
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 14 October 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.883904
This article is part of the Research TopicThe Role of Vitamin D in Metabolic and Cardiovascular HealthView all 14 articles
 Xin Yin1,2
Xin Yin1,2 Jia-Yu Chen3
Jia-Yu Chen3 Xiang-Jie Huang4
Xiang-Jie Huang4 Jia-Hong Lai3
Jia-Hong Lai3 Chang Huang3
Chang Huang3 Wang Yao5
Wang Yao5 Nan-Xi Li6
Nan-Xi Li6 Wei-Chao Huang7
Wei-Chao Huang7 Xu-Guang Guo1,3,8,9,10*
Xu-Guang Guo1,3,8,9,10*Insulin resistance, a pathological response to insulin hormone in insulin-dependent cells, is characterized by the presence of high glucose and insulin concentrations. The homeostasis model of insulin resistance (HOMA-IR) is one of the most used indexes to estimate insulin resistance by assessing the fasting glucose and insulin levels. An association was observed between vitamin D levels and insulin resistance, which varied in different ethnic groups, and there is some evidence that vitamin D supplementation could contribute to the improvement of insulin resistance. This study assessed the association between 25-hydroxyvitamin D (25[OH]D) concentration and HOMA-IR in American adults aged 20 years and older, without diabetes and other chronic diseases that can influence insulin resistance. The data from the National Health and Nutrition Examination Survey (NHANES) 2007–2014 were used by exploiting the free and publicly-accessible web datasets. Linear regression models were performed to evaluate the association between serum 25(OH)D concentration and HOMA-IR, and a negative association was observed, which remained significant following the adjustment for age, gender, race/ethnicity, education, body mass index (BMI), physical activity, the season of examination, current smoking, hypertension, the use of drugs which can influence insulin resistance, serum bicarbonates, triglycerides, and calcium and phosphorus levels. Only in non-Hispanic Blacks was this inverse association between vitamin D and HOMA-IR not observed in the fully adjusted model. Further studies are needed to explain the mechanisms of the observed ethnic/racial differences in the association of vitamin D levels with HOMA-IR.
Insulin resistance is identified as an underlying and partly modifiable pathogenic factor of type 2 diabetes mellitus (T2DM) and many related conditions (1). Even though hyperinsulinemic-euglycemic clamp is a gold standard for estimating insulin resistance, it is a quite expensive, invasive, and time-consuming method, which requires trained staff, and therefore, the homeostasis model of insulin resistance (HOMA-IR) presents one of the most simple and suitable substitutes to estimate IR, by assessing the fasting glucose and insulin levels (2).
Vitamin D is the collective name for vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) (3). Surveys from across the globe have shown that vitamin D deficiency was a global health problem that affects people of various ages and nationalities (4, 5). Numerous illnesses, including T2DM (6), obesity (7–9), metabolic syndrome (9, 10), chronic kidney disease (CKD) (11), infective diseases (including COVID-19) (12), autoimmune disorders (13), and infertility (14, 15), have been associated with insufficient vitamin D levels. Many cross-sectional surveys and meta-analyses indicated vitamin D deficiency to be inversely related to HOMA-IR (8, 16), and some meta-analyses (but not all) have shown that supplementation with vitamin D may help control glycemic response and can improve insulin resistance in patients with T2DM (17–19). Additionally, vitamin D receptor (VDR) polymorphisms are associated with insulin resistance and abnormal glucose metabolism, particularly in some ethnic groups (20, 21). Furthermore, a cross-sectional study in the USA, which was performed based on the National Health and Nutrition Examination Survey (NHANES) 2001–2006, found that Non-Hispanic Black people were at a greater risk for insulin resistance compared to White people (22), which may be due to lower serum vitamin D levels.
In this study, we aimed to examine the associations between 25-hydroxyvitamin D (25[OH]D) and HOMA-IR in American adults without diabetes and explore the factors that impact insulin resistance in particular ethnics, using the available data from NHANES 2007–2014, a large-scale and nationally representative cross-sectional surveys of the U.S. population. We hypothesized that the association between insulin resistance and vitamin D would differ across the ethnic groups.
The National Health and Nutrition Examination Survey is an ongoing, health-related survey that assesses the nutritional and health status of the American population. Survey participants were recruited by a stratified multistage probability sampling method to ensure the sample was nationally representative (23).
The original study protocol was available on the website of the ethics review board of the national center for health statistics research (https://www.cdc.gov/nchs/nhanes/irba98.htm), which was further approved by the ethical review committee (protocol # 2005–06; protocol # 2011–17). The current study was based on the existing data retrieved from NHANES, and the details were extracted from the official website (24).
This study used public data retrieved from four cycles of NHANES (2007–2008, 2009–2010, 2011–2012, and 2013–2014). Adult patients aged 20 or older with available data for HOMA-IR and vitamin D were included. The exclusion criteria were the presence of Type 1 diabetes mellitus (T1DM) and T2DM (since in patients with diabetes, HOMA-IR may not be a representative indicator of insulin resistance due to diminished insulin secretion) (25), CKD, and the use of drugs that can influence insulin sensitivity, including antidiabetic drugs, glucose elevating agents, antineoplastics and anti-retroviral agents, adrenal cortical steroids, selective estrogen receptor modulators, parathyroid hormone and analogs, antiandrogens, aromatase inhibitors, calcimimetics, antipsychotics, other metabolic agents, bone resorption inhibitors (bisphosphonates, etc.), and niacin. Although some anti-hypertensive drugs, sex hormones (including contraceptives), and statins can influence insulin sensitivity, the subjects who used those medications were not excluded from the study, because a substantial number of the subjects were using these agents (N = 1,081, N = 269, and N = 561, respectively). Nevertheless, to account for their potential influence on insulin sensitivity, the usage of these drugs was included in covariates in our regression analyses. Participants with any covariates missing were excluded.
Type 2 diabetes mellitus is diagnosed based on plasma glucose levels, including either the fasting plasma glucose value or the 2-h plasma glucose value during a 75 g oral glucose tolerance test or the glycosylated hemoglobin A1c criteria (26). However, either doctor-diagnosed or self-reported diabetes is included for certain. The participants with impaired glucose tolerance or impaired fasting glucose, in case they were not using antidiabetic drugs, were included. CKD was diagnosed based on an increased albumin/creatinine ratio (≥30 mg/g) and a decreased estimated glomerular filtration rate (<60 ml/min/1.73m2) (27). The data on the prescription medications were inquired and collected by trained interviewers.
Plasma and serum samples for fasting plasma glucose, serum insulin, 25(OH)D, bicarbonates, total calcium, phosphorus, and triglycerides were obtained and stored in the Mobile Examination Center until shipped to the Centers for Disease Control and Prevention Environmental Health Laboratory (Atlanta, Georgia). The HOMA-IR model was used to evaluate insulin resistance, calculated using the following formula: fasting serum insulin (μU/L) × fasting plasma glucose (mmol/L)/22.5 (28). Concentrations of 25(OH)D3 and 25(OH)D2 in the serum samples were analyzed using super high-ultra performance liquid chromatography-tandem mass spectrometry. Total 25(OH)D (or vitamin D) was defined as the sum of 25(OH)D3 and 25(OH)D2. In terms of the serum total vitamin D levels, the participants were classified as deficient (<50 nmol/L), suboptimal (50–75 nmol/L), and sufficient (>75 nmol/L), as recommended by the American Endocrine Society (29).
We tested all covariates if they were associated with HOMA-IR or vitamin D levels, and the significantly associated covariates were included in the adjusted linear regression models. The eligible covariates included age, gender, race/ethnicity, education, body mass index (BMI), physical activity level (PAL), the season of examination, current smoking, hypertension, the usage of antihypertensive drugs, sex hormones (30, 31), statins (32, 33), serum bicarbonates (34, 35), triglycerides (36, 37), and calcium and phosphorus levels (38–40). The race/ethnicity was divided into five groups: Mexican Americans, Other Hispanics, Non-Hispanic Whites, Non-Hispanic Blacks, and Other races/ethnicities (including Asians and mixes). Education levels were categorized as < 9th grade, 9th−11th grade (including 12th grade with no diploma), high school graduate/general educational development (GED) or equivalent, college/associate of arts (AA) degree, college graduate or above, refused, and unknown. The season of examinations was classified into November to April and May to October. The current smokers were separated from the former and never smokers. Participants who reported smoking either some days or every day at the time of the interview were considered current smokers. Participants who smoked more than 100 cigarettes during their lifetime but did not smoke currently were former smokers. Body mass index (BMI, kg/m2) was defined as body weight in kilograms divided by squared body height in meters. Physical activity level (PAL) scores were calculated to assess physical activity based on the different levels of activity, including vigorous (2 points) or moderate (1 point) work-related activity, vigorous (2 points) or moderate (1 point) leisure-time physical activity, and walking or bicycling for transportation (1 point). The minimum PAL score was 0, and the maximum PAL score was 5. Hypertension was defined as having systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg, which were measured on more than or equal to two occasions to acquire an average (41).
Median and interquartile range (IQR) were used to describe a non-normal distribution. The mean and standard deviation (SD) were used to describe a normal distribution. To compare the differences between various vitamin D status categories, the χ2 test (for nominal data), the one-way analysis of variance (ANOVA) (for continuous variables with normal distribution), and the Kruskal-Wallis's test (for continuous variables with non-normal distribution) were used. In linear correlation analyses, any continuous variable that was not normally distributed underwent log 10 transformation to ensure its normal distribution (HOMA-IR, triglycerides). Pearson correlation coefficient (r) was used for normally distributed continuous variables, while Spearman correlation coefficient (rs) was used for non-normally distributed continuous variables or ordered categorical variables. The Point-biserial correlation coefficient (rpb) was used for dichotomous variables.
The association between total vitamin D and HOMA-IR was evaluated by employing the enter-type linear regression models. Standardized beta was utilized to compare the relative predictive strength of different covariates in the regression models. The variance inflation factor (VIF) was used to assess the multicollinearity of all covariates in the regression model. In linear regression analyses, HOMA-IR and triglycerides underwent log 10 transformation. Stratified regression analyses were used to account for differences between races. Two-tail p < 0.05 were considered statistically significant. All analyses were performed using Empower stats (http://www.empowerstats.net/cn/) and SPSS software Version 21.0.
Following the exclusions, this study included a total of 6,079 participants aged 20 years or older (Figure 1). Baseline characteristics of the selected participants were classified according to varying serum vitamin D status categories as provided in Table 1.
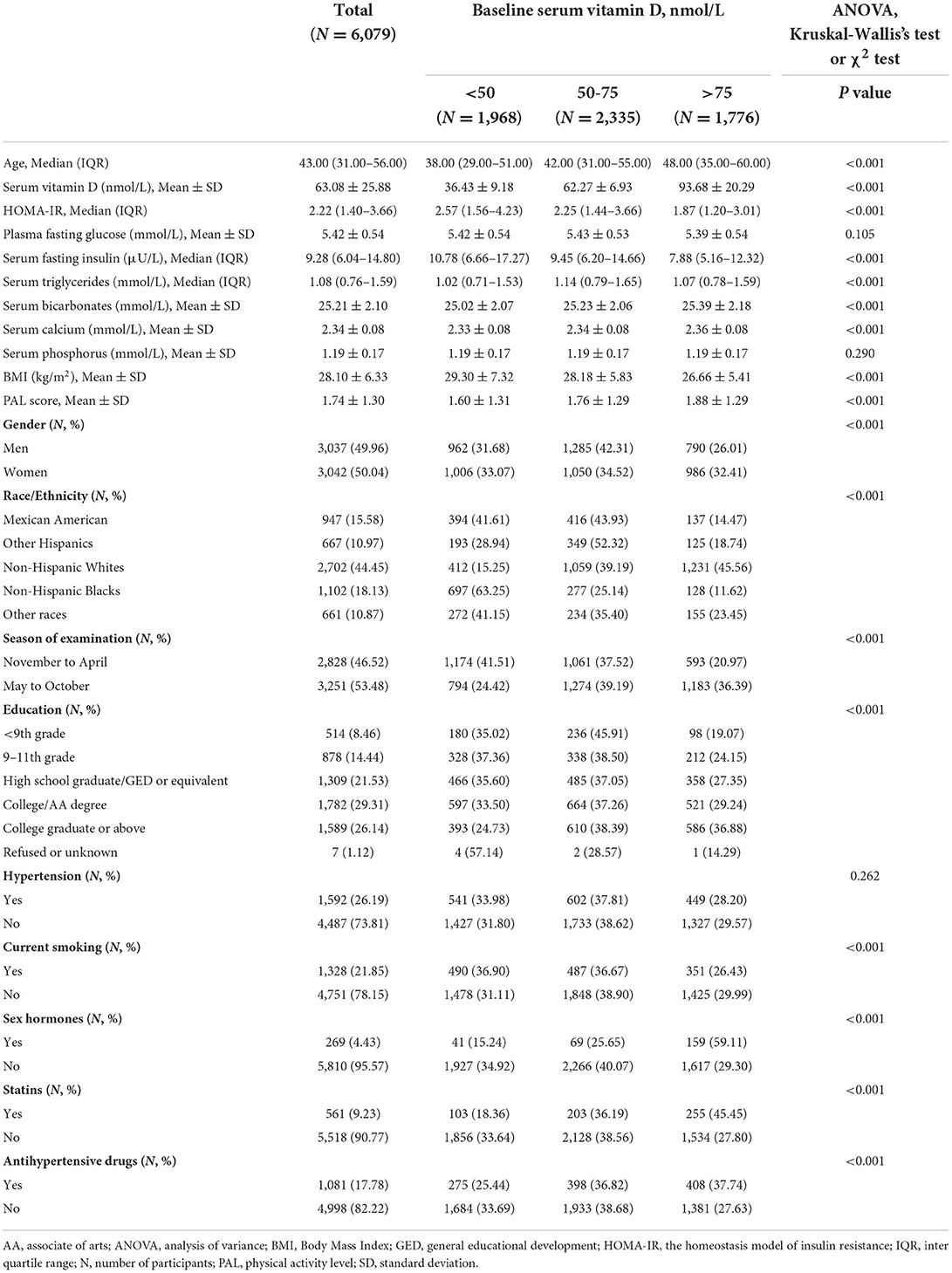
Table 1. Baseline characteristics of participants and distribution across different vitamin D categories.
The proportion of vitamin D deficiency did not differ between men and women; however, a higher proportion of vitamin D suboptimal and a lower proportion of vitamin D sufficient was found among men. The highest proportion of vitamin D deficiency was among the Non-Hispanic Blacks (63.25%), followed by Mexican Americans, other races-ethnicities (including Asians and mixes), other Hispanics, and finally, the Non-Hispanic Whites (15.25%). Meanwhile, the highest proportion of vitamin D sufficiency was observed among the non-Hispanic Whites (45.56%), while the lowest was among the non-Hispanic Blacks (11.62%).
As expected, a higher prevalence of vitamin D deficiency was observed in samples collected during the winter period (from November to April). With the exception of those who refused to answer or were left unknown about their education levels, the group with the most vitamin D deficiency belonged to the education groups of 9th−11th grade, whereas the group with the most vitamin D sufficiency was the group of college graduates or above. In the present study population, patients with hypertension and current smokers were more vitamin D deficient. Compared with the vitamin D sufficient subgroup, participants in the deficient subgroup were at the highest HOMA-IR.
Serum vitamin D levels were related to age, sex, race, educational level, BMI, PAL score, the season of examination, smoking status, usage of antihypertensive drugs, sex hormones, and statins, serum fasting insulin, triglycerides, bicarbonates, and calcium levels, as well as HOMA-IR. There was no association found between serum vitamin D levels and plasma fasting glucose, serum phosphorus, and hypertension (Table 1).
Table 2 shows that all covariates, except age and serum calcium, were linearly related to HOMA-IR. Regarding the stratified racial analysis, insulin resistance was found to be different among various races: Mexican Americans and other Hispanics were more prone to higher HOMA-IR, while the Non-Hispanic Whites and other races/ethnicities (including Asiatic) were less susceptible, while the Non-Hispanic Blacks were in the middle (Table 2).
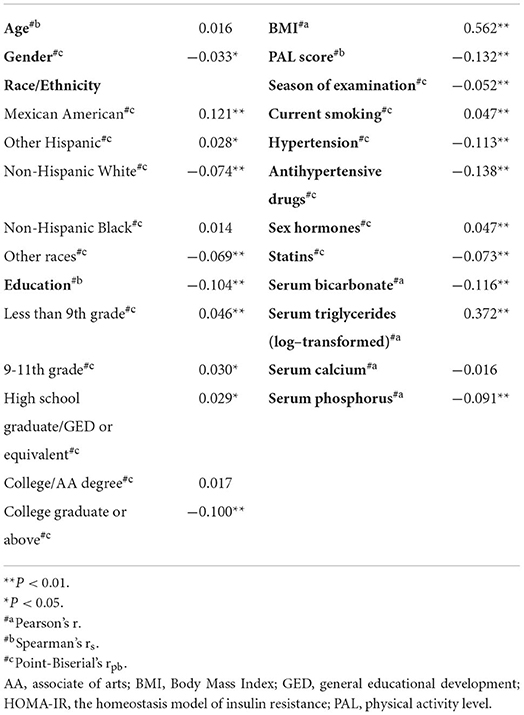
Table 2. Screening of covariates based on statistically significant association with log-transformed HOMA-IR.
Linear regression analysis (Table 3) revealed that HOMA-IR was inversely associated with vitamin D levels prior to the adjustments for covariates (Model 1). The unadjusted model described only a small variance in HOMA-IR by using only vitamin D levels (2.8%). Following the adjustments of covariates that included age, gender, specific race/ethnicity, education, BMI, physical activity, the season of examination, current smoking, hypertension, the usage of antihypertensive drugs, sex hormones, and statins, as well as serum bicarbonates, calcium, and phosphorus levels (Model 2), this inverse association between vitamin D and HOMA-IR remained significant, although it decreased. This model explained a much higher variance in HOMA-IR (36.1%). The association of vitamin D with HOMA-IR in the fully adjusted model with added log-transformed triglycerides was even more significant since the standardized regression coefficient for vitamin D increased (Model 3). This model explained the highest percentage of variance in HOMA-IR (41.3%).
In stratified regression analyses (Table 4), only in the Non-Hispanic Blacks, there was no significant inverse association between vitamin D and insulin resistance in the fully adjusted model with serum triglycerides included (Model 3).
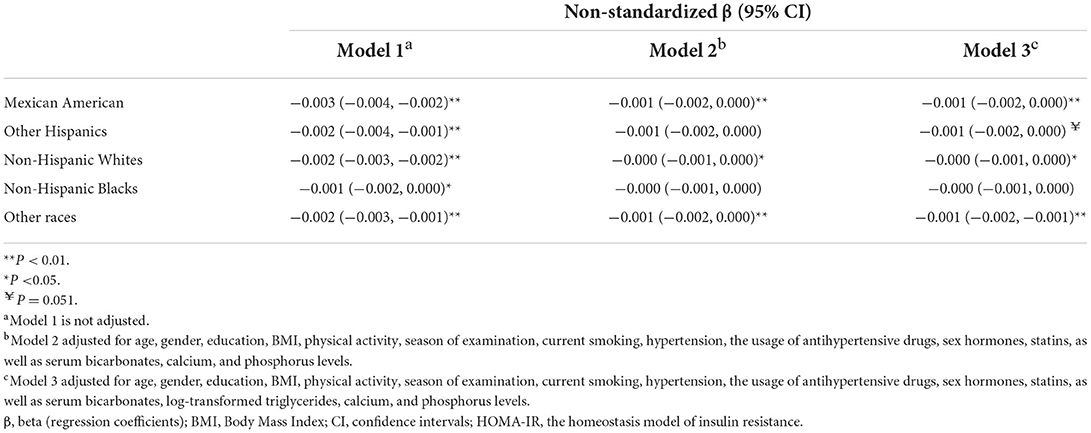
Table 4. Linear regression relationship for serum vitamin D and log-transformed HOMA-IR in stratification analysis of race/ethnicity.
In the general population or ethnic subgroups, BMI contributed the most to HOMA-IR, as shown in Supplementary Tables S1, S2. The influence of vitamin D on HOMA-IR was the strongest among other races/ethnicities (including Asiatic) compared to Mexicans and other Hispanics and the Non-Hispanic Whites, while the association was not observed in the Non-Hispanic Blacks (Supplementary Table S2).
The present study confirmed the inverse association between vitamin D and insulin resistance in accordance with many studies in different countries (42–45). However, the direct effect of vitamin D on insulin sensitivity is still controversial, since some meta-analyses indicated that vitamin D supplementation did not have the expected beneficial effects, which could be attributed to suboptimal dosing and short duration of follow-up (46, 47).
The mechanisms by which vitamin D can influence insulin sensitivity are various, and some of them are still unknown. Some studies showed that vitamin D by interacting with VDR in insulin-responsive tissues increased the transcription and number of insulin receptors (48, 49). Also, vitamin D can influence the extracellular calcium concentration and influx through the insulin-responsive cell, subsequently activating the glucose transporters, thus enhancing the response to insulin (50, 51). In addition, vitamin D could block the effect of inflammatory cytokines on insulin signaling by modulating the innate immune system and decreasing inflammatory cytokine secretion (52, 53). It is known that reactive oxygen species (ROS) can trigger insulin resistance (54), while vitamin D accelerates ROS catabolism by enhancing the synthesis of antioxidants and anti-inflammatory cytokines (55). Vitamin D can also modulate insulin sensitivity by activating peroxisome proliferator-activated receptors-δ, which reduces free fatty acid-induced insulin resistance (56, 57). Parathyroid hormone (PTH) can mediate insulin resistance by inhibiting insulin signaling and reducing glucose uptake, while vitamin D could exert an insulin-improving effect by reducing PTH levels (58). Moreover, higher PTH and vitamin D insufficiency can be jointly associated with higher HOMA-IR: the effect of PTH on insulin release from islets depends on vitamin D-related calcium and phosphorus (59, 60).
Regarding racial/ethnic differences in the association of vitamin D with HOMA-IR, one population-based investigation showed that the association between circulating 25(OH)D concentrations and insulin resistance did not differ within race (16). Conversely, other studies demonstrated that vitamin D was inversely associated with fasting insulin and insulin resistance in the Non-Hispanic Whites and Mexican Americans, but not in the Non-Hispanic Blacks (61, 62).
The reason for the lack of this association among the Non-Hispanic Blacks is still not clear. Black people have lower levels of vitamin D and higher levels of PTH compared to White people, so the negative association between vitamin D and insulin resistance should be stronger. However, in the Non-Hispanic Blacks, a decreased sensitivity to the effects of decreased vitamin D and elevated PTH was hypothesized (61, 63). Regarding 25(OH)D clearance, Black people had higher 25(OH)D clearance and lower 25(OH)D levels compared to White people, probably owing to lower levels of vitamin D binding protein (22, 62, 64, 65). The threshold for a sufficient 25(OH)D levels is the lowest among the Non-Hispanic Blacks (44), and the inverse association between 25(OH)D and PTH levels were only observed below a much lower cutoff point for vitamin D in Black people (66–68). As a result, the combined effect of PTH and vitamin D lacks in Black people.
In addition, in one study, it was observed that African Americans had significantly lower triglyceride levels for any given level of insulin sensitivity, compared with other races/ethnicities (69, 70), and in another study, it was observed that low levels of triglyceride could slightly modify the association of 25(OH)D with insulin resistance (71), which probably could explain why there was no significant association between HOMA-IR and vitamin D in the Non-Hispanic Blacks. Nonetheless, even though adding the triglyceride levels in the regression model slightly increased the association between vitamin D and HOMA-IR (as assessed by standardized beta coefficients) in the whole studied sample, adding triglyceride levels in the model still did not make this association significant in the Non-Hispanic Blacks. Therefore, other factors can contribute more to the observed disparities in the Non-Hispanic Blacks. Various types of VDR genotypes and their related variants were related to the development of insulin resistance, which may potentially affect the individual response to vitamin D supplements (72, 73), and there probably could be racial disparities in the VDR polymorphism responsible for the lower association of vitamin D levels with HOMA-IR (21, 74–77). In addition, HOMA-IR mainly reflects hepatic insulin resistance, whereas vitamin D is more associated with insulin-mediated peripheral glucose uptake (78). Similarly, as for lower levels of serum triglycerides, intrahepatic fat, and intraperitoneal fat (70), it was shown that Black people have lower hepatic glucose production compared with other races/ethnicities, despite decreased whole-body insulin sensitivity and decreased peripheral (glucose disposal) and hepatic (suppression of glucose production) insulin sensitivity, compared with White people with the same body composition (79). Additionally, they have lower hepatic insulin clearance and increased insulin production, which probably could lead to increased insulin resistance, since chronic hyperinsulinemia can lead to insulin receptor desensitization (80, 81). Therefore, studies that include hyperinsulinemic-euglycemic clamp in the Non-Hispanic Blacks are needed to test if they will reveal different results compared with our study.
This study also found that Mexican Americans were more prone to be resistant to insulin, while non-Hispanic Whites were less susceptible. Mexican Americans have higher blood glucose levels and a greater family history of obesity, diabetes, and insulin resistance compared with the Non-Hispanic Whites (82). Mexican Americans with higher insulin levels were more likely to develop T2DM about 3–5 times more than the non-Hispanic Whites (82). A study about the genetics of variation in Mexican Americans demonstrated the importance of identifying HOMA-IR linkage on chromosome 12q24, as this region contained multiple candidate genes associated with obesity and diabetes (83).
This study has some potential limitations. The inherent properties of cross-sectional designs did not allow for verifying the causal relationships between vitamin D and insulin resistance. The study was limited to the non-diabetic population in the US, and the results cannot be extrapolated to the world; hence, larger multicenter analyses included would be more universally applicable. Additionally, HOMA-IR is only a substitute for a gold standard–a hyperinsulinemic-euglycemic clamp. The strengths are that we controlled for possible confounders and that we used a large scale and representative sample with precise super high-ultra performance liquid chromatography-tandem mass spectrometry for measurements of vitamin D serum levels.
In conclusion, race/ethnicity affected the negative association of vitamin D with insulin resistance assessed by HOMA-IR among the USA non-diabetic adults, as the negative association was not seen among the Non-Hispanic Blacks. While additional studies are required to verify the results of this study and explain the racial disparities, monitoring serum 25(OH)D may be useful in detecting those with vitamin D deficiency, starting with timely and adequate supplementation to prevent possible negative metabolic consequences.
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
The studies involving human participants were reviewed and approved by Ethics Review Board of the National Center for Health Statistics Research (https://www.cdc.gov/nchs/nhanes/irba98.htm). The patients/participants provided their written informed consent to participate in this study.
XY: conceptualization, methodology, formal analysis, investigation, and data curation, writing—original draft, writing—review and editing, visualization, supervision, and project administration. J-YC: formal analysis, software, validation, investigation, visualization, and writing—original draft. X-JH: programming, data curation, and writing—review and editing. JH-L, CH, WY, and N-XL: writing—original draft and writing—review and editing. W-CH: conceptualization. X-GG: project design and administration. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.883904/full#supplementary-material
AA, associate of arts; ANOVA, analysis of variance; BMI, body mass index; CKD, chronic kidney disease; GED, general educational development; HOMA-IR, homeostasis model of insulin resistance; IQR, interquartile range; NHANES, National Health and Nutrition Examination Survey; PAL, physical activity level; PTH, parathyroid hormone; ROS, reactive oxygen species; SD, standard deviation; T2DM, type 2 diabetes mellitus; VDR, vitamin D receptor; VIF, variance inflation factor; 25(OH)D, 25-hydroxyvitamin D.
1. Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab Vasc Dis Res. (2019) 16:118–27. doi: 10.1177/1479164119827611
2. Rudvik A, Månsson M. Evaluation of surrogate measures of insulin sensitivity - correlation with gold standard is not enough. BMC Med Res Methodol. (2018) 18:64. doi: 10.1186/s12874-018-0521-y
3. Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proc Nutr Soc. (2017) 76:392–9. doi: 10.1017/S0029665117000349
4. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154-017-9424-1
5. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. (2014) 144:138–45. doi: 10.1016/j.jsbmb.2013.11.003
6. Lips P, Eekhoff M, van Schoor N, Oosterwerff M, de Jongh R, Krul-Poel Y, et al. Vitamin D and type 2 diabetes. J Steroid Biochem Mol Biol. (2017) 173:280–5. doi: 10.1016/j.jsbmb.2016.11.021
7. Rafiq S, Jeppesen PB. Body mass index, vitamin D, and type 2 diabetes: a systematic review and meta-analysis. Nutrients. (2018) 10:1182. doi: 10.3390/nu10091182
8. Rafiq S, Jeppesen PB. Vitamin D deficiency is inversely associated with homeostatic model assessment of insulin resistance. Nutrients. (2021) 13:4358. doi: 10.3390/nu13124358
9. Kauser H, Palakeel J, Ali M, Chaduvula P, Chhabra S, Lamichhane S, et al. Factors showing the growing relation between vitamin D, metabolic syndrome, and obesity in the adult population: a systematic review. Cureus. (2022) 14:e27335. doi: 10.7759/cureus.27335
10. Melguizo-Rodríguez L, Costela-Ruiz VJ, García-Recio E, De Luna-Bertos E, Ruiz C, Illescas-Montes R. Role of vitamin D in the metabolic syndrome. Nutrients. (2021) 13:830. doi: 10.3390/nu13030830
11. Jean G, Souberbielle JC, Chazot C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients. (2017) 9:328. doi: 10.3390/nu9040328
12. Martineau AR, Cantorna MT. Vitamin D for COVID-19: where are we now? Nat Rev Immunol. (2022) 22:529–30. doi: 10.1038/s41577-022-00765-6
13. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12:209. doi: 10.3390/nu12072097
14. Bosdou JK, Konstantinidou E, Anagnostis P, Kolibianakis EM, Goulis DG. Vitamin D and obesity: two interacting players in the field of infertility. Nutrients. (2019) 11:1455. doi: 10.3390/nu11071455
15. Šarac I. The Influence of Metabolic Syndrome on Reproductive Health—The Impact of Low Vitamin D. Reference Module in Food Science: Elsevier. Dutch: Elsevier (2019). doi: 10.1016/B978-0-08-100596-5.22524-9
16. Jackson JL, Judd SE, Panwar B, Howard VJ, Wadley VG, Jenny NS, et al. Associations of 25-hydroxyvitamin D with markers of inflammation, insulin resistance and obesity in black and white community-dwelling adults. J Clin Transl Endocrinol. (2016) 5:21–5. doi: 10.1016/j.jcte.2016.06.002
17. Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The effect of improved serum 25-hydroxyvitamin D status on glycemic control in diabetic patients: a meta-analysis. J Clin Endocrinol Metab. (2017) 102:3097–110. doi: 10.1210/jc.2017-01024
18. Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. (2018) 10:375. doi: 10.3390/nu10030375
19. Geng J, Qiu Y, Li Y, Li J, Liao R, Du H, et al. Associations between 25-hydroxyvitamin D, kidney function, and insulin resistance among adults in the United States of America. Frontiers in nutrition. (2021) 8:716878. doi: 10.3389/fnut.2021.716878
20. Chiu KC, Chuang LM, Yoon C. The vitamin D receptor polymorphism in the translation initiation codon is a risk factor for insulin resistance in glucose tolerant Caucasians. BMC Med Genet. (2001) 2:2. doi: 10.1186/1471-2350-2-2
21. Aravindhan S, Almasoody MFM, Selman NA, Andreevna AN, Ravali S, Mohammadi P, et al. Vitamin D Receptor gene polymorphisms and susceptibility to type 2 diabetes: evidence from a meta-regression and meta-analysis based on 47 studies. J Diabetes Metab Disord. (2021) 20:845–67. doi: 10.1007/s40200-020-00704-z
22. Williams SK, Fiscella K, Winters P, Martins D, Ogedegbe G. Association of racial disparities in the prevalence of insulin resistance with racial disparities in vitamin D levels: National Health and Nutrition Examination Survey (2001-2006). Nutrition Res (New York, NY). (2013) 33:266–71. doi: 10.1016/j.nutres.2013.02.002
23. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1. (2013) 2013:1–37.
24. Centers for Disease Control Prevention (CDC). National Health and Nutrition Examination Survey. Available online at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed May 1, 2022).
25. Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. (2015) 19:160–4. doi: 10.4103/2230-8210.146874
26. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. (2019) 42 (Suppl 1):s13–28. doi: 10.2337/dc19-S002
27. KDIGO 2021. clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:s1–s276. doi: 10.1016/j.kint.2021.05.021
28. Zhao G, Ford ES, Li C. Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among U.S. adults without physician-diagnosed diabetes: NHANES, 2003-2006. Diabetes Care. (2010) 33:344–7. doi: 10.2337/dc09-0924
29. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
30. Gasparini SJ, Swarbrick MM, Kim S, Thai LJ, Henneicke H, Cavanagh LL, et al. Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation. Diabetologia. (2019) 62:1463–77. doi: 10.1007/s00125-019-4887-0
31. De Paoli M, Zakharia A, Werstuck GH. The role of estrogen in insulin resistance: a review of clinical and preclinical data. Am J Pathol. (2021) 191:1490–8. doi: 10.1016/j.ajpath.2021.05.011
32. Henriksbo BD, Tamrakar AK, Xu J, Duggan BM, Cavallari JF, Phulka J, et al. Statins promote interleukin-1β-dependent adipocyte insulin resistance through lower prenylation, not cholesterol. Diabetes. (2019) 68:1441–8. doi: 10.2337/db18-0999
33. Abbasi F, Lamendola C, Harris CS, Harris V, Tsai MS, Tripathi P, et al. Statins are associated with increased insulin resistance and secretion. Arterioscler Thromb Vasc Biol. (2021) 41:2786–97. doi: 10.1161/ATVBAHA.121.316159
34. Mandel EI, Curhan GC, Hu FB, Taylor EN. Plasma bicarbonate and risk of type 2 diabetes mellitus. Can Med Assoc J. (2012) 184:E719–25. doi: 10.1503/cmaj.120438
35. Bellasi A, Di Micco L, Santoro D, Marzocco S, De Simone E, Cozzolino M, et al. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. (2016) 17:158. doi: 10.1186/s12882-016-0372-x
36. Moro E, Gallina P, Pais M, Cazzolato G, Alessandrini P, Bittolo-Bon G. Hypertriglyceridemia is associated with increased insulin resistance in subjects with normal glucose tolerance: evaluation in a large cohort of subjects assessed with the 1999 World Health Organization criteria for the classification of diabetes. Metabolism. (2003) 52:616–9. doi: 10.1053/meta.2003.50102
37. Zheng S, Xu H, Zhou H, Ren X, Han T, Chen Y, et al. Associations of lipid profiles with insulin resistance and β cell function in adults with normal glucose tolerance and different categories of impaired glucose regulation. PLoS ONE. (2017) 12:e0172221. doi: 10.1371/journal.pone.0172221
38. Akter S, Eguchi M, Kochi T, Kabe I, Nanri A, Mizoue T. Association of serum calcium and phosphate concentrations with glucose metabolism markers: the furukawa nutrition and health study. Nutrients. (2020) 12:2344. doi: 10.3390/nu12082344
39. Yamaguchi T, Kanazawa I, Takaoka S, Sugimoto T. Serum calcium is positively correlated with fasting plasma glucose and insulin resistance, independent of parathyroid hormone, in male patients with type 2 diabetes mellitus. Metabolism. (2011) 60:1334–9. doi: 10.1016/j.metabol.2011.02.003
40. Lorenzo C, Hanley AJ, Rewers MJ, Haffner SM. Calcium and phosphate concentrations and future development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetologia. (2014) 57:1366–74. doi: 10.1007/s00125-014-3241-9
41. Prevention D. Evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2018) 138:e426–e83. doi: 10.1161/CIR.0000000000000597
42. Han B, Wang X, Wang N, Li Q, Chen Y, Zhu C, et al. Investigation of vitamin D status and its correlation with insulin resistance in a Chinese population. Public Health Nutr. (2017) 20:1602–8. doi: 10.1017/S1368980017000490
43. Pham NM, Akter S, Kurotani K, Nanri A, Sato M, Hayabuchi H, et al. Serum 25-hydroxyvitamin D and markers of insulin resistance in a Japanese working population. Eur J Clin Nutr. (2012) 66:1323–8. doi: 10.1038/ejcn.2012.169
44. Al-Khalidi B, Rotondi MA, Kimball SM, Ardern CI. Clinical utility of serum 25-hydroxyvitamin D in the diagnosis of insulin resistance and estimation of optimal 25-hydroxyvitamin D in U. S adults. J Diabetes Res. (2017) 134:80–90. doi: 10.1016/j.diabres.2017.09.010
45. Kim H, Lee H, Yim HW, Kim HS. Association of serum 25-hydroxyvitamin D and diabetes-related factors in Korean adults without diabetes: the Fifth Korea National Health and Nutrition Examination Survey 2010-2012. Prim Care Diabetes. (2018) 12:59–65. doi: 10.1016/j.pcd.2017.07.002
46. Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, et al. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2014) 99:3551–60. doi: 10.1210/jc.2014-2136
47. George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabetic Med. (2012) 29:e142–50. doi: 10.1111/j.1464-5491.2012.03672.x
48. Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct. (2002) 20:227–32. doi: 10.1002/cbf.951
49. Nicholls DG. The pancreatic β-Cell: a bioenergetic perspective. Physiol Rev. (2016) 96:1385–447. doi: 10.1152/physrev.00009.2016
50. Szymczak-Pajor I, Drzewoski J, Sliwińska A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int J Mol Sci. (2020) 21:6644. doi: 10.3390/ijms21186644
51. Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol. (2018) 175:177–89. doi: 10.1016/j.jsbmb.2016.09.017
52. Sassi F, Tamone C, D'Amelio P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients. (2018) 10:1656. doi: 10.3390/nu10111656
53. Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. (2016) 167:228–56. doi: 10.1016/j.trsl.2015.08.011
54. Burgos-Morón E, Abad-Jiménez Z, Marañón AM, Iannantuoni F, Escribano-López I, López-Domènech S, et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clini Med. (2019) 8:1385. doi: 10.3390/jcm8091385
55. Wimalawansa SJ. Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. biology. (2019) 8:30. doi: 10.3390/biology8020030
56. Dunlop TW, Väisänen S, Frank C, Molnár F, Sinkkonen L, Carlberg C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. (2005) 349:248–60. doi: 10.1016/j.jmb.2005.03.060
57. Szymczak-Pajor I, Sliwińska A. Analysis of association between vitamin D deficiency and insulin resistance. Nutrients. (2019) 11:794. doi: 10.3390/nu11040794
58. Teegarden D, Donkin SS. Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev. (2009) 22:82–92. doi: 10.1017/S0954422409389301
59. Xia J, Tu W, Manson JE, Nan H, Shadyab AH, Bea JW, et al. Race-specific associations of 25-hydroxyvitamin D and parathyroid hormone with cardiometabolic biomarkers among US white and black postmenopausal women. Am J Clin Nutr. (2020) 112:257–67. doi: 10.1093/ajcn/nqaa121
60. Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Prospective associations of vitamin D status with β-cell function, insulin sensitivity, and glycemia: the impact of parathyroid hormone status. Diabetes. (2014) 63:3868–79. doi: 10.2337/db14-0489
61. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. (2004) 27:2813–8. doi: 10.2337/diacare.27.12.2813
62. Christensen MHE, Scragg RK. Consistent ethnic specific differences in diabetes risk and vitamin D status in the National Health and Nutrition Examination Surveys. J Steroid Biochem Mol Biol. (2016) 164:4–10. doi: 10.1016/j.jsbmb.2015.09.023
63. Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. (1997) 12:958–66. doi: 10.1359/jbmr.1997.12.6.958
64. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. (2013) 369:1991–2000. doi: 10.1056/NEJMoa1306357
65. Hsu S, Zelnick LR, Lin YS, Best CM, Kestenbaum B, Thummel KE, et al. Differences in 25-hydroxyvitamin D clearance by eGFR and race: a pharmacokinetic study. JASN. (2021) 32:188–98. doi: 10.1681/ASN.2020050625
66. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporosis Int. (2011) 22:1745–53. doi: 10.1007/s00198-010-1383-2
67. Xia J, Tu W, Manson JE, Nan H, Shadyab AH, Bea JW, et al. Combined associations of 25-hydroxivitamin D and parathyroid hormone with diabetes risk and associated comorbidities among U. S white and black women. Nutr Diabetes. (2021) 11:29. doi: 10.1038/s41387-021-00171-2
68. Cândido FG, Bressan J. Vitamin D: link between osteoporosis, obesity, and diabetes? Int J Mol Sci. (2014) 15:6569–91. doi: 10.3390/ijms15046569
69. Raygor V, Abbasi F, Lazzeroni LC, Kim S, Ingelsson E, Reaven GM, et al. Impact of race/ethnicity on insulin resistance and hypertriglyceridaemia. Diabetes Vascular Dis Res. (2019) 16:153–9. doi: 10.1177/1479164118813890
70. Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology (Baltimore, Md). (2009) 49:791–801. doi: 10.1002/hep.22726
71. Gong R, Tang X, Jiang Z, Luo G, Dong C, Han X. Serum 25(OH)D levels modify the association between triglyceride and IR: a cross-sectional study. Int J Endocrinol. (2022) 2022:5457087. doi: 10.1155/2022/5457087
72. Pramono A, Jocken JWE, Adriaens ME, Hjorth MF, Astrup A, Saris WHM, et al. The association between vitamin D receptor polymorphisms and tissue-specific insulin resistance in human obesity. Int J Obesity (2005). (2021). 45:818–27. doi: 10.1038/s41366-021-00744-2
73. Jain R, von Hurst PR, Stonehouse W, Love DR, Higgins CM, Coad J. Association of vitamin D receptor gene polymorphisms with insulin resistance and response to vitamin D. Metabolism. (2012) 61:293–301. doi: 10.1016/j.metabol.2011.06.018
74. Swamy GK, Garrett ME, Miranda ML, Ashley-Koch AE. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. Am J Med Genet. (2011) 155:1264–71. doi: 10.1002/ajmg.a.33583
75. Sarkissyan M, Wu Y, Chen Z, Mishra DK, Sarkissyan S, Giannikopoulos I, et al. Vitamin D receptor FokI gene polymorphisms may be associated with colorectal cancer among African American and Hispanic participants. Cancer. (2014) 120:1387–93. doi: 10.1002/cncr.28565
76. Nelson DA, Vande Vord PJ, Wooley PH. Polymorphism in the vitamin D receptor gene and bone mass in African-American and white mothers and children: a preliminary report. Ann Rheum Dis. (2000) 59:626–30. doi: 10.1136/ard.59.8.626
77. Meyer V, Saccone DS, Tugizimana F, Asani FF, Jeffery TJ, Bornman L. Methylation of the vitamin d receptor (VDR) gene, together with genetic variation, race, and environment influence the signaling efficacy of the toll-like receptor 2/1-VDR pathway. Front Immunol. (2017) 8:1048. doi: 10.3389/fimmu.2017.01048
78. Hoffman RP. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatr Diabetes. (2008) 9:57–61. doi: 10.1111/j.1399-5448.2007.00350.x
79. Ellis AC, Alvarez JA, Granger WM, Ovalle F, Gower BA. Ethnic differences in glucose disposal, hepatic insulin sensitivity, and endogenous glucose production among African American and European American women. Metabolism. (2012) 61:634–40. doi: 10.1016/j.metabol.2011.09.011
80. Ladwa M, Bello O, Hakim O, Boselli ML, Shojaee-Moradie F, Umpleby AM, et al. Exploring the determinants of ethnic differences in insulin clearance between men of Black African and White European ethnicity. Acta Diabetol. (2022) 59:329–37. doi: 10.1007/s00592-021-01809-4
81. Haffner SM, Howard G, Mayer E, Bergman RN, Savage PJ, Rewers M, et al. Insulin sensitivity and acute insulin response in African-Americans, non-Hispanic whites, and Hispanics with NIDDM: the Insulin Resistance Atherosclerosis Study. Diabetes. (1997) 46:63–9. doi: 10.2337/diab.46.1.63
82. Lorenzo C, Hazuda HP, Haffner SM. Insulin resistance and excess risk of diabetes in Mexican-Americans: the San Antonio Heart Study. J Clin Endocrinol Metab. (2012) 97:793–9. doi: 10.1210/jc.2011-2272
Keywords: vitamin D, 25-hydroxyvitamin D, insulin resistance, NHANES, cross-sectional
Citation: Yin X, Chen J-Y, Huang X-J, Lai J-H, Huang C, Yao W, Li N-X, Huang W-C and Guo X-G (2022) Association between vitamin D serum levels and insulin resistance assessed by HOMA-IR among non-diabetic adults in the United States: Results from NHANES 2007–2014. Front. Nutr. 9:883904. doi: 10.3389/fnut.2022.883904
Received: 25 February 2022; Accepted: 08 August 2022;
Published: 14 October 2022.
Edited by:
Ivana Šarac, University of Belgrade, SerbiaReviewed by:
Majid Hajifaraji, National Nutrition and Food Technology Research Institute, IranCopyright © 2022 Yin, Chen, Huang, Lai, Huang, Yao, Li, Huang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu-Guang Guo, Z3lzeWd4Z0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.