
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 09 August 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.886224
 Alireza Jafari1,2
Alireza Jafari1,2 Sina Naghshi3
Sina Naghshi3 Hossein Shahinfar4,5
Hossein Shahinfar4,5 Sayed Omid Salehi3
Sayed Omid Salehi3 Fateme Kiany6
Fateme Kiany6 Mohammadreza Askari1
Mohammadreza Askari1 Pamela J. Surkan7
Pamela J. Surkan7 Leila Azadbakht1,8,9*
Leila Azadbakht1,8,9*Background: Numerous studies report an association between coffee or caffeine consumption and pregnancy loss; however, the nature and strength of this relationship have not been clearly established. Based on recent studies, our meta-analysis aimed to test whether a dose–response relationship between coffee or caffeine consumption and pregnancy loss exists.
Methods: We searched for articles in PubMed, Web of Science, and Scopus published until May 2022. Two independent reviewers extracted data and rated the quality of the evidence using the GRADE approach. We applied a random-effects, one-stage dose–response meta-analysis.
Results: A total of 34 articles (18 cohort studies and 16 case-control studies) were included in this review. Results showed a significantly higher risk of pregnancy loss for coffee consumption before (Pooled ES: 1.21; 95% CI: 1.01–1.43) and during pregnancy (Pooled ES: 1.26; 95% CI: 1.04–1.57), and for coffee consumption during pregnancy in case-control studies (Pooled ES: 1.20; 95% CI: 1.19–6.41). Findings from this meta-analysis demonstrated that caffeine intake during pregnancy was associated with a significantly higher risk of pregnancy loss in cohort (Pooled ES: 1.58; 95% CI: 1.23–2.01) and case-control studies (Pooled ES: 2.39; 95% CI: 1.69–3.37, P < 0.001), respectively. A dose–response analysis suggested that an increase of a cup of coffee per day during pregnancy was associated with 3% increased risk of pregnancy loss; 100 mg of caffeine per day during pregnancy was also associated with 14 and 26% increased risk of pregnancy loss in cohort and case-control studies, respectively. A non-linear dose–response association was observed between coffee intake and the risk of pregnancy loss.
Conclusion: This study confirms that coffee or caffeine consumption raises the risk of pregnancy loss. Researchers are encouraged to conduct more studies to explore the underlying mechanisms and active compounds in coffee and caffeine.
Systematic Review Registration: [www.crd.york.ac.uk/prospero/], identifier [CRD42021267731].
Fetal deaths account for a high percentage of perinatal deaths and can be categorized into stillbirth and spontaneous abortion (or miscarriage) (1, 2). Miscarriage or spontaneous abortion is defined as the involuntary termination of a pregnancy leading to fetal death before week 20 of gestation. Stillbirth refers to the death of a fetus after 20 weeks of pregnancy or after attaining 14 oz in weight. In other situations, the fetus is alive at the beginning of labor but dies during delivery (3). An estimated 26% of all pregnancies and up to 10% of clinically recognized pregnancies result in pregnancy loss (4), whereas the global stillbirth rate was 18.4 per 1,000 total births in 2015 (5). Risk factors for fetal death include advanced maternal age, obesity/overweight, low socioeconomic status, history of fetal loss, smoking and alcohol consumption, and caffeine intake during pregnancy (6).
Caffeine is a 1,3,7-trimethylxanthine; in the world, it is one of the most common substances consumed in pharmacologically active amounts and is found in beverages like coffee, tea, soda, solid milk chocolate, and products containing cacao (7, 8). Hoyt et al. reported that 82% of pregnant women in the United States consumed caffeine (9). In another study, the National Institute of Child Health and Human Development (NICHD) reported that 35% of participants consumed coffee and 41% drank soda daily (10). A safe dose of daily caffeine intake in pregnancy is not more than 200 mg (or two cups) of coffee (11). In adults, caffeine is metabolized in the liver mainly by cytochrome P450 enzymes (monooxygenase and xanthine oxidase enzymes). However, since the P450 enzyme system remains undeveloped until infancy, there is a low metabolite rate in the fetus (12). Caffeine has delayed clearance in the second and third trimesters of pregnancy compared with clearance in non-pregnant women. Ingested caffeine by pregnant women is rapidly absorbed from the digestive tract and readily passes through the placenta (13, 14). In pregnant women, caffeine has a long clearance compared with non-pregnant women, and the fetus has a low metabolite rate because of the lack of enzymes required in caffeine metabolism (6, 15, 16). The half-life of caffeine is tripled in the second and third trimesters of pregnancy compared to in non-pregnant women, and therefore the fetus is more exposed to caffeine and its metabolites (6, 13). Caffeine raises cellular cyclic adenosine monophosphate levels, which can accelerate cell growth; it also raises levels of circulating catecholamine that could interfere with placental blood flow through vasoconstriction and lead to fetal hypoxia (13, 17, 18). During pregnancy, caffeine consumption has been connected to different adverse outcomes, including spontaneous abortion, congenital disabilities, and low birth weight (19). Although some observational studies have been carried out to investigate how caffeine intake is associated with the risk of fetal loss, the results have not been consistent due to difficulties in measuring self-reported caffeine intakes, different caffeine metabolisms, and differences in study settings and genetics of participants (8, 16, 20).
The previous meta-analysis including studies published until 2015 (2, 21, 22) was limited in scope due to inclusion restrictions. We had fewer limitations on inclusion criteria, and we also included more recent studies (8, 16, 20, 23–27). Moreover, we use a new one-stage random effect dose–response analytic approach. Therefore, in the present meta-analysis, we aimed to update and expand the current literature on the association of caffeine and coffee consumption with the risk of miscarriage.
This systematic review and meta-analysis were performed based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-analyses Of Observational Studies in Epidemiology) guidelines (28, 29). The corresponding checklists are shown in Supplementary Tables 1, 2.
A systematic literature search was conducted on Medline/PubMed, ISI Web of Knowledge, and Scopus to include observational studies published until May 2022. The following keywords were used in a comprehensive literature search: [“caffeine*” OR “coffee*”] in combination with [“miscarriage*,” “abortion*,” “stillbirth*,” “still birth*,” “fetal loss*,” “fetal death*,” “misbirth*,” and “pregnancy loss*”] AND [“prospective*” OR “retrospective*” OR “observational” OR “longitudinal” OR “cohort*” OR “relative risk” OR “hazard ratio” OR “odds ratio” OR “follow-up” OR “follow up” OR “population-based” OR “hr” OR “rr”]. The reference lists of full-text publications were also screened to identify any relevant studies. The search was not restricted by publication date or language. Supplementary Table 3 provides more details on the search terms.
Endnote (version 20) was used for the management of records downloaded from databases. Two reviewers (AJ and MA) screened the titles and abstracts of each relevant study. Potentially eligible studies were reviewed independently by the reviewers. Discrepancies were resolved by discussion, and if necessary, a third author (LA) was consulted to reach consensus.
The two researchers independently checked the studies’ eligibility based on our inclusion and exclusion criteria, and differences were resolved by arbitration or consensus. Original studies were selected if they met the following inclusion criteria:
1. Study design: cohort or case-control studies.
2. Participants: pregnant women.
3. Exposure: dietary caffeine or coffee intake.
4. Outcome: pregnancy loss including miscarriage, abortion, and stillbirth (RR, OR, HR with 95% confidence interval [CI]).
Two independent reviewers (AJ and SN) extracted the relevant data independently. Any disagreements and differences were resolved by the study supervisor (LA). The following data were extracted from each included study by use of a standardized data-collection form: the first author’s last name, publication year, country, study design, length of follow-up, sample size, the incident of pregnancy loss, measurement of exposure, method of dietary intake assessment and outcome, comparison categories, and effect sizes (RR, OR, HR) with 95% CI with the maximum number of adjustments. Disagreements were resolved through discussion with the senior author (LA).
The risk of bias in the included studies was assessed by two authors using the ROBINS-I tool (30). This tool consists of seven questions aimed at determining bias based on confounding, participant selection, exposure classification, bias due to departures from intended exposures, missing data, outcomes measurement, and selection of the reported result. Discrepancies were resolved through discussion with the senior author. We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to evaluate the quality of the evidence (31).
In this meta-analysis, the common measure of association was OR in case-control studies and RR in cohort studies. Due to the low incidence of pregnancy loss, ORs and RRs are considered nearly equivalent in cohort studies (32). If an estimate was reported for the lowest category of caffeine or coffee intake compared with the highest category, we computed the highest vs. lowest estimates using the Orsini method (33). Results from case-control and cohort studies were presented separately. The Q-statistic and I2 were used as indicators of heterogeneity. We used a random-effects model (n > 5) to assess heterogeneity (34) for more conservative results than a fixed-effects model (35). We conducted a series of subgroup analyses to identify potential sources of heterogeneity based on the study design while controlling for BMI, alcohol consumption, smoking, education, and vitamin supplementation. We also performed an analysis that, excluded or included studies one-by-one to assess the influence overall estimate by a single study. When at least 10 primary studies had available data, we used Egger’s regression asymmetry test (36) and/or used a visual examination of counter-enhanced funnel plots (37) to detect the effects of potential publication bias from these studies (38).
Greenland and Orsini’s (39, 40) method was used to compute the trend based on the odds ratios, relative risks, or hazard ratios estimates and their respective 95% confidence intervals across categories of 100 mg/day increments of caffeine and intake of one cup of coffee per day. This method requires the distribution of cases and the odds ratios, relative risks, or hazard ratios with the variance estimates for three or more quantitative categories of exposure. We used mean or median intake, midpoint, and estimated the midpoint (if the mean was not presented) to derive the dose–response trend.
We applied a one-stage weighted, random-effects dose–response meta-analysis to investigate possible associations between caffeine or coffee intake and pregnancy loss. This non-linear dose–response analysis was done by modeling caffeine intake with a restricted cubic spline with 3 knots fixed at 10, 50, and 90% (41) using a generalized least squares trend estimation method. Furthermore, we combined the study-specific estimates by the restricted maximum likelihood method into a random-effects model. A probability value for non-linearity was estimated by testing the null hypothesis, in which the coefficient of the second spline was considered equal to zero (33). Statistical analyses were conducted using STATA version 16.0 (StataCorp, College Station, TX, United States), and meta and dr meta were used for analysis. A two-sided P-value < 0.05 was considered significant for all tests, including Cochran’s Q-test.
We identified 2,253 records in our initial search of three databases. We removed 245 duplicates and 1,912 non-relevant articles via title and abstract screening. After excluding those that did not meet the inclusion criteria, we identified 46 full-text publications of potentially relevant studies. After a full-text review, we excluded an additional nine studies (42–50). The flowchart is shown in Figure 1.
Finally, 18 cohort studies and 16 case-control studies were included in the current systematic and meta-analysis: seven studies reported effect sizes for coffee consumption, 25 for caffeine intake, and 2 studies reported both. Of these publications, 6 reported effect sizes for coffee consumption before pregnancy, 10 reported effect sizes for caffeine consumption before pregnancy, 12 reported effect sizes for coffee consumption during pregnancy, and 24 reported effect sizes for caffeine intake during pregnancy.
Characteristics of the studies are provided in Tables 1, 2. Participants in these studies ranged from 66 to 90,086 people. In total, 292,795 participants were included in the 34 publications in the systematic review. In total, 12 (31,544 participants) publications were conducted in the United States and 22 (261,251 participants) in non- United States countries.
To examine coffee and caffeine intake, 26 studies used dietary records or recall, and 8 studies used a food frequency questionnaire.
Most cohorts controlled for some conventional risk factors, including age (n = 30), body mass index (n = 7), smoking (n = 17), education (n = 13), alcohol consumption (n = 17), and previous spontaneous abortion (SAB; n = 12). Some others also adjusted for energy intake (n = 1) and other dietary variables (n = 1).
Supplementary Figure 1 displays the results of the risk of bias assessment. Most of the review studies had a serious risk of bias, and only seven studies (8, 15, 20, 24, 51–53) showed a moderate risk of bias. Regarding confounding, bias was considered serious (6, 24, 54–63) or moderate. Selection bias was considered moderate in two studies (19, 61) and serious in one study (25). One study (19) had low bias in the misclassification domain, since bias in the remaining studies was moderate. Missing data were measured in four studies (15, 27, 59, 61). Measurement bias was determined to be low and moderate (13, 19, 24, 52, 57, 64–66). As for reporting bias, only one article (54) showed a moderate risk of bias.
The GRADE assessment was very low for caffeine intake during pregnancy, low for coffee and caffeine intake before pregnancy, and moderate for coffee intake during pregnancy (Supplementary Table 4).
Coffee consumption before pregnancy, which was examined in four cohort and two case-control studies with a total of 26,748 participants and 4,817 pregnancy losses, was associated with an increased risk of pregnancy loss (Pooled ES comparing the highest and lowest intakes: 1.21; 95% CI: 1.01–1.43, P < 0.001), with no significant heterogeneity among the studies (τ2 = 0, I2 = 0%; Pheterogenity = 0.68; Figure 2A).
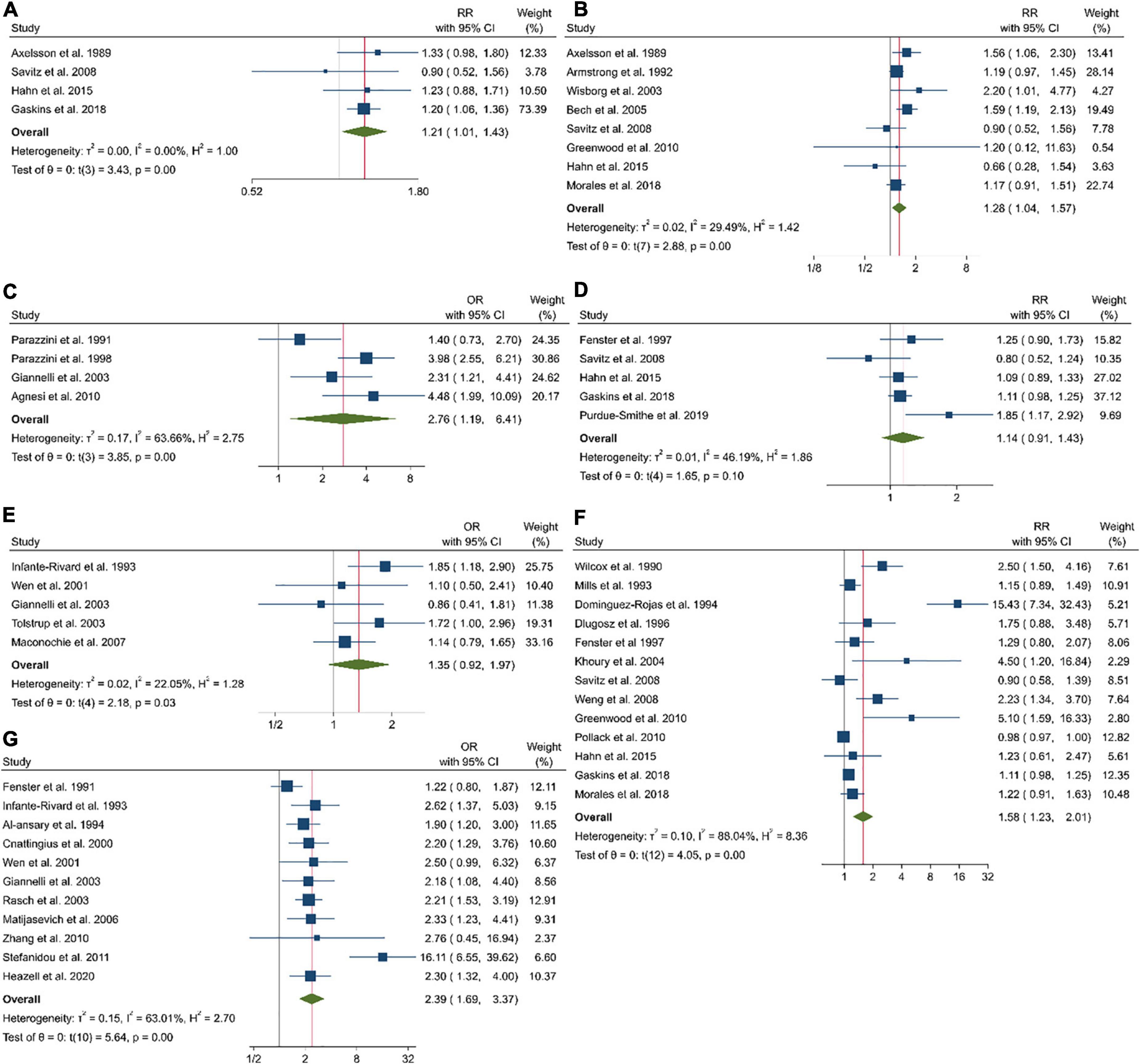
Figure 2. Forest plots of the estimated relative risk (RR) of pregnancy loss related to coffee intake before pregnancy in cohort studies (A), coffee intake during pregnancy in cohort studies (B), coffee intake during pregnancy in case-control studies (C), caffeine intake before pregnancy in cohort studies (D), caffeine intake before pregnancy in case-control studies (E), caffeine intake during pregnancy in cohort studies (F), and caffeine intake during pregnancy in case-control studies (G).
Examining the association between coffee consumption and the risk of pregnancy loss in eight cohorts and four case-control studies that involved a total of 246,770 participants and 12,409 abortions, we found a significantly higher risk (Pooled ES comparing the highest and lowest intakes: 1.28; 95% CI: 1.04–1.57, P < 0.001), with no significant heterogeneity among the cohort studies (τ2 = 0.02, I2 = 24.49%; Pheterogenity < 0.001; Figure 2B) and case-control studies (2.76; 95% CI: 1.19–6.41; τ2 = 0.17, I2 = 63.66%; Figure 2C).
The association between caffeine intake before pregnancy and the risk of pregnancy loss was examined in five cohort and five case-control studies that included 40,712 participants with 5,716 cases, we found a non-significant association (Pooled ES comparing the highest and lowest intakes: 1.14; 95% CI: 0.91–1.43, P = 0.02), with moderate heterogeneity among the cohort studies (τ2 = 0.01, I2 = 46.19%; Pheterogenity = 0.1; Figure 2D) and case-control studies (1.35; 95% CI: 0.92–1.97, P = 0.03; τ2 = 0.02, I2 = 22.05; Figure 2E).
A total of 13 cohort and 11 case-control studies examined associations between caffeine intake during pregnancy and the risk of pregnancy loss. These studies included a total of 137,128 participants among them, 9,666 cases of pregnancy loss were found. The summary effect size for abortion, comparing highest and lowest caffeine intake, was 1.58 (95% CI: 1.23–2.01, P < 0.001), indicated a significant positive association between caffeine intake and the risk of pregnancy loss (Figure 2F). High heterogeneity among the studies was observed (τ2 = 0.10, I2 = 88.04%; Pheterogenity < 0.001). Notably, 11 studies reported on the association in case-control studies (2.39; 95% CI: 1.69–3.37; τ2 = 0.15, I2 = 63.01; Figure 2G).
In the dose–response analysis of coffee intake before pregnancy and abortion risk based on four studies, we found a significant positive non-linear association (Pnon–linearity = 0.976; Figure 3A). A linear dose–response analysis revealed a significantly higher risk of abortion with each additional cup per day (Pooled ES: 1.03; 95% CI: 0.99–1.07, P = 0.002; Figure 2A).
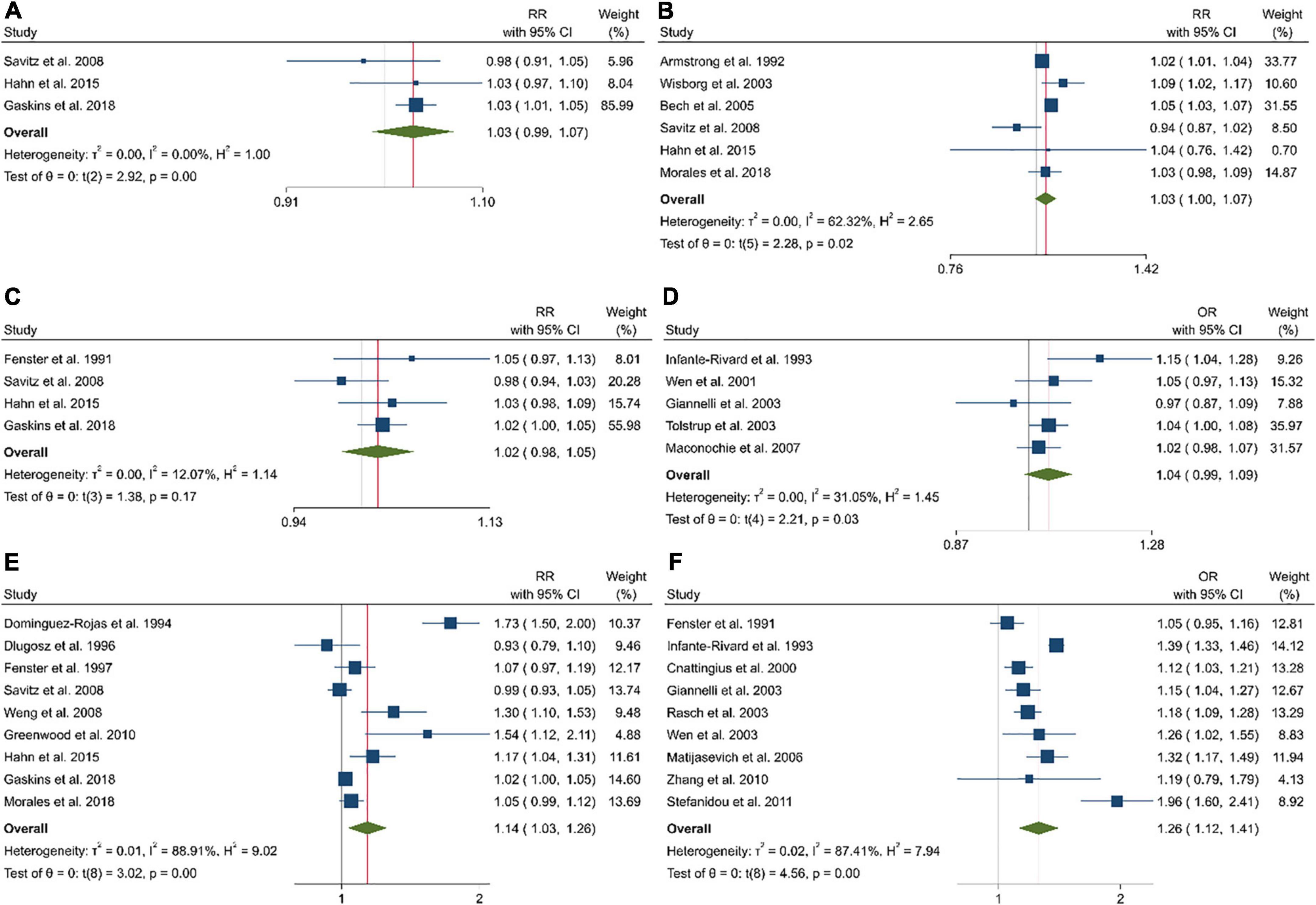
Figure 3. The linear dose–response analysis between the risk of pregnancy loss and one cup of coffee intake before pregnancy in cohort studies (A), one cup of coffee intake during pregnancy in cohort studies (B), 100 mg caffeine intake before pregnancy in cohort studies (C), 100 mg caffeine intake before pregnancy in case-control studies (D), 100 mg caffeine intake during pregnancy in cohort studies (E), and 100 mg caffeine intake during pregnancy in case-control studies (F).
Eight of 10 studies that had sufficient data to examine the association between coffee intake during pregnancy and the risk of pregnancy loss were included in the dose–response analysis (Figure 3B). Coffee intake was associated with a higher risk of pregnancy loss (Pnon–linearity = 0.036). This was also the case in the linear dose–response meta-analysis when examining an additional one cup of coffee per day (Pooled ES: 1.03; 95% CI: 1.00–1.07, P = 0.019; Figure 2B).
A non-linear dose–response analysis of eight studies revealed a significant positive association between caffeine intake before pregnancy and abortion (Pnon–linearity = 0.929; Figure 3C). Based on the linear dose–response analysis, an additional 100 mg of caffeine per day was associated with a higher risk of abortion in the cohort (Pooled ES: 1.02; 95% CI: 0.98–1.05, P = 0.11; Figure 2C) and case-control studies (Pooled ES: 1.04; 95% CI: 0.99–1.09; Figure 2D).
Combining data from 16 (out of 20 studies) in the dose–response analysis of caffeine intake during pregnancy and abortion risk, a significant non-linear association was observed in both cohort (Pnon–linearity = 0.085; Figure 4E) and case-control studies (Pnon–linearity = 0.372; Figure 4F). Moreover, the linear association between an increase of 100 mg of caffeine per day was associated with 14% and 26% increased risk of pregnancy loss in the cohort (Pooled ES: 1.14; 95% CI: 1.03–1.26, P < 0.001; Figure 2E) and case-control studies (Pooled ES: 1.26; 95% CI: 1.12–1.41; Figure 2F). No other association was observed (Figures 3D–F, 4A–D).
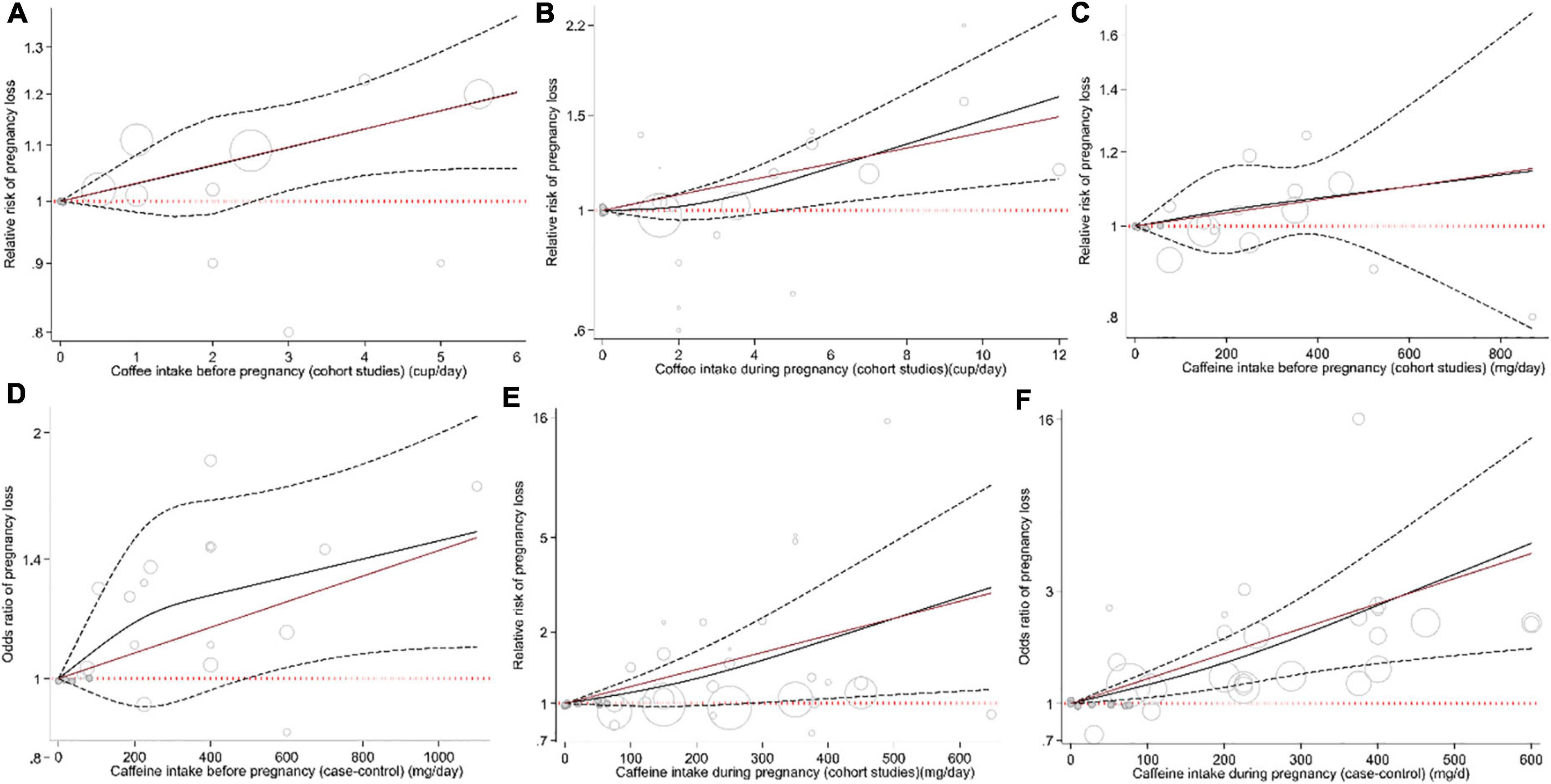
Figure 4. Non-linear dose–response association between the risk of pregnancy loss and intake of coffee before pregnancy in cohort studies (A), intake of coffee during pregnancy in cohort studies (B), intake of caffeine before pregnancy in cohort studies (C), intake of caffeine before pregnancy in case-control studies (D), intake of caffeine during pregnancy in cohort studies (E), and intake of caffeine during pregnancy in case-control studies (F).
To test the robustness of the findings, we conducted subgroup analyses to find possible sources of heterogeneity. These analyses were performed based by controlling for history of pregnancy loss, BMI, alcohol consumption, smoking, education, marital status, employment, nausea, race, and vitamin intake. Supplementary Figures 2–8 present findings for the different subgroups. A non-significant association was seen between coffee consumption before pregnancy and the risk of pregnancy loss in studies that controlled for education but did not control for the history of pregnancy loss (Supplementary Figure 2). A significant positive association was seen between coffee consumption during pregnancy and the risk of pregnancy loss among studies that controlled for pregnancy loss history, smoking, employment, and studies that did not control for BMI, education, nausea, and vitamin intake (Supplementary Figure 3). In terms of coffee intake during pregnancy and the risk of pregnancy loss, a significant positive risk was seen in case-control studies that did not control for education and nausea (Supplementary Figure 4). A non-significant association was observed between caffeine consumption before pregnancy and the risk of pregnancy loss in cohort studies that controlled for alcohol, marital status, BMI, education, nausea, race, and vitamin intake and did not control for pregnancy loss history, smoking, alcohol, marital status, employment, and race (Supplementary Figure 5). A significant positive association was seen between caffeine consumption before pregnancy and the risk of pregnancy loss in case-control studies among those that controlled for smoking, alcohol, employment, education, and studies that did not control for marital status and nausea (Supplementary Figure 6). In terms of caffeine intake during pregnancy and the risk of pregnancy loss in cohort studies, a non-significant positive association was seen in case-control studies that did control for education, nausea, and vitamin intake (Supplementary Figure 7). A non-significant association was seen between caffeine consumption during pregnancy and the risk of pregnancy loss in case-control studies that controlled for marital status (Supplementary Figure 8).
Regarding the significant positive association between caffeine intake and the risk of pregnancy loss, findings from the sensitivity analyses indicated that this association was dependent on particular studies. For example, exclusion of studies by Gaskins et al. (Supplementary Figure 9a), Wen et al., Giannelli et al., and Maconochie et al. (Supplementary Figure 9e) resulted in a non-significant association between caffeine intake and pregnancy loss. When we excluded the study by Savitz et al., pooled effect estimates resulted in a significant association (Supplementary Figure 9d).
Findings from another sensitivity analysis revealed that excluding any single study from the analysis did not appreciably alter the pooled effect sizes (Supplementary Figures 9b,c,f,g). No publication bias was found based on Egger’s regression asymmetry test (Supplementary Figures 10, 11). In terms of caffeine intake during pregnancy and pregnancy loss, Egger’s linear regression test indicated some degree of publication bias; however, the trim and fill methods’ application did not change the average effect size, further suggesting that results were not affected by publication bias. Three missing studies were imputed in regions of the contour-enhanced funnel plots to adjust for asymmetry (Supplementary Figure 9a).
In summary, these meta-analysis results show pregnancy loss was associated with coffee intake before and during pregnancy, with caffeine during pregnancy, but not with caffeine intake before pregnancy. Based on a linear dose–response analysis, increased intake of one cup of coffee during pregnancy was associated with 3% increased risk of pregnancy loss. Likewise, increased intake of 100 mg of caffeine per day during pregnancy increased the risk of pregnancy loss by 14 and 26% based on cohort studies and case-control studies, respectively.
In this study, we found that higher coffee intake before pregnancy was associated with an increased risk of pregnancy loss, which is consistent with the cohort study by Gaskins et al. that showed that higher coffee consumption before pregnancy could increase the risk of miscarriage (RR = 1.21; 95% CI: 1.07–1.37, P = 0.002) and that consuming ≥4 servings/day had 20% increased risk of SAB (20). However, Savitz et al. observed a null relationship between coffee consumption before pregnancy and the risk of miscarriage (18).
Our findings revealed that increased coffee consumption during pregnancy was related to an increase in the risk of pregnancy loss in both cohort and case-control studies (8). However, Morales et al.’s cohort study found no significant association (8). Our results are consistent with the meta-analysis study by Li et al., who discovered a significant positive association between coffee intake and the risk of pregnancy loss (RR = 1.31; 95% CI: 1.15–1.50, P < 0.001) but not for low (<2 cups) and moderate (2–3 cup) consumption in subgroup analyses (22). Likewise, in both meta-analyses, no significant heterogeneity was observed. On the contrary, Savitz et al.’s cohort study showed no association between coffee consumption during pregnancy and pregnancy outcomes (18). Although the harmful effects of coffee on pregnancy loss appear to be due to caffeine, it should be noted that in the case of low coffee intake, other coffee compounds such as amino acids, carbohydrates, vitamins, and minerals can reduce caffeine’s harmful effects (8). Our results showed that an increment of one cup of coffee was correlated with 3% increased risk of pregnancy loss. Similar to our results, Li et al. revealed that every increase of two cups of coffee was associated with a 3% increase in the risk of pregnancy loss (22).
With regard to caffeine, we found no significant association between its intake before pregnancy and the risk of pregnancy loss in either cohort or case-control studies. In line with our study, in the studies by Gaskins et al. and Tolstrup et al., caffeine consumption before pregnancy increased the risk of miscarriage (20, 52). Regarding the dose–response analysis, we found no significant association between caffeine intake and the risk of pregnancy loss. Consistent with our study, Gaskins et al.’s study showed no evidence of a non-linear association (P = 0.06) (20). Our inclusion of both cohort and case-control studies, more recent studies (than in prior reviews), and no evidence of publication bias may have led to the differences between studies.
We found a positive association between caffeine consumption during pregnancy and the risk of pregnancy loss. Our findings are in agreement with several past studies (2, 16, 21). Li et al. found that caffeine consumption was associated with the risk of pregnancy loss (22). Both meta-analyses by Greenwood et al. and Li et al. included cohort and case-control studies (21, 22). On the contrary, we performed subgroup and sensitivity analysis to reduce heterogeneity. Additionally, 100 mg of additional caffeine per day during pregnancy was associated with an increased risk of pregnancy loss by 14%, which is in agreement with the findings of Greenwood et al. On the contrary, Greenwood et al. observed a non-linear relationship between caffeine consumption and abortion risk (21). Conversely, caffeine intake during pregnancy was not related to abortion risk in two cohort studies (18, 66); this may be because of the small sample sizes and/or because of including only miscarriage.
Smoking is related to caffeine intake and is a known risk factor for pregnancy loss. Therefore, smoking is a potentially important confounder of the association between caffeine intake and pregnancy loss. Pregnancy symptoms (including nausea, vomiting, and aversions to smells and tastes) are more common in healthy pregnancies than in pregnancies that end in spontaneous abortion. Women with viable pregnancies may be more likely to reduce their caffeine consumption in response to these symptoms during pregnancy. Symptoms during pregnancy can affect the interpretation of the relationship between caffeine and pregnancy loss.
As mentioned, the main component of coffee is caffeine, which has several effects on the human body, especially pronounced during the pregnancy period. Caffeine can increase catecholamine secretion and reduce uterine and placental blood flow due to its vasoconstrictive effects, resulting in fetal hypoxia and growth and developmental defects. Moreover, caffeine can directly affect the fetal cardiovascular function and initiate tachycardia (2). However, some studies did not emphasize the harm of caffeine consumption during pregnancy, especially for pregnancy loss (18). Despite such contradictory findings, caffeine restriction may help inform this debate; however, in a systematic review of RCTs, Jahanfar et al. did not conclude that caffeine restriction had an effect on pregnancy outcomes (67). Caffeine consumption can reduce nausea and discomfort in pregnancy by lowering estrogen levels in the blood, but lowering blood estrogen levels can increase the risk of miscarriage. However, several factors can affect the relationship between caffeine consumption and the risk of pregnancy loss, nausea severity, and the amount of caffeine consumption for reducing nausea (59). Another important confounder between caffeine consumption and the risk of pregnancy loss is smoking, because smoking is associated with coffee consumption (2). In addition, other factors like circulating caffeine levels and their metabolites, genetic differences in caffeine metabolism, and different lifestyles may confound the relationship between caffeine consumption and the risk of pregnancy loss (67).
Our study is a comprehensive and up-to-date meta-analysis that investigated the association of maternal coffee and caffeine with the risk of pregnancy loss. Also, we used the one-stage weighted effect method. There are several strengths of our study. We tried to reduce the effects of confounding, searched databases without language restrictions, and included a large number of studies by modulating the effects of confounders in the meta-analysis. Additionally, we evaluated coffee and caffeine consumption separately and evaluated time points during and before pregnancy. Moreover, we conducted a one-stage dose–response analysis to determine linear and non-linear relationships between the variables, performed a subgroup analysis to eliminate the possible effects of confounders (such as BMI, alcohol consumption, smoking, education, and taking vitamins), and performed Egger’s asymmetry test to assess the effect of publication bias.
The limitations of this study include potential measurement error of caffeine intake in the primary studies, the use of case-control studies, the possibility of recall and selection bias, and evaluation of coffee consumption as a source of caffeine. Regarding the measurement error of caffeine intake, the studies examined in our meta-analysis used interviews and self-report as well as a food frequency questionnaire (FFQ), which are accurate and reliable (68).
This meta-analysis suggests that increased coffee and caffeine consumption in pregnancy may be associated with an increased risk of pregnancy loss. More research is needed to explore the underlying mechanisms and active compounds in coffee and caffeine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AJ and MA designed the study and independently carried out the literature search and screening of manuscripts. SN analyzed the data. AJ and HS assessed risk of bias and quality of evidence. AJ, SN, SS, and FK wrote the manuscript. PS edited the English and commented on the manuscript. LA supervised and revised the study. All authors have read and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.886224/full#supplementary-material
1. Feresu SA, Harlow SD, Welch K, Gillespie BW. Incidence of and socio-demographic risk factors for stillbirth, preterm birth and low birthweight among Zimbabwean women. Paediatr Perinatal Epidemiol. (2004) 18:154–63. doi: 10.1111/j.1365-3016.2003.00539.x
2. Chen LW, Wu Y, Neelakantan N, Chong MF, Pan A, van Dam RM. Maternal caffeine intake during pregnancy and risk of pregnancy loss: a categorical and dose-response meta-analysis of prospective studies. Public Health Nutr. (2016) 19:1233–44. doi: 10.1017/s1368980015002463
3. Robinson GE. Pregnancy loss. Best Pract Res Clin Obstet Gynaecol. (2014) 28:169–78. doi: 10.1016/j.bpobgyn.2013.08.012
5. Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. (2016) 4:e98–108. doi: 10.1016/s2214-109x(15)00275-2
6. Matijasevich A, Barros FC, Santos IS, Yemini A. Maternal caffeine consumption and fetal death: a case-control study in Uruguay. Paediatr Perinat Epidemiol. (2006) 20:100–9. doi: 10.1111/j.1365-3016.2006.00706.x
7. Fenster L, Hubbard AE, Swan SH, Windham GC, Waller K, Hiatt RA, et al. Caffeinated beverages, decaffeinated coffee, and spontaneous abortion. Epidemiology. (1997) 8:515–23. doi: 10.1097/00001648-199709000-00008
8. Morales-Suárez-Varela M, Nohr EA, Olsen J, Bech BH. Potential combined effects of maternal smoking and coffee intake on foetal death within the Danish National Birth Cohort. Eur J Public Health. (2018) 28:315–20. doi: 10.1093/eurpub/ckx222
9. Hoyt AT, Browne M, Richardson S, Romitti P, Druschel C. Maternal caffeine consumption and small for gestational age births: results from a population-based case-control study. Matern Child Health J. (2014) 18:1540–51. doi: 10.1007/s10995-013-1397-4
10. Gleason JL, Tekola-Ayele F, Sundaram R, Hinkle SN, Vafai Y, Buck Louis GM, et al. Association between maternal caffeine consumption and metabolism and neonatal anthropometry: a secondary analysis of the NICHD fetal growth studies–singletons. JAMA Network Open. (2021) 4:e213238.
11. Acog. CommitteeOpinion No. 462: moderate caffeine consumption during pregnancy. Obstet Gynecol. (2010) 116:467–8. doi: 10.1097/AOG.0b013e3181eeb2a1
12. James JE. Maternal caffeine consumption and pregnancy outcomes: a narrative review with implications for advice to mothers and mothers-to-be. BMJ Evid Based Med. (2021) 26:114–5. doi: 10.1136/bmjebm-2020-111432
13. Bech BH, Nohr EA, Vaeth M, Henriksen TB, Olsen J. Coffee and fetal death: a cohort study with prospective data. Am J Epidemiol. (2005) 162:983–90. doi: 10.1093/aje/kwi317
14. Wierzejska R, Jarosz M, Wojda B. Caffeine intake during pregnancy and neonatal anthropometric parameters. Nutrients. (2019) 11:11040806. doi: 10.3390/nu11040806
15. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. (2008) 198:.e1–8.
16. Hahn KA, Wise LA, Rothman KJ, Mikkelsen EM, Brogly SB, Sørensen HT, et al. Caffeine and caffeinated beverage consumption and risk of spontaneous abortion. Hum Reprod. (2015) 30:1246–55. doi: 10.1093/humrep/dev063
17. Wisborg K, Kesmodel U, Bech BH, Hedegaard M, Henriksen TB. Maternal consumption of coffee during pregnancy and stillbirth and infant death in first year of life: prospective study. BMJ. (2003) 326:420. doi: 10.1136/bmj.326.7386.420
18. Savitz DA, Chan RL, Herring AH, Howards PP, Hartmann KE. Caffeine and miscarriage risk. Epidemiology. (2008) 19:55–62. doi: 10.1097/EDE.0b013e31815c09b9
19. Mills JL, Holmes LB, Aarons JH, Simpson JL, Brown ZA, Jovanovic-Peterson LG, et al. Moderate caffeine use and the risk of spontaneous abortion and intrauterine growth retardation. JAMA. (1993) 269:593–7. doi: 10.1001/jama.1993.03500050071028
20. Gaskins AJ, Rich-Edwards JW, Williams PL, Toth TL, Missmer SA, Chavarro JE. Pre-pregnancy caffeine and caffeinated beverage intake and risk of spontaneous abortion. Eur J Nutr. (2018) 57:107–17. doi: 10.1007/s00394-016-1301-2
21. Greenwood DC, Thatcher NJ, Ye J, Garrard L, Keogh G, King LG, et al. Caffeine intake during pregnancy and adverse birth outcomes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. (2014) 29:725–34. doi: 10.1007/s10654-014-9944-x
22. Li J, Zhao H, Song JM, Zhang J, Tang YL, Xin CM. A meta-analysis of risk of pregnancy loss and caffeine and coffee consumption during pregnancy. Int J Gynaecol Obstet. (2015) 130:116–22. doi: 10.1016/j.ijgo.2015.03.033
23. Axelsson G, Rylander R, Molin I. Outcome of pregnancy in relation to irregular and inconvenient work schedules. Br J Ind Med. (1989) 46:393–8. doi: 10.1136/oem.46.6.393
24. Parazzini F, Bocciolone L, Fedele L, Negri E, La Vecchia C, Acaia B. Risk factors for spontaneous abortion. Int J Epidemiol. (1991) 20:157–61. doi: 10.1093/ije/20.1.157
25. Khoury JC, Miodovnik M, Buncher CR, Kalkwarf H, McElvy S, Khoury PR, et al. Consequences of smoking and caffeine consumption during pregnancy in women with type 1 diabetes. J Matern Fetal Neonatal Med. (2004) 15:44–50. doi: 10.1080/14767050310001650716
26. Purdue-Smithe A, Kim K, Schisterman E, Schliep K, Perkins N, Sjaarda L, et al. Caffeinated beverage intake and serum caffeine metabolites and risk of pregnancy loss (OR17-04-19). Curr Dev Nutrit. (2019) 3:19. doi: 10.1093/cdn/nzz039.OR17-04-19
27. Heazell AEP, Timms K, Scott RE, Rockliffe L, Budd J, Li M, et al. Associations between consumption of coffee and caffeinated soft drinks and late stillbirth-Findings from the Midland and North of England stillbirth case-control study. Eur J Obstet Gynecol Reprod Biol. (2021) 256:471–7. doi: 10.1016/j.ejogrb.2020.10.012
28. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Internal Med. (2009) 151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136
29. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
30. Sterne JA, Hernán MA, Reeves BC, Savoviæ J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
31. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
32. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
33. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. doi: 10.1093/aje/kwr265
34. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons (2019).
35. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
36. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
37. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. (2008) 61:991–6. doi: 10.1016/j.jclinepi.2007.11.010
38. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
39. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
40. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. (2006) 6:40–57.
41. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. (2019) 28:1579–96. doi: 10.1177/0962280218773122
42. Alomar MJ. Evaluation of caffeine consumption and effect during pregnancy among women in the UAE. Int J Pharm Pharmaceut Sci. (2016) 8:101–3.
43. Little RE, Weinberg CR. Risk-factors for antepartum and intrapartum stillbirth. Am J Epidemiol. (1993) 137:1177–89. doi: 10.1093/oxfordjournals.aje.a116620
44. Andersen AM, Andersen PK, Olsen J, Grønbæk M, Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. Int J Epidemiol. (2012) 41:405–13. doi: 10.1093/ije/dyr189
45. Buck Louis GM, Sapra KJ, Schisterman EF, Lynch CD, Maisog JM, Grantz KL, et al. Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: the LIFE Study. Fertil Steril. (2016) 106:180–8. doi: 10.1016/j.fertnstert.2016.03.009
46. Kline J, Levin B, Silverman J, Kinney A, Stein Z, Susser M, et al. Caffeine and spontaneous abortion of known karyotype. Epidemiology. (1991) 2:409–17. doi: 10.1097/00001648-199111000-00004
47. Nonaka T, Takakuwa K, Tanaka K. Analysis of the polymorphisms of genes coding biotransformation enzymes in recurrent miscarriage in the Japanese population. J Obstet Gynaecol Res. (2011) 37:1352–8. doi: 10.1111/j.1447-0756.2011.01529.x
48. Setti AS, Braga DPDAF, Halpern G, Figueira RDCS, Iaconelli A Jr., Borges E Jr. Is there an association between artificial sweetener consumption and assisted reproduction outcomes? Reproduct BioMed Online. (2018) 36:145–53. doi: 10.1016/j.rbmo.2017.11.004
49. Srisuphan W, Bracken MB. Caffeine consumption during pregnancy and association with late spontaneous abortion. Am J Obstet Gynecol. (1986) 154:14–20. doi: 10.1016/0002-9378(86)90385-6
50. Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am J Epidemiol. (2001) 154:322–7. doi: 10.1093/aje/154.4.322
51. Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. (2003) 82:182–8. doi: 10.1034/j.1600-0412.2003.00078.x
52. Tolstrup JS, Kjaer SK, Munk C, Madsen LB, Ottesen B, Bergholt T, et al. Does caffeine and alcohol intake before pregnancy predict the occurrence of spontaneous abortion? Hum Reprod. (2003) 18:2704–10. doi: 10.1093/humrep/deg480
53. Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health. (1992) 82:85–7. doi: 10.2105/ajph.82.1.85
54. Wilcox AJ, Weinberg CR, Baird DD. Risk factors for early pregnancy loss. Epidemiology. (1990) 1:382–5. doi: 10.1097/00001648-199009000-00008
55. al-Ansary LA, Babay ZA. Risk factors for spontaneous abortion: a preliminary study on Saudi women. J R Soc Health. (1994) 114:188–93. doi: 10.1177/146642409411400403
56. Domínguez-Rojas V, de Juanes-Pardo JR, Astasio-Arbiza P, Ortega-Molina P, Gordillo-Florencio E. Spontaneous abortion in a hospital population: are tobacco and coffee intake risk factors? Eur J Epidemiol. (1994) 10:665–8. doi: 10.1007/bf01719278
57. Dlugosz L, Belanger K, Hellenbrand K, Holford TR, Leaderer B, Bracken MB. Maternal caffeine consumption and spontaneous abortion: a prospective cohort study. Epidemiology. (1996) 7:250–5. doi: 10.1097/00001648-199605000-00006
58. Cnattingius S, Signorello LB, Annerén G, Clausson B, Ekbom A, Ljunger E, et al. Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med. (2000) 343:1839–45. doi: 10.1056/nejm200012213432503
59. Wen W, Shu XO, Jacobs DR Jr., Brown JE. The associations of maternal caffeine consumption and nausea with spontaneous abortion. Epidemiology. (2001) 12:38–42. doi: 10.1097/00001648-200101000-00008
60. Giannelli M, Doyle P, Roman E, Pelerin M, Hermon C. The effect of caffeine consumption and nausea on the risk of miscarriage. Paediatr Perinatal Epidemiol. (2003) 17:316–23. doi: 10.1046/j.1365-3016.2003.00523.x
61. Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage–results from a UK-population-based case-control study. BJOG. (2007) 114:170–86. doi: 10.1111/j.1471-0528.2006.01193.x
62. Agnesi R, Valentini F, Fedeli U, Rylander R, Meneghetti M, Fadda E, et al. Maternal exposures and risk of spontaneous abortion before and after a community oriented health education campaign. Eur J Public Health. (2011) 21:282–5. doi: 10.1093/eurpub/ckq073
63. Stefanidou EM, Caramellino L, Patriarca A, Menato G. Maternal caffeine consumption and sine causa recurrent miscarriage. Eur J Obstet Gynecol Reprod Biol. (2011) 158:220–4. doi: 10.1016/j.ejogrb.2011.04.024
64. Infante Rivard C, Fernández A, Gauthier R, David M, Rivard GE. Fetal loss associated with caffeine intake before and during pregnancy. JAMA. (1993) 270:2940–3. doi: 10.1001/jama.1993.03510240052031
65. Greenwood DC, Alwan N, Boylan S, Cade JE, Charvill J, Chipps KC, et al. Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol. (2010) 25:275–80. doi: 10.1007/s10654-010-9443-7
66. Pollack AZ, Buck Louis GM, Sundaram R, Lum KJ. Caffeine consumption and miscarriage: a prospective cohort study. Fertil Steril. (2010) 93:304–6. doi: 10.1016/j.fertnstert.2009.07.992
67. Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochr Datab Syst Rev. (2015) 2015:Cd006965. doi: 10.1002/14651858.CD006965.pub4
68. Grosso LM, Triche E, Benowitz NL, Bracken MB. Prenatal caffeine assessment: fetal and maternal biomarkers or self-reported intake? Ann Epidemiol. (2008) 18:172–8. doi: 10.1016/j.annepidem.2007.11.005
69. Fenster L, Eskenazi B, Windham GC, Swan SH. Caffeine consumption during pregnancy and spontaneous abortion. Epidemiology. (1991) 2:168–74. doi: 10.1097/00001648-199105000-00002
70. Parazzini F, Chatenoud L, Di Cintio E, Mezzopane R, Surace M, Zanconato G, et al. Coffee consumption and risk of hospitalized miscarriage before 12 weeks of gestation. Hum Reprod. (1998) 13:2286–91. doi: 10.1093/humrep/13.8.2286
Keywords: caffeine, coffee, risk, meta-analysis, pregnancy loss
Citation: Jafari A, Naghshi S, Shahinfar H, Salehi SO, Kiany F, Askari M, Surkan PJ and Azadbakht L (2022) Relationship between maternal caffeine and coffee intake and pregnancy loss: A grading of recommendations assessment, development, and evaluation-assessed, dose-response meta-analysis of observational studies. Front. Nutr. 9:886224. doi: 10.3389/fnut.2022.886224
Received: 28 February 2022; Accepted: 04 July 2022;
Published: 09 August 2022.
Edited by:
Arindam Basu, University of Canterbury, New ZealandReviewed by:
Hadith Tangestani, Bushehr University of Medical Sciences, IranCopyright © 2022 Jafari, Naghshi, Shahinfar, Salehi, Kiany, Askari, Surkan and Azadbakht. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leila Azadbakht, YXphZGJha2h0bGVpbGFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.