
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 14 July 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.885412
This article is part of the Research TopicPhytochemical Changes in Vegetables During Post-harvest Storage and Processing, and Implications for Consumer BenefitsView all 17 articles
 Kazutoshi Shindo1,2*†
Kazutoshi Shindo1,2*† Yuka Sakemi1†
Yuka Sakemi1† Saki Shimode1
Saki Shimode1 Chiharu Takagi1
Chiharu Takagi1 Yohei Uwagaki3
Yohei Uwagaki3 Jun-ichiro Hattan3
Jun-ichiro Hattan3 Miu Akao1
Miu Akao1 Shiori Usui1
Shiori Usui1 Ayako Kiyokawa4
Ayako Kiyokawa4 Masako Komaki4
Masako Komaki4 Minoru Murahama4
Minoru Murahama4 Miho Takemura3
Miho Takemura3 Isamu Ishikawa2
Isamu Ishikawa2 Norihiko Misawa2,3*
Norihiko Misawa2,3*Crocetin glycosides such as crocin are noted as functional food materials since the preventive effects of crocin have been reported against chronic disease and cancer. However, it is unclear how these apocarotenoids are structurally changed through cooking for our intake. We examined such changes in crocetin glycosides (crocin, tricrocin, and crocin-3) contained in saffron (stigmas of Crocus sativus) through cooking models. These glycosides were almost kept stable in boiling for 20 min (a boiled cooking model), while hydrolysis of the ester linkage between glucose and the crocetin aglycone occurred in a grilled cooking model (180°C, 5 min), along with a 13-cis isomerization reaction in a part of crocetin subsequently generated. We further here revealed that the yellow petals of freesia (Freesia x hybrida) with yellow flowers accumulate two unique crocetin glycosides, which were identified to be crocetin (mono)neapolitanosyl ester and crocetin dineapolitanosyl ester. A similar result as above was obtained on their changes through the cooking models. Utility applications of the freesia flowers as edible flowers are also suggested in this study. Additionally, we evaluated singlet oxygen (1O2)-quenching activities of the crocetin glycosides contained in saffron and freesia, and crocetin and 13-cis crocetin contained in the grilled saffron, indicating that they possessed moderate 1O2-quenching activities (IC50 24–64 μM).
Crocetin is an apocarotenoid composed of 20 carbons, and its digentiobiosyl ester is called crocin, which is the major pigment contained in the dried red stigmas of saffron plants (Crocus sativus L.) and gardenia fruits (Gardenia jasminoides Ellis), which belong to the Iridaceae and Rubiaceae families, respectively (1). Along with crocin, these plant organs have been reported to accompany several minor components of crocetin glycosides, i.e., crocetin (mono)glucosyl ester, crocin-3 [crocetin (mono)gentiobiosyl ester], tricrocin (crocetin gentiobiosyl glucosyl ester), and crocetin gentiobiosyl neapolitanosyl ester (1, 2). The biosynthetic pathways of these apocarotenoids are shown in Figure 1A (3, 4). Saffron is an expensive spice, which has widely been used in cooking as a water-soluble yellow colorant with a distinctive flavor (5). Crocin and relevant glycosides are noted as functional food materials since the preventive effects of crocin have been reported against chronic diseases such as diabetes, fatty liver, and autoimmune diseases, and cancer such as colon cancer (6–9). It is however unclear how crocin and the relevant glycosides are structurally changed through cooking (boiling and grilling) for our intake, although changes of carotenoids that are contained in maize and vegetables through cooking were examined in a few reports (10, 11). In this study, such a change is examined on saffron. In addition, we evaluate singlet oxygen (1O2)-quenching activities of the generated compounds.
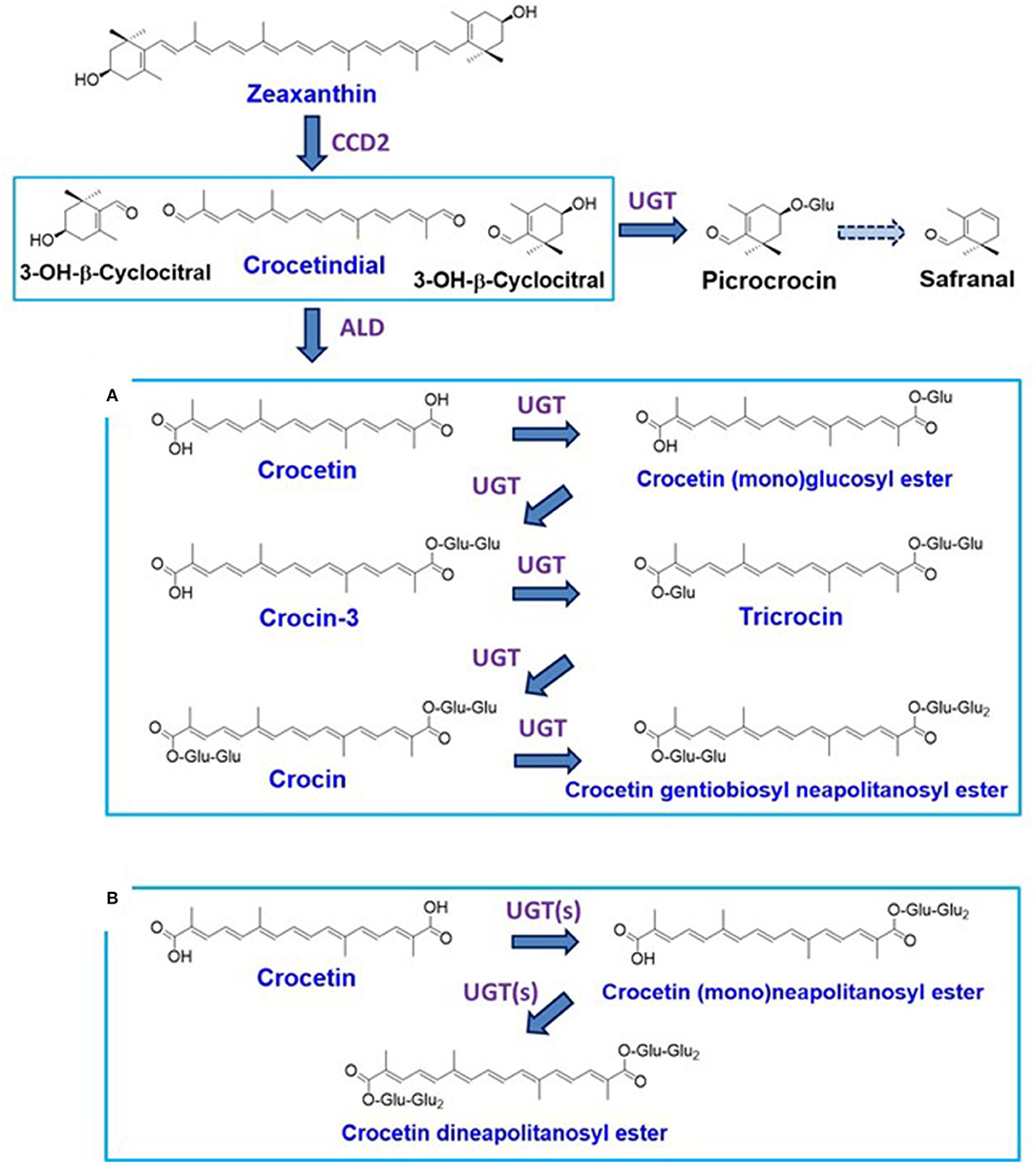
Figure 1. Carotenoid metabolism in saffron [stigmas of Crocus sativus; (A)] and the yellow flowers of freesia [Freesia x hybrida; (B)].
Freesia (Freesia x hybrida; the Iridaceae family) is a popular flowering plant of South African (the Cape Province area) origin as the cut flowers (12). Many cultivars of freesia plants, whose flowers retain a variety of colors, shapes, and fragrances, have been generated up to the present (13). In Japan, freesia cultivars with yellow flowers maintain an 80% share of the market. Among them, “Aladin” (Figure 2A), which retains big yellow flowers, has been the most cultivated freesia cultivar since the 1990s. Cultivar “Ishikawa f2 go” (“f2”; Figure 2B) was established by crossing cultivars, “Aladin” and “Rapid Yellow” that retains a characteristic suitable for early forcing, and by selecting one seedling among consequently generated 222 seedlings (14). This study reveals that yellow pigments in the yellow petals of freesia [cultivars “f2” and “Aladin” in addition to cultivar “Kayak” (Figure 2C)] are unique apocarotenoids, crocetin (mono)neapolitanosyl ester and crocetin dineapolitanosyl ester (Figure 1B). Plants that produce such apocarotenoids have not been reported so far. Moreover, this is the first report on plants that include the (mono)neapolitanosyl ester. According to Edible Flowers Guide by Thompson & Morgan (https://www.thompson-morgan.com/edible-flowers), freesia is positioned as an unusual edible flower, and described to be “Infused in a tisane with lemon juice and zest. The peppery scent and bold color are a perfect pick-me-up.” Utility applications of the freesia flowers as edible flowers are also suggested in this study.
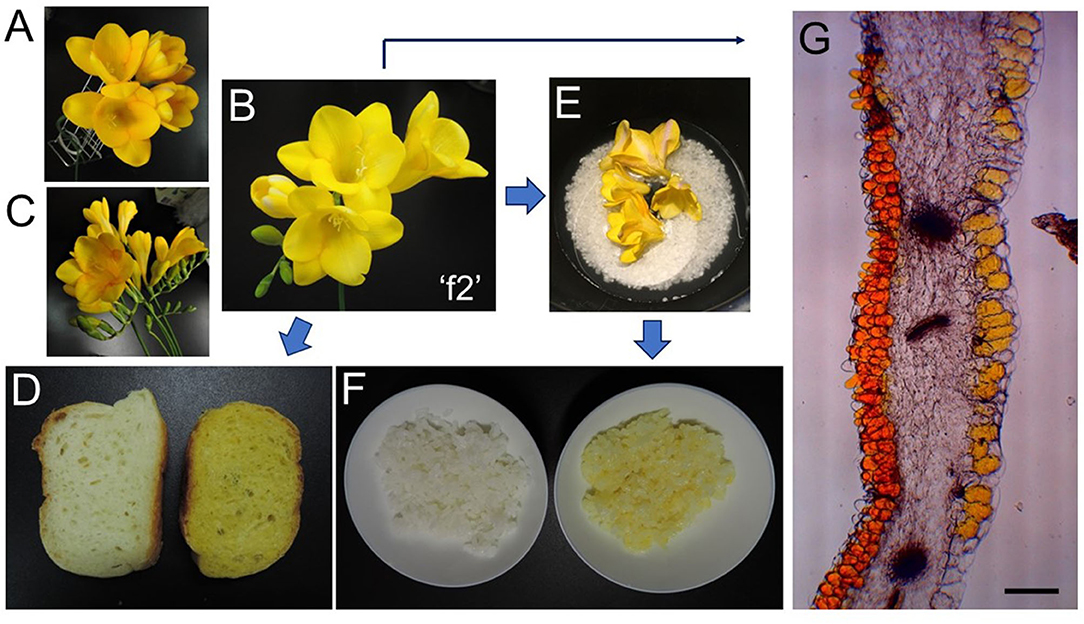
Figure 2. Freesia-relevant pictures: (A), freesia (Freesia x hybrida L.) cultivar “Aladin;” (B), freesia cultivar “Ishikawa f2 go” (“f2”); (C), freesia cultivar “Kayak;” (D), bread baked without (left) and with (right) freesia “f2” flowers; (E), before cooking with freesia “f2” flowers by a rice cooker; (F), rice cooked without (left) and with (right) freesia “f2” flowers; (G), microscopy image of a freesia “f2” petal (Bar represents 200 μm).
Dried red pistils containing stigmas (called “saffron”) from saffron plants (Crocus sativus L.) that had been cultivated in Spain were purchased at a spice-special store in Tokyo, Japan. As for freesia (Freesia x hybrida), we used cultivars “Ishikawa f2 go” (“f2”), “Kayak,” and “Aradin.” Cultivars “f2” and “Kayak” were grown in a greenhouse, and their flowers were harvested in middle and late March, respectively. Cultivar “Aladin” was purchased at a floral shop in Ibaragi, Japan.
Microscopy pictures of the “f2” petal were recorded by BH2 (Olympus) after cutting it in 3% agar with MicroSlicer ZERO-1 (Dosaka EM, Kyoto, Japan).
Dichloromethane (CH2Cl2), methanol (MeOH), acetonitrile (CH3CN), ethyl acetate (EtOAc), and chloroform of analytical grade were purchased from Wako Pure Chemicals Industries, Ltd. (Osaka, Japan) or Kanto Chemical Co. Inc. (Tokyo, Japan). The other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, United States).
In order to detect volatile components of saffron and freesia “f2” flowers, we carried out a gas chromatography-mass spectrometry (GC-MS) analysis. Each 0.1 g of their dried materials was ground with an addition of 0.3 ml of water (H2O) and 0.3 ml of MeOH, subsequently extracted with 0.6 ml of chloroform (by mixing and centrifugation), and the chloroform extraction was repeated by adding 0.5 ml of H2O, and finally, each chloroform phase was concentrated to threefold by decompression. The prepared samples were subjected to GC-MS using Shimadzu GCMS-QP5050 (Shimadzu, Kyoto, Japan), as described (15).
Nuclear magnetic resonance (NMR) spectra (1H and 13C NMR, 1H-1H DQF COSY, HMQC, HMBC, and NOESY) were measured using an AVANCE 400 MHz NMR spectrometer (Bruker, Rheinstettern, Germany) utilizing standard programs in TopSpin1.3. Chemical shifts were referenced to solvent signals. HR-ESI-MS (+) was measured using a JMS-T100LP mass spectrometer (JEOL, Tokyo, Japan) and accumulated mass calibration was performed using reserpine [C33H41N2O9, m/z 609.2812044 (M+H)+].
As a boiled cooking model, saffron (dried; 0.08 g) or freesia petals [1.8 g (fresh weight)] were added to boiled water (50 ml) and boiled for 20 min. As a grilled cooking model, saffron (dried; 0.08 g) or freesia petals [1.8 g (fresh weight)] were added to olive oil (7.5 ml) and grilled at 180°C for 5 min.
A paella-type cooking was adopted as an example of the boiled dishes with saffron, as follows: Saffron (0.08 g)-including water (300 ml), polished rice (500 g), and olive oil (30 ml) were put together on a frying pan, and the materials were boiled under medium heat for 20 min. A pilaf-type cooking was adopted as an example of the grilled dishes with saffron, as follows: Olive oil (30 ml), polished rice (500 g), and saffron (0.08 g) were grilled on a frying pan under medium heat for 10 min. Then, water (300 ml) was added to the frying pan and the materials were boiled for 15 min.
Rice was cooked in a rice cooker, using polished rice (Japonica; 75 g), water (90 ml), and “f2” flowers (5 g fresh weight) (Figure 2E). After cooking, the flower debris was eliminated (Figure 2F right). Bread was baked as follows: Wheat powder (100 g), water (60 ml), NaCl (1.5 g), budding yeast (powder; 1 g), and dried powder from “f2” flowers (10 g fresh weight) by microwave were mixed and left for 2 h 20 min. The material was incubated for 1 h at 37°C, and baked for 21 min at 220°C (Figure 2D right).
Each sample obtained by cooking had an addition of 100 μL of 9,10-dimethylanthracene solution (10 mg/1 ml CH2Cl2) as an internal standard to confirm the recovery rate of apocarotenoids. Each of the cooked materials as the cooking models was extracted by CH2Cl2-MeOH (1:1) (V:V) (20 ml), MeOH (20 ml), and 50% (V/V) MeOH (20 ml) in a stepwise manner by stirring for 30 min at 20°C. All the extracts (total 60 ml) were combined and concentrated to dryness in vacuo.
Each of the actually cooked materials [the paella (boiled) and pilaf (grilled) types] was freeze-dried and powdered by a mill. The powder was extracted by CH2Cl2-MeOH (1:1) (1 L), MeOH (1 L), and 50% (V/V) MeOH (1 L) in a stepwise manner by stirring for 30 min at 20°C, and all the extracts (total 3 L) were combined and concentrated to dryness in vacuo.
Each extract was dissolved into MeOH (10 ml), and 2.5 μL (saffron) or 20μL (freesia) of the supernatant was analyzed by high-performance liquid chromatography-diode array detector (HPLC-DAD) [column, PEGASIL ODS SP100 (4.6 × 150 mm, Senshu Scientific Co. Ltd., Tokyo, Japan); solvents: A 5% (V/V) CH3CN containing 20 mM phospholic acid, B 95% (V/V) CH3CN containing 20 mM phospholic acid. 0 → 5 min 100% A, 5 → 18 min 100% A → 100% B linear gradient, 18–30 min 100% B, flow rate, 3.0 ml/min; detection, DAD; monitored at 250–600 nm].
Saffron (0.22 g) was extracted for 30 min at 20°C by stirring with CH2Cl2-MeOH (1:1) (V:V) (100 ml), MeOH (100 ml), and 50% (V/V) MeOH (100 ml) in a stepwise manner. CH2Cl2-MeOH (1:1) extract was concentrated to dryness to give a yellow oil, and the yellow oil was applied on a diol column chromatography (10 × 150 mm, CHROMATOREX DIOL MB 100-40/75, Fuji Silysia Chemical Ltd., Aichi, Japan) with n-hexane-CH2Cl2 (5:1) (V:V) and developed with the same solvent. The elution fractions containing yellow pigment were combined and concentrated to dryness (4.7 mg), and further purified by ODS preparative HPLC [column, Develosil C30-UG-5 (10 × 250 mm, Nomura Chemical Co. Ltd., Aichi, Japan); solvent, CH2Cl2-CH3CN (95:5) (V:V); flow rate, 3.0 ml/min; detection, DAD; monitored at 250–600 nm]. The peak eluted at 9.5 min was collected to afford pure 4 (0.4 mg).
MeOH and 50% MeOH extracts were combined and concentrated to dryness (38.2 mg), and 1–3 in the extract were purified by ODS preparative HPLC [column, ADME ODS (10 × 250 mm, Osaka Soda Co. Ltd., Osaka, Japan); solvent, 50% (V/V) CH3CN containing 0.1% (V/V) trifluoro acetic acid (TFA); flow rate, 3.0 ml/min; detection, DAD; monitored at 250–600 nm]. In this HPLC, the mixture of 1 and 2 was eluted at 3.0 min, and 3 was eluted at 5.0 min. The peak of 3 eluted at 5.0 min was collected and concentrated in a small volume to remove CH3CN and finally freeze-dried to afford pure 3 (7.1 mg). The peak eluted at 3.0 min was also collected and freeze-dried to give yellow oil containing 1 and 2. This yellow oil containing 1 and 2 was further purified by ODS preparative HPLC [column, PEGASIL ODS SP100 (20 × 250 mm); solvent, 30% (V/V) CH3CN; flow rate, 8.0 ml/min; detection, DAD; monitored at 250–600 nm]. In this HPLC, 1 and 2 were eluted at 8.0 min (12.8 mg) and 13.0 min (11.4 mg), respectively, as pure compounds.
Saffron (0.22 g) in olive oil (10 ml) was grilled for 1 min in a frying pan with medium heat and extracted with CH2Cl2-MeOH (1:1) (V:V) (100 ml) twice. The extract (200 ml) was concentrated to dryness, added n-hexane (30 ml), and mixed well. The precipitate containing apocarotenoids was purified by ODS preparative ODS HPLC [column, Develosil C30-UG-5 (20 × 250 mm); solvent, 85% (V/V) CH3CN containing 0.1% (V/V) TFA; flow rate, 8.0 ml/min; detection, DAD; monitored at 250-600 nm]. In this HPLC, 5 and 6 were eluted at 12.0 min (3.2 mg) and 17.0 min (2.1 mg), respectively, as pure compounds.
Freesia (Freesia x hybrida) “f2” flowers (179.3 g) were freeze-dried and powdered by mill (18.6 g). This powder was extracted for 30 min by stirring with CH2Cl2-MeOH (1:1) (V:V) (1 L), MeOH (1 L), 80% (V/V) MeOH (1 L), and 50% (V/V) MeOH (1 L) in a stepwise manner.
The CH2Cl2-MeOH (1:1) and MeOH extracts were combined and concentrated to dryness in vacuo (14.50 g). This extract was dissolved in H2O (300 ml) and partitioned with EtOAc (300 ml) twice, and a water layer containing yellow pigment was collected. The water layer was concentrated to 200 ml to remove EtOAc, 1 M HCl (10 ml) was added to adjust at pH3, and adsorbed to HP-20 column chromatography (50 × 150 mm, Mitsubishi Chemical Corporation, Tokyo, Japan). The HP-20 column was washed with H2O (750 ml) and 50% (V/V) MeOH (750 ml), and the yellow compound was eluted with MeOH (750 ml). The MeOH eluate was concentrated to dryness to obtain yellow oil (1.11 g). The yellow oil was mixed with 15 ml of CH2Cl2, and the insoluble yellow sediment was collected (repeated twice). The obtained red sediment was dried (0.91 g), dissolved in 3 ml of 50% MeOH, and further purified using preparative ODS HPLC (column, Develosil C30-UG-5 (10 × 250 mm); solvent, 30% (V/V) CH3CN + 0.1% (V/V) TFA; flow rate, 3.0 ml/min; detection, DAD; monitored at 200–600 nm). The yellow compound was eluted at 17.8 min as pure compound (7) (27.1 mg).
The 80% MeOH (including compound 7 also) and 50% MeOH extracts were combined and concentrated in a small volume (300 ml) to remove MeOH and partitioned with EtOAc (300 ml) twice, and the water layer containing yellow pigment was collected. The water layer was concentrated to 200 ml to remove EtOAc, and adsorbed to HP-20 column chromatography (50 × 150 mm). The HP-20 column was washed with H2O (750 ml) and the yellow compound was eluted with 50% (V/V) MeOH (750 ml). The 50% MeOH eluate was concentrated to dryness to obtain yellow oil (0.75 g). The yellow oil was then purified using preparative ODS HPLC [column, Develosil C30-UG-5 (10 × 250 mm); solvent, 20% (V/V) CH3CN + 0.1% (V/V) TFA; flow rate, 3.0 ml/min; detection, DAD; monitored at 200–600 nm]. The yellow compound was eluted at 15.3 min as pure compound (8) (14.0 mg).
HR-ESI-MS (+) m/z 837.31834 (C38H54O19Na calcd. for 837.31570, Δ 3.16 ppm). UV-Vis λmax (ε) in MeOH 254 (9200), 315 (6800), 407 (43000), 428 (62000), 452 (56000). 1H NMR (CD3OD) δ: 1.96 (3H, s, H-19), 2.00 (9H, s, H-20, H-19', and H-20'), 3.15 (1H, m, H-5”'), 3.17 (1H, m, H-2”'), 3.21 (1H, m, H-2””), 3.24 (2H, H-4”” and H-5””), 3.36 (2H, H-3”' and H-3””), 3.37 (1H, m, H-4”'), 3.54 (1H, m, H-4”), 3.57 (1H, m, H-5”), 3.65 (2H, H-6”'b and H-6””b), 3.73 (1H, m, H-2”), 3.78 (1H, m, H-3”), 3.80 (1H, m, H-6”b), 3.83 (2H, H-6”'a and H-6””a), 4.15 (1H, m, H-6”a), 4.32 (1H, d, J = 7.6 Hz, H-1””), 4.59 (1H, d, J = 7.8 Hz, H-1”'), 5.65 (1H, d, J = 7.8 Hz, H-1”), 6.47 (3H, H-14, H-9', and H-14'), 6.62 (1H, m, H-11'), 6.64 (1H, m, H-11), 6.70 (1H, m, H-8'), 6.78 (1H, m, H-12), 6.80 (2H, H-15 and H-15'), 7.29 (1H, d, J = 9.1 Hz, H-10'), 7.42 (1H, d, J = 11.9 Hz, H-10). 13C NMR (CD3OD) δ: 12.7 (C-19), 12.7 (C-19'), 12.9 (C-20), 12.9 (C-20'), 62.2 (C-6”'), 62.6 (C-6””), 69.4 (C-6”), 70.6 (C-4”), 71.0 (C-4”'), 71.5 (C-4””), 75.0 (C-2””), 76.0 (C-2”'), 77.6 (C-3”), 77.6 (C-3””), 77.7 (C-5”), 77.9 (C-5”'), 77.9 (C-5””), 78.0 (C-3”'), 82.4 (C-2”), 94.6 (C-1”), 104.5 (C-1””), 105.6 (C-1”'), 124.6 (C-11), 125.0 (C-11'), 126.3 (C-9'), 127.5 (C-14'), 127.7 (C-9), 132.6 (C-12'), 133.1 (C-12), 136.7 (C-14), 137.9 (C-13), 138.2 (C-13'), 140.4 (C-10'), 142.0 (C-10), 145.0 (C-15'), 146.4 (C-15), 168.1 (C-8). 171.9 (C-8').
Methylene Blue (80 μL, 25 μM in ethanol) and linoleic acid (100 μL, 0.24 M in ethanol) were added to 5 ml glass test tubes with and without 40 μl of an apocarotenoid (final concentration, 1–100 μM in ethanol). The tubes were mixed well and then illuminated at 7,000 lux and 22°C for 3 h in a Styrofoam box, after which 50 μl of the reaction mixture was removed and diluted to 1.5 ml with ethanol. OD235 was then measured to estimate the levels of conjugated dienes formed in the reaction (16). OD235 in the absence of the carotenoid was also measured as a negative control (no 1O2-quenching activity), and the 1O2 quenching activity of each apocarotenoid was then calculated relative to this reference value. The activity was determined as the IC50 value, representing the concentration at which 50% inhibition was observed.
Figures 3A,B show the results of GC analysis of the MeOH-chloroform extracts of saffron (A) and freesia “f2” flowers (B). Peak 1, one of the main peaks in saffron, was found to exist in freesia as peak 3. MS spectra of peak 1 and peak 3 had the highest similarity to safranal in the database of the GC-MS system (Figure 3C). Peak 4 appearing in freesia, which corresponded to peak 2 in saffron, was considered 3-hydroxy-β-cyclocitral (C10H16O2; MW 168.23) by their MS spectra (C), which should be biosynthesized from zeaxanthin with carotenoid cleavage dioxygenase 2 (CCD2) (3, 17), as shown in Figure 1. It is thus likely that these two volatile compounds are present not only in saffron but also in freesia yellow flowers.
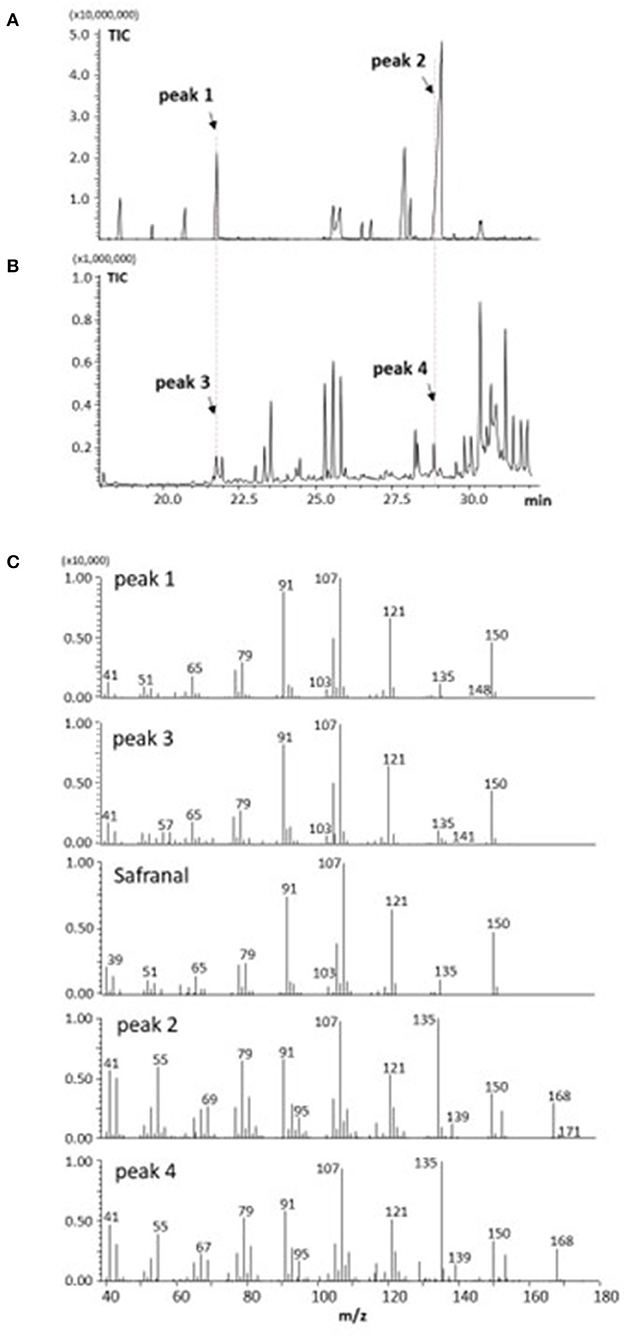
Figure 3. GC-MS analysis of volatile components from saffron (A,C) and freesia “f2” yellow-flowers (B,C).
We extracted apocarotenoids from saffron (dried; 0.08 g) as well as its boiled and grilled samples by the cooking models, and analyzed them by HPLC-DAD (Figures 4A–C). Six apocarotenoids corresponding to peaks 1–4 and peaks 5, 6 were isolated from saffron and the grilled saffron, respectively, by the methods described in Material and methods, and subjected to HR-ESI-MS, 1H and 13C NMR analyses. The results, 1–6 were identified as crocin (2), tricrocin (18), crocin-3 (18), crocetindial (19), crocetin (20), and 13-cis crocetin (21), respectively (their 1H and 13C NMR spectra of 1–6 are shown in Supplementary Figures S1–S12). The chemical structures of 1–6 are exhibited in Figure 5.
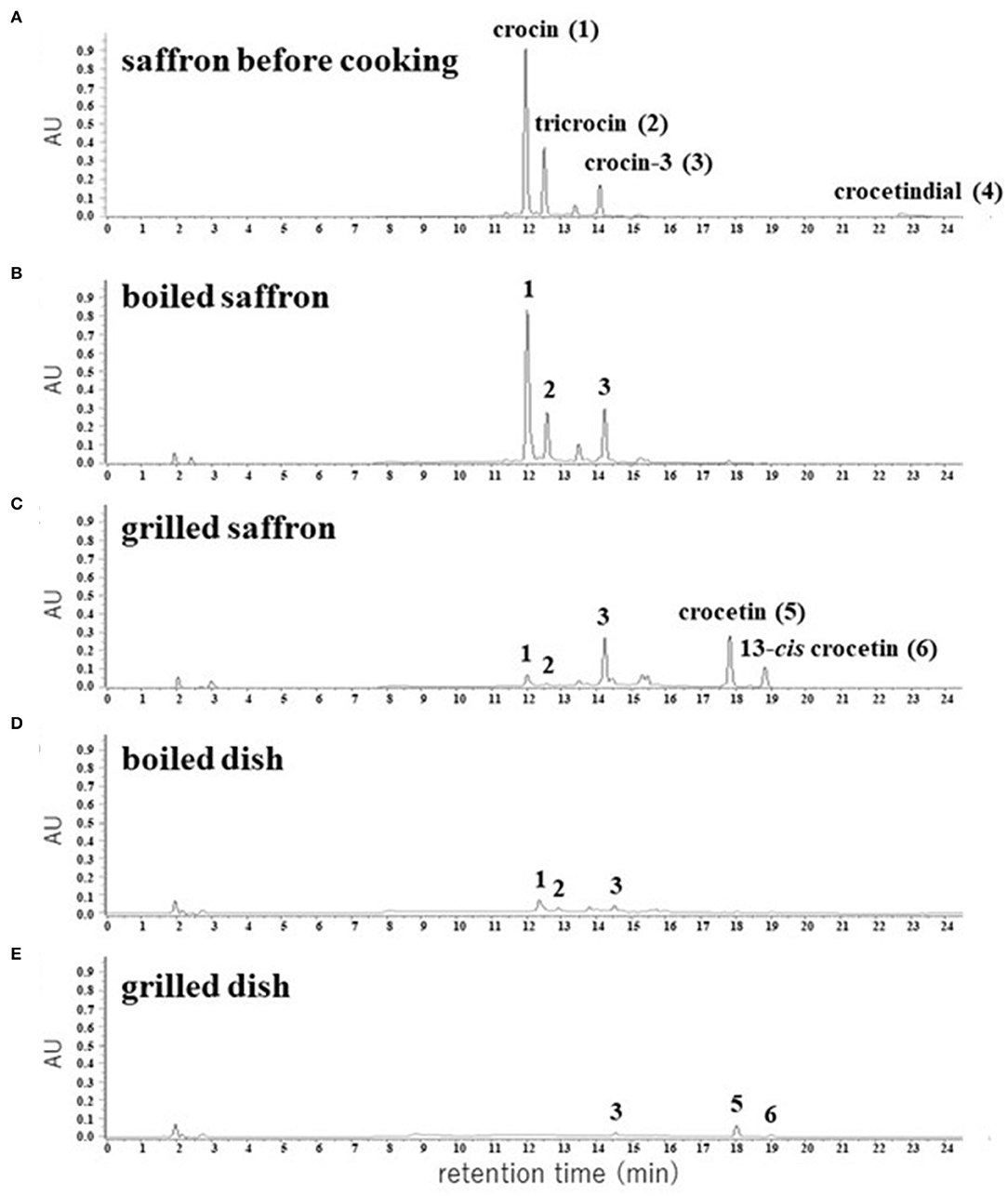
Figure 4. HPLC chromatograms of apocarotenoids extracted from the dried pistils of saffron (A) and their cooked materials (B–E). The detection wavelength was 440 nm.
The predominant apocarotenoid 1 and the other main apocarotenoids 2 and 3 showed the same UV-Vis spectra that possess λmax at 440 nm depending on the same aglycone, and minor apocarotenoids 4–6 also exhibited λmax around 440 nm (Supplementary Figure S13). Thus, we calculated the peak areas of individual apocarotenoids (1–6) and total apocarotenoids (1 + 2 + 3 + 4 + 5 + 6) at 440 nm in saffron, the boiled saffron, and the grilled saffron in HPLC-DAD analyses, as listed in Table 1. In the boiled cooking model of saffron, the amount of crocin (1), tricrocin (2), and crocin-3 (3) almost did not change, while crocetindial (4) disappeared, presumably due to its decomposition or a chemical reaction to other compounds. In the grilled cooking model of saffron, the amount of crocin (1) and tricrocin (2) decreased, the amount of crocin-3 (3) slightly increased, and crocetin (5) and 13-cis crocetin (6) appeared (Figure 4C). In addition, the total amount of apocarotenoids decreased to about 1/3 (Table 1). Supplementary Figure S18 also shows that the purified crocin (1) and crocetin (2) were partially changed into crocetin (5) and 13-cis crocetin (6), and into 6, respectively, at 180°C for 5 min. These observations indicated that hydrolysis of the ester linkage between glucose—the crocetin aglycone occurred, presumably by conversion from crocin and tricrocin to crocin-3 and further to crocetin, along with the subsequent trans to 13-cis isomerization of a part of crocetin. It is also likely that the degradation of the apocarotenoids occurred through strong heat cooking like grilling.
In order to confirm whether the same chemical changes occur in actual cooking, saffron was mixed with rice and a typical boiling and grilling cooking process to prepare dishes was performed. The apocarotenoids contained in the dishes were extracted and subjected to HPLC-DAD analyses (Figures 4D,E; Table 1). These results apparently showed that the same chemical changes occurred in the actual cooking. However, the total peak area of detected apocarotenoids in both dishes has decreased to about 1/5-−1/10. It is currently under investigation what happens to such disappearing apocarotenoids.
The apocarotenoids in freesia “f2” flowers (7 and 8) were isolated through chromatographic methods as described in Material and method.
The structures of 7 were analyzed by HR-ESI-MS and 1D (1H and 13C) and 2D (1H-1H DQF COSY, HMQC, and HMBC) NMR spectra. HR-ESI-MS analysis of 7 showed (M + Na)+ at m/z 837.31834, and the molecular formula of 7 was determined as C38H54O19 [(M + Na)+ C38H54O19Na calcd. for 837.31570 (Δ3.16 ppm)]. The molecular formula of 7 was identical to that of tricrocin, and the 1H and 13C NMR spectra of 7 in CD3OD (Supplementary Figures S13, S14) were closely related to tricrocin but not identical. The carotenoid aglycone of 7 proved to be identical to that of tricrocin (=crocetin) by comparison the 1H and 13C NMR with those of crocetin. Since three anomeric signals [δH 4.32 (J = 7.6 Hz), δH 4.59 (J = 7.8 Hz), δH 5.65 (J = 7.8 Hz)] with β configuration were observed in the 1H NMR spectrum of 7 and since the molecular formula of 7 (C38H54O19) was [crocetin (C20H24O4) + 3 hexose (C18H36O18)−3H2O], 7 was proposed to possess a structure in which 3 β-hexoses were bound to crocetin. To determine the type of hexose, 7 (5.0 mg) was hydrolyzed in 2 M HCl (5 ml) under reflux for 2 h, and the hydrolysate was partitioned with EtOAc (5 ml) twice. The H2O layer containing hexose was concentrated to dryness (2.0 mg), and analyzed by 1H and 13C NMR in D2O, and [α]D [+68.3° (c 0.1, H2O)]. These data clearly proved that hexoses in 7 were all D-glucose. The linkages of 3 β-D-glucose to crocetin in 7 were analyzed by 1H-1H DQF COSY, HSQC, and HMBC, and the linkages among glucoses were proved to be glucoseB(1 → 2)glucoseA and glucoseC(1 → 6)glucoseA and the ester linkage of glucoseA to crocetin was proved by the key 1H-13C long-range couplings observed in HMBC spectrum (from H-1”' (δH 4.59) to C-2” (δC 82.4), from H-1”” (δH 4.32) to C-6” (δ C 69.4), and from H-1” (δ H 5.65) to C-8 (δ C 168.1) as shown in Figure 6. From these observations, the structure of 7 was determined to be crocetin neapolitanosyl ester (Figure 6).
The production of 7 by artificial manufacturing processing was reported in two previous studies (22, 23), while this is the first report finding 7 in natural flowers.
HR-ESI-MS analysis of 8 showed (M-H)− at m/z 1299.47367, and the molecular formula of 8 was determined as C56H84O34 [(M-H)− C56H84O33 calcd. for 1299.47657 (Δ 2.23 ppm), and this formula was identical with that of crocetin dineapolitanosyl ester which was reported as a minor yellow pigment produced by Crocus sativus. Thus, we compared 1H and 13C NMR spectrum of 8 (Supplementary Figures S15, S16) with those of reported crocetin dineapolitanosyl ester (2), and identified 8 as crocetin dineapolitanosyl ester (Figure 6).
We extracted apocarotenoids from the yellow petals of freesia cultivar “Kayak” [1.8 g (fresh weight)] as well as their boiled and grilled samples by cooking models, and analyzed them by HPLC-DAD (Figure 7). Figure 7A (Table 2) revealed that the petals of freesia “Kayak,” like the case of “f2,” possessed the dineapolitanosyl ester and (mono)neapolitanosyl ester of crocetin dominantly, which were 48 and 52% of whole apocarotenoids when calculated by peak areas at 440 nm, respectively. In the boiled cooking model in freesia, the amount of crocetin dineapolitanosyl ester was reduced to 59% and a small amount of crocetin appeared, while the level of crocetin (mono)neapolitanosyl ester was not changed (Table 2; Figure 7B). In the grilled cooking model in freesia, the amounts of crocetin dineapolitanosyl ester and crocetin neapolitanosyl ester were reduced to 11 and 23%, respectively, and a small amount of crocetin also appeared (Table 2; Figure 7C). These results indicated that hydrolysis of the ester linkage between glucose—the crocetin aglycone occurred in both cooking models, presumably by conversion from crocetin dineapolitanosyl ester to crocetin neapolitanosyl ester and further to crocetin. The degradation of the apocarotenoids is likely to occur specifically through strong heat cooking like grilling.
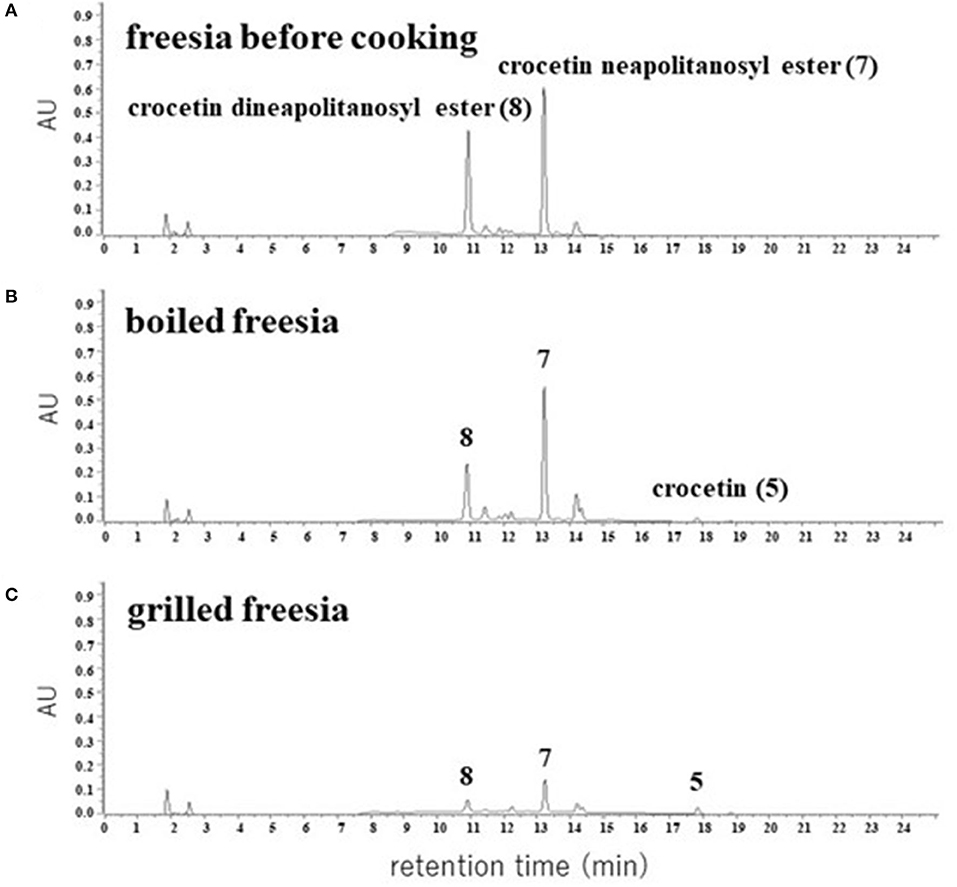
Figure 7. HPLC chromatograms of apocarotenoids extracted from the yellow petals of freesia “Kayak” (A) and their cooked materials (B,C). The detection wavelength was 440 nm.
We also extracted and analyzed apocarotenoids from the flowers of freesia cultivar “Aladin” in the same way, and found that freesia “Aladin” possessed the dineapolitanosyl ester and (mono)neapolitanosyl ester of crocetin dominantly, like “f2” and “Kayak.”
This study revealed that the main pigments in the yellow flowers of freesia (Freesia x hybrida) were unique apocarotenoids, crocetin (mono)neapolitanosyl ester, and crocetin dineapolitanosyl ester. Plants that include the (mono)neapolitanosyl ester of crocetin have not been reported so far. The stigmas of Crocus sativus (saffron) and the fruits of Gardenia jasminoides, retain crocin predominantly (1, 2). Recently, fully open mature flowers of G. jasminoides were shown to accumulate crocin-3 and crocetin as main apocarotenoids (24). These findings suggest that, among plants producing crocetin glycosides, only freesia retains UDP-glucose transferase(s) [UGT(s)] that has activity sufficient for constructing the neapolitanosyl group composed of three molecules of glucose, as shown in Figure 1B. The microscopy image of the petal of cultivar “f2” showed that such yellow apocarotenoids accumulate not in plastids (chromoplasts) but vacuoles (Figure 2G).
In addition, we demonstrated that the freesia apocarotenoids can keep yellow color after bread and rice cooking, as shown in Figures 2D,F. This study opens the feasibility of freesia yellow flowers as new edible flowers with beneficial functions for human health.
Many carotenoids were reported to possess 1O2-quenching activity depending on their olefin structures (14). Although such activity of crocin (1) was reported (25), 1O2-quenching activity of 2–8 has not been reported so far. Thus, we examined these 1O2-quenching activities and proved that 1–8 possessed almost equivalent moderate 1O2-quenching activity (IC50 24–64 μM), presumably due to their same conjugation number (=9) (Table 3). On the other hand, apocarotenoids 1–8 did not show DPPH radical scavenging activities and lipid peroxidation-inhibiting activities even at 100 μM, according to our experiment.
In this study, we first reported chemical changes of apocarotenoids in saffron and freesia through cooking models as well as identification of unique apocarotenoids in freesia flowers. We also evaluated the 1O2-quenching activities of apocarotenoids in saffron and freesia.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
This research was conceived and supervised by KS and NM. Laboratory experiments were done by KS, YS, SS, CT, MA, SU, YU, J-iH, and II. Field experiments were carried out by AK, MK, and MM. Data were analyzed by KS, J-iH, MT, and NM. Pictures were recorded by NM. The manuscript was written by KS and NM. All authors contributed to the article and approved the submitted version.
KS, II, and NM were employed by CaroProTech Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Ryusei Watanabe, Ishikawa Prefectural University, for taking the microscopy picture of freesia.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.885412/full#supplementary-material
1. Britton G, Liaaen-Jensen S, Pfander H, Carotenoids Handbook. Basel; Boston; Berlin: Birkhäuser (2004).
2. Pfister S, Meyer P, Steck A, Pfander H. Isolation and structure elucidation of carotenoid–glycosyl esters in gardenia fruits (Gardenia jasminoides Ellis) and saffron (Crocus sativus Linne). J Agr Food Chem. (1996) 44:2612–5. doi: 10.1021/jf950713e
3. Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, et al. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. P Natl Acad Sci. (2014) 111:12246–51. doi: 10.1073/pnas.1404629111
4. López-jimenez AJ, Frusciante S, Niza E, Ahrazem O, Rubio-Moraga Á, Diretto G, et al. A new glycosyltransferase enzyme from family 91, UGT91P3, is responsible for the final glucosylation step of crocins in saffron (Crocus sativus L.). Int J Mol Sci. (2021) 22:8815. doi: 10.3390/ijms22168815
5. Carmona M, Zalacain A, Salinas MR, Alonso GL. A new approach to saffron aroma. Crit Rev Food Sci. (2007) 47:145–59. doi: 10.1080/10408390600626511
6. Garc-Olmo DC, Riese HH, Escribano J, Ontan J, Fernandez JA, Atiénzar MM, Garcí-Olmo D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): an experimental study in the rat. Nutr Cancer. (1999) 35:2; 120–6. doi: 10.1207/S15327914NC352_4
7. Colapietro A, Mancini A, D'Alessandro AM, Festuccia C. Crocetin and crocin from saffron in cancer chemotheraphy and chemoprevention. Anti-cancer Agent Me. (2019) 19:38–47. doi: 10.2174/1871520619666181231112453
8. Korani S, Korani M, Sathyapalan T, Sahebkar A. Therapeutic effects of crocin in autoimmune diseases: a review. Bio Factors. (2019) 45:835–43. doi: 10.1002/biof.1557
9. Pashirzad M, Shafiee M, Avan A, Ryzhikov M, Fiuji H, Bahreyni A, et al. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J Cell Physiol. (2019) 234:14601–11. doi: 10.1002/jcp.28177
10. Zhang S, Ji J, Zhang S, Guan C, Wang G. Effects of three cooking methods on content changes and absorption efficiencies of carotenoids in maize. Food Funct. (2020) 11:944–54. doi: 10.1039/C9FO02622C
11. Miglio C, Chiavaro E, Visconti A, Fogliano V, Pellegrini N. Effect of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J Agri Food Chem. (2008) 56:139–47. doi: 10.1021/jf072304b
12. Gilbertson-Ferriss TL. Freesia × Hybrida En., Fr., Ge., Sp., Freesia. Halevy AH, editor. CRC Handbook of Flowering Vol III. Boca Raton: CRC Press (2019). p. 4.
14. Murahama M, Matsuda E, Hirano H, Isu H. Breeding of a new freesia cultivar ‘Ishikawa f2 go' (Freesia spp). Hort Res. (2020) 19:309–11. doi: 10.2503/hrj.19.309
15. Hattan J, Shindo K, Sasaki T, Ohno F, Tokuda H, Ishikawa K, et al. Identification of novel sesquiterpene synthase genes that mediate the biosynthesis of valerianol, which was an unknown ingredient of tea. Sci Rep. (2018) 8:12474. doi: 10.1038/s41598-018-30653-w
16. Hirayama O, Nakamura K, Hamada S, Kobayashi Y. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids. (1994) 29:149–50. doi: 10.1007/BF02537155
17. Fang Q, Li Y, Liu B, Meng X, Yang Z, Yang S et al. Cloning and functional characterization of a carotenoid cleavage dioxygenase 2 gene in safranal and crocin biosynthesis from Freesia hybrida. Plant Physiol Biochem. (2020) 154:439–50. doi: 10.1016/j.plaphy.2020.06.035
18. Zhang H, Zeng Y, Yan F, Chen F, Zhang X, Liu M. Semi-preparative isolation of crocins from saffron (Crocus sativus L). Chromatographia. (2004) 59:691–6. doi: 10.1365/s10337-004-0311-z
19. Frederico D, Donate PM, Constantino MG, Bronze ES, Sairre MI. A short and efficient synthesis of crocetin-dimethyl ester and crocetindial. J Org Chem. (2003) 68:9126–8. doi: 10.1021/jo034545y
20. Pfander H, Schurtenberger H. Biosynthesis of C20-carotenoids in Crocus sativus. Phytochemistry. (1982) 21:1039–42. doi: 10.1016/S0031-9422(00)82412-7
21. Samaha H, Chahine N, Sobolev AP, Menghini L, Makhlouf H. 1H-NMR metabolic profiling and antioxidant activity of saffron (Crocus sativus) cultured in Lebanon. Molecules. (2021) 26:4906. doi: 10.3390/molecules26164906
22. Dufresne D, Cormier F, Dorion S, Niggli UA, Pfister S, Pfander H. Glycosylation of encapsulated crocetin by a Crocus sativus L. cell culture Enzyme. Microb Tech. (1999) 24:453–62. doi: 10.1016/S0141-0229(98)00143-4
23. Zareena AV, Variyar PS, Gholap AS, Rongirwar DR. Chemical investigation of gamma-irradiated saffron (Crocus sativus L.). J Agr Food Chem. (2001) 49:687–91. doi: 10.1021/jf000922l
24. Sommano SR, Suppakittpaisam P, Sringarm K, Junmahasathien T, Ruksiriwanich W. Recovery of crocins from floral tissue of Gardenia jasminoides Ellis. Front Nutrition. (2020) 7:106. doi: 10.3389/fnut.2020.00106
Keywords: saffron, freesia, crocin, crocetin neapolitanosyl ester, cooking
Citation: Shindo K, Sakemi Y, Shimode S, Takagi C, Uwagaki Y, Hattan J-i, Akao M, Usui S, Kiyokawa A, Komaki M, Murahama M, Takemura M, Ishikawa I and Misawa N (2022) Changes of Crocin and Other Crocetin Glycosides in Saffron Through Cooking Models, and Discovery of Rare Crocetin Glycosides in the Yellow Flowers of Freesia Hybrida. Front. Nutr. 9:885412. doi: 10.3389/fnut.2022.885412
Received: 28 February 2022; Accepted: 15 June 2022;
Published: 14 July 2022.
Edited by:
Dharini Sivakumar, Tshwane University of Technology, South AfricaReviewed by:
Amit Jaisi, Walailak University, ThailandCopyright © 2022 Shindo, Sakemi, Shimode, Takagi, Uwagaki, Hattan, Akao, Usui, Kiyokawa, Komaki, Murahama, Takemura, Ishikawa and Misawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazutoshi Shindo, a3NoaW5kb0BmYy5qd3UuYWMuanA=; Norihiko Misawa, bi1taXNhd2FAaXNoaWthd2EtcHUuYWMuanA=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.