
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 17 June 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.888898
This article is part of the Research Topic Dietary Intake, Eating Behavior and Health Outcomes View all 36 articles
Background: The evidence of dietary carrot/carotene intake's effect on the association with colorectal cancer (CRC) risk is conflicted. We sought to examine the association of carrot/carotene intake with CRC incidence and mortality in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening cohort.
Methods: In all, 101,680 participants were enrolled between November 1993 and July 2001 from the PLCO cohort. We employed the multivariable Cox regression analyses to estimate the hazard ratios and 95% confidence interval. Subgroup analyses and interaction tests were performed to examine the potential effect modifiers. We further applied the generalized additive model to explore the non-linear trend of the exposure to cancer-related outcomes.
Results: A total of 1,100 CRC cases and 443 cancer-related deaths were documented. We noted that the 4th quintile of dietary carrot intakes was associated with a 21% lower risk of CRC incidence, compared with the lowest quintile group (full-adjusted HRquintile4vs.quintile1 = 0.79, 95%CI = 0.65–0.97, p for trend = 0.05), while the adjusted-HR was 0.95 (95%CI = 0.89–1.02) with per SD increment of carrot intakes, and no statistically significant associations were detected between dietary α-, and β-carotene intake and CRC incidence. There were no statistically significant associations observed between carrot/carotene intakes and CRC mortality. Furthermore, there were no non-linear dose-response relationships between dietary carrot, α-, and β-carotene intake and CRC incidence and mortality (all pnonlinearity > 0.05). Of note, smoking status as a modifier on the association of dietary carrot intakes with CRC incidence but not mortality was observed.
Conclusions: In summary, this large U.S. prospective cohort study indicated that a moderate consumption of carrots was associated with a lower CRC incidence, which suggested that a certain dose-range of carrots consumed might contribute to a potential cancer-prevention effect, not the more the better.
Colorectal cancer (CRC) is the third most common cause of cancer-related death in the United States, with nearly 147,950 incident cases and 53,200 cancer deaths in 2020 (1). Apart from well-established risk factors (i.e., environmental and genetic factors) that play a crucial role in the pathogenesis of CRC (2, 3), more than half of patients can be attributed to other risk factors including smoking, diet, drinking, obesity, and thus may be potentially preventable (4). Although emerging evidence implies that cancer prevention dietary nutrients or food, including calcium (5, 6), fiber (7), dairy products (8), and whole grain (9) have been associated with a lower risk of colorectal cancer, it remains controversial.
Carrots are rich in high amounts of carotenoid antioxidants (α- and -β-carotene) that might have a potential role in cancer prevention (10, 11). Several meta-analyses on carrot consumption have indicated that carrot intake was inversely associated with the risk of several cancers, including gastric (12), lung (13), prostate (14), breast (15), and urothelial cancer (16). However, epidemiological studies depicted an inverse (17–20) or a null association (21–23) of dietary α-/β-carotene intakes with the risk of CRC. Recently, a prospective cohort study of 57,053 Danes has shown that the consumption of raw carrots was associated with a 17% decrease in the risk of CRC (24). Given the differences in geography and eating habits, it remains unclear whether the results would be stable for U.S. adults. Meanwhile, there is no evidence of the effect of dietary carrot intake on mortality of CRC.
Therefore, to provide the most reliable prospective evidence on the association of dietary carrot intake with the risk of CRC, we, respectively, analyze the association of dietary carrot, α- and -β-carotene intakes with the risk of CRC incidence and mortality using a multicenter randomized controlled trial data from the prostate, lung, colorectal, and ovarian (PLCO) screening trial.
The PLCO Cancer Screening Trial is a randomized, controlled trial conducted to investigate whether certain screening examinations would reduce the mortality from PLCO cancers. The specific study design and methods were previously illustrated elsewhere (25). Approximately 155,000 participants were recruited from 1993 to 2001 via 10 screening centers across the United States, through a detailed recruitment plan. Then, they were randomly assigned to two groups (the control group receiving usual care, whereas the intervention arm receiving screening tests). The PLCO study was approved by the Institutional Review Boards of the US National Cancer Institute and each study center, and written informed consents were obtained from all eligible participants.
We established inclusion criteria to identify eligible cases in our final cohort. They would be further excluded as follows: (1) no baseline questionnaire returned (BQ) (n = 4,918) and any history of colorectal cancer before BQ (n = 34); (2) did not complete Diet History Questionnaire (DHQ) (n = 33,230), and invalid DHQ for missing the date of DHQ completion, the date of DHQ completion before the date of death, the presence of ≥8 missing frequency responses or extreme values of calorie intake [i.e., top 1% or bottom 1%]) (n = 5,221); (3) a history of any cancer before DHQ entry (n = 9,682), and no follow-up time after the DHQ (n = 122). At last, 101,680 subjects were included in our cohort.
The self-reported information of sex, race, trial arm, body mass index (BMI), educational level, marital status, aspirin use, cigarette smoking, family history of colorectal cancer, and diabetes history, were collected from the BQ. Dietary data, including age at DHQ, alcohol drinking, dietary carrot/carotene intake, energy intake from diet, and supplemental nutrients (Beta-Carotene, Calcium, Vitamin A, Vitamin C, Vitamin D, and Vitamin E), were collected from the DHQ (version 1.0, National Cancer Institute 2007), which was a self-administered food frequency questionnaire (FFQ) designed to assess the portion size and consumption frequency of 124 food items and supplement use over the past year, and has been validated with better performance in estimating dietary intake than two widely used FFQs (26) at the time of PLCO study carried out. The 1994–96 Continuing Survey of Food Intakes by Individuals, available from the USDA Food Surveys Research Group, and the Nutrition Data Systems for Research (NDS-R) from the University of Minnesota was applied to calculate the daily intake of all nutrients in the database (27). Three exposure variables (dietary carrot, α-, and dietary β-carotene intakes) were acquired in this study.
The time metric was followed up from the date of DHQ completion to the date of events that firstly occurred, including colorectal cancer diagnosis, dropout, colorectal cancer death, or the end of follow-up (incidence through December 31, 2009; and mortality through December 31, 2015). CRC diagnosis was ascertained via an annually updated medical record. Deaths were mainly identified by (1) annually mailed questionnaires, (2) reports from relatives, friends, or physicians, and (3) periodic linkage to the National Death Index. Our interested endpoints were the incidence and mortality of colorectal cancer.
Dietary carrot, α-, and β-carotene intakes were adjusted for energy intake with the residual method (28). The distributions of them were transformed with Z-score as continuous variables, and then were divided into quintiles as categorical variables, and the lowest quintile was as the referent. Continuous variables are expressed as median (IQR, interquartile range), and categorical variables are presented as numbers (frequency). We applied the Kruskal–Wallis H test and Pearson's chi-squared test to compare the baseline differences, if appropriate. Multivariable Cox regression analyses were employed to estimate the hazard ratios (HR) and 95% confidence interval (CI). The proportional hazard assumption of baseline covariates for the Cox model was verified using the Schoenfeld residuals (29) (all p > 0.05). Potential confounders were selected according to the change-in-estimate strategy (30) (more than 10% change in effect estimates) and literature-known risk factors. Missing values of covariates were treated as dummy variables in the multivariable Cox regression analyses. Specifically, the full-adjusted model included age, sex, race, trial arm, marital status, BMI, educational level, aspirin use, cigarette smoking, alcohol drinking, diabetes, family history of colorectal cancer, and energy intake from the diet, and supplemental use of Beta-Carotene, Calcium, Vitamin A, Vitamin C, Vitamin D, and Vitamin E. We also analyzed the linear trend of each quintile of energy-adjusted dietary carrot, α-, and β-carotene intakes, by entering the median value as a continuous variable in the model.
Subgroup analyses were performed, including age, sex, race, trial arm, marital status, BMI, educational level, aspirin use, cigarette smoking, alcohol drinking, diabetes, and family history of colorectal cancer. The interaction effect on each stratum was compared using likelihood-ratio tests. To address the dose-response trend between dietary carrot/carotene intakes and colorectal cancer incidence and mortality, the smooth curve fitting was conducted with a multivariable Cox regression model using the generalized additive model (GAM, Restricted Cubic Spline Functions). Here, we excluded subjects with <1st or >90th percentile values of energy-adjusted dietary carrot/carotene intakes (i.e., to reduce the potential impacts on the association of dose-response analyses).
The following sensitivity analyses were conducted: (1) excluding cases diagnosed or died within the first 5 years of follow-up; (2) excluding the extreme values of energy intake from the diet (<800/>4,000 kcal/day for men and <500/>3,500 kcal/day for women); (3) additional adjusted for other factors, including fruit (continuous), dietary Magnesium (continuous), dietary Sodium (continuous), dietary Potassium (continuous), whole grain (continuous), vegetables (continuous), added sugars (continuous), fiber (continuous), and saturated fatty acids (continuous). All analyses were performed using R Statistical Software (http://www.R-project.org, The R Foundation) and the Free Statistics analysis platform (31). Tests were two-tailed, and the significance level was set at 0.05.
Accordingly, 101,680 subjects were selected for the final data analysis (Figure 1). The baseline characteristics are presented in Table 1. The median value of dietary carrot intake (without adjustment for energy intake) was 4.3 g/day, ranging from 0 to 205.3 g/day. Compared to participants with the highest quintile (Q5) of dietary carrot consumption, participants with the lowest quintile (Q1) showed a higher proportion of males (72.8% vs. 36.2%), Black race, aspirin usage, higher BMI (70.4% vs. 60.4%), and higher energy intake (2132.0 kcal/day vs. 1597.0 kcal/day), and former/current cigarette smoking (48.2% vs. 39.3%;15.4% vs. 5.0%).
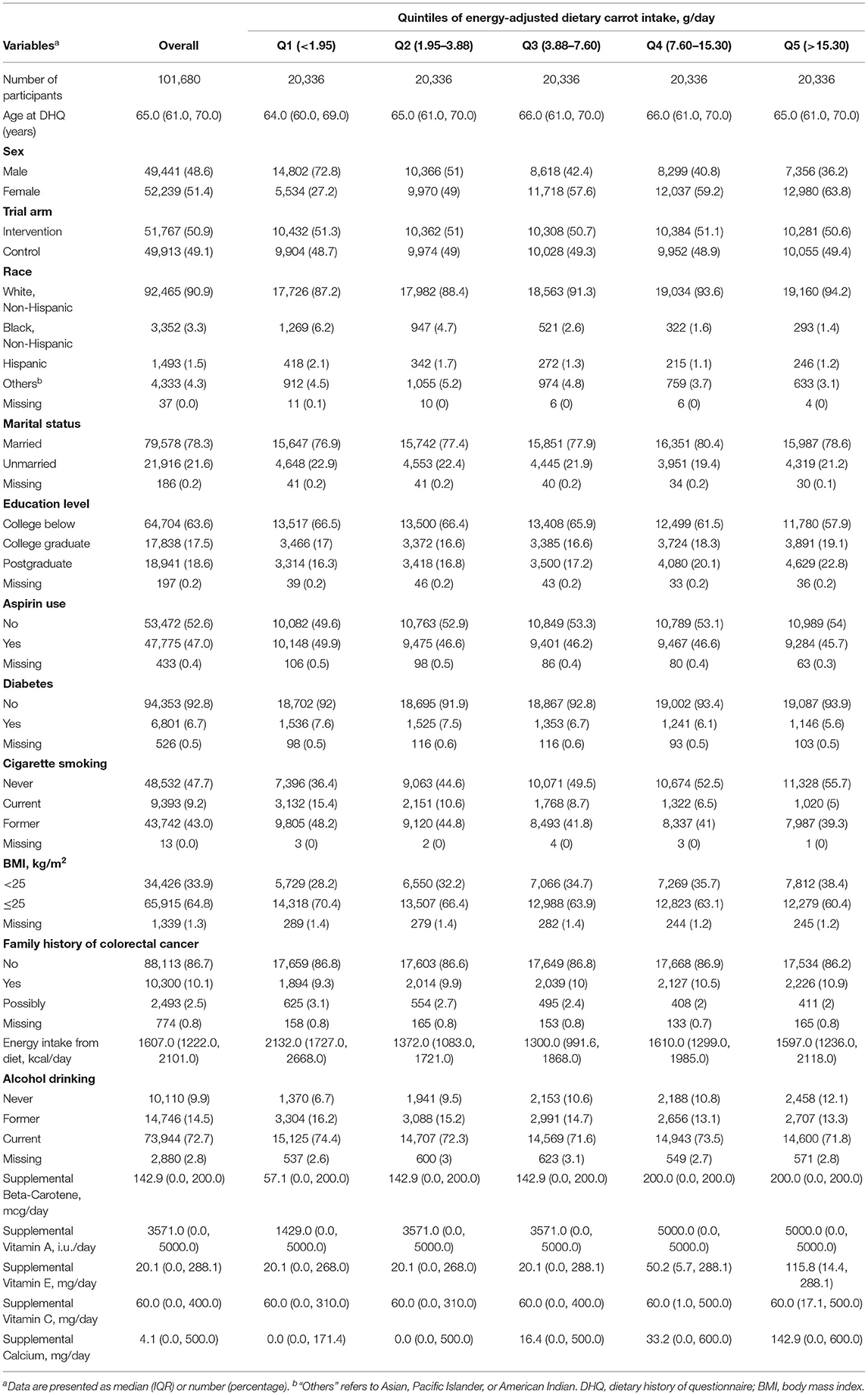
Table 1. Baseline characteristics of study population according to quintiles of energy-adjusted dietary carrot intake in 101680 US participants.
A total of 1,100 participants were diagnosed with CRC after a median follow-up of 9.4 years (896,327.8 person-years), and the incidence rate was 12.27 per 10,000 person-years. As shown in Table 2, we observed an inverse effect on the association between carrot intakes and CRC incidence at the quintile 4 level, which showed the multivariable-adjusted HR was 0.79 (95%CI = 0.65–0.97, p trend = 0.05), compared with the referent group; and corresponding adjusted HR of cancer risk was 0.95 (95%CI = 0.89–1.02) with per SD increment of carrot intakes. However, no statistical association was detected between dietary α- and β-carotene intakes and CRC incidence after adjusting for covariates.
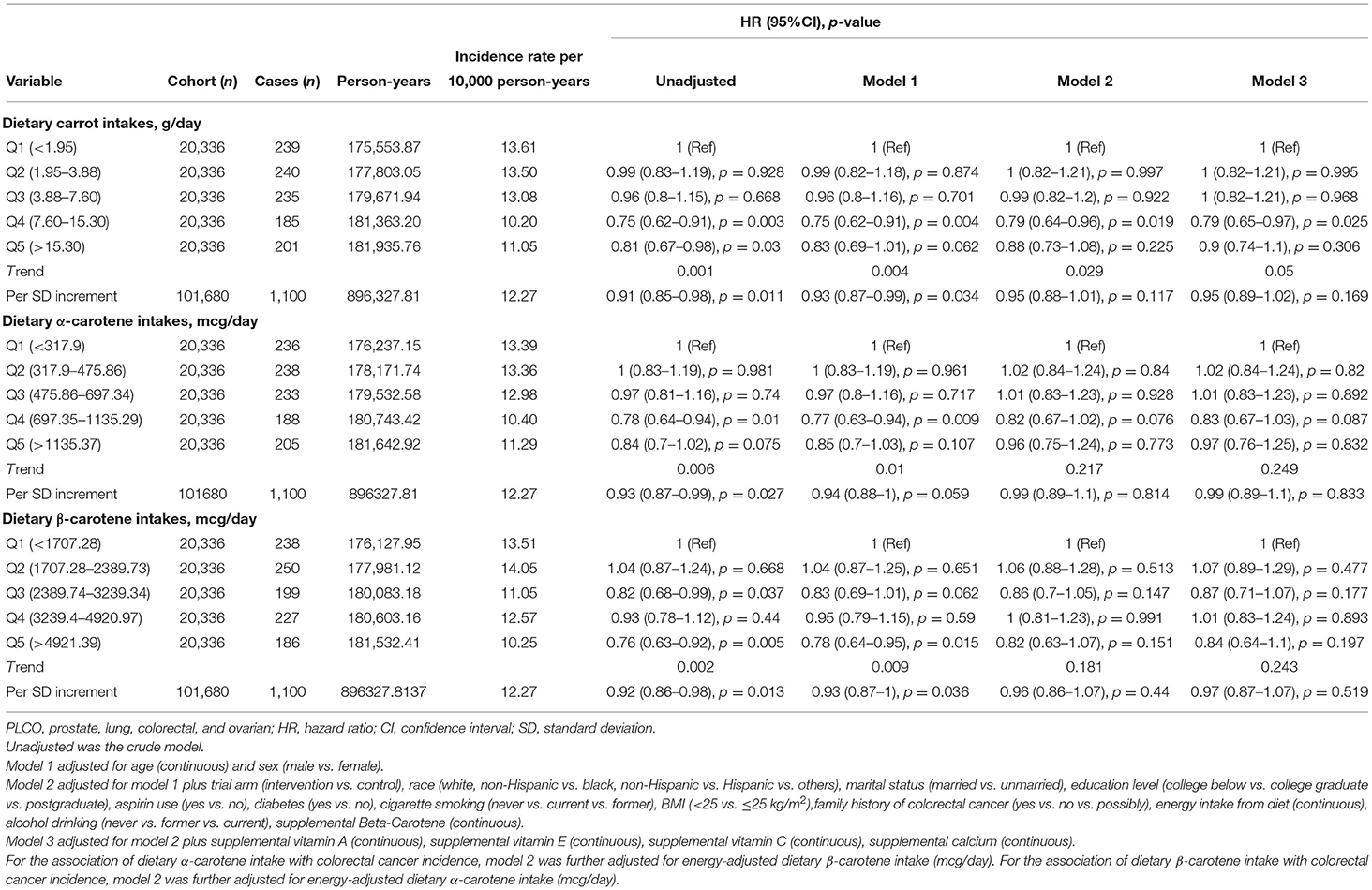
Table 2. Association between energy-adjusted dietary carrot/carotene intakes and colorectal cancer incidence risk in the PLCO cancer screening trial.
The results of subgroup analyses indicated that the associations between dietary carrot intakes and the risk of CRC were stable in almost all subgroups (Supplementary Table 1). An exception of significant effect modifier was represented by smoking status, which showed a stronger association for the participants who never smoked than those with a history of smoking (p for interaction = 0.027). Sensitivity analyses revealed that the results of the correlation between carrot/carotene intake and CRC risk were substantially robust to the findings in Table 2 and Supplementary Table 3. The smooth curve fitting plots of carrot/carotene intakes with CRC risk revealed no evidence of a non-linear trend (all p for non-linearity > 0.05, Figures 2A–C).
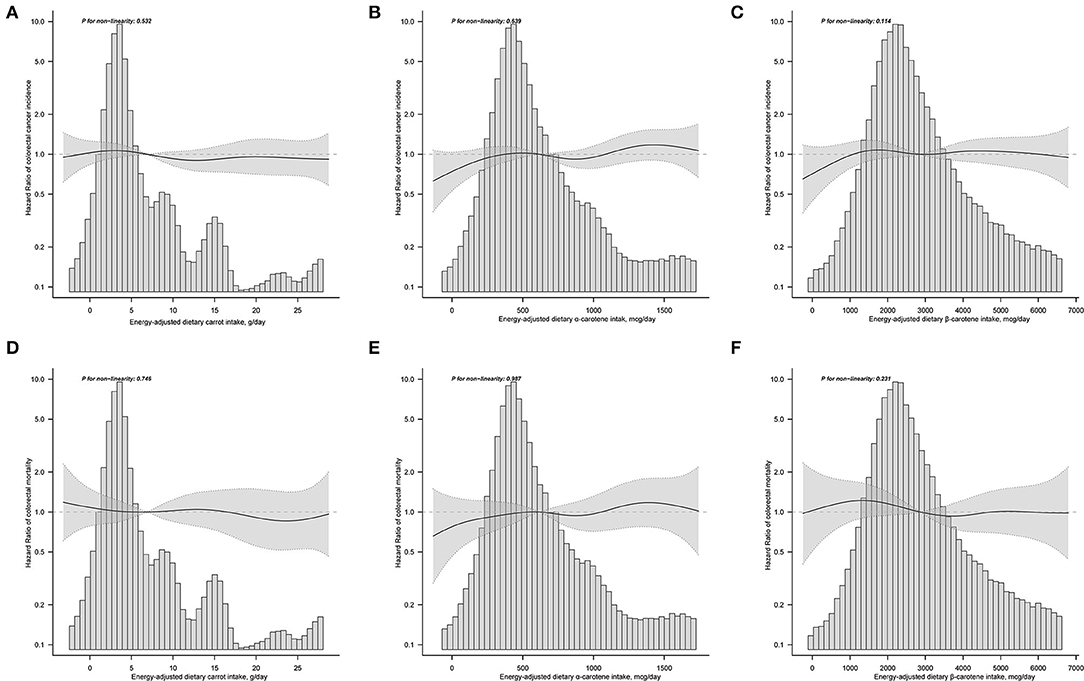
Figure 2. Does-response analyses for the associations between energy-adjusted dietary carrot intake (A), dietary α-carotene intake (B), dietary β-carotene intake (C) and colorectal cancer incidence; and the associations between energy-adjusted dietary carrot intake (D), dietary α-carotene intake (E), dietary β-carotene intake (F) and colorectal cancer mortality were performed with smooth curve fitting using the generalized additive model. Hazard ratios and 95% confidence interval were calculated by the fully-adjusted multivariable Cox regression model, including age, sex, race, trial arm, marital status, BMI, educational level, aspirin use, cigarette smoking, alcohol drinking, diabetes, family history of colorectal cancer, energy intake from diet, supplemental Beta-Carotene, supplemental Calcium, supplemental Vitamin A, supplemental Vitamin C, supplemental Vitamin D, and Supplemental Vitamin E. Solid lines represent point estimates and dashed lines represent corresponding 95% confidence intervals. The histograms show the percentage of participants belonging to each level of specific energy-adjusted dietary carrot/carotene intake.
A total of 443 cases died from CRC after a median follow-up of 14.5 years (1,353,326.28 person-years), and the mortality rate was 3.27 per 10,000 person-years. As shown in Table 3, in the fully-adjusted Cox model, only a suggestive but no significant association with cancer mortality was noted for carrot intakes (HRquintile5vs.quintile1 = 0.87, 95%CI = 0.64–1.18, ptrend = 0.297), and corresponding adjusted HR was 0.94 (95%CI = 0.84–1.05), with per SD increment of carrot intakes. Similar results on the association of dietary α-carotene intake with colorectal mortality were obtained (model 3: HRquintile5vs.quintile1 = 0.91, 95%CI = 0.62–1.35, p trend = 0.417; and HR with per SD increment = 0.94, 95%CI = 0.8–1.11); and for dietary β-carotene intake (model 3: HR quintile 5 vs. quintile 1 = 0.91, 95%CI = 0.61–1.36, p trend = 0.344; and HR with per SD increment = 1.00, 95%CI = 0.86–1.17). Results of subgroup analyses showed no significant effect modifies in the prespecified groups (all p for interaction > 0.05, Supplementary Table 2). In the sensitivity analyses, the null associations of dietary carrot/carotene intakes were robust to colorectal cancer mortality (Supplementary Table 4). Dose-response analyses suggested no non-linear relationship between carrot/carotene intakes and colorectal cancer mortality (all p for non-linearity > 0.05, Figures 2D–F).
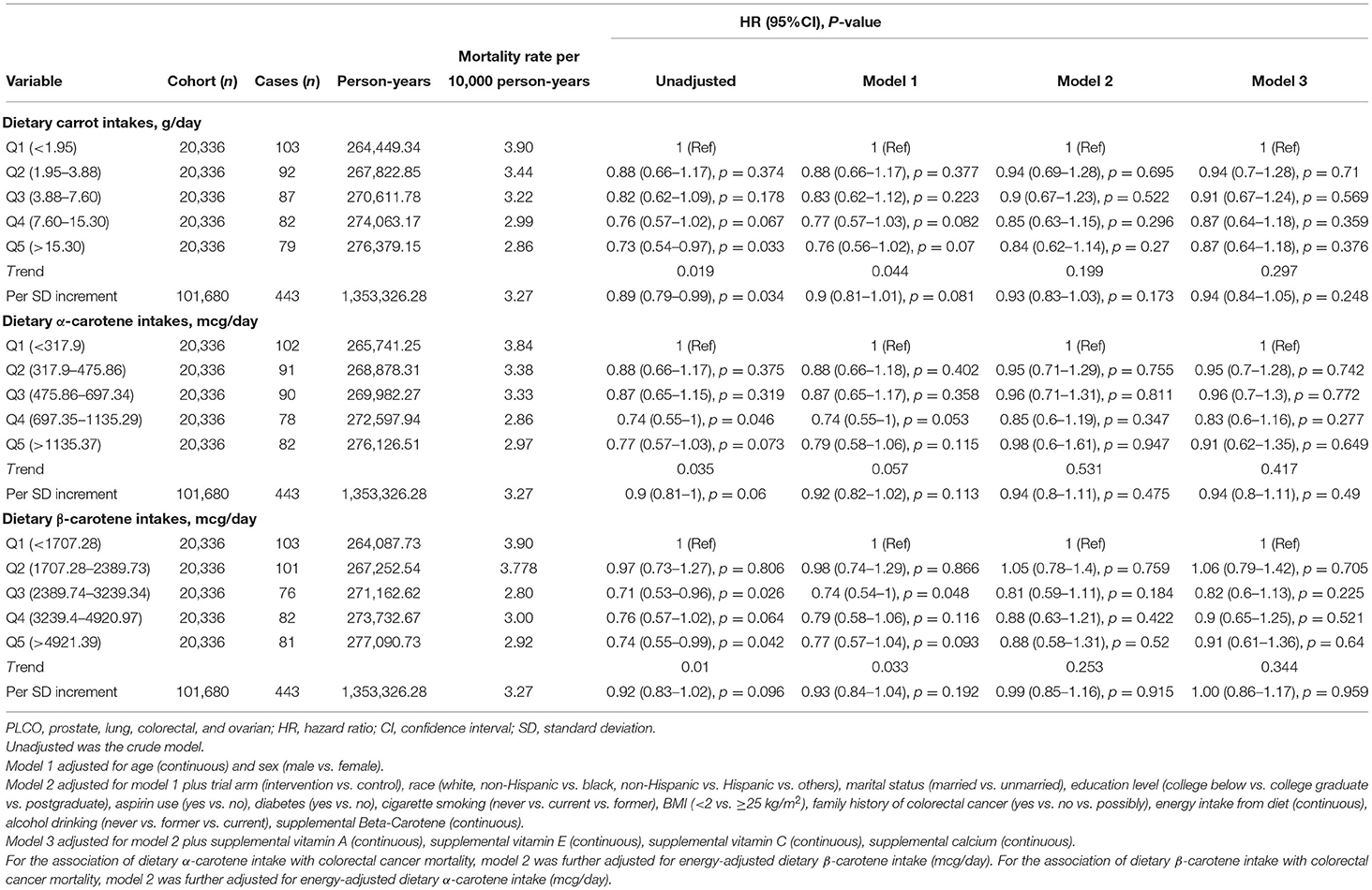
Table 3. Association between energy-adjusted dietary carrot/carotene intakes and colorectal cancer mortality risk in the PLCO cancer screening trial.
In this prospective cohort of 101,680 U.S. adults, we found that only the quintile 4 group of dietary carrot intakes but not dietary α-, and β-carotene intakes, had a lower risk of colorectal cancer, compared with the referent group. Meanwhile, the null associations were also detected between dietary carrot, α-, and β-carotene intakes and colorectal cancer mortality. Similar results were supported in several sensitivity analyses. Of note, the association of dietary carrot intakes was modified by smoking status with colorectal cancer incidence but not mortality.
Although the effect of carrot consumption on multiple types of cancer risk has been widely investigated, the findings were mixed. In our analysis, except for the quintile 4 group of carrot intakes, however, there were no significant associations shown in other quintile groups. The observed inverse association of our study was close to a previous prospective cohort from Danes (17), in which they illustrated that a higher intake of raw carrots (>32 g/day) was related to a 17% lower risk of CRC, compared with no intake of raw carrots (HR = 0.83, 95%CI = 0.71–0.98), but as for the raw carrot intakes <32 g/day, no significant association was observed with decreased risk of CRC (HR = 0.93, 95%CI = 0.82–1.06). Of note, there were also some differences. Our results, based on a large prospective cohort of 101,680 US adults, found that moderate consumption of carrots (Q4, >7.60–15.30 g/day) but not the highest consumption of carrots (Q5, >15.30 g/day) was associated with a lower risk of CRC incidence, compared with the lowest consumption of carrot (Q1, <1.95 g/day), while the Danes cohort showed that a higher raw carrots intake was associated with a lower risk of CRC incidence. We further categorized the dietary carrot intake into three groups (0, 0–32 g/day, and ≥32 g/day), and the highest carrot intake was also null associated with CRC incidence (HR ≥32 g/day vs. none = 0.75, 95%CI = 0.46–1.24) after fully-adjustment. The small size of events might reduce the statistical power, in which only 56 (0.8%) incident cases and 26 (0.4%) CRC deaths were in the group of carrot intake ≥32 g/day, and lower the association with CRC risk. Some residual confounders, such as dietary habits, and assessment of exposure may account for the difference between the dose-response analyses of carrot consumption and CRC incidence in a different population and thus needed to interpret cautiously. In addition, we explored the association of carrot intake with CRC mortality, but no significant correlation was detected in the multivariable Cox models and other analyses.
The cancer-prevention effect of carrot intake might be explained that carrots are rich in carotenoid antioxidants such as α- and β-carotene, which showed a potential prevention effect on cancer development. Hence, we further examined the association between dietary α-, and β-carotene intakes and CRC incidence, and mortality, but no statistically significant inverse associations were observed, respectively. That was in line with the results of a meta-analysis of nine randomized controlled trials (32), in which no significant association was found between dietary intakes of α- and β-carotene and colorectal cancer incidence. However, another two case-control studies in the Chinese population (18, 19) noted an inverse association of dietary α- and β-carotene intake with the risk of colorectal cancer. The differences in retrospective study design, number of samples, exposure assessment, potential recall bias, and the unadjusted confounders may contribute to the conflict findings. Another biological interpretation might be that carrots are also a major source of falcarinol (FaOH), falcarindiol (FaDOH), which have been demonstrated to inhibit neoplastic transformations in the rat models with a prevention effect on the development of colorectal cancer (33, 34). Thus, our findings supported that the potential cancer-prevention effects on CRC might derive from other specific components, rather than α-, and β-carotene.
Interestingly, we found that smoking status was an effect modifier on the relationship between carrot intake and colorectal cancer incidence (p for interaction = 0.027). In the strata of never smokers, the HR of the risk of CRC was 0.99 (95%CI = 0.99–1, p < 0.01), even though the association was weak. The result was constant with the previous research that smoking as a well-established risk factor could attenuate or reversed the observed protective effect of dietary carotenoids on CRC occurrence (17).
There have several inevitable limitations in the current study. First, the nature of observational studies resulted in residual confounding that could not be fully ruled out. Second, the generalizability of conclusions may limit by the geographical location and population differences. Third, we could not take into account the dynamic change data because of the absence of repeated measurements of dietary nutrients. Forth, we lack the data on raw, cooked carrots, and carrot juice due to the limited raw data, thus we were impossible to further distinguish and analyze their effect on colorectal cancer risk. In addition, some epidemiological studies published inconsistent results about the association of serum α-, and β-carotene concentration with CRC incidence (18, 35). We were interested in this, but for the limited raw data, thus we were unable to further analyze the association between serum carrot/carotene and the risk of CRC.
In summary, this U.S. prospective cohort indicated that moderate consumption of dietary carrots was associated with a lower risk of CRC incidence, while no statistically significant associations were observed between dietary α-, and β-carotene intakes and CRC incidence. Dietary carrot, α-, and β-carotene intakes were null associated with CRC mortality. Furthermore, we found smoking status modified the association of dietary carrot intakes with CRC incidence but not mortality. The results added evidence for the potential cancer-prevention effect of dietary carrot intakes on CRC incidence but should be cautiously interpreted. More large, prospective, well-designed cohort studies are warranted to verify the findings in other populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The PLCO study was approved by the Institutional Review Boards of the US National Cancer Institute and each study center and written informed consents were obtained from all eligible participants. The patients/participants provided their written informed consent to participate in this study.
ZJ: conception, design, and acquisition of data. WW, CF, and FL: administrative support. ZJ and HC: data analysis and interpretation. ZJ, CF, and ML: manuscript revising. All authors: manuscript writing, final approval, and accountable for all aspects of work ensuring integrity and accuracy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the National Cancer Institute for access to NCI's data collected by the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.888898/full#supplementary-material
1. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. (2020) 70:145–64. doi: 10.3322/caac.21601
2. Thomas D. Gene–environment-wide association studies: emerging approaches. Nat Rev Genet. (2010) 11:259–72. doi: 10.1038/nrg2764
3. de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. (2004) 4:769–80. doi: 10.1038/nrc1453
4. Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. (2018) 68:31–54. doi: 10.3322/caac.21440
5. Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. (1985) 1:307–9. doi: 10.1016/S0140-6736(85)91082-7
6. Park SY, Murphy SP, Wilkens LR, Nomura AM, Henderson BE, Kolonel LN. Calcium and vitamin D intake and risk of colorectal cancer: the Multiethnic Cohort Study. Am J Epidemiol. (2007) 165:784–93. doi: 10.1093/aje/kwk069
7. Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. (2020) 49:246–58. doi: 10.1093/ije/dyz064
8. Barrubés L, Babio N, Becerra-Tomás N, Rosique-Esteban N, Salas-Salvadó J. Association between dairy product consumption and colorectal cancer risk in adults: a systematic review and meta-analysis of epidemiologic studies. Adv Nutr. (2019) 10:S190–211. doi: 10.1093/advances/nmy114
9. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. (2011) 343:d6617. doi: 10.1136/bmj.d6617
10. Huang J, Weinstein SJ, Yu K, Männistö S, Albanes D. Serum beta carotene and overall and cause-specific mortality. Circ Res. (2018) 123:1339–49. doi: 10.1161/CIRCRESAHA.118.313409
11. Buijsse B, Feskens EJ, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, et al. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am J Clin Nutr. (2005) 82:879–86. doi: 10.1093/ajcn/82.4.879
12. Fallahzadeh H, Jalali A, Momayyezi M, Bazm S. Effect of carrot intake in the prevention of gastric cancer: a meta-analysis. J Gastric Cancer. (2015) 15:256–61. doi: 10.5230/jgc.2015.15.4.256
13. Xu H, Jiang H, Yang W, Song F, Yan S, Wang C, et al. Is carrot consumption associated with a decreased risk of lung cancer? A meta-analysis of observational studies. Br J Nutr. (2019) 122:488–98. doi: 10.1017/S0007114519001107
14. Xu X, Cheng Y, Li S, Zhu Y, Xu X, Zheng X, et al. Dietary carrot consumption and the risk of prostate cancer. Eur J Nutr. (2014) 53:1615–23. doi: 10.1007/s00394-014-0667-2
15. Chen H, Shao F, Zhang F, Miao Q. Association between dietary carrot intake and breast cancer: a meta-analysis. Medicine. (2018) 97:e12164. doi: 10.1097/MD.0000000000012164
16. Luo X, Lu H, Li Y, Wang S. Carrot intake and incidence of urothelial cancer: a systematic review and meta-analysis. Oncotarget. (2017) 8:77957–62. doi: 10.18632/oncotarget.19832
17. Chaiter Y, Gruber SB, Ben-Amotz A, Almog R, Rennert HS, Fischler R, et al. Smoking attenuates the negative association between carotenoids consumption and colorectal cancer risk. Cancer Causes Control. (2009) 20:1327–38. doi: 10.1007/s10552-009-9354-7
18. Huang J, Lu MS, Fang YJ, Xu M, Huang WQ, Pan ZZ, et al. Serum carotenoids and colorectal cancer risk: a case-control study in Guangdong, China. Mol Nutr Food Res. (2017) 61:1700267. doi: 10.1002/mnfr.201700267
19. Lu MS, Fang YJ, Chen YM, Luo WP, Pan ZZ, Zhong X, et al. Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: a case-control study. Eur J Nutr. (2015) 54:619–28. doi: 10.1007/s00394-014-0743-7
20. Okuyama Y, Ozasa K, Oki K, Nishino H, Fujimoto S, Watanabe Y. Inverse associations between serum concentrations of zeaxanthin and other carotenoids and colorectal neoplasm in Japanese. Int J Clin Oncol. (2014) 19:87–97. doi: 10.1007/s10147-013-0520-2
21. Männistö S, Yaun SS, Hunter DJ, Spiegelman D, Adami HO, Albanes D, et al. Dietary carotenoids and risk of colorectal cancer in a pooled analysis of 11 cohort studies. Am J Epidemiol. (2007) 165:246–55. doi: 10.1093/aje/kwk009
22. Park SY, Nomura AM, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Carotenoid intake and colorectal cancer risk: the multiethnic cohort study. J Epidemiol. (2009) 19:63–71. doi: 10.2188/jea.JE20080078
23. Slattery ML, Benson J, Curtin K, Ma KN, Schaeffer D, Potter JD. Carotenoids and colon cancer. Am J Clin Nutr. (2000) 71:575–82. doi: 10.1093/ajcn/71.2.575
24. Deding U, Baatrup G, Christensen LP, Kobaek-Larsen M. Carrot intake and risk of colorectal cancer: a prospective cohort study of 57,053 danes. Nutrients. (2020) 12:332. doi: 10.3390/nu12020332
25. Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Prostate, lung, colorectal and ovarian cancer screening trial project team. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. (2000) 21:273–9S. doi: 10.1016/S0197-2456(00)00098-2
26. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. (2001) 154:1089–99. doi: 10.1093/aje/154.12.1089
27. Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. (2000) 152:279–86. doi: 10.1093/aje/152.3.279
28. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. (1997) 65:1220–28S; discussion 1229S−1231S. doi: 10.1093/ajcn/65.4.1229S
29. Tahir MR, Tran QX, Nikulin MS. Comparison of hypertabastic survival model with other unimodal hazard rate functions using a goodness-of-fit test. Stat Med. (2017) 36:1936–45. doi: 10.1002/sim.7244
30. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. (1993) 138:923–36. doi: 10.1093/oxfordjournals.aje.a116813
31. Su NJ, Huang CY, Liu J, Kang DY, Wang SL, Liao LJ, et al. Association between baseline LH/FSH and live-birth rate after fresh-embryo transfer in polycystic ovary syndrome women. Sci Rep. (2021) 11:20490. doi: 10.1038/s41598-021-99850-4
32. Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, et al. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. (2010) 127:172–84. doi: 10.1002/ijc.25008
33. Kobaek-Larsen M, Baatrup G, KhataeiNotabi M, El-Houri RB, Pipó-Ollé E, Christensen Arnspang E, et al. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: a mechanistic and dose-response study in a rat model. Nutrients. (2019) 11:2223. doi: 10.3390/nu11092223
34. Kobaek-Larsen M, El-Houri RB, Christensen LP, Al-Najami I, Fretté X, Baatrup G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. (2017) 8:964–74. doi: 10.1039/C7FO00110J
Keywords: carrot, carotene, colorectal cancer, cohort, PLCO
Citation: Jiang Z, Chen H, Li M, Wang W, Fan C and Long F (2022) Association of Dietary Carrot/Carotene Intakes With Colorectal Cancer Incidence and Mortality in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Front. Nutr. 9:888898. doi: 10.3389/fnut.2022.888898
Received: 03 March 2022; Accepted: 09 May 2022;
Published: 17 June 2022.
Edited by:
Rafaela Rosário, University of Minho, PortugalCopyright © 2022 Jiang, Chen, Li, Wang, Fan and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feiwu Long, bG9uZ2Z3MTk3OEBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.