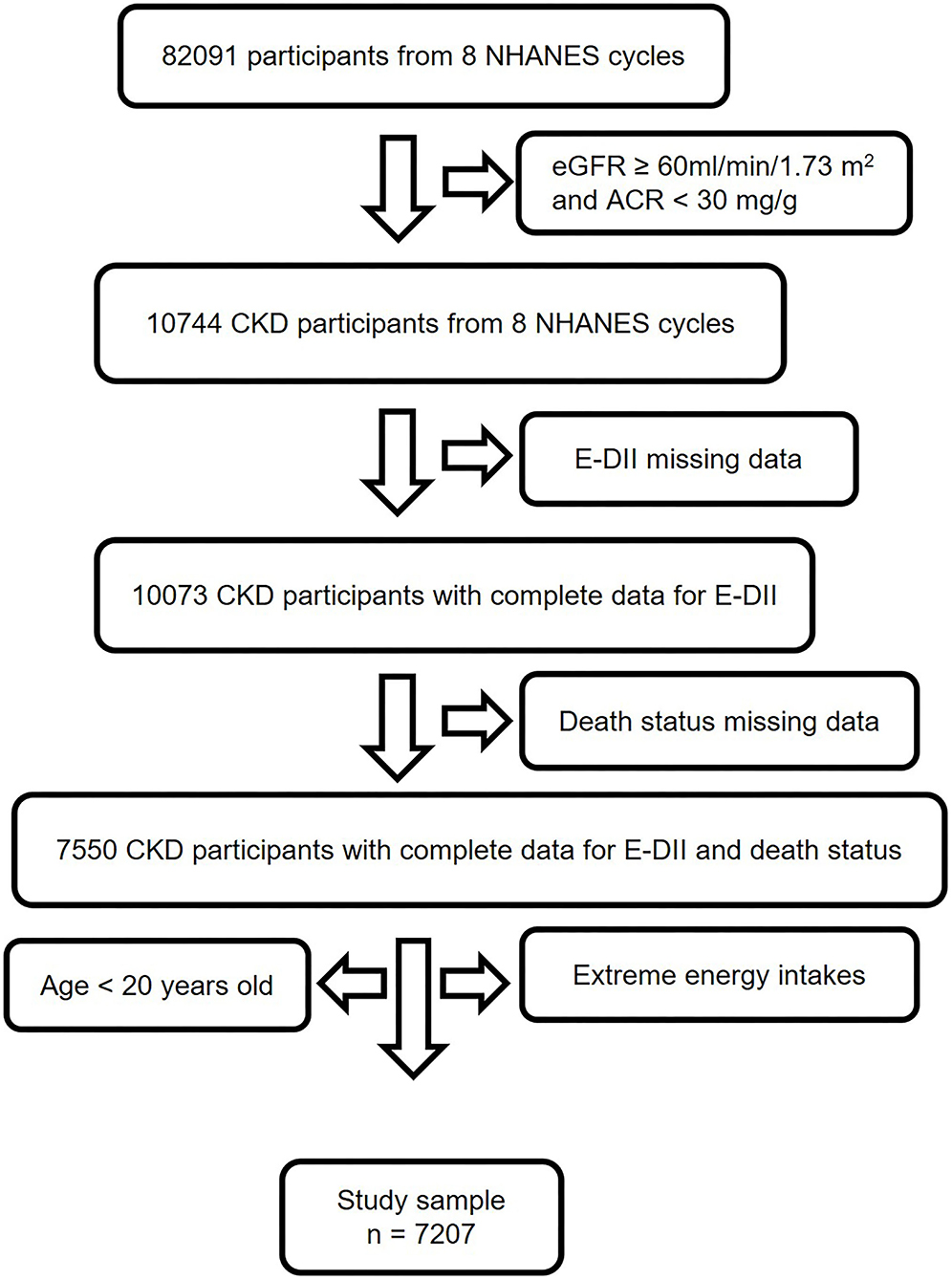
Figure 1. Study flow chart. A total of 82,091 participants from NHANES 1999–2014 were involved in this study. Individuals with eGFR ≥ 60 ml/min/1.73 m2, ACR < 30 mg/g and age < 20 years old didn't meet the inclusion criteria. After excluding subjects with missing data for E-DII, mortality, and extreme energy intake, 7,207 participants were finally used for analysis. CKD, chronic kidney disease; NHANES, National Health and Nutrition Examination Survey; E-DII, energy adjusted dietary inflammatory index; eGFR, estimate glomerular filtration rate; ACR, urine albumin to creatinine ratio.
Energy-Adjusted DII
Dietary inflammatory index is a method for nutritional evaluation to estimate the influence of diet on systematic inflammatory biomarkers (7). DII is calculated based on 45 different food components' pro- and anti-inflammatory properties. Pro-inflammatory diets display higher and positive DII scores, whereas anti-inflammatory diets show negative DII (7). We collected dietary intake information from 24 h dietary recalls (24 h) in this study. DII scores were calculated based on 27 or 28 food components listed below (16): energy, carbohydrate, protein, fat, dietary fiber, saturated, monounsaturated, and polyunsaturated fatty acids, ω-3 and ω-6 polyunsaturated fatty acids, cholesterol, vitamin A, B1, B2, B3, B6, B12, C, D (missing in NHANES 1999–2006), and E, folic acid, alcohol, beta-carotene, caffeine, iron, magnesium, zinc, and selenium. The details of DII calculation are provided in the Supplementary Material. To minimize the influence of total energy intake, we designed an E-DII (E-DII, DII/total energy intake) as the exposure variable in this study (10).
Outcome Variable
The outcome of interest was the 5-year all-cause and cardiovascular mortality, which were collected from the NHANES linked mortality file. Reason of death was classified by the NCHS based on the Tenth Revision of International Classification of Diseases (ICD-10). In this study, cardiovascular mortality was defined as death caused by heart and cerebrovascular diseases (6).
Potential Confounders
Potential confounders included age, gender, race, physical activity, smoking, alcohol consumption, comorbidities, the eGFR categories of chronic kidney disease, and NHANES release cycles. The age was classified to 2 groups: <65 years old (non-elderly) and ≥ 65 years old (elderly). Gender was categorized into “male” and “female.” The race was assigned to 4 groups: Mexican American, non-Hispanic white, non-Hispanic black, and others (17). Physical activity was defined as “inactive” or “active” according to the average physical activity time (“active” was defined as getting more than 75 min/week vigorous or 150 min/week moderate physical activity) (18). Smokers were participants who answered “Yes” for the question “whether smoke more than 100 cigarettes in life.” Alcohol consumers were individuals who drink alcohol more than 0 g/day. NHANES release cycle was categorized as 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007-2008, 2009-2010, 2011-2012, or 2013-2014. Comorbidities included diabetes, hypertension, overweight, central obesity, dyslipidemia, cardiovascular diseases, and cancer. Diabetes was identified by self-reported physician's diagnosis or application of hypoglycemic drugs or glycated hemoglobin level ≥ 6.5% or fasting blood glucose ≥ 7 mmol/L or blood glucose examined 2 h after Oral Glucose Tolerance Test (OGTT) ≥ 11.1 mmol/L (6). Hypertension was self-reported physician's diagnosis or application of hypertensive drugs or blood pressure ≥ 140/90 mm Hg (6). Overweight was defined as BMI≥ 25 kg/m2 (11), central obesity was confirmed by waist circumference ≥ 88 cm (female)/102 cm (male) (19). Dyslipidemia was self-reported physician's diagnosis or application of anti-dyslipidemia drugs or HDL cholesterol level < 1.0 mmol/L (male)/1.3 mmol/L (female) or triacylglycerol ≥ 1.7 mmol/L or LDL cholesterol ≥ 3.0 mmol/L (11, 19). Cancer was recognized by self-reported physician's diagnosis (6). Heart disease was identified by self-reported physician's diagnosis of congestive heart failure or coronary heart disease or angina pectoris or heart attack or stroke (11). CKD eGFR categories: G1, eGFR ≥ 90 ml/min per 1.73 m2, G2, eGFR < 90 but ≥ 60 ml/min per 1.73 m2, G3, eGFR < 60 but ≥ 30 ml/min per 1.73 m2, G4-5, eGFR < 30ml/min per 1.73 m2 (20).
Statistical Analyses
All the analyses were conducted with Empower (R) (21) and RStudio (22). Continuous variable with irregular distribution was described by median and interquartile range (IQR). Categorical variables were presented as frequency and percentage and comparisons among different groups were performed by chi-square tests. Missing values for confounders were set as another level of the categorical covariate. Smooth curve fitting (penalized spline method) was used to evaluate the non-linear association between E-DII and 5-year all cause or cardiovascular mortality. Survival by tertiles of E-DII was determined by the Kaplan–Meier survival analysis, and the log-rank test was used to compare the differences between groups. Then univariate and multivariate Cox proportional hazards models was applied to evaluate the independent association between E-DII and 5-year mortality. In model 1, no covariate was adjusted. In model 2, age, gender, and race were adjusted and in model 3 age, gender, race, physical activity, smoking, alcohol drinking, comorbidities, CKD G categories, and NHANES cycle were adjusted. In model 3, we selected these confounders due to their associations with mortality or a change in effect estimate of more than 10%. We also performed subgroup analyses. Interaction effect was assessed via likelihood ration test.
Results
Participants Characteristics
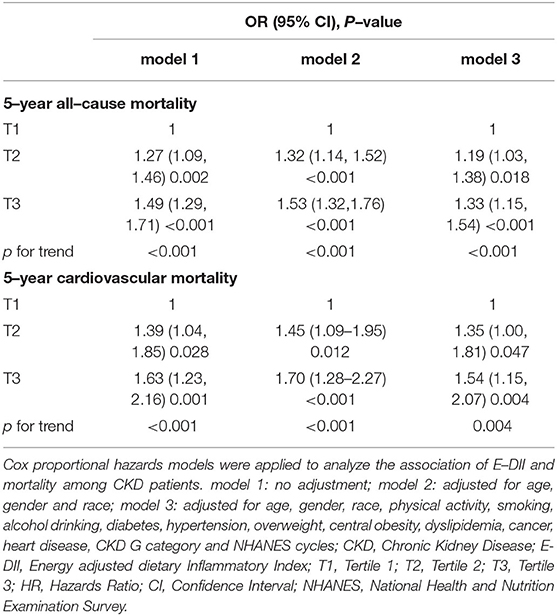
The baseline characteristics of participants were summarized in Table 1. In total, 55.46% participants were elderly patients and 46.54% participants were men. The overall E-DII score ranged from −2.51 to 9.00 (Table 1). Participants were equally distributed into 3 groups according to their E-DII score: the first tertile group (T1, E-DII = −2.51 to 0.37), the second one (T2, DII = 0.37 to 1.62), and the third one (T3, DII = 1.62–9.00). Compared with T1, subjects in T3 group tended to be elderly, female, inactive subjects, and non-alcohol consumers. They also had a higher incidence of diabetes, hypertension, central obesity, dyslipidemia, and heart disease than those in T1 group (p < 0.05). Notably, participants who consumed more pro-inflammatory diets were more likely to be in CKD G3-5 groups. Besides, blood inflammatory marker CRP concentration increased along with the E-DII (Table 1), suggesting that the dietary inflammatory potential was correlated to systemic inflammation. The overall 5-year all-cause and cardiovascular mortality among CKD patients were 16.86 and 4.32%, respectively. Moreover, the 5-year all-cause and cardiovascular mortality significantly increased in T3 groups, compared with T1.
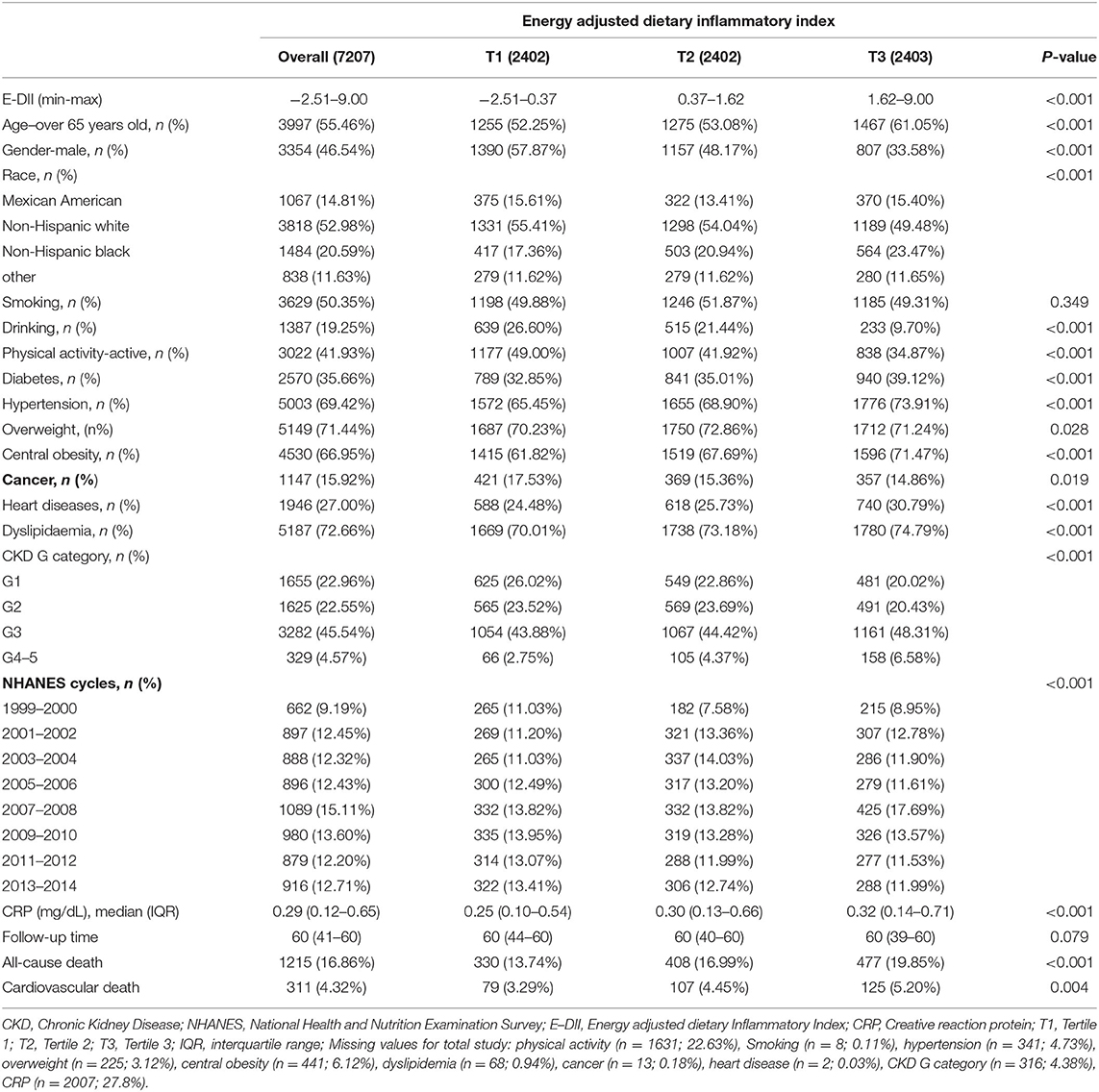
Table 1. Characteristics of 7,207 chronic kidney disease (CKD) patients aged ≥20 years from 8 National Health and Nutrition Examination Survey (NHANES) cycles overall and by tertile of energy adjusted dietary inflammatory index (E–DII).
Energy-Adjusted DII and Mortality
The smooth curve fitting results showed a “J” shape relationship between E-DII score and the all-cause 5-year mortality and a near-linear association between E-DII and the 5-year cardiovascular mortality without adjustment (Figures 2A,B). The Kaplan–Meier survival curves displayed significant differences in all-cause and cardiovascular survival by tertiles of the E-DII, p < 0.05 (Figure 3). Univariate and multivariate Cox proportional hazards model analysis further confirmed that the 5-year all-cause and cardiovascular mortality is positively related with the E-DII scores (Table 2). In crude model, the hazards ratio (HR) for 5-year all-cause and cardiovascular mortality were 1.49 (1.29–1.71) and 1.63 (1.23–2.16) for the highest vs. lowest tertile of the E-DII, respectively. Besides, after adjusting for all confounders, the risk of 5-year all-cause mortality increased by 33% in E-DII T3 groups, compared with that in T1 (p < 0.001). As expected, the risk of cardiovascular mortality was also enhanced by 54%.
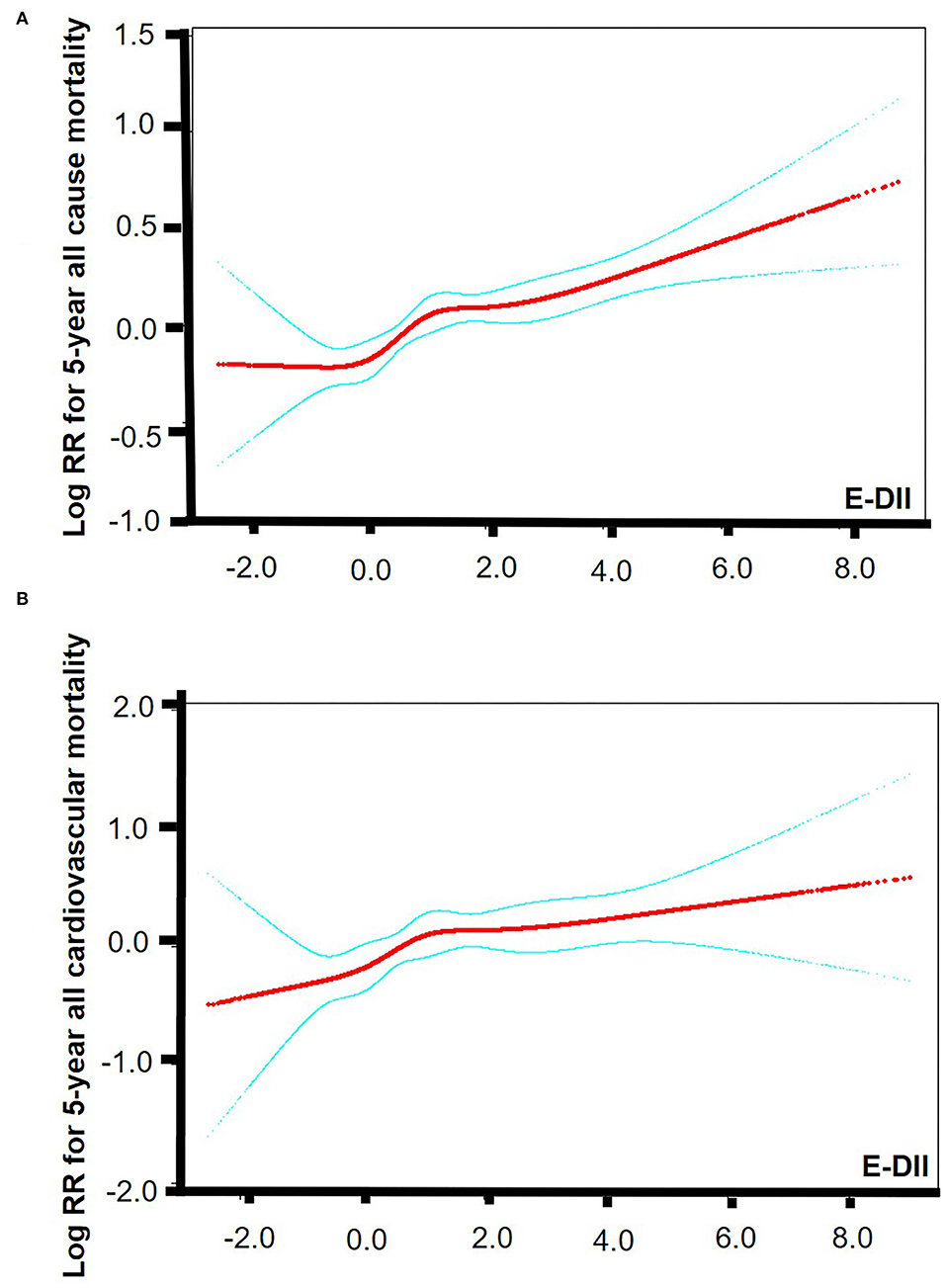
Figure 2. Smooth curve fitting results. (A) Smooth curve fitting results between E-DII and 5-year all-cause mortality. (B) Smooth curve fitting results between E-DII and 5-year cardiovascular mortality. Risk of death (red) with 95% CIs (blue) determined using the Cox proportional Hazard Model. The p value for linear is < 0.0001. E-DII, Energy adjusted dietary inflammatory index.
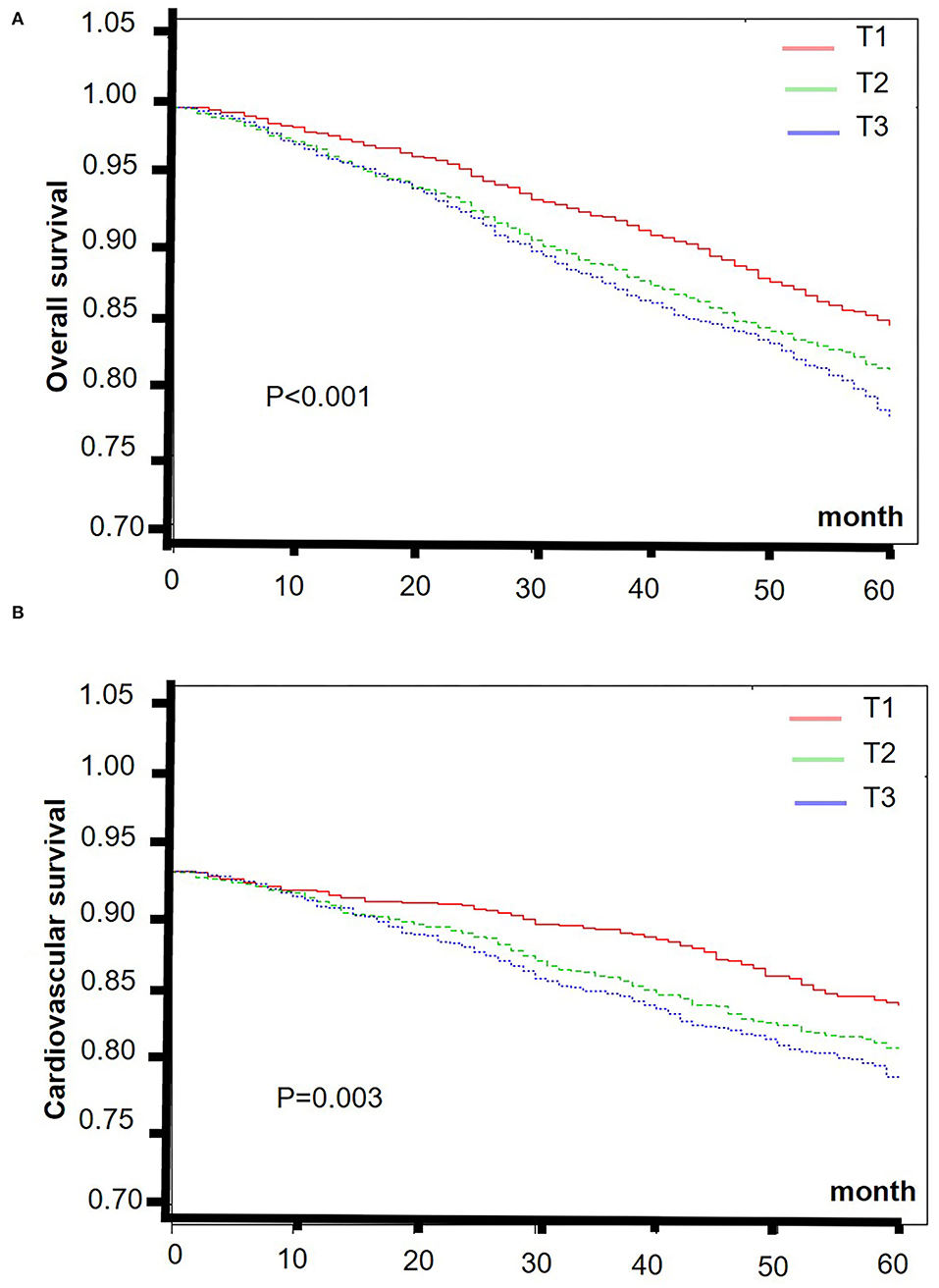
Figure 3. The Kaplan–Meier curves of 5-year overall (A) and cardiovascular (B) survival rates of the lowest and highest tertiles of E-DII among CKD patients. E-DII, Energy adjusted dietary inflammatory index; CKD, chronic kidney disease.
Subgroup Analyses
Subgroup analyses results were summarized in Supplementary Tables S2, S3. For the 5-year all-cause mortality, relatively stronger associations between E-DII and mortality were observed among inactive subjects, smokers, patients with dyslipidemia, and patients without cancers. CKD patients in G3-5 also tended to suffer higher risk of death if they adherent to a more pro-inflammatory diet pattern. But no significant interaction effect was identified. Interestingly, the risk of pro-inflammatory diet-associated cardiovascular death was relatively higher in participants who were elderly, female, non-Hispanic White, overweight, with dyslipidemia, with heart disease and without cancers. Notably, compared with CKD G1, patients with worse kidney function were more likely to be influenced by E-DII, p for interaction < 0.05.
Discussion
In this cohort study, we found that E-DII is independently associated with 5-year all cause and cardiovascular mortality. Overall, the results appeared to be more remarkable among CKD patients with other risk factors, including dyslipidemia and worse kidney function.
DII was developed according to the influence of food parameter on blood inflammation markers such as IL-1β/4/6/10, TNF-α, and CRP (7). The previous studies confirmed that DII is positively correlated with TNF-α, IL-1/6/7, and IFN-γ level (23, 24). We and other group also displayed participants with high E-DII scores exhibited increased CRP level (25). Thus, DII is a reliable tool to estimate the dietary inflammatory effect.
The influence of DII on all-cause and cardiovascular death risk has been documented in general population (26, 27), overweight and obese population (28), cancer patients (29, 30), and prediabetics (31). Interestingly, the previous publications reported DII was also associated with renal function, CKD prevalence and progression and CKD-associated complication including hyperparathyroidism (32–34). However, the influence of DII on mortality among CKD patients remained unclear. To our best knowledge, this is the first study to investigate the relationship between DII and the mortality in CKD patients.
The idea of “food as medicine” is widely spread among CKD patients and nephrology physicians (35). Moreover, numerous studies still focus on the diet management in CKD patients. Researchers believe that diet manage could help to delay the progression of kidney function deterioration and prevent bad outcomes. Although limited study about diet management in CKD focused on DII, previous studies could prompt us to connect diet intervention with DII in CKD. Typically, dietary patterns rich in red meat, saturated fats, and simple carbohydrates, higher and positive DII scores (pro-inflammatory diets) (10), while diets composed of vegetables, fruits, whole grains, legumes, nuts, and fish show more negative DII scores (anti-inflammatory diets) (36). The KIDIGO guideline recommended patients with glomerular disease to develop a plant-based diet and restrict sodium, fats and red meat intake (20). An observational cohort study reported that dietary pattern rich in processed and fried foods was independently related with death risk in CKD. In contrast, diets rich in fruits and vegetables tended to be protective (37). A cross-section study involving 2,403 CKD participants also revealed that patients who follow healthy diet patterns would show around 25% lower risk of CKD progression and 24–31% lower all-cause mortality, compared with participants with the lowest adherence (38). Besides, population with medium and high Mediterranean diet (typical anti-inflammatory diet) compliance displayed a 25 and 23% lower mortality risk than population with low compliance (39). Notably, white wine and olive oil, important components of the Mediterranean diet, consumption decreased the plasma levels of CRP and IL-6 in CKD patients (40). All of these reports support our findings on the association between the pro-inflammatory diet adherence and the risk of death.
The mechanisms of the association between DII and the mortality are unknown. Individuals who adhere to diets with high DII score displayed high levels of systematic inflammation biomarkers, such as CRP, IL-6, and TNF-α (7). Inflammation not only increases the death risk directly but also promotes the protein-energy wasting, sarcopenia, vascular calcification and cardiovascular events in CKD patients (41). It was reported that high levels of IL-6, TNF-α, fibrinogen, and albumin increased the risk of the all-cause death and the atherosclerotic vascular events 3.1 fold among CKD patients (4). Besides, proinflammatory diet which is lack of plant fiber, may alter the normal gut microbiome, leading to accumulation of toxic bacterial byproducts. Toxic bacterial byproducts may further promote systemic inflammation, atheromatous changes in arteries, CKD progression and finally adverse outcomes (42, 43).
There are some limitations in this study. Firstly, CKD was judged by one-time UACR and creatinine measurement. Participants might be falsely classified as CKD, especially as early stage of CKD. Secondly, we calculated E-DII based on a recall survey because NHANES didn't perform food frequency questionnaires (FFQs), the most used tool for DII. It may produce recall bias. Besides, participants may underreport their diet information, especially for unhealthy diet components (44). Furthermore, most of the comorbidities were self-reported, which may lead to recall and misclassification bias. Thirdly, participants may change their diet behaviors during follow-up, resulting in over or under-estimation of the association between DII and mortality. Fourthly, DII should be calculated based on 45 food parameters, but only 27 or 28 food components were available in the NHANES data (Vitamin D was missing in NHANES 1999–2006). Although it was demonstrated that DII scores based on 27 or 28 food parameters will not influence its predictive ability (10, 45), we still need to consider the impact on the accuracy. Fifth, we excluded individuals under 20 years and with extreme energy intake. The findings may not be generalized to them. Despite the limitations, there are some strengths in this study. Notably, this is the first time to confirm the relationship between E-DII and mortality among CKD patients based on public NHANES database. The nationwide and non-institutionalized samples make our results more representative. Furthermore, we performed multivariate regression and subgroup analysis to exclude the influence of demographics, behaviors, and other death risk factors. Besides, subgroup analysis revealed that CKD patients with some death risk factors appeared to be easily influenced by inflammatory diets.
Conclusion
This study demonstrates that E-DII is independently related to the 5-year all-cause and cardiovascular mortality in CKD patients. We should recommend healthy and anti-inflammatory diet patterns to CKD patients to achieve a better outcome.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx and https://www.cdc.gov/nchs/data-linkage/mortality-public.htm.
Ethics Statement
The studies involving human participants were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YH, YL, and LX designed the study. YH, LZ, and MZ collected and organized the original data. YH and LZ analyzed the data. YH, MZ, LZ, LS, FL, YL, and LX assisted in the interpretation of the results and writing the manuscript. All authors contributed to the article and approved the submitted manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 82170744].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the NHANES participants and staff for their contributions of the data and data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.899004/full#supplementary-material
References
1. Global Regional. National burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
2. Jager DJde, Vervloet MG, Dekker FW. Noncardiovascular mortality in CKD: an epidemiological perspective. Nat Rev Nephrol. (2014) 10:208–14. doi: 10.1038/nrneph.2014.8
3. Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW. the systemic nature of CKD. Nat Rev Nephrol. (2017) 13:344–58. doi: 10.1038/nrneph.2017.52
4. Amdur RL, Feldman HI, Dominic EA, Anderson AH, Beddhu S, Rahman M, et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis. (2019) 73:344–53. doi: 10.1053/j.ajkd.2018.09.012
5. Munoz Mendoza J, Isakova T, Cai X, Bayes LY, Faul C, Scialla JJ, et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. (2017) 91:711–9. doi: 10.1016/j.kint.2016.10.021
6. Zeng M, Liu Y, Liu F, Peng Y, Sun L, Xiao L. J-shaped association of platelet-to-lymphocyte ratio with 5-year mortality among patients with chronic kidney disease in a prospective cohort study. Int Urol Nephrol. (2020) 52:1943–57. doi: 10.1007/s11255-020-02548-1
7. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
8. Hariharan R, Odjidja EN, Scott D, Shivappa N, Hébert JR, Hodge A, et al. The dietary inflammatory index, obesity, type 2 diabetes, cardiovascular risk factors and diseases. Obes Rev. (2022) 23:e13349. doi: 10.1111/obr.13349
9. Xu H, Sjögren P, Ärnlöv J, Banerjee T, Cederholm T, Risérus U, et al. A proinflammatory diet is associated with systemic inflammation and reduced kidney function in elderly adults. J Nutr. (2015) 145:729–35. doi: 10.3945/jn.114.205187
10. Li A, Chen Y, Schuller AA, van der Sluis LWM, Tjakkes GE. Dietary inflammatory potential is associated with poor periodontal health: a population-based study. J Clin Periodontol. (2021) 48:907–18. doi: 10.1111/jcpe.13472
11. Han E, Lee YH, Kim YD, Kim BK, Park JY, Kim DY, et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am J Gastroenterol. (2020) 115:584–95. doi: 10.14309/ajg.0000000000000572
12. N.C.f.H.S. US CENTERS FOR DISEASE CONTROL AND PREVENTION, NATIONAL HEALTH AND NUTRITION EXAMINATION SURVEY 1999-2000, 2001-2002, 2003-2004, 2005-2006, 2011-2012, 2013-2014, 2015-2016, 2017-2018 Documentation Files. Available online at: https://www.cdc.gov/nchs/nhanes/ (accessed December 15, 2021).
13. N.C.f.H.S.U.C.f.D.C.a. Prevention, NCHS Research Ethics Review Board (ERB) Approval. Available online at: https://www.cdc.gov/nchs/nhanes/irba98.htm (Accessed at March 15, 2022).
14. Jespersen T, Kruse N, Mehta T, Kuwabara M, Noureddine L, Jalal D. Light wine consumption is associated with a lower odd for cardiovascular disease in chronic kidney disease. Nutr Metab Cardiovasc Dis. (2018) 28:1133–9. doi: 10.1016/j.numecd.2018.06.018
15. Wang T, Jiang H, Wu Y, Wang W, Zhang D. The association between dietary inflammatory index and disability in older adults. Clin Nutr. (2021) 40:2285–92. doi: 10.1016/j.clnu.2020.10.017
16. Geng J, Deng L, Qiu S, Bian H, Cai B, Jin K. Dietary inflammatory potential and risk of sarcopenia: data from national health and nutrition examination surveys. Aging (Albany NY). (2020) 13:1913–28. doi: 10.18632/aging.202141
17. Sun Y, Chen C, Mustieles V, Wang L, Zhang Y, Wang YX, et al. Association of blood trihalomethane concentrations with risk of all-cause and cause-specific mortality in us adults: a prospective cohort study. Environ Sci Technol. (2021) 55:9043–51. doi: 10.1021/acs.est.1c00862
18. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. Jama. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
19. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
20. KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–s276. doi: 10.1016/j.kint.2021.05.021
21. X&Y Solutions Empower. Available online at: www.empowerstats.com (accessed December 6, 2020).
22. M.R. RStudio. Boston, Inc; 2009-2020., RStudio: integrated development for R. https://rstudio.com/ (Accessed at January 1, 2022).
23. Cervo MMC, Scott D, Seibel MJ, Cumming RG, Naganathan V, Blyth FM, et al. Proinflammatory diet increases circulating inflammatory biomarkers and falls risk in community-dwelling older men. J Nutr. (2020) 150:373–81. doi: 10.1093/jn/nxz256
24. Shivappa N, Hebert JR, Marcos A, Diaz LE, Gomez S, Nova E. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. (2017) 1:61. doi: 10.1002/mnfr.201600707
25. Shivappa N, Wirth MD, Hurley TG, Hébert JR. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National health and nutrition examination survey-1999-2002. Mol Nutr Food Res. (2017) 1:61. doi: 10.1002/mnfr.201600630
26. Shivappa N, Godos J, Hébert JR., Wirth MD, Piuri G, Speciani AF, et al. Dietary inflammatory index and cardiovascular risk and mortality-a meta-analysis. Nutrients. (2018) 10:2001. doi: 10.3390/nu10020200
27. Okada E, Shirakawa T, Shivappa N, Wakai K, Suzuki K, Date C, et al. Dietary inflammatory index is associated with risk of all-cause and cardiovascular disease mortality but not with cancer mortality in middle-aged and older Japanese adults. J Nutr. (2019) 149:1451–9. doi: 10.1093/jn/nxz085
28. Park YM, Choi MK, Lee SS, Shivappa N, Han K, Steck SE, et al. Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin Nutr. (2019) 38:682–8. doi: 10.1016/j.clnu.2018.04.002
29. Fowler ME, Akinyemiju TF. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int J Cancer. (2017) 141:2215–27. doi: 10.1002/ijc.30922
30. Zahedi H, Djalalinia S, Asayesh H, Mansourian M, Esmaeili Abdar Z, Mahdavi Gorabi A, et al. A higher dietary inflammatory index score is associated with a higher risk of incidence and mortality of cancer: a comprehensive systematic review and meta-analysis. Int J Prev Med. (2020) 11:15. doi: 10.4103/ijpvm.IJPVM_332_18
31. Deng FE, Shivappa N, Tang Y, Mann JR, Hebert JR. Association between diet-related inflammation, all-cause, all-cancer, cardiovascular disease mortality, with special focus on prediabetics: findings from NHANES III. Eur J Nutr. (2017) 56:1085–93. doi: 10.1007/s00394-016-1158-4
32. Mazidi M, Shivappa N, Wirth MD, Hebert JR, Kengne AP. Greater Dietary Inflammatory Index score is associated with higher likelihood of chronic kidney disease. Br J Nutr. (2018) 120:204–9. doi: 10.1017/S0007114518001071
33. Rouhani MH, Najafabadi MM, Surkan PJ, Esmaillzadeh A, Feizi A, Azadbakht L. Dietary inflammatory index and its association with renal function and progression of chronic kidney disease. Clin Nutr ESPEN. (2019) 29:237–41. doi: 10.1016/j.clnesp.2018.09.001
34. Qin Z, Yang Q, Liao R, Su B. The association between dietary inflammatory index and parathyroid hormone in adults with/without chronic kidney disease. Front Nutr. (2021) 8:688369. doi: 10.3389/fnut.2021.688369
35. Mafra D, Borges NA, Lindholm B, Shiels PG, Evenepoel P, Stenvinkel P. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol. (2021) 17:153–71. doi: 10.1038/s41581-020-00345-8
36. Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. (2013) 39:99–110. doi: 10.1016/j.diabet.2012.08.007
37. Gutiérrez OM, Muntner P, Rizk DV, McClellan WM, Warnock DG, Newby PK, et al. Dietary patterns and risk of death and progression to ESRD in individuals with CKD: a cohort study. Am J Kidney Dis. (2014) 64:204–13. doi: 10.1053/j.ajkd.2014.02.013
38. Hu EA, Coresh J, Anderson CAM, Appel LJ, Grams ME, Crews DC, et al. Adherence to healthy dietary patterns and risk of ckd progression and all-cause mortality: findings from the cric (chronic renal insufficiency cohort) study. Am J Kidney Dis. (2021) 77:235–44. doi: 10.1053/j.ajkd.2020.04.019
39. Huang X, Jiménez-Moleón JJ, Lindholm B, Cederholm T, Arnlöv J, Risérus U. Mediterranean diet, kidney function, mortality in men with CKD. Clin J Am Soc Nephrol. (2013) 8:1548–55. doi: 10.2215/CJN.01780213
40. Migliori M, Panichi VR, Fitó M, Covas M, Bertelli A, Muñoz-Aguayo D. Anti-inflammatory effect of white wine in CKD patients and healthy volunteers. Blood Purif. (2015) 39:218–23. doi: 10.1159/000371570
41. Voelkl J, Egli-Spichtig D, Alesutan I, Wagner CA. Inflammation: a putative link between phosphate metabolism and cardiovascular disease. Clin Sci (Lond). (2021) 135:201–27. doi: 10.1042/CS20190895
42. Jovanovich A, Isakova T, Stubbs J. Microbiome and cardiovascular disease in CKD. Clin J Am Soc Nephrol. (2018) 13:1598–604. doi: 10.2215/CJN.12691117
43. Lau WL, Kalantar-Zadeh K, Vaziri ND. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron. (2015) 130:92–8. doi: 10.1159/000381990
44. Samuel-Hodge CD, Fernandez LM, Henríquez-Roldán CF, Johnston LF, Keyserling TC. A comparison of self-reported energy intake with total energy expenditure estimated by accelerometer and basal metabolic rate in African-American women with type 2 diabetes. Diabetes Care. (2004) 27:663–9. doi: 10.2337/diacare.27.3.663
Keywords: energy adjusted dietary inflammation index, 5-year all-cause mortality, 5-year cardiovascular mortality, chronic kidney disease, National Health and Nutrition Examination Survey
Citation: Huang Y, Zhang L, Zeng M, Liu F, Sun L, Liu Y and Xiao L (2022) Energy-Adjusted Dietary Inflammatory Index Is Associated With 5-Year All Cause and Cardiovascular Mortality Among Chronic Kidney Disease Patients. Front. Nutr. 9:899004. doi: 10.3389/fnut.2022.899004
Received: 18 March 2022; Accepted: 16 May 2022;
Published: 14 June 2022.
Edited by:
Caterina Conte, Università telematica San Raffaele, ItalyReviewed by:
Te-Chih Wong, Chinese Culture University, TaiwanAlice Sabatino, University of Parma, Italy
Liliana Garneata, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2022 Huang, Zhang, Zeng, Liu, Sun, Liu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Xiao, eGlhb2xpem5keEBjc3UuZWR1LmNu; Yu Liu, cm9yeUBjc3UuZWR1LmNu
 Ying Huang
Ying Huang Li Xiao
Li Xiao