- 1Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, China
- 2Department of Global Health, Ningbo Institute of Life and Health Industry, University of Chinese Academy of Sciences, Ningbo, China
- 3Department of Neurology, Shenzhen Qianhai Shekou Free Trade Zone Hospital, Shenzhen, China
- 4Department of Child and Adolescent Health and Social Medicine, School of Public Health, Medical College of Soochow University, Suzhou, China
Objective: The purpose of this study was to evaluate the associations of serum biomarkers of fruit and vegetable intake (vitamin C and carotenoids) with cause–specific mortality and all–cause mortality in a nationally representative sample of US adults.
Methods: We analyzed data from 12,530 participants from the National Health and Nutrition Examination Survey III (1988–1994). The Cox proportional hazards models with restricted cubic spline were used for the analysis.
Results: During 246,027 person–years of follow–up, 4,511 deaths occurred, including 1,395 deaths from cardiovascular disease, 1,072 deaths from heart disease, 323 deaths from cerebral disease, and 954 deaths from cancer. The serum vitamin C was significantly associated with the cancer and all–cause mortality, with hazard ratios (HRs) (95% CIs) for each one SD of 0.80 (0.71–0.91) and 0.91 (0.86–0.96). The serum alpha–carotene was significantly associated with the cancer mortality, with HRs (95% CIs) of 0.70 (0.54–0.90), 0.68 (0.48–0.95), 0.64 (0.43–0.95), and 0.44 (0.33–0.60) for comparisons of groups 2–5 with group 1 in model 2, respectively. The change for each one SD in the composite biomarker score, equivalent to a 0.483 times/month difference in total fruits and vegetables intake, gave an HR of 0.79 (0.69–0.90) for cancer mortality.
Conclusion: Inverse associations were found between serum vitamin C, carotenoids, and composite biomarker score and outcomes expect for cerebral disease, heart disease, and cardiovascular disease mortality. This finding supports an increase in dietary fruit and vegetable intake as a primary prevention strategy for cancer and all–cause mortality.
Introduction
Cardiovascular disease (CVD) and cancer are the major causes of deaths globally, causing an estimated 25.5 million deaths annually (1). Previously, evidence indicated that a higher intake of fruit and vegetable is associated with a reduced risk of CVD and cancer mortality (2, 3). However, fruit and vegetable intake has traditionally been assessed using dietary food frequency questionnaires, which are easily susceptible to measurement error and reporting biases (4). Thus, objective serum biomarkers of fruit and vegetable intake are indispensable to confirm these associations.
Vitamin C and carotenoids are considered as objective serum biomarkers of fruit and vegetable intake (4–6). Negative associations between the concentrations of these markers and CVD mortality or all–cause mortality risk in different populations were observed (7, 8). However, there is a lack of evidence for these associations in the general population of the United States (US), which has different lifestyles and dietary behaviors compared to other countries. Moreover, the associations between serum biomarkers of fruit and vegetable intake and cerebral disease, heart disease, CVD, and cancer mortality risk have not been previously evaluated.
Thus, we evaluated the associations between the baseline concentrations of serum biomarkers of fruit and vegetable intake (vitamin C and carotenoids) and cause–specific and all–cause mortality in the US nationally representative sample using data from the Third National Health and Nutrition Examination Survey (NHANES III) (1988–1994), which is a nationally representative cohort of the US population (9). We also constructed a composite biomarker score to systematically examine potential associations.
Methods
Study Population Sources
Participants in this study were recruited through the NHANES III (1988–1994), a nationally representative sample of noninstitutionalized civilians in the US (10). The design of the NHANES III used a multistage, stratified, clustered, and probability sampling method. It combines the results of home interviews and physical examinations and includes demographic, socioeconomic, dietary, health–related, and laboratory data. The survey was reviewed and approved by the National Health Statistics Research Ethics Review Board. Details of the survey design have been described elsewhere (10). All the participants of the survey signed an informed consent form. We conducted prospective analyses of adult participants (≥18 years). A flowchart of the participant inclusion process is shown in Supplementary Figure 1.
Ascertainment of Outcomes
The survival and cause of death information of participants were obtained by a prospective follow–up survey that was implemented by the National Center for Health Statistics until 31 December 2015 (11). Mortality data were obtained from the National Death Index death certificate records, based on the participants' social security number, name, date of birth, etc. (12). The leading causes of death (UCOD_LEADING) are provided and based on the UCOD_113 variable, which was identified according to the International Classification of Diseases, Tenth revision (cancer: C00–C97; CVD: I00–I09, I11, I13, I20–I51, and I60–I69; heart disease: I00–I09, I11, I13, and I20–I51; and cerebral diseases: I60–I69) (13).
Measurement of Serum Vitamin C and Carotenoid Concentrations
For the NHANES III, serum concentrations of vitamin C and carotenoids were measured using high–performance liquid chromatography with electrochemical detection (9). The lower limits of detection of alpha–carotene, beta–carotene, beta–cryptoxanthin, lycopene, and lutein/zeaxanthin were 0, 0.67, 0, 0.63, and 0.43 μg/dl, respectively (9).
Demographic Characteristics
The demographic characteristics of the participants, including age (<40, 40– <60, and ≥ 60 years), sex (female, male), marital status (married/living as married, widowed/divorced, and separated/never married), body mass index (BMI) (<25, 25– <30, and ≥ 30 kg/m2), educational status (less than high school, high school, and more than high school), smoking status (current smoker, former smoker, and never smoker), alcohol consumption (0, 0– <6, 6– <12, 12– <24, and ≥ 24 times/week), physical activity (ideal, intermediate, and poor) (14), and income (<$20,000, $20,000–50,000, and > $50,000) were collected. Serum concentrations of high–density lipoprotein cholesterol (HDL–C) and low–density lipoprotein cholesterol (LDL–C) were calculated using the Friedewald equation (9). Total energy intake was calculated by summing the calories (kcal) from all the foods for every participant (15). Participants were classified into the two groups (low or high) according to HDL–C (<1.4 mmol/l and ≥ 1.4 mmol/l) and LDL–C (<3.7 mmol/l and ≥ 3.7 mmol/l) concentrations (16). Hypertension was defined as any self–reported history, medication history, a systolic blood pressure ≥ 130 mm Hg, or a diastolic blood pressure ≥ 80 mm Hg (17). Diabetes was defined as any self–reported history, medication history, or a serum glucose concentration ≥ 7.1 mmol/l (18).
Statistical Analysis
Baseline information regarding serum biomarkers of fruit and vegetable intake was divided into quintiles (from the lowest to the highest concentration, group 1 to group 5). The sum of the five individual carotenoid concentrations was regarded as the serum total carotenoid concentration. The value was considered as composite biomarker score, which was generated by calculating the average of the standardized values of serum concentrations of vitamin C and the five individual carotenoids (19). We calculated the Spearman's correlations between serum concentrations of vitamin C, other vitamins (vitamin A, B12, and E), and carotenoids. Exposure factors, including serum vitamin C, carotenoid concentrations, and their composite biomarker score, were standardized. We used linear regression to evaluate the relationship and output standardized regression coefficients between serum vitamin C, carotenoids, and their composite biomarker scores and dietary covariates such as soft drink, fruit and vegetable juice, and red meat intake. Age, sex, physical activity, smoking status, marital status, educational status, alcohol consumption, total energy intake, HDL–C concentration, and BMI were adjusted in above analyses (16). We also evaluated the differences in total fruits and vegetable intake for each one SD higher composite biomarker score using linear regression.
Hazard ratios (HRs) and 95% CIs for all–cause and cause–specific mortality were determined by the Cox proportional hazards models. Serum biomarkers were divided into quintiles (group 1 to group 5, from the lowest to the highest concentration) and for each one SD change. Three models were used in this study. In model 1a, adjustments were made for age and sex. Model 1b included adjustments for age, sex, physical activity, smoking status, marital status, educational status (low, middle, and high), alcohol consumption (never, 0 to <6, 6– <12, 12– <24, and ≥ 24 times/week), total energy intake (continuous), and HDL–C concentration (continuous; only for carotenoids analyses). Model 2 included the same adjustments as model 1b, but was also adjusted for BMI (16). In model 2, we also used restricted cubic spline (four knots) to evaluate the relationship between serum vitamin C, carotenoids, and their composite biomarker scores and outcomes.
We excluded participants recruited within the first 2 years or the first 4 years or those with baseline cancer or CVD for sensitivity analyses. We further separately adjusted for hypertension, diabetes, and those with a history of cancer or CVD. The composite biomarker scores were also amended in this study, by excluding one biomarker at a time. Finally, we explored unmeasured confounding by calculating E–values (20). All the statistical analyses were performed using SAS version 9.4 statistical software (SAS Institute, Cary, North Carolina, USA). All the tests were two–sided and P < .05 was considered as statistically significant.
Results
Baseline Characteristics
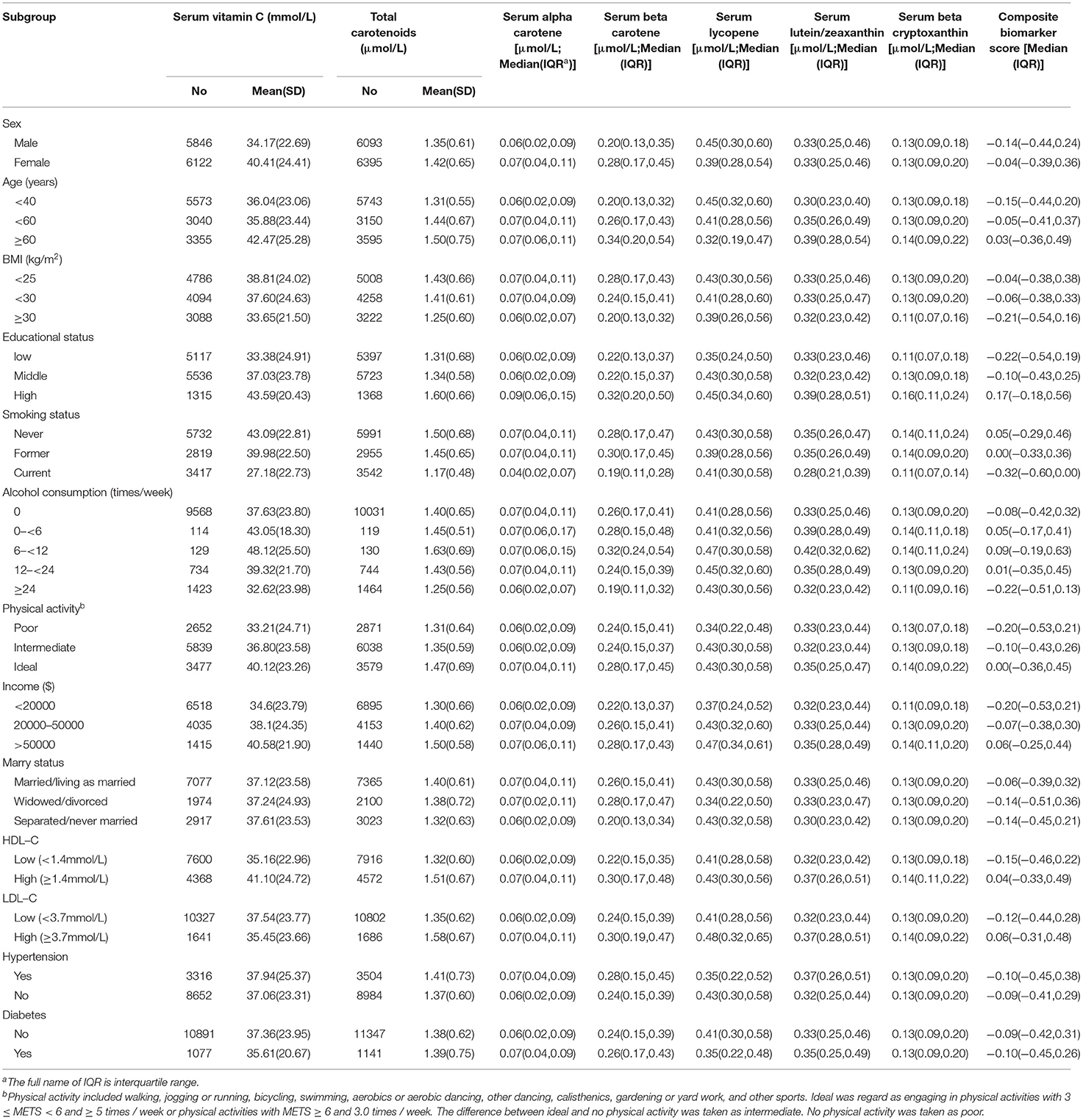
Table 1 shows the baseline characteristics of serum biomarkers. In this study, the mean SD concentrations of serum vitamin C and total carotenoids in females were 40.41 (24.41) and 1.42 (0.65) μmol/l and in males were 34.17 (22.69) and 1.35 (0.61) μmol/l, respectively. The mean (SD) concentrations of serum vitamin C for the three age groups (<40, 40– <60, and ≥ 60 years) were 36.04 (23.06), 35.88 (23.44), and 42.47 (25.28) mmol/l, respectively, and those of serum total carotenoids for the three age groups were 1.31 (0.55), 1.44 (0.67), and 1.50 (0.75) μmol/l, respectively (Table 1). The serum concentrations of all the biomarkers of fruit and vegetable intake were all positively correlated with each other (Supplementary Table 1).
Associations of Serum Concentrations of Biomarkers and Demographic Characteristics
After multivariate adjustment, educational status was positively associated with serum vitamin C, total carotenoid concentration, and composite biomarker score. Meanwhile, the inverse associations were found between BMI and current smoking status (compared with never smoking) and the serum concentrations of vitamin C, total carotenoid concentration, and composite biomarker score (Supplementary Table 2).
The positive association was found between monthly intake of fruit juices and serum vitamin C, beta–cryptoxanthin, and the composite biomarker score (P < 0.05, Figure 1). The number of times per month that soft drinks were consumed was negatively associated with serum vitamin C, total carotenoids, and the composite biomarker score (P < 0.05, Figure 1). Each one time/month higher intake of total fruits and vegetables was associated with an increase of 0.282 in the composite biomarker score. Conversely, each one (SD) increase in the composite biomarker score was associated with a 0.483 times/month increase in the intake of total fruits and vegetables (Figure 1 and Supplementary Table 2). Each one time/month increase in the intake of total fruits and vegetables was associated with a 0.283 μmol/l increase in serum vitamin C concentration. Conversely, each one SD increase in serum vitamin C concentration was associated with a 0.435 times/month increase in the intake of total fruits and vegetables (Figure 1 and Supplementary Table 2).
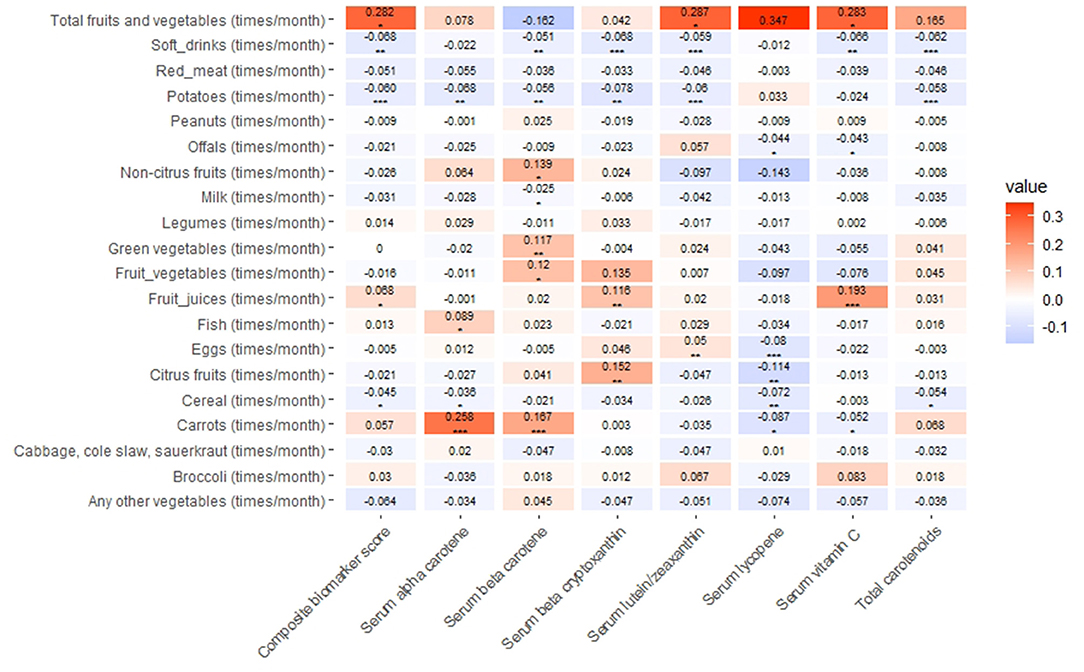
Figure 1. Association of dietary factors with serum vitamin C, carotenoids, and their composite biomarker scores in the Third National Health and Nutrition Examination Survey (NHANES III). Linear regression was used to obtain the estimate values of an association between dietary factors and vitamin C, carotenoids, or the composite biomarker score (in SD units), adjusting for demographic and lifestyle factors. The positive associations and negative associations of values were expressed in a red scale and blue scale, respectively. *P < 0.05; **P < 0.01; ***P < 0.001. Each one time/month higher intake of total fruits and vegetables was associated with 0.282 higher level of the composite biomarker score; conversely, every one SD higher composite biomarker score was associated with a 0.483 times/months higher intake of total fruits and vegetables. Each one time/month higher intake of fruits and vegetable was associated with 0.283 higher level of serum vitamin C; conversely, every one SD serum vitamin C was associated with a 0.435 times/months higher intake of fruits and vegetables in this figure.
Associations of Serum Biomarkers of Fruit and Vegetable Intake With Outcomes
Table 2 shows the HRs and 95% CIs for the associations between serum concentrations of biomarkers of fruit and vegetable intake and outcomes. The medians for total fruit and vegetable intake were 39, 50, 57, 68, and 89 times/month for lowest concentrations (group 1), 2, 3, 4, and 5 (highest concentrations) of the composite biomarker score, respectively (Table 2). Higher serum concentrations of vitamin C were associated with lower cancer mortality. The HRs (95% CIs) for each one SD increase in serum vitamin C concentration were 0.70 (0.62–0.78), 0.80 (0.71–0.91), and 0.80 (0.71–0.91) in models 1a, 1b, and 2, respectively. The serum vitamin C concentration was significantly associated with the cancer mortality, with HRs (95% CIs) of 0.68 (0.49–0.93), 0.51 (0.35–0.75), and 0.53 (0.38–0.75) for comparisons of groups 3, 4, and 5, respectively, with group 1 in model 2 (P < 0.001 for the trend; Table 2).
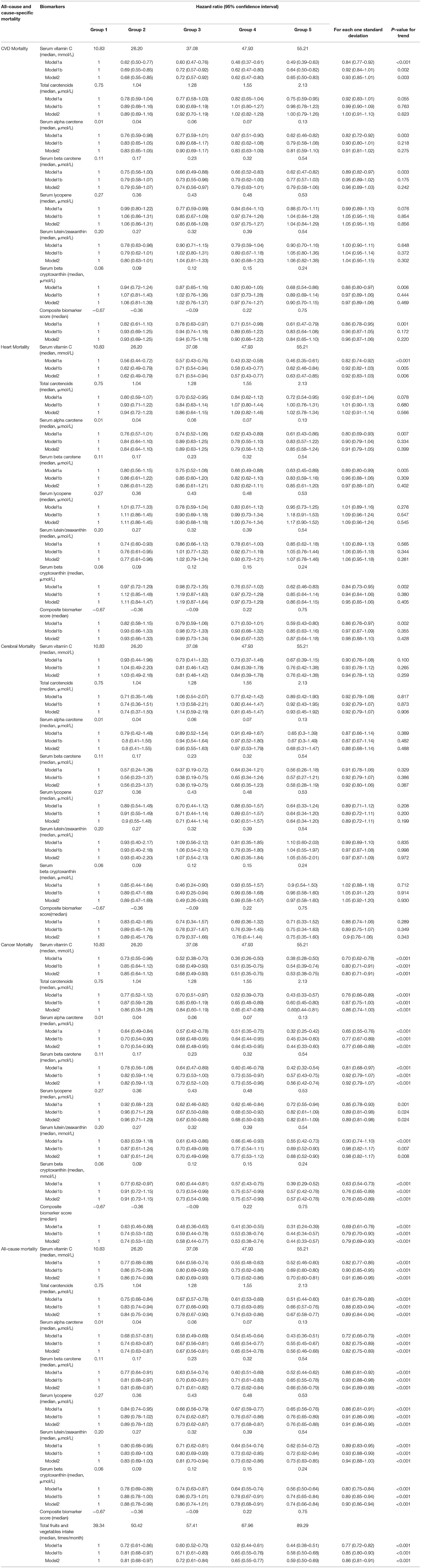
Table 2. Prospective associations between serum biomarkers levels of fruit and vegetable intake and outcomes.
The serum alpha–carotene concentration was significantly associated with the cancer mortality, with HRs (95% CIs) of 0.70 (0.54–0.90), 0.68 (0.48–0.95), 0.64 (0.43–0.95), and 0.44 (0.33–0.60) for comparisons of groups 2, 3, 4, and 5, respectively, with group 1 in model 2 (P < .001 for the trend; Table 2). The HRs (95% CIs) for the association between each one SD increase in serum alpha–carotene, lycopene, beta–cryptoxanthin concentrations, and composite biomarker score and cancer mortality in model 2 were 0.77 (0.66–0.89), 0.89 (0.81–0.98), 0.76 (0.65–0.89), and 0.79 (0.69–0.90), respectively (Table 2). The composite biomarker score was significantly associated with cancer mortality, with HRs (95% CIs) of 0.58 (0.44–0.77), 0.53 (0.38–0.74), and 0.44 (0.33–0.57) when comparing groups 3 to 5, respectively, with group 1 in model 2 (P < 0.001 for the trend; Table 2).
The relationships were not statistically significant between each one SD increase in serum vitamin C, carotenoids, and their composite biomarker score and CVD mortality, heart disease mortality, and cerebral disease mortality in model 2 (Table 2). Serum vitamin C concentration was inversely associated with all–cause mortality in model 2, with HRs (95% CIs) for each one SD of 0.91 (0.86–0.96). The HRs (95% CIs) for the association between each one SD increase in total carotenoid, serum alpha–carotene, beta–carotene, lycopene, lutein/zeaxanthin, and beta–cryptoxanthin concentrations and all–cause mortality were 0.89 (0.84–0.94), 0.82 (0.75–0.89), 0.94 (0.89–0.99), 0.91 (0.86–0.96), 0.94 (0.88–1.00), and 0.90 (0.86–0.94) in model 2, respectively. A higher composite biomarker score was associated with a decrease in all–cause mortality, with an HR (95% CI) of 0.86 (0.81–0.91) for each one SD increase in the score (Table 2). The HRs (95% CIs) for the association between the composite biomarker score and all–cause mortality were 0.81 (0.68–0.97), 0.72 (0.61–0.84), 0.65 (0.55–0.77), and 0.59 (0.50–0.69) when comparing groups 2, 3, 4, and 5, respectively, compared with the lowest concentrations (group 1) in model 2 (P < .001 for the trend; Table 2).
We found that non–linear associations were shown between cancer mortality and serum concentrations of vitamin C, total carotenoids and individual carotenoids, and composite biomarker score, expect for serum lutein/zeaxanthin (P–value for nonlinearity <0.05) (Figure 2). U–shaped relationships were found between serum vitamin C, total carotenoid concentrations, and composite biomarker score and cancer mortality (Figure 2). The null effect between very low and very high levels of serum vitamin C, total carotenoid concentrations, and composite biomarker score is shown in Figure 2. The figures of associations between serum biomarkers of fruit and vegetable intake and CVD, heart disease, cerebral disease, and all–cause mortality are shown in Supplementary Materials (Supplementary Figures 2–5).
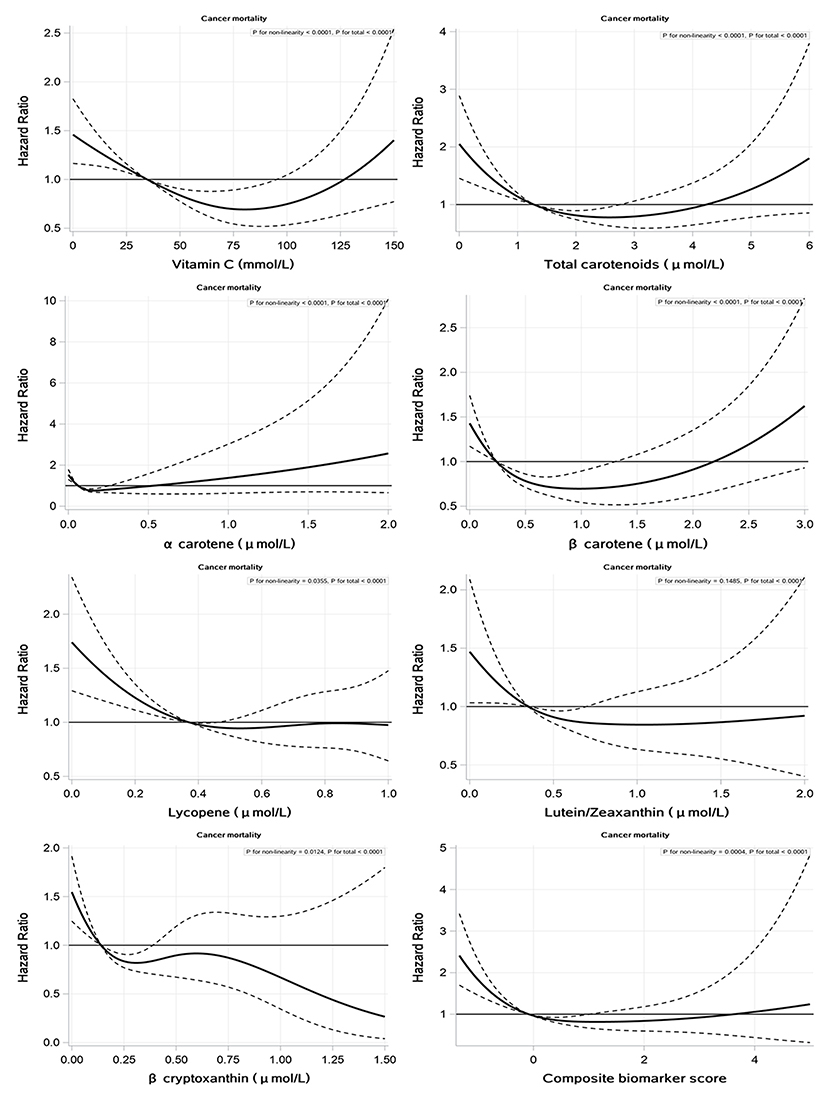
Figure 2. Associations of serum vitamin C, carotenoids, and composite biomarker scores with cancer mortality.
Sensitivity Analysis
The results did not change after excluding participants recruited within the first 2 years and the first 4 years and those with baseline cancer or CVD (Supplementary Table 3). The results also remain unchanged after further adjustments for hypertension, diabetes, and a history of cancer or CVD (Supplementary Table 3). Moreover, excluding one biomarker at a time to amend the composite biomarker score did not change the results (Supplementary Table 4). Furthermore, we calculated the E–value to evaluate the effect of unmeasured confounding between serum vitamin C concentration (E–value for each one SD, 1.81) and composite biomarker score (E–value for each one SD, 1.85) and cancer mortality. E–values for each one SD change in serum vitamin C concentration, total carotenoid concentration, and composite biomarker score with all–cause mortality were 1.43, 1.50, and 1.60, respectively (Supplementary Table 5).
Discussion
In this prospective study, we found that inverse association between each one SD increases in serum vitamin C and cancer and all–cause mortality. Both the total carotenoids and the five individual carotenoids, expect for serum lutein/zeaxanthin, were negatively associated with all–cause mortality, whereas serum alpha–carotene, lycopene, and beta–cryptoxanthin concentrations were negatively associated with cancer mortality. Moreover, composite biomarker score was inversely associated with cancer mortality and all–cause mortality. The change for each one SD in the composite biomarker score, which is equivalent to an increase of approximately one time per quarter (0.483 times/month) in fruits and vegetables intake, the HR of cancer mortality was reduced by 21%. The relationships were not statistically significant between each one SD increase in serum vitamin C, carotenoids and their composite biomarker score and CVD, heart disease, and cerebral mortality.
A British study also shown an inverse association between serum vitamin C concentration and cancer mortality in older people (21). Vitamin C is a dietary antioxidant that can protect against oxidative stress and the harmful effects of reactive oxygen species (22). Consistent with our results, a meta–analysis showed a negative association between serum carotenoid concentration and cancer mortality (23). A positive association between the number of times per month that total fruits and vegetables were consumed and serum vitamin C was also observed in this study. Carotenoids are sources of various immune–protective substances and they are reported to have the protective effects on human health (24). Although studies of the individual carotenoids have given inconsistent results (6, 25). Serum carotenoids are powerful antioxidants that are important for the immune system (26). One of the most plentiful dietary carotenoids, alpha–carotene, inhibit the proliferation of human cancer cells (27). Similarly, a negative association between alpha–carotene levels and cancer mortality was found in this study. Previous studies have shown that root vegetable and carrot intake is a good proxy for serum alpha–carotene concentration (28). All of the above evidence indicates that strengthening the consumption of fruit and vegetable intake is beneficial to prevent cancer mortality.
In previous study of Americans, the relationship between vitamin C and all–cause mortality was consistent with this study (29). This study further explored the relationship between total carotenoids and individual carotenoids and all–cause mortality, as serum carotenoid levels are thought to reflect the intake of vegetables and fruits in an individual's diet (28, 30). Previous studies have found a negative association between serum total carotenoid, alpha–carotene, beta–carotene, lycopene, lutein/zeaxanthin, and beta–cryptoxanthin and all–cause mortality (24, 31, 32). Lycopene, a non–provitamin A carotenoid, has been shown to have direct anti–inflammatory effects (33, 34). Beta–carotene is a provitamin A carotenoid that contributes indirectly to the biological functions of vitamin A in human body and has been shown to have protective effects against chronic disease (35, 36).
We found that the composite biomarker score was negatively associated with cancer mortality and all–cause mortality, but it was not statistically significant with cerebral disease, heart disease, and CVD mortality. However, the association between participants with excessive composite biomarker score levels and cancer and all–cause mortality is viewed with caution, as a null effect of increased risk can be seen in the restricted cubic splines. The composite biomarker score allowed us to analyze individual serum biomarkers of fruit and vegetable intake using a minimum number of variables (37). A previous randomized controlled trial also found that a combined biomarker approach may be better than a single biomarker at determining the effect of a mixed fruit and vegetable diet (38). Also, the WHO encourages an increase intake of fruits and vegetables and recommends at least five servings (~400 g) of fruit or vegetables per day (39). Therefore, our findings support the intake of fruits and vegetables together.
The strengths of this study include the analysis of objective serum biomarkers of fruit and vegetable intake (serum vitamin C and carotenoid concentrations and their composite biomarker scores) with heart, CVD, cerebral, cancer, and all–cause mortality for the first time in a nationally representative sample of US population. Furthermore, we performed a number of sensitivity analyses, which showed robust findings, by excluding participants or adjusting for additional variables. Meanwhile, we quantified the potential effects of unmeasured confounders by the E–value sensitivity analysis and found that the results were unlikely affected by unmeasured confounders. However, this study also has some limitations. First, we were not able to classify the specific types of cancer due to limitations of the available data. Second, this study only analyzed serum biomarkers of fruit and vegetable intake in the general US population, which may limit the applicability of these findings to other populations. Finally, causality cannot be inferred because this study used an observational design.
Conclusion
In this prospective study, inverse associations were found between serum vitamin C, carotenoids, and composite biomarker scores and outcomes expect for CVD, heart disease, and cerebral mortality in US adults. Our results indicate that the dietary intake of fruits and vegetables is crucial to promote public health, as it reduces the risk of all–cause mortality and cancer mortality.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: the Third National Health and Nutrition Examination Survey, [NHANES III (1988–1994) (cdc.gov).
Ethics Statement
The studies involving human participants were reviewed and approved by the National Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LP: software, validation, and writing—original draft preparation. RZ: visualization and writing—reviewing. XW: supervision and writing—reviewing and editing. TZ: data curation and writing—reviewing. HS: methodology, software, and writing—reviewing and editing. LH: conceptualization, funding acquisition, and writing—reviewing and editing. The first draft of the manuscript was written by LP and all the authors commented on previous versions of the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
The study is supported by grants from the National Natural Science Foundation of China (Grant 82173648), Internal Fund of Ningbo Institute of Life and Health Industry, University of Chinese Academy of Sciences (2020YJY0212), the Public Welfare Foundation of Ningbo (2021S108), the Innovative Talent Support Plan of the Medical and Health Technology Project in Zhejiang province (2021422878), the Zhejiang Provincial Public Service and Application Research Foundation (LGF20H250001 and GC22H264267), Ningbo Health Branding Subject Fund (PPXK2018-01), and Sanming Project of Medicine in Shenzhen (SZSM201803080). The funders had no role in the design of the study or in the collection, analysis, and interpretation of data or in writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.874943/full#supplementary-material
References
1. Global regional and national age-sex specific all-cause and cause-specific mortality for 240 causes of death 1990-2013: 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. (2015) 385:117–71. doi: 10.1016/S0140-6736(14)61682-2
2. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. (2014) 349:g4490. doi: 10.1136/bmj.g4490
3. Aune D, Chan DSM, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, et al. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. (2012) 96:356–73. doi: 10.3945/ajcn.112.034165
4. Pennant M, Steur M, Moore C, Butterworth A, Johnson L. Comparative validity of vitamin C and carotenoids as indicators of fruit and vegetable intake: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. (2015) 114:1331–40. doi: 10.1017/S0007114515003165
5. Baldrick FR, Woodside JV, Elborn JS, Young IS, McKinley MC. Biomarkers of fruit and vegetable intake in human intervention studies: a systematic review. Crit Rev Food Sci Nutr. (2011) 51:795–815. doi: 10.1080/10408398.2010.482217
6. Hofe CR, Feng L, Zephyr D, Stromberg AJ, Hennig B, Gaetke LM. Fruit and vegetable intake, as reflected by serum carotenoid concentrations, predicts reduced probability of polychlorinated biphenyl-associated risk for type 2 diabetes: national health and Nutrition examination survey 2003–2004. Nutr Res. (2014) 34:285–93. doi: 10.1016/j.nutres.2014.02.001
7. Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary and circulating vitamin C, vitamin E, β-carotene and risk of total cardiovascular mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutr. (2019) 22:1872–87. doi: 10.1017/S1368980018003725
8. Fletcher AE, Breeze E, Shetty PS. Antioxidant vitamins and mortality in older persons: findings from the nutrition add-on study to the medical research council trial of assessment and management of older people in the community. Am J Clin Nutr. (2003) 78:999–1010. doi: 10.1093/ajcn/78.5.999
9. Third National Health and Nutrition Examination Survey (NHANES III), 1988–94 lab-acc.pdf (cdc.gov). Available online at: https://wwwn.cdc.gov/nchs/data/nhanes3/1a/lab-acc.pdf (accessed December 11, 2021).
10. Plan and operation of the Third National Health and Nutrition Examination Survey 1988-94. series 1: programs and collection procedures. Vital Health Stat 1. (1994) 1–407.
11. National Center for Health Statistics. Office of Analysis and Epidemiology, Public-use Linked Mortality File. (2015). Hyattsville, Maryland. Available online at: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm
12. National Center for Health Statistics Office of Analysis Epidemiology. National Death Index. Available online at: https://www.cdc.gov/nchs/ndi/index.htm (accessed October 31, 2018).
13. National Center for Health Statistics. Office of Analysis and Epidemiology, Public-use Linked Mortality File. (2015). Hyattsville, Maryland. Available online at: https://www.cdc.gov/nchs/data/datalinkage/backeding_and_multiple_cause_of_death_codes.pdf
14. Han L, You D, Ma W, Astell-Burt T, Feng X, Duan S, et al. National trends in American heart association revised life's simple 7 metrics associated with risk of mortality among US adults. JAMA Netw open. (2019) 2:e1913131. doi: 10.1001/jamanetworkopen.2019.13131
15. Third National Health and Nutrition Examination Survey (NHANES III), 1988–94 EXAMDR-acc.pdf (cdc.gov). Available online at: https://wwwn.cdc.gov/nchs/data/nhanes3/2a/EXAMDR-acc.pdf (accessed December 11, 2021).
16. Zheng J-S, Sharp SJ, Imamura F, Chowdhury R, Gundersen TE, Steur M, et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ. (2020) 370:m2194. doi: 10.1136/bmj.m2194
17. McEvoy JW, Daya N, Rahman F, Hoogeveen RC, Blumenthal RS, Shah AM, et al. Association of isolated diastolic hypertension as defined by the 2017 ACC/AHA blood pressure guideline with incident cardiovascular outcomes. JAMA. (2020) 323:329–38. doi: 10.1001/jama.2019.21402
18. Avilés-Santa ML, Hsu LL, Arredondo M, Menke A, Werner E, Thyagarajan B, et al. Differences in hemoglobin A1c between hispanics/latinos and non-hispanic whites: an analysis of the hispanic community health study/study of latinos and the 2007–2012 national health and nutrition examination survey. Diabetes Care. (2016) 39:1010–7. doi: 10.2337/dc15-2579
19. Cooper AJM, Sharp SJ, Luben RN, Khaw K-T, Wareham NJ, Forouhi NG. The association between a biomarker score for fruit and vegetable intake and incident type 2 diabetes: the EPIC-Norfolk study. Eur J Clin Nutr. (2015) 69:449–54. doi: 10.1038/ejcn.2014.246
20. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA. (2019) 321:602–3. doi: 10.1001/jama.2018.21554
21. Bates CJ, Hamer M, Mishra GD. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the national diet and nutrition survey of people aged 65 years and over. Br J Nutr. (2011) 105:123–32. doi: 10.1017/S0007114510003053
22. Halliwell B, Murcia MA, Chirico S, Aruoma OI. Free radicals and antioxidants in food and in vivo: what they do and how they work. Crit Rev Food Sci Nutr. (1995) 35:7–20. doi: 10.1080/10408399509527682
23. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr. (2018) 108:1069–91. doi: 10.1093/ajcn/nqy097
24. Fujii R, Tsuboi Y, Maeda K, Ishihara Y, Suzuki K. Analysis of repeated measurements of serum carotenoid levels and all-cause and cause-specific mortality in Japan. JAMA Netw open. (2021) 4:e2113369. doi: 10.1001/jamanetworkopen.2021.13369
25. Macready AL, George TW, Chong MF, Alimbetov DS, Jin Y, Vidal A, et al. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease–FLAVURS: a randomized controlled trial. Am J Clin Nutr. (2014) 99:479–89. doi: 10.3945/ajcn.113.074237
26. Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. (2006) 50:85–94. doi: 10.1159/000090495
27. Murakoshi M, Takayasu J, Kimura O, Kohmura E, Nishino H, Iwashima A, et al. Inhibitory effects of alpha-carotene on proliferation of the human neuroblastoma cell line GOTO. J Natl Cancer Inst. (1989) 81:1649–52. doi: 10.1093/jnci/81.21.1649
28. Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr. (2005) 59:1387–96. doi: 10.1038/sj.ejcn.1602252
29. Goyal A, Terry MB. Siegel AB. Serum antioxidant nutrients, vitamin A, and mortality in US Adults Cancer. Cancer Epidemiol Biomarkers Prev. (2013) 22:2202–11. doi: 10.1158/1055-9965.EPI-13-0381
30. Jenab M, Riboli E, Ferrari P, Friesen M, Sabate J, Norat T, et al. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. Br J Cancer. (2006) 95:406–15. doi: 10.1038/sj.bjc.6603266
31. Akbaraly TN, Favier A, Berr C. Total plasma carotenoids and mortality in the elderly: results of the Epidemiology of Vascular Ageing (EVA) study. Br J Nutr. (2009) 101:86–92. doi: 10.1017/S0007114508998445
32. Huang J, Weinstein SJ Yu K, Männistö S, Albanes D. Serum beta carotene and overall and cause-specific mortality. Circ Res. (2018) 123:1339–49. doi: 10.1161/CIRCRESAHA.118.313409
33. Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Adv Nutr. (2018) 9:701–16. doi: 10.1093/advances/nmy040
34. Cha JH, Kim WK, Ha AW, Kim MH, Chang MJ. Anti-Inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr Res Pract. (2017) 11:90–6. doi: 10.4162/nrp.2017.11.2.90
35. Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. (2012) 96:1204S–6S. doi: 10.3945/ajcn.112.034868
36. Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. (2004) 44:275–95. doi: 10.1080/10408690490468489
37. Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, et al. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr. (2014) 112:1341–52. doi: 10.1017/S0007114514001962
38. McGrath AJ, Hamill LL, Cardwell CR, Draffin CR, Neville CE, Appleton KM, et al. Combining vitamin C and carotenoid biomarkers better predicts fruit and vegetable intake than individual biomarkers in dietary intervention studies. Eur J Nutr. (2016) 55:1377–88. doi: 10.1007/s00394-015-0953-7
39. World Health Organization. Fruit and Vegetables for Health. Report of the Joint FAO/WHO Workshop on Fruit and Vegetables for Health. Kobe, Japan: World Health Organization (2004). Available online at: https://apps.who.int/iris/handle/10665/43143
Keywords: fruit, vegetables, biomarkers, vitamin C, cancer mortality, all–cause mortality
Citation: Pu L, Zhang R, Wang X, Zhao T, Sun H and Han L (2022) Associations of Serum Biomarkers of Fruit and Vegetable Intake With the Risk of Cause–Specific Mortality and All–Cause Mortality: A National Prospective Cohort Study. Front. Nutr. 9:874943. doi: 10.3389/fnut.2022.874943
Received: 13 February 2022; Accepted: 06 April 2022;
Published: 11 May 2022.
Edited by:
Manfred Eggersdorfer, University of Groningen, NetherlandsReviewed by:
Meng Wang, Zhejiang Center for Disease Control and Prevention (Zhejiang CDC), ChinaJie Li, Guangdong Provincial People's Hospital, China
Copyright © 2022 Pu, Zhang, Wang, Zhao, Sun and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongpeng Sun, aHBzdW5Ac3VkYS5lZHUuY24=; Liyuan Han, aGFubGl5dWFuQHVjYXMuYWMuY24=
†These authors have contributed equally to this work and share last authorship
 Liyuan Pu1,2
Liyuan Pu1,2 Hongpeng Sun
Hongpeng Sun Liyuan Han
Liyuan Han