- 1School of Public Health, Global Health Institute, Xi’an Jiaotong University Health Science Center, Xi’an, China
- 2School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, China
- 3The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 4Beijing Key Laboratory of Behavior and Mental Health, School of Psychological and Cognitive Sciences, Peking University, Beijing, China
- 5Department of Cardiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 6Key Laboratory of Environment and Genes Related to Diseases, Ministry of Education, Xi’an, China
- 7Key Laboratory of Molecular Cardiology, Xi’an, China
- 8Department of Cardiology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 9Community Health Sciences Division, School of Public Health, University of California, Berkeley, Berkeley, CA, United States
This study examined associations between hair, salivary, serum, and urinary cortisol concentration with adiposity-related indicators in children, and explored their potential effects modification by age, sex, cortisol measurement method, and country developmental context. We systematically searched PubMed, Web of Science, and Embase for studies examining at least one of the four aforementioned cortisol with objectively measured adiposity-related outcomes in children. Meta-analyses of cross-sectional studies revealed that hair cortisol concentration was associated with fat mass index (FMI)-standard deviation score (SDS)/FMI z-score (pooled-β = 0.04, 95% CI: 0.01, 0.08) and BMI/BMI z-score (pooled-β = 0.15, 95% CI: 0.06, 0.25), and these associations were significant among children aged ≤ 12 years (pooled-β = 0.15, 95% CI: 0.05, 0.26) and >12 years (pooled-β = 0.13, 95% CI: 0.04, 0.22), children from developed countries (pooled β = 0.12, 95% CI: 0.03, 0.21) and developing countries (pooled-β = 0.193, 95% CI: 0.188, 0.198), and in studies extracting cortisol via LC-MS/MS (pooled-β = 0.18, 95% CI: 0.06, 0.29) but not ELISA (pooled-β = 0.08, 95% CI: −0.06, 0.22). Meta-analyses of both cohort and cross-sectional studies revealed non-significant associations of morning salivary cortisol concentration and total daily cortisol output with BMI/BMI z-score. Serum cortisol concentration was not associated with BMI or waist circumference. Meta-analysis of urinary cortisol concentration and adiposity was hindered by insufficient data. These findings further corroborate understanding of chronic stress’ physiological contribution to increased pediatric obesity risk.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/#recordDetails], identifier [CRD42020215111].
Introduction
Childhood obesity persists as a global public health crisis (1–4). Recent research has identified stress as an important risk factor for childhood adiposity (5–8). Stress is a negative emotional experience accompanied by predictable biochemical, physiological, cognitive, and behavioral changes directed toward altering the stressful event or accommodating to its effects (7); such changes may further serve to increase childhood obesity risk (7). Measurement of stress is inherently complex and requires consideration of multiple dimensions, including the social, psychological, and physiological (9). Given the inherent limitations of using subjective, self-reported measures for stress, considerable literature has established the use of physiological biomarkers for the objective assessment of stress for research. However, associations between physiological measures of stress and adiposity-related indicators in children are inconsistent, preventing a unified understanding of the stress processes in childhood obesity and subsequent design of related interventions.
The hypothalamic-pituitary-adrenal (HPA) axis is the most widely studied physiological stress system. When an individual perceives stress, a physiological cascade occurs in the HPA axis, and its main downstream hormone “cortisol” has been viewed as the “gold standard” biomarker with which to assess stress (6, 10). Alterations in HPA axis may be reflected in changes in the level and diurnal trajectory of cortisol secretion (11). Cortisol can facilitate obesity by stimulating unhealthy eating behaviors and promoting fat deposition (7). Moreover, visceral adipose tissue itself is rich in 11β-hydroxysteroid dehydrogenase type I, which converts inactive cortisone to cortisol (12). Therefore, a potential bidirectional relationship between cortisol and adiposity outcomes may exist. However, in this study, our primary focus will be placed on examinations of cortisol on adiposity outcomes in children.
It is possible for laboratories to utilize blood, urine, saliva, and hair to measure cortisol (13). For many years, cortisol was obtained primarily from serum or urine, but more recent approaches have sampled saliva and hair for less invasive monitoring of HPA functioning, and each measure reflects bodily cortisol levels. Serum cortisol concentration measures the total cortisol (14). Salivary cortisol concentration is usually used to assess the circadian rhythm of cortisol (e.g., cortisol awakening response) and the secretion of cortisol under stress-induced conditions (e.g., the total output of cortisol) (15). Urine samples will generally capture HPA activity over a period of only 24 h or less. In contrast, hair cortisol concentration (HCC) have increasingly been used to assess the long-term presence and/or accumulation of cortisol in children (16, 17).
More research is needed to evaluate and understand the associations between different cortisol measures for stress with adiposity-related outcomes in children. However, the literature on such associations is very limited (18–20). To date, only one systematic review (of n = 26 studies) has provided the evidence on associations between HCC and obesity in children, finding a modest positive correlation between HCC and anthropometric measures including body mass index (BMI), BMI z-score, waist circumference (WC), and body fat (21). However, the meta-analyses of reviewed studies did not exclude those relying on self-reported weight status and did not distinguish between cross-sectional and longitudinal studies. Moreover, studies have suggested that individual (e.g., age and sex) and environmental contextual factors (e.g., country development status) may modify associations between cortisol and adiposity outcomes in children (22). For example, a study found that association between cortisol and increased BMI were stronger in early adolescence than in late adolescence (23). Another study showed that altered cortisol balance modified the net lipogenetic/lipolytic in various adipose tissue depots in a sex-dependent manner in the periphery, therefore contributing to the differential associations between cortisol and adiposity outcomes (24). Furthermore, lower socio-economic status of a country was a predictor of higher cortisol levels and obesity risk (25, 26). These findings indicate that these background factors may modify the associations between cortisol and adiposity-related outcomes in children. Interestingly, no studies have heretofore examined how different cortisol measures may vary in their associations with obesity by different sociodemographic or socio-economic factors in children.
Therefore, this systematic review and meta-analysis aimed to examine associations of different cortisol measures – hair, saliva, serum, and urine – with various adiposity-related outcomes in children, and to further explore the potential modification of these associations by external contextual factors including child age, sex, cortisol measurement method, and country developmental context. These findings will synthesize the body of evidence surrounding associations between different cortisol measures and pediatric obesity, and advance the understanding of child stress biomarker research.
Methods
This study was developed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and other recommended practice standards (e.g., Johnson and Hennessy, 2019).
Literature Search
A systematic search was performed in three electronic bibliographic databases-PubMed, Web of Science, and Embase-for relevant studies published from inception to October 2021. We developed a search strategy for databases based on keywords of seminal articles we had previously identified. Search strategies included all combinations of terms related to cortisol, anthropometric measures, and children (Supplementary Table 1).
Hand searching of references was conducted to uncover any potentially overlooked studies. Articles identified from the reference lists were further screened and evaluated using the same study criteria. Reference searching was repeated on all newly identified articles until no additionally relevant articles were found.
Study Selection
Studies that met all of the following criteria were included: (1) was cross-sectional, case-control, or longitudinal; (2) studied children under 18 years old without mental disorders or any diagnosed chronic conditions (e.g., hypertension, cardiovascular disease); (3) examined naturally occurring cortisol, assayed from either urine, saliva, hair or blood, as exposure variables; (4) analyzed objectively measured adiposity-related outcomes; (5) reported statistical associations between cortisol and adiposity-related outcomes; (6) were published in English; and (7) were peer-reviewed publications. When multiple articles reported on the same data, the article with the largest sample size and results most relevant to this review was retained. Two authors assessed all identified studies for eligibility independently and disagreements were resolved through discussion.
Data Extraction and Preparation
A standardized form was developed to collect information from selected studies. Data extracted included that on: (1) the study (e.g., first author, publication year, study design, cortisol measure[s] used, adiposity-related outcome[s] assessed, the country site of study, and the country site’s developmental context [developed vs. developing]), (2) the sample (e.g., participant age, sex, race/ethnicity), and (3) effect sizes. Acceptable adiposity-related outcomes included BMI/BMI z-score/BMI-standard deviation score (BMI-SDS), waist circumference (WC), percentage body fat (PBF), fat mass index (FMI)-SDS/FMI z-score, free fat mass index (FFMI), and waist to height ratio (WtHR), and truncal distribution of fat mass (TDFM). Data were extracted independently by two authors and discrepancies were resolved through discussion.
Study Quality Assessment
Two authors independently assessed the quality of eligible articles using the U.S. National Heart, Lung, and Blood Institute’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (27). This assessment tool rates studies based on 14 criteria. For each criterion, a score of one was assigned for “yes” and zero otherwise (i.e., “no,” “not applicable,” “not reported,” or “cannot determine”). Overall quality was rated based on the total score of the scale, with 0–3, 4–7, and 7–14 reflecting poor, fair, and good quality, respectively. Discrepancies on study quality ratings were also resolved through discussion (Supplementary Table 2).
Statistical Analysis
A meta-analysis was performed to estimate the pooled associations between different cortisol measures and adiposity-related outcomes in children. Study heterogeneity was assessed using the I2 index and Tau-squared (T2). The level of heterogeneity represented by I2 was interpreted as modest (I2 ≤ 25%), moderate (25% < I2 ≤ 50%), substantial (50% < I2 ≤ 75%), or considerable (I2 > 75%) (28). A random-effects model was applied because of assumed clinical and methodological heterogeneity among the studies (29).
Pre-specified subgroup analyses were conducted to test potential modifying effects of age, sex, country developmental context (i.e., developed vs. developing), and cortisol measurement method [i.e., enzyme-linked immunosorbent assay (ELISA) vs. liquid chromatography tandem-mass spectrometry (LC-MS/MS) vs. chemiluminescence immunoassay (CLIA) vs. electrochemiluminescence immunoassay (ECLIA) vs. Radioimmunoassay (RIA) vs. dissociation-enhanced lanthanide fluorescence immunoassay (DELFIA) vs. a time-resolved fluorescence immunoassay (TRFIA)]. Sensitivity analyses were conducted to investigate the influence of a single study on the overall pool estimation by omitting one study at a time.
Publication bias was assessed by visual inspection for symmetry/asymmetry of contour-enhanced funnel plots and Egger’s tests. All statistical analyses were conducted in Stata 14 with specific meta-analysis commands (i.e., metan and metareg) (College Station, TX, United States). All analyses used two-sided tests and p < 0.05 was considered statistically significant.
Results
Study Selection
The search identified 8,627 articles of which 38 (31 cross-sectional articles and seven longitudinal articles) were included in this systematic review, with a sample size of 18,667 children. Twenty-four articles were included in the meta-analysis (Figure 1). For testing potential modifying effects, nine (20, 30–37) of the 24 articles were further divided into 18 separate studies given differences in age, sex, indicators of adiposity, and cortisol measurement method, thus, in sum, 33 separated studies were included for meta-analysis. Study characteristics are shown in Table 1.
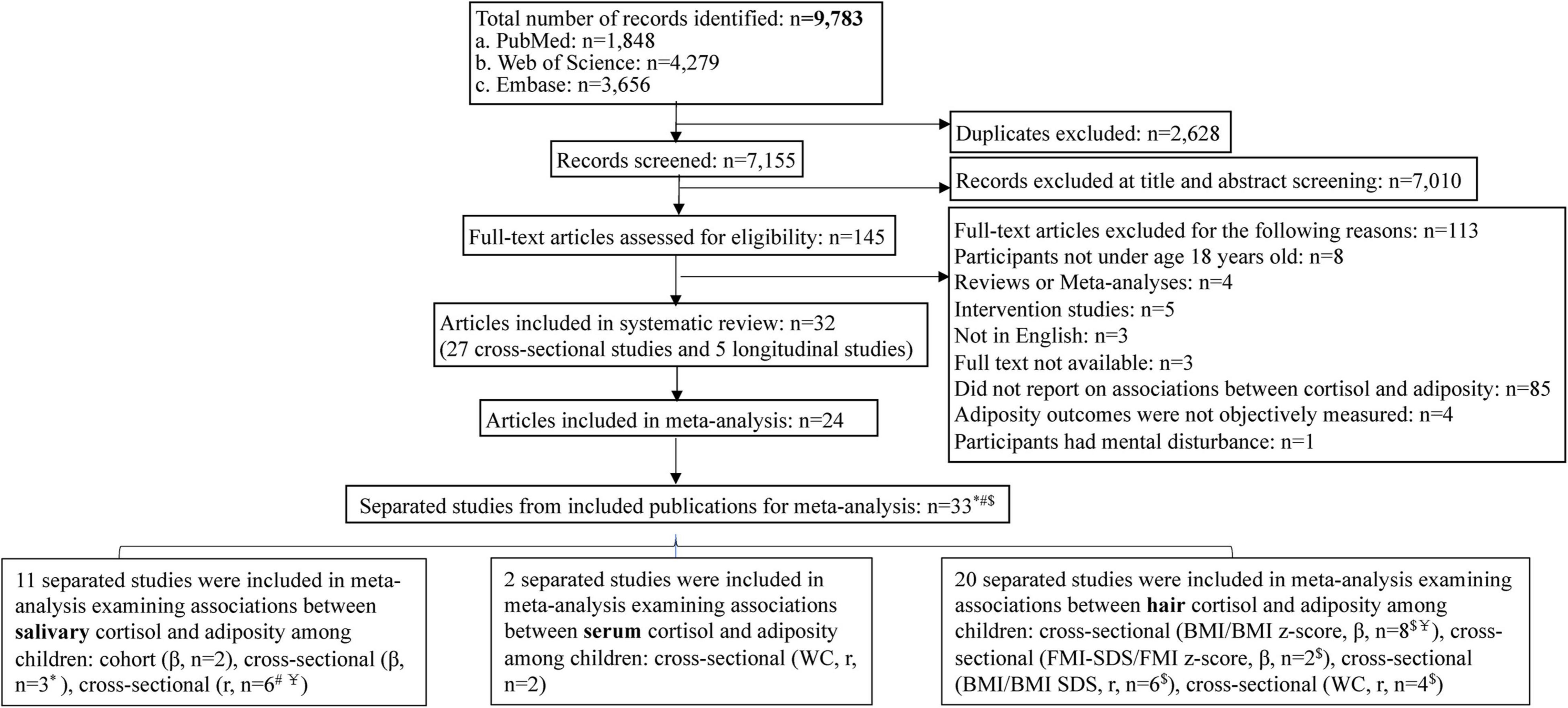
Figure 1. Flowchart of the literature search and study selection procedures. *Included articles were divided into separate studies by different age groups. #Included publications were divided into separate studies by gender. $Included articles were divided into separate studies by different indicators of adiposity. Y=Included articles were divided into separate studies by different measurement of cortisol.
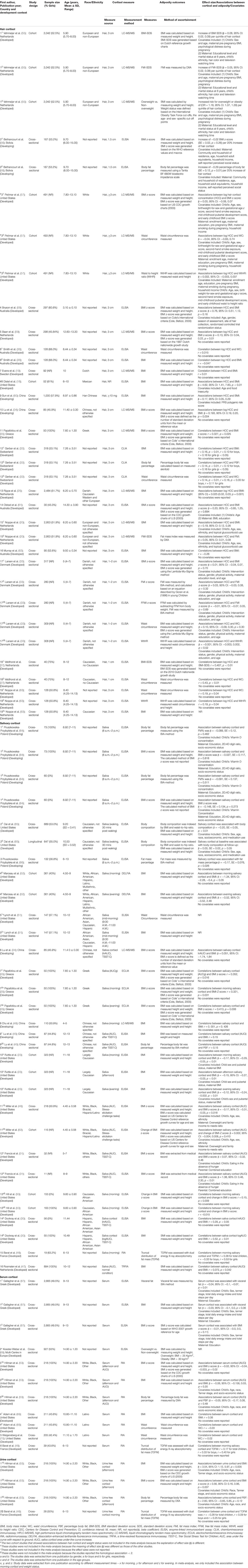
Table 1. Summary of main characteristics of 33 studies reporting on associations between hair, salivary, serum, and/or urinary cortisol concentration with adiposity-related outcomes in children.
Hair Cortisol Concentration and Adiposity-Related Outcomes Among Children
Nineteen articles encompassing 11,067 children reported on associations between HCC and adiposity-related outcomes, with three longitudinal articles, 11 articles among children aged ≤ 12 years old, 16 from developed countries, 13 using 3 cm hair samples, and ten extracting cortisol by ELISA and eight by LC-MS/MS. All articles measured BMI/BMI z-score/BMI-SDS, and six of them also measured WC, PBF, FMI-SDS/FMI z-score, and WtHR (Table 1).
Unadjusted correlations (r) between HCC and WC were significant (n = 4 studies, pooled-r = 0.16, 95% CI: 0.03, 0.28; Figure 2C). Similar unadjusted correlations were found for studies extracting HCC by ELISA (n = 3 studies, pooled-r = 0.19, 95% CI: 0.03, 0.40) and for studies by CLIA (n = 1 study, r = 0.14, 95% CI: 0.03, 0.25). However, the unadjusted correlations between HCC and BMI/BMI z-score/BMI-SDS were not significant (Figure 2D). Significant unadjusted correlations between HCC and BMI/BMI z-score were found for girls (n = 2 studies, pooled-r = 0.20, 95% CI: 0.07, 0.34) but not for boys (n = 1 study, r = 0.13, 95% CI: −0.03, 0.29; Table 2).
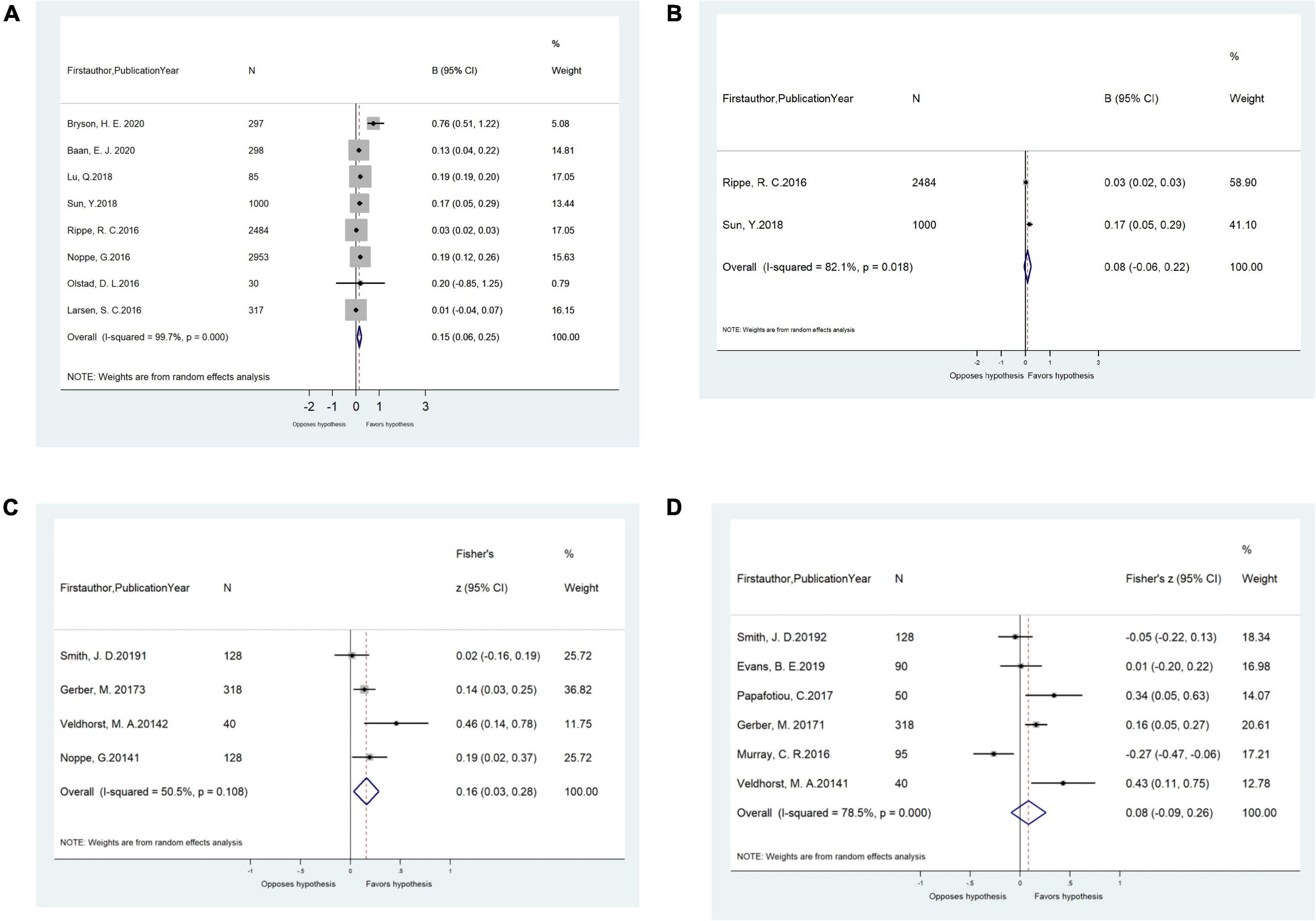
Figure 2. (A) Meta-analysis of the adjusted associations (β, 95% CI) between hair cortisol and BMI/BMI z-score in cross-sectional studies (n = 8). (B) Meta-analysis of the adjusted associations (β, 95% CI) between hair cortisol and FMI-SDS/FMI z-score in cross-sectional studies (n = 2). (C) Meta-analysis of the unadjusted correlations (r, 95% CI) between hair cortisol and waist circumference in cross-sectional studies (n = 4). (D) Meta-analysis of the unadjusted correlations (r, 95% CI) between hair cortisol and BMI/BMI z-score/BMI-SDS in cross-sectional studies (n = 6). BMI, body mass index; BMI SDS, BMI standard deviation scores.
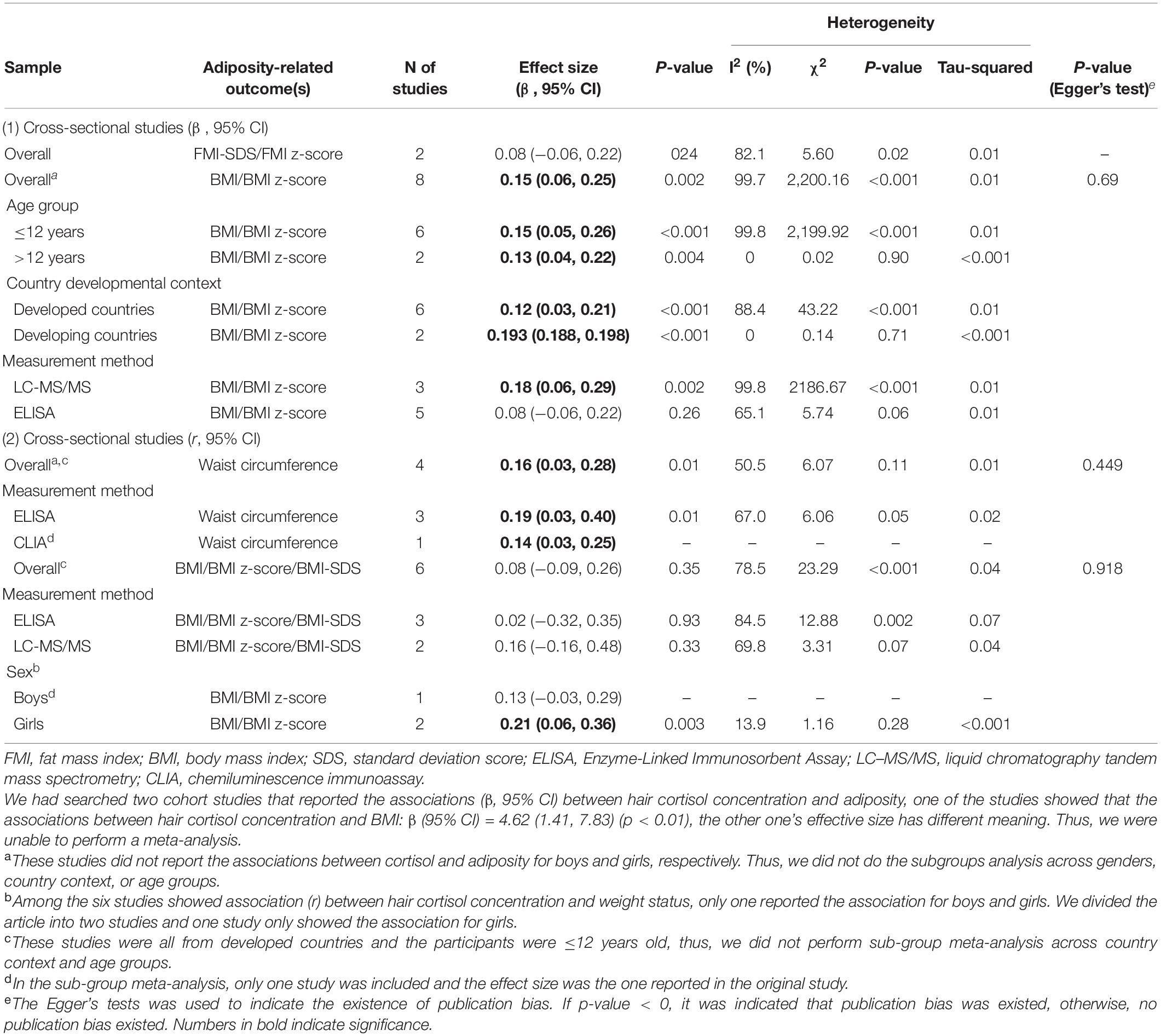
Table 2. Overall and sub-group meta-analysis of the associations between hair cortisol concentration and adiposity-related outcome(s) among children based on 20 included studies.
In meta-analyses, the pooled adjusted associations from cross-sectional studies revealed that HCC was positively associated with FMI-SDS/FMI z-score (n = 2 studies, pooled-β = 0.04, 95% confidence interval [CI]: 0.01, 0.08) and BMI/BMI z-score (n = 8 studies, pooled-β = 0.15, 95% CI: 0.06, 0.25; Figures 2A,B). Such adjusted associations varied by cortisol measurement method. Significant effects were found for studies extracting HCC by LC-MS/MS (n = 3 studies, pooled-β = 0.18, 95% CI: 0.06, 0.29) but not for those by ELISA (n = 5 studies, pooled-β = 0.08, 95% CI: −0.06, 0.22). Similar adjusted associations were observed for children aged ≤ 12 years old (n = 6 studies, pooled-β = 0.15, 95% CI: 0.05, 0.26) and children > 12 years old (n = 2 studies, pooled-β = 0.13, 95% CI: 0.04, 0.22), and for studies from developing countries (n = 2 studies, pooled-β = 0.193, 95% CI: 0.188, 0.198) and those from developed countries (n = 6 studies, pooled-β = 0.12, 95% CI: 0.03, 0.21; Table 2).
Salivary Cortisol Concentration and Adiposity-Related Outcomes Among Children
Sixteen articles with 3,462 children examined associations between salivary cortisol concentration and adiposity-related outcomes, including 13 cross-sectional articles and five longitudinal articles (two articles reported both cross-sectional and longitudinal results). Fourteen of the 16 articles examined children ≤ 12 years old, twelve articles took place in developed countries, five articles examined cortisol as AUCi (area-under-the-curve-increase) and two reported AUCg (area under the curve with respect to ground), and eleven articles used ELISA for cortisol extraction. All these articles measured BMI/BMI z-score and four also measured WC and PBF (Table 1).
In meta-analyses, the total daily cortisol output of salivary cortisol (as AUCi) was positively correlated with BMI among all children (n = 4 studies, pooled-r = 0.25, 95% CI: 0.04, 0.46) in cross-sectional studies (Figure 3B). Age and country developmental context modified such unadjusted correlations. Significant correlations were found for studies among children aged ≤ 12 years old (n = 3 studies, pooled-r = 0.30, 95% CI: 0.02, 0.61) but not for children > 12 years old (n = 1 study, r = 0.15, 95% CI: −0.06, 0.37), and for studies from developed countries (n = 3 studies, pooled-r = 0.30, 95% CI: 0.02, 0.61) but not for the study from developing country (n = 1 study, r = 0.15, 95% CI: −0.06, 0.37). The significant pooled correlations were similar for studies extracting salivary cortisol using ELISA (n = 3 studies, pooled-r = 0.33, 95% CI: 0.09, 0.58) and using TRFIA (n = 1 study, r = 0.07, 95% CI: 0.01, 0.14), and for study among boys (n = 1 study, r = 0.30, 95% CI: 0.03, 0.57) and girls (n = 2 studies, pooled-r = 0.10, 95% CI: 0.04, 0.16; Table 3).
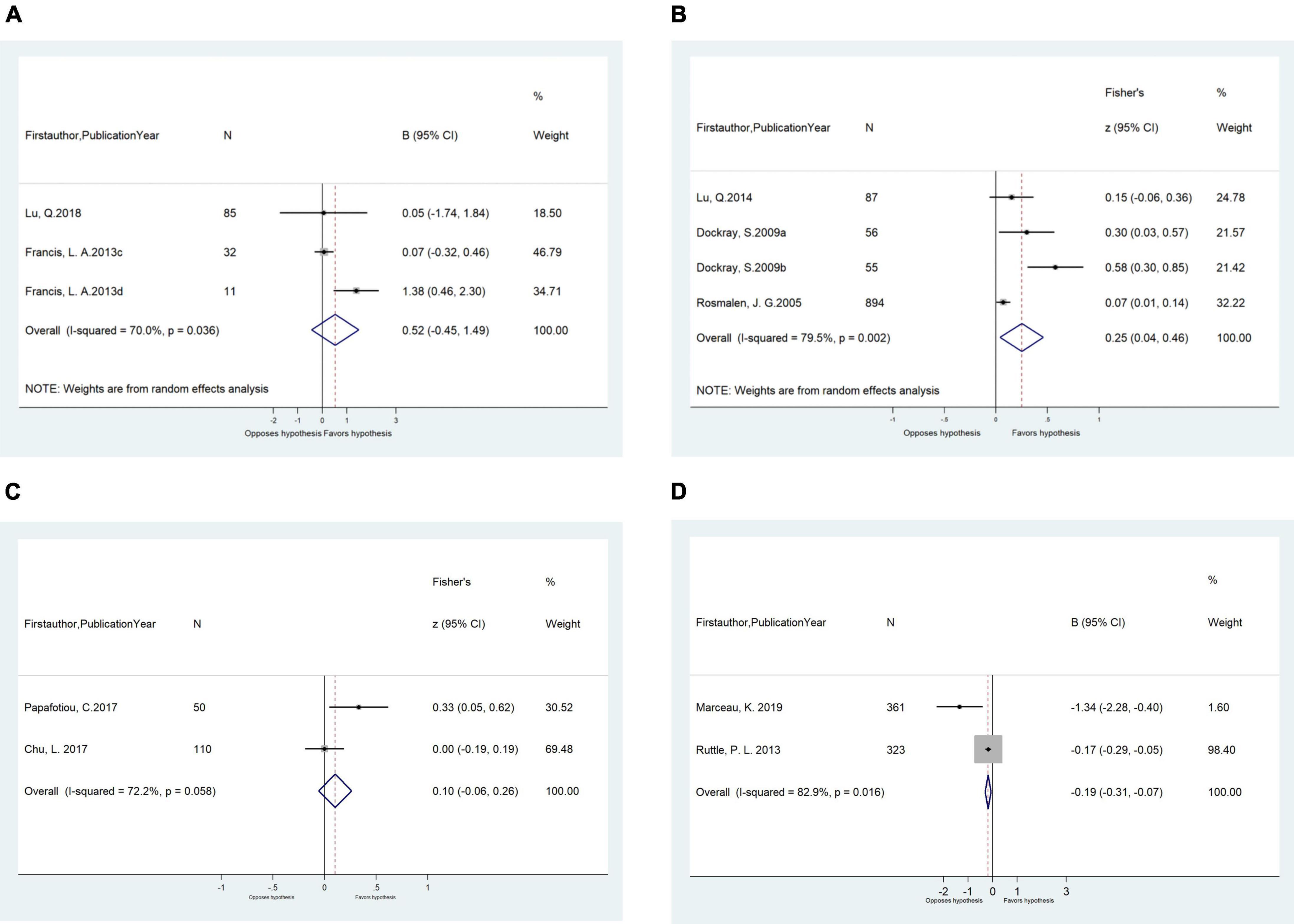
Figure 3. (A) Meta-analysis of the associations (β, 95% CI) between salivary cortisol (lnAUCi) and BMI z-score in cross-sectional studies (n = 3). (B) Meta-analysis of the unadjusted correlations (r, 95% CI) between salivary cortisol (log AUCi) and BMI in cross-sectional studies (n = 4). (C) Meta-analysis of the unadjusted correlations (r, 95% CI) between morning salivary cortisol and BMI/BMI z-score in cross-sectional studies (n = 2). (D) Meta-analysis of the longitudinal adjusted effects (β, 95% CI) of morning salivary cortisol on BMI in longitudinal studies (n = 2). BMI, body mass index.
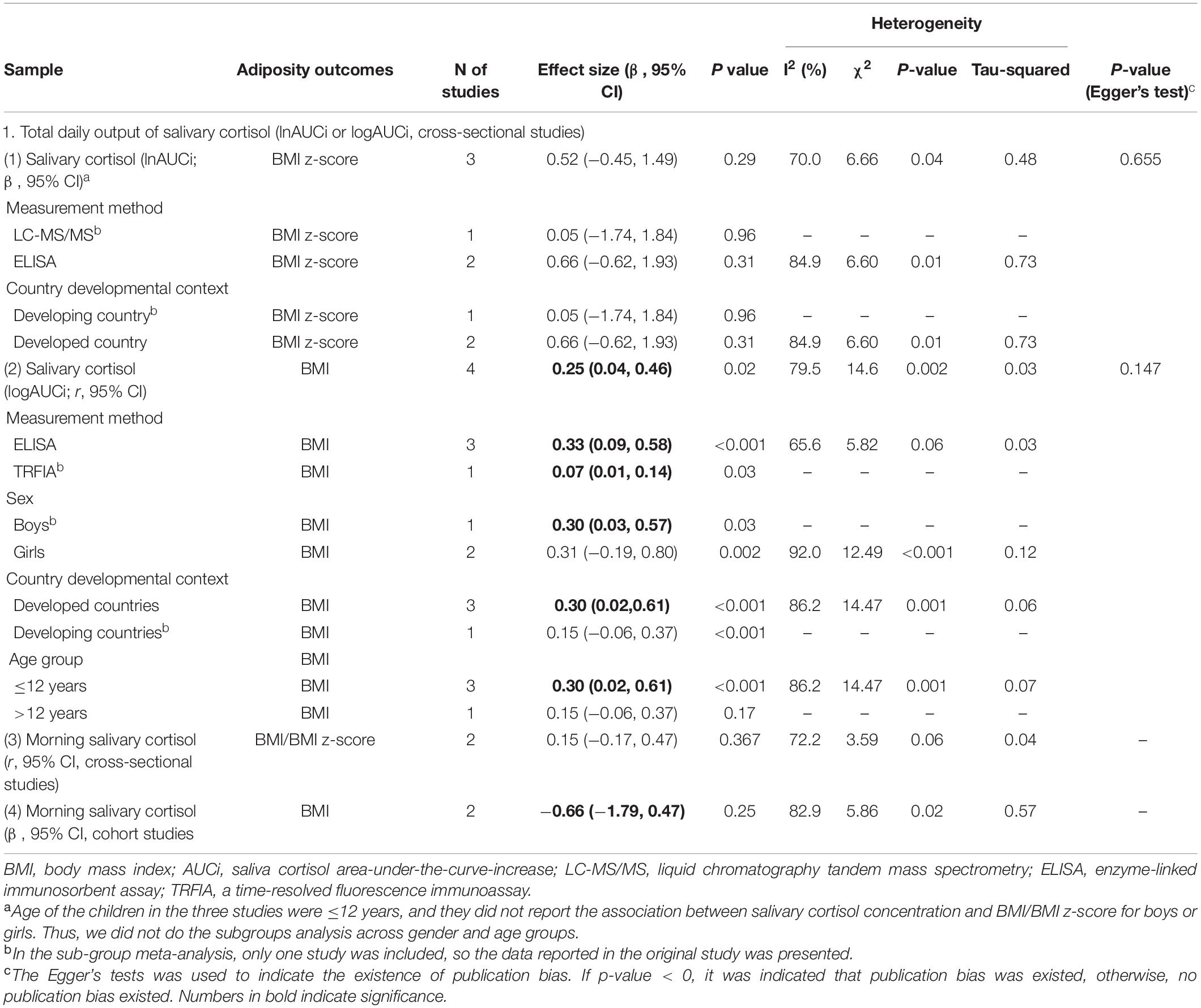
Table 3. Overall and sub-group meta-analysis of the associations between salivary cortisol concentration and adiposity-related outcomes among children based on 11 included studies.
However, the adjusted association between salivary cortisol concentration (as AUCi) and BMI z-score was non-significant (n = 3 studies, pooled-β = 0.52, 95% CI: −0.45, 1.49; Figure 3A). The associations were also non-significant stratifying by cortisol measurement method (LC-MS/MS vs. ELISA) and country developmental context (developing country vs. developed country; Table 3).
Regarding morning salivary cortisol, neither its correlations with BMI/BMI z-score from two cross-sectional studies (pooled-r = 0.10 95% CI: r = −0.06, 0.26) nor the adjusted associations from two cohort studies were significant (pooled-β = −0.19, 95% CI: −0.31, −0.07; Table 3 and Figures 3C,D).
Serum Cortisol Concentration and Adiposity-Related Outcomes Among Children
Six cross-sectional articles encompassing 4,265 children examined associations between serum cortisol concentration and adiposity-related outcomes. All were based in developed countries. Three articles were among children aged ≤ 12 years old and four articles extracted cortisol by RIA. Two articles measured BMI/BMI z-score, while others measured WC, PBF, visceral fat, and TDFM (Table 1). Pooled results showed that serum cortisol concentration was not correlated with WC (pooled-r = −0.01, 95% CI: −0.10, 0.09) from two cross-sectional studies (Table 4 and Figure 4). Meta-analysis of serum cortisol concentration and other adiposity-related outcomes were not possible due to insufficient statistical data.
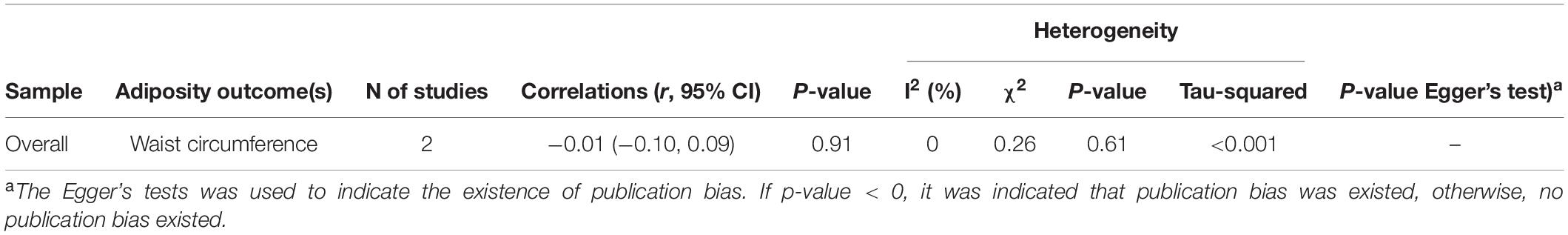
Table 4. Overall meta-analysis of the correlations (r, 95% CI) between serum cortisol concentration and waist circumference among children based on cross-sectional studies.
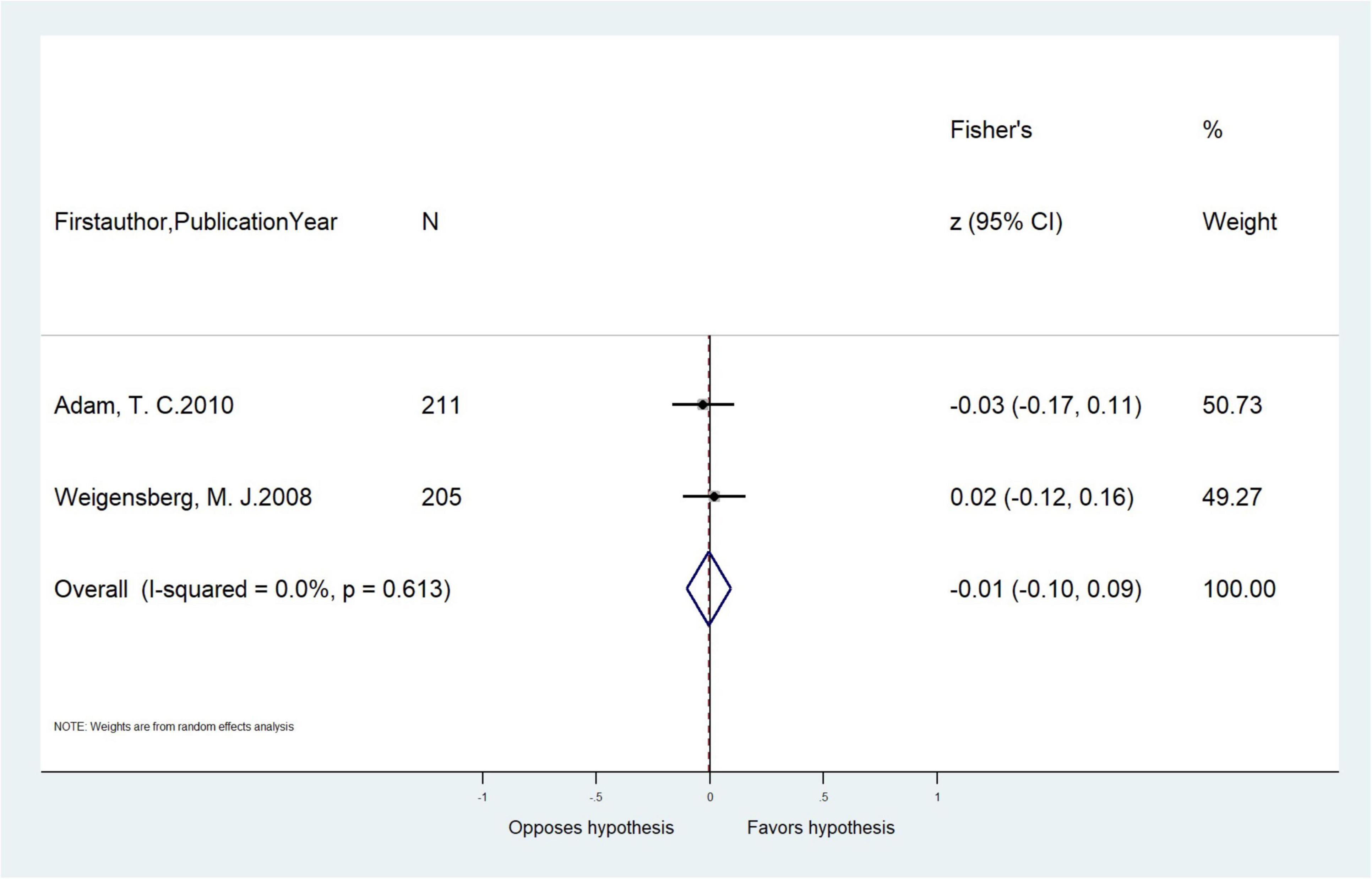
Figure 4. Meta-analysis of the unadjusted correlations (r, 95% CI) between serum cortisol concentration and waist circumference in cross-sectional studies (n = 2).
Urinary Cortisol Concentration and Adiposity-Related Outcomes Among Children
Two articles of 246 children examined associations between urinary cortisol and adiposity-related outcomes. Both were from developed countries and extracted cortisol by RIA. One study measured TDFM while the other measured BMI/BMI z-score and PBF (Table 1). Of the two articles, one reported that the correlations between urinary cortisol and TDFM was r = 0.14 (−0.24, 0.49) for all children and by sex, for boys: r = 0.09, and for girls: r = 0.25. The other article reported that urinary cortisol to be positively associated with BMI (β = 3.54, 95% CI: 1.12, 5.97) and BMI z-score (β = 0.56, 95% CI: 0.16, 0.96), but not with PBF (β = 2.60, 95% CI: −0.65, 5.85). Further subgroup meta-analysis was not possible because necessary statistics were not available.
Sensitivity Analysis and Assessment of Publication Bias
Respective sensitivity analyses were conducted to examine associations of HCC, salivary cortisol, and serum cortisol concentration with adiposity-related outcomes. Only when the study by Chu et al., 2017 was removed from the meta-analyses of cross-sectional studies did the non-significant correlations between morning salivary cortisol concentration and BMI/BMI z-score become significant (r = 0.35, 95% CI: 0.10, 0.60; Supplementary Table 3). The Egger’s tests and funnel plots indicated no publication bias within our evaluated study parameters (Table 2 and Supplementary Figure 1).
Discussion
This is the first systematic review and meta-analysis to synthesize and evaluate the associations between different cortisol measures and adiposity-related outcomes in children. We found that most of our included studies examined the associations of either HCC or salivary cortisol concentration with adiposity-related outcomes, and most studies were from developed countries. However, results from our meta-analysis indicated that only HCC, the cortisol measure that serves as an indicator of long-term stress and cumulative cortisol activity, was positively associated with objectively measured adiposity-related outcomes (i.e., FMI-SDS/FMI z-score, BMI, BMI z-score) in children. Salivary, serum, and urinary cortisol measures were not consistently associated with these adiposity-related outcomes, especially after adjustment for covariates, and/or lacked sufficient data for meta-analyses.
For HCC, meta-analysis of results from cross-sectional studies showed it to be robustly and positively associated with objectively measured adiposity-related outcomes in children, including FMI-SDS/FMI z-score and BMI/BMI z-score. The age- (≤12 years vs. >12 years) and country developmental context-stratified (developing countries vs. developed countries) analyses also supported these positive adjusted associations. Our meta-analyses result also revealed HCC to be positively correlated with WC without adjusting for covariates. These observations support the role of chronic stress or chronically elevated levels of cortisol in the development and maintenance of both general and central obesity in children. These findings are consistent with the results of a previous systematic review (21). Cortisol increases fat accumulation via glucocorticoid receptors, which have a particularly high density in visceral adipose tissue (38). Moreover, cortisol can increase food intake, especially of energy dense “comfort foods”(39), which can further contribute to increased obesity risk. The positive pooled effect sizes between HCC and adiposity-related outcomes corroborate the importance of considering chronic stress exposures over more acute stress measures when designing or evaluating childhood obesity interventions as well as in treating obesity (7).
Notably, our meta-analyses revealed the novel importance of HCC measurement method, the choice of which modified adjusted cross-sectional associations between HCC and BMI/BMI z-score in children. Only HCC extracted by LC-MS/MS, not ELISA, was associated with BMI/BMI z-scores. Immunoassays such as ELISA tend to yield higher but less accurate HCC than LC-MS/MS, possibly because ELISA overestimate steroid content given antibody cross-reactivity (40). Rather, LC-MS/MS offers superior specificity and sensitivity with its multi-analyte capabilities, making it the preferred method for HCC analysis in high-quality clinical research (41). Additionally, thanks to the high sensitivity for cortisol in hair provided by mass spectrometers, only small samples of hair are needed to run LC-MS/MS, which is conducive for large epidemiological studies among pediatric populations. Future studies should measure HCC by LC-MS/MS, and more longitudinal work is necessary to examine long-term associations.
Twelve of the 17 studies measuring HCC used hair 3 cm proximal to the scalp. Based on an average hair growth rate of 1 cm per month, such samples can reflect the cumulative cortisol and cortisone secretion of HPA axis in the previous 3 months (42). It follows then that most studies using HCC are, either consciously or not, accounting for chronic stress over the past 3-months in children. Other studies have also suggested that researchers could retrospectively examine cortisol production for a particular preceding time period when stress could have been more salient (43). However, other studies have observed HCC to decrease gradually along the length of hair shaft as distal hair samples may suffer more insults (e.g., repeated water and soap exposure) (44). Future study designs should consider these attributes and explore ways to incorporate HCC measures so as to capture cortisol levels encompassing several months. This will serve to further elucidate associations between chronic stress and childhood obesity.
In contrast to the long-term inference enabled by HCC, salivary cortisol concentration is more reflective of HPA reactivity and the stress response facilitated by laboratory settings (30). Seven (30, 31, 34, 35, 45–47) of the 13 studies (23, 30, 31, 34, 35, 45–52) used AUCi to assess increases in salivary cortisol after administering the Trier Social Stress Test for Children (TSST-C) (53). Though AUCi of salivary cortisol was correlated with BMI prior to adjusting for covariates, the adjusted associations were not significant for cross-sectional or longitudinal studies, for studies that measured salivary cortisol by ELISA or LC-MS/MS or for studies from developing or developed countries.
Rather than AUCi of salivary cortisol, the other six studies (23, 48–52) measured morning salivary cortisol to indicate the cortisol awakening response (54). However, we found neither unadjusted nor adjusted associations between morning salivary cortisol concentration and adiposity-related outcomes to be significant. These findings suggest that both cortisol awakening response and cortisol reactivity to acute stress challenge tasks are not associated with adiposity-related outcomes in children. Correspondingly, recent longitudinal studies found that obesity predicted greater changes in cortisol awakening response and cortisol reactivity to challenge in early to middle childhood, not that cortisol awakening response and cortisol reactivity predicted increased likelihood of obesity over the same time period (31). In our review, only four of the 13 included studies were longitudinal, precluding similar inferences on the direction of these associations. More longitudinal studies are needed to understand these associations.
Given the current evidence base, serum cortisol concentration was not observed to be correlated with WC and BMI in children. For urinary cortisol and adiposity-related outcomes, limited studies and data precluded further meta-analyses. However, we did have two studies examine these associations, both supporting significant positive associations between urinary cortisol and BMI (34). Still, these studies’ cross-sectional designs and solitary existence demonstrate the need for more efforts to confirm serum and urinary cortisol associations in childhood obesity.
The present systematic review and meta-analysis expands the knowledge base concerning stress biomarker utility in pediatric adiposity research by providing pooled effect sizes for different cortisol measures against objectively measured adiposity-related outcomes. These findings may help health professionals and policymakers better understand how different cortisol measures reflect underlying stress processes and how stress may contribute to adiposity in children. This review also comprehensively investigated the effects of potential moderators on cortisol-adiposity associations, such as age, sex, cortisol measurement method, and country development context. These latter findings provide insights on how to measure HCC more precisely, and how to better understand obesogenic effects of stress in different socio-demographic and economic contexts. Furthermore, examining the pooled effect sizes separately using unadjusted and adjusted models provides a more comprehensive picture of the cortisol with adiposity.
Nonetheless, some limitations should be considered in the interpretation of our results. First, sex-stratified analyses of adjusted associations between HCC and adiposity-related outcomes were not possible given limited statistics available. Second, the generalizability of our findings is limited as we included only studies published in English, most of our included studies were from developed countries, and we excluded studies focusing on children with mental disorders or chronic diseases. Third, most studies were observational in nature, precluding causal interpretations. Fourth, while our findings provide insights on physiological stress processes and adiposity-related outcomes, the sources of stress could not be identified beyond chronicity and acuteness and are thus unable to inform actionable recommendations for childhood obesity prevention efforts; such can be the efforts of future work. Fifth, the number of studies included in some subgroup analyses were small as only limited eligible studies were available, especially for salivary and serum cortisol; more studies utilizing these biomarkers are needed. Last, as several original studies with <50 participants were included in the meta-analysis, the small samples reduced the power to find significant associations between cortisol and adiposity-related outcomes.
After consideration of the four cortisol measures of hair, saliva, serum, and urine in children, this study provides important evidence supporting a positive relationship between HCC and objectively measured adiposity-related outcomes. Similar findings were found for children aged ≤12 years and >12 years, and for children from developing and developed countries. These findings provide direct evidence of the physiological stress processes that contribute to increased risk of adiposity-related outcomes in children, and corroborate the need to focus on chronic stress in childhood obesity intervention efforts.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
LuM and LeM designed the research. XL, LuM, NY, and MC conducted the literature search, data screening, and extraction. XL performed the meta-analysis. LuM, XL, and DTC drafted the manuscript. LeM and DTC provided administrative support for the project and had primary responsibility for the final manuscript. All authors read and approved the final manuscript. All authors revised the manuscript, critically helped in the interpretation of results, provided relevant intellectual input, and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2019YFA0802300), the National Natural Science Foundation of China (8210120946), Natural Science Basic Research Program of Shaanxi (2020JQ-094), and China Postdoctoral Science Foundation (2019M653669). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.879256/full#supplementary-material
References
1. GBD 2015 Obesity Collaborators Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 Years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. (2014) 311:806–14. doi: 10.1001/jama.2014.732
3. Puhl RM, Latner JD. Stigma, Obesity, and the health of the Nation’s children. Psychol Bull. (2007) 133:557–80. doi: 10.1037/0033-2909.133.4.557
4. Sutaria S, Devakumar D, Yasuda SS, Das S, Saxena S. Is obesity associated with depression in children? Systematic review and meta-analysis. Arch Dis Child. (2019) 104:64–74. doi: 10.1136/archdischild-2017-314608
5. Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. (2001) 2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x
6. Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. (2000) 16:924–36. doi: 10.1016/s0899-9007(00)00422-6
7. Tomiyama AJ. Stress and obesity. Annu Rev Psychol. (2019) 70:703–18. doi: 10.1146/annurev-psych-010418-102936
8. van der Valk ES, Savas M, van Rossum EFC. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. (2018) 7:193–203. doi: 10.1007/s13679-018-0306-y
9. Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, et al. More than a feeling: a unified view of stress measurement for population science. Front Neuroendocrinol. (2018) 49:146–69. doi: 10.1016/j.yfrne.2018.03.001
10. Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology. (2015) 62:301–18. doi: 10.1016/j.psyneuen.2015.08.014
11. Mikkelsen S, Forman JL, Fink S, Vammen MA, Thomsen JF, Grynderup MB, et al. Prolonged perceived stress and saliva cortisol in a large cohort of danish public service employees: cross-sectional and longitudinal associations. Int Arch Occup Environ Health. (2017) 90:835–48. doi: 10.1007/s00420-017-1241-z
12. Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. (2014) 1842:473–81. doi: 10.1016/j.bbadis.2013.05.029
13. Vanaelst B, De Vriendt T, Huybrechts I, Rinaldi S, De Henauw S. Epidemiological approaches to measure childhood stress. Paediatr Perinat Epidemiol. (2012) 26:280–97. doi: 10.1111/j.1365-3016.2012.01258.x
14. El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva–are our assays good enough? Ann Clin Biochem. (2017) 54:308–22. doi: 10.1177/0004563216687335
15. Powell DJ, Schlotz W. Daily life stress and the cortisol awakening response: testing the anticipation hypothesis. PLoS One. (2012) 7:e52067. doi: 10.1371/journal.pone.0052067
16. Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. (1983) 20:329–35. doi: 10.1177/000456328302000601
17. Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. (1994) 19:313–33. doi: 10.1016/0306-4530(94)90013-2
18. Distel LML, Egbert AH, Bohnert AM, Santiago CD. Chronic stress and food insecurity: examining key environmental family factors related to body mass index among low-income mexican-origin youth. Fam Community Health. (2019) 42:213–20. doi: 10.1097/FCH.0000000000000228
19. Evans BE, Beijers R, Hagquist C, de Weerth C. Childhood urbanicity and hair steroid hormone levels in ten-year-old children. Psychoneuroendocrinology. (2019) 102:53–7. doi: 10.1016/j.psyneuen.2018.11.039
20. Noppe G, van den Akker EL, de Rijke YB, Koper JW, Jaddoe VW, van Rossum EF. Long-term glucocorticoid concentrations as a risk factor for childhood obesity and adverse body-fat distribution. Int J Obes (Lond). (2016) 40:1503–9. doi: 10.1038/ijo.2016.113
21. Ling J, Kao TSA, Robbins LB. Body mass index, waist circumference and body fat are positively correlated with hair cortisol in children: a systematic review and meta-analysis. Obes Rev. (2020) 21:e13050. doi: 10.1111/obr.13050
22. Biggs A, Brough P, Drummond S. Lazarus and Folkman’s psychological stress and coping theory. In: Cooper CL, Quick JC editors. The Handbook Of Stress And Health: A Guide To Research And Practice. Hoboken, NJ: Wiley Blackwell (2017). p. 351–64.
23. Ruttle PL, Javaras KN, Klein MH, Armstrong JM, Burk LR, Essex MJ. Concurrent and longitudinal associations between diurnal cortisol and body mass index across adolescence. J Adolesc Health. (2013) 52:731–7. doi: 10.1016/j.jadohealth.2012.11.013
24. Pasquali R, Vicennati V, Gambineri A, Pagotto U. Sex-dependent role of glucocorticoids and androgens in the pathophysiology of human obesity. Int J Obes (Lond). (2008) 32:1764–79. doi: 10.1038/ijo.2008.129
25. Rippe RC, Noppe G, Windhorst DA, Tiemeier H, van Rossum EF, Jaddoe VW, et al. Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology. (2016) 66:56–64. doi: 10.1016/j.psyneuen.2015.12.016
26. Doom JR, Lumeng JC, Sturza J, Kaciroti N, Vazquez DM, Miller AL. Longitudinal associations between overweight/obesity and stress biology in low-income children. Int J Obes (Lond). (2020) 44:646–55. doi: 10.1038/s41366-019-0447-4
27. National Heart, Lung, and Blood Institute.Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Bethesda: National Institutes of Health, Department of Health and Human Services (2014). p. 103–11.
28. Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. (2017) 8:5–18.
29. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. doi: 10.1136/bmj.d549
30. Dockray S, Susman EJ, Dorn LD. Depression, Cortisol Reactivity, and Obesity in Childhood and Adolescence. J Adolesc Health. (2009) 45:344–50. doi: 10.1016/j.jadohealth.2009.06.014
31. Francis LA, Granger DA, Susman EJ. Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite. (2013) 64:32–8. doi: 10.1016/j.appet.2012.11.008
32. Gerber M, Endes K, Brand S, Herrmann C, Colledge F, Donath L, et al. In 6- to 8-year-old children, hair cortisol is associated with body mass index and somatic complaints, but not with stress, health-related quality of life, blood pressure, retinal vessel diameters, and cardiorespiratory fitness. Psychoneuroendocrinology. (2017) 76:1–10. doi: 10.1016/j.psyneuen.2016.11.008
33. Larsen SC, Fahrenkrug J, Olsen NJ, Heitmann BL. Association between hair cortisol concentration and adiposity measures among children and parents from the “healthy start” study. PLoS One. (2016) 11:e0163639. doi: 10.1371/journal.pone.0163639
34. Lu Q, Pan F, Ren L, Xiao J, Tao F. Sex differences in the association between internalizing symptoms and hair cortisol level among 10-12 year-old adolescents in China. PLoS One. (2018) 13:e0192901. doi: 10.1371/journal.pone.0192901
35. Papafotiou C, Christaki E, van den Akker EL, Wester VL, Apostolakou F, Papassotiriou I, et al. Hair cortisol concentrations exhibit a positive association with salivary cortisol profiles and are increased in obese prepubertal girls. Stress. (2017) 20:217–22. doi: 10.1080/10253890.2017.1303830
36. Smith JD, Johnson KA, Whittle S, Allen NB, Simmons JG. Measurement of cortisol, dehydroepiandrosterone, and testosterone in the hair of children: preliminary results and promising indications. Dev Psychobiol. (2019) 61:962–70. doi: 10.1002/dev.21807
37. Veldhorst MA, Noppe G, Jongejan MH, Kok CB, Mekic S, Koper JW, et al. Increased scalp hair cortisol concentrations in obese children. J Clin Endocrinol Metab. (2014) 99:285–90. doi: 10.1210/jc.2013-2924
38. Jackson SE, Kirschbaum C, Steptoe A. Hair cortisol and adiposity in a population-based sample of 2,527 men and women aged 54 to 87 years. Obesity. (2017) 25:539–44. doi: 10.1002/oby.21733
39. Finch LE, Cummings JR, Tomiyama AJ. Cookie or clementine? psychophysiological stress reactivity and recovery after eating healthy and unhealthy comfort foods. Psychoneuroendocrinology. (2019) 107:26–36. doi: 10.1016/j.psyneuen.2019.04.022
40. Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. (2017) 77:261–74. doi: 10.1016/j.psyneuen.2016.12.017
41. Marceau K, Wang W, Robertson O, Shirtcliff EA. A systematic review of hair cortisol during pregnancy: reference ranges and methodological considerations. Psychoneuroendocrinology. (2020) 122:104904. doi: 10.1016/j.psyneuen.2020.104904
42. Staufenbiel SM, Penninx BW, de Rijke YB, van den Akker EL, van Rossum EF. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology. (2015) 60:182–94. doi: 10.1016/j.psyneuen.2015.06.011
43. Garcia-Leon MA, Peralta-Ramirez MI, Arco-Garcia L, Romero-Gonzalez B, Caparros-Gonzalez RA, Saez-Sanz N, et al. Hair cortisol concentrations in a spanish sample of healthy adults. PLoS One. (2018) 13:e0204807.
44. Noppe G, de Rijke YB, Dorst K, van den Akker EL, van Rossum EF. Lc-Ms/Ms-Based method for long-term steroid profiling in human scalp hair. Clin Endocrinol (Oxf). (2015) 83:162–6. doi: 10.1111/cen.12781
45. Lu Q, Tao F, Hou F, Zhang Z, Sun Y, Xu Y, et al. Cortisol reactivity, delay discounting and percent body fat in chinese urban young adolescents. Appetite. (2014) 72:13–20. doi: 10.1016/j.appet.2013.09.019
46. Miller AL, Clifford C, Sturza J, Rosenblum K, Vazquez DM, Kaciroti N, et al. Blunted cortisol response to stress is associated with higher body mass index in low-income preschool-aged children. Psychoneuroendocrinology. (2013) 38:2611–7. doi: 10.1016/j.psyneuen.2013.06.014
47. Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10-12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. (2005) 30:483–95. doi: 10.1016/j.psyneuen.2004.12.007
48. Barat P, Gayard-Cros M, Andrew R, Corcuff JB, Jouret B, Barthe N, et al. Truncal distribution of fat mass, metabolic profile and hypothalamic-pituitary adrenal axis activity in prepubertal obese children. J Pediatr. (2007) 150:535–9,539.e1. doi: 10.1016/j.jpeds.2007.01.029
49. Chu L, Shen K, Liu P, Ye K, Wang Y, Li C, et al. Increased cortisol and cortisone levels in overweight children. Med Sci Monit Basic Res. (2017) 23:25–30. doi: 10.12659/msmbr.902707
50. Hill EE, Eisenmann JC, Gentile D, Holmes ME, Walsh D. The association between morning cortisol and adiposity in children varies by weight status. J Pediatr Endocrinol Metab. (2011) 24:709–13. doi: 10.1515/jpem.2011.267
51. Lynch T, Azuero A, Lochman JE, Park NJ, Turner-Henson A, Rice M. The influence of psychological stress, depressive symptoms, and cortisol on body mass and central adiposity in 10- to-12-year-old children. J Pediatr Nurs. (2019) 44:42–9. doi: 10.1016/j.pedn.2018.10.007
52. Marceau K, Abel EA, Duncan RJ, Moore PJ, Leve LD, Reiss D, et al. Longitudinal associations of sleep duration, morning and evening cortisol, and BMI during childhood. Obesity (Silver Spring). (2019) 27:645–52. doi: 10.1002/oby.22420
53. Hillman JB, Dorn LD, Loucks TL, Berga SL. Obesity and the hypothalamic-pituitary-adrenal axis in adolescent girls. Metabolism. (2012) 61:341–8. doi: 10.1016/j.metabol.2011.07.009
54. Petrowski K, Schmalbach B, Stalder T. Morning and evening type: the cortisol awakening response in a sleep laboratory. Psychoneuroendocrinology. (2020) 112:104519. doi: 10.1016/j.psyneuen.2019.104519
55. Vehmeijer FOL, Santos S, Gaillard R, de Rijke YB, Voortman T, van den Akker ELT, et al. Associations of hair cortisol concentrations with general and organ fat measures in childhood. J Clin Endocrinol Metab. (2021) 106:e551–61. doi: 10.1210/clinem/dgaa785
56. Bethancourt HJ, Ulrich MA, Almeida DM, Rosinger AY. Household food insecurity, hair cortisol, and adiposity among tsimane’ hunter-forager-horticulturalists in Bolivia. Obesity (Silver Spring). (2021) 29:1046–57. doi: 10.1002/oby.23137
57. Petimar J, Rifas-Shiman SL, Hivert MF, Fleisch AF, Tiemeier H, Oken E. Childhood hair cortisol concentration and early teen cardiometabolic outcomes. Pediatr Obes. (2020) 15:e12592. doi: 10.1111/ijpo.12592
58. Bryson HE, Mensah F, Goldfeld S, Price AMH. Using hair cortisol to examine the role of stress in children’s health inequalities at 3 years. Acad Pediatr. (2020) 20:193–202. doi: 10.1016/j.acap.2019.05.008
59. Baan EJ, van den Akker ELT, Engelkes M, de Rijke YB, de Jongste JC, Sturkenboom M, et al. Hair cortisol and inhaled corticosteroid use in asthmatic children. Pediatr Pulmonol. (2020) 55:316–21. doi: 10.1002/ppul.24551
60. Sun Y, Fang J, Wan Y, Hu J, Xu Y, Tao F. Polygenic differential susceptibility to cumulative stress exposure and childhood obesity. Int J Obes. (2018) 42:1177–84. doi: 10.1038/s41366-018-0116-z
61. Olstad DL, Ball K, Wright C, Abbott G, Brown E, Turner AI. Hair cortisol levels, perceived stress and body mass index in women and children living in socioeconomically disadvantaged neighborhoods: the readi study. Stress. (2016) 19:158–67. doi: 10.3109/10253890.2016.1160282
62. Murray CR, Simmons JG, Allen NB, Byrne ML, Mundy LK, Seal ML, et al. Associations between dehydroepiandrosterone (DHEA) levels, pituitary volume, and social anxiety in children. Psychoneuroendocrinology. (2016) 64:31–9. doi: 10.1016/j.psyneuen.2015.11.004
63. Noppe G, Van Rossum EF, Koper JW, Manenschijn L, Bruining GJ, de Rijke YB, et al. Validation and reference ranges of hair cortisol measurement in healthy children. Horm Res Paediatr. (2014) 82:97–102. doi: 10.1159/000362519
64. Pruszkowska-Przybylska P, Sitek A, Rosset I, Sobalska-Kwapis M, Slomka M, Strapagiel D, et al. Associations between Second to Fourth digit ratio, cortisol, vitamin D, and body composition among polish children. Sci Rep. (2021) 11:7029. doi: 10.1038/s41598-021-86521-7
65. Dai W, Wagh SA, Chettiar S, Zhou GD, Roy R, Qiao X, et al. Blunted circadian cortisol in children is associated with poor cardiovascular health and may reflect circadian misalignment. Psychoneuroendocrinology. (2021) 129:105252. doi: 10.1016/j.psyneuen.2021.105252
66. Pruszkowska-Przybylska P, Sitek A, Rosset I, Sobalska-Kwapis M, Slomka M, Strapagiel D, et al. Cortisol concentration affects fat and muscle mass among polish children aged 6-13 years. BMC Pediatr. (2021) 21:365. doi: 10.1186/s12887-021-02837-3
67. Gallagher C, Moschonis G, Lambert KA, Karaglani E, Mavrogianni C, Gavrili S, et al. Sugar-sweetened beverage consumption is associated with visceral fat in children. Br J Nutr. (2021) 125:819–27. doi: 10.1017/S0007114520003256
68. Koester-Weber T, Valtuena J, Breidenassel C, Beghin L, Plada M, Moreno S, et al. Reference values for leptin, cortisol, insulin and glucose, among European adolescents and their association with adiposity: the helena study. Nutr Hosp. (2014) 30:1181–90. doi: 10.3305/nh.2014.30.5.7982
69. Adam TC, Hasson RE, Ventura EE, Toledo-Corral C, Le K-A, Mahurkar S, et al. Cortisol is negatively associated with insulin sensitivity in overweight latino youth. J Clin Endocrinol Metabol. (2010) 95:4729–35. doi: 10.1210/jc.2010-0322
Keywords: hair cortisol concentration, salivary cortisol, serum cortisol, urinary cortisol, obesity, children
Citation: Ma L, Liu X, Yan N, Gan Y, Wu Y, Li Y, Chu M, Chiu DT and Ma L (2022) Associations Between Different Cortisol Measures and Adiposity in Children: A Systematic Review and Meta-Analysis. Front. Nutr. 9:879256. doi: 10.3389/fnut.2022.879256
Received: 19 February 2022; Accepted: 30 May 2022;
Published: 23 June 2022.
Edited by:
Andre Pascal Kengne, South African Medical Research Council, South AfricaReviewed by:
Jean De Schepper, Free University of Brussels, BelgiumRosaura Leis, University of Santiago de Compostela, Spain
Li Cai, Sun Yat-sen University, China
Copyright © 2022 Ma, Liu, Yan, Gan, Wu, Li, Chu, Chiu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorothy T. Chiu, dtchiu.79@gmail.com; Le Ma, male@mail.xjtu.edu.cn
†These authors have contributed equally to this work
 Lu Ma1†
Lu Ma1†