- 1Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3Changsha Social Work College, Changsha, China
Objective: To clarify the associations of dietary vitamin A and beta-carotene intake with depression based on a meta-analysis of observational studies.
Methods: An extensive literature search on February 2022 (PubMed, Web of Science and Embase) was employed to identify observational studies on the associations of dietary vitamin A and beta-carotene intake with depression. The pooled relative risk (RR) of depression for the highest vs. lowest dietary vitamin A and beta-carotene intake category, and the standard mean difference (SMD) of dietary vitamin A and beta-carotene intake for depression vs. control subjects, were calculated.
Results: A total of 25 observational studies (100,955 participants), which included 24 cross-sectional/case-control and 1 prospective cohort study, were included in this study. The overall multi-variable adjusted RR demonstrated that dietary vitamin A intake was inversely associated with depression (RR = 0.83, 95%CI: 0.70–1.00; P = 0.05). In addition, the combined SMD showed that the dietary vitamin A intake in depression was also lower than that in control subjects (SMD = −0.13, 95%CI: −0.18 to −0.07; P < 0.001). On the other hand, the overall multi-variable adjusted RR indicated that dietary beta-carotene intake was negatively associated with depression (RR = 0.63, 95%CI: 0.55–0.72; P < 0.001). The combined SMD showed that the dietary beta-carotene intake in depression was also lower than that in control subjects (SMD = −0.34, 95%CI: −0.48 to −0.20; P < 0.001).
Conclusion: Our results suggest that both dietary vitamin A and beta-carotene intake is inversely associated with depression. However, due to the limited evidence, further prospective cohort studies are still needed.
Introduction
Depression, one of the most common global mental disorders, affects females twice as much as males (1). The usual symptoms of depression are exhaustion, sadness, lack of interest in daily activities and suicide (2). Depression has affected approximately 300 million people (3), and is estimated to be the leading cause of disability worldwide by 2030 (4). Most importantly, low- and middle-income countries (LMICs) may be disproportionally suffered from depression. More than 80% of global disability due to depression comes from LMICs, and the majority of subjects suffered from depression in LMICs do not receive appropriate treatment (5). Since emerging evidence has indicated the significant role of dietary factors in depression (6, 7), the identification of affordable and accessible dietary factors for depression is important in its clinical management, especially in LMICs.
Vitamin A, a generic term for compounds with retinol biological activity, is usually found in foods derived from animal products (8, 9). Generally speaking, vitamin A is related to several physiological processes, such as differentiation and function of immune system, embryo development, vision, and energy metabolism (10). On the other hand, synthesized by photosynthetic organisms, carotenoids are served as light-harvesting scavengers during photosynthesis. Beta-carotene, the most common carotene in nature (10), is served as an important vitamin A precursor. On the contrary to vitamin A, beta-carotene is mainly derived from plant products (8). As a natural antioxidant, carotenoids protect organisms from oxidative damage via removing reactive oxygen species (ROS) and other free radicals (11). Interestingly, fundamental evidence has indicated the antidepressant property of beta-carotene, which may be associated with the reduced levels of tumor necrosis factor-a (TNF-α) and interleukin-6 (IL-6), and increased levels of brain-derived neurotrophic factor (BDNF) (12). Therefore, it seems naturally that dietary vitamin A and beta-carotene intake is negatively associated with depression.
To our best knowledge, a number of observational studies have investigated the associations of dietary vitamin A and beta-carotene intake with depression (13–37). However, no final conclusion is obtained. The present meta-analysis is therefore employed to clarify the issue. It is hypothesized that both dietary vitamin A and beta-carotene intake is inversely associated with depression.
Materials and Methods
Search Strategy
This meta-analysis study was employed according with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (38). The electronic database of PubMed, Web of Science and Embase were searched through during February 2022 (no restriction was set for the initiate time) using a combination of keywords and in-text words related to depression (“depression,” “depressive”), vitamin A (“vitamin A,” “retinol,” “prepalin”) and beta-carotene (“carotene,” “carotin,” “carotenoid”). No language restrictions were imposed in the search. To identify eligible studies, the titles and abstracts of all articles were first screened. Then, the full articles were read to include the eligible studies. Moreover, the references of the retrieved articles and reviews were also evaluated.
Study Selection
Two researchers reviewed the titles, abstracts and full texts of the retrieved studies independently for relevance evaluation, and disagreements (if any) were resolved by discussions. The included studies were required to meet the following criteria: (1) observational studies; (2) the associations of dietary vitamin A and beta-carotene intake with depression; (3) odds ratio (OR), relative risk (RR) or standard mean difference (SMD) reported. The exclusion criteria were listed as follows: (1) duplicated or irrelevant articles; (2) reviews, letters or case reports; (3) randomized controlled trials; and (4) non-human studies.
Data Extraction
The quality of each included study was evaluated in accordance with the Newcastle-Ottawa (NOS) criteria for non-randomized studies. It contains 8 items categorized into three dimensions: (1) the selection of study groups; (2) the comparability among different groups; (3) the identification of exposure or outcome of study cohorts, respectively. The included cross-sectional/case-control studies were assessed by using NOS for case-control studies, whereas cohort studies were assessed by using NOS for cohort studies. Disagreements (if any) were resolved through discussions until a consensus was reached.
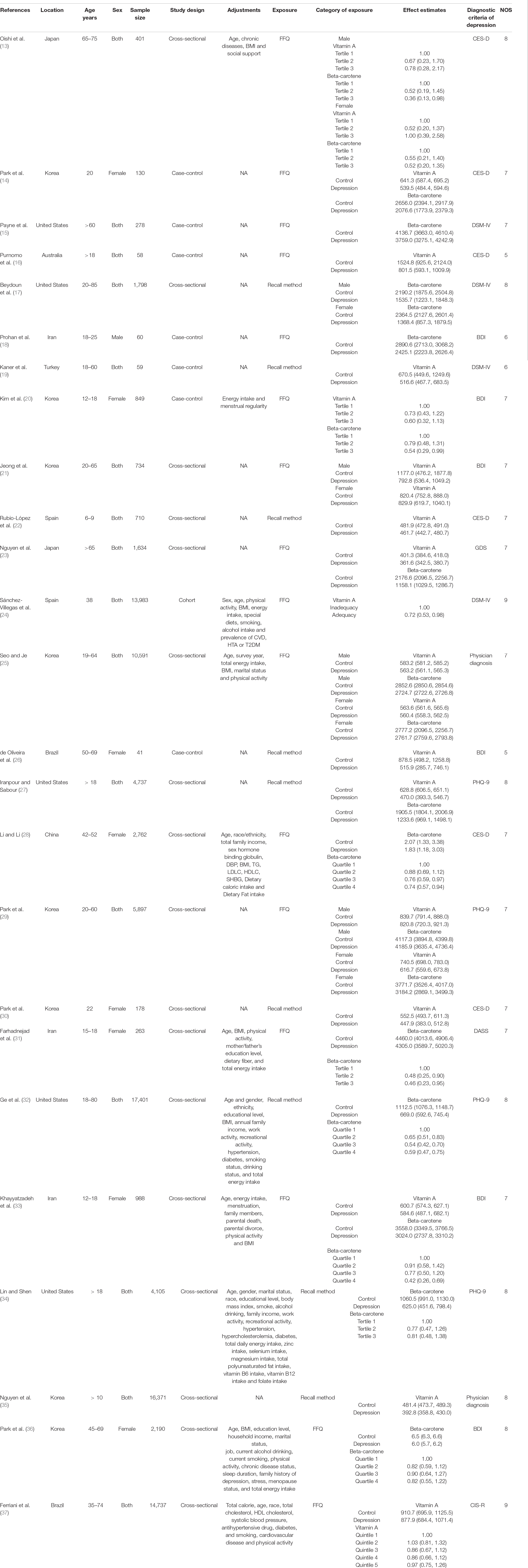
The extracted data included the first author, year of publication, location, age, sex, sample size, study design, adjustments, exposure assessment, category of exposure, effect estimates, and diagnostic criteria of depression. The corresponding effect estimates with 95% CIs for the highest vs. lowest dietary vitamin A and carotene intake category were extracted (adjusted for the maximum number of confounding variables). Moreover, the dietary vitamin A and beta-carotene intake (mean ± SD) was also extracted for depression vs. control subjects to calculate the SMD.
Statistical Analyses
The RR for depression and SMD for dietary vitamin A and beta-carotene intake were the outcome measures in our study. The I2 statistic was examined to measure the percentage of total variation across studies due to heterogeneity (I2 > 50% was considered as heterogeneity). The random-effects model was accepted if significant heterogeneity was observed among the studies; otherwise, the fixed effects model was utilized. The publication bias was assessed by Begg’s test (39). A p-value < 0.05 was considered as statistically significant. Moreover, subgroup analysis was employed for geographical region, exposure assessment, sex, population, sample size, study design, and adjustment of BMI and energy intake.
Results
Study Identification and Selection
Figure 1 presents the study screening process. During the initial literature search, a total of 1,514 potentially relevant articles (295 for PubMed, 353 for Embase and 866 for Web of Science) were retrieved. After eliminating 340 duplicated articles, 1,174 articles were screened according to the titles and abstracts. 745 irrelevant studies were removed. Then, 208 reviews, case reports or letters, 99 non-human studies and 97 randomized control trials studies were excluded. Eventually, 25 studies (24 cross-sectional/case-control and 1 prospective cohort study) were selected for this meta-analysis (13–37).
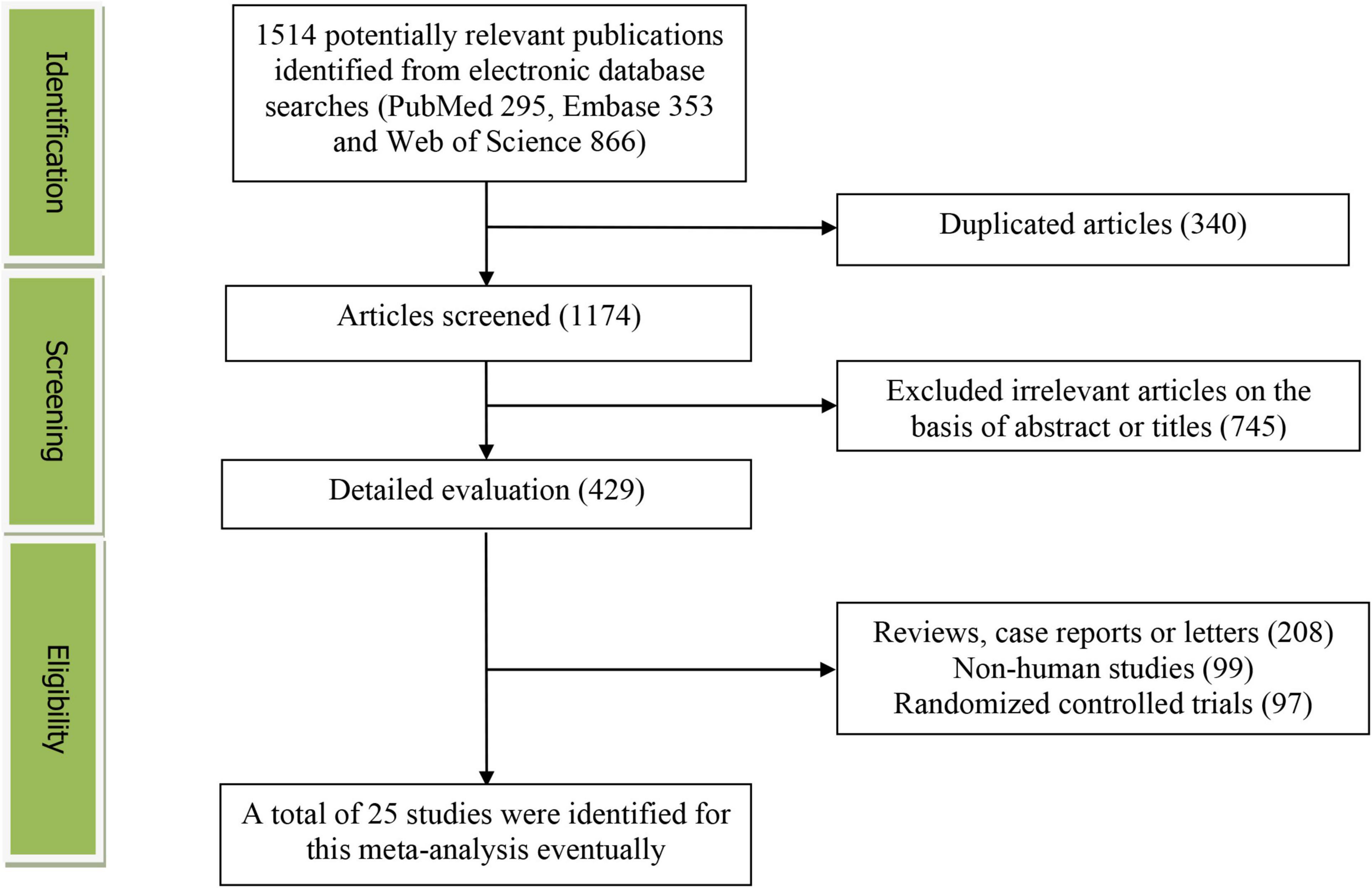
Figure 1. The detailed flow diagram of the study identification and selection in this meta-analysis.
Study Characteristics
The characteristics and NOS score of all the included studies are shown in Table 1. These studies were published between 2009 and 2022. 14 of the included studies were performed in Asian countries [Korea (14, 20, 21, 25, 29, 30, 35, 36), Iran (18, 31, 33), Japan (13, 23) and China (28)], and the other ones were conducted in United States (15, 17, 27, 32, 34), Brazil (26, 37), Australia (16), Spain (22, 24), and Turkey (19). Male, female and both male and female participants were recruited in 1 (18), 8 (14, 20, 26, 28, 30, 31, 33, 36), and 16 (13, 15–17, 19, 21–25, 27, 29, 32, 34, 35, 37) studies, respectively. The sample size ranged from 41 to 17,401 for a total number of 100,955. The exposure was assessed by food-frequency questionnaire (FFQ) in 16 studies (13–16, 18, 20, 21, 23–25, 28, 29, 31, 33, 36, 37), and recall method in 9 studies (17, 19, 22, 26, 27, 30, 32, 34, 35). The diagnostic criteria of depression were Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (15, 17, 19, 24), Patient Health Questionnaire-9 (PHQ-9) (27, 29, 32, 34), Center for Epidemiological Studies Depression Scale (CES-D) (13, 14, 16, 22, 28, 30), Beck Depression Inventory (BDI) (18, 20, 21, 26, 33, 36), Geriatric Depression Scale (GDS) (23), Clinical Interview Schedule Revised (CIS-R) (37), and Depression, Anxiety, Stress Scale (DASS) (31), respectively.
Relative Risk of Depression for the Highest vs. Lowest Category of Dietary Vitamin A Intake
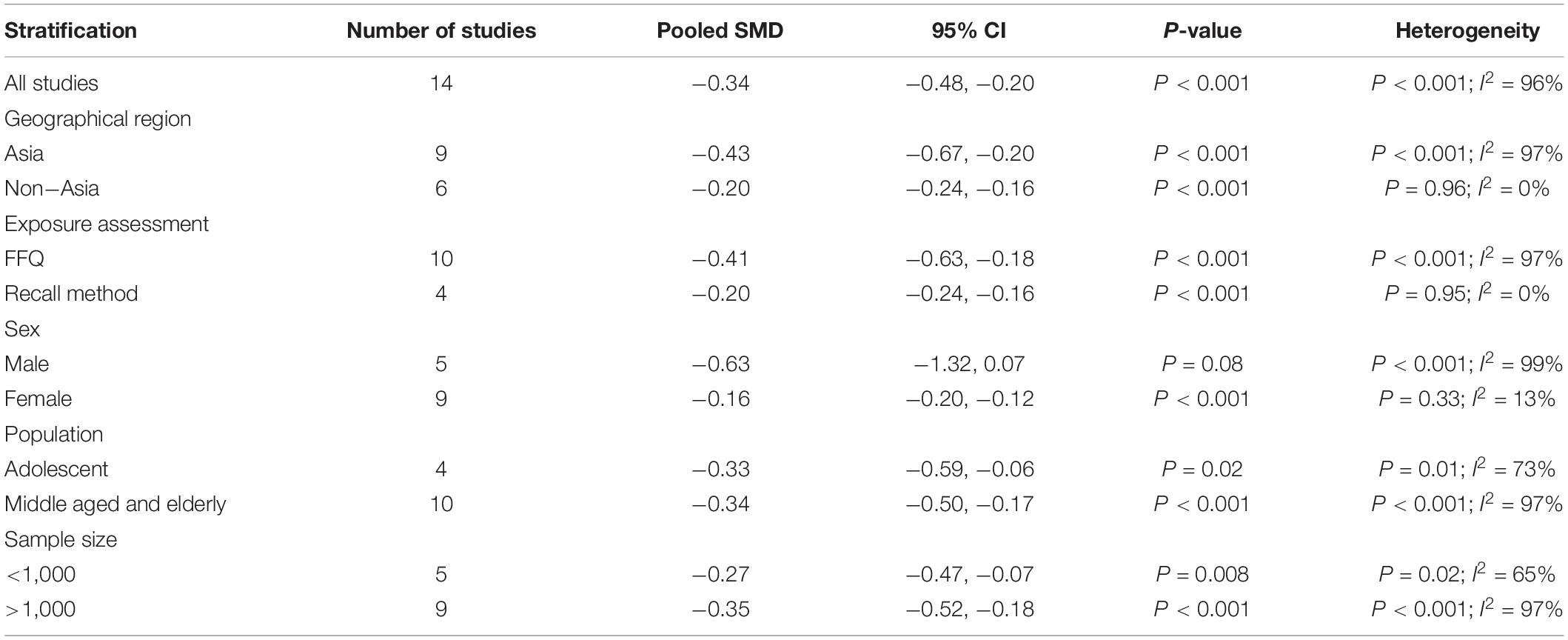
The overall multi-variable adjusted RR demonstrated that dietary vitamin A intake was negatively associated with depression (RR = 0.83, 95%CI: 0.70–1.00; P = 0.05) (Figure 2). No substantial level of heterogeneity was observed among various studies (P = 0.49, I2 = 0%). No evidence of publication bias existed according to the Begg’s rank-correlation test (P = 1.00). Table 2 presents the results of subgroup analysis. Such results only existed in females (RR = 0.75, 95%CI: 0.58–0.98; P = 0.03), cohort (RR = 0.72, 95%CI: 0.53–0.98) and adjustment of BMI (RR = 0.75, 95%CI: 0.56–0.99; P = 0.04) and energy intake (RR = 0.70, 95%CI: 0.53–0.92; P = 0.01) studies.
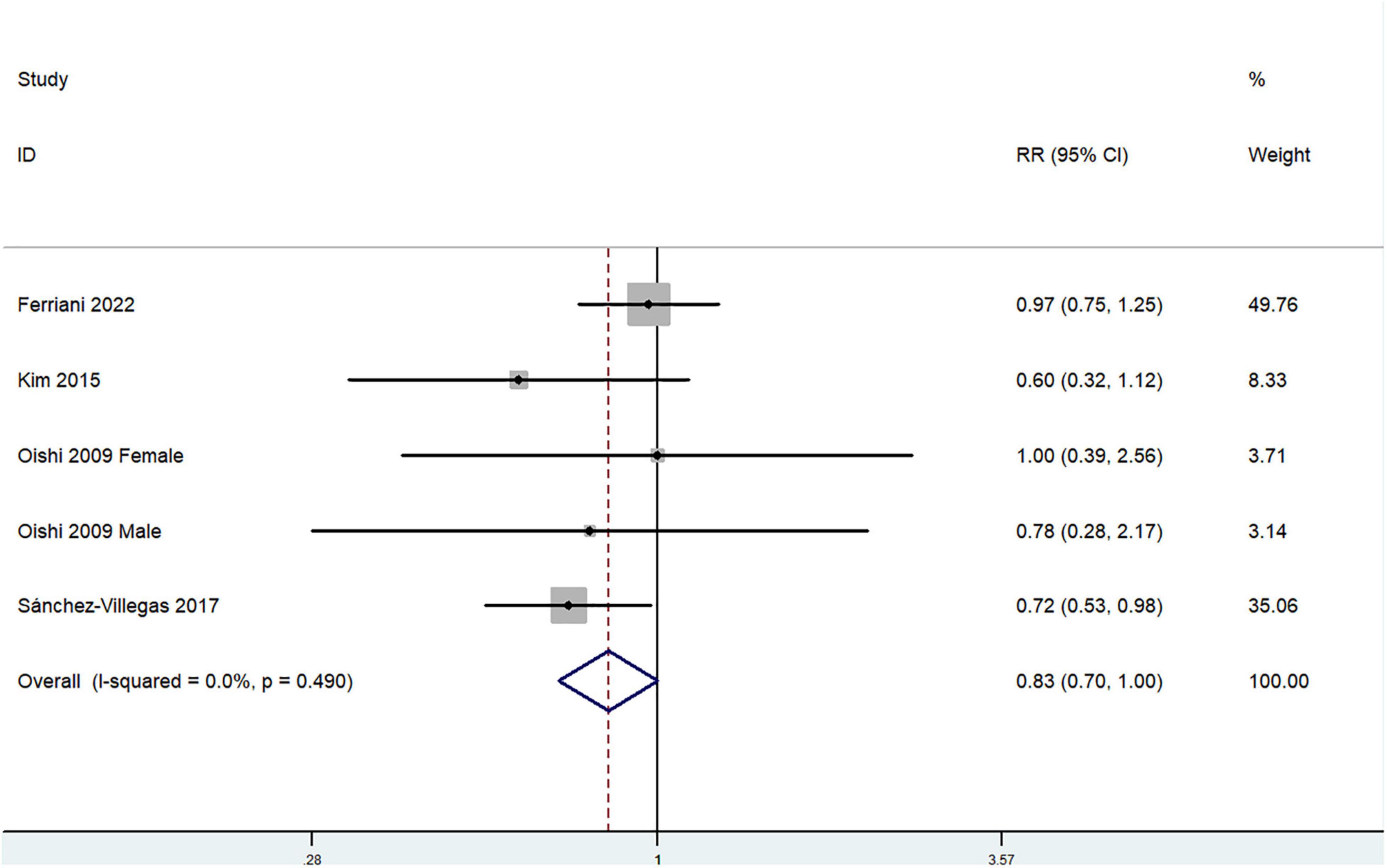
Figure 2. Forest plot of meta-analysis: Overall multi-variable adjusted RR of depression for the highest vs. lowest category of dietary vitamin A intake.
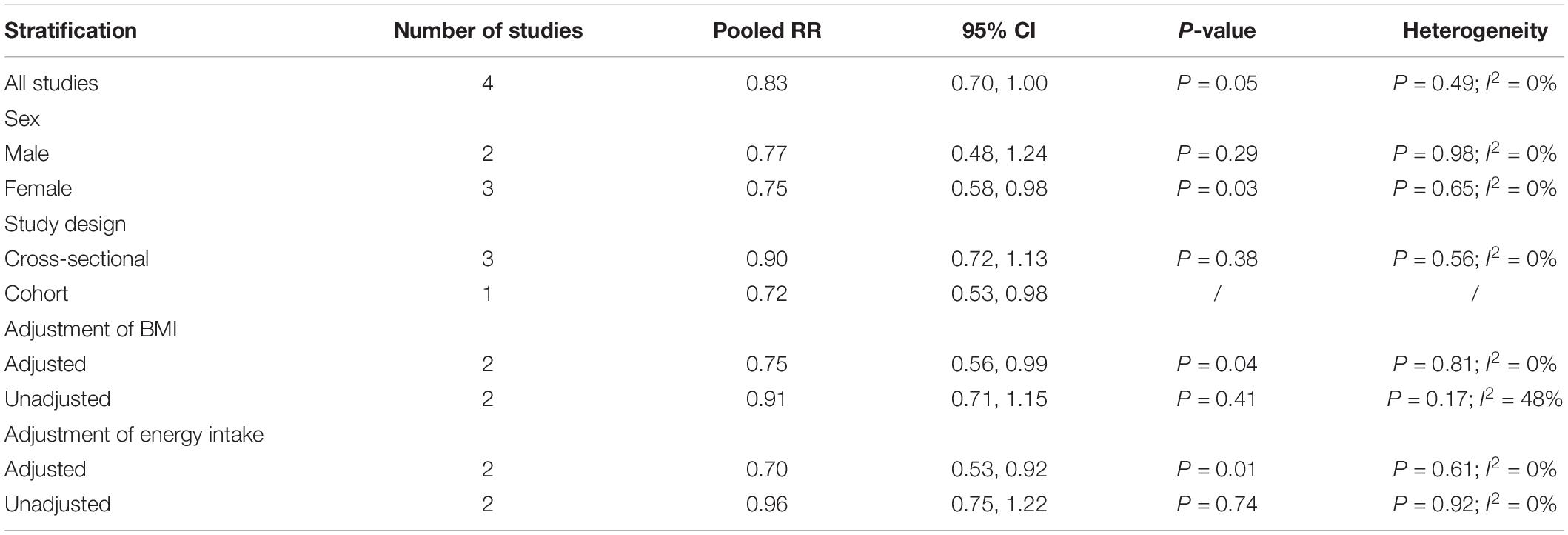
Table 2. Subgroup analysis of depression for the highest vs. lowest category of dietary vitamin A intake.
Standard Mean Difference of Dietary Vitamin A Intake for Depression vs. Control Subjects
The overall combined SMD showed that dietary vitamin A intake in depression was lower than that in control subjects (SMD = −0.13, 95%CI: −0.18 to −0.07; P < 0.001) (Figure 3). A substantial level of heterogeneity was observed among the various studies (P = 0.005, I2 = 53.4%). No evidence of publication bias existed according to the Begg’s rank-correlation test (P = 0.149). Table 3 presents the results of subgroup analysis.
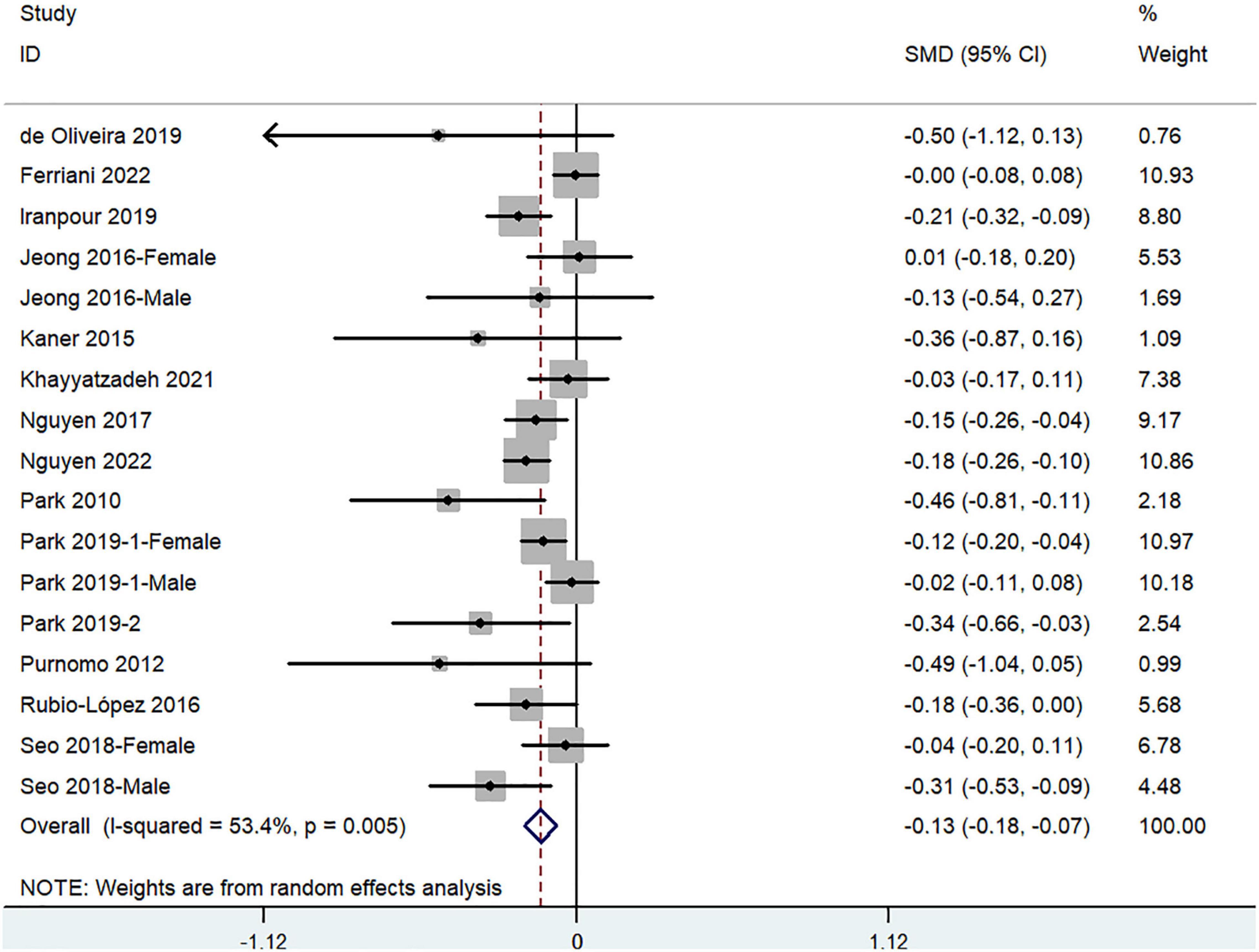
Figure 3. Forest plot of meta-analysis: SMD of dietary vitamin A intake for depression vs. control subjects.
Relative Risk of Depression for the Highest vs. Lowest Category of Dietary Beta-Carotene Intake
The overall multi-variable adjusted RR demonstrated that dietary beta-carotene intake was negatively associated with depression (RR = 0.63, 95%CI: 0.55–0.72; P < 0.001) (Figure 4). No substantial level of heterogeneity was observed among various studies (P = 0.308, I2 = 15.1%). No evidence of publication bias existed according to the Begg’s rank-correlation test (P = 0.251). Table 4 presents the results of subgroup analysis. Such results only existed in adjustment of BMI (RR = 0.62, 95%CI: 0.54–0.72; P < 0.001) studies.
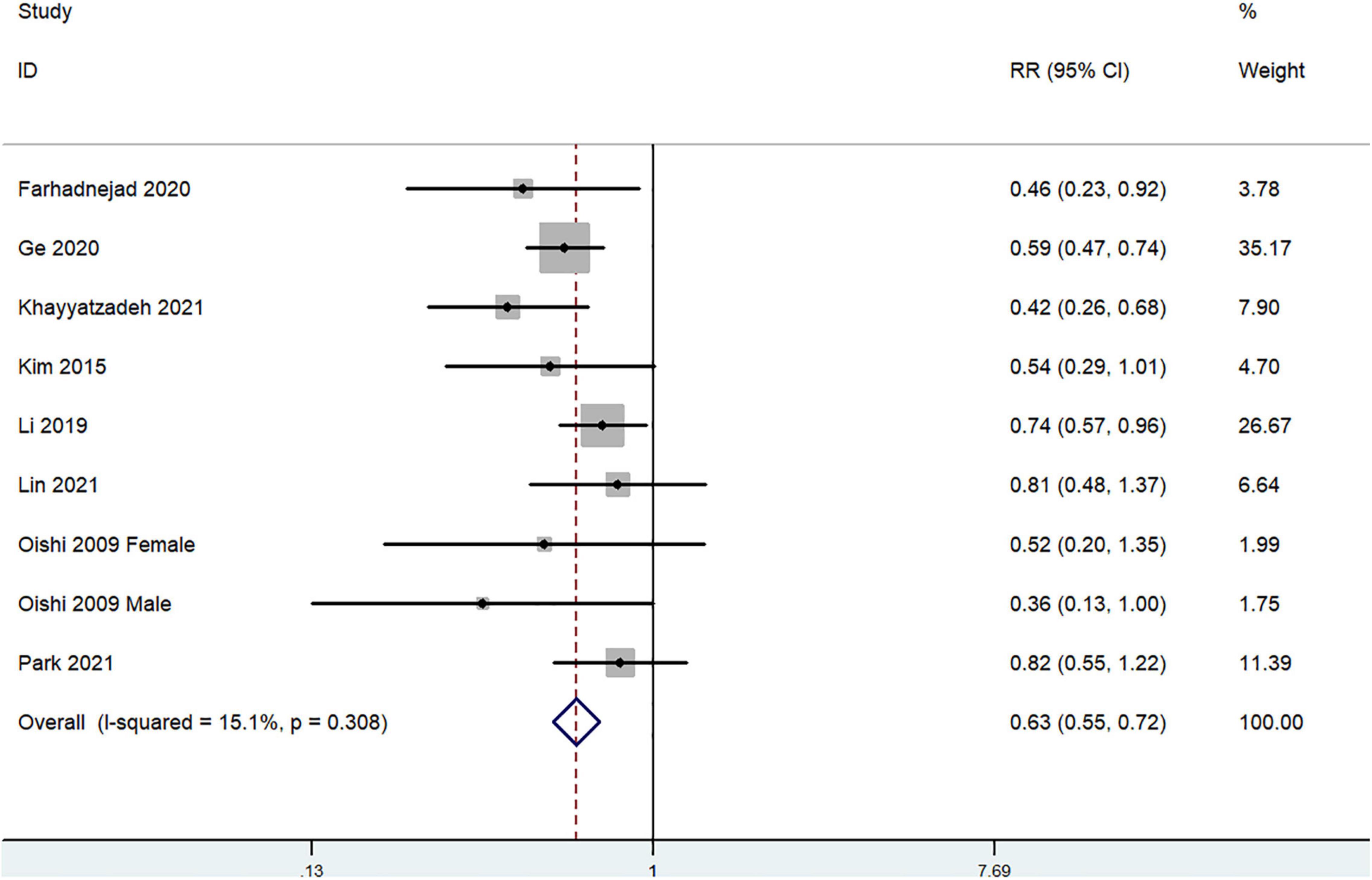
Figure 4. Forest plot of meta-analysis: Overall multi-variable adjusted RR of depression for the highest vs. lowest category of dietary beta-carotene intake.
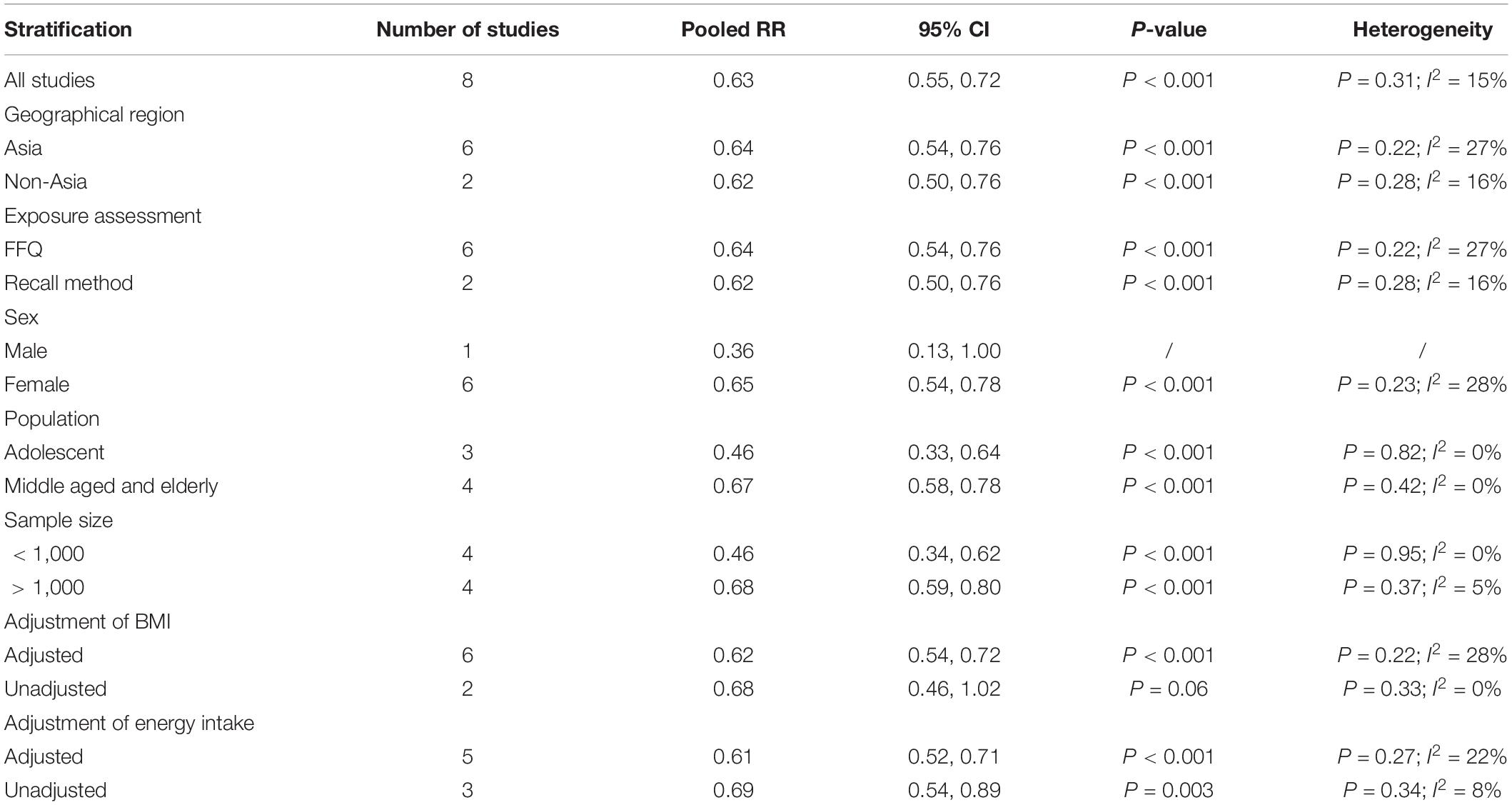
Table 4. Subgroup analysis of depression for the highest vs. lowest category of dietary beta-carotene intake.
Standard Mean Difference of Dietary Beta-Carotene Intake for Depression vs. Control Subjects
The overall combined SMD showed that dietary beta-carotene intake in depression was lower than that in control subjects (SMD = −0.34, 95%CI: −0.48 to −0.20; P < 0.001) (Figure 5). A substantial level of heterogeneity was observed among the various studies (P < 0.001, I2 = 95.7%). A publication bias existed according to the Begg’s rank-correlation test (P = 0.044). Table 5 presents the results of subgroup analysis. Such results only existed in females (SMD = −0.16, 95%CI: −0.12 to −0.20; P < 0.001) study.

Figure 5. Forest plot of meta-analysis: SMD of dietary beta-carotene intake for depression vs. control subjects.
Discussion
A total of 25 observational studies were included in the present meta-analysis. The pooled analysis showed that both dietary vitamin A and beta-carotene intake was inversely associated with depression.
The negative associations of dietary vitamin A and beta-carotene intake with depression can be explained as follow. First, oxidative stress plays a significant role in the pathophysiology of depression (40, 41). Equipped with extended π-electron system, carotenoids stabilize unpaired electrons after radical quenching. As potent scavengers for singlet oxygen and peroxyl radicals, carotenoids act through hydrogen acceptance/abstraction, donation, electron acceptance, or physical quenching (42, 43). Second, the levels of IL-6 and TNF-α are significantly increased in depression, which impairs the expression of BDNFs and then contributes to depression (44). Beta-carotene may lead to a reduction in levels of IL-6 and TNF-α mRNA in vivo (12). Third, carotenoids may act through indirect pathways and cellular signaling cascades, such as nuclear factor κB (NF-κB), mitogen-activated protein kinase (MAPK) and nuclear factor erythroid 2-related factor 2 (Nrf2) (45, 46), which are closely associated with the pathology of depression (47–50). On the other hand, randomized controlled trials have indicated the potential therapeutic effect of vitamin A supplementation on depression (51), and the dietary pattern rich in vitamin A may also exert beneficial effect on depression (52–54). Taken together, current fundamental and clinical evidence is consistence with our results.
Interestingly, some of our findings are only obtained in females [the females may be more precise and reliable in the exposure assessment (55)], it may be attributed to the potential genetic sexual differences in diet-related pathology of depression (56, 57). Importantly, the inverse relationship between dietary vitamin A intake and depression only exists in prospective cohort study, but not cross-sectional study. Although the number of prospective cohort studies is rather limited (only 1), the factors that matter the dietary vitamin A and beta-carotene intake may change after depression. For instance, depressive subjects may consume less dietary vitamin A and beta-carotene due to the reduced appetite (reversed causality). Moreover, the result of subgroup analysis suggests that BMI and energy intake may also influence the overall result. Taken together, more well-designed prospective cohort studies with sexual specification are still needed.
Since vitamin A and beta-carotene are affordable and accessible nutritional factors, our findings may build an awareness with the potential collaboration between physicians and nutritionists (especially in LMICs). Nevertheless, the safety issue should also be emphasized. For instance, excessive carotenoid intake may lead to orange/yellowish skin coloration (carotenoderma or carotenemia) (58). Moreover, long-term intake of vitamin A for several months can lead to a chronic toxicity (10 mg/day for adults and 7.5–15 mg/day in children) (59). In addition, acute vitamin A toxicity cannot be ignored neither (more than 500 mg/day in adults, and 100 mg/day in children or 30 mg/day in infants) (58). The main symptoms include irritability, nausea, blurry vision, vomiting, reduced appetite, hair loss, headaches, papilledema, hemorrhage, muscle pain, weakness, altered mental status, and drowsiness (60, 61). Therefore, a careful validation for its clinical application is still needed.
Several strengthens in our study should be emphasized. First, this is the first meta-analysis study on the associations of dietary vitamin A and beta-carotene intake with depression based on observational studies. Moreover, our findings may encourage to build the potential collaboration between physicians and nutritionists for depression management (especially in LMICs). Our study is also restricted to the following issues. First, due to the limited evidence, only 1 prospective cohort studies were identified (precludes causal relationships). Second, our results may be influenced by the substantial level of heterogeneity. Third, the classification of exposure and diagnostic criteria of depression varies greatly among individuals. Forth, the adjusted factors were not uniform. Fifth, the circulating level of vitamin A and beta-carotene is not considered due to the limited evidence. The significance of our study may be weakened by these limitations.
Conclusion
Our results suggest that both dietary vitamin A and beta-carotene intake is inversely associated with depression. However, due to the limited evidence, further well-designed prospective cohort studies with sexual specification are still needed.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YZ and JL conceived the idea and drafted this manuscript and guarantor of the overall content. JD and JL selected and retrieved relevant manuscript, and assessed each study. All authors revised and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82102581), the National Postdoctoral Science Foundation of China (2021M693562), the Provincial Outstanding Postdoctoral Innovative Talents Program of Hunan (2021RC2020), the Provincial Natural Science Foundation of Hunan (2019JJ40517), the Young Investigator Grant of Xiangya Hospital, the Central South University (2020Q14), and the FuQing Postdoc Program of Xiangya Hospital, Central South University (176).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kessler RC. Epidemiology of women and depression. J Affect Disord. (2003) 74:5–13. doi: 10.1016/s0165-0327(02)00426-3
2. Yary T, Aazami S. Dietary intake of zinc was inversely associated with depression. Biol Trace Elem Res. (2012) 145:286–90. doi: 10.1007/s12011-011-9202-y
3. Jung A, Spira D, Steinhagen-Thiessen E, Ilja Demuth I, Norman K. Zinc deficiency is associated with depressive symptoms-results from the Berlin aging study II. J Gerontol A Biol Sci Med Sci. (2017) 72:1149–54. doi: 10.1093/gerona/glw218
4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
5. Smith L, Shin J, McDermott D, Jacob L, Barnett Y, López-Sánchez GF, et al. Association between food insecurity and depression among older adults from low- and middle-income countries. Depress Anxiety. (2021) 38:439–46. doi: 10.1002/da.23147
6. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. (2014) 99:181–97. doi: 10.3945/ajcn.113.069880
7. Zhang Y, Yang Y, Xie MS, Ding X, Li H, Liu ZC, et al. Is meat consumption associated with depression? A meta-analysis of observational studies. BMC Psychiatry. (2017) 17:409. doi: 10.1186/s12888-017-1540-7
8. Hinds TS, West WL, Knight EM. Carotenoids and retinoids: a review of research, clinical, and public health applications. J Clin Pharmacol. (1997) 37:551–8. doi: 10.1002/j.1552-4604.1997.tb04336.x
9. Zhang X, Zhang R, Moore JB, Wang YQ, Yan HY, Wu YR, et al. The effect of vitamin a on fracture risk: a meta-analysis of cohort studies. Int J Environ Res Public Health. (2017) 14:1043. doi: 10.3390/ijerph14091043
10. Miller AP, Coronel J, Amengual J. The role of ββ-carotene and vitamin A in atherogenesis: evidences from pre-clinical and clinical studies. Biochim Biophys Acta Mol Cell Biol Lipids. (2020) 1865:158635. doi: 10.1016/j.bbalip.2020.158635
11. Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. (2014) 6:466–88. doi: 10.3390/nu6020466
12. Kim NR, Kim HY, Kim MH, Kim HM, Jeong HJ. Improvement of depressive behavior by Sweetme sweet pumpkin™ and its active compound, β-carotene. Life Sci. (2016) 147:39–45. doi: 10.1016/j.lfs.2016.01.036
13. Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. (2009) 63:9–17. doi: 10.18926/AMO/31854
14. Park JY, You JS, Chang KJ. Dietary taurine intake, nutrients intake, dietary habits and life stress by depression in Korean female college students: a case-control study. J Biomed Sci. (2010) 17(Suppl. 1):S40. doi: 10.1186/1423-0127-17-S1-S40
15. Payne ME, Steck SE, George RR, Steffens DC. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. (2012) 112:2022–7. doi: 10.1016/j.jand.2012.08.026
16. Purnomo J, Jeganathan S, Begley K, Houtzager L. Depression and dietary intake in a cohort of HIV-positive clients in Sydney. Int J STD AIDS. (2012) 23:882–6. doi: 10.1258/ijsa.2012.012017
17. Beydoun MA, Beydoun HA, Boueiz A, Shroff MR, Zonderman AB. Antioxidant status and its association with elevated depressive symptoms among US adults: national health and nutrition examination surveys 2005-6. Br J Nutr. (2013) 109:1714–29. doi: 10.1017/S0007114512003467
18. Prohan M, Amani R, Nematpour S, Jomehzadeh N, Haghighizadeh MH. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-CRP levels in relation to depression scales in university male students. Redox Rep. (2014) 19:133–9. doi: 10.1179/1351000214Y.0000000085
19. Kaner G, Soylu M, Yüksel N, Inanç N, Ongan D, Başmısırlı E. Evaluation of nutritional status of patients with depression. Biomed Res Int. (2015) 2015:521481. doi: 10.1155/2015/521481
20. Kim TH, Choi JY, Lee HH, Park YS. Associations between dietary pattern and depression in Korean adolescent girls. J Pediatr Adolesc Gynecol. (2015) 28:533–7. doi: 10.1016/j.jpag.2015.04.005
21. Jeong YJ, Han AL, Shin SR, Lee SY, Kim JH. Relationship between diet and prevalence of depression among Korean adults: Korea national health and nutrition examination survey 2010. J Agric Med Community Health. (2016) 41:75–84. doi: 10.5393/jamch.2016.41.2.075
22. Rubio-López N, Morales-Suárez-Varela M, Pico Y, Livianos-Aldana L, Llopis-González A. Nutrient Intake and depression symptoms in Spanish children: the ANIVA study. Int J Environ Res Public Health. (2016) 13:352. doi: 10.3390/ijerph13030352
23. Nguyen T, Tsujiguchi H, Kambayashi Y, Hara A, Miyagi S, Yamada Y. Relationship between vitamin intake and depressive symptoms in elderly Japanese individuals: differences with gender and body mass index. Nutrients. (2017) 9:1319. doi: 10.3390/nu9121319
24. Sánchez-Villegas A, Pérez-Cornago A, Zazpe I, Santiago S, Lahortiga F, Martínez-González MA. Micronutrient intake adequacy and depression risk in the SUN cohort study. Eur J Nutr. (2018) 57:2409–19. doi: 10.1007/s00394-017-1514-z
25. Seo YR, Je YJ. A comparative study of dietary habits and nutritional intakes among Korean adults according to current depression status. Asia Pac Psychiatry. (2018) 10:e12321. doi: 10.1111/appy.12321
26. de Oliveira NG, Teixeira IT, Theodoro H, Branco CS. Dietary total antioxidant capacity as a preventive factor against depression in climacteric women. Dement Neuropsychol. (2019) 13:305–11. doi: 10.1590/1980-57642018dn13-030007
27. Iranpour S, Sabour S. Inverse association between caffeine intake and depressive symptoms in US adults: data from national health and nutrition examination survey (NHANES) 2005-2006. Psychiatry Res. (2019) 271:732–9. doi: 10.1016/j.psychres.2018.11.004
28. Li D, Li Y. Associations of α-carotenoid and β-carotenoid with depressive symptoms in late midlife women. J Affect Disord. (2019) 256:424–30. doi: 10.1016/j.jad.2019.06.003
29. Park SH, Oh EY, Kim SH, Chang KJ. Relationship among dietary taurine intake, dietary attitudes, dietary behaviors, and life stress by depression in Korean female college students. Adv Exp Med Biol. (2019) 1155:293–300. doi: 10.1007/978-981-13-8023-5_28
30. Park SY, Lum HA, Shin SR, Eo JE. Relationship between Dietary Intake and depression among Korean adults: Korea national health and nutrition examination survey 2014. Korean J Fam Pract. (2019) 9:139–46. doi: 10.21215/kjfp.2019.9.2.139
31. Farhadnejad H, Tehrani AN, Salehpour A, Hekmatdoost A. Antioxidant vitamin intakes and risk of depression, anxiety and stress among female adolescents. Clin Nutr ESPEN. (2020) 40:257–62. doi: 10.1016/j.clnesp.2020.09.010
32. Ge HH, Yang TT, Sun J, Zhang DF. Associations between dietary carotenoid intakes and the risk of depressive symptoms. Food Nutr Res. (2020) 64. doi: 10.29219/fnr.v64.3920
33. Khayyatzadeh SS, Omranzadeh A, Miri-Moghaddam MM, Arekhi S, Naseri A, Ziaee A, et al. Dietary antioxidants and fibre intake and depressive symptoms in Iranian adolescent girls. Public Health Nutr. (2021) 24:5650–6. doi: 10.1017/S1368980020004838
34. Lin S, Shen Y. Dietary carotenoids intake and depressive symptoms in US adults, NHANES 2015-2016. J Affect Disord. (2021) 282:41–5. doi: 10.1016/j.jad.2020.12.098
35. Nguyen HD, Oh HJ, Hoang N, Jo WH, Kim MS. Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: a national cross-sectional study (2009-2017). Environ Sci Pollut Res Int. (2022) 29:4574–86. doi: 10.1007/s11356-021-15986-w
36. Park SJ, Jaiswal V, Lee HJ. Dietary intake of flavonoids and carotenoids is associated with anti-depressive symptoms: epidemiological study and in silico-mechanism analysis. Antioxidants (Basel). (2021) 11:53. doi: 10.3390/antiox11010053
37. Ferriani LO, Silva DA, Molina M, Mill JG, Brunoni AR, da Fonseca M. Associations of depression and intake of antioxidants and vitamin B complex: results of the Brazilian longitudinal study of adult health (ELSA-Brasil). J Affect Disord. (2022) 297:259–68. doi: 10.1016/j.jad.2021.10.027
38. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
39. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
40. Bajpai A, Verma AK, Srivastava M, Srivastava R. Oxidative stress and major depression. J Clin Diagn Res. (2014) 8:CC04–7.
41. Liu T, Zhong SM, Liao XX, Chen J, He TT, Lai SK. A meta-analysis of oxidative stress markers in depression. PLoS One. (2015) 10:e0138904. doi: 10.1371/journal.pone.0138904
42. Woodall AA, Lee SW, Weesie RJ, Jackson MJ, Britton G. Oxidation of carotenoids by free radicals: relationship between structure and reactivity. Biochim Biophys Acta. (1997) 1336:33–42. doi: 10.1016/s0304-4165(97)00006-8
43. Woodall AA, Britton G, Jackson MJ. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim Biophys Acta. (1997) 1336:575–86. doi: 10.1016/s0304-4165(97)00007-x
44. Numakawa T, Richards M, Nakajima S, Adachi N, Furuta M, Odaka H, et al. The role of brain-derived neurotrophic factor in comorbid depression: possible linkage with steroid hormones, cytokines, and nutrition. Front Psychiatry (2014) 5:136. doi: 10.3389/fpsyt.2014.00136
45. Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, et al. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. (2005) 4:177–86.
46. Palozza P, Serini S, Torsello A, Nicuolo FD, Piccioni E, Ubaldi V, et al. Beta-carotene regulates NF-kappaB DNA-binding activity by a redox mechanism in human leukemia and colon adenocarcinoma cells. J Nutr. (2003) 133:381–8. doi: 10.1093/jn/133.2.381
47. Chao B, Zhang LL, Pan JH, Zhang Y, Chen YX, Xu MM, et al. Stanniocalcin-1 overexpression prevents depression-like behaviors through inhibition of the ROS/NF-κB signaling pathway. Front Psychiatry. (2021) 12:644383. doi: 10.3389/fpsyt.2021.644383
48. Humo M, Ayazgök B, Becker LJ, Waltisperger E, Rantamäki T, Yalcin I. Ketamine induces rapid and sustained antidepressant-like effects in chronic pain induced depression: role of MAPK signaling pathway. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 100:109898. doi: 10.1016/j.pnpbp.2020.109898
49. Huang XZ, Qu Q, Ren DM. Nrf2 Alleviates cognitive dysfunction and brain inflammatory injury via mediating Wfs1 in Rats with depression-like behaviors. Inflammation. (2022) 45:399–413. doi: 10.1007/s10753-021-01554-4
50. Zhu X, Zhang YM, Zhang MY, Chen YJ, Liu YW. Hesperetin ameliorates diabetes-associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab Brain Dis. (2021) 36:1969–83. doi: 10.1007/s11011-021-00785-6
51. Bitarafan S, Saboor-Yaraghi A, Sahraian M, Soltani D, Nafissi S, Togha M, et al. Effect of Vitamin A Supplementation on fatigue and depression in multiple sclerosis patients: a double-blind placebo-controlled clinical trial. Iran J Allergy Asthma Immunol. (2016) 15:13–9.
52. Tanprasertsuk J, Scott TM, Barbey AK, Barger K, Wang XD, Johnson MA, et al. Carotenoid-rich brain nutrient pattern is positively correlated with higher cognition and lower depression in the oldest old with no dementia. Front Nutr. (2021) 8:704691. doi: 10.3389/fnut.2021.704691
53. Das A, Cumming RG, Naganathan V, Ribeiro RV, Couteur D, Handelsman DJ, et al. The association between antioxidant intake, dietary pattern and depressive symptoms in older Australian men: the concord health and ageing in men project. Eur J Nutr. (2021) 60:443–54. doi: 10.1007/s00394-020-02255-8
54. Shakya PR, Melaku YA, Page AJ, Gill TK. Nutrient patterns and depressive symptoms among Australian adults. Eur J Nutr. (2021) 60:329–43. doi: 10.1007/s00394-020-02243-y
55. Shin S, Lee HW, Kim CE, Lim JY, Lee JK, Lee SA, et al. Egg consumption and risk of metabolic syndrome in Korean adults: results from the health examinees study. Nutrients. (2017) 9:687. doi: 10.3390/nu9070687
56. Chang CC, Chang HA, Fang WH, Chang TC, Huang SY. Gender-specific association between serotonin transporter polymorphisms (5-HTTLPR and rs25531) and neuroticism, anxiety and depression in well-defined healthy Han Chinese. J Affect Disord. (2017) 207:422–8. doi: 10.1016/j.jad.2016.08.055
57. Wurtman JJ. Depression and weight gain: the serotonin connection. J Affect Disord. (1993) 29:183–92. doi: 10.1016/0165-0327(93)90032-f
58. Carazo A, Macáková K, Matoušová K, Krèmová LK, Protti M, Mladìnka P. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. (2021) 13:1703. doi: 10.3390/nu13051703
59. Beltrán-de-Miguel B, Estévez-Santiago R, Olmedilla-Alonso B. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the national survey of dietary intake in Spain (2009-2010). Int J Food Sci Nutr. (2015) 66:706–12. doi: 10.3109/09637486.2015.1077787
Keywords: dietary vitamin A intake, dietary beta-carotene intake, depression, meta-analysis, observational studies
Citation: Zhang Y, Ding J and Liang J (2022) Associations of Dietary Vitamin A and Beta-Carotene Intake With Depression. A Meta-Analysis of Observational Studies. Front. Nutr. 9:881139. doi: 10.3389/fnut.2022.881139
Received: 22 February 2022; Accepted: 24 March 2022;
Published: 25 April 2022.
Edited by:
Mainul Haque, National Defence University of Malaysia, MalaysiaReviewed by:
Sameer Dhingra, National Institute of Pharmaceutical Education and Research, IndiaIffat Jahan, Eastern Medical College and Hospital, Bangladesh
Copyright © 2022 Zhang, Ding and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieyu Liang, amFtZXNsaWFuZzhAYWxpeXVuLmNvbQ==
 Yi Zhang
Yi Zhang Jun Ding3
Jun Ding3