- 1Department of Biological Chemistry and Pharmacology, College of Medicine, The Ohio State University, Columbus, OH, United States
- 2AdventHealth Translational Research Institute, Orlando, FL, United States
The Ile191Val variant of the TAS1R2 gene of sweet taste receptors causes a partial loss-of-function and is associated with reduced glucose excursions in a healthy lean cohort. However, it is unclear whether this polymorphism contributes to the regulation of glucose homeostasis in metabolically unhealthy individuals. Thus, we used participants with variable glycemic profiles and obesity to assess the effects of the TAS1R2-Ile191Val variant. We found that the Val minor allele carriers had lower HbA1c at all levels of fasting glucose and glucose tolerance. These effects were not due to differences in beta-cell function or insulin sensitivity assessed with a frequently sampled intravenous glucose tolerance test. This study extends our previous findings and provides further evidence that sweet taste receptor function may contribute to glucose regulation in humans.
Introduction
Sweet taste receptors (STR; TAS1R2/TAS1R3 heterodimer) belong to the nutrient-sensing class of novel G-protein coupled receptors (GPCRs) that includes free fatty acid (i.e., GPR40,GPR120, CD36) and amino acid receptors (TAS1R1/TAS1R3; umami receptors). Among various tissues, these GPCRs are expressed in the gastrointestinal tract to integrate local and peripheral signals that modulate nutrient digestion and absorption (1). For instance, activation of STR can stimulate peptide release from mouse and human intestinal L-cells (2–4). In addition, STR promote glucose absorption in response to high concentrations of luminal glucose dependent on GLP-2 secretion and the apical translocation of GLUT2 transporter. Thus, genetic ablation of Tas1r2 gene of STR in mice (TAS1R2-KO)reduces glucose absorption and its plasma excursions (5). Although there is some evidence that a similar mechanism may be present in humans (6, 7), the direct involvement of STR has not been clearly demonstrated. Interestingly, intestinal expression of STR in individuals with type 2 diabetes is linked to glucose absorption, suggesting that the levels and function of STR may contribute to postprandial hyperglycemia (8, 9).
We recently demonstrated that the TAS1R2 (Ile191Val) polymorphism reduces the levels of STR in the plasma membrane, causing partial loss-of-function (10). Consequently, Val carriers had a mild reduction in glucose excursions in response to the ingestion of a glucose load, recapitulating the phenotype seen in TAS1R2-KO mice (5). Notably, the TAS1R2 (Ile191Val) variant is also associated with carbohydrate intake (11–13) and fasting insulin (11). Although these observations establish a strong link between STR function and glucose control, their clinical significance for the regulation of glycemia cannot be adequately assessed in metabolically healthy lean participants. To address this limitation and further explore the physiological manifestations of STR loss-of-function, we performed a retrospective observational study to assess the effects of Ile191Val polymorphism in a cohort of individuals with variable metabolic and obesity profiles.
Methods
This prospective observational study was performed in accordance with the requirements of Good Clinical Practice and the Revised Declaration of Helsinki. Recruitment, enrollment and all study-related visits, including specimen collection and point-of-care laboratory testing, took place at Advent-Health Translational Research Institute (TRI) Clinical Research Unit (CRU), as previously described (NCT02226640) (14). The study was approved by the Institutional Review Board at Advent-Health and all participants signed an informed consent. All subjects were required to be at least 18 years of age, in general good health, with BMI <25 or > 30 kg/m2, but weight stable (<3 kg change within the last 8 weeks) and within 10% of their lifetime heaviest body weight. Non-diabetic participants were not taking medications known to affect glucose metabolism. Individuals with diabetes (HbA1c <8.0%) were either non-treated or were on monotherapy using either a sulfonylurea, metformin, or GLP-1 analog and were able to maintain accurate and reliable home glucose monitoring logs. Participants were excluded if one of the following conditions applied: Treatment with more than 2 of the following: metformin (Fortamet, Glucophage, Glumetza, Riomet), sulfonylureas (Glucotrol, Diabeta, Glynase, Micronase), Glucagon-like peptide-1 analogs (Byetta) and/or Dipeptidyl peptidase IV inhibitors (Januvia, Onglyza); Treatment with long acting Glucagon-like peptide-1 agonists within the last 3 months (i.e., exenatide once weekly), Treatment with thiazolidinediones (TZDs) (i.e. Avandia, Actos, Rezulin) within the last 3 months; Known, untreated thyroid disease or abnormal thyroid function blood test; Known diagnosis of liver disease (except NASH) or elevated liver function blood test; Known diagnosis of kidney disease or elevated kidney function blood test; Uncontrolled high blood pressure (BP > 140 systolic or > 90 diastolic); Start of or changes in oral contraceptives or hormone replacement therapy within the last 3 months; Use of drugs or alcohol (>3 drinks per day) within the last 5 years; Uncontrolled psychiatric disease that would interfere with study participation; History of cancer within the last 5 years (skin cancers, with the exception of melanoma, may be acceptable); History of organ transplant; History of heart attack within the last 6 months; Current treatment with blood thinners or antiplatelet medications that cannot be safely stopped for testing procedures; Current anemia; History of HIV, active Hepatitis B or C, or Tuberculosis; Presence of clinically significant abnormalities on electrocardiogram; Current smokers (smoking any nicotine or non-nicotine product within the past 3 months); Use of any medications known to influence glucose, fat and/or energy metabolism within the last 3 months (e.g., growth hormone therapy, glucocorticoids [steroids], etc.).
Participants were genotyped and retrospectively grouped according to rs35874116 (Ile191Val) or rs9701796 (Cys9Ser) TAS1R2 non-synonymous single nucleotide polymorphism (SNP). Mathematical modeling was performed as previously described for (a) beta-cell function, insulin sensitivity and insulin clearance (7), and (b) insulin sensitivity (Si) and the acute insulin response to glucose (AIRg) (15).
Statistical Analysis
All data are represented as mean +/- standard error and plotted with Prism 9 (GraphPad Software). All participants were retrospectively assigned to two groups based on TAS1R2 genotypes. Statistical analyses were performed using jamovi 2.2.5 (jamovi team). Allele equilibrium, frequency, and SNP linkage were analyzed by Chi-square tests. Baseline characteristics and metabolic responses to the oral glucose tolerance test (OGTT) and frequently sampled intravenous glucose tolerance test (FSIVGTT) were analyzed with a general linear model approach using sex, age, BMI, fasting glucose, and 2 h glucose during the OGTT as covariates. Area under curve (AUC) glucose, insulin, and C-peptide were adjusted for baseline values. Non-parametric variables were log-transformed prior to analysis and all models were checked for multicolinearity and normal distribution of the residuals. Possible confounding effects were analyzed by introducing the variables of interest as covariates in a hierarchical model. Relationships between glycated hemoglobin (HbA1c) and other variables were analyzed as partial correlations after adjustment for sex, age and BMI.
Data and Resource Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
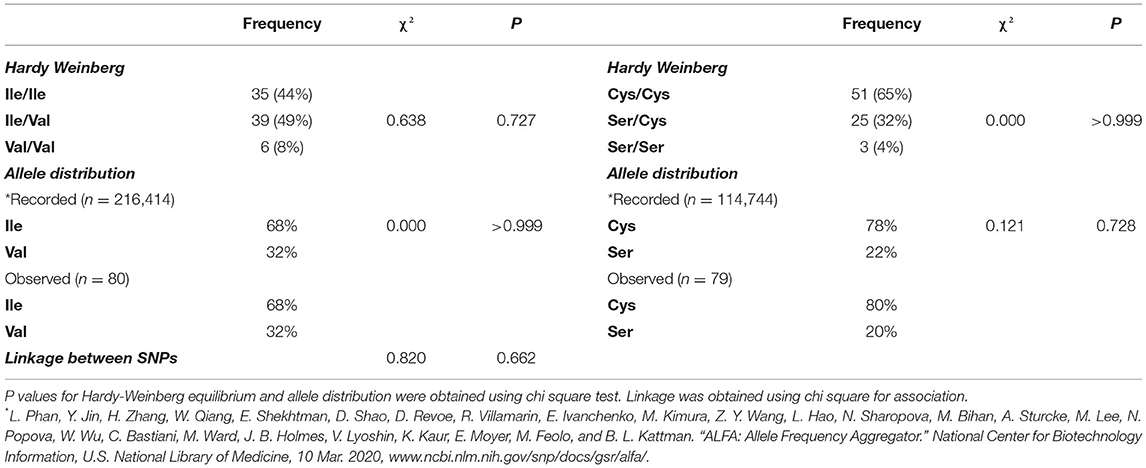
The cohort of participants had the expected Hardy-Weinberg equilibrium and minor allele frequency (Table 1). We specifically considered glucose tolerance along with gender, age, and BMI and performed multiple regression analysis between Ile/Ile and Val carriers (Val/_). We found that, at various levels of fasting glucose or glucose tolerance, Val carriers had lower HbA1c (Table 2 and Figures 1A,B). The genotype effect on HbA1c persisted even when the population was grouped according to their diabetes status (p = 0.040) based on American Diabetes Association (ADA) classification criteria (16) (i.e. normal glucose tolerance, pre- type 2 diabetes (T2D) and T2D) or when we only analyzed participants with normal fasting glucose and glucose tolerance (Ile/Ile 5.55 ± 0.07 vs Val/_ 5.34 ± 0.08, p = 0.046). Val/Val participants trended to have lower HbA1c, but the number of participants (total n=6) was inadequate to demonstrate statistical differences (Supplementary Table 1). Nevertheless, even after omitting Val/Val participants from the analysis, the HbA1c differences between Ile/Ile and Val/Ile genotypes persisted (5.83 ± 0.05 vs 5.65 ± 0.05, respectively; p = 0.011). This suggests that heterozygosity is sufficient for the SNP effect on HbA1c. In contrast, the rs9701796 (Ser9Cys) polymorphism of TAS1R2, which has comparable allele frequency to Ile191Val (17), had no associations with HbA1c (Table 2 and Figures 1C,D). No genotype differences were noted in OGTT variables or in insulin sensitivity or pancreatic beta-cell responsiveness during a FSIVGTT (Table 2).
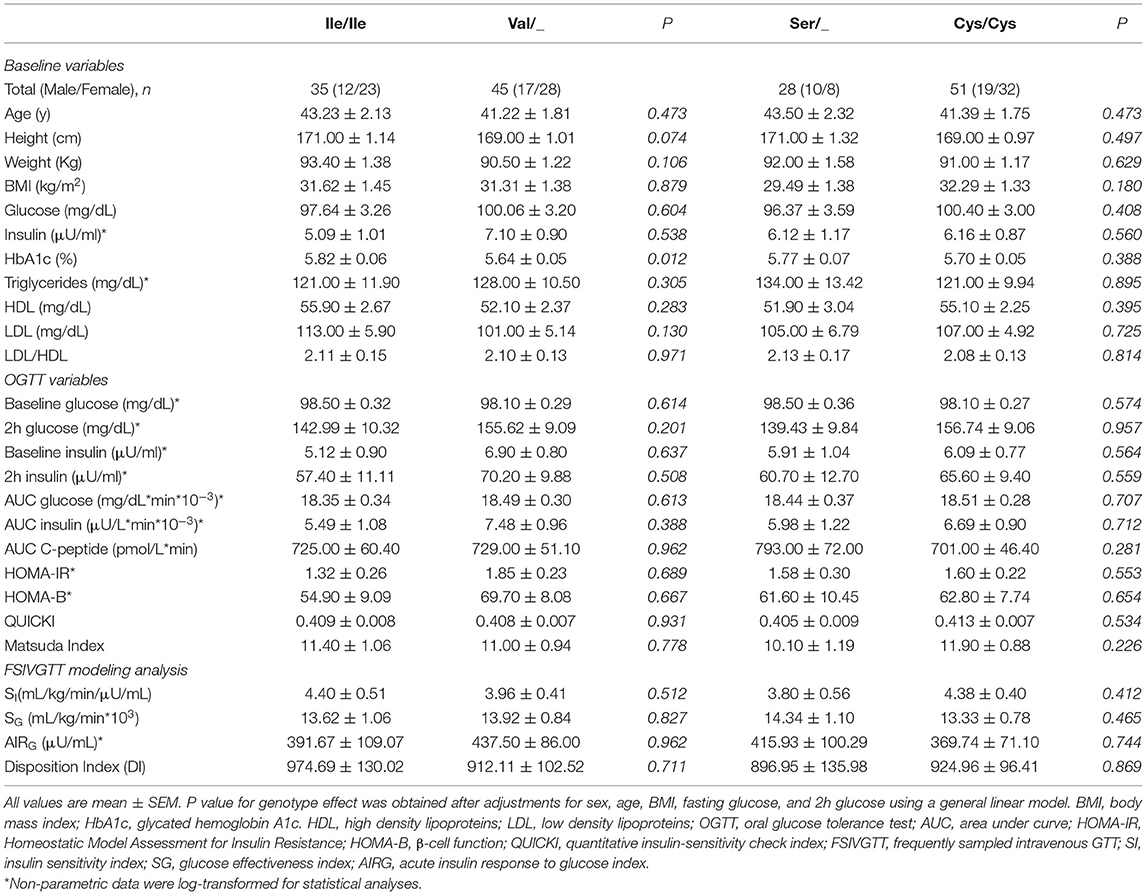
Table 2. Baseline and metabolic responses to an OGTT and FSIVGTT in adults with various levels of BMI and glucose control grouped by two common TAS1R2 polymorphisms.
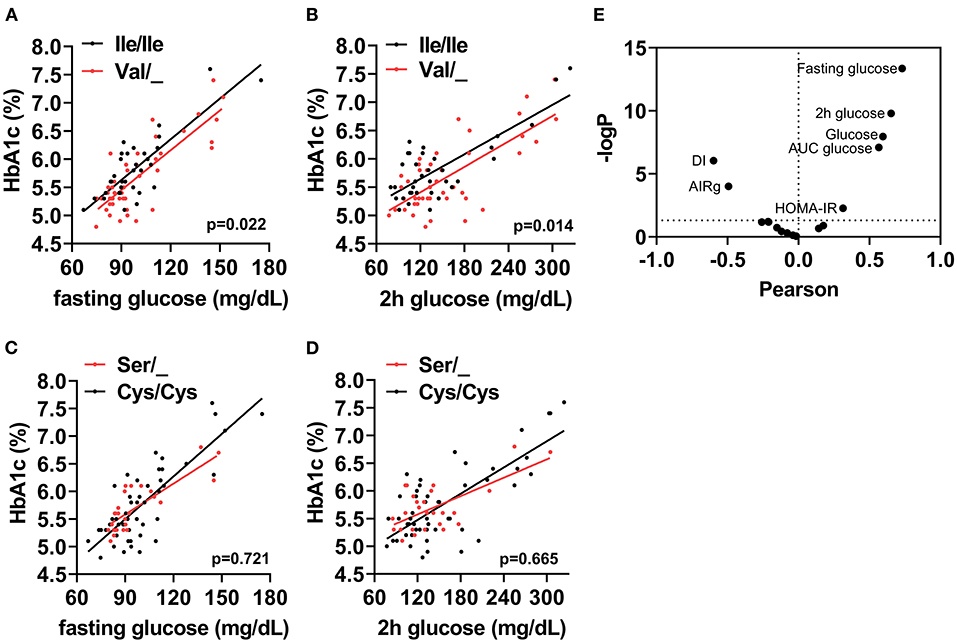
Figure 1. The TAS1R2-(Val) variant is associated with HbA1c in humans. Association between (A) Fasting glucose, or (B) 2h glucose post OGTT (2h glucose) with percent glycated hemoglobin (HbA1c) in Ile/Ile (black) and Val carriers (red) using a linear regression model (p value for intercepts). (C) Fasting glucose, or (D) 2h glucose post OGTT (2h glucose) with percent glycated hemoglobin (HbA1c) in Cys/Cys (black) and Ser carriers (red) using a linear regression model (p value for intercepts). All values shown in A-D are unadjusted. (E) Correlation coefficient (Pearson) and statistical significance [–Log(p)] volcano plot for all assessed variables (i.e., baseline, OGTT and FSIVGTT) with HbA1c. Horizontal dotted line shows statistical significance of p <0.05 or higher. Only variables with p <0.05 are labeled. AUC, area under curve; DI, disposition index; AIRg, acute insulin response to glucose index; HOMA-IR, homeostatic model assessment for insulin resistance.
Therefore, we reasoned that HbA1c levels might represent cumulative differences in other parameters related to glucose metabolism. Partial correlation analysis, adjusted for sex, age and BMI, demonstrated anticipated relationships between HbA1c and basal, OGTT, or FSIVGTT parameters. Fasting and 2h glucose showed the strongest and most significant correlations with HbA1c (Fasting: r = 0.75, p <0.001 and 2h: r = 0.65, p ≤ 0.001), suggesting that small changes in these variables, which are also descriptors of diabetes status, could explain cumulative differences in HbA1c. We also noted significant negative correlations with the disposition index (DI) and the AIRg of the FSIVGTT, and significant positive correlations with the homeostatic model assessment for insulin resistance (HOMA-IR), AUC glucose, fasting glucose and 2h glucose of the OGTT (Figure 1E). Similar correlations were found for both Ile/Ile and Val carriers (Supplementary Figures 1A,B). However, the addition of any of these parameters to our full regression model did not significantly decrease the standardized estimate for the SNP effect. Instead, the addition of DI further improved the regression model (Δ r2 = 0.04, model p = 0.014).
Discussion
There is accumulating evidence to suggest that taste receptors, including STRs, regulate endocrine function (18). For instance, STRs regulate GLP-1 and GLP-2 secretion from intestinal L-cells to regulate incretin responses and glucose absorption (2, 5). In addition, STRs regulate insulin secretion directly on beta-cells in response to ingested sugars (19, 20) and artificial sweeteners (21, 22). These findings suggest that receptor-mediated “sweet” nutrient sensing is part of an intestinal-pancreatic axis that coordinates nutrient absorption and disposal.
These studies were primarily performed in cells and mice, so the direct involvement of STRs in human endocrine physiology is still ambiguous. This is primarily due to the absence of specific and potent pharmacological inhibitors or methods that directly assess STR function. Nevertheless, genomics approaches using SNPs has allowed scientists and clinicians alike to identify genetic markers that predict the present and development of a disease or screen for potential novel gene functions through various associations. STRs (TAS1R2/TAS1R3) are highly polymorphic (23), but TAS1R2 in particular is characterized by high levels of nucleotide diversity (24). TAS1R2 also confers specificity to sweet taste, since TAS1R3 is involved in both sweet and umami taste (i.e. amino acid) (25). Out of the nine TAS1R2 nonsynonymous SNPs, the rs35874116 (Ile191Val) and rs9701796 (Cys9Ser) have a minor allele frequency >0.2 and are associated with different nutritional and metabolic variables (17). TAS1R2-(Ile191Val) in particular is associated with sugar and carbohydrate consumption in adults (11, 13) and in children (12), but these effects are not due to differences in taste sensitivity (26). Taken together these observations suggest that, like in mice, TAS1R2 may have functional roles in peripheral tissues beyond taste perception. We recently used biochemical approaches to show that the Ile191Val substitution causes a partial loss-of-function of TAS1R2 by reducing the availability of the STR dimer in the plasma membrane (10). Healthy lean Val carriers had reduced glucose excursions during an OGTT (10), which resembles the effects seen in mice with a genetic loss-of-function of TAS1R2 (5, 27), confirming that the Val substitution causes a partial loss-of-function of STRs.
Because the rate of glucose excursions can affect the duration and magnitude of postprandial hyperglycemia (28), we explored contributions of TAS1R2-(Ile191Val) at baseline and during an OGTT or an FSIVGTT in a cohort of adults with various degrees of glucose control. We found that TAS1R2-(Val) carriers had reduced HbA1c, a measure that assesses progression of glycemic burden and predicts diabetic complications. The Ser9Cys substitution is located in the putative signal peptide of TAS1R2 and has been associated with dietary and anthropometric variables in children (17). However, the Ser9Cys variant did not affect HbA1c levels or any other assessed variable. Although we cannot exclude the possibility of linkage disequilibrium with another causal polymorphism, the interactions of HbA1c with the Ile191Val are not linked to Ser9Cys polymorphism. Unlike direct measures of fasting or postprandial plasma glucose, HbA1c reflects mean glycaemia in the past 2–3 months, integrating total glucose exposure during fed and fasted states (29). Postprandial hyperglycemia significantly contributes to total daytime hyperglycemia and strongly correlates with HbA1c (30). This finding is aligned with the reduced OGTT glucose excursions seen in metabolically healthy lean Val/_ participants (10). However, we did not observe a direct genotype effects in glucose or insulin responses during an OGTT. This may be partially explained by the population characteristics and the physiological factors affecting an OGTT. Previously, we used healthy lean adults with very homogeneous metabolic characteristics. This was deliberate in order to make phenotypic comparisons with corresponding healthy lean mouse models. Presently, the objective was to retrospectively assess the effects of TAS1R2 SNPs in a population with variable glucose status. This may have slightly reduced the power of our study considering that OGTT responses are not homogeneous across different levels of glucose intolerance and obesity status. This is likely due to the many factors that contribute to the development of glucose dysregulation (i.e., beta-cell function, insulin sensitivity, rate of glucose absorption) (31). Thus, interactions between these parameters can have differential effects on the OGTT responses.
To overcome this limitation, we reasoned that the HbA1c differences might represent cumulative effects, so we set to identify which set of variables from the OGTT and FSIVGTT can account for the genotype association with HbA1c. The strongest correlations were noted with fasting and postprandial glucose (i.e., 2h post OGTT and AUC) along with indices of beta-cell function (i.e., AIRG and DI). Although this is predictable, none of these variables reduced the regression coefficient of the model when added as covariate. Instead, adding DI as a covariate magnified the genotype effect. This suggests that the reduced HbA1c in Val carriers could be mediated through amelioration of postprandial hyperglycemia linked to mechanisms that alter glucose absorption (32), instead of beta-cell function or insulin sensitivity. Although this is consistent with finding from animal models (5), to confirm this hypothesis in humans, clinical studies that directly measure glucose absorption are required. Notably, the genotype effect on HbA1c persisted in normoglycemic participants, after exclusion of participants with abnormal glucose control (16). In addition, the Val allele is associated with lower consumption of sugars in obese (11), which could ameliorate the magnitude of postprandial hyperglycemia in this population. Therefore, although food intake was not recorded in this study, habitual differences in food choices and consumption may partially explain the lower HbA1c. Regardless of the associated mechanism, loss-of-function of STRs may predispose individuals to lower HbA1c levels and confer a mild protective effect in daily glycaemia during the development of diabetes. This hypothesis should be confirmed through direct experimental evidence, such as in patients with continuous glucose monitors (33).
In conclusion, our studies highlight that, beyond taste perception, STR can act as peripheral carbohydrate sensors for the regulation of glucose homeostasis in humans. Particularly, partial loss-of-function of STRs through the TAS1R2-(Ile191Val) variant may confer beneficial effects in the regulation of daily glucose control. Our study was not adequately powered or designed to identify the mechanisms, but the genotype effects may be linked to differences in food preference and consumption, glucose excursions or other, yet unknown, peripheral mechanisms of glucose disposal. Notably, genome-wide association studies have yet to reveal independent contributions of TAS1R2 polymorphisms on metabolic dysregulation, but careful consideration of appropriate covariates may be required to evaluate undelaying associations.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board at Advent-Health, FL. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JSe, RP, and GK designed experiments and interpreted data. JSe, JSm, and FY analyzed data. JSe and GK wrote manuscript. RP edited manuscript. GK conceived studies. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute of Food and Agriculture (NIFA-2018-67001-28246 to GK), the National Institutes of Health (R01DK127444 to GK), the American Heart Association (AHA-904048 to JSe), and institutional support from the Ohio State University (to GK) and AdventHealth (to GK and RP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.896205/full#supplementary-material
References
1. Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. (2013) 10:729–40. doi: 10.1038/nrgastro.2013.180
2. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. ProcNatlAcadSciUSA. (2007) 104:15069–74. doi: 10.1073/pnas.0706890104
3. Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clinical nutrition. (2011) 30:524–32. doi: 10.1016/j.clnu.2011.01.007
4. Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci. (2009) 1170:91–4. doi: 10.1111/j.1749-6632.2009.04485.x
5. Smith K, Karimian Azari E, LaMoia TE, Hussain T, Vargova V, Karolyi K, et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol Metab. (2018) 17:98–111. doi: 10.1016/j.molmet.2018.08.009
6. Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metabol. (2011) 301:E317–25. doi: 10.1152/ajpendo.00077.2011
7. Karimian Azari E, Smith KR, Yi F, Osborne TF, Bizzotto R, Mari A, et al. Inhibition of sweet chemosensory receptors alters insulin responses during glucose ingestion in healthy adults: a randomized crossover interventional study. Am J Clin Nutr. (2017) 105:1001–9. doi: 10.3945/ajcn.116.146001
8. Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. (2009) 58:337–46. doi: 10.1136/gut.2008.148932
9. Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. (2013) 62:3532–41. doi: 10.2337/db13-0581
10. Serrano J, Seflova J, Park J, Pribadi M, Sanematsu K, Shigemura N, et al. The Ile191Val is a partial loss-of-function variant of the TAS1R2 sweet-taste receptor and is associated with reduced glucose excursions in humans. Mol Metab. (2021) 54:101339. doi: 10.1016/j.molmet.2021.101339
11. Eny KM, Wolever TM, Corey PN, El-Sohemy A. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am J Clin Nutr. (2010) 92:1501–10. doi: 10.3945/ajcn.2010.29836
12. Melo SV, Agnes G, Vitolo MR, Mattevi VS, Campagnolo PDB, Almeida S. Evaluation of the association between the TAS1R2 and TAS1R3 variants and food intake and nutritional status in children. Genet Mol Biol. (2017) 40:415–20. doi: 10.1590/1678-4685-gmb-2016-0205
13. Ramos-Lopez O, Panduro A, Martinez-Lopez E, Roman S. Sweet taste receptor TAS1R2 polymorphism (Val191Val) is associated with a higher carbohydrate intake and hypertriglyceridemia among the population of West Mexico. Nutrients. (2016) 8:101. doi: 10.3390/nu8020101
14. Pachori AS, Madan M, Nunez Lopez YO, Yi F, Meyer C, Seyhan AA. Reduced skeletal muscle secreted frizzled-related protein 3 is associated with inflammation and insulin resistance. Obesity. (2017) 25:697–703. doi: 10.1002/oby.21787
15. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. (1979) 236:E667–77. doi: 10.1152/ajpendo.1979.236.6.E667
16. American Diabetes 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15–S33. doi: 10.2337/dc21-S002
17. Smith NJ, Grant JN, Moon JI, So SS, Finch AM. Critically evaluating sweet taste receptor expression and signaling through a molecular pharmacology lens. FEBS J. (2021) 288:2660–72. doi: 10.1111/febs.15768
18. Calvo SS, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol. (2015) 11:213–27. doi: 10.1038/nrendo.2015.7
19. Kyriazis GA, Smith KR, Tyrberg B, Hussain T, Pratley RE. Sweet taste receptors regulate basal insulin secretion and contribute to compensatory insulin hypersecretion during the development of diabetes in male mice. Endocrinology. (2014) 155:2112–21. doi: 10.1210/en.2013-2015
20. Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. (2012) 109:E524–32. doi: 10.1073/pnas.1115183109
21. Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE. (2009) 4:e5106. doi: 10.1371/journal.pone.0005106
22. Serrano J, Meshram NN, Soundarapandian MM, Smith KR, Mason C, Brown IS, et al., Saccharin stimulates insulin secretion dependent on sweet taste receptor-induced activation of PLC signaling axis. Biomedicines. (2022) 10:120. doi: 10.3390/biomedicines10010120
23. Chamoun E, Mutch DM, Allen-Vercoe E, Buchholz AC, Duncan AM, Spriet LL, et al. Family Health, A review of the associations between single nucleotide polymorphisms in taste receptors, eating behaviors, and health. Crit Rev Food Sci Nutr. (2018) 58:194–207. doi: 10.1080/10408398.2016.1152229
24. Valente C, Alvarez L, Marques PI, Gusmao L, Amorim A, Seixas S, et al. Genes from the TAS1R and TAS2R families of taste receptors: looking for signatures of their adaptive role in human evolution. Genome Biol Evol. (2018) 10:1139–52. doi: 10.1093/gbe/evy071
25. Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. (2003) 115:255–66. doi: 10.1016/S0092-8674(03)00844-4
26. Dias AG, Eny KM, Cockburn M, Chiu W, Nielsen DE, Duizer L, et al. Variation in the TAS1R2 gene, sweet taste perception and intake of sugars. J Nutrigenet Nutrigenomics. (2015) 8:81–90. doi: 10.1159/000430886
27. Smith KR, Hussain T, Karimian Azari E, Steiner JL, Ayala JE, Pratley RE, et al. Disruption of the sugar-sensing receptor T1R2 attenuates metabolic derangements associated with diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. (2016) 310:E688–98. doi: 10.1152/ajpendo.00484.2015
28. J.E. Gerich. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. (2003) 163:1306–16. doi: 10.1001/archinte.163.11.1306
29. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. (2003) 26:881–5. doi: 10.2337/diacare.26.3.881
30. Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health. (2015) 73:43. doi: 10.1186/s13690-015-0088-6
31. Jagannathan R, Neves JS, Dorcely B, Chung ST, Tamura K, Rhee M, et al. The Oral glucose tolerance test: 100 Years Later. Diabetes Metab Syndr Obes. (2020) 13:3787–805. doi: 10.2147/DMSO.S246062
32. Anderwald C, Gastaldelli A, Tura A, Krebs M, Promintzer-Schifferl M, Kautzky-Willer A, et al. Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab. (2011) 96:515–24. doi: 10.1210/jc.2010-1398
Keywords: HbA1C, TAS1R2 gene, glucose homeostasis, sweet taste receptors, polymorphism, oral glucose tolerance test (OGTT), frequently sampled intravenous glucose tolerance test (FSIVGTT), diabetes risk
Citation: Serrano J, Yi F, Smith J, Pratley RE and Kyriazis GA (2022) The Ile191Val Variant of the TAS1R2 Subunit of Sweet Taste Receptors Is Associated With Reduced HbA1c in a Human Cohort With Variable Levels of Glucose Homeostasis. Front. Nutr. 9:896205. doi: 10.3389/fnut.2022.896205
Received: 14 March 2022; Accepted: 05 May 2022;
Published: 19 May 2022.
Edited by:
Erwin Ilegems, Karolinska Institutet (KI), SwedenReviewed by:
Peihua Jiang, Monell Chemical Senses Center, United StatesKazuki Yasuda, Kyorin University, Japan
Copyright © 2022 Serrano, Yi, Smith, Pratley and Kyriazis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George A. Kyriazis, R2Vvcmdpb3MuS3lyaWF6aXNAb3N1bWMuZWR1
 Joan Serrano
Joan Serrano Fanchao Yi2
Fanchao Yi2 Richard E. Pratley
Richard E. Pratley George A. Kyriazis
George A. Kyriazis