- Department of Pancreatic and Gastric Surgery, National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: The present work focused on assessing the role of computed tomography (CT)-determined sarcopenia in the prognosis of patients with gastric cancer liver metastases (GCLM) receiving hepatectomy.
Methods: We analyzed data collected from GCLM cases that underwent hepatectomy between March 2011 and July 2017. The third lumbar vertebra (L3) level skeletal muscle index (SMI) was analyzed by abdominal CT to determine the sarcopenia before surgery. The thresholds for CT-based sarcopenia of sex-specific L3 SMI were ≤ 34.9 cm2/m2 and ≤ 40.8 cm2/m2 for female and male, separately We determined overall survival (OS) and recurrence-free survival (RFS)by univariate and multivariate analyses.
Results: The cohort enrolled altogether 114 patients with GCLM receiving hepatectomy (average age: 62.6 years, male: 79.8%), and 58 (50.8%) patients had sarcopenia. The mean SMI was 34.2 in patients with sarcopenia compared to 42.7 in patients without sarcopenia (p < 0.001). The 1-, 3-, and 5-year OS rates in patients with GCLM after hepatectomy were 78.1, 43.7, and 34.3%, respectively. The 1-, 3-, and 5-year RFS rates in patients were 49.8, 33.6, and 29.3%, respectively. Sarcopenia was related to an advanced age (≥65.0 years) (p = 0.009), reduced BMI (<18.5 kg/m2) (p < 0.001) and number of liver metastases (>1) (p = 0.025). Sarcopenia had a significant associated with the patterns of recurrence (p < 0.001). In addition, patients with sarcopenia had a significant difference in number of liver metastases in comparison with those without sarcopenia (p = 0.025). We discovered from multivariate analysis that sarcopenia independently predicted RFS [hazard ratio (HR) = 1.76; 95% confidence interval (CI)= 1.18–2.35, p = 0.007]. Nevertheless, sarcopenia was not the prognostic factors that independently predicted OS (HR = 1.62; 95% CI = 0.57–2.73; p = 0.330).
Conclusions: In conclusion, we showed that CT-determined sarcopenia was the facile and effective prognostic factor for RFS inpatients with GCLM after hepatectomy. Patients with sarcopenia are associated with an increased tumor recurrence risk, and thereby customized treatment should be applied.
Introduction
Gastric cancer (GC) ranks the 5th place among cancers in terms of its morbidity, and there are 1,033,701 patients being diagnosed annually. Also, GC is the 3rd most common reason for cancer-associated mortality, which causes about 782,685 deaths every year (1). Although great achievements have been made in diagnosing and treating GC, distant metastases are related to reduced survival. The liver is the common organ of distant metastases from gastric cancer and the incidence of GC liver metastases (GCLM) is 9.9–18.7% (2, 3). The median survival time of patients with GCLM is about 7–12 months (4). Even with first-line chemotherapy, it is difficult for patients with GCLM to achieve long-term survival (4). Considering the dismal overall survival results receiving palliative chemotherapy alone, hepatectomy has been investigated as a suitable measure to improve outcome. Many studies have described the advantage of liver resection for GCLM over the past 2 decades (5–7). Recently, several large-scale studies have reported that 5-year overall survival rates were 31.1–39.5% of liver resection for GCLM (8, 9). Nevertheless, due to the extremely high recurrence rate after hepatectomy, it is difficult to determine the indication and timing of hepatectomy for GCLM. Therefore, assessment approaches for hepatectomy for GCLM are necessary.
Sarcopenia, which is a kind of age-associated losses of muscle strength, mass, as well as function, has become a serious medical issue in aging societies (10). Sarcopenia is significantly related to non-alcoholic fatty liver disease, liver cirrhosis or cardiovascular events (11–13). It is recognized that more and more attention has been paid to the impact of sarcopenia on the prognosis of patients with cancer, since low muscularity represents an important predicting factor for dismal survival of different tumors (14, 15). Sarcopenia is frequently seen among patients with GC, with a prevalence of over 6.8–57.7% in the patients with GC (16). And it is markedly related to the dismal long-run prognostic outcome in GC cases undergoing surgical resection (17). Nevertheless, the importance of sarcopenia in predicting the prognostic outcome of patients with GCLM after hepatectomy has not been reported.
In this study, the aim of the present work was to assess the significance of sarcopenia in patients with GCLM after hepatectomy, and exploring the relation of sarcopenia with additional clinicopathological characteristics. We also examined whether sarcopenia could become one of the assessment approach for hepatectomy.
Methods
The Institutional Review Board of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College approved our study (NCC2020C-220). This work was performed following the Declaration of Helsinki and the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.
Study Design and Population
The present work evaluated all cases receiving liver resection for GCLM at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College from March 2011 to July 2017. Patients conforming to the criteria below were excluded: (1) unresected extrahepatic disease, (2) repeated hepatectomy for recurrent liver metastases, (3) those receiving R1/R2 resection, (4) those with insufficient/inexact medical records, (5) those with inadequate skeletal muscle index (SMI) measurement, (6)died because of postoperative complications, and (7) patients who had insufficient follow-up data. Finally, we enrolled 114 cases into the cohort (Figure 1). Additionally, we also analyzed patients' laboratory, demographic and histopathological data and collected related data based on patient records in this institute and relevant databases. The collected data included age, sex, body mass index (BMI, kg/m2), CEA levels, serum albumin, American society of anesthesiologists (ASA) score, location of gastric cancer, Lauren classification, tumor differentiation, timing of liver metastases (metachronous or synchronous), number of liver metastases, maximum diameter of the liver metastases, type of hepatectomy, neoadjuvant chemotherapy, adjuvant chemotherapy, and survival. Major hepatectomy was considered as the number of resection liver segments ≥3, where as minor hepatectomy was considered as the number of resection liver segments <3 (18). Post-operative follows-up were conducted at 3-month intervals in initial 2 years postoperatively, and every 6 months since then. We conducted the final follow-up visit in April 2021. In follow-up visits, we examined tumor markers (CA19-9, CEA, AFP), annual endoscopy, abdomino pelvic computed tomography, and chest X-ray. The present work defined overall survival (OS) as duration between surgery date and final follow-up or all-cause mortality, which served as a primary endpoint, while recurrence-free survival (RFS) as duration between surgery date and disease recurrence or mortality, and it served as a secondary endpoint. We recorded all-cause mortality as an event.
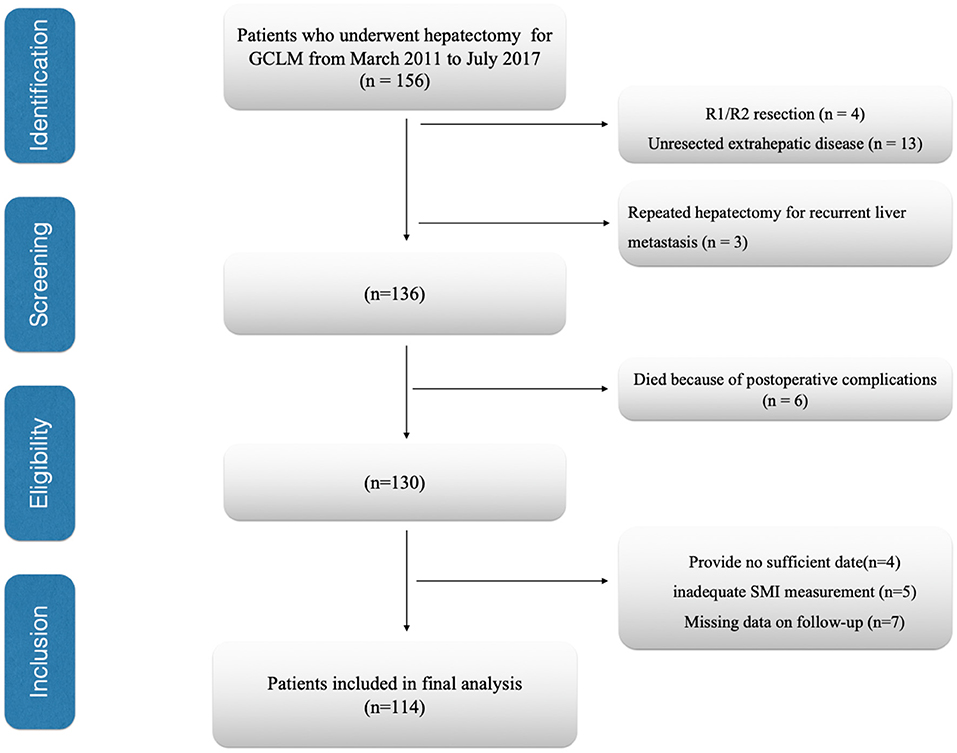
Figure 1. Flow diagram of patients. GCLM, gastric cancer liver Metastasis; SMI, skeletal muscle index.
Definition of Sarcopenia
According to latest Asian Working Group for Sarcopenia (AWGS) guideline, sarcopenia was considered as low muscle quality plus low grip strength or slow gait speed (19). Because our study design was retrospective in nature, it was not possible to collect information on muscle function (muscle strength or physical performance). Therefore, we preoccupied with muscle quality assessment to identify patients with sarcopenia. Computed tomography (CT) is used as a means to accurately assess muscle mass. CT images at the beginning of the treatment (before neoadjuvant chemotherapy) were retrieved for analysis. By adopting a public semi-automatic software (BMI measurement approach, version 1.0; https://sourceforge.net/projects/ muscle-fat-area-measurement/), we determined the cross-section areas (CSAs) of paraspinal muscles, psoas muscles, as well as rectus, oblique and transverse abdominal muscles at the third lumbar vertebra (L3) level with the threshold being−29–150 Hounsfield units (HU) (20, 21). The radiologist who had 5-year experience of abdominal imaging and was blinded to subject information was invited for analyses by the de-identified Digital Imaging and Communications in Medicine files. We later normalized the L3 skeletal muscle index (SMI) to patient stature below: lumbar total muscle CSA (cm2)/height (m2). In addition, the thresholds for CT-based sarcopenia of Sex-specific L3 SMI were ≤ 34.9 cm2/m2 and ≤ 40.8 cm2/m2 for female and male, separately, which was created by the Zhuang and colleagues for the Chinese population (22).
Statistical Methods
Chi-square tests and t tests were used to analyze categorical and continuous data, respectively. Thereafter, we plotted the Kaplan–Meier (K-M) survival curve and examined heterogeneities in curves by log-rank test. Upon univariate analysis, we included significant variables for multivariate analysis by using Cox regression model. p < 0.05 (two-sided) stood for statistical significance. Statistical analyses were completed using Rver. 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7 software (GraphPad Software, CA, USA).
Results
Patient Features
The cohort enrolled altogether114 patients with GCLM receiving hepatectomy, including 91 (79.9%), men along with23 (20.1%) women. Their mean age when the liver resection was performed was 62.6 years [interquartile range: 57.5–70.2 years]. According to our thresholds, 58 (50.9%) cases had sarcopenia. Concerning tumor location of the primary GC, 32 patients (28.1%) had an upper lesion, and 82 patients (71.9%) had a middle or lower lesion. Concerning Lauren classification of the primary GC, 55 patients (48.3%) had anintestinal-type, 38 patients (33.3%) had a diffused-type, and 21 patients (18.4%) had mixed-type. Concerning liver metastases, 45 patients (39.5%) had synchronous metastases, and 69 patients (60.5%) had metachronous metastases. About 71 patients (62.3%) had solitary liver metastases and 43 patients (39.4%) had multiple liver metastases. About 64 patients (56.2%) received neoadjuvant chemotherapy and 75 patients (65.7%) received adjuvant chemotherapy. The 1-, 3-, and 5-year OS rates in patients with GCLM receiving hepatectomy were 78.1, 43.7, and 34.3%, respectively. The 1-, 3-, and 5-year RFS rates in patients with GCLM receiving hepatectomy were 49.8, 33.6, and 29.3%, respectively.
Associations Between CT-Determined Sarcopenia and Clinicopathological Features
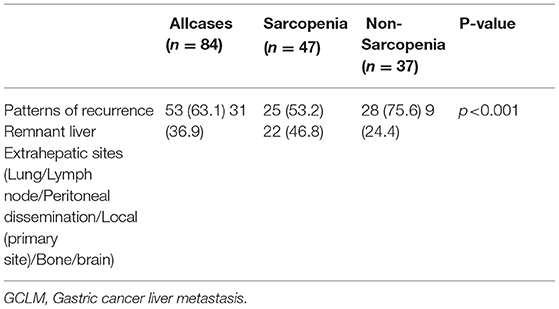
We classified patients into 2 groups based on whether they had sarcopenia. The mean SMI was 34.2 in patients with sarcopenia compared to 42.7 in patients without sarcopenia (p < 0.001). Table 1 displays the associations between sarcopenia and clinicopathological features in the cohort. Sarcopenia was markedly related to an advanced age (≥65.0 years) (p = 0.009) and reduced BMI (<18.5 kg/m2) (p < 0.001). In addition, patients with sarcopenia had a significant difference in number of liver metastases in comparison with those without sarcopenia (p = 0.025). The incidence rate of recurrence in the remnant liver was 53.2 in patients with sarcopenia compared to 75.6 in patients without sarcopenia (Table 2). Sarcopenia had a significant associated with the patterns of recurrence (p < 0.001) (Table 2).
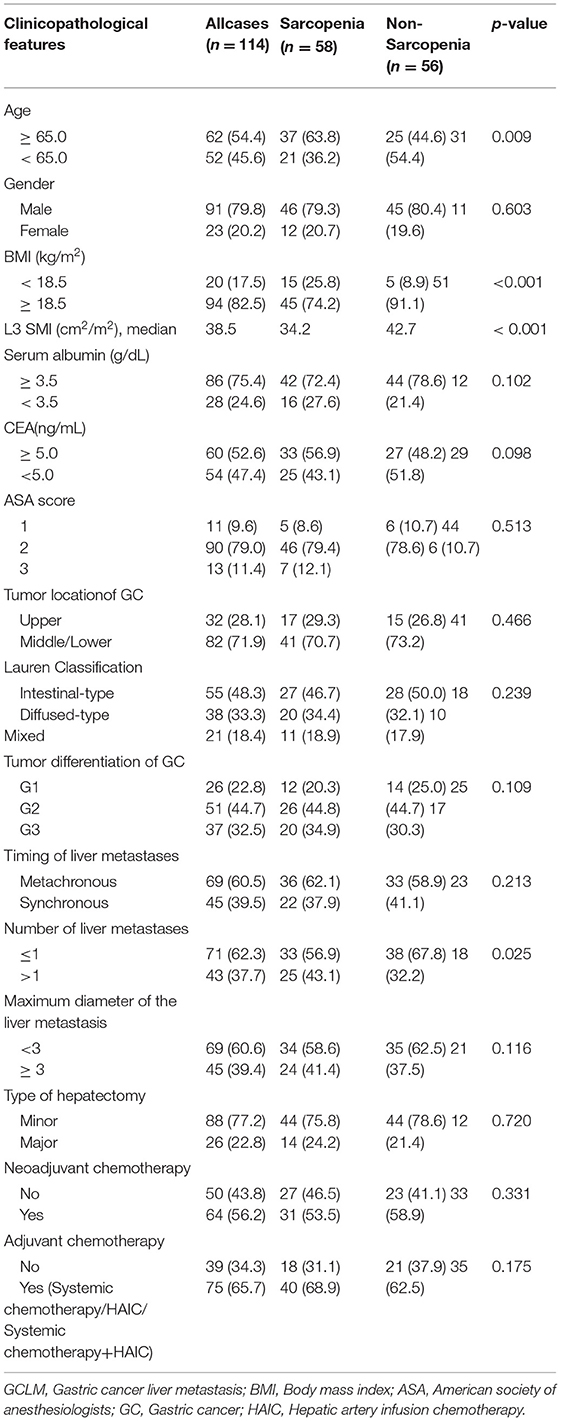
Table 1. Association of Sarcopeniaand clinicopathological characteristics in patients with GCLM after hepatectomy.
Prognostic Factors for OS and RFS After Hepatectomy
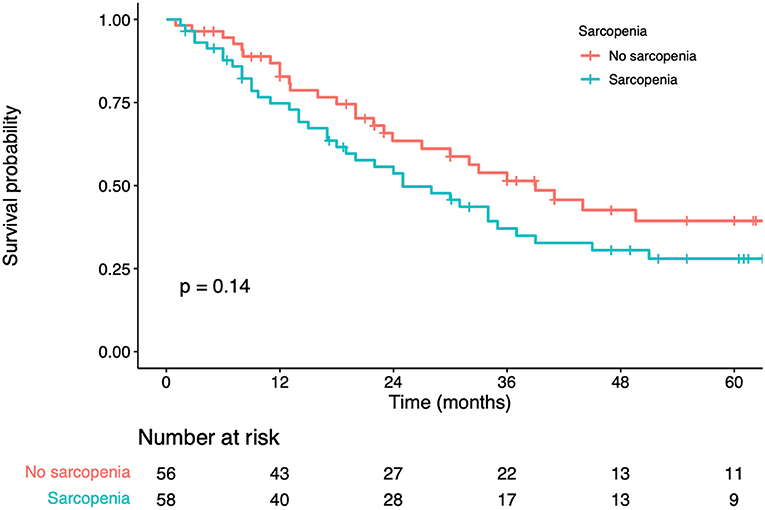
In univariate analysis, significant factors closely associated with OS included BMI (p = 0.010), serum albumin (p < 0.001), CEA levels (p < 0.001), number of liver metastases (p < 0.001), maximum diameter of the liver metastases (p < 0.001), adjuvant chemotherapy (p < 0.001), and sarcopenia status (p = 0.129) (Table 3). In multivariate analysis, serum albumin (hazard ratio [HR] = 1.63; 95% confidence interval [CI] = 1.12–2.48; p = 0.005), CEA levels (HR = 1.75; 95% CI = 1.26–2.29; p = 0.003), number of liver metastases (HR = 2.12; 95% CI=1.26–3.08; p < 0.01), maximum diameter of the liver metastases (HR = 1.54; 95% CI=1.09–2.85; p = 0.014) were the prognostic factors that independently predicted OS (Table 3). K-M curves displayed no significant difference in OS between sarcopenia and non-sarcopenia groups (log-rank test, p> 0.05) (Figure 2). We discovered that sarcopenia was not an independent prognostic factor for OS (HR = 1.62; 95% CI= 0.57–2.73; p = 0.330) (Table 3).
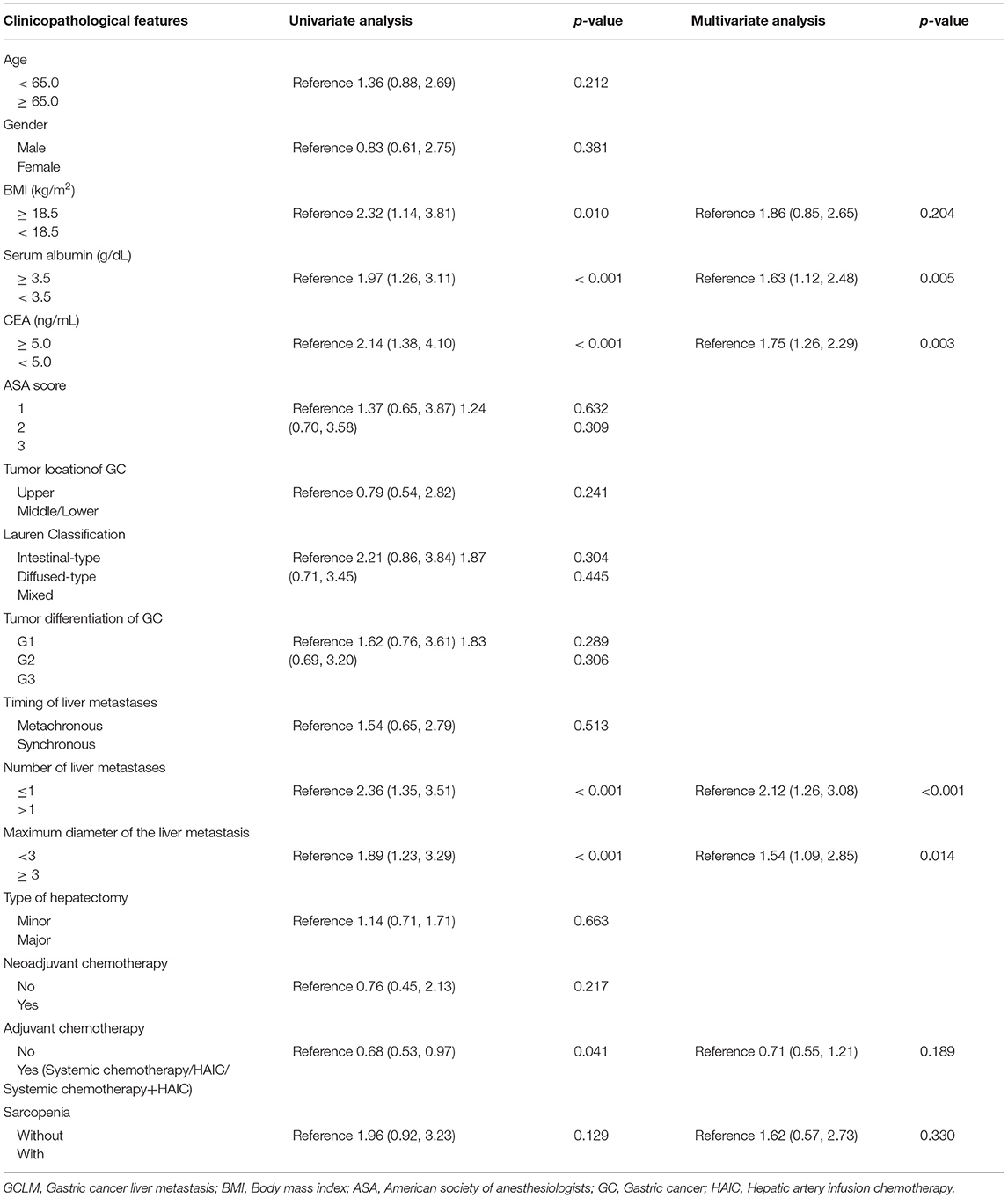
Table 3. Univariate and multivariate analysis of clinicopathologic variablesin relation to overall survivalin patients with GCLM after hepatectomy.
In univariate analysis, significant factors closely associated with RFS included serum albumin (p = 0.034), CEA levels (p = 0.011), number of liver metastases (p < 0.001), maximum diameter of the liver metastases (p = 0.002), sarcopenia status (p = 0.024) (Table 4). In multivariate analysis, number of liver metastases (HR = 1.88; 95% CI=1.12–2.72; p = 0.018), maximum diameter of the liver metastases (HR = 1.56; 95% CI=1.19–2.80; p = 0.011) were the prognostic factors that independently predicted RFS (Table 4). As revealed by K-M survival curves, sarcopenia predicted the dismal RFS (log-rank test, p < 0.001) (Figure 3). We discovered from multivariate analysis that sarcopenia independently predicted RFS (HR= 1.76; 95% CI= 1.18–2.35, p = 0.007) (Table 4).
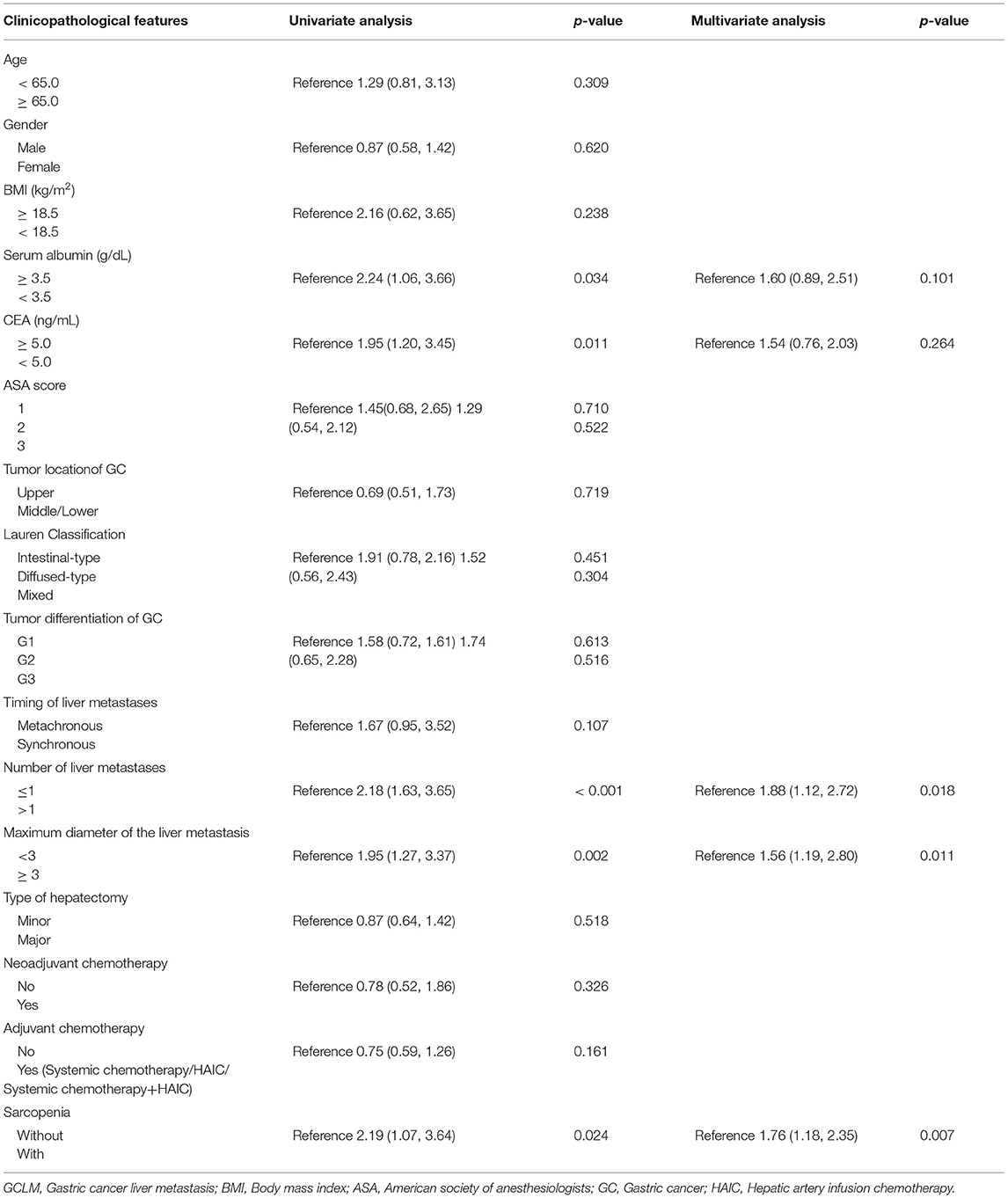
Table 4. Univariate and multivariate analysis of clinicopathologic variables in relation to recurrence-free survival in patients with GCLM after hepatectomy.
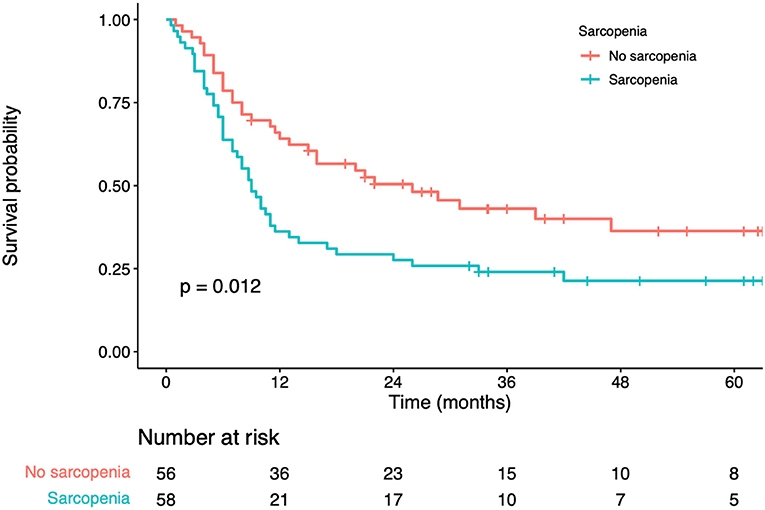
Figure 3. Kaplan-Meier curves for recurrence-free survival according to the preoperative sarcopenia status.
Discussion
This study explored the prognostic significance of CT-determined sarcopenia among GCLM cases receiving liver resection. As a result, sarcopenia was identified as the prognostic index for predicting RFS for GCLM cases receiving liver resection independently. Based on our results, Sarcopenia was related to an advanced age (≥65.0 years), reduced BMI (<18.5 kg/m2) and number of liver metastases (>1).
Liver metastases can occur in approximately 5–14% of patients undergoing gastric cancer surgery (2). It is well known that the treatment of liver metastases is incredibly important for prognostic improvement in patients with GC. GC patients with liver metastases are traditionally receiving with palliative chemotherapy (23). Nevertheless, many studies report that surgical resection of liver metastases was related to a markedly improved survival in selected cases in the last couple of years. Survival rates of GCLM after hepatectomy from Far Eastern studies were higher than those from Western studies (2). Nonetheless, 5-year overall survival rates after hepatectomy range from 27.7 to 42.3 %, and recurrence rates range from 60 to 75 % (9, 24, 25). As a consequence, prediction and assessment of recurrence have become the extremely important considerations in patients with GCLM after liver resection. Sarcopenia, which has been confirmed as the losses of function and mass of skeletal muscle, predicts the dismal nutritional status. And it has been currently regarded as the tumor cachexia hallmark. Sarcopenia is clinically important among cancer cases, which arouses more and more interests from researchers in the last decade. Sarcopenia's prognostic significance is identified within different tumors. It was independently related to dismal prognosis of cases having GC (17). In addition, sarcopenia closely related to shorter progression-free survival following receiving immune checkpoint inhibitors in patients with malignancy, such as non-small cell lung cancer or renal cell carcinoma (26, 27). Sarcopenia was found to be an independent, unfavorable prognostic index for progression-free survival in advanced patients with GC receiving programmed death-1 inhibitor (28). Sarcopenia was confirmed as the independent prognostic index for OS and RFS in patients with hepato cellular carcinoma undergoing hepatectomy (29, 30). Previous studies indicate that colorectal cancer patients with sarcopenia have an inferior OS (31). Moreover, some of the studies indicated that sarcopenia was an independent predictor of long-term survival in colorectal liver metastases (CRLM) receiving liver resection (32–34). Additionally, van Dijk et al. found sarcopenia accompanied by elevated C-reactive protein was significantly related to a truncated OS in CRLM (35). It seems that these results contribute to the decisions concerning the timing of and the indications for liver resection. Our study showed that sarcopenia was not an independent prognostic factor for OS, although patients with sarcopenia had a trend toward dismal OS (log-rank test, p = 0.14). Although previous research indicated that sarcopenia can independently predicted OS among GC patients, this might not entirely be applicable in patients with GC, especially in patients with GCLM (22, 36). Our study confirmed the prognostic significance of sarcopenia in GCLM cases receiving liver resection, that sarcopenia independently predicted RFS. Sarcopenia contributes to accurately predicting the prognosis and assisting decision-making among GCLM cases. Meanwhile, it can be utilized as one part of prognosis stratification before surgery, and sarcopenia predicts an increased tumor recurrence risk and the necessity for customized treatment.
The mechanism by which sarcopenia increases the risk of tumor recurrence is still obscure. The following reasons can be assumed. First, sarcopenia may reflect increased metabolic activity of more aggressive tumor biology, which leads to more severe systemic inflammation and subsequent muscle wasting (37). Second, previous studies reported that myokines secreted by muscle cells can inhibit the growth of cancer cells. Therefore, we speculate that a reduction in muscle mass may lead to an impaired myokine response and increases the risk of tumor recurrence (38).
There are some limitations in the present work. First, the AWGS (2019 edition) suggests using the presence of loss of muscle quality plus low muscle function (strength or performance) to determine sarcopenia. Because our study design was retrospective in nature, it was not possible to collect information on muscle function (muscle strength or physical performance). Therefore, we preoccupied with muscle quality assessment to identify patients with sarcopenia. CT is used as a means to accurately assess muscle mass. There are several advantages to the use of CT. CT is widely used as a routine examination and staging method for patients with gastric cancer and can accurately quantify muscle mass. The definition of sarcopenia put forward by Zhuang et al. was utilized in the present work, which defined sarcopenia criteria for Chinese population (22). The L3-SMI thresholds for diagnosing CT-based sarcopenia were 34.9 cm2/m2 and 40.8 cm2/m2 for women and men, separately. This study showed limited generalizability to western populations, since our adopted L3 SMI thresholds showed high specificity to geographic location. Secondly, the effect of preoperative sarcopenia on predicting the prognosis of GCLM cases receiving hepatectomy was evaluated, but selection bias still existed due to the retrospective nature. And we only recruited cases at a single center in China, showing ethic homogeneity. For overcoming the above limitations, more large-scale multicenter prospective studies should be conducted.
Conclusions
In conclusion, we suggest that CT-determined sarcopenia is a meaningful predictor of recurrence after hepatectomy in patients with GCLM, and it should be regarded as one of the assessment criteria of hepatectomy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JX conceived the study and wrote the manuscript. YW, WK, and HH searched the database, reviewed the studies and collected the data. XS and JX performed the statistical analyses. YL, PJ, and WL performed revision of the manuscript. YT arranged for and provided the funding for this work. All authors reviewed the manuscript and participated in its revision. YT had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors of this manuscript have read and approved the final submitted version and are aware that they are listed as an author on this paper.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81772642).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank to Zhisong Liu for his help in the statistical analyses of our manuscript data. We are very grateful for the valuable support of the Radiology Department.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.878791/full#supplementary-material
Abbreviations
GC, Gastric cancer; GCLM, Gastric cancer liver metastasis; BMI, Body mass index; ASA, American society of anesthesiologists; GC, Gastric cancer; HAIC, Hepatic artery infusion chemotherapy; OS, Overall survival; RFS, Recurrence-Free survival; AWGS, Asian working group for sarcopenia; SMI, Skeletal muscle index; CT, Computed tomography; HU, Hounsfield units; L3, Third lumbar vertebra; CSA, Cross-Section area; HR, Hazard ratio; 95% CI, 95% Confidence interval; CRLM, Colorectal liver metastases.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. (2008) 19:1146–53. doi: 10.1093/annonc/mdn026
3. Zhang K, Chen L. Chinese consensus on the diagnosis and treatment of gastric cancer with liver metastases. Ther Adv Med Oncol. (2020) 12:1758835920904803. doi: 10.1177/1758835920904803
4. Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, eta al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. (2019) 30:19–33. doi: 10.1093/annonc/mdy502
5. Takemura N, Saiura A, Koga R, Arita J, Yoshioka R, Ono Y, et al. Long-term outcomes after surgical resection for gastric cancer liver metastasis: an analysis of 64 macroscopically complete resections. Langenbecks Arch Surg. (2012) 397:951–7. doi: 10.1007/s00423-012-0959-z
6. Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. (2002) 235:86–91. doi: 10.1097/00000658-200201000-00011
7. Oki E, Tokunaga S, Emi Y, Kusumoto T, Yamamoto M, Fukuzawa K, et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer. (2016) 19:968–76. doi: 10.1007/s10120-015-0530-z
8. Markar SR, Mackenzie H, Mikhail S, Mughal M, Preston SR, Maynard ND, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer. (2017) 20:379–86. doi: 10.1007/s10120-016-0604-6
9. Kinoshita T, Kinoshita T, Saiura A, Esaki M, Sakamoto H, Yamanaka T. Multicentre analysis of long-term outcome after surgical resection for gastric cancer liver metastases. Br J Surg. (2015) 102:102–7. doi: 10.1002/bjs.9684
10. Cruz-Jentoft J, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
11. Seo DH, Lee YH, Park SW, Choi YJ, Huh BW, Lee E, et al. Sarcopenia is associated with non-alcoholic fatty liver disease in men with type 2 diabetes. Diabetes Metab. (2020) 46:362–69. doi: 10.1016/j.diabet.2019.10.004
12. Han E, Lee YH, Kim YD, Kim BK, Park JY, Kim DY, et al. Nonalcoholic fatty liver disease and Sarcopenia are independently associated with cardiovascular risk. Am J Gastroenterol. (2020) 115:584–95. doi: 10.14309/ajg.0000000000000572
13. Zeng X, Shi ZW, Yu JJ, Wang LF, Luo YY, Jin SM, et al. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. (2021) 9:629–35. doi: 10.1002/jcsm.12797
14. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. (2008) 9:629–35. doi: 10.1016/S1470-2045(08)70153-0
15. Yang M, Shen Y, Tan L, Li W. Prognostic value of Sarcopenia in lung cancer: a systematic review and meta-analysis. Chest. (2019) 156:101–11. doi: 10.1016/j.chest.2019.04.115
16. Yang Z, Zhou X, Ma B, Xing Y, Jiang X, Wang Z. Predictive value of preoperative Sarcopenia in patients with gastric cancer: a meta-analysis and systematic review. J Gastrointest Surg. (2018) 22:1890–1902. doi: 10.1007/s11605-018-3856-0
17. Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer. (2019) 22:10–22. doi: 10.1007/s10120-018-0882-2
18. Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. (2013) 257:377–82. doi: 10.1097/SLA.0b013e31825a01f6
19. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: (2019). consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
20. Kim SS, Kim JH, Jeong WK, Lee J, Kim YK, Choi D, et al. Semiautomatic software for measurement of abdominal muscle and adipose areas using computed tomography: a STROBE-compliant article. Medicine (Baltimore). (2019) 98: e15867. doi: 10.1097/MD.0000000000015867
21. Kang SH, Jeong WK, Baik SK, Cha SH, Kim YM. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle. (2018) 9:860–70. doi: 10.1002/jcsm.12333
22. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). (2016) 95:e3164. doi: 10.1097/MD.0000000000003164
23. Wagner D, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. (2010) 3:Cd004064. doi: 10.1002/14651858.CD004064.pub3
24. Markar SR, Mikhail S, Malietzis G, Athanasiou T, Mariette C, Sasako M, et al. Influence of surgical resection of hepatic metastases from gastric adenocarcinoma on long-term survival: systematic review and pooled analysis. Ann Surg. (2016) 263:1092–101. doi: 10.1097/SLA.0000000000001542
25. Montagnani F, Crivelli F, Aprile G, Vivaldi C, Pecora I, De Vivo R Clerico MA, et al. Long-term survival after liver metastasectomy in gastric cancer: systematic review and meta-analysis of prognostic factors. Cancer Treat Rev. (2018) 69:11–20. doi: 10.1016/j.ctrv.2018.05.010
26. Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle. (2021) 12:1122–35. doi: 10.1002/jcsm.12755
27. Deng HY, Chen ZJ, Qiu XM, Zhu DX, Tang XJ, Zhou Q. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: A comprehensive systematic review and meta-analysis. Nutrition. (2021) 90:111345. doi: 10.1016/j.nut.2021.111345
28. Kim YY, Lee J, Jeong WK, Kim ST, Kim JH, Hong JY, et al. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer. (2021) 24:457–66. doi: 10.1007/s10120-020-01124-x
29. Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. (2015) 261:1173–83. doi: 10.1097/SLA.0000000000000743
30. Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. (2020) 55:927–43. doi: 10.1007/s00535-020-01711-w
31. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle. (2018) 9:53–9. doi: 10.1002/jcsm.12234
32. Waalboer RB, Meyer YM, Galjart B, Olthof PB, van Vugt JLA, Grünhagen DJ. Sarcopenia and long-term survival outcomes after local therapy for colorectal liver metastasis: a meta-analysis. HPB (Oxford). (2021) 24:9–16. doi: 10.1016/j.hpb.2021.08.947
33. van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. (2012) 99:550–7. doi: 10.1002/bjs.7823
34. Bajrić T, Kornprat P, Faschinger F, Werkgartner G, Mischinger JH, Wagner D. Sarcopenia and primary tumor location influence patients outcome after liver resection for colorectal liver metastases. Eur J Surg Oncol. (2021) 48:615–20. doi: 10.1016/j.ejso.2021.09.010
35. van Dijk DPJ, Krill M, Farshidfar F, Li T, Rensen SS, Olde Damink S, et al. Host phenotype is associated with reduced survival independent of tumour biology in patients with colorectal liver metastases. J Cachexia Sarcopenia Muscle. (2019) 10:123–30. doi: 10.1002/jcsm.12358
36. Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, et al. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin Nutr. (2020) 39:2045–54. doi: 10.1016/j.clnu.2019.10.021
37. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. (2011) 62:265–79. doi: 10.1146/annurev-med-061509-131248
Keywords: gastric cancer, liver metastases, hepatectomy, sarcopenia, prognostic factors
Citation: Xiong J, Wu Y, Hu H, Kang W, Li Y, Jin P, Shao X, Li W and Tian Y (2022) Prognostic Significance of Preoperative Sarcopenia in Patients With Gastric Cancer Liver Metastases Receiving Hepatectomy. Front. Nutr. 9:878791. doi: 10.3389/fnut.2022.878791
Received: 18 February 2022; Accepted: 11 April 2022;
Published: 10 May 2022.
Edited by:
Nada Rotovnik Kozjek, Institute of Oncology Ljubljana, SloveniaReviewed by:
Erik Brecelj, Institute of Oncology Ljubljana, SloveniaYoshihiko Yano, Kobe University, Japan
Copyright © 2022 Xiong, Wu, Hu, Kang, Li, Jin, Shao, Li and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yantao Tian, dGlhbnlhbnRhb0BjaWNhbXMuYWMuY24=
†These authors have contributed equally to this work
 Jianping Xiong
Jianping Xiong Yunzi Wu†
Yunzi Wu† Wenzhe Kang
Wenzhe Kang Yantao Tian
Yantao Tian