
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 15 August 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.885364
This article is part of the Research TopicInsights in Clinical NutritionView all 30 articles
 Anton Franz Fliri*
Anton Franz Fliri* Shama Kajiji
Shama KajijiIt is well recognized that redox imbalance, nitric oxide (NO), and vitamin D deficiencies increase risk of cardiovascular, metabolic, and infectious diseases. However, clinical studies assessing efficacy of NO and vitamin D supplementation have failed to produce unambiguous efficacy outcomes suggesting that the understanding of the pharmacologies involved is incomplete. This raises the need for using systems pharmacology tools to better understand cause-effect relationships at biological systems levels. We describe the use of spectral clustering methodology to analyze protein network interactions affected by a complex nutraceutical, Cardio Miracle (CM), that contains arginine, citrulline, vitamin D, and antioxidants. This examination revealed that interactions between protein networks affected by these substances modulate functions of a network of protein complexes regulating caveolae-mediated endocytosis (CME), TGF beta activity, vitamin D efficacy and host defense systems. Identification of this regulatory scheme and the working of embedded reciprocal feedback loops has significant implications for treatment of vitamin D deficiencies, atherosclerosis, metabolic and infectious diseases such as COVID-19.
The term “nutraceuticals” includes dietary supplements, functional foods, vitamins, and nutritional products. These products are generally mixtures of natural products, vitamins, minerals and/or herbal ingredients. For the most part, clinical evidence is generally limited at the ingredient level since in the United States it is optional to make claims of clinical benefit to bring a nutraceutical to market. Further, unlike pharmaceuticals, nutraceuticals offer narrow profit margins; this in-conjunction with the non-stringent global regulatory environment allows manufacturers to avoid running expensive and time-consuming clinical trials for demonstrating health benefits for gaining marketing approval. However, with a plethora of products in the marketplace, it is becoming more and more important to competitively position the nutraceutical in terms of its health and wellness benefits.
Critical for predicting health effects of the nutraceuticals is the understanding of how complex mixtures of substances (ingredients, probes) influence the propagation of information in biological networks (1). While the structures and functions of cellular components are relatively well understood, very little is known on how system components work or cease to work together in case of injuries, medications or diets (1). This knowledge gap can largely be attributed to the complexity of network-network interactions giving rise to system plasticity and emergent properties (2). Thus, system perturbations affecting behavior frequently display “modularity” and “interdependence” wherein modularity refers to effects on system components that by working together deliver well defined outputs, and interdependence refers to effects of the perturbations on the organization of components necessary for delivering optimal end results (3). In the framework of network biology, interdependence results from interactions between networks of tissues, cells and proteins (4). However, predicting properties regulated by interacting network systems in real-world settings requires large amounts of data and, if absent, escapes the reach of even the most sophisticated statistical methodologies (3). For addressing this gap, applications of various spectral clustering methodologies have been explored (6, 7).
We have developed a novel spectral clustering methodology for advancing these efforts. It allows tracking of perturbation-induced information flows through multiple interacting network systems and facilitates determination of cause-effect relationships for even complex mixtures (see section “Materials and methods”).
The aim of this study was to use spectral clustering for determining cause-effect relationships of the nutraceutical, Cardio Miracle (CM) marketed as a nitric oxide booster (see Supplementary Data 1), containing amongst its 50 + ingredients arginine, citrulline, vitamin D and antioxidants, which have recently been shown to increase the bioavailability of NO and decrease oxidative stress in vitro (8). Previous studies have linked vitamin D and NO deficiencies to nutrient-sensing (10, 11). It has also been shown that the addition of antioxidants to a combination of arginine, citrulline and vitamin D synergistically increases the ratio between NO and peroxynitrite production in endothelial cells (9). Critical for endothelial cell function; these signaling systems are important for health: endothelial cells dysfunction (ED) plays a key role in development of cardiovascular diseases, diabetes, obesity, and inflammatory conditions (12).
We describe the use of spectral clustering for identifying how CM affects the propagation of signals and impacts biological processes. We present evidence that interaction(s) between arginine, citrulline, vitamin D3 and antioxidants not only synergistically balance NO and peroxynitrate generation, but also affect functions of a protein network that regulates caveolae mediated endocytosis (CME), TGF beta activity, and vitamin D efficacy. Identification of this regulatory scheme and the working of embedded reciprocal feedback loops advances our understanding of how the various signaling systems/biological processes interact at a body-wide scale and generates meaningful hypotheses for bridging the gap between preclinical and clinical studies.
Cardio miracle, marketed by Evolution Nutraceuticals (12), is a NO supplement. It is a mixture of arginine, citrulline (13), cholecalciferol vitamin D, various vitamins, quercetin, minerals and over 700 natural products that can be isolated from herbal and vegetable product constituents (see Supplementary Material Section) (13–15).1
Spectral Clustering Methodology, specifically designed to discover emergent properties resulting from network-network interactions, has been described in detail in PCT/US2016/06379. Its application for analysis of CM is detailed in the work-flow below.
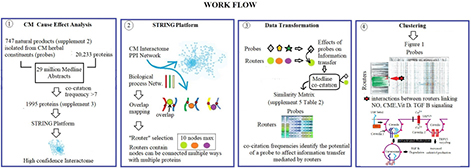
Specifically,
(1) Protein network components affected by CM ingredients were identified by determining co-occurrence frequencies of 700 natural products isolated from its herbal constituents with 20,233 proteins in over 17 million Medline abstracts. This data-gathering step resulted in the selection of 1,995 proteins with a co-occurrence frequency count of more than seven.
(2) Use of the STRING platform’s highest confidence in network connectivity level for protein network construction delineated a high confidence CM interactome (see Supplementary Material Section) (2). STRING’s gene enrichment analysis using biological process networks as background was used to divide the 1,995 protein-containing CM interaction network into smaller network fragments. This step resulted in > 4,000 protein network fragments that overlap with biological process networks regulating functions throughout the body. Selection of network fragments with < 10 network nodes (associated with a strength of > 0.9 and a p value of < 0.0001) produced 1,373 biological structure-function constraint network fragments.
(3) These 1,373 network fragments were used as topological descriptors for determining information densities of 747 CM ingredients associated with these 1,373 fragments in the Medline database resulting in the generation of a similarity matrix containing 1,373 × 747 information density measurements (Supplementary Material Section: Supplementary Table 2).
(4) Clustering of this similarity matrix using the TIBCO Spotfire platform (15)2, and cosine correlation as similarity measure provided Figure 1.
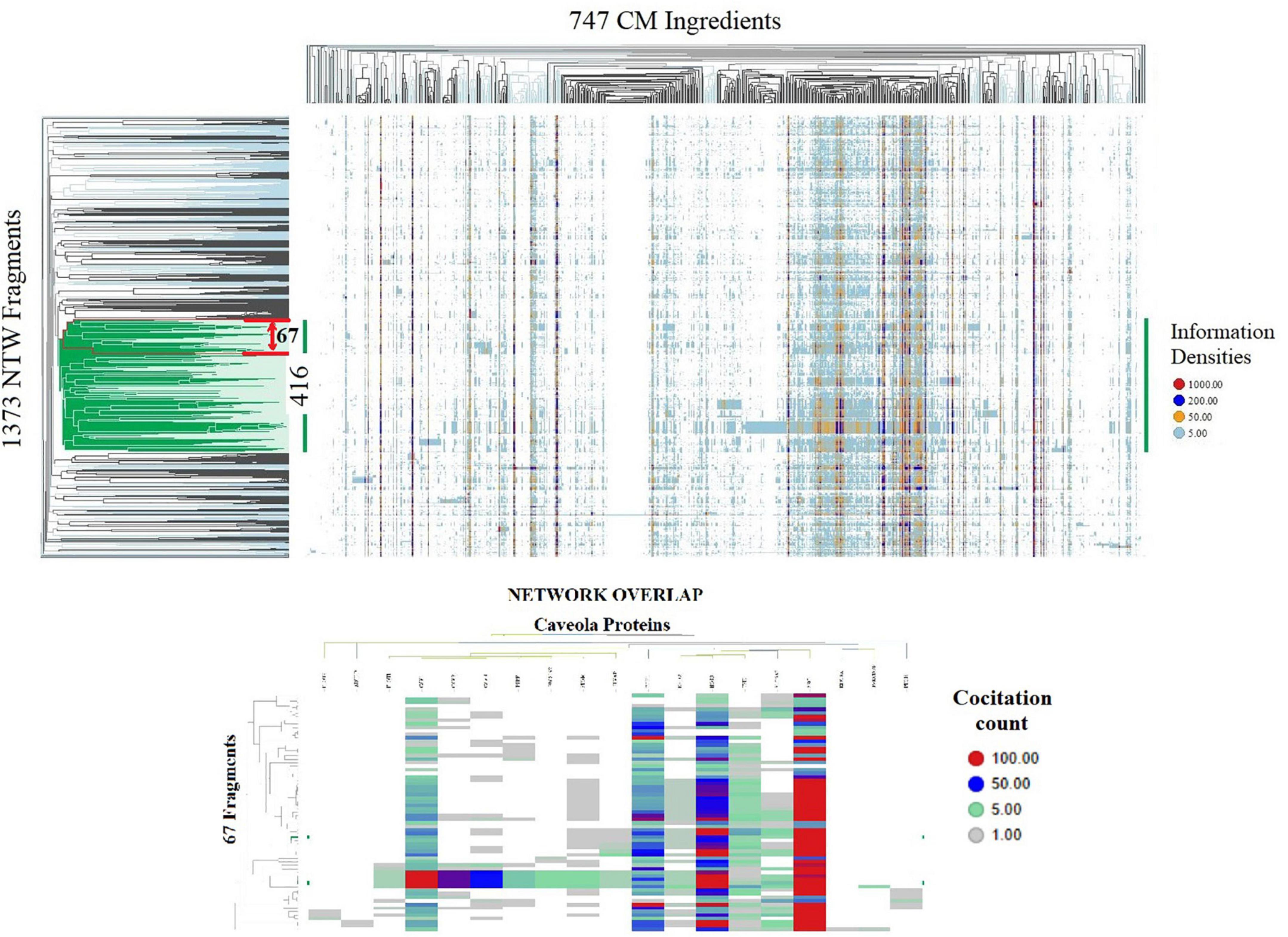
Figure 1. Spotfire generated heatmaps. TOP shows on the vertical dendrogram axis the organization of 1,373 CM interactome fragments overlapping with biological process network and identifies network-network interactions; the horizontal dendrogram axis identifies phenotypes of 747 natural products and CM ingredients containing substance groups inducing similar interactions in network fragment phenotypes; the vertical dendrogram section highlighted in green identifies network-network connectivity of core group of 416 network fragments induced by all 747 CM ingredients. BOTTOM identifies that a phenotype of 67 Fragment within the red boundary of the top heatmap containing biological process regulating vitamin D metabolism, Nitric oxide production and redox balance overlap with a network regulating caveola functions. Shown are the information densities of proteins CAV1, CAV3, PTRF, CAV2, FLOT1, FLOT2, NOS3, SRC, STOM, PRKCDBP, EHD2, SLC6A3, KIF18A, PACSIN2, CDH1, ADTRP, TFPI, PTGIS, PLVA with 67 network fragments.
Identification of molecular underpinnings of the observation that interaction(s) between arginine, citrulline, vitamin D3 and antioxidants synergistically balance NO and peroxynitrate generation, using hierarchical clustering of the 1,373 × 747 similarity matrix in SPOTFIRE revealed that 747 CM ingredients induce network-network interactions involving a core group of 416 network fragments (Figure 1).
Using STRING’s highest confidence level for investigating the connectivity between these 416 network fragments identified networks of protein complexes containing 1,320 proteins overlapping with networks involved in the regulation of caveolae-associated functions, redox stress (17), and nutrient sensing. Of relevance to the regulation of endothelial functions is that the flattening of the curvature of caveolae under mechanical pressure (e.g., shear stress, blood pressure) that functions as a stress sensor (18) and modulates CME (18, 19). CME, by connecting mechanical input signals to the nucleus, regulates system-wide responses to ED associated with NO and vitamin D deficiency (20–24).
Determination of the connectivity between CM interactome fragments overlapping with biological process networks involved in NO and vitamin D signaling (Figures 1, 2) identified caveolin 1 (caveolin 1), a major structural component of caveolae, as a key regulator of endothelial nitric oxide synthetase (NOS3) activity, vitamin D activation (VDR/CYP27B1), TGF beta activity and CME. Evidence discussed below supports the premise that this functional relationship represents a novel mechanism for regulating vitamin D efficacy.
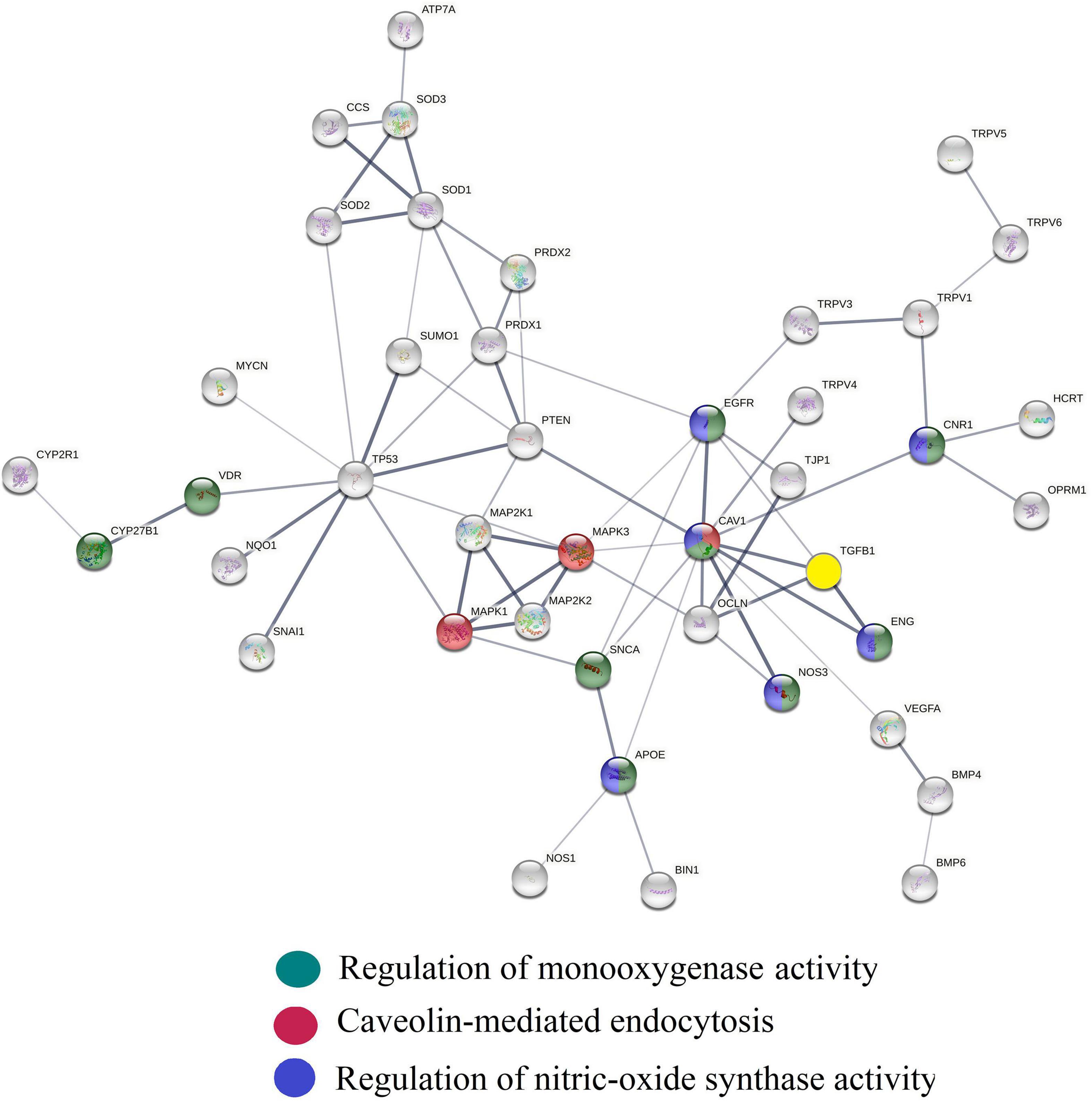
Figure 2. Protein interaction network generated using the String platform’s highest confidence level (0.9) and CM interactome proteins: ADCYAP1, ADRB1, ADRBK1, AKAP5, AKT1, APOE, ATF4, ATP7A, BIN1, BMP2, BMP4, BMP6, CACNA1C, CACNA1D, CASQ2, caveolin1, CCK, CCS, CD320, CD9, CEACAM1, CNR1, CUBN, CYBA, CYP19A1, CYP1A1, CYP1A2, CYP1B1, CYP24A1, CYP26A1, CYP27B1, CYP2R1, CYP3A4, CYP3A4, CYP7A1, ENG, ENO1, EREG, F2, FGF19, FGF23, FGFR1, FOXO1, FOXO3, HCRT, HMOX1, IFNG, IL1B, IL4, ISCU, JAG1, KLF4, LEP, LMNA, MAPK3, MPO, MTOR, NF1, NFATC1, NFKB1, NOS1, NOS3, NOTCH1, NPY, NQO1, OCLN, OPRM1, ORAI1, PARK7, PDGFRA, PGR, PIAS4, PINK1, PLTP, POR, PPARA, PPARGC1A, PRDX1, PRDX2, PRKCD, PTEN, PTGS2, RBPJ, RORA, SCARB1, SIRT1, SMAD3, SNAI1, SNCA, SOD1, SOD2, SOD3, SPP1, STAT3, STIM1, STRA6, SUMO1, TGFB1, TJP1, TLR2, TNF, TNFRSF1A, TREM2, TRPV4, UBIAD1, UCN, UGT1A1, UGT1A8, VDR, VEGFA. Edges show physical interactions between proteins. Biological processes overlapping with this network fragment regulate caveola mediated endocytosis (yellow), NO biosynthesis (green), vitamin D activation (red) and TGF beta activity (light blue). Caveolin 1 (caveolin 1) serves as a hub protein connecting these biological process networks.
Spectral clustering based functional analysis of CM provides valuable insights regarding supplement-mediated regulation of vitamin D in cardiovascular diseases and for exploring ED. CM delivers vitamin D in form of pure cholecalciferol and as a mixture of cholecalciferol and 25-hydroxycholecalciferol (25(OH)D3) in the form of shitake and maitake mushroom powders. Cholecalciferol and 25-hydroxycholecalciferol are precursors of the hormone 1,25 dihydroxyvitamin D3 (calcitriol) and reach the bloodstream via intestinal absorption. The oral bioavailability of vitamin D precursors is limited by the ABCA1 transport protein which functions as an intestinal absorption barrier (25–27). Determination of co-investigation frequencies of 747 CM ingredients and ABCA1 transport protein was used to establish that the natural product, quercetin (28) and catechins (found in abundance in CM’s antioxidant ingredient group) can inhibit ABCA1 transport and thereby, increase the oral bioavailability of vitamin D (29). Clinical observations provide strong evidence that co-administration of quercetin with vitamin D increases the oral bioavailability and efficacy of vitamin D and support the premise that CM ingredients enhance the oral bioavailability of cholecalciferol vitamin D (30, 31).
As shown in Figure 3, cytochrome CYP2R1 and CYP27A1 convert cholecalciferol into 25-hydroxyvitamin D (32) and which, upon binding to vitamin D binding protein and albumin (33, 34) circulates in the bloodstream. Vitamin D binding protein regulates circulating free and total levels of vitamin D metabolites, where ∼0.03% of 25(OH)D is free and 99.97% is bound to the vitamin D binding protein and to albumin. Cytochrome CYP24A1 transforms 25 (OH)D3 into an inactive metabolite 24,25-di-hydroxyvitamin D. Activation of 25 (OH)D3 bound to vitamin D binding protein requires active transport via megalin-mediated endocytosis into kidney proximal tubule cells and conversion by CYP27B1 into calcitriol. Calcitriol is metabolized by cytochrome CYP24A1 into its inactive form, 1,24,25 (OH)3 vitamin D (35) and has a half-life of ∼ 6 h vs. the half-life of the inactive form (25 (OH)D3) which is up to 3 weeks (36). Considering the plethora of vitamin D effects, the balance between vitamin D inactivating and activating metabolic enzymes and the speed with which calcitriol and its precursors can enter cells determines overall efficacy profile of vitamin D – and is therefore, dynamically regulated (37).
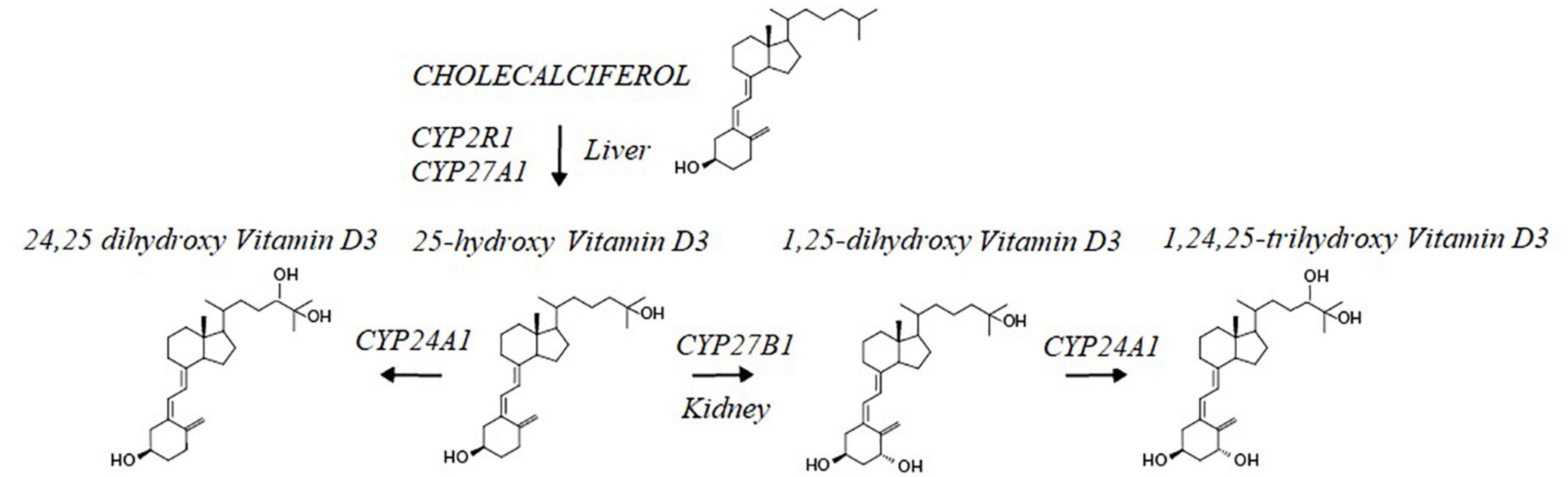
Figure 3. Cholecalciferol Vit D is metabolized in the liver by CYP2R1 and CYP27a1 into the Vitamin D3 precursor, 25 hydroxy Vitamin D3. This intermediate is further metabolized by CYP24A1 into a hormonal inactive form of Vit D3; 24,25 dihydroxy vitamin D3 and in the kidney by CYP27B1 into the active from of Vit D3, 1,25 dihydroxy-Vitamin D3. Levels of the hormonal active form of Vit D3 are decreased through the action of CYP24A1 converting the hormone into 1,25,25-trihydroxy Vitamin D3.
Amongst the many vitamin D efficacy regulators is calcitriol itself; it adjusts the expressions of CYP27B1, CYP24A1 and the vitamin D receptor (VDR) (38, 39). Calcitriol upon binding to VDR (enriched in caveolae) (40) is transported via CME across cell membranes (41). This caveolae-mediated active transport of receptor bound vitamin D3 is activated by NO (42) and inhibited by peroxynitrite (43, 44). Since CM has been documented to increase levels of bioavailable NO and lower peroxynitrate concentrations, it has therefore the capacity to activate and stabilize of CME leading to increased cellular uptake and genomic activity of vitamin D3 (41, 45–47).
The premise that CME activation increases calcitriol production is grounded in observations that CME regulates activity of a vanillin-type selective calcium channel TRPV5, present on the apical membrane of distal kidney tubule epithelial cells (48, 49), and that the loss of TRPV5 channel activity causes calcitriol overproduction and vitamin D hypervitaminosis (50, 51). Hence CME activation, by removing TRPV5 from the cell surface, decreases TRPV5 activity and increases calcitriol production (52, 53). This fine-tuning of CME-mediated vitamin D activation involves protein kinase C (PKC) and phospholipase D (PLD) wherein the activation of PKC inhibits CME and the inhibition of PKC activates CME (54). Since vitamin D3 (55) in combination with PKC inhibitors quercetin (56), oleanolic acid (57, 58), and curcumin (59) in CM’s herbal constituents reinforces CME activation, this ingredient combination is projected to increase calcitriol production. The vitamin D efficacy of CM is further enhanced by oleanic acid, a natural product isolated from CM’s hawthorn and mango extracts, which decreases the expression and protein levels of the calcitriol inactivator CYP24A1 (60).
Activation of CME also increases the degradation of TGF beta (61), an immunosuppressive cytokine that upregulates ROS production (62), arginases expression, and decreases NO production (63, 64). Thus, CM-mediated activation of CME is expected to reduce TGF beta signaling; this inhibitory effect is enhanced by hesperidin, a natural product isolated from CM’s citrus extracts, which downregulates TGF beta expression (65). CME-mediated decrease in TGF beta activity adds to CM’s projected capacity to increase vitamin D efficacy (66). This is because TGF beta has been shown to inhibit the expression of megalin, the intracellular protein essential for uptake of 25 hydroxyvitamin D by kidney proximal tubule cells and its subsequent conversion to calcitriol by CYP27B1 (67, 68). Accordingly, the experimental observations summarized in Figure 4, provides strong support for the existence of a CME-based regulatory scheme that upon activation controls functions of reciprocal feedback loops that decrease ROS, decrease TGF beta activity, and increase NO and calcitriol production (45).
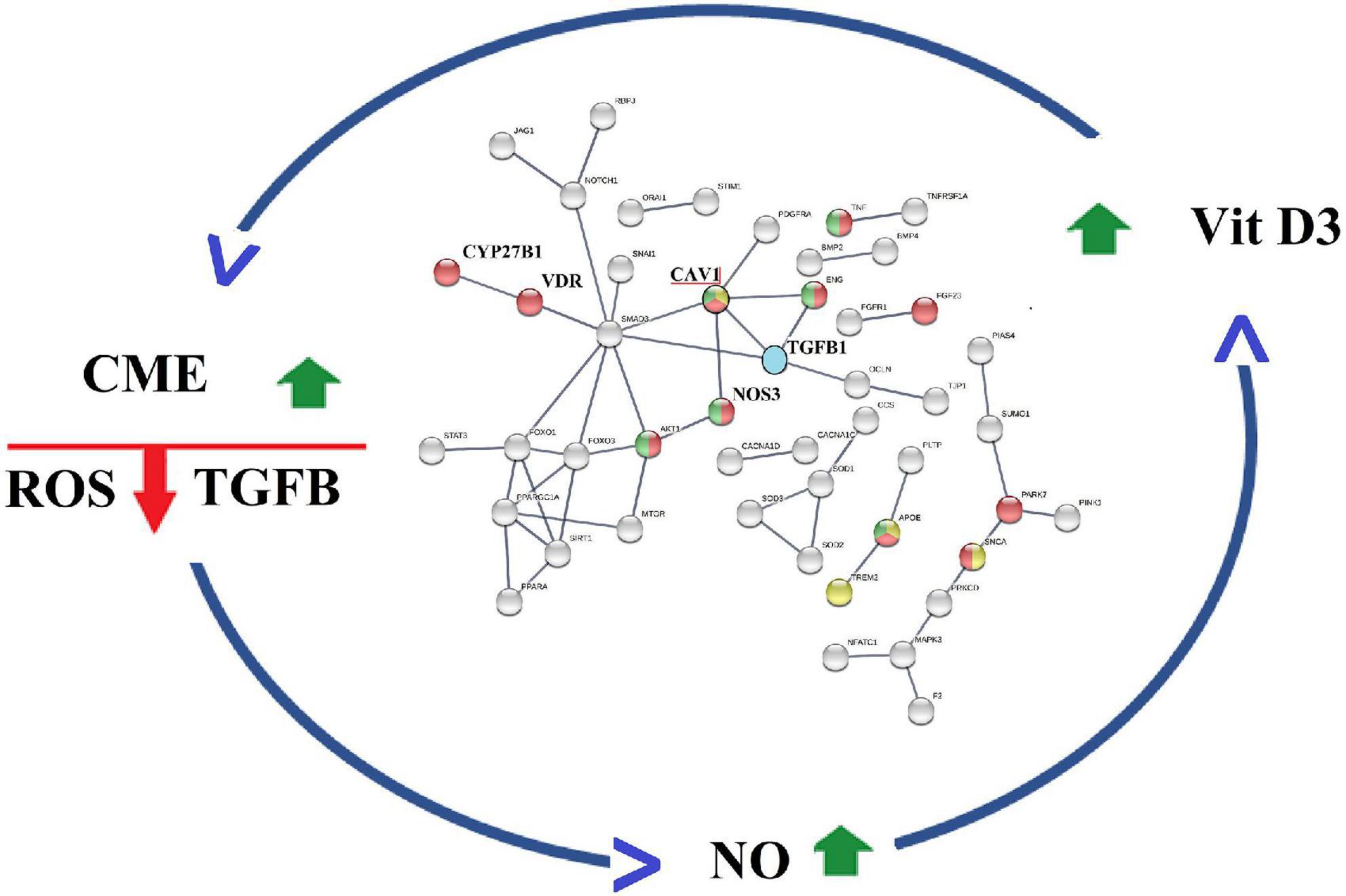
Figure 4. Caveola mediated endocytosis (CME) modulates activities of a reciprocal feedback loops that finetunes ROS production, TGF beta activity, Nitric oxide levels O and Calcitriol production.
Inhibition of TGF beta signaling has been shown to impede progression of atherosclerosis and results in regression of established disease (69). Thus, CM-mediated activation of CME in combination with hesperidin’s capacity to downregulate TGF beta expression is projected to have beneficial effects in atherosclerosis. Secondly, CM-mediated increase in calcitriol production via CME-activation is projected to lower caveolin 1 level. This is therefore expected to also intercept development of atherosclerosis since increased caveolin 1 expression is linked to disease progression (70–73). The mechanistic rationale is that caveolin 1 levels are regulated by autophagy and prevent its own degradation since it is itself an autophagy inhibitor (64, 74, 75). Caveolin 1-mediated inhibition of autophagy is reduced by calcitriol which activates tyrosine kinase activity of pp60src and results in the phosphorylation of caveolin 1 at tyrosine 14 and increased autophagy (76–78).
Phosphorylation of caveolin 1 at tyrosine 14 also increases endothelial nitric oxide synthase activity and increases NO production to further reinforce the CM feedback loop. Thus, the capacity of CM to enhance TGF beta and caveolin 1 degradation is projected to enhance the anti-atherosclerotic efficacy of this nutraceutical (79–82).
It is also important to note that CM’s projected pharmacology to lower caveolin 1 level by increasing autophagic degradation is enhanced by other autophagy-activating CM ingredients: palmitic acid (83), resveratrol (84), pterostilbene (85), quercetin (86), piceatannol (87), delphinidin (88), cyanidin-3-o-glucoside (89), and sulforaphane (89).
Identification of network-network interactions regulating reciprocal feedback loops advances our understanding of how ingredients/natural products of nutraceuticals interact within a system and provide guidance for product optimization and improving preclinical and clinical outcomes. Spectral clustering of protein interaction information associated with ingredients of a complex nutraceutical supplement, CM, uncovered several biological functions supported by its ability to correct cellular redox imbalance. These include its ability to increase oral bioavailability of cholecalciferol vitamin D3 and to activate and stabilize caveolin-mediated endocytosis. The combination of these functionalities infers involvement of reciprocal cellular feedback loops that increase NO production and vitamin D3 efficacy, decrease TGF beta signaling and oxidative stress, and activate autophagy. Since down-regulation of TGF beta activity and activation of autophagy is anticipated to intercept/reverse endothelial dysfunction associated diseases including atherosclerosis (90–93), diabetic kidney disease (94–96), and COVID-19 (97–99), supplementation with CM-like functionalities is projected to benefit treatment of these diseases. For validating these effect predictions, clinical trials are warranted.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
AF developed the spectral clustering methodology and performed the data analysis of this study. SK contributed to the interpretation of analysis outcomes. Both authors contributed to the manuscript and agreed to be accountable for the content of the work.
John Hewlett contributed valuable information on CM ingredients and suggestions to the study.
AF and SK are the founders of Emergent System Analytics LLC.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.885364/full#supplementary-material
1. Santolini M, Barabási AL. Predicting perturbation patterns from the topology of biological networks. Proc Natl Acad Sci U.S.A. (2018) 115:E6375–83. doi: 10.1073/pnas.1720589115
2. Liu W, Suzumura T, Ji H, Hu G. Finding overlapping communities in multilayer networks. PLoS One. (2018) 13:e0188747. doi: 10.1371/journal.pone.0188747
3. Noell G, Faner R, Agustí A. From systems biology to P4 medicine: applications in respiratory medicine. Eur Respir Rev. (2018) 27:170110. doi: 10.1183/16000617.0110-2017
4. Sun PG, Quan Y, Miao Q. Interdependent patterns in protein-protein interaction networks. IEEE Trans Network Sci Eng. (2020) 7:3257–65. doi: 10.1109/TNSE.2020.3022170
5. Ahn YY, Bagrow JP, Lehmann S. Link communities reveal multiscale complexity in networks. Nature. (2010) 466:761–4. doi: 10.1038/nature09182
6. Liu W, Chang Z, Jia C, Zheng Y. A generative node-attribute network model for detecting generalized structure and semantics. Phys A Statist Mech Appl. (2022) 588:126557. doi: 10.1016/j.physa.2021.126557
7. Sadikaj Y, Velaj Y, Behzadi S, Plant C. “Spectral clustering of attributed multi-relational graphs,” in Proceedings of the 27th ACM SIGKDD Conference on Knowledge Discovery & Data Mining, Singapore (2021). doi: 10.1145/3447548.3467381
8. Zhang H, Wang CD, Lai JH. Modularity in complex multilayer networks with multiple aspects: a static perspective. Appl Inform. (2017) 4:7. doi: 10.1186/s40535-017-0035-4
9. Dawoud H, Malinski T. Vitamin D3, L-Arginine, L-Citrulline, and antioxidant supplementation enhances nitric oxide bioavailability and reduces oxidative stress in the vascular endothelium–Clinical implications for cardiovascular system. Pharmacogn Res. (2020) 12:17–23. doi: 10.4103/pr.pr_79_19
10. Hsu CN, Tain YL. Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int J Mol Sci. (2019) 20:681. doi: 10.3390/ijms20030681
11. El Maaty MAA, Gad MZ. Vitamin D deficiency and cardiovascular disease: potential mechanisms and novel perspectives. J Nutr Sci vitaminol. (2013) 59:479–88. doi: 10.3177/jnsv.59.479
12. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. (2007) 87:315–424. doi: 10.1152/physrev.00029.2006
13. Cieri-Hutcherson NE, Jaenecke A, Bahia A, Lucas D, Oluloro A, Stimmel L, et al. Systematic review of L-arginine for the treatment of hypoactive sexual desire disorder and related conditions in women. Pharmacy. (2021) 9:71. doi: 10.3390/pharmacy9020071
14. Rashid J, Kumar SS, Job KM, Liu X, Fike CD, Sherwin CM. Therapeutic potential of citrulline as an arginine supplement: a clinical pharmacology review. Pediatr Drugs. (2020) 22:279–93. doi: 10.1007/s40272-020-00384-5
15. Afendi FM, Okada T, Yamazaki M, Hirai-Morita A, Nakamura Y, Nakamura K, Ikeda S, et al. KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. (2012) 53:1–12. doi: 10.1093/pcp/pcr165
16. Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. (2019) 47:D607–13. doi: 10.1093/nar/gky1131
17. Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radical Biol Med. (2015) 88:108–46. doi: 10.1016/j.freeradbiomed.2015.06.021
18. Joseph JG, Liu AP. Mechanical regulation of endocytosis: new insights and recent advances. Adv Biosyst. (2020) 4:1900278. doi: 10.1002/adbi.201900278
19. Del Pozo MA, Lolo FN, Echarri A. Caveolae: mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr Opin Cell Biol. (2021) 68:113–23. doi: 10.1016/j.ceb.2020.10.008
20. Xu Y, Buikema H, van Gilst WH, Henning RH. Caveolae and endothelial dysfunction: filling the caves in cardiovascular disease. Eur J Pharmacol. (2008) 585:256–60. doi: 10.1016/j.ejphar.2008.02.086
21. Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. (2009) 32(suppl. 2):S314–21. doi: 10.2337/dc09-S330
22. Majkova Z, Toborek M, Hennig B. The role of caveolae in endothelial cell dysfunction with a focus on nutrition and environmental toxicants. J Cell Mol Med. (2010) 14:2359–70. doi: 10.1111/j.1582-4934.2010.01064.x
23. Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. (2003) 285:L1179–83. doi: 10.1152/ajplung.00242.2003
24. Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. (2011) 57:63–9. doi: 10.1161/HYPERTENSIONAHA.110.160929
25. Margier M, Collet X, Le May C, Desmarchelier C, André F, Lebrun C, et al. ABCB1 (P-glycoprotein) regulates vitamin D absorption and contributes to its transintestinal efflux. FASEB J. (2019) 33:2084–94. doi: 10.1096/fj.201800956R
26. Shekhawat PB, Pokharkar VB. Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm Sin B. (2017) 7:260–80. doi: 10.1016/j.apsb.2016.09.005
27. Maurya VK, Aggarwal M. Factors influencing the absorption of vitamin D in GIT: an overview. J Food Sci. Technol. (2017) 54:3753–65. doi: 10.1007/s13197-017-2840-0
28. Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: targeting cancer cells death and MDR. Food Chem. Toxicol. (2012) 50:3375–83. doi: 10.1016/j.fct.2012.06.035
29. Kitagawa S, Nabekura T, Kamiyama S. Inhibition of P-glycoprotein function by tea catechins in KB-C2 cells. J Pharm Pharmacol. (2004) 56:1001–5. doi: 10.1211/0022357044003
30. Paller CJ, Kanaan YM, Beyene DA, Naab TJ, Copeland RL, Tsai HL, et al. Risk of prostate cancer in African-American men: evidence of mixed effects of dietary quercetin by serum vitamin D status. Prostate. (2015):1376–83. doi: 10.1002/pros.23018
31. Hassan JK, Sharrad AK, Sheri FH. Effect of quercetin supplement on some bone mineralization biomarkers in diabetic type 2 patients. Adv Pharmacol Pharm. (2018) 6:43–9. doi: 10.13189/app.2018.060202
32. Bikle DD. Vitamin D: newer concepts of its metabolism and function at the basic and clinical level. J Endocr Soc. (2020) 4:bvz038. doi: 10.1210/jendso/bvz038
33. Daiger SP, Schanfield MS, Cavalli-Sforza LL. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci U.S.A. (1975) 72:2076–80. doi: 10.1073/pnas.72.6.2076
34. Bikle D. Vitamin D: production, metabolism, and mechanisms of action and clinical applications. Chem Biol. (2015) 21:319–29. doi: 10.1016/j.chembiol.2013.12.016
35. Meyer MB, Lee SM, Carlson AH, Benkusky NA, Kaufmann M, Jones G, et al. A chromatin-based mechanism controls differential regulation of the cytochrome P450 gene Cyp24a1 in renal and non-renal tissues. J Biol Chem. (2019) 294:14467–81. doi: 10.1074/jbc.RA119.010173
36. Mosekilde L. Vitamin D and the elderly. Clin Endocrinol. (2005) 62:265–81. doi: 10.1111/j.1365-2265.2005.02226.x
37. Meyer MB, Pike JW. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. J Steroid Biochem Mol Biol. (2020) 196:105500. doi: 10.1016/j.jsbmb.2019.105500
38. Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. (2014) 55:13–31. doi: 10.1194/jlr.R031534
39. Lu X, Chen Z, Watsky MA. Effects of 1, 25 and 24, 25 vitamin D on corneal fibroblast VDR and vitamin D metabolizing and catabolizing enzymes. Current Eye Res. (2021) 46:1271–82. doi: 10.1080/02713683.2021.1884726
40. Donati S, Palmini G, Romagnoli C, Aurilia C, Miglietta F, Falsetti I, et al. In vitro non-genomic effects of calcifediol on human preosteoblastic cells. Nutrients. (2021) 13:4227. doi: 10.3390/nu13124227
41. Schaefer R. Calcitriol Conjugated Quantum Dots, An Innovative Tool As Both Probe And Treatment. Doctoral dissertation, University of Delaware, Newark, DE (2012).
42. Chen Z, Ds Oliveira S, Zimnicka AM, Jiang Y, Sharma T, Chen S, et al. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol Biole Cell. (2018) 29:1190–202. doi: 10.1091/mbc.E17-01-0049
43. Mougeolle A, Poussard S, Decossas M, Lamaze C, Lambert O, Dargelos E. Oxidative stress induces caveolin 1 degradation and impairs caveolae functions in skeletal muscle cells. PLoS One. (2015) 10:e0122654. doi: 10.1371/journal.pone.0122654
44. Shamsaldeen YA, Lione LA, Benham CD. Dysregulation of TRPV4, eNOS and caveolin-1 contribute to endothelial dysfunction in the streptozotocin rat model of diabetes. Eur J Pharmacol. (2020) 888:173441. doi: 10.1016/j.ejphar.2020.173441
45. Martínez-Miguel P, Valdivielso JM, Medrano-Andrés D, Román-García P, Cano-Peñalver JL, Rodríguez-Puyol M, et al. The active form of vitamin D, calcitriol, induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells. Am J Physiol Endocrinol Metab. (2014) 307:E1085–96. doi: 10.1152/ajpendo.00156.2014
46. Pike JW, Christakos S. Biology and mechanisms of action of the vitamin D hormone. Endocrinol Metab Clin. (2017) 46:815–43.
47. Christakos S. In search of regulatory circuits that control the biological activity of vitamin D. J Biol Chem (2017) 292:17559–60. doi: 10.1074/jbc.H117.806901
48. Cha SK., Wu T, Huang CL. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol. (2008) 294:F1212–21. doi: 10.1152/ajprenal.00007.2008
49. Gkika M, Hoenderop JG, Bindels RJ. The epithelial Ca2+ channel TRPV5 in health and disease. Naunyn Schmiedebergs Arch Pharmacol. (2004) 371:295–306.
50. Jacquillet G, Unwin RJ. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflügers Arch. (2019) 471:83–98.
51. Renkema KY, Nijenhuis T, van der Eerden BC, van der Kemp AW, Weinans H, van Leeuwen JP, et al. Hypervitaminosis D mediates compensatory Ca2+ hyperabsorption in TRPV5 knockout mice. J Am Soc Nephrol. (2005) 16:3188–95. doi: 10.1681/ASN.2005060632
52. Estadella I, Pedrós-Gámez O, Colomer-Molera M, Bosch M, Sorkin A, Felipe A. Endocytosis: a turnover mechanism controlling ion channel function. Cells. (2020) 9:1833.
53. De Groot T, Bindels RJ, Hoenderop JG. TRPV5: an ingeniously controlled calcium channel. Kidney Int. (2008) 74:1241–6. doi: 10.1038/ki.2008.320
54. Kim JH, Han JM, Lee S, Kim Y, Lee TG, Park JB, et al. Phospholipase D1 in caveolae: regulation by protein kinase Cα and caveolin-1. Biochemistry. (1999) 38:3763–9. doi: 10.1021/bi982478+
55. Lin LM, Peng F, Liu YP, Chai DJ, Ning RB, Xu CS, et al. Coadministration of VDR and RXR agonists synergistically alleviates atherosclerosis through inhibition of oxidative stress: an in vivo and in vitro study. Atherosclerosis. (2016) 251:273–81. doi: 10.1016/j.atherosclerosis.2016.06.005
56. Kang TB, Liang NC. Effect of quercetin on activities of protein kinase C and tyrosine protein kinase from HL-60 cells. Acta Pharmacol Sin. (1997) 18:374–6.
57. Mooi LY, Yew WT, Hsum YW, Soo KK, Hoon LS, Chieng YC. Suppressive effect of maslinic acid on PMA-induced protein kinase C in human B-lymphoblastoid cells. Asian Pac J Cancer Prev. (2012) 13:1177–82. doi: 10.7314/apjcp.2012.13.4.1177
58. Li S, Wang YN, Wan ZL, Zhang XW, Cong PJ. Study on extraction and purification of ursolic acid and oleanolic acid from hawthorn fruits. Food Sci. (2007) 28:141–4.
59. Mahmmoud YA. Modulation of protein kinase C by curcumin; inhibition and activation switched by calcium ions. Br J Pharmacol. (2007) 150:200–8. doi: 10.1038/sj.bjp.0706970
60. Cao S, Dong XL, Ho MX, Yu WX, Wong KC, Yao XS, et al. Oleanolic acid exerts osteoprotective effects and modulates vitamin D metabolism. Nutrients. (2018) 10:247.
62. Krstić J, Trivanović D, Mojsilović S, Santibanez JF. Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. (2015) 2015:654594. doi: 10.1155/2015/654594
63. Salminen A. Immunosuppressive network promotes immunosenescence associated with aging and chronic inflammatory conditions. J Mol Med. (2021) 99:1553–69.
64. Zhang X, Ramírez CM, Aryal B, Madrigal-Matute J, Liu X, Diaz A, et al. Cav-1 (Caveolin-1) deficiency increases autophagy in the endothelium and attenuates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. (2020) 40:1510–22. doi: 10.1161/ATVBAHA.120.314291
65. Maneesai P, Bunbupha S, Potue P, Berkban T, Kukongviriyapan U, Kukongviriyapan V, et al. Hesperidin prevents nitric oxide deficiency-induced cardiovascular remodeling in rats via suppressing TGF-β1 and MMPs protein expression. Nutrients. (2018) 10:1549. doi: 10.3390/nu10101549
66. Gliozzi ML, Rbaibi Y, Long KR, Vitturi DA, Weisz OA. Metabolism, oxidative stress and cell signaling: hemoglobin alters vitamin carrier uptake and vitamin D metabolism in proximal tubule cells: implications for sickle cell disease. Am J Physiol Cell Physiol. (2019) 317:C993. doi: 10.1152/ajpcell.00287.2019
67. Cabezas F, Farfán P, Marzolo MP. Participation of the SMAD2/3 signalling pathway in the down regulation of megalin/LRP2 by transforming growth factor beta (TGF-ß1). PLoS One. (2019) 14:e0213127. doi: 10.1371/journal.pone.0213127
68. Shankar AS, van den Berg SA, Tejeda Mora H, Du Z, Lin H, Korevaar SS, et al. Vitamin D metabolism in human kidney organoids. Nephrol Dial Transpl. (2022) 37:190–3. doi: 10.1093/ndt/gfab264
69. Chen PY, Qin L, Li G, Wang Z, Dahlman JE, Malagon-Lopez J, et al. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat Metab. (2019) 1:912–26. doi: 10.1038/s42255-019-0102-3
70. Zhang X, Fernández-Hernando C. Transport of LDLs into the arterial wall: impact in atherosclerosis. Curr Opin Lipidol. (2020) 31:279–85.
71. Deng L, Vrieling F, Stienstra R, Hooiveld G, Feitsma AL, Kersten S. Caveolae mediated endocytosis of VLDL particles in macrophages requires NPC1 and STARD3 for further lysosomal processing. bioRxiv [Preprint] (2021): doi: 10.1101/2021.12.16.473074
72. Shiroto T, Romero N, Sugiyama T, Sartoretto JL, Kalwa H, Yan Z, et al. Caveolin-1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS One. (2014) 9:e87871. doi: 10.1371/journal.pone.0087871
73. Pavlides S, Gutierrez-Pajares JL, Iturrieta J, Lisanti MP, Frank PG. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. (2014) 356:147–57. doi: 10.1007/s00441-013-1767-7
74. Poredos P, Poredos AV, Gregoric I. Endothelial dysfunction and its clinical implications. Angiology. (2021) 72:604–15.
75. Münzel T, Templin C, Cammann VL, Hahad O. Takotsubo syndrome: impact of endothelial dysfunction and oxidative stress. Free Radical Biol Med. (2021) 169:216–23. doi: 10.1016/j.freeradbiomed.2021.03.033
76. Nah J, Yoo SM, Jung S, Jeong EI, Park M, Kaang BK, et al. Phosphorylated caveolin1 activates autophagy through an interaction with BECN1 under oxidative stress. Cell Death Dis. (2017) 8:e2822. doi: 10.1038/cddis.2017.71
77. Buitrago C, Boland R. Caveolae and caveolin-1 are implicated in 1α, 25 (OH) 2-vitamin D3-dependent modulation of Src. MAPK cascades and VDR localization in skeletal muscle cells. J Steroid Biochem Mol Biol. (2010) 121:169–75. doi: 10.1016/j.jsbmb.2010.03.002
78. Wang RC, Levine B. Calcipotriol induces autophagy in HeLa cells and keratinocytes. J Invest Dermatol. (2011) 131:990. doi: 10.1038/jid.2010.423
80. Bennett AL, Lavie CJ. Vitamin D metabolism and the implications for atherosclerosis. Adv Exp Med Biol. (2017) 996:185–92.
81. Miao J, Zang X, Cui X, Zhang J. Autophagy, hyperlipidemia, and atherosclerosis. Adv Exp Med Biol. (2020) 1207:237–64.
82. Layne J, Majkova Z, Smart EJ, Toborek M, Hennig B. Caveolae: a regulatory platform for nutritional modulation of inflammatory diseases. J Nutr Biochem. (2011) 22:807–11.
83. Tan SH, Shui G, Zhou J, Li JJE, Bay BH, Wenk MR, et al. Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin). J Biol Chem. (2012) 287:14364–76. doi: 10.1074/jbc.M111.294157
84. Tian Y, Song W, Li D, Cai L, Zhao Y. Resveratrol as a natural regulator of autophagy for prevention and treatment of cancer. OncoTargets Ther. (2019) 12:8601.
85. Chen RJ, Lee YH, Yeh YL, Wu WS, Ho CT, Li CY, et al. Autophagy-inducing effect of pterostilbene: a prospective therapeutic/preventive option for skin diseases. J Food Drug Anal. (2017) 25:125–33. doi: 10.1016/j.jfda.2016.10.022
86. Ashrafizadeh M, Ahmadi Z, Farkhondeh T, Samarghandian S. Autophagy as a molecular target of quercetin underlying its protective effects in human diseases. Arch Physiol Biochem. (2019) 128:200–8.
87. Siedlecka-Kroplewska K, Ślebioda T, Kmieć Z. Induction of autophagy, apoptosis and aquisition of resistance in response to piceatannol toxicity in MOLT-4 human leukemia cells. Toxicol Vitro. (2019) 59:12–25. doi: 10.1016/j.tiv.2019.03.040
88. Lee DY, Park YJ, Song MG, Kim DR, Zada S, Kim DH. Cytoprotective effects of delphinidin for human chondrocytes against oxidative stress through activation of autophagy. Antioxidants. (2020) 9:83. doi: 10.3390/antiox9010083
89. Wang S, Huang Y, Luo G, Yang X, Huang W. Cyanidin-3-O-glucoside attenuates high glucose–induced podocyte dysfunction by inhibiting apoptosis and promoting autophagy via activation of SIRT1/AMPK pathway. Can J Physiol Pharmacol. (2021) 99:589–98. doi: 10.1139/cjpp-2020-0341
90. Matthaeus C. Caveolae mediated lipid uptake and trafficking in health and disease. FASEB J. (2021) 35. doi: 10.1096/fasebj.2021.35.S1.01983
91. Martelli A, Citi V, Calderone V. Recent efforts in drug discovery on vascular inflammation and consequent atherosclerosis. Expert Opin Drug Discov. (2021) 16:411–27. doi: 10.1080/17460441.2021.1850688
92. Pillai SC, Borah A, Jacob EM, Kumar DS. Nanotechnological approach to delivering nutraceuticals as promising drug candidates for the treatment of atherosclerosis. Drug Deliv. (2021) 28:550–68. doi: 10.1080/10717544.2021.1892241
93. Poznyak AV, Nikiforov NG, Wu WK, Kirichenko TV, Orekhov AN. Autophagy and mitophagy as essential components of atherosclerosis. Cells. (2021) 10:443. doi: 10.3390/cells10020443
94. Zhao L, Zou Y, Liu F. Transforming growth factor-beta1 in diabetic kidney disease. Front Cell Dev Biol. (2020) 8:187. doi: 10.3389/fcell.2020.00187
95. Shi GJ, Shi GR, Zhou JY, Zhang WJ, Gao CY, Jiang YP, et al. Involvement of growth factors in diabetes mellitus and its complications: a general review. Biomed Pharmacother. (2018) 101:510–27. doi: 10.1016/j.biopha.2018.02.105
96. Wu M, Zhang M, Zhang Y, Li Z, Li X, Liu Z, et al. Relationship between lysosomal dyshomeostasis and progression of diabetic kidney disease. Cell Death Dis. (2021) 12:1–10. doi: 10.1038/s41419-021-04271-w
97. Chen W. A potential treatment of COVID-19 with TGF-β blockade. Int J Biol Sci. (2020) 16:1954. doi: 10.7150/ijbs.46891
98. Shen WX, Luo RC, Wang JQ, Chen ZS. Features of cytokine storm identified by distinguishing clinical manifestations in COVID-19. Front Public Health. (2021) 9:614. doi: 10.3389/fpubh.2021.671788
Keywords: atherosclerosis, cardio miracle, caveolae-mediated endocytosis, endothelial cells dysfunction, nitric oxide, TGF beta degradation, spectral clustering of protein swarms, vitamin D efficacy
Citation: Fliri AF and Kajiji S (2022) Functional characterization of nutraceuticals using spectral clustering: Centrality of caveolae-mediated endocytosis for management of nitric oxide and vitamin D deficiencies and atherosclerosis. Front. Nutr. 9:885364. doi: 10.3389/fnut.2022.885364
Received: 07 March 2022; Accepted: 14 July 2022;
Published: 15 August 2022.
Edited by:
Maurizio Muscaritoli, Sapienza University of Rome, ItalyReviewed by:
Simone Potje, Minas Gerais State University, BrazilCopyright © 2022 Fliri and Kajiji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anton Franz Fliri, YW50b24uZmxpcmlAZW1lcmdlbnRzYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.